Malaria vaccine and preparation method thereof
A technology of microbial strains and recombinant baculovirus, applied in the field of biomedicine, can solve the problems of high transmission density of malaria, long half-life of drugs, insufficient drug dosage, etc., and achieve the effect of high application value, less pyrogenicity, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
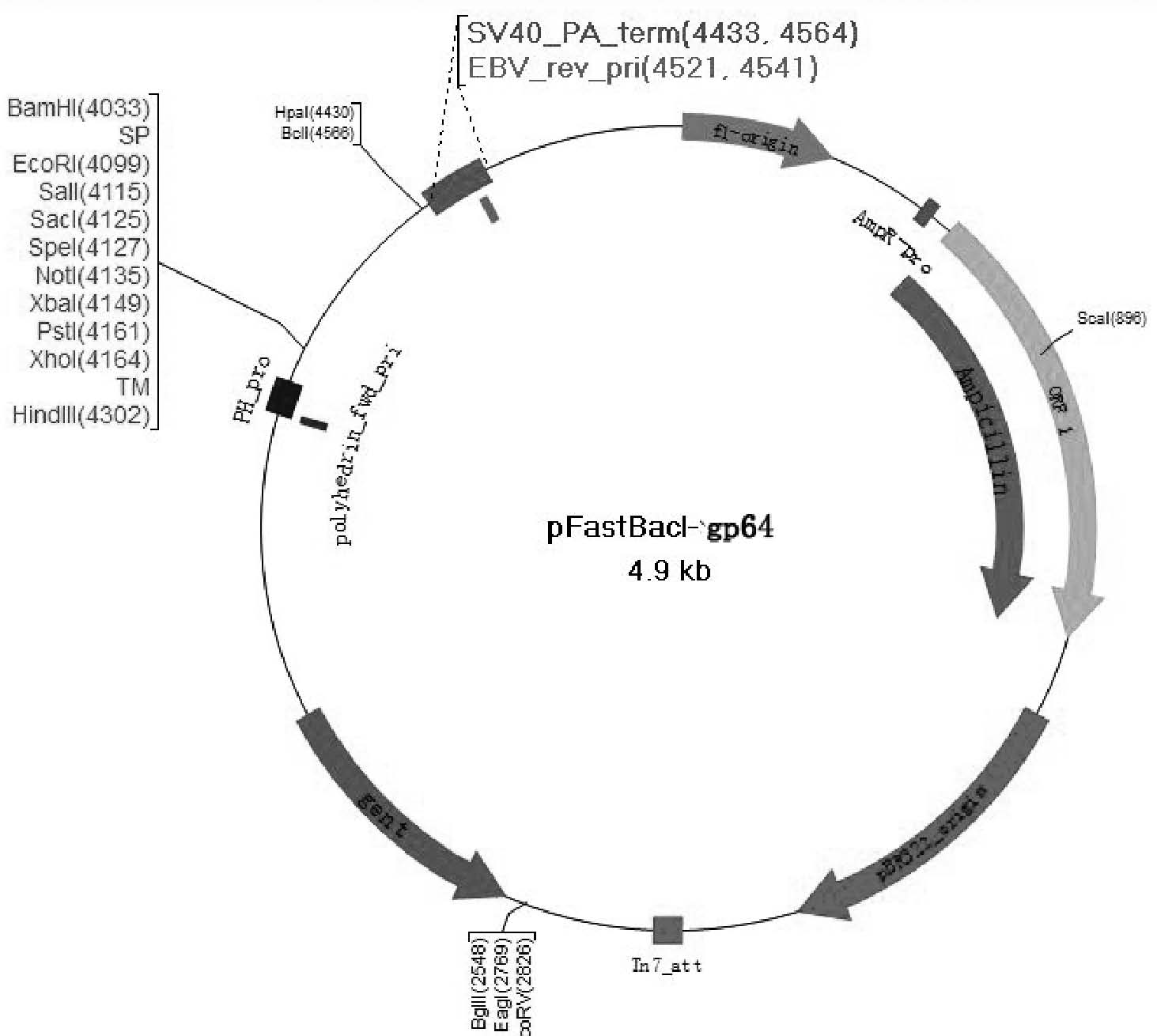
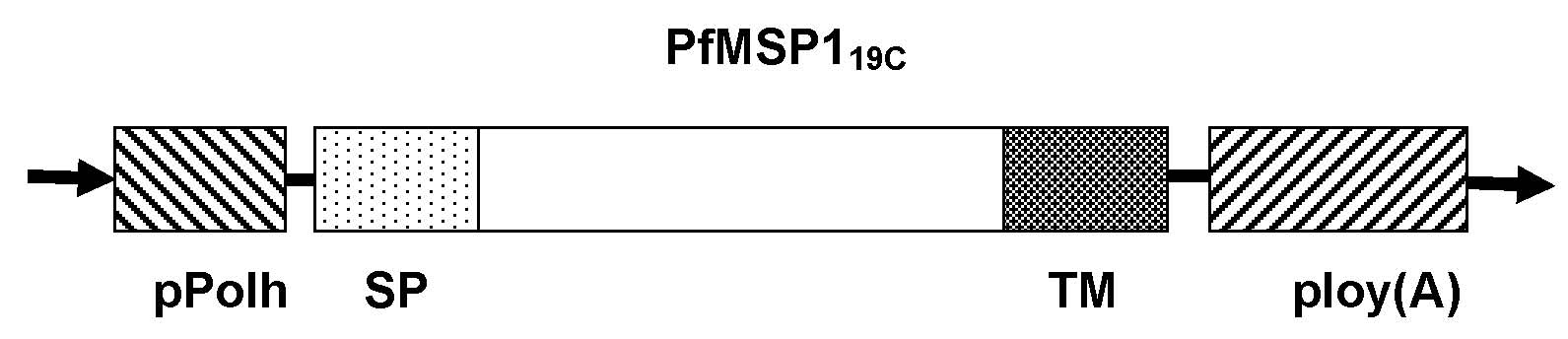
[0041] Example 1: Recombinant transfer plasmid pFastBacI-gp64-MSP1 19c build
[0042] Using the baculovirus gp64 sequence as a template, primers P1 (as shown in SEQ ID NO: 1), P2 (as shown in SEQ ID NO: 2), P3 (as shown in SEQ ID NO: 3), and P4 ( As shown in SEQ ID NO: 4), the signal peptide (SP) sequence and the transmembrane region sequence (TM) of gp64 were amplified by PCR, and the PCR product was passed through Bam H I. EcoR I and xho I. Hind III (both purchased from Fermentas Company) were double digested and inserted into the upstream and downstream ends of the multiple cloning site of the pFastBacI vector to construct the display vector pFstBacI-gp64. Using Plasmodium falciparum 3D7 standard strain cDNA (gifted by Professor Yang Zhaoqing, Kunming Academy of Sciences, Yunnan) as a template, PCR amplification of malignant The DNA sequence of the 19kD antigenic site of the Plasmodium merozoite surface membrane protein 1 C-terminus, the PCR product passed EcoR ...
Embodiment 2
[0075] Embodiment 2: Bombyx mori recombinant baculovirus BmNPV-gp64-MSP1 19c acquisition and expansion of
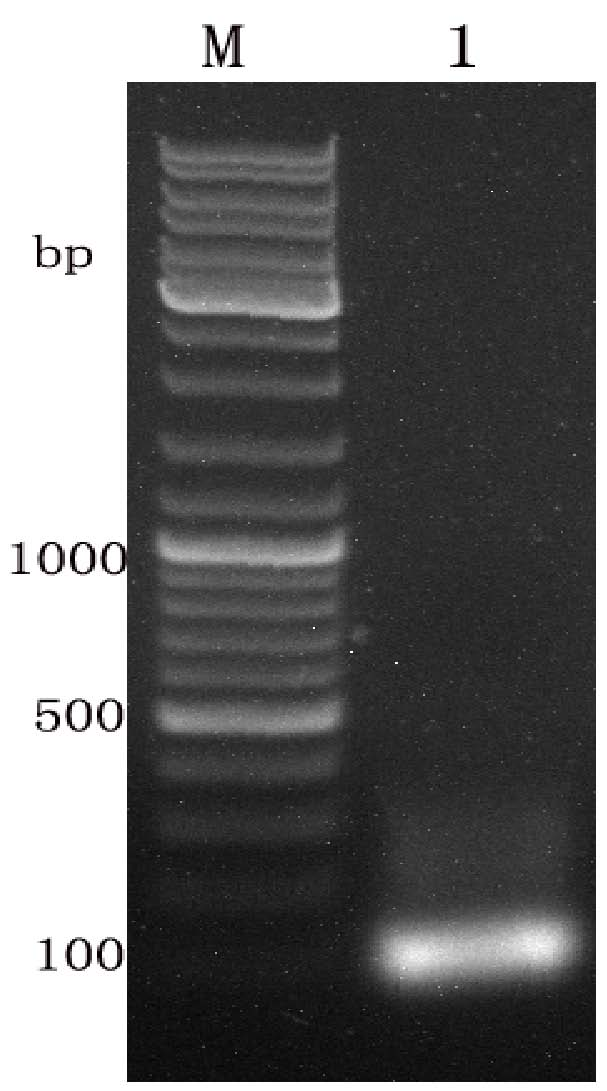
[0076]Escherichia coli DH10Bac competent cells (purchased from Invitrogen) were successfully identified by recombinant pFastBacI-gp64-MSP119c, in the presence of kanamycin (Kan), gentamycin (Gen), tetracycline (Tet), X-gal and IPTG (isopropylthio-β-D-galactoside) LB culture plate was used for blue and white spot screening, and the white spot was picked after 48 hours of dark culture, and the genome was extracted with isopropanol after 24 hours of culture, and the M13 universal primer was used and MSP119c primers for PCR identification, the results showed that specific bands appeared between 2500bp~3000bp and 400bp~500bp respectively (see Figure 7 ).
[0077] The successfully identified recombinant virus genome was transfected into silkworm BmN cells by liposome-mediated method (refer to the Invitrogen company's liposome transfection reagent Cellfectin Ⅱ Reagent manual...
Embodiment 3
[0078] Embodiment 3: Bombyx mori recombinant baculovirus BmNPV-gp64-MSP1 19c Expression and identification in silkworm chrysalis
[0079] Select the silkworm chrysalis on the 6th to 9th day after clustering (the first-generation hybrid Jingsong × Haoyue, produced by Zhejiang Zhongqi Biological Pharmaceutical Co., Ltd.), and remove necrotic, septic, and bad silkworm chrysalis to reduce the number of silkworm chrysalis after injection. Bacterial infection rate, after disinfection with 70% infiltration alcohol, inoculate 1~5 μl of the fourth-generation recombinant virus obtained in Example 3 on the intersegmental membrane, place it in an artificial climate box after injection, and protect it at 25°C for 4-5 days, let The recombinant virus was fully propagated, and then the infected silkworm chrysalis were collected, and some of them were used for detection, and the rest were stored at -80°C for later use.
[0080] 10 g of the above-mentioned diseased silkworm chrysalis were resp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com