Nasal influenza vaccine composition
A technology of influenza vaccine and composition, applied in the direction of drug combination, microorganism, drug delivery, etc., to achieve the effects of small side effects, long-term distribution, and induction of immune response
Inactive Publication Date: 2019-07-23
JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES +1
View PDF5 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0008] As mentioned above, it is urgently desired to develop and put into practical use a nasal administration type vaccine as a next-generation influenza vaccine based on existing influenza vaccines administered subcutaneously or intramuscularly. There are various problems in the face of practical application, such as the toxicity of the adjuvant
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0064]
Embodiment 2
[0066]
Embodiment 3
[0068]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Login to View More
Abstract
The present invention relates to a nasal mucosal spray delivery type influenza vaccine composition which is characterized in that the composition comprises a gel base including an influenza virus inactivated whole particle antigen and a carboxyl vinyl polymer but does not comprise an adjuvant.
Description
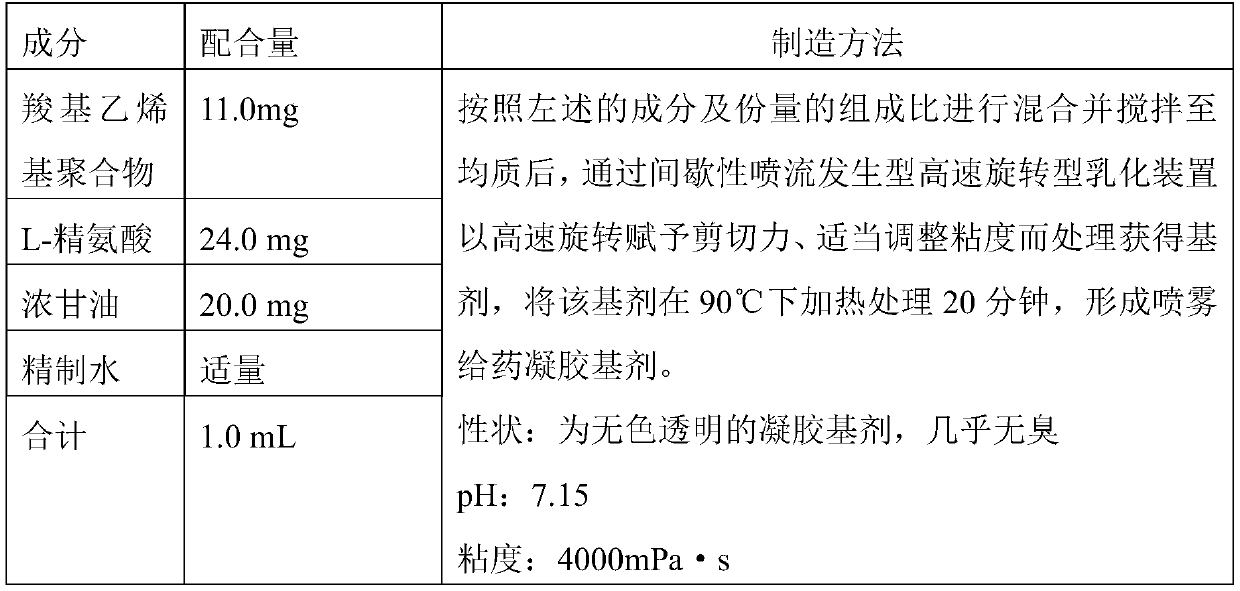
[0001] This application is a divisional application of the original application of the PCT international application PCT / JP2013 / 078721 with the international filing date of October 23, 2013, which entered the Chinese national phase with the application number 201380068031.4 and the invention name "nasal influenza vaccine composition" . technical field [0002] The invention relates to a nasal mucosa spray administration type influenza vaccine composition. Background technique [0003] Influenza is an acute respiratory infectious disease caused by influenza virus, especially in winter, which is prevalent every year. In addition, sometimes it becomes a worldwide pandemic, and there are many cases where the disease becomes severe and leads to death. Influenza is known to have a certain preventive effect through influenza vaccination, and the vaccination is widely performed before the epidemic period. [0004] In Japan, influenza vaccines are approved only for subcutaneous adm...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K39/145A61K9/06A61K47/32A61P31/16
CPCA61K9/06A61K47/32A61K9/0043A61K2039/543C12N2760/16134A61K2039/5252A61K2039/55555C12N2760/16234A61K39/12A61K39/145A61P31/00A61P31/16A61P37/04A61K47/30C12N7/00C12N2760/16121
Inventor 长谷川秀树铃木忠树相内章上下泰造宫崎隆
Owner JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com