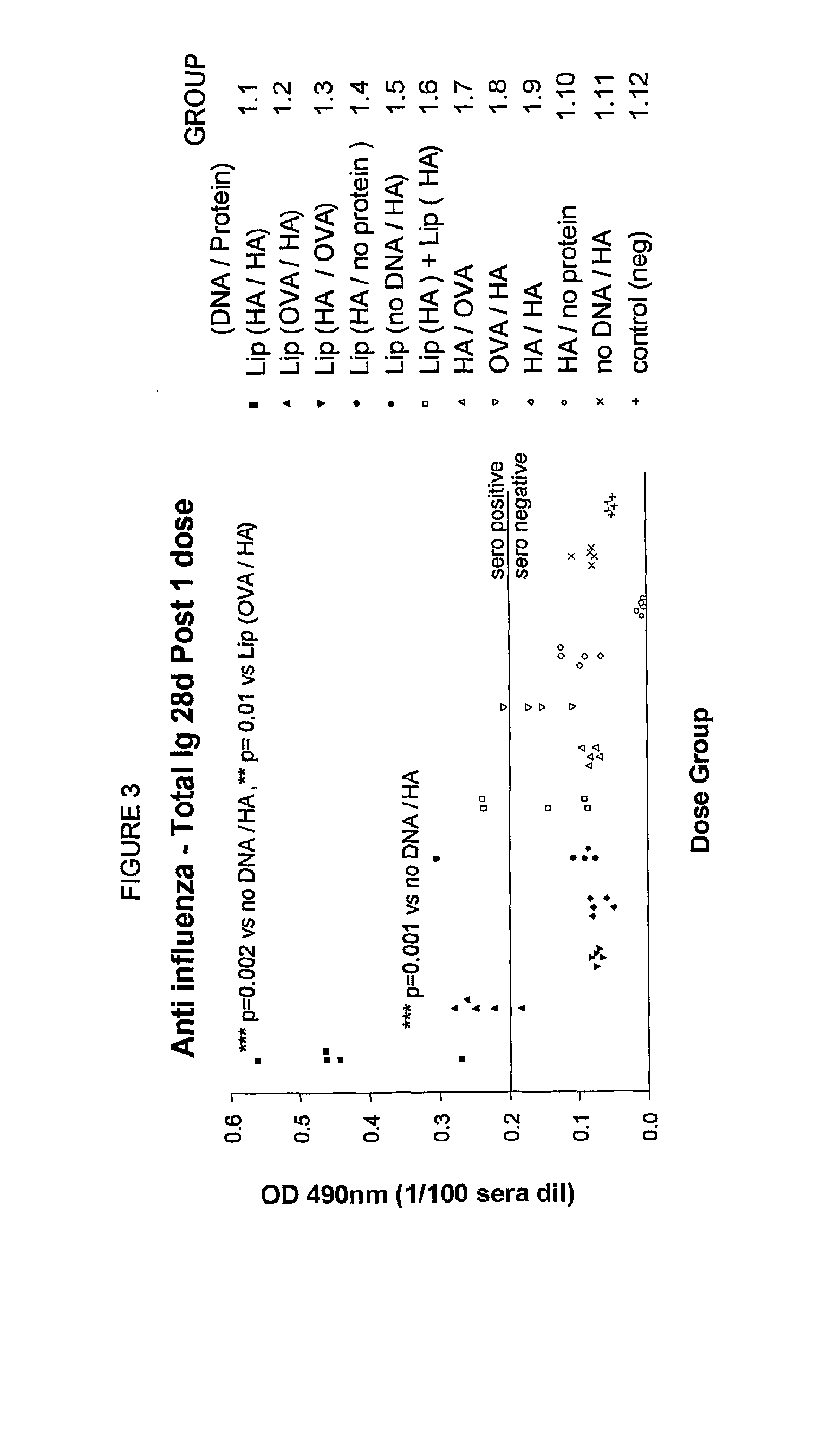
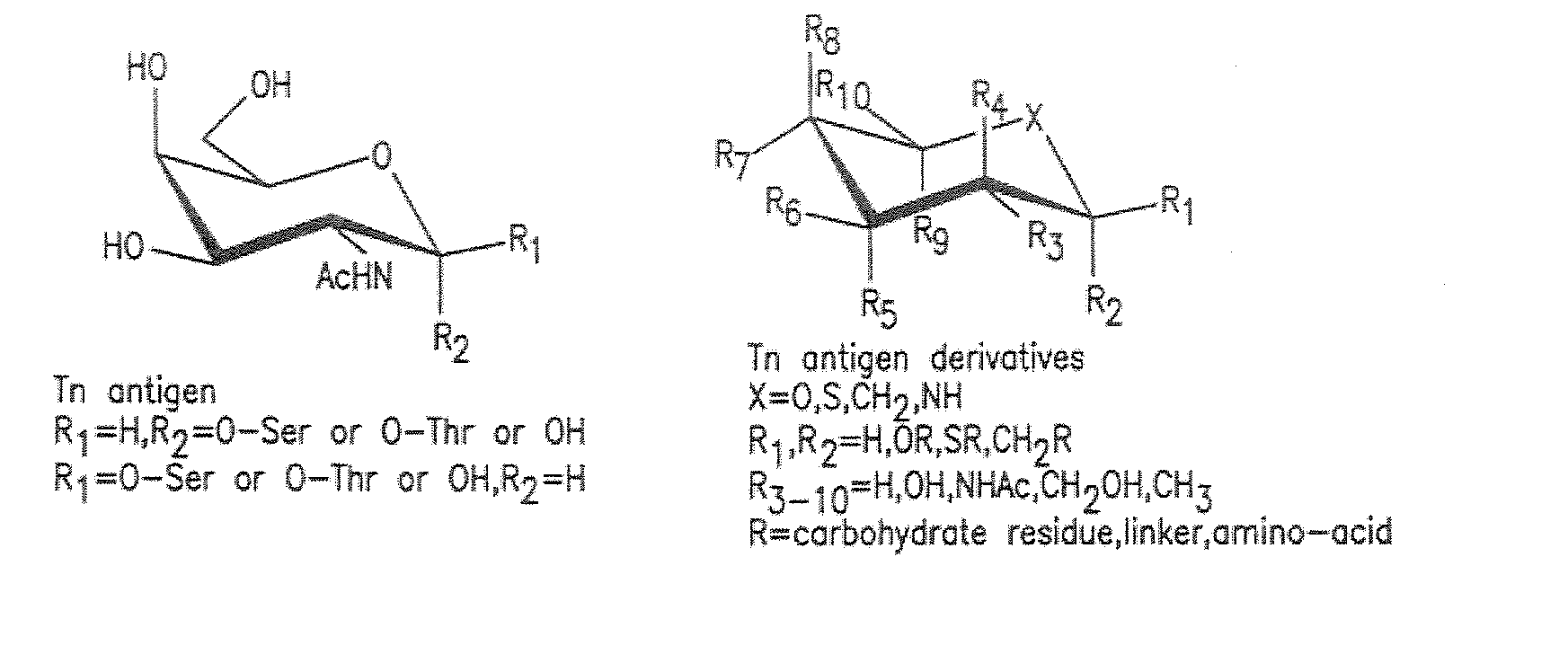
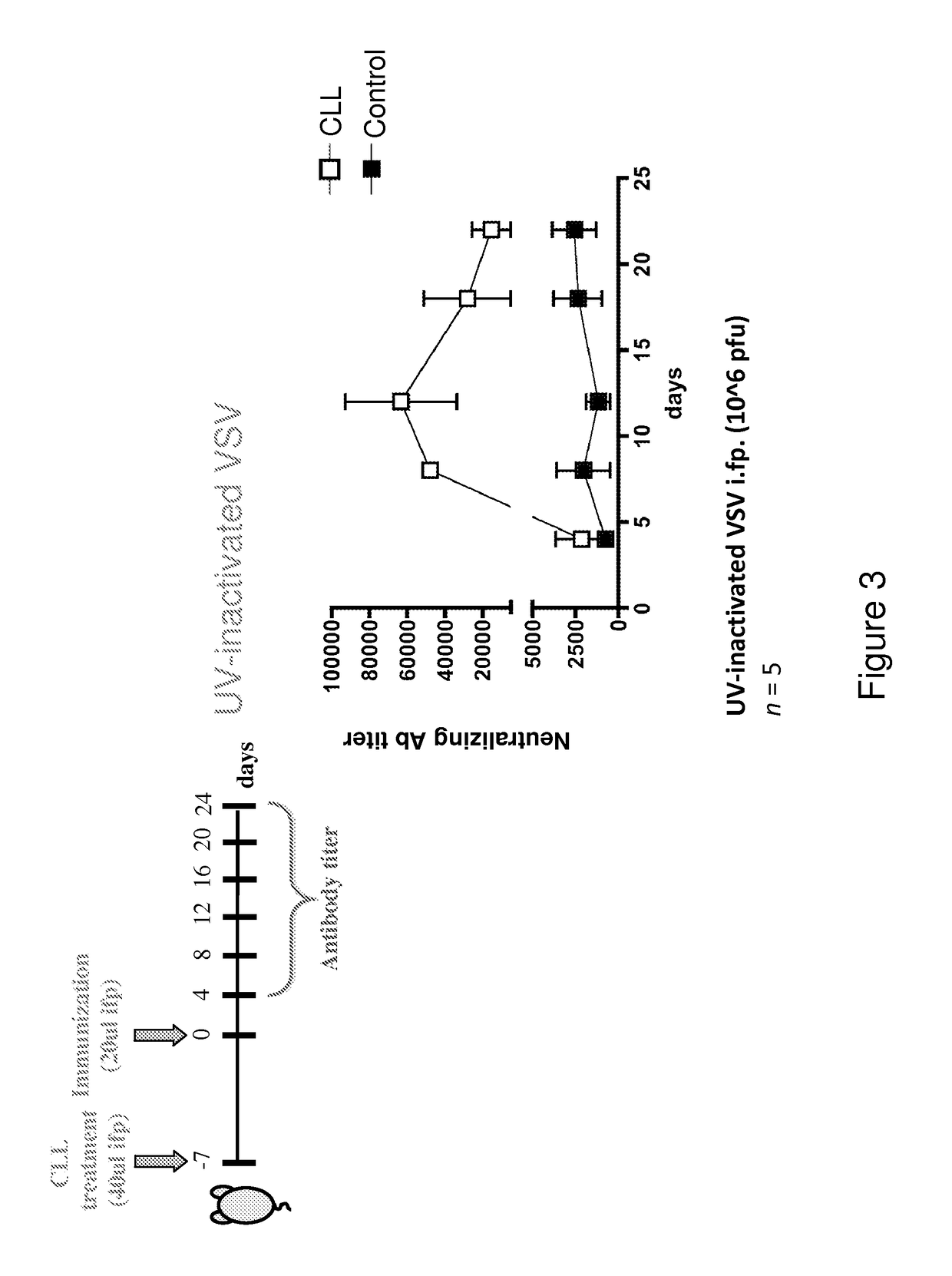
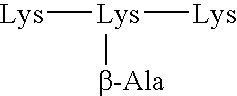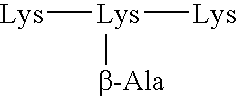Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49results about How to "Enhance antibody response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
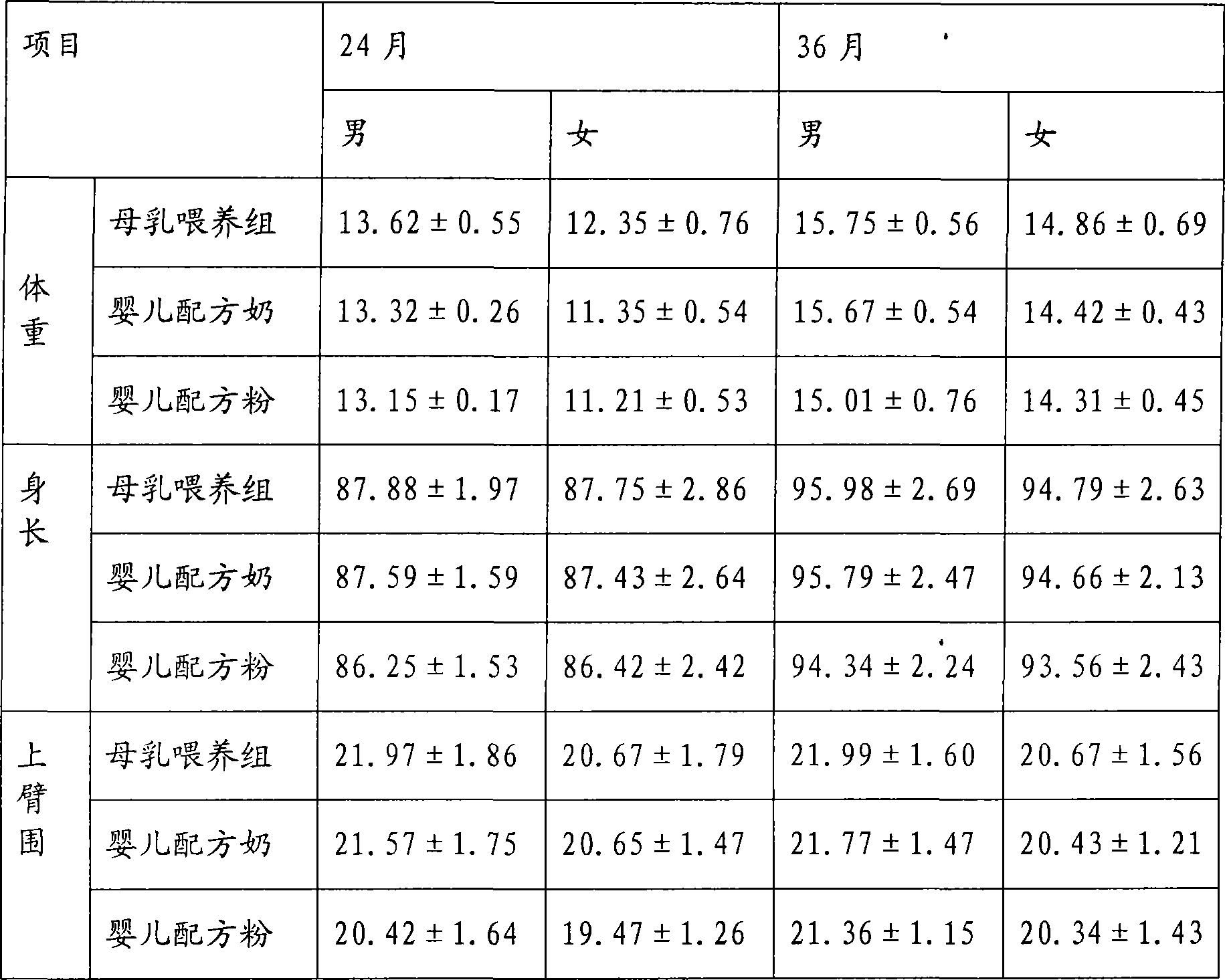
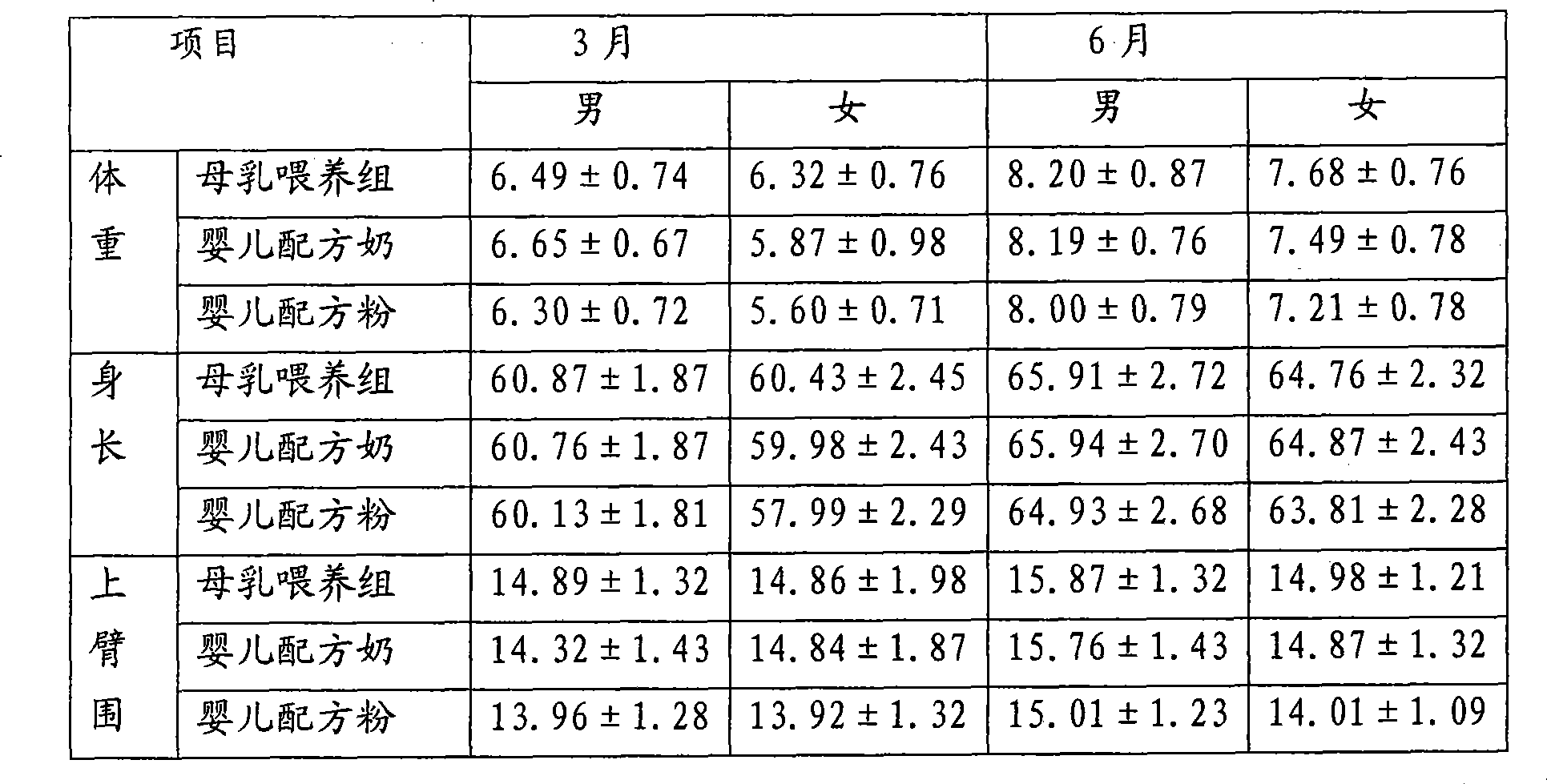
Infant Formula
InactiveUS20080125346A1Good effectIncreased proliferationAntibacterial agentsOrganic active ingredientsGlycanPolyphosphate
The present invention relates to a nutritional composition containing protein, fat and carbohydrate; and a. a nucleotide component selected from the group consisting of nucleic acid, nucleic acid derivatives, nucleotides, nucleoside polyphosphates, polynucleotides, nucleosides, ribose, desoxyribose, and dinucleosidpolyphosphates (NpxN); and b. a non-proteinaceous negatively charged, glycan or glycoconjugate component with a molecular weight between 200 and 20.000 dalton. The present nutritional composition is particularly suited for feeding infants as it mimics the protective effects of human milk, in particular against allergies and infections.
Owner:NUTRICIA
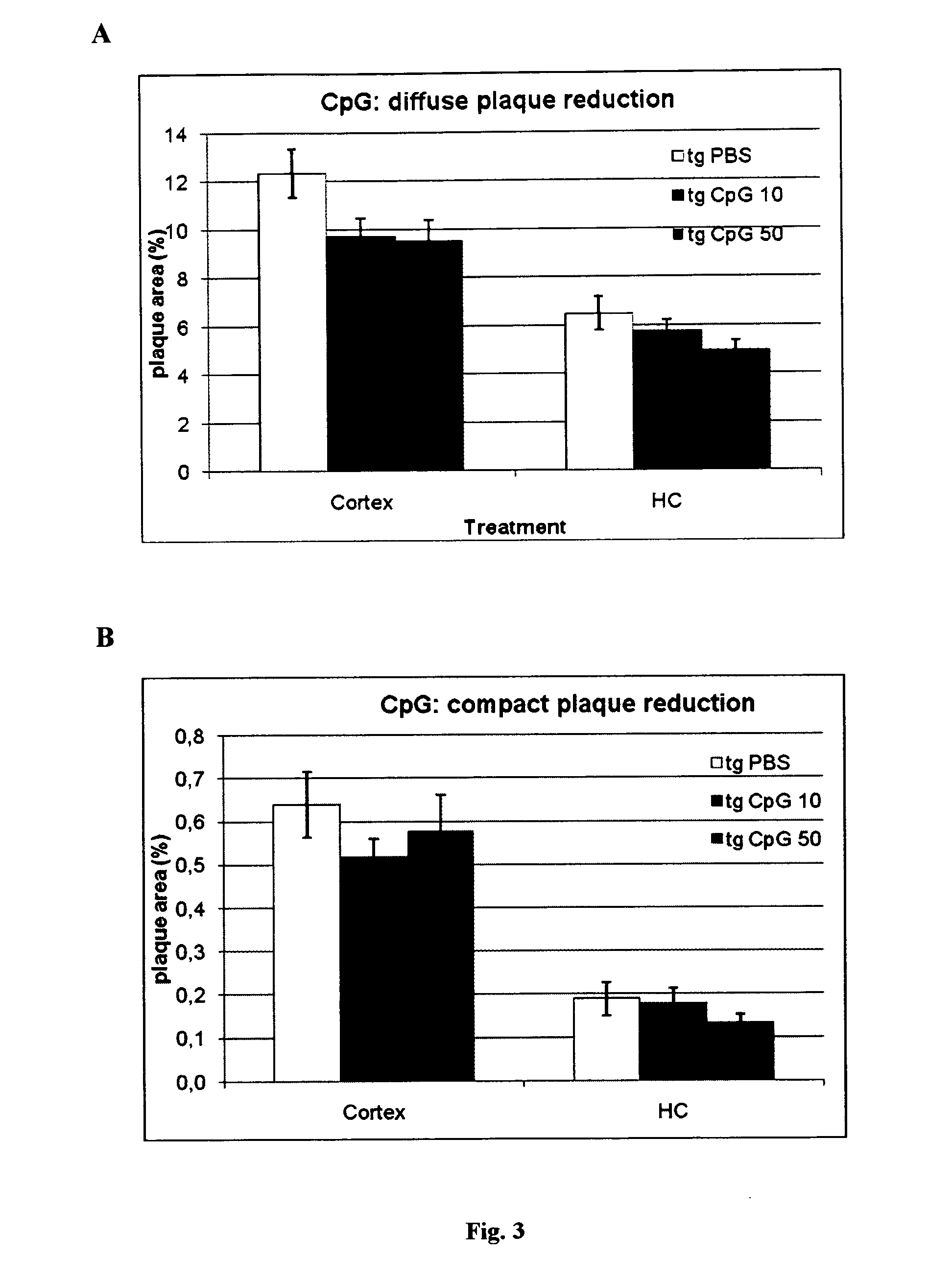
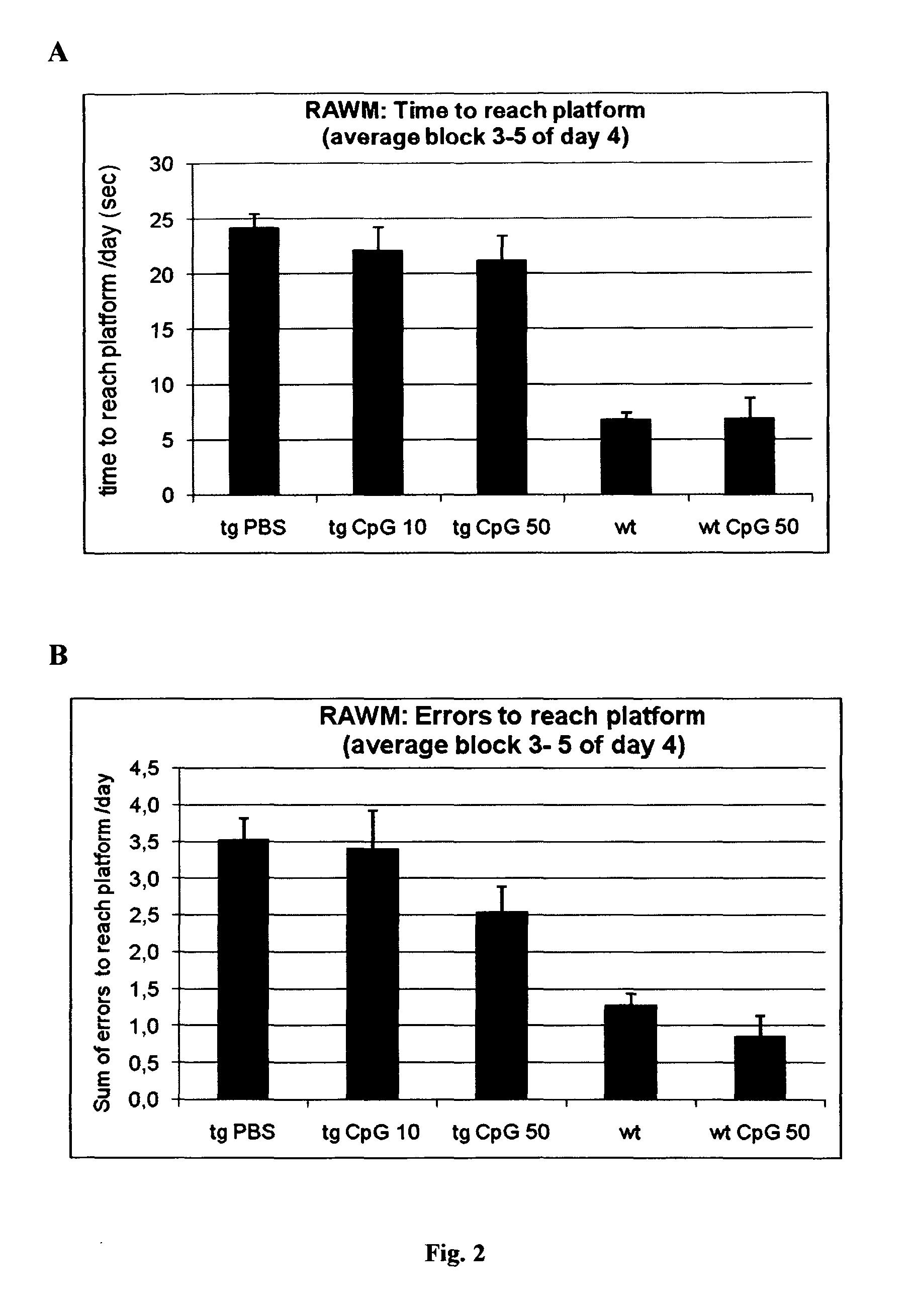
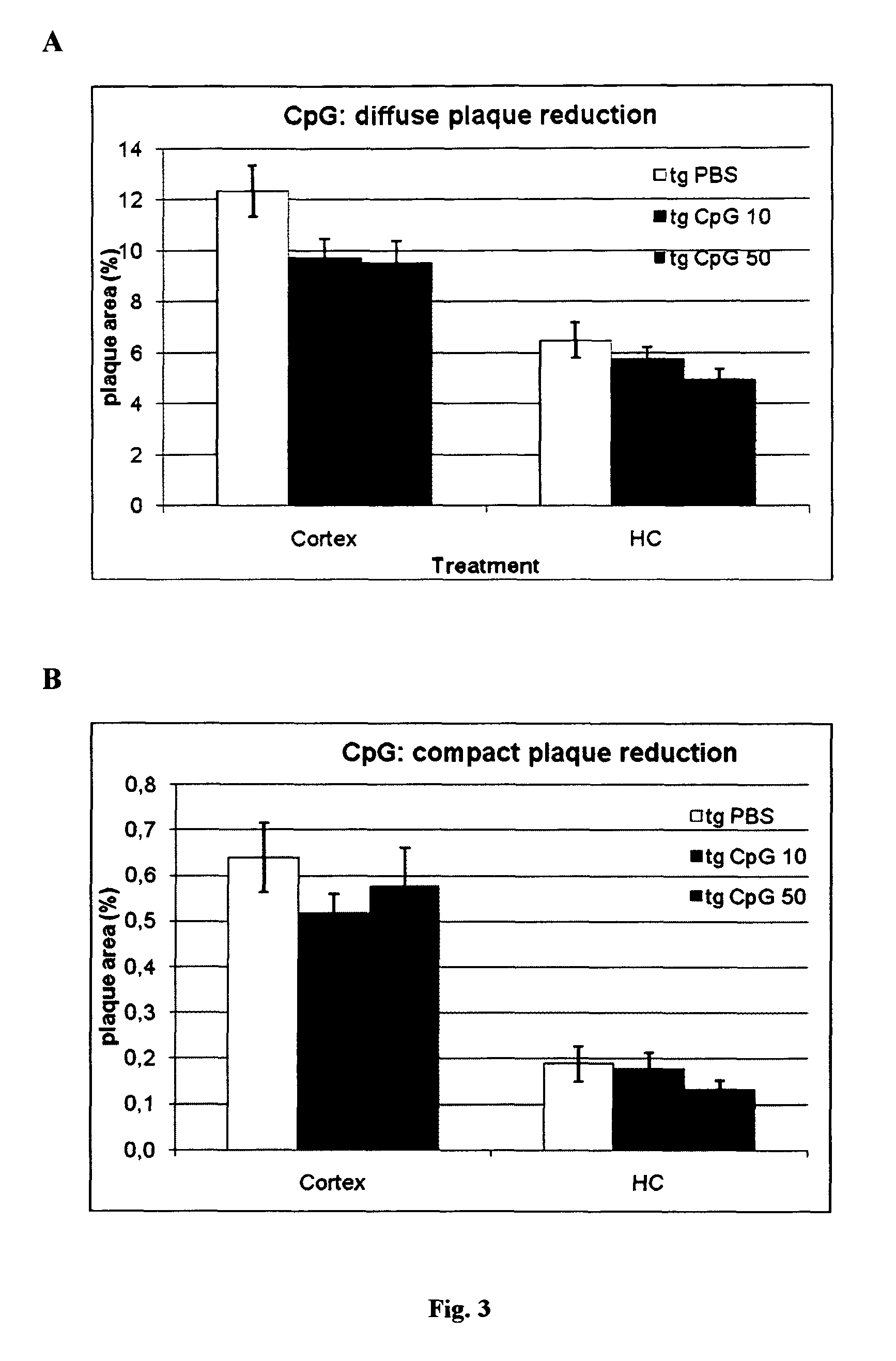
Method of providing patient specific immune response in amyloidoses and protein aggregation disorders
ActiveUS20100297108A1Increase heightFavor selective antibody responseOrganic active ingredientsSenses disorderCell AggregationsSpecific immunity
A novel treatment of Alzheimer's disease and other disorders involving protein misfolding or aggregation is provided by enhancing or sustaining an antibody response against predominantly directed against pathological protein aggregates or neo-epitopes present on pathogenic forms of said protein or protein complex. Furthermore, therapeutic methods are also described, wherein ex vivo stimulated antigen-selected peripheral blood lymphocytes are regrafted into the cognate donor.
Owner:NEW YORK UNIV
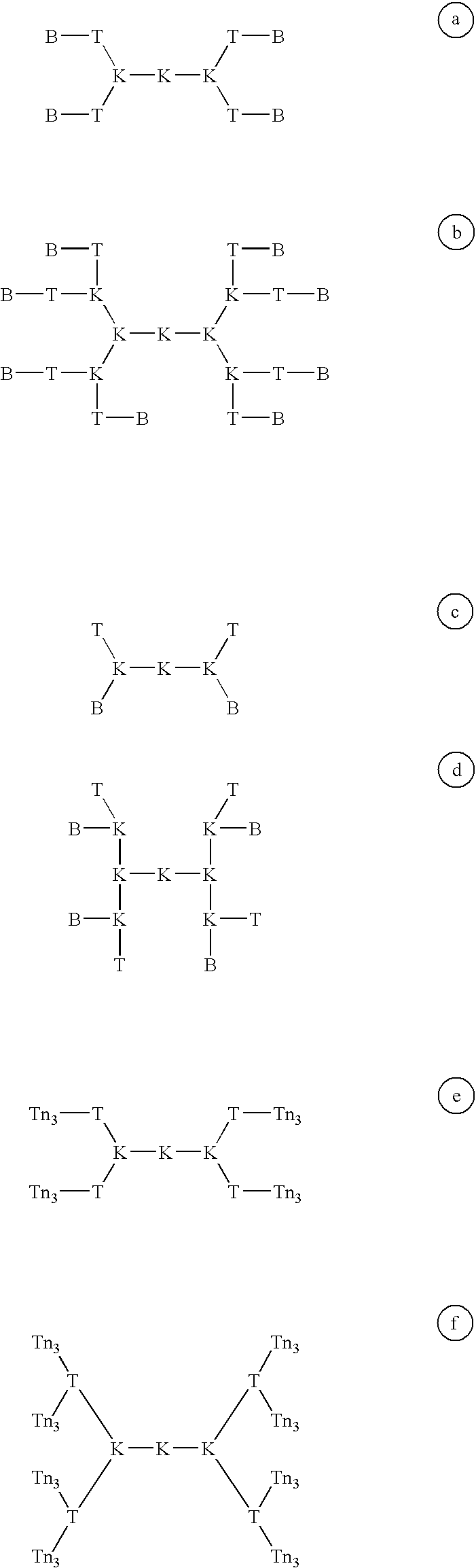
Multiple antigen glycopeptide carbohydrate vaccine comprising the same and use thereof
InactiveUS6676946B2Enhance antibody responseEfficient T cell helpPeptide/protein ingredientsBacteria peptidesT epitopeGlycopeptide
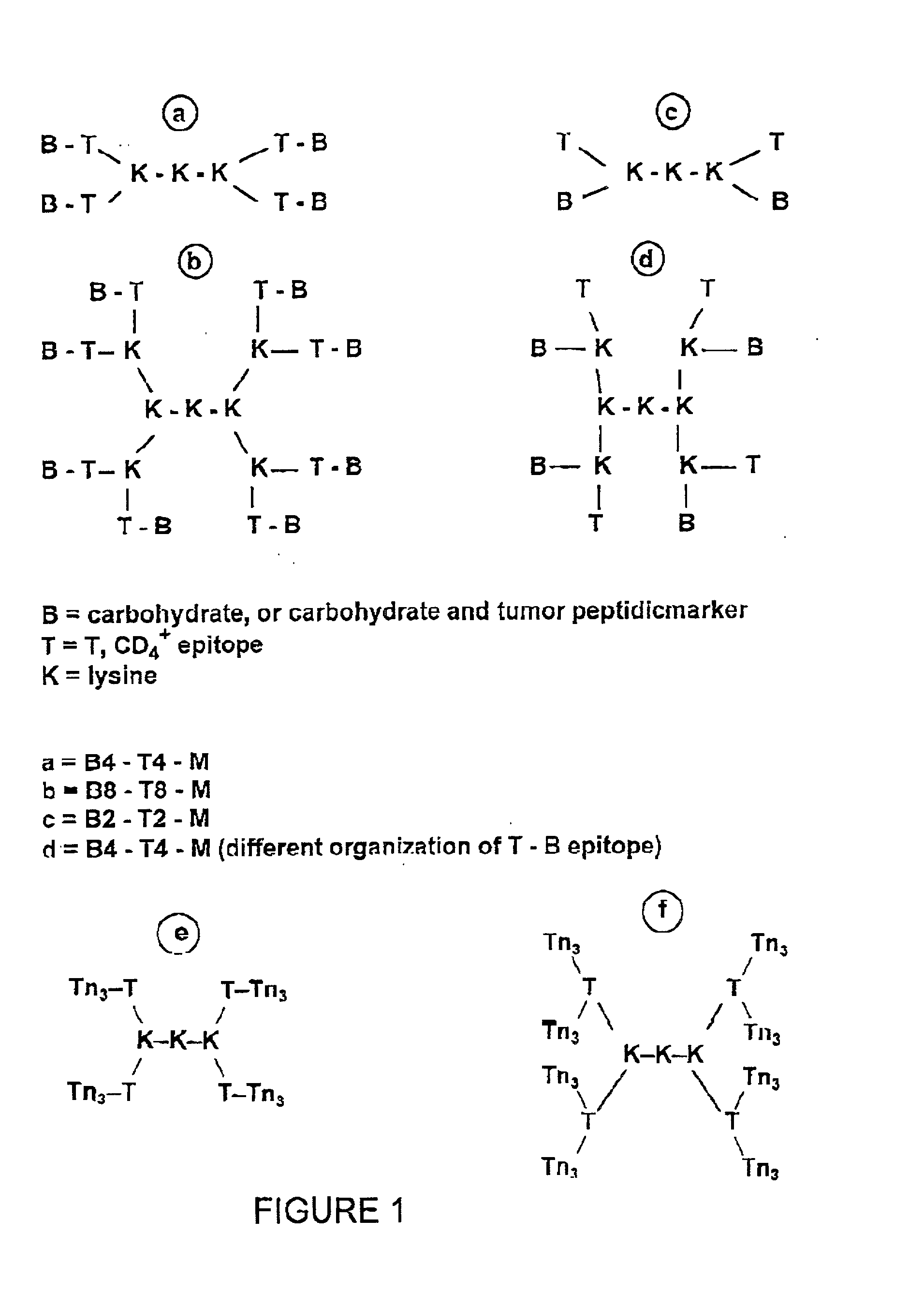
A carbohydrate peptide conjugate containing:(i) a carrier containing a dendrimeric poly-lysine enabling multiple epitopes to be covalently attached thereto,(ii) at least one peptide containing one T epitope or several identical or different T-epitopes,(iii) at least one carbohydrate moiety which is tumor antigen, or a derivative thereof, containing a B epitope, provided it is not a sialoside, or several identical or different epitopes, wherein said conjugate containing at least 3-lysines and up to 15 lysine covalently linked to one another, and wherein:(a) to the NH2 and of at least two lysine residues is bound at least one carbohydrate residue being not a sialoside, optionally substituted and containing an epitope and wherein the peptide containing one T epitope is covalently bound to the end of said carbohydrate which induces immune responses.
Owner:INST PASTEUR
Multiple antigen glycopeptide carbohydrate vaccine comprising the same and use thereof
InactiveUS20030157115A1Enhance antibody responseEfficient T cell helpPeptide/protein ingredientsVirus peptidesT epitopeGlycopeptide
A carbohydrate peptide conjugate comprising: a carrier comprising a dendrimeric poly-Lysine enabling multiple epitopes to be covalently attached thereto, at least one peptide comprising one T epitope or several identical or different T epitopes, at least one carbohydrate moiety, or a derivative thereof, containing B epitope, provided it is not a sialoside, or several identical or different epitopes. Use of this conjugate for inducing immune response.
Owner:INST PASTEUR
Compounds and methods to inhibit or augment an inflammatory response
InactiveUS20060073114A1Efficacious to preventEfficacious to treatPeptide/protein ingredientsAntiviralsStereochemistryPeptide analog
Owner:CAMBRIDGE ENTERPRISE LTD
Use of hemagglutinin of the african swine fever virus as an adjuvant
InactiveUS20100086556A1Enhance immune responseHigh homologyOrganic active ingredientsVirusesHemagglutininAdjuvant
The invention generally relates to the use of the hemagglutinin (HA) of African swine fever virus (ASFV) as an adjuvant to enhance the immune response against an antigen in a subject. The invention provides a gene construct comprising all or part of the encoding sequence of said HA fused to the encoding sequence of an antigen. The invention is applicable in human and animal health.
Owner:FUNDACIO CENT DE RECERCA & SANITAT ANIMAL
Compound
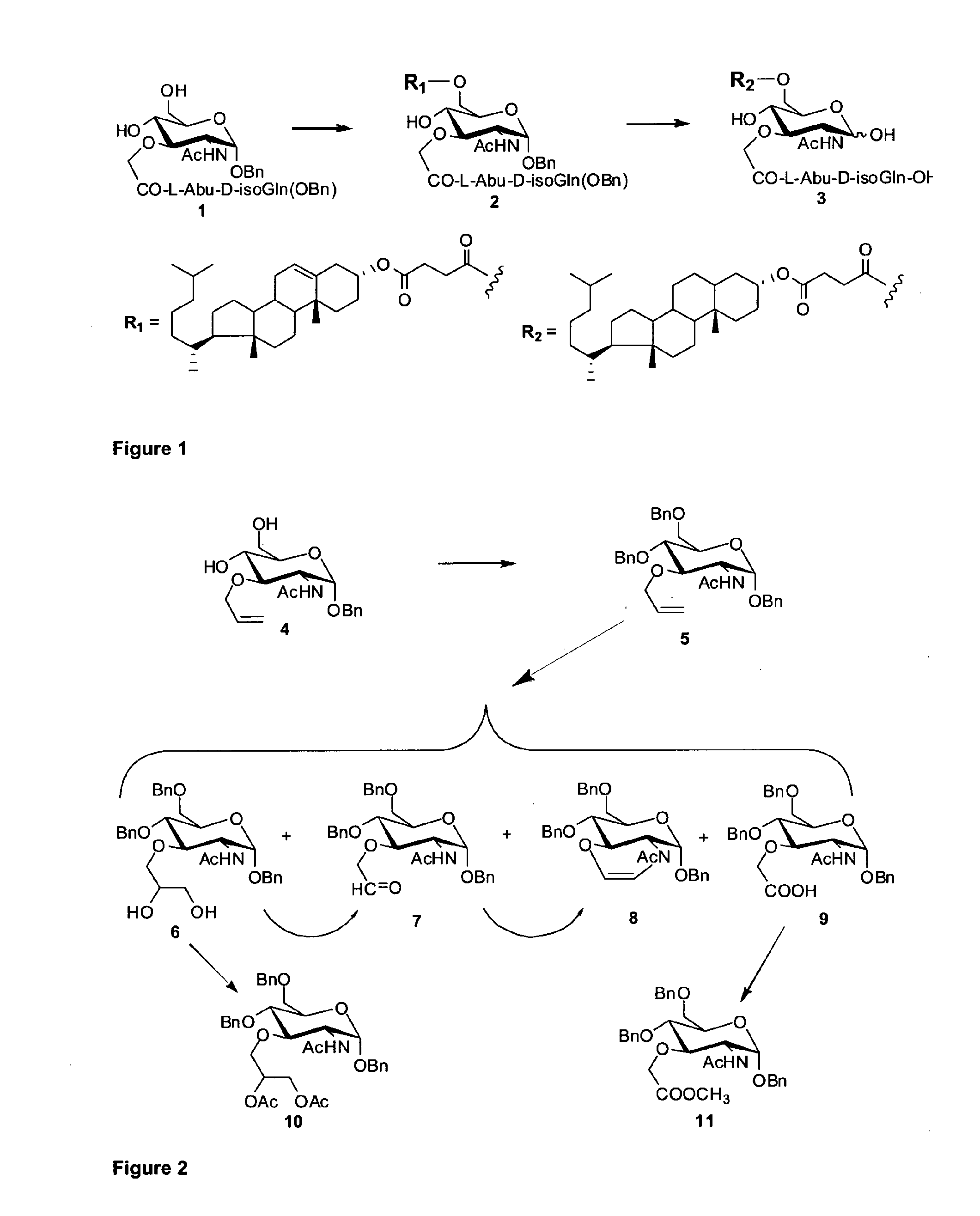
ActiveUS20110002983A1Reduce capacityEnhance antibody responseOrganic active ingredientsAntimycoticsAntigenAdjuvant
The invention provides a compound (which can act as an adjuvant) of Formula I or Formula (II), wherein R1 R4 R5 R6 and R7 are each independently selected from hydrogen, acetyl, hydrocarbyl, a lipid moiety and a lipid acyl moiety; R2 is a hydroxyl, a hydrocarbyl, a lipid moiety, a lipid acyl moiety; or an amino hydrocarbyl group optionally substituted with a hydrocarbyl, a lipid moiety or a lipid acyl moiety; R3 and R8 are each independently selected from acetyl, a hydrocarbyl, a lipid moiety and a lipid acyl moiety; X is a peptide chain; The above normuramylglycopeptide compounds can be located in liposomes and micelles and can function as immunomodulators, along with a desired antigen (or DNA encloding the antigen), in (e.g. DNA) vaccines.
Owner:IMUTHES LTD +2
Human cytomegalovirus vaccine compositions and method of producing the same
InactiveUS20170119874A1Efficient infectionNot impair balanced expressionViral antigen ingredientsVirus peptidesGlycoproteinGene expression
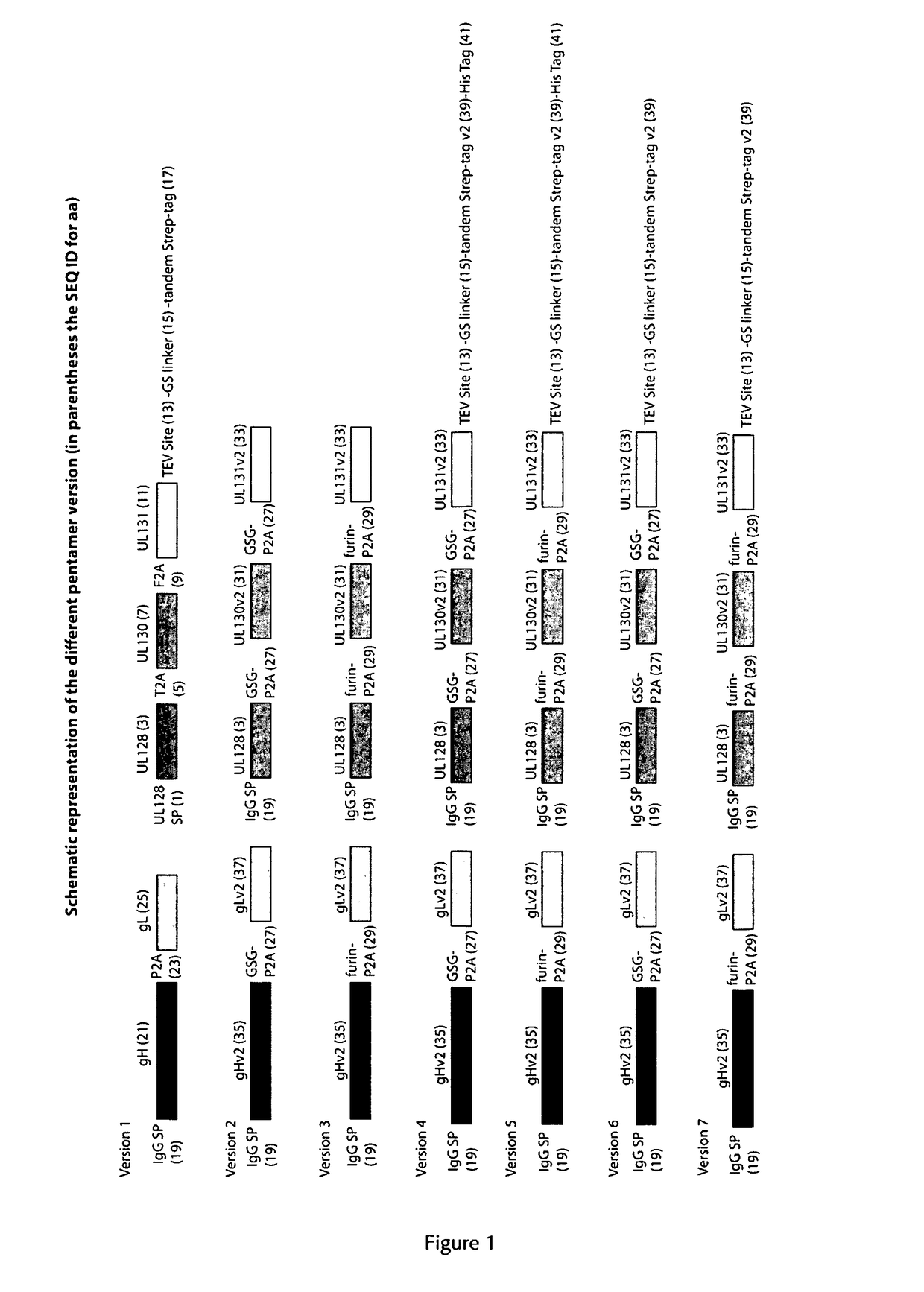
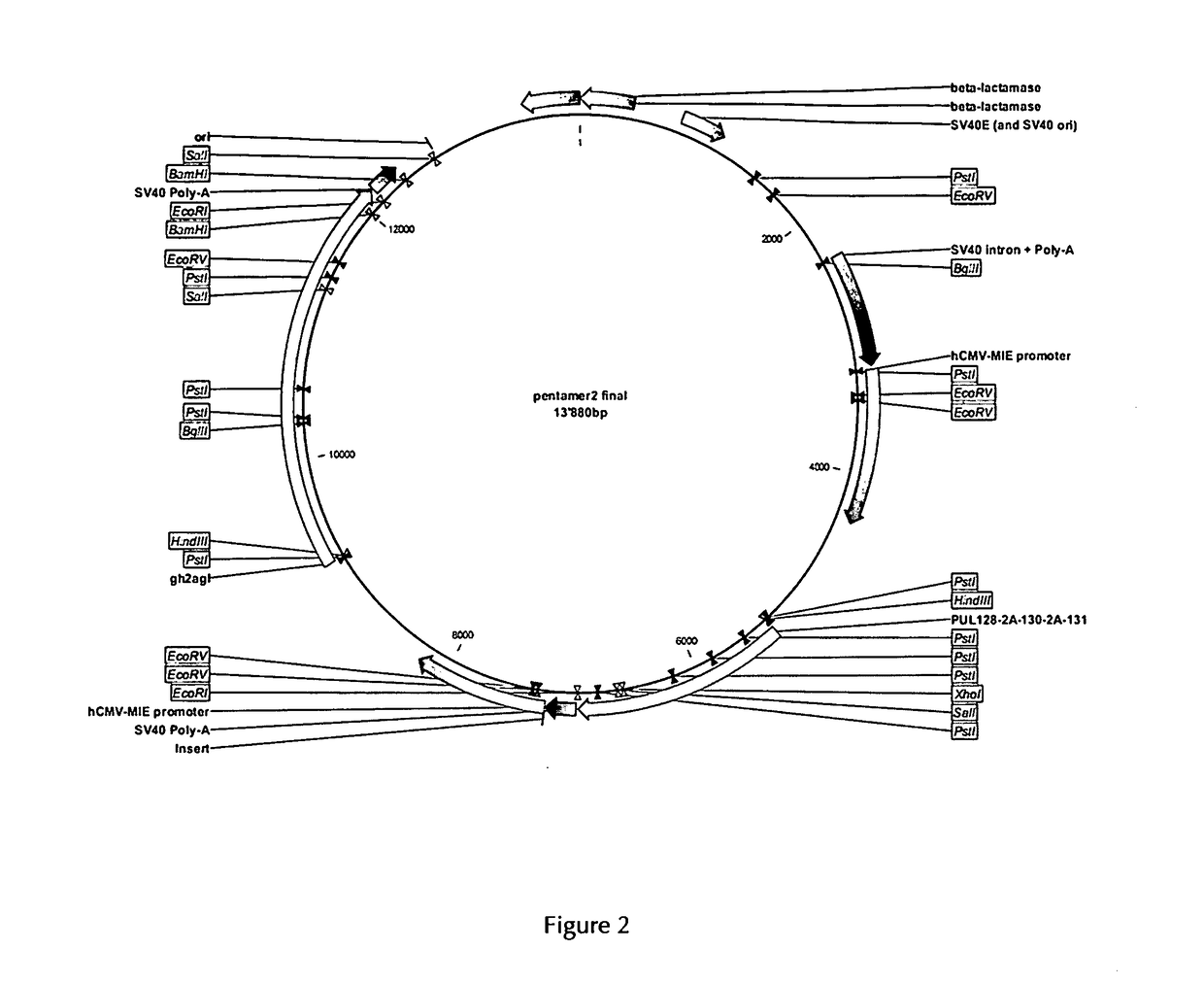
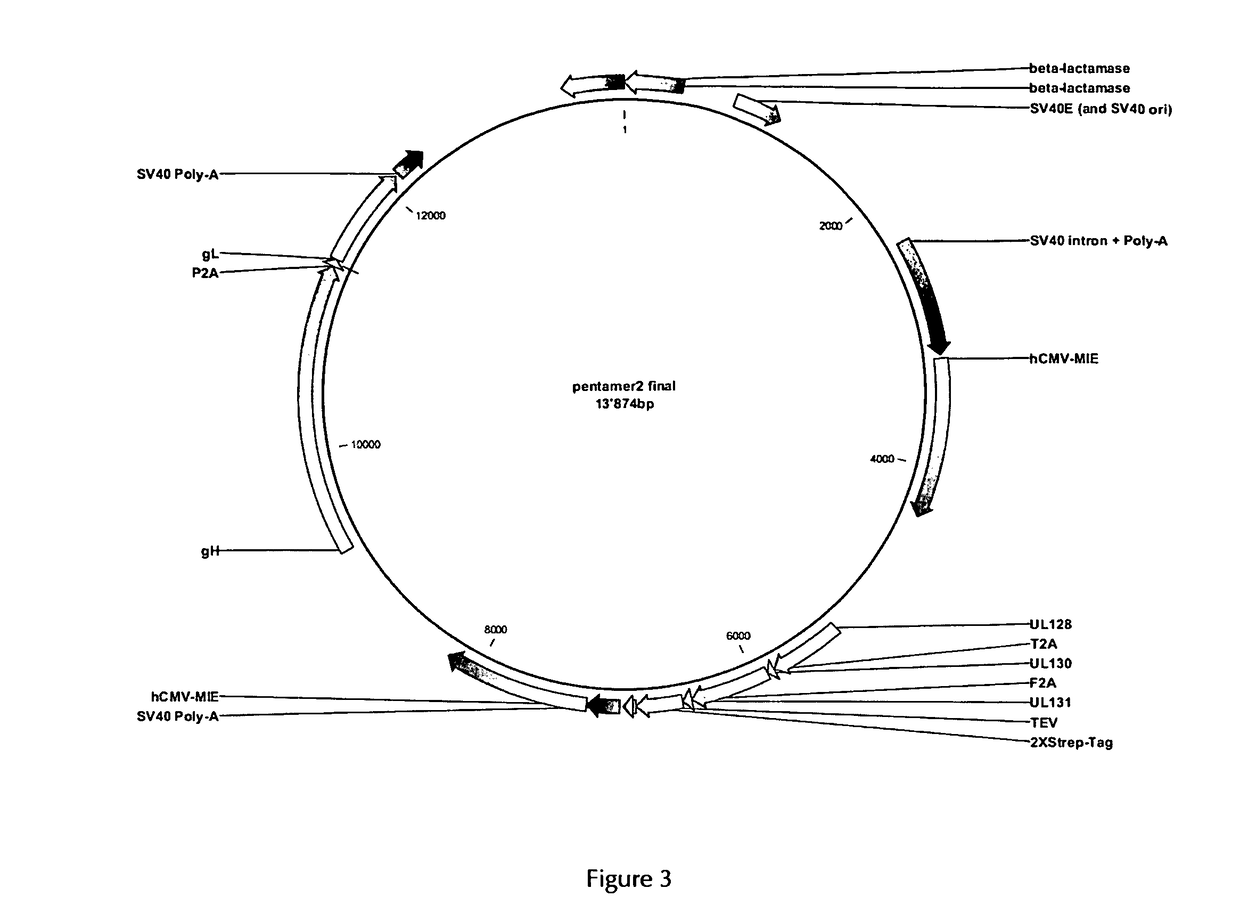
The present invention provides for a vector and a gene expression system for producing a soluble pentameric protein complex comprising the HCMV glycoproteins UL128, UL130, UL131, gH and gL or sequence variants thereof, as well as vaccine compositions comprising the same. The present invention further provides for a vaccine composition for use in prophylactically or therapeutically vaccinating against HCMV infections. Also disclosed are methods of producing the inventive vaccine. Furthermore, the present invention pertains to methods of vaccination of humans with the inventive vaccine composition.
Owner:INSTITUTE FOR RESEARCH IN BIOMEDECINE +1
Meningococcal vaccines formulated with interleukin-12
InactiveUS6841160B2Increasing cell-mediated immunityModulate immune responseBiocideOrganic active ingredientsWhite blood cellInterleukin I
This invention pertains to vaccine compositions comprising a mixture of antigen, such as pneumococcal or meningococcal antigen, and interleukin IL-12, which may be adsorbed onto a mineral in suspension. The pneumococcal or meningococcal antigen may be conjugated to a carrier molecule. These vaccine compositions modulate the protective immune response to the antigen.
Owner:WYETH HOLDINGS CORP
Use of B Cell Expansion Agents in Generating Antibodies
InactiveUS20080286289A1Enhance antibody responseIncrease the number ofAntibacterial agentsSenses disorderTarget antigenDisease
A method for efficiently generating antibodies immunizes an animal with a target antigen and a B cell expansion agent, such as an anti-CD40 agonist. The antibodies generated from this method are useful as therapeutic agents, diagnostic agents or research reagents in a variety of diseases and conditions.
Owner:DUCHALA CYNTHIA +2
Compounds and methods to inhibit or augment an inflammatory response
InactiveUS7067117B1Alter chemokine expressionSufficient sequence complementarityBiocideAntipyreticPeptide analogStereochemistry
Owner:CAMBRIDGE ENTERPRISE LTD
Immunogenic HPV L2-Containing VLPs and Related Compositions, Constructs, and Therapeutic Methods
ActiveUS20140105924A1Highly immunogenicReduce the possibilityViral antigen ingredientsDsDNA virusesImmunogenicityImmunotherapy
The invention provides immunotherapeutic and prophylactic bacteriophage viral-like particle (VLPs) which are useful in the treatment and prevention of human papillomavirus (HPV) infections and related disorders, including cervical cancer and persistent infections associated with HPV. Related compositions (e.g. vaccines), nucleic acid constructs, and therapeutic methods are also provided. VLPs and related compositions of the invention induce high titer antibody responses against HPV L2 and protect against HPV challenge in vivo. VLPs, VLP-containing compositions, and therapeutic methods of the invention induce an immunogenic response against HPV infection, confer immunity against HPV infection, protect against HPV infection, and reduce the likelihood of infection by HPV infection.
Owner:STC UNM
Method of providing patient specific immune response in amyloidoses and protein aggregation disorders
ActiveUS9370531B2Favor selective antibody responseImmune responseOrganic active ingredientsSenses disorderCell AggregationsSpecific immunity
Owner:NEW YORK UNIV
Preparation process of avian influenza virus split vaccine containing MF59 adjuvant
InactiveCN102600468AReduce antigen contentLow protein contentAntiviralsAntibody medical ingredientsVirulenceImmunogenicity
The invention discloses a preparation process of an avian influenza virus split vaccine containing an MF59 adjuvant. The preparation process comprises the following steps of: inoculating an R1203 avian influenza virus strain onto a chicken embryo; inoculating viruses and culturing to obtain a virus liquid; inactivating viruses and purifying; cracking; purifying once again; removing bacteria and filtering, and the like. The preparation process has the beneficial effects that: the vaccine prepared from the MF59 adjuvant has high immunogenicity, and can generate higher antibody response; the immunity of children and the old who cannot generate immune response or have low immune response after inoculation of the conventional vaccine can be enhanced; cross immunity can be generated for different influenza virus subtypes; and on the premise of keeping constant immunity, the antigen content can be lowered, the content of foreign proteins capable of causing side reactions is lowered simultaneously, the side reaction of the vaccine is lower, higher safety is achieved, and the risks of virulence returning strength and variation are avoided.
Owner:ZHEJIANG TIANYUAN BIO PHARM CO LTD
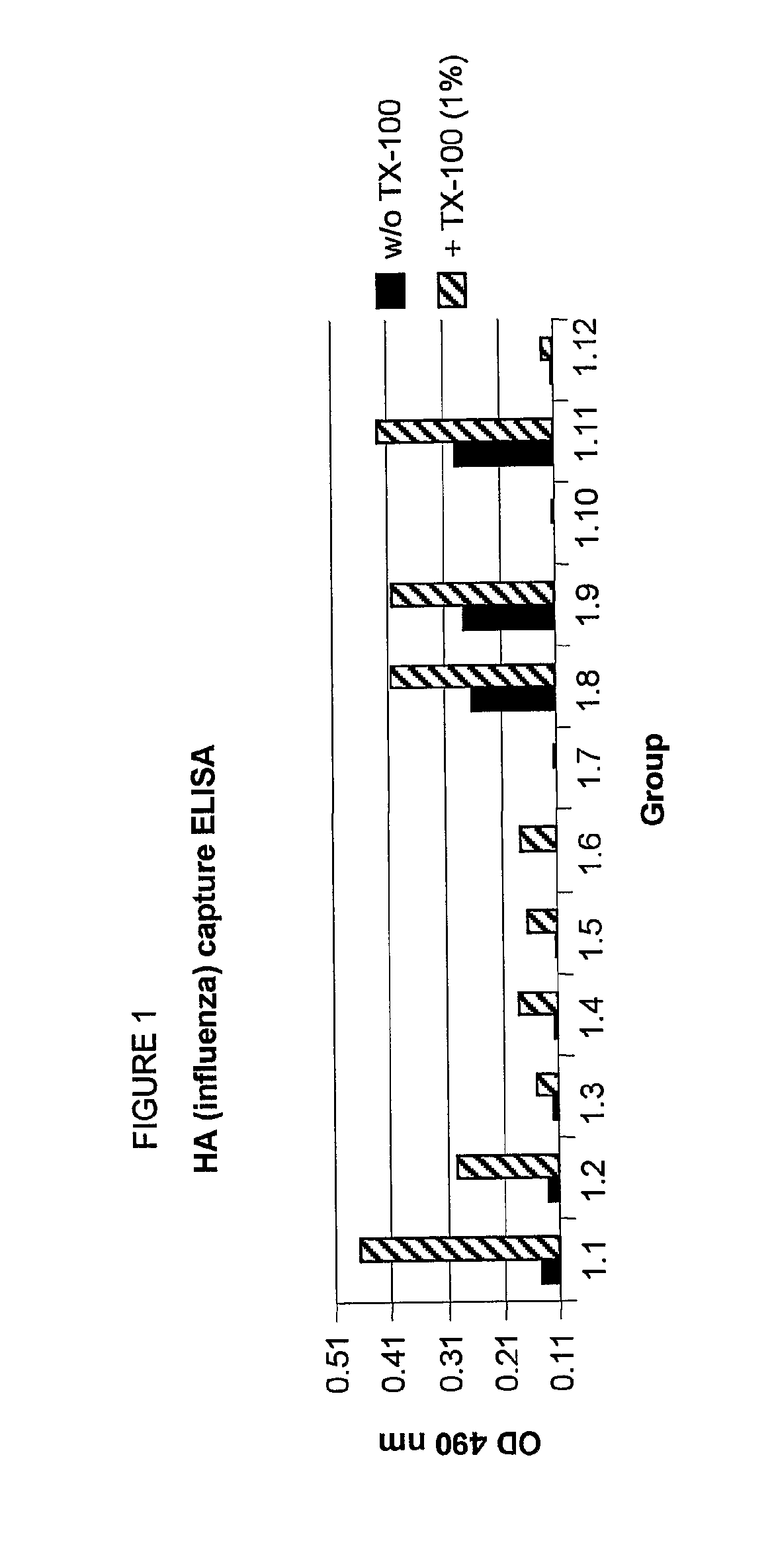
Method to enhance an immune response of nucleic acid vaccination
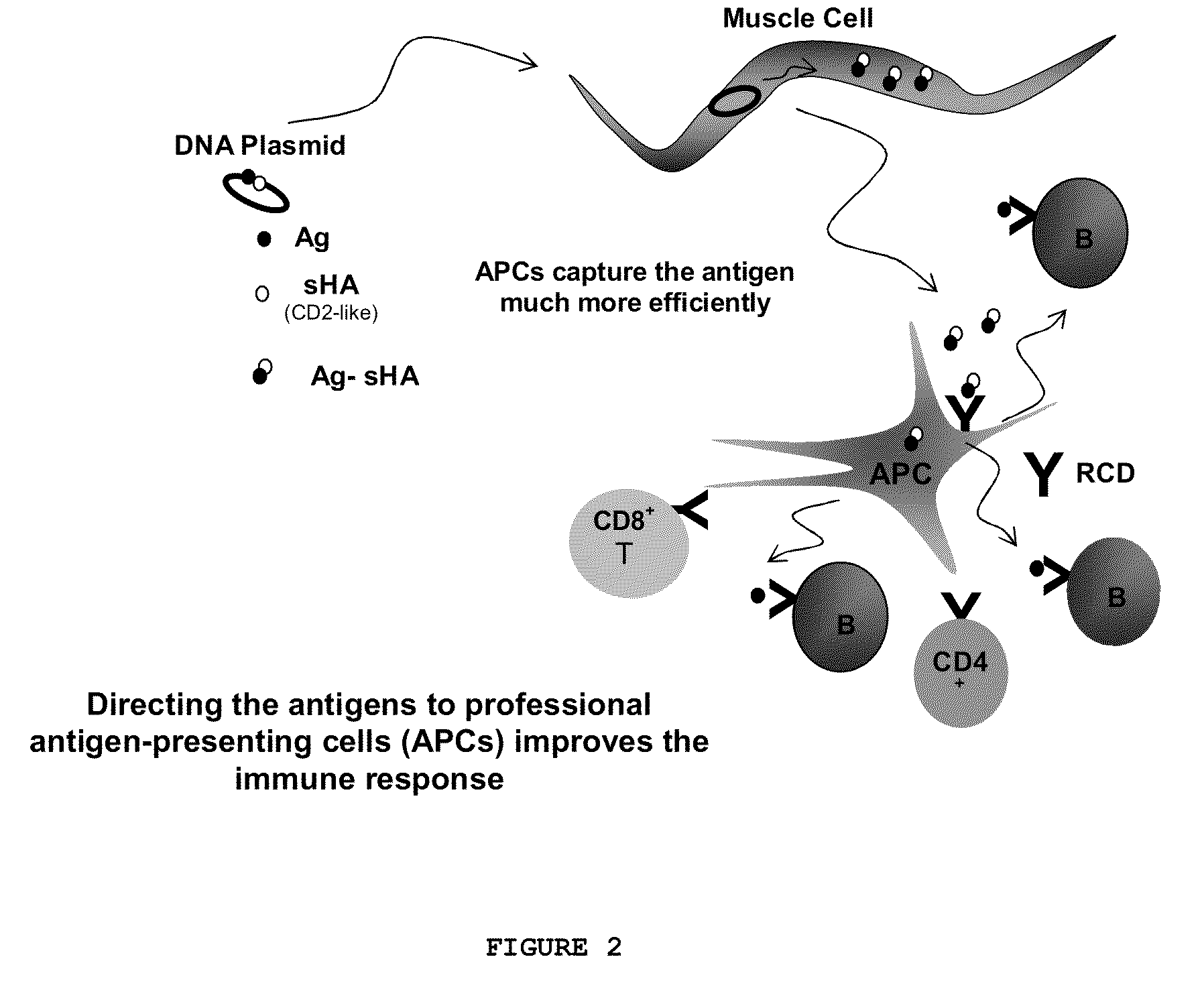
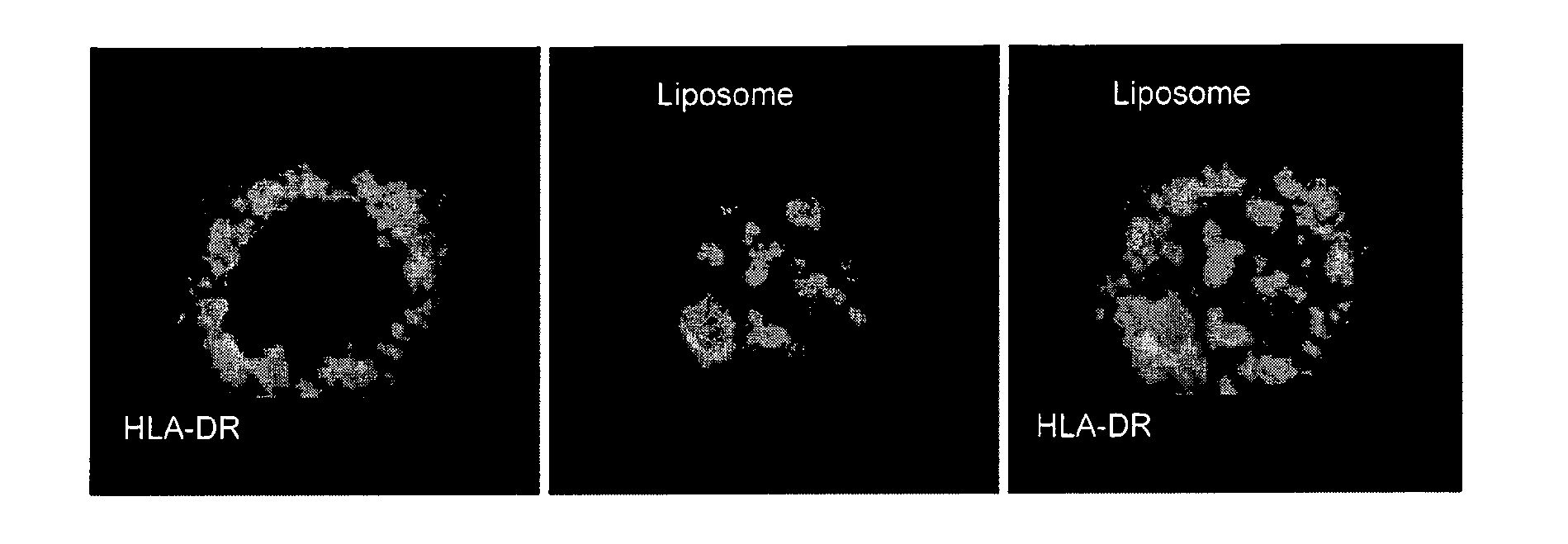
InactiveUS8075896B2Generate efficientlyEnhance antibody responseSsRNA viruses negative-senseGenetic material ingredientsEpitopeVaccination
A composition comprising liposomes associated with a nucleic acid operatively encoding an antigenic protein and with an assistor protein, wherein the assistor protein shares at least one epitope with the antigenic protein, and wherein the nucleic acid and said assistor protein are associated with the same liposomes is described. The composition provides an improved immune response compared to mixtures of liposomes some of which are associated with the nucleic acid and some of which are associated with the assistor protein.
Owner:LIPOXEN TECHNOLOGIES LTD
Multiple antigen glycopeptide carbohydrate, vaccine comprising the same and use thereof
InactiveUS20070237785A1Increase survivalEnhance antibody responsePeptide-nucleic acidsPeptide/protein ingredientsCell immune responseAntigen
The present invention relates to a glycoconjugate, a composition and vaccine comprising the same and to the use thereof for enhancing the immune response in cancer therapy wherein the induction of a humoral or a cellular immune response is sought. The invention also relates to a diagnosis kit and to a method for the diagnosis of a cancer.
Owner:INST PASTEUR
Method for producing anti-human C-reaction protein antiserum for sheep
InactiveCN104987417AStable productionLow costSerum immunoglobulinsImmunoglobulins against animals/humansInjection siteAntibody level
The invention provides a method for producing anti-human C-reaction protein antiserum for sheep. The method comprises the following steps of: (1) preparing an antigen; (2) performing injection for the first time: using the antigen emulsion to inject the sheep, wherein injection parts are soles of feet, inguinal regions, armpits, necks, backs and subcutaneous multipoint injection; (3) performing injection for the second time; (4) performing injection for the third time; (5) performing injection for the fourth time; (6) performing injection for the fifth time; and (7) detecting serum titer: drawing blood from the vein of the sheep after 8-10 days from the injection each time, separating the serum and detecting dual-extending titer. Compared with the prior art, the method for producing the anti-human C-reaction protein antiserum for the sheep, disclosed by the invention, is low in cost, and simple in process; the method not only can stably produce the antibodies, but also is high in antibody producing speed; the antibody level is high, and the dosage of the antigen is small.
Owner:海奥斯生物科技镇江有限公司
Preparation method and applications of anti-pseudorabies virus pig origin monoclonal antibody
InactiveCN108203716AMake up for the damageEnhance phagocytosisHybrid immunoglobulinsImmunoglobulins against virusesPig farmsPlasmid dna
The invention belongs to the field of gene engineering, and discloses a preparation method and applications of an anti-pseudorabies virus pig origin monoclonal antibody. The preparation method comprises following steps: positive hybridoma cells capable of realizing stable secretion of anti-pseudorabies virus (PRV) glycoprotein monoclonal antibodies are screened; secretion of the monoclonal antibodies is carried out; enlarge culture is carried out; enrichment, purification, and sequencing are carried out; design of corresponding specific primers is carried out, pig origin modification is carried out, cloning is carried out using a virus gene carrier or a non-virus gene carrier (including plasmid DNA and minicircle DNA), large scale production of different pig origin monoclonal antibodies iscarried out using insect cells or CHO cell fermentation, or the above pig origin monoclonal antibody gene carrier is introduced into pigs; injection, or feeding through nasal feeding and spraying areadopted for neutralization of pseudorabies virus with the anti-pseudorabies virus pig origin monoclonal antibodies, so that passive immunity is adopted for purifying of pseudorabies virus positive pig farms, and preventing and treating of porcine pseudorabies.
Owner:SHENZHEN JASON INTELLIGENT BIOTECH CO LIMLTED PRC
Immunogenic HPV L2-Containing VLPs and Related Compositions, Constructs, and Therapeutic Methods
ActiveUS20120308592A1Improving immunogenicityReduce the possibilityViral antigen ingredientsVirus peptidesHuman papillomavirusBacteriophage
The invention provides immunotherapeutic and prophylactic bacteriophage viral-like particle (VLPs) which are useful in the treatment and prevention of human papillomavirus (HPV) infections and related disorders, including cervical cancer and persistent infections associated with HPV. Related compositions (e.g. vaccines), nucleic acid constructs, and therapeutic methods are also provided. VLPs and related compositions of the invention induce high titer antibody responses against HPV L2 and protect against HPV challenge in vivo. VLPs, VLP-containing compositions, and therapeutic methods of the invention induce an immunogenic response against HPV infection, confer immunity against HPV infection, protect against HPV infection, and reduce the likelihood of infection by HPV infection.
Owner:STC UNM
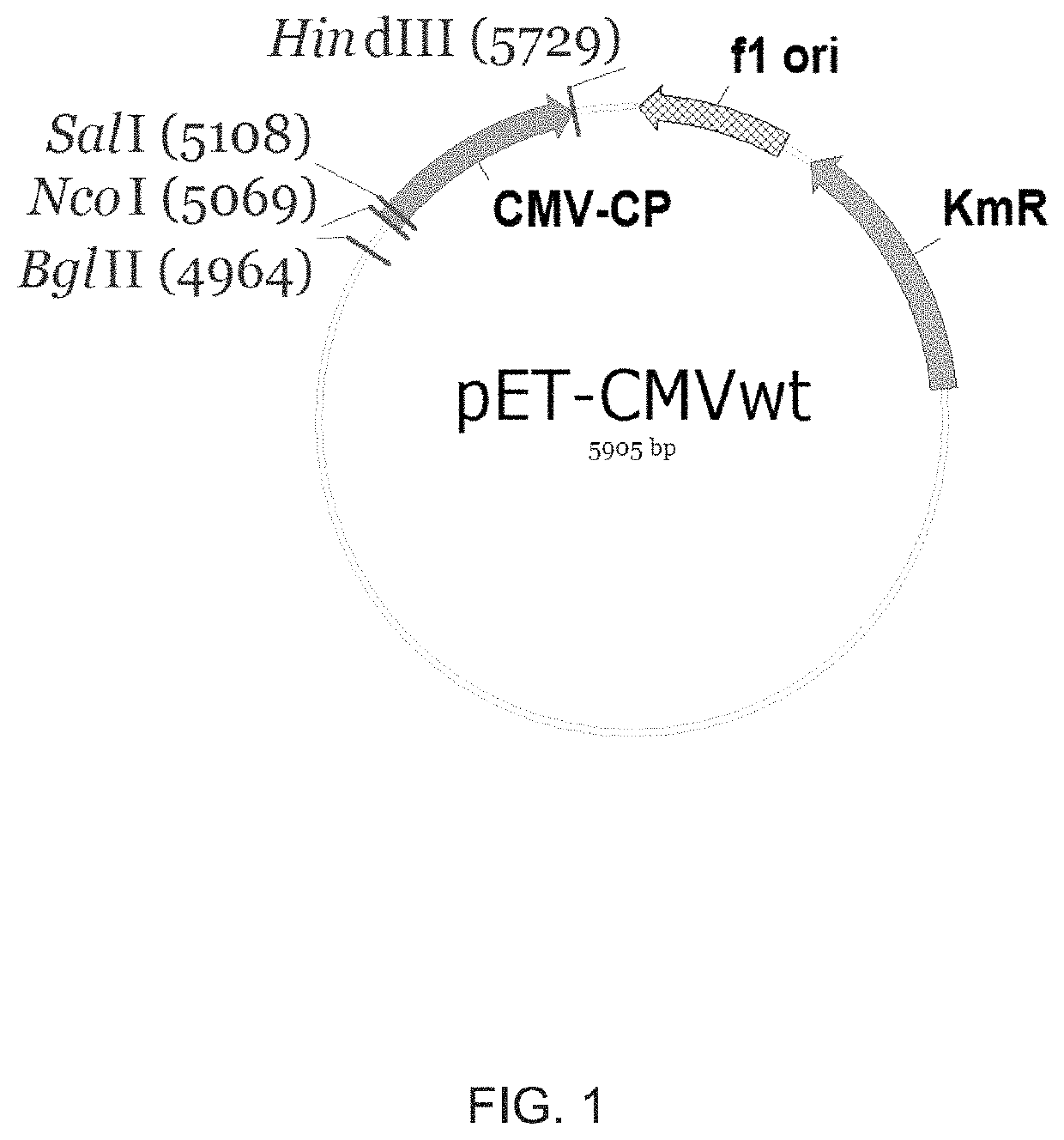
Modified virus-like particles of CMV
ActiveUS10532107B2Hinder self-assemblyImproving immunogenicitySsRNA viruses negative-senseSsRNA viruses positive-senseEpitopeVirus-like particle
The present invention relates to virus-like particles of plant virus Cucumber Mosaic Virus (CMV), and in particular to modified VLPs of CMV comprising Th cell epitopes, in particular universal Th cell epitopes. Furthermore, these modified VLPs serve as, preferably, vaccine platform, for generating immune responses, in particular antibody responses, against antigens linked to said modified VLPs. The presence of the Th cell epitopes, in particular universal Th cell epitopes, led to a further increase in the generated immune response.
Owner:SAIBA AG
Immunomodulators and immunomodulator conjugates
ActiveUS20140212442A1Enhance antibody responseImproved ability to combat infectionBiocideOrganic chemistryStereochemistry
Owner:RGT UNIV OF MINNESOTA
Vaccination Regimen for B-Cell Vaccines
InactiveUS20100119540A1High antibody titer achievableHigh antibody responseNervous disorderCarrier-bound antigen/hapten ingredientsDiseaseRegimen
This invention relates to the field of vaccination and treatment or prevention diseases. In particular this invention relates to the treatment or prevention of diseases by inducing hapten-specific antibody responses in a vaccinated subject. The invention further provides a method for prevention or treatment of a disease by inducing hapten-specific antibodies in a subject comprising administering into said subject a composition comprising a virus-like particle of an RNA bacteriophage and at least one hapten linked thereto.
Owner:BACHMANN MARTIN +4
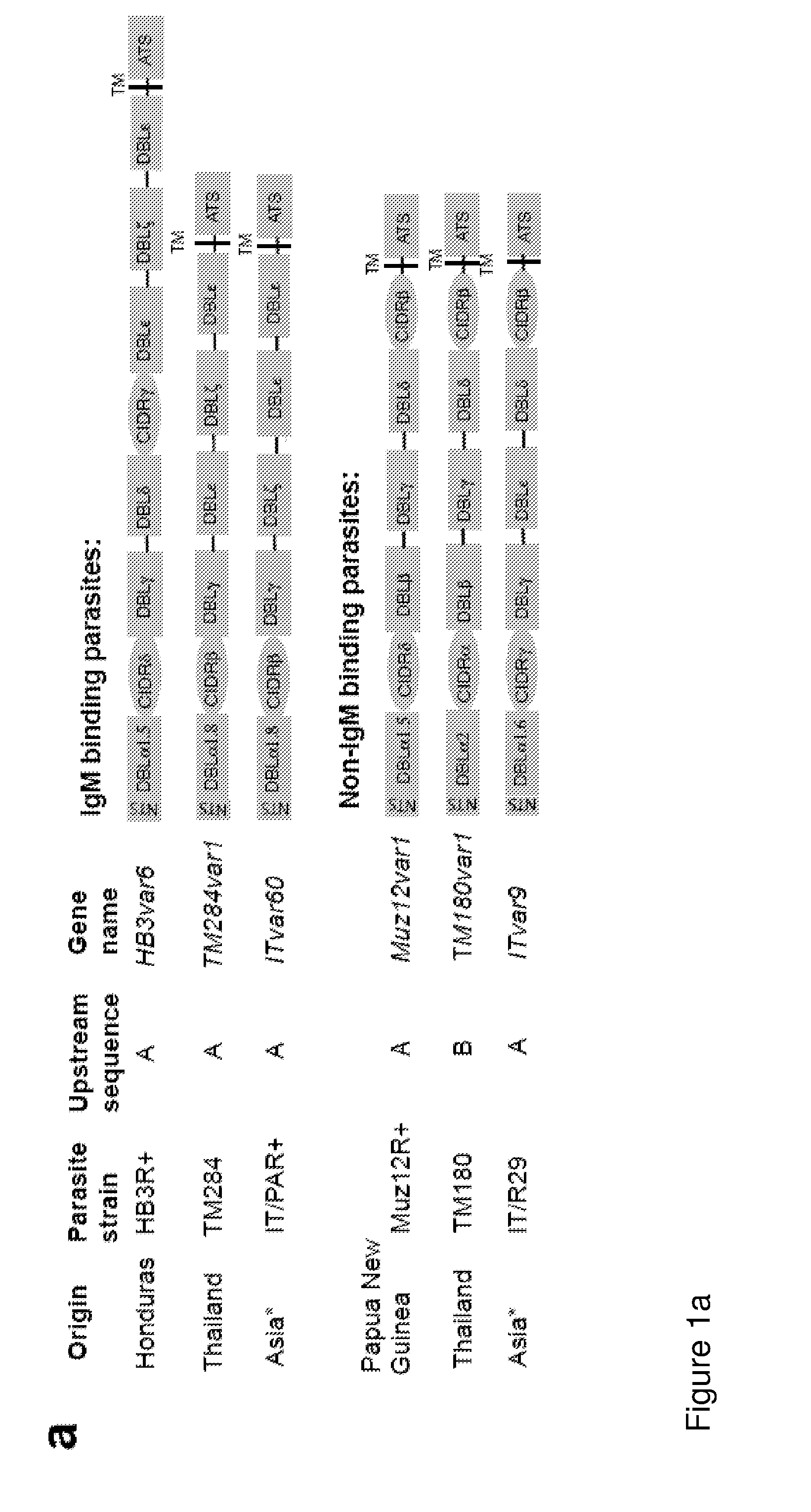
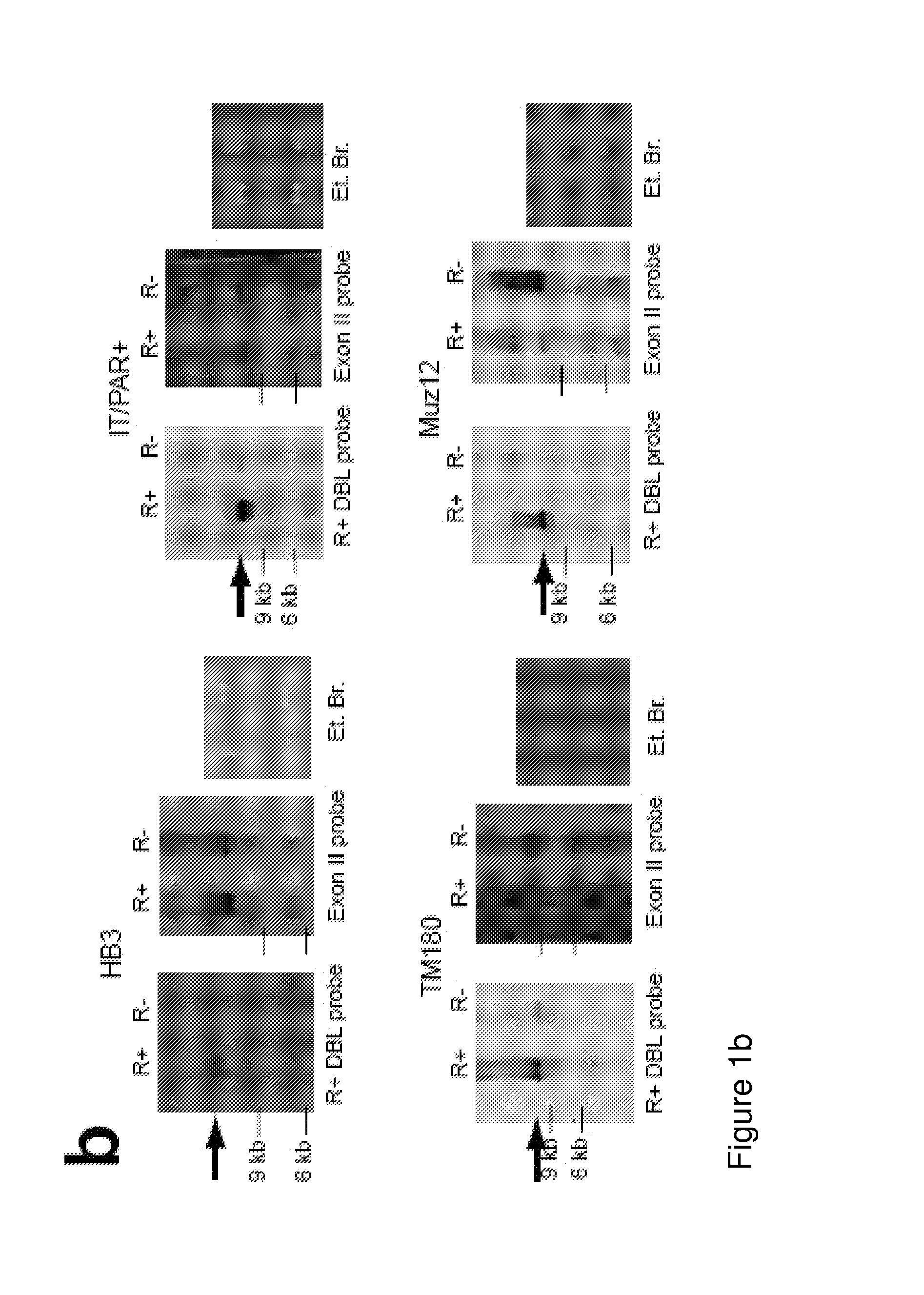
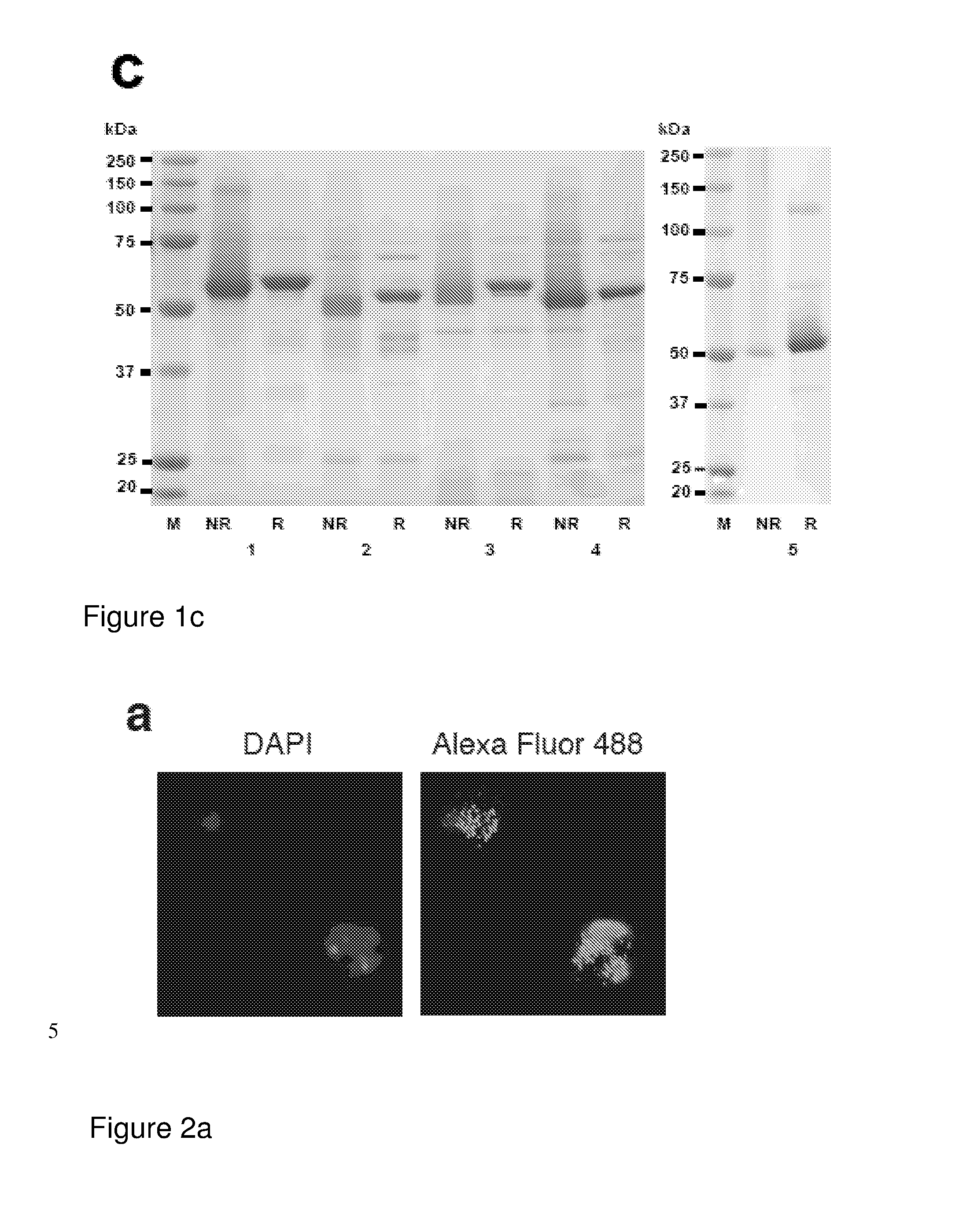
Malaria vaccine
ActiveUS20140322240A1Enhance antibody responseAlleviating and reducing and eliminating symptomAntibody ingredientsImmunoglobulinsEpitopeErythrocyte membrane
The present invention provides an antigenically restricted subset of the highly variant PfEMP1 rosetting antigen which possess epitopes which may be exploited to raise immune responses effective against many diverse strains and isolates of the malaria parasite, Plasmodium falciparum. In this regard, the invention provides one or more P. falciparum Erythrocyte Membrane Protein-1 (PfEMP1) antigen(s) or a fragment or fragments thereof, for use in raising immune responses in humans.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
Liquid milk with additive nucleotide suitable for baby of 6 to 12 months old
InactiveCN101427794APromote growthPromote growth and developmentMilk preparationFood preparationCow milkingNucleotide
The invention relates to milk and a preparation method thereof, in particular to infant liquid milk supplemented with nucleotides suitable for 6 to 12 months babies, which belongs to the technical field of dairy products. The ingredients (in weight ratios) of the infant liquid milk are as follows: 400Kg to 600Kg of cow milk, 10Kg to 50Kg of skimmed milk, 2Kg to 10Kg of whey protein concentrate 34 (whey protein content of 33%), 5Kg to 20Kg of desalted whey powder D70 (whey protein content of 12%), 10Kg to 50Kg of vegetable fat powder, 3Kg to 10Kg of sucrose, 5Kg to 20Kg of dextrin, 100g to 500g of vitamin complex, 200g to 600g of complex minerals, 1Kg to 8Kg of stabilizers, 200Kg to 500Kg of water, and 0.1Kg to 1Kg of nucleotides.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Liquid milk with additive nucleotide suitable for baby of 12 to 36 months old
InactiveCN101427789APromote growthPromote growth and developmentMilk preparationFood preparationCow milkingSucrose
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Liquid milk with additive nucleotide suitable for baby of 0 to 6 months old
InactiveCN101427798APromote growthPromote growth and developmentMilk preparationFood preparationCow milkingAdditive ingredient
The invention relates to milk and a preparation method thereof, in particular to infant liquid milk supplemented with nucleotides suitable for newborn to 6 months babies, which belongs to the technical field of dairy products. The ingredients (in weight ratios) of the infant liquid milk are as follows: 200Kg to 500Kg of cow milk, 1Kg to 10Kg of whey protein concentrate 34 (whey protein content of 33%), 20Kg to 100Kg of desalted whey powder D70 (whey protein content of 12%), 20Kg to 100Kg of vegetable fat powder, 1Kg to 10Kg of lactose, 100g to 500g of vitamin complex, 200g to 600g of complex minerals, 1Kg to 8Kg of stabilizers, 300Kg to 700Kg of water, and 0.1Kg to 1Kg of nucleotides. The infant liquid milk has balanced nutrition and fine and smooth mouthfeel, is easy to absorb, and can meet the needs for normal growth and development of infants and simultaneously promote the intelligence development of infants.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
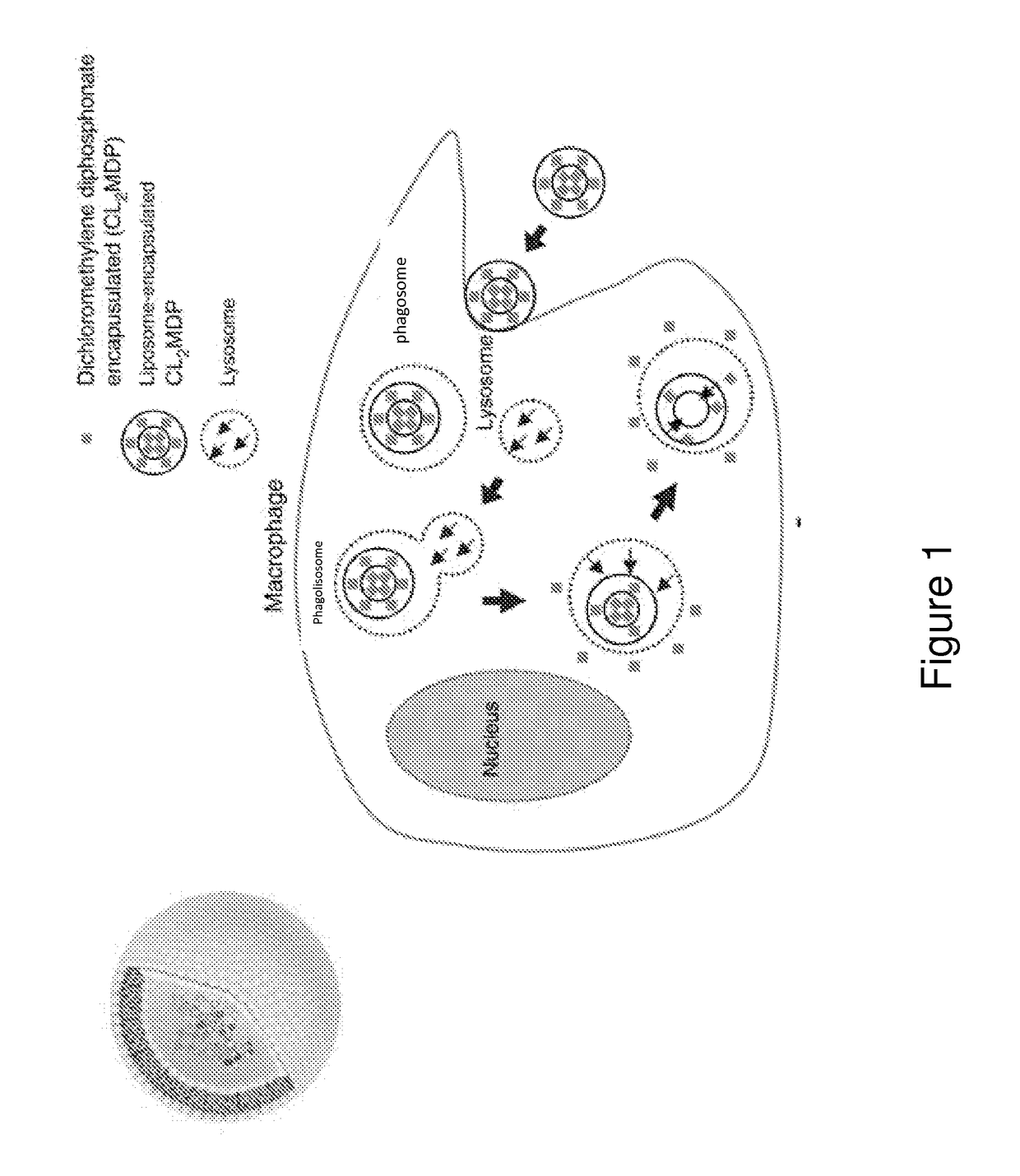
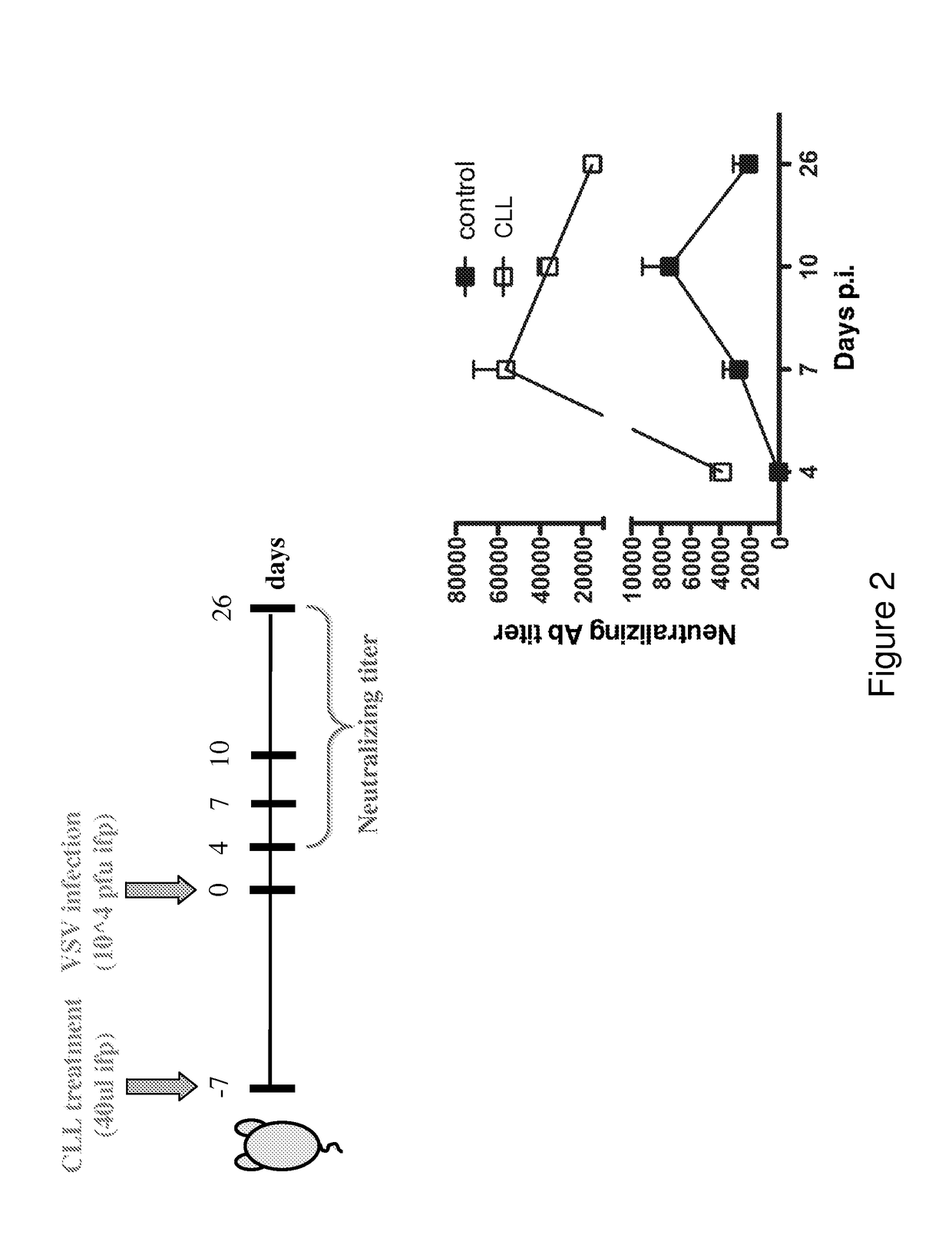
Vaccines comprising bisphosphonate and methods of use thereof
ActiveUS10188733B2Increasing antigen availabilityEnhance antibody responseAnimal cellsInorganic non-active ingredientsDiseaseAdjuvant
The present invention demonstrates that bisphosphonates have an intrinsic adjuvant activity and directly stimulate B cell antibody secretion. Accordingly, the present invention provides vaccines comprising a bisphosphonate, methods for stimulating an immune response, enhancing the immunogenicicty of an immunogen, and methods of treating an infection, an autoimmune disease, an allergy, and / or a cancer using the same.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Influenza vaccines
InactiveUS20150098966A1Increased cross-reactive immune responseEnhance antibody responseSsRNA viruses negative-senseViral antigen ingredientsHemagglutininStrain specificity
An influenza vaccine comprising an influenza hemagglutinin-containing antigen which is subjected to a treatment at a suitable low pH or other suitable conditions to obtain a suitable degree of loss of potency, and the method of making it are provided. The vaccine not only induces an increased cross-reactive immune response and cross protection, but can also induce a strain-specific immune response and protection like current inactivated vaccines. A method of administering influenza vaccines is also provided to induce an increased cross-reactive immune response and cross protection, which is especially suitable for use in emergency situations such as a pandemic.
Owner:KJ BIOSCI
Immunogenic HPV L2-containing VLPs and related compositions, constructs, and therapeutic methods
ActiveUS9533057B2Reduce the possibilityEnhance antibody responseViral antigen ingredientsVirus peptidesHuman papillomavirusBacteriophage
The invention provides immunotherapeutic and prophylactic bacteriophage viral-like particle (VLPs) which are useful in the treatment and prevention of human papillomavirus (HPV) infections and related disorders, including cervical cancer and persistent infections associated with HPV. Related compositions (e.g. vaccines), nucleic acid constructs, and therapeutic methods are also provided. VLPs and related compositions of the invention induce high titer antibody responses against HPV L2 and protect against HPV challenge in vivo. VLPs, VLP-containing compositions, and therapeutic methods of the invention induce an immunogenic response against HPV infection, confer immunity against HPV infection, protect against HPV infection, and reduce the likelihood of infection by HPV infection.
Owner:STC UNM
Influenza Vaccines
ActiveUS20190142930A1Increased cross-reactive immune responseLoss of potencySsRNA viruses negative-senseViral antigen ingredientsHemagglutininStrain specificity
An influenza vaccine comprising an influenza hemagglutinin-containing antigen which is subjected to a treatment at a suitable low pH or other suitable conditions to obtain a suitable degree of loss of potency, and the method of making it are provided. The vaccine not only induces an increased cross-reactive immune response and cross protection, but can also induce a strain-specific immune response and protection like current inactivated vaccines. A method of administering influenza vaccines is also provided to induce an increased cross-reactive immune response and cross protection, which is especially suitable for use in emergency situations such as a pandemic.
Owner:KJ BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com