Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
93 results about "Bordetella pertussis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bordetella pertussis is a Gram-negative, aerobic, pathogenic, encapsulated coccobacillus of the genus Bordetella, and the causative agent of pertussis or whooping cough. Like B. bronchiseptica, B. pertussis is motile and expresses a flagellum-like structure. Its virulence factors include pertussis toxin, adenylate cyclase toxin, filamentous hæmagglutinin, pertactin, fimbria, and tracheal cytotoxin.
Agents and methods for treatment of disease by oligosaccharide targeting agents
InactiveUS20050026866A1Reduce incidenceSpecific susceptibilityOrganic active ingredientsBiocideTolerabilityMammal
A method for targeting, treating, or diagnosing malignant mammalian tumor cells, comprising administering an effective amount of a β1,6-branched oligosaccharide specific binding agent to the mammal. As a treatment, the binding agent may be intrinsically cytotoxic, initiate an endogenous cytotoxic cascade, or play a role in a cytotoxic cascade involving exogenous factors. A preferred binding agent is Bordetella pertussis, which is both specific for the β1,6-branched oligosaccharide and well tolerated. Genetically engineered organisms may also be employed. Pharmaceutical compositions may also serve as binding agents.
Owner:YALE UNIV
Vaccine
InactiveUS6040427AReduce the binding forceLow toxicityBacterial antigen ingredientsBacteriaToxin proteinSaxitoxin
The Bordetella pertussis toxin is genetically modified to express a toxin protein which is deficient in target-cell receptor binding and is used in a vaccine for protection against whooping cough.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine for Prophylaxis or Treatment of an Allergen-Driven Airway Pathology
ActiveUS20130183336A1Reduce harmLow costAntibacterial agentsBacterial antigen ingredientsToxinPathology diagnosis
The present invention relates to a life attenuated Bordetella pertussis vaccine which is deficient for tracheal cytotoxin (TCT), pertussis toxin (PTX), and dermonecrotic toxin (DNT) for prophylaxis or treatment of an allergen-driven airway pathology.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Multi-component antigen acellular pertussis vaccine and preparation method thereof
ActiveCN101507814ABroad-spectrum antigenicityAntibacterial agentsBacterial antigen ingredientsCell freeType antigen
The invention relates to a multi-component antigen cell-free pertussis vaccine for preventing Bordetella pertussis infection and a preparation method thereof. The cell-free pertussis vaccine comprises multi-component cell structure antigen which is obtained by cracking, separating, extracting and purifying thalli cells of Bordetella pertussis liquid cultures, and can further comprise multi-component cell secretion-type antigen which is obtained by separating, extracting and purifying the supernatant part of the Bordetella pertussis liquid cultures. The combined vaccine is suitable for infants, children, teenagers and adults for preventing the Bordetella pertussis infection.
Owner:复星安特金(成都)生物制药有限公司
Recombinant adenylate cyclase of Bordetella sp. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase
InactiveUS20060019323A1Enhanced T cell responseImprove responseMicrobiological testing/measurementBiological material analysisAntigenCyaA
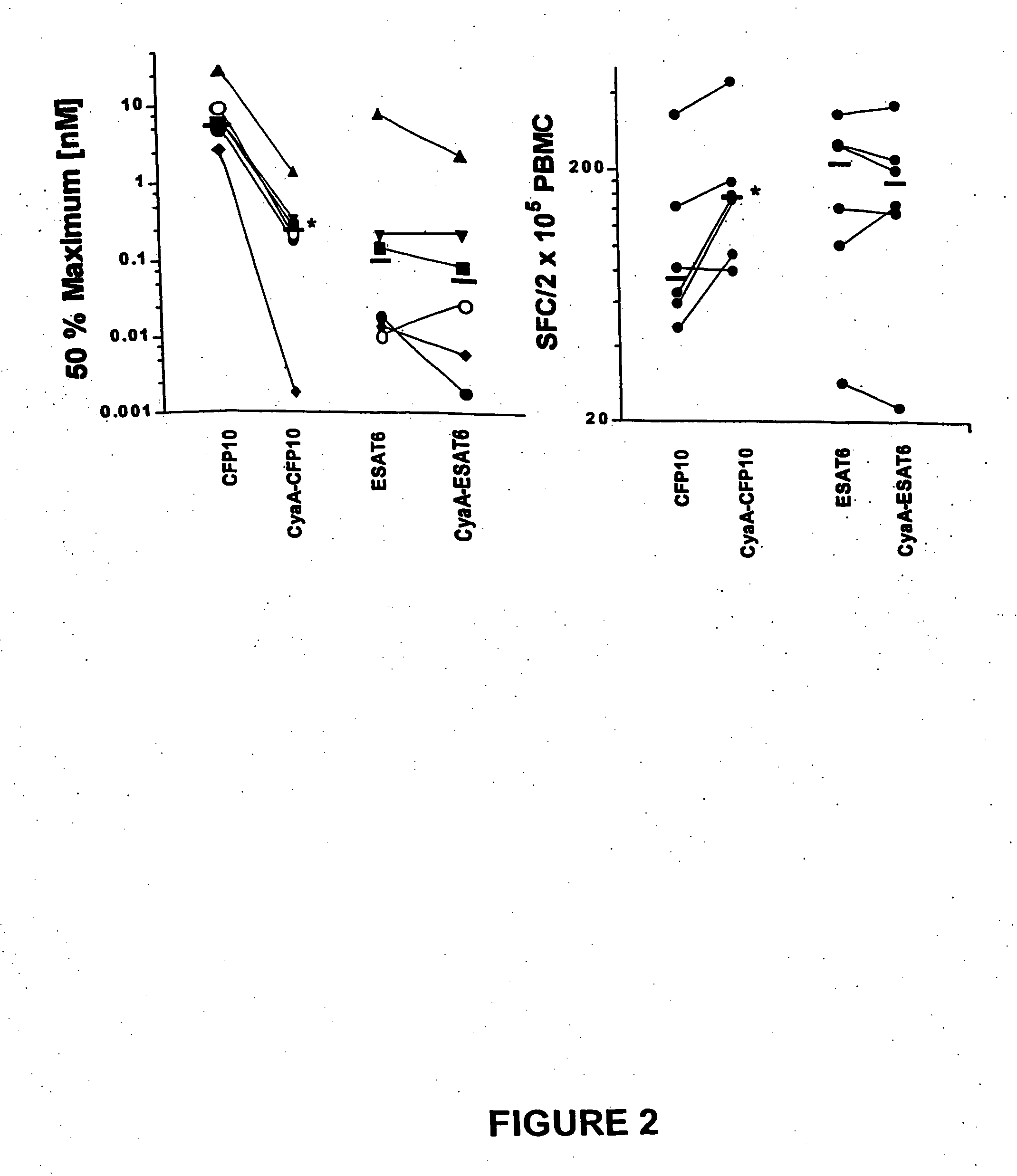
Diagnostic testing and immunomonitoring that uses genetically detoxified Bordetella pertussis CyaA as a delivery system are effective in tracking any immune responses, such as those generated by infectious and non-infectious diseases, or vaccinations, for example. T cells previously stimulated by a given antigen can be restimulated in vitro by the same antigen fused or chemically coupled to CyaA or a fragment thereof. The invention includes diagnostic tests and immunomonitoring for tuberculosis by providing a delivery system, which can deliver the M. tuberculosis immunodominant proteins ESAT-6 and CFP-10, to human cells and non-human animal cells, such as cattle. In addition, fusion proteins between CyaA and cancer antigens are also provided as diagnostic tests and immunomonitoring systems for cancers, such as melanoma.
Owner:INST PASTEUR
Recombinant expression and use for pertussis vaccine protective antigen
InactiveCN101074442AGood immune protectionIn line with the development trendBacterial antigen ingredientsDepsipeptidesProtective antigenEscherichia coli
A method for cloning PRN FIM2 and FIM3 genes from Bordetella pertussis CS strain and prokaryotic expressed recombinant protein by expression carrier are disclosed. The procedure is carried out by cloning CS strain to obtain PRN, FIM2 and FIM3 genes, sub-cloning to expression carriers separately, constructing FIM2-FIM3 fused gene expression plasmid, inducing and expressing in colibacillus. It has excellent immune protection and immunogenicity. They can be used as pertussis vaccine antigen active ingredients.
Owner:NAT INST FOR THE CONTROL OF PHARMA & BIOLOGICAL PROD +2
IPV-DPT vaccine
InactiveUS8753646B2Efficient productionAntibacterial agentsBacterial antigen ingredientsProtective antigenTetanus toxoids
The invention provides a process for producing a combined vaccine containing an inactivated Sabin strain of poliovirus, a Bordetella pertussis protective antigen, a diphtheria toxoid and a tetanus toxoid, the process including a step of producing a high-titer Sabin strain poliovirus. The inventive process for producing a combined vaccine, including a step of culturing, in the presence of from about 4 g / L to about 6 g / L of a microcarrier, Vero cells to be inoculated with a Sabin strain of poliovirus, is useful as a process for efficiently producing a combined vaccine containing an inactivated Sabin strain of poliovirus.
Owner:TAKEDA PHARMA CO LTD +1
Primer combination for detecting PCR of Elizabethkingia meningoseptica
ActiveCN107083443AImprove featuresIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseStaphylococcus aureus
The invention discloses a primer sequence combination and a method for applying the primer sequence combination in PCR amplification. The amplification target in the method is specific fragments EMA inside the genome of Elizabethkingia meningoseptica, and the method shows excellent specificity and sensibility; and moreover, by using the method, the neisseria meningitidis, haemophilus influenzae, staphylococcus aureus, streptococcus pneumoniae, escherichia coli, listeria monocytogenes, mycoplasma pneumoniae, bordetella pertussis, klebsiella pneumoniae and mycobacterium tuberculosis and the other pathogenic bacteria which are likely to cause meningitis and diseases of the upper respiratory tract, and easy to be confused with the Elizabethkingia meningoseptica in an identification of bacteria, can be distinguished at one time. Additionally, the lower detection limit of the method can reach 10-4 ng per Mul.
Owner:ICDC CHINA CDC
Primer group for detecting Bordetella pertussis, detection test kit and detection method
InactiveCN101875974AReduced Pollution ChancesIncrease costMicrobiological testing/measurementDNA/RNA fragmentationNucleotide sequencingGel electrophoresis
The invention discloses a primer group for detecting Bordetella pertussis, a detection test kit and a detection method. The primer group comprises an IS481 gene amplification pair and / or a PT gene amplification primer pair, wherein the primer pair sequence of the IS481 gene amplification pair is a nucleotide sequence shown as SEQ IDNO:1 and SEQ IDNO:2, and the primer pair sequence of the PT gene amplification primer pair is a nucleotide sequence shown as SEQ IDNO:3 and SEQ IDNO:4. The invention detects two genes simultaneously in the same reaction system, and the two genes can be detected through gel electrophoresis. The specificity of detection is guaranteed, and the sensitivity of detection is increased; meanwhile, detection efficiency is greatly increased, and detection cost is saved.
Owner:SHENZHEN CHILDRENS HOSPITAL
Kit for rapidly detecting bordetella pertussis and detection method
PendingCN111635950ASimplify detection stepsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPertusariaThermal stability
The invention discloses a kit for rapidly detecting bordetella pertussis and a detection method thereof. A specific probe is combined with a high-tolerance polymerase technology, a sample can be directly amplified after simple heating treatment, a nucleic acid extraction step is not needed, and the sample treatment time of a pertussis sample is shortened. The components used for detecting bordetella pertussis are prepared into a dry powder preparation, so that the thermal stability of the kit is remarkably improved, and the kit can be used for normal-temperature transportation. The single-tubepackaging of the multi-component dry powder reduces the step of liquid preparation. Experimental results prove that the kit and the detection method thereof are strong in specificity, high in sensitivity and simple to operate, and can be used for directly, quickly and accurately detecting the bordetella pertussis.
Owner:SHENZHEN YILIFANG BIOTECH CO LTD
Purification method for 69KD outer membrane protein of pertussis bacillus
InactiveCN102584958AEfficient inoculationLow costPeptide preparation methodsDepsipeptidesPurification methodsIon exchange
Owner:北京天坛生物制品股份有限公司
Suspension microbead array system for detecting common pathogens of respiratory tract infection
ActiveCN107254554AConducive to targeted treatmentLow costMicrobiological testing/measurementMicroorganism based processesBacteroidesMoraxella catarrhalis
The invention relates to a suspension microbead array system for detecting common pathogens of the respiratory tract infection. The system detects influenza A virus, influenza B virus, parainfluenza virus, human metapneumovirus, rhinovirus, bocavirus, adenovirus, respiratory syncytial virus, mycoplasma pneumoniae, moraxella catarrhalis, haemophilus influenza, streptococcus pneumoniae and bordetella pertussis in one experiment. The suspension microbead array system finishes the detection of 8 viruses, 4 bacteria and 1 mycoplasma in one experiment, prevents leak detection to the greatest extent, and is beneficial for the targeted clinical treatment; the cost of reagents, if divided on the detection of each index, is greatly lowered, so that the medical cost is reduced.
Owner:广东辉锦创兴生物医学科技有限公司
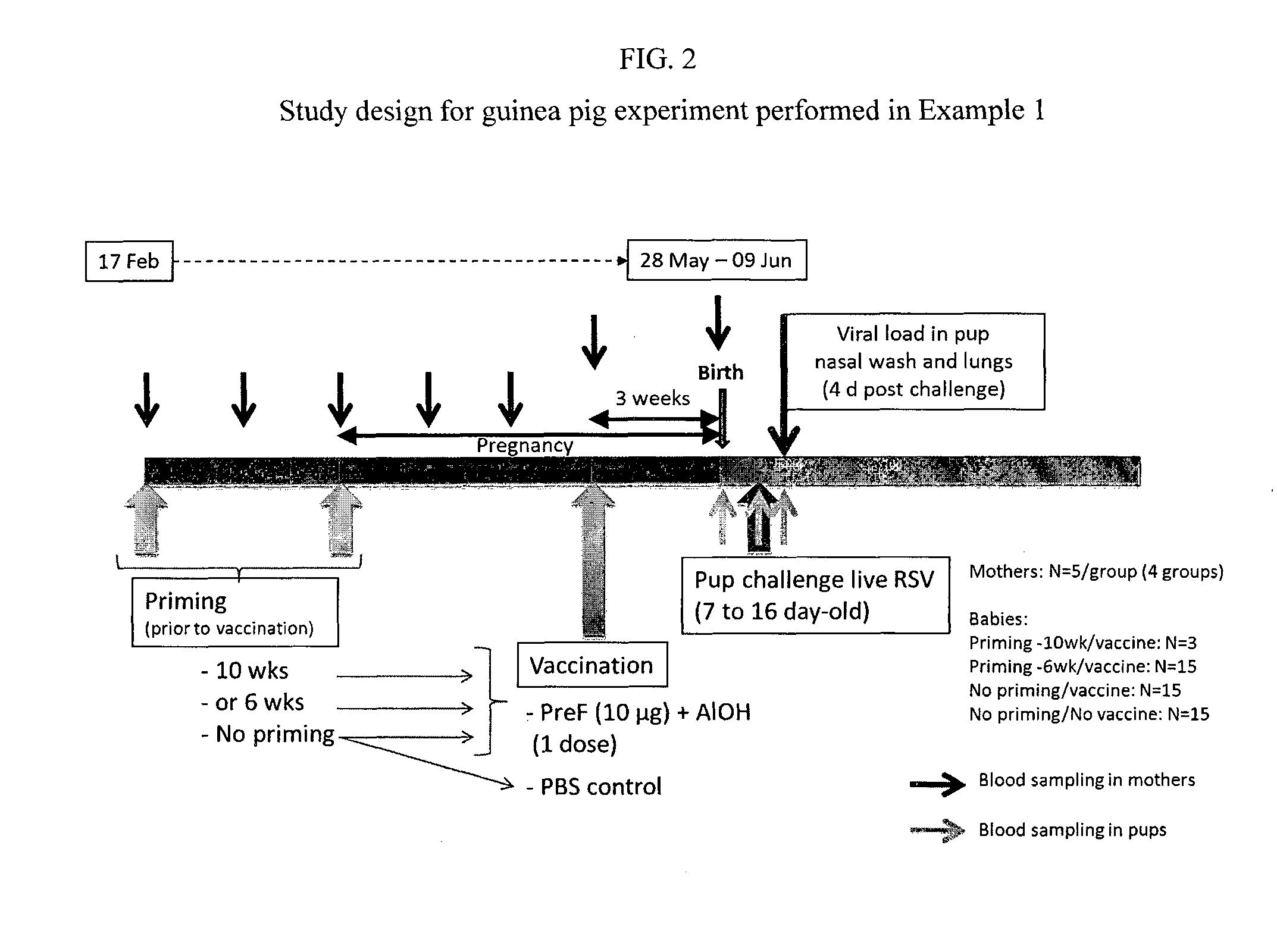
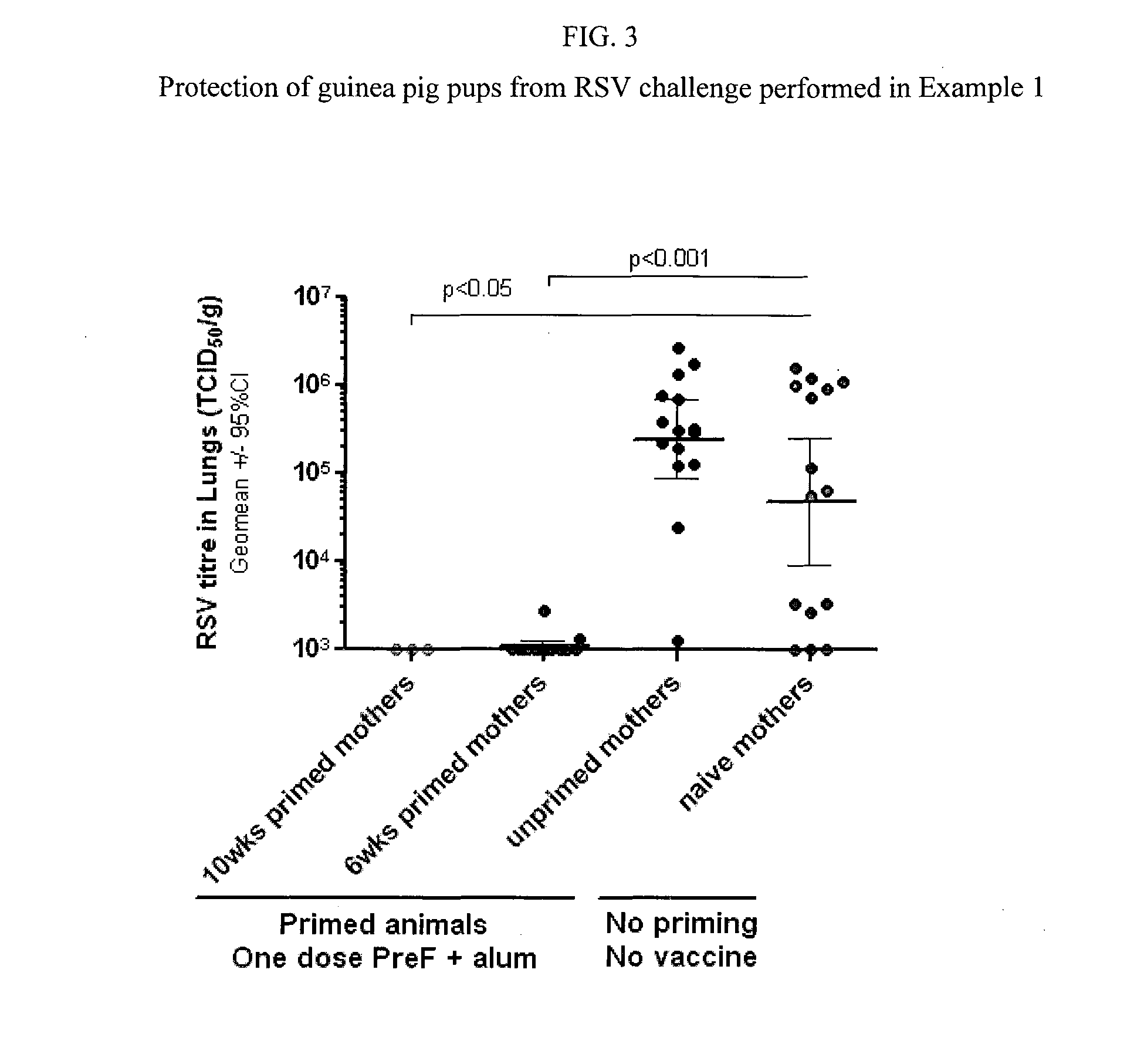
Method for eliciting in infants an immune response against rsv and b. pertussis
InactiveUS20150202283A1Antibacterial agentsSsRNA viruses negative-senseAntigenBordetella pertussis DNA
This disclosure provides methods for protecting infants against disease caused by respiratory syncytial virus (RSV) and Bordetella pertussis through maternal immunization using recombinant respiratory syncytial virus (RSV) and B. pertussis antigens to reduce the incidence or severity of RSV and pertussis infection in young infants.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
CRISPR detection primer group for bordetella pertussis and application of CRISPR detection primer group
ActiveCN110791578AShorten detection timeIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPertusariaSequenceome
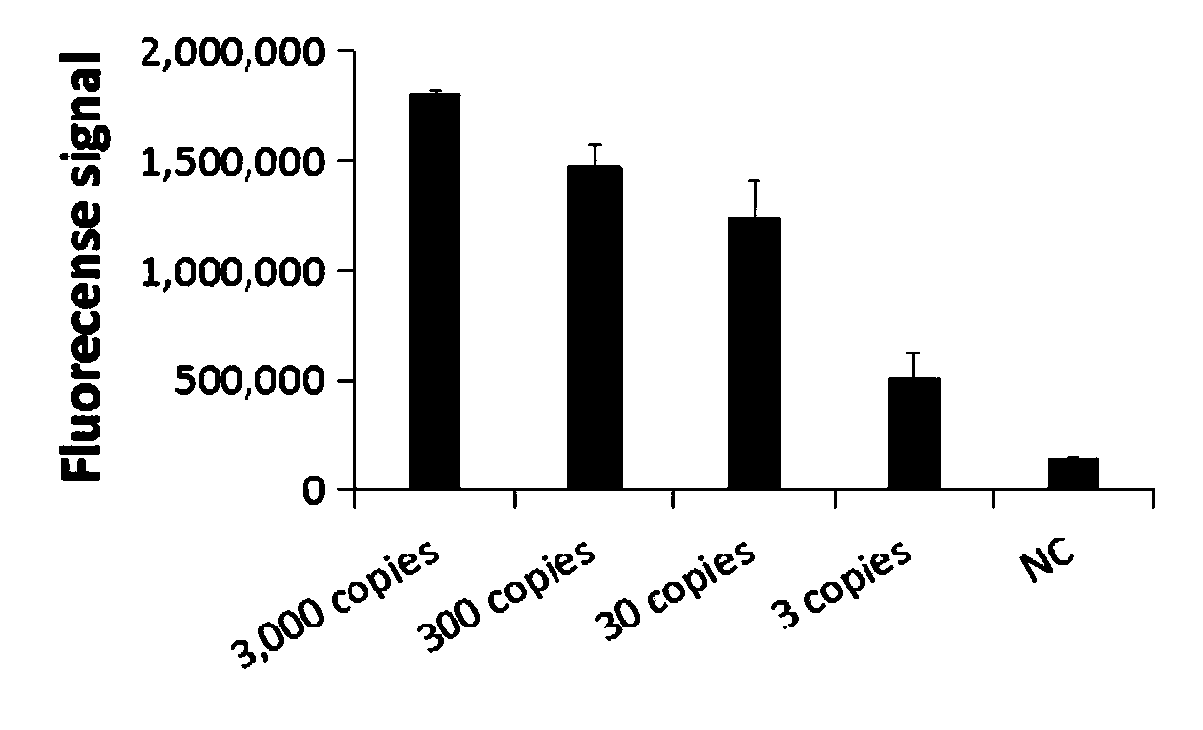
The invention relates to a CRISPR detection primer group for bordetella pertussis and an application of the CRISPR detection primer group, which belong to the technical field of gene detection of CRISPR technologies. The primer group comprises an amplification primer pair and crRNA, wherein the amplification primer pair is used for amplifying a sequence of bordetella pertussis as shown in SEQ ID NO.1; wherein the crRNA comprises an anchoring sequence and a guide sequence, the anchoring sequence and the Cas protein are specifically recognized, and the guide sequence is matched with the targeting sequence fragment in the SEQ ID NO.1 sequence. By adopting the primer group, the bordetella pertussis is detected by a CRISPR technology, so that the detection time of the bordetella pertussis is shortened, and the detection can be completed within 60 minutes. According to the invention, a specific sequence combination obtained by screening is used as the primer group for detection, so that theprimer group has the advantages of high sensitivity and strong specificity, and the detection limit can reach 3 copies. The primer group is used for CRISPR detection of bordetella pertussis, dependence on qPCR instruments and other complex variable-temperature amplification instruments is avoided, and the primer group has wide application prospects.
Owner:广州微远医疗器械有限公司 +3
Bordetella pertussis and primer probe combination and kit for specific detection of bordetella parapertussis
ActiveCN102634596ALow detection costStrong specificityMicrobiological testing/measurementMicroorganism based processesBordetella pertussis DNABordetella parapertussis infection
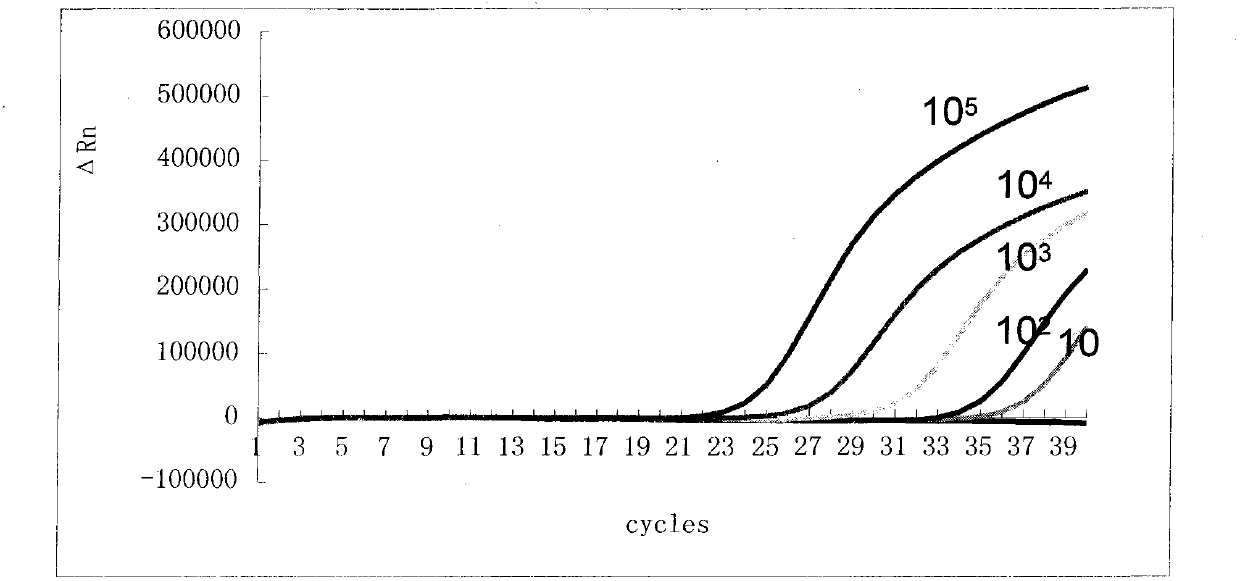
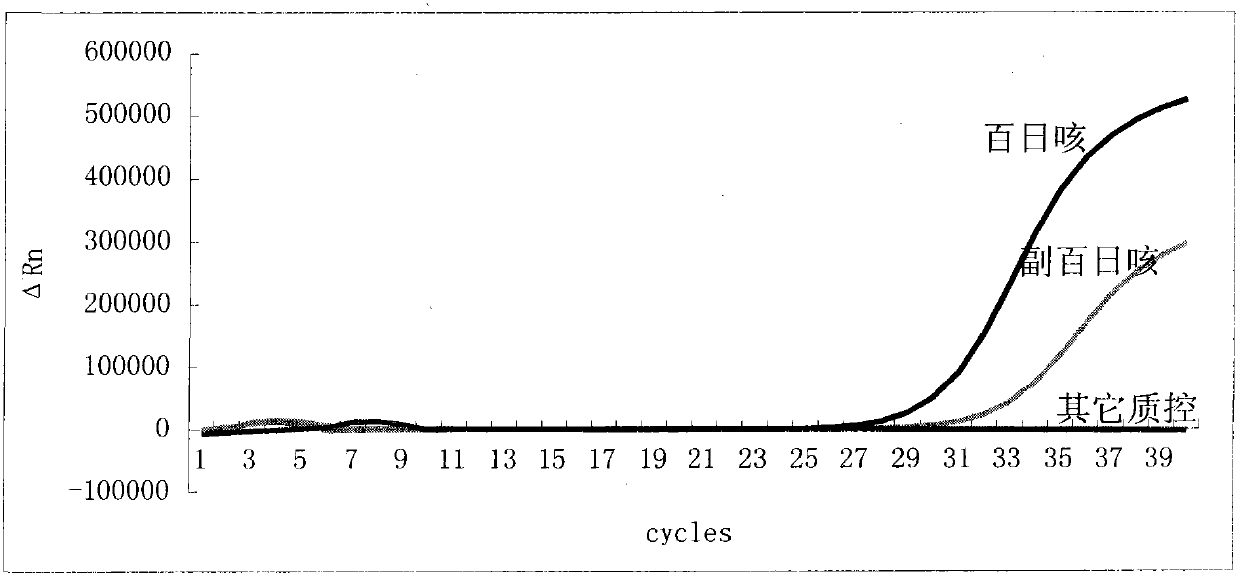
The invention relates to an oligonucleotide sequence combination for specific detection of bordetella pertussis and bordetella parapertussis nucleic acids existing in a sample with the adoption of fluorescent PCR (polymerase chain reaction), and a kit containing the combination. The kit can sensitively detect the bordetella pertussis nucleic acid and the bordetella parapertussis nucleic acid existing in the sample with a detection lower limit of 20 copies in each reaction system, and has important application value in the fields such as disease surveillance and clinical diagnosis.
Owner:JIANGSU UNINOVO BIOLOGICAL TECH
Primer group, kit and method for detecting respiratory pathogens
InactiveCN109457053AAvoid the cons of being limitedReduce testing costsMicrobiological testing/measurementAgainst vector-borne diseasesEnterovirusBordetella parapertussis infection
The invention discloses a primer group, a kit and a method for detecting respiratory pathogens and relates to the technical field of respiratory pathogen detection. The primer group for detecting therespiratory pathogens comprises, such as, one or a combination of several of a first primer to a thirteenth primer. The primer group can realize detection of common respiratory pathogens such as a respiratory syncytial virus, an adenovirus, a human metapneumovirus, a rhinovirus / enterovirus, a parainfluenza virus, a coronavirus, a bocavirus, an influenza virus A, an influenza virus B, bordetella pertussis, bordetella parapertussis, mycoplasma pneumoniae and chlamydia pneumoniae and has the characteristics of relatively high sensitivity and specificity, low cost, convenience in detection, relatively good repeatability and the like.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Protective epitopes of adenyl cyclase-haemolysin (AC-Hly), their application to the treatment or to the prevention of bordetella infections
Owner:INST PASTEUR
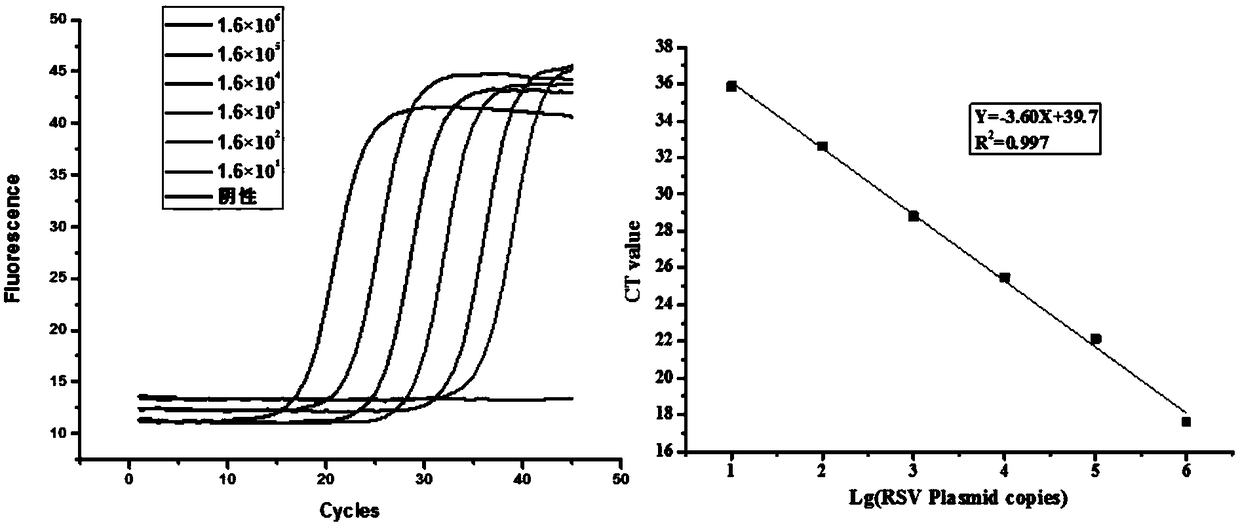
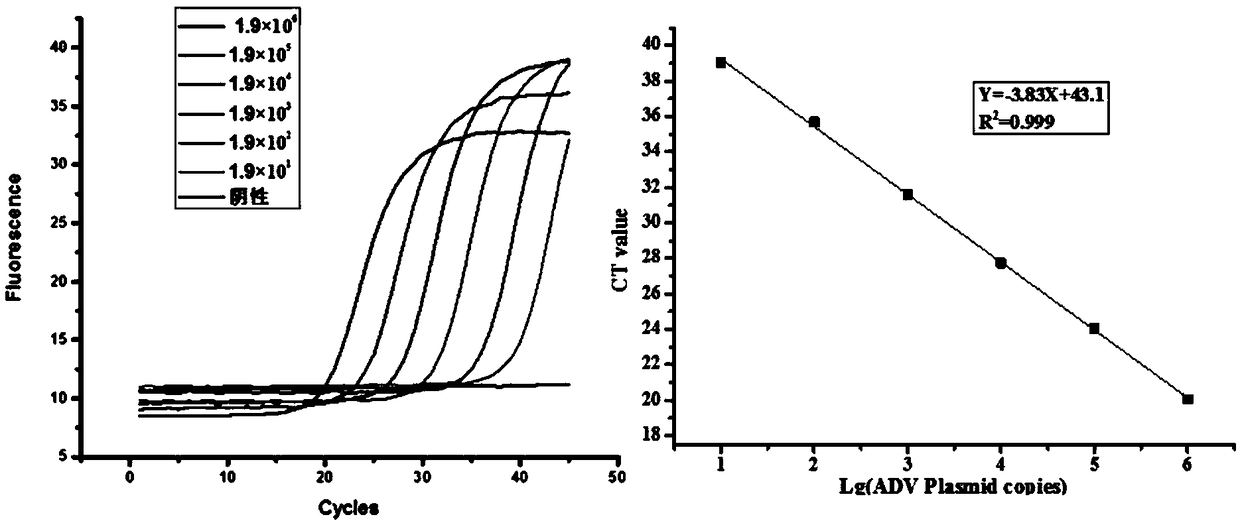
Method for removing pertussis component pilin 2/3 endotoxin
ActiveCN110358802AMaintain biological activityMaintain recoveryMicroorganism based processesDepsipeptidesPhosphateBordetella pertussis
The invention relates to the technical field of biologic pharmacy, and discloses a method for removing pertussis component pilin 2 / 3 endotoxin. The method comprises the steps that after bordetella pertussis is fermented and cultured, thalli are centrifugally collected, thalli are extracted through a urea phosphate buffer solution, a supernate is centrifugally collected, and PEG-8000 and ammonium sulfate are used for performing precipitation; precipitates are extracted by the phosphate buffer solution, then, a supernate is centrifugally collected, a Q-Sepharose chromatographic column is adoptedfor processing, and a phosphate buffer solution containing sodium chloride is adopted for eluting the Fim 2 / 3 component. By combining PEG8000 precipitating, ammonia sulfate precipitating and Q-Sepharose chromatographic column processing are combined, endotoxin in pilin 2 / 3 is removed through repeated means, on the premise of not introducing allogenic materials, the effective ingredient Fim 2 / 3 biologic activity and recycling rate are kept, and meanwhile the endotoxin in Fim 2 / 3 can be removed to 10 EU / mg or lower.
Owner:CHANGCHUN BCHT BIOTECH
Whooping cough genetic engineering blending second unit vaccine and preparing method thereof
InactiveCN101199843AEfficient expressionOvercome technical difficulties that are difficult to expressAntibacterial agentsBacterial antigen ingredientsSide effectPertussis toxin
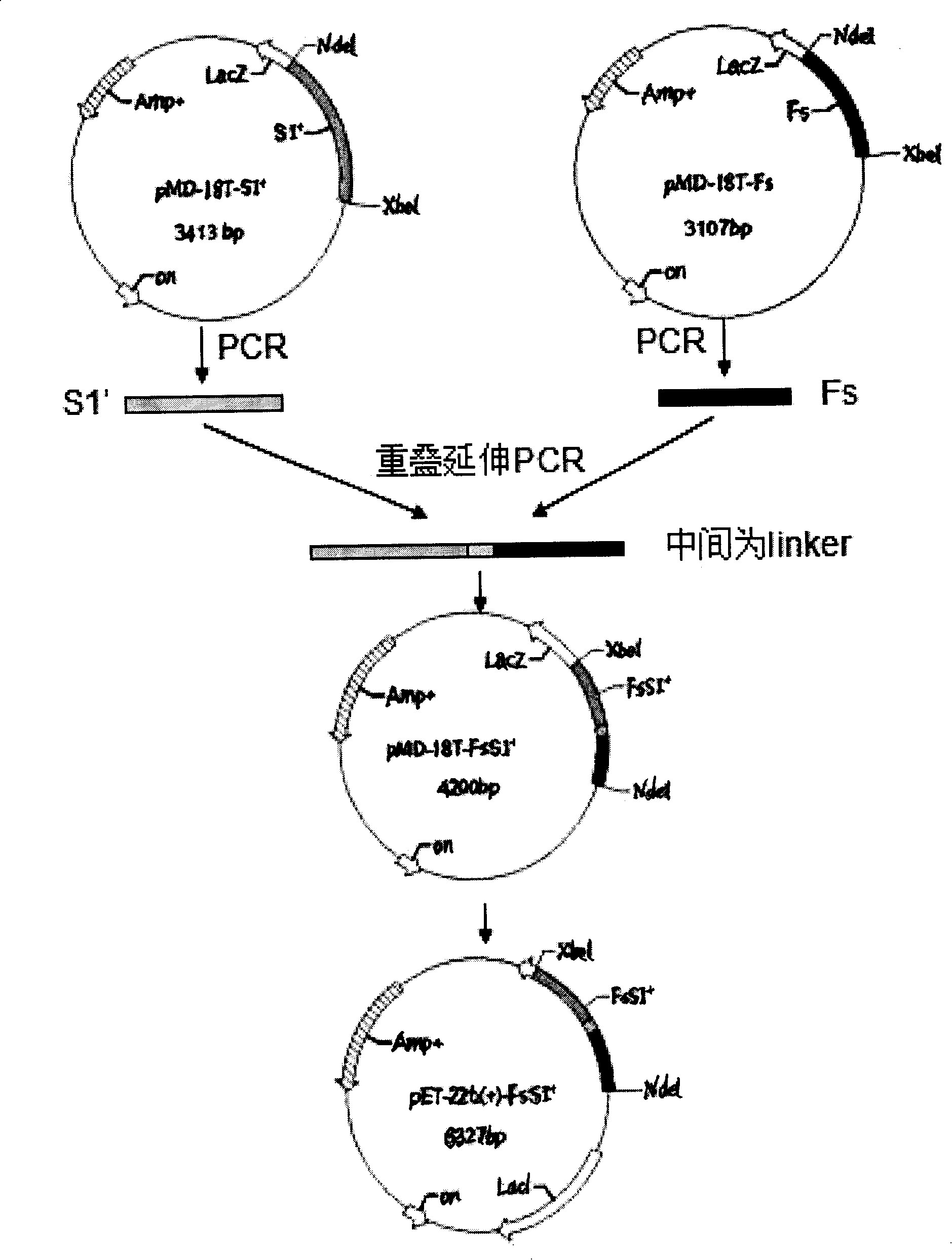
The invention relates to a pertussis genetic-engineering fused subunit vaccine, which is fused protein produced by connecting pertussis toxin S1 subunit mutant with a peptide Fs. The invention also relates to a preparation method of bordetella pertussis double-subunit genetic engineering vaccine. The invention, which overcomes a variety of deficiencies of the traditional vaccine and the traditional preparation method, has the advantages of mass production potential, easy operation, high security, small side effect and no reverse mutation.
Owner:ARMY MEDICAL UNIV
Bordetella pertussis PT (pertussis toxin) antigen monoclonal antibody and application thereof
InactiveCN105039259AIntegrity guaranteedImproving immunogenicityAntibacterial agentsImmunoglobulins against bacteriaAntigenImmune monitoring
The invention relates to a bordetella pertussis PT (pertussis toxin) antigen monoclonal antibody and application thereof, and belongs to the field of preparation of biological products. The bordetella pertussis PT antigen monoclonal antibody is secreted by hybridoma cell strains capable of stably secreting bordetella pertussis FT antigen monoclonal antibodies, and a preservation number of the hybridoma cell strains is CGMCC No.10588. The bordetella pertussis PT antigen monoclonal antibody and the application have the advantages that the monoclonal antibody is high in potency and good in specificity, and can be applied to monitoring the quality in bordetella pertussis vaccine production procedures, carrying out enzyme-linked immunosorbent assay on contents of PT components in finished vaccine and preparing bordetella pertussis PT antigen enzyme-linked immune monitoring reagents or enzyme-linked immune monitoring reagent kits.
Owner:SINOVAC RES & DEV
Vaccine for prophylaxis or treatment of an allergen-driven airway pathology
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Methods of identifying compounds that inhibit the activation of a biomolecule and methods of treatment using the compounds
The present invention relates to methods of identifying compounds that inhibit the activation between a biomolecule, pharmaceutical compositions comprising such compounds, and methods of treating and / or to reducing the risk of Bacillus anthracis and Bordetella pertussis infection by administering such pharmaceutical compositions.
Owner:INST PASTEUR
Kit for detecting three respiratory pathogens based on micro-fluidic chip and use method of kit
ActiveCN108559790AStrong specificityIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesConserved sequenceFluorescence
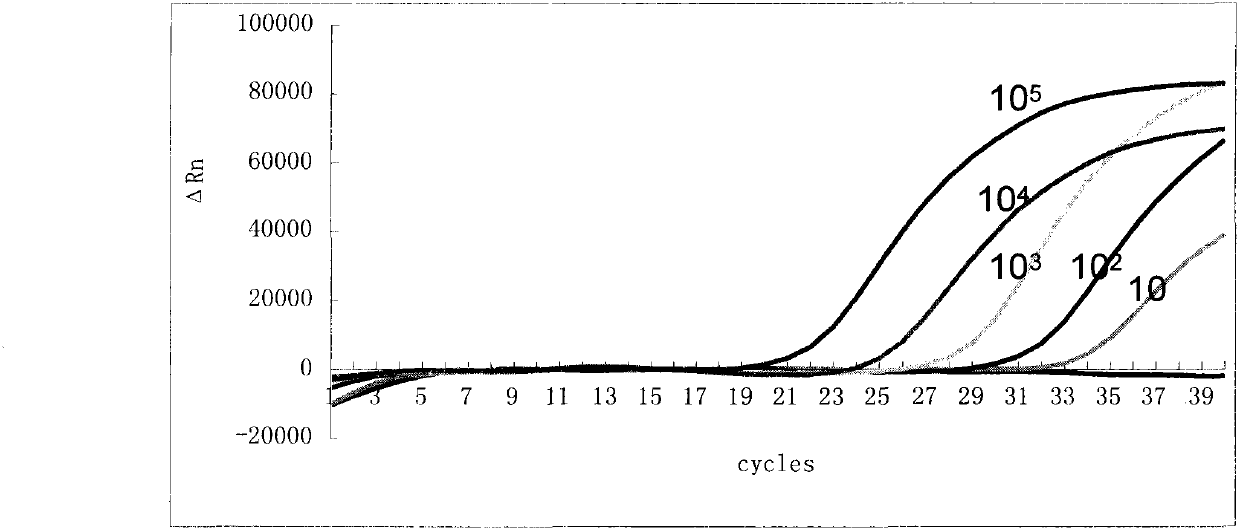
The invention discloses a kit for detecting respiratory adenoviruses, streptococcus pneumoniae and bordetella pertussis based on a micro-fluidic chip and a use method of the kit. The kit comprises a multi-flux micro-fluidic nucleic acid detection chip for actively controlling flow paths, a pre-filled dry powder detection reagent and a positive quality control product, wherein the pre-filled dry powder detection reagent contains primers with specific conserved sequences for the respiratory adenoviruses, the streptococcus pneumoniae and the bordetella pertussis and a TaqMan fluorescent probe. The kit disclosed by the invention adopts a micro-fluidic chip technology, all operation processes are completed by instruments after application of a sample, the operation is simple and convenient, thespeed is high, the detection can be completed within 30-60 min, and pollution can be avoided; the fluorescent PCR technology of the TaqMan probe is adopted to detect the respiratory adenoviruses, thestreptococcus pneumoniae and the bordetella pertussis; the sequences of the designed primers and probe are very conserved in genes of the respiratory adenoviruses, the streptococcus pneumoniae and the bordetella pertussis, and the designed primers and probe have high specificity; and detection results can be obtained within 2h, and the sensitivity can reach 10 copies / mu L.
Owner:NANJING LANSION BIOTECH CO LTD
Recombinant adenylate cyclase of BORDETELLA SP. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase
InactiveUS20090317854A1Improve responseEasy to testMicrobiological testing/measurementMaterial analysisAntigenCyaA
Owner:INST PASTEUR INSERM +3
Production method of acellular pertussis vaccine
ActiveCN102793915AAvoiding pitfalls in the detoxification processMaintain immunogenicityAntibacterial agentsBacterial antigen ingredientsAcellular pertussis vaccinesGenetic engineering
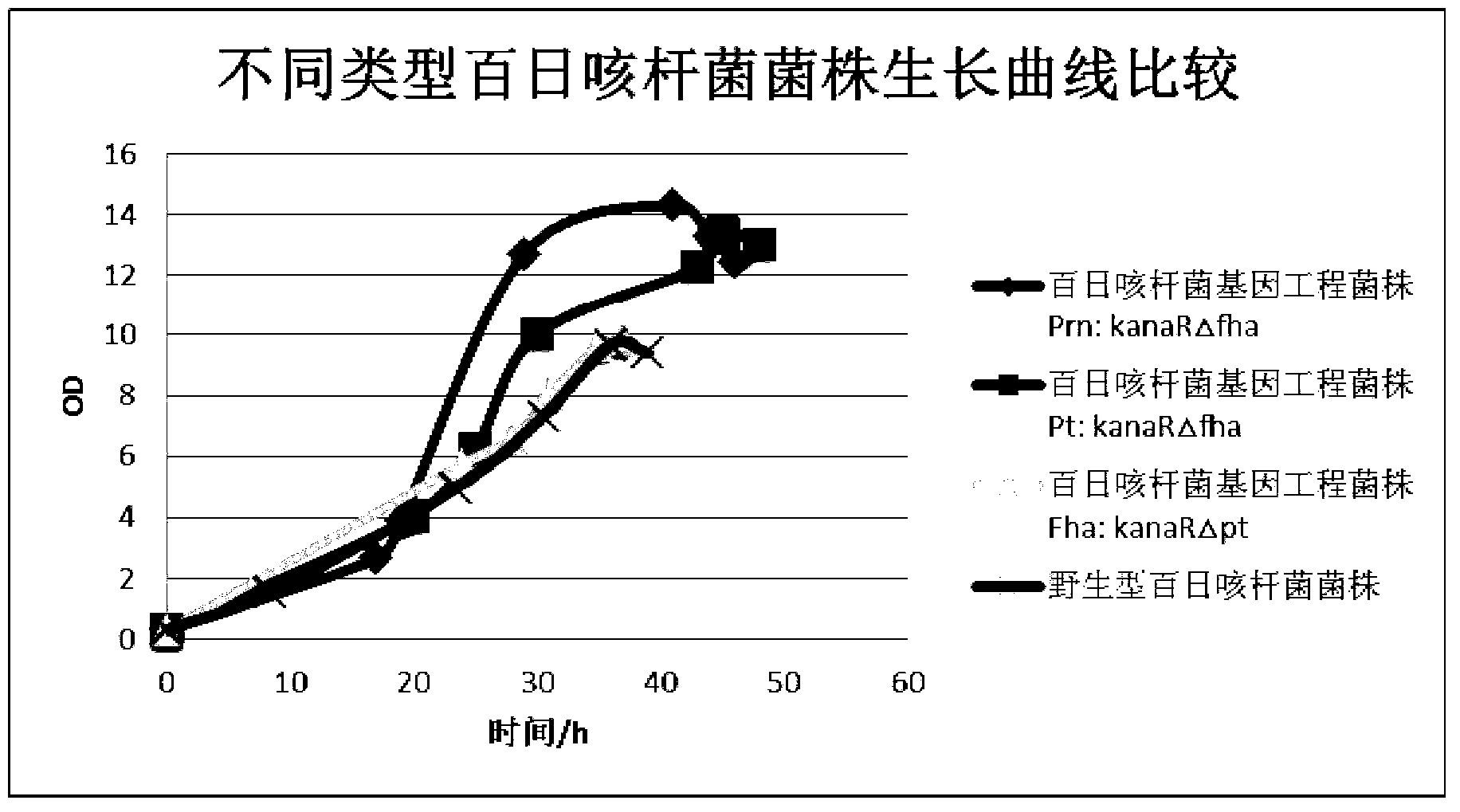
The invention discloses a production method of an acellular pertussis vaccine. The production method comprises the following steps of: fermenting and purifying a Fha bordetella pertussis genetic engineering strain in which pt gene is knocked out to obtain an antigen component fha; fermenting and purifying the Pt bordetella pertussis genetic engineering strain in which fha gene is knocked out to obtain an antigen component pt; fermenting and purifying Prn bordetella pertussis genetic engineering strain in which fha gene is knocked out to obtain an antigen component prn; and preparing the acellular pertussis vaccine from the antigen components fha, pt and prn. According to the method, high-efficiency expression of a single target antigen can be realized, so that the growing speed of the bordetella pertussis genetic engineering strain is remarkably accelerated, and the production time and production cost can be reduced.
Owner:CANSINO BIOLOGICS INC
Kit and method for fluorescent quantitative detection of Bordetella pertussis
InactiveCN107267653ARapid clear infectionIncrease usageMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceBiology
The invention discloses a kit and a method for fluorescent quantitative detection of Bordetella pertussis, and provides a fluorescent quantitative reagent for detecting the Bordetella pertussis. The fluorescent quantitative reagent comprises a primer 1 for detecting the Bordetella pertussis, a primer 2 for detecting the Bordetella pertussis and a probe 1 for detecting the Bordetella pertussis. Experimental results prove that the kit and the detection method is based on Taqman fluorescent quantitative detection technique, pathogens of infection can be rapidly defined, detection expense is decreased, use efficiency of instruments is improved, and the method solves the problem of flux of clinical respiratory fungus infection pathogen detection and has important guide significance for clinical early diagnosis and treatment.
Owner:BEIJING FUANHUA BIOLOGICAL TECH CO LTD
High yield pertussis vaccine production strain and method for making same
InactiveUS20060154354A1Increase chanceOvercomes shortcomingBiocideBacterial antigen ingredientsHemagglutininAntibiotic resistance
The present invention provides a vaccine production strain of Bordetella bronchiseptica that produces a pertussis toxin in high yield. The present invention provides a method for creating a Bordetella bronchiseptica cell line which produces a Bordetella pertussis toxin comprising the steps of introducing a plasmid containing a DNA encoding antibiotic resistance into a Bordetella bronchiseptica strain, selecting for isolates in which the DNA encoding antibiotic resistance is recombinantly incorporated into the chromosome in place of the Bordetella bronchiseptica toxin gene, introducing a plasmid containing DNA encoding subunits of the Bordetella pertussis toxins into the Bordetella bronchiseptica isolates; and, selecting for isolates in which DNA encoding Bordetella pertussis toxin subunit is recombinantly incorporated into the chromosome, the resulting cells producing the Bordetella pertussis toxin. The present invention further provides a method for creating a Bordetella bronchiseptica cell line which produces a Bordetella pertussis toxin and does not express filamentous hemagglutinin.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Primer probe combination, PCR reaction fluid, kit and application thereof
InactiveCN107338286AReduce false negative rateStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceBiology
The invention discloses a primer probe combination. The primer probe combination includes primers and a probe for detecting Bordetella pertussis, the primers for detecting the Bordetella pertussis includes an upstream primer and a downstream primer, wherein the upstream primer for detecting Bordetella pertussis is shown as SEQ ID NO. 1 in a sequence table; the downstream primer for detecting Bordetella pertussis is shown as SEQ ID NO. 2 in the sequence table; the probe used for detecting Bordetella pertussis is shown as SEQ ID NO. 3 in the sequence table. Based on the primer probe combination, the invention further provides a PCR reaction liquid, a kit and an application thereof. According to the primer probe combination and the kit, the primer pair and the probe with good specificity are designed based on the gene sequence of Bordetella pertussis, the kit can only detect Bordetella pertussis, and the specificity of the kit is good.
Owner:SHENZHEN CHILDRENS HOSPITAL +1
Purification of a pertussis outer membrane protein
InactiveUS6444211B2Bacterial antigen ingredientsSnake antigen ingredientsUltrafiltrationIon exchange
Pertactin (formerly 69 kDa protein) is recovered in stable biologically pure form having no detectable adenylate cyclase activity from fermentation broth from the fermentation of Bordetella pertussis as well as from the cells. The broth is processed to selectively remove pertussis toxin (PT) and filamentous haemagglutinin (FHA), the pertactin is precipitated by ammonium sulphate and the precipitate is dissolved in buffer at pH 6.0 to 8.5, the solution then is passed through hydroxyapatite and ion-exchange chromatograph columns before final ultrafiltration. Cells are extracted with urea and the extract ultrafiltered and diafiltered. The pertactin is precipitated from the extract and the precipitate processed as above. In a variation, the broth is contacted with ammonium sulphate to precipitate pertactin, PT and FHA, the precipitate is dissolved and the PT and FHA selectively removed, before the solution is passed to the chromatograph columns.
Owner:CONNAUGHT LAB
Deacylation of LPS in gram negative bacteria
ActiveUS8048433B2Low toxicityReduce in quantityAntibacterial agentsBiocideBordetella parapertussis infectionGram
The current invention provides new Gram negative polypeptides exhibiting lipid A 3-O-deacylase activity and are capable of modifying and / or detoxifying gram negative LPS. The present invention also provides Gram negative bacteria, Gram negative bacterial lipopolysaccharides (LPS) and compositions comprising LPS, which are provided with or treated with a 3-O-deacylase activity according to the invention and which may be used for pharmaceutical and / or veterinary purposes, in particular for the preparation of whole cell or acellular vaccines against pathogenic Gram negatives such as Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica.
Owner:NVI NEDERLANDS VACCININSTITUUT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com