Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Immune monitoring" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune monitoring provides insight into a patient’s own immune response over the course of a treatment. Information such as whether immune cells are functional or non-functional, and what specific proteins the immune cells can recognize on the cancer cell, can be essential in understanding if (and how)...
Method for differentially quantifying naturally processed hla-restricted peptides for cancer, autoimmune and infectious diseases immunotherapy development
ActiveUS20130096016A1Efficient use ofBiological material analysisLibrary member identificationDiseaseAntigen
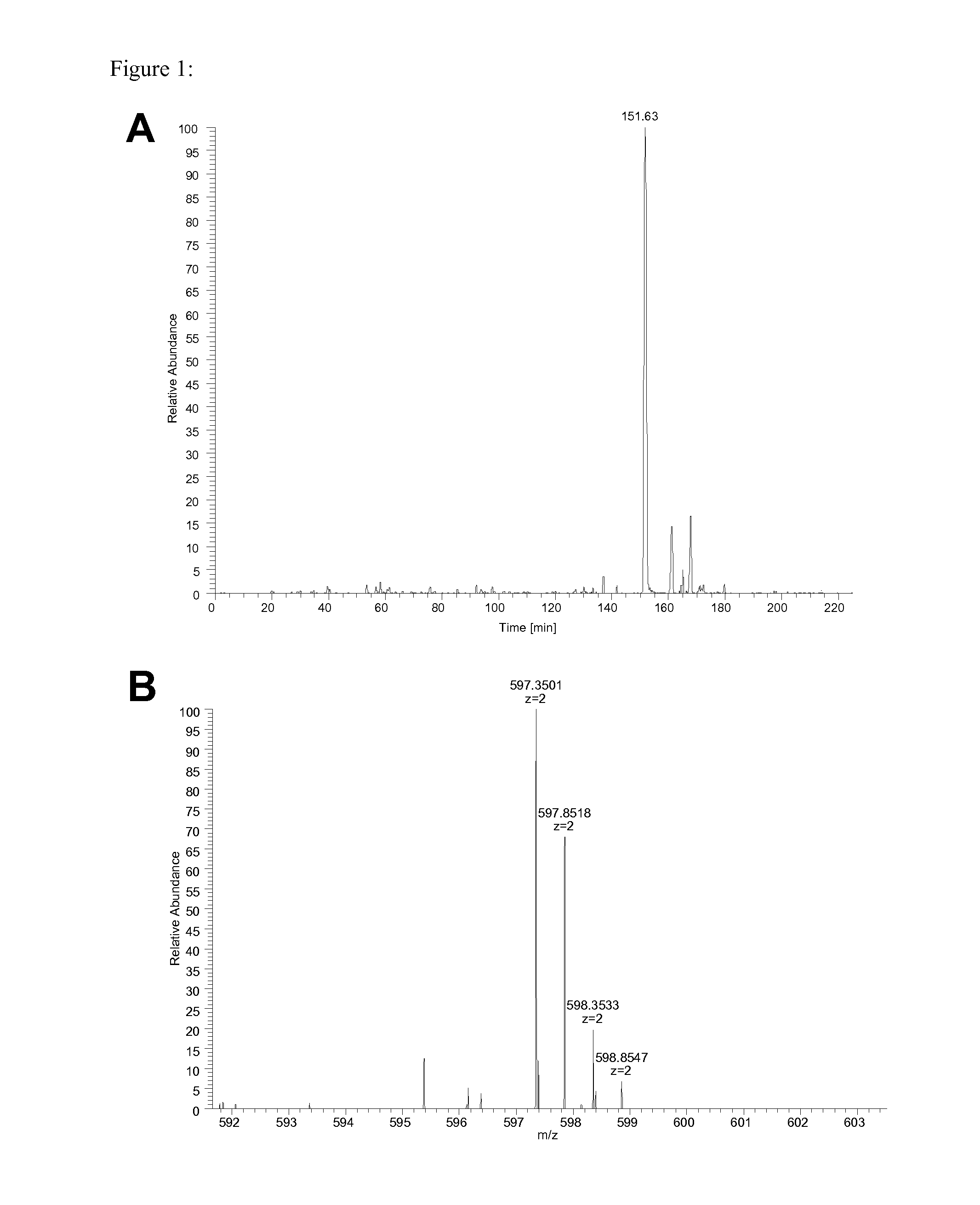
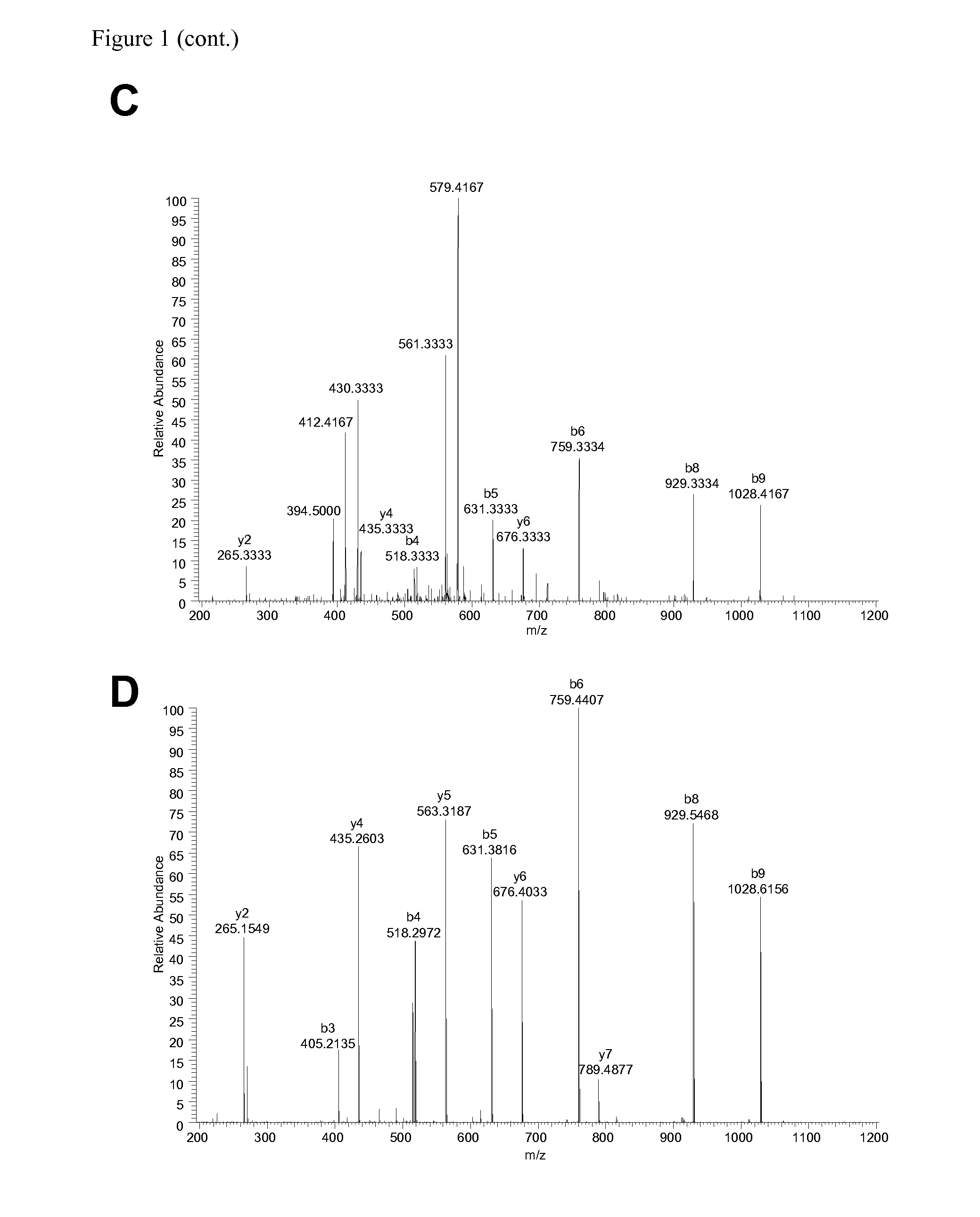
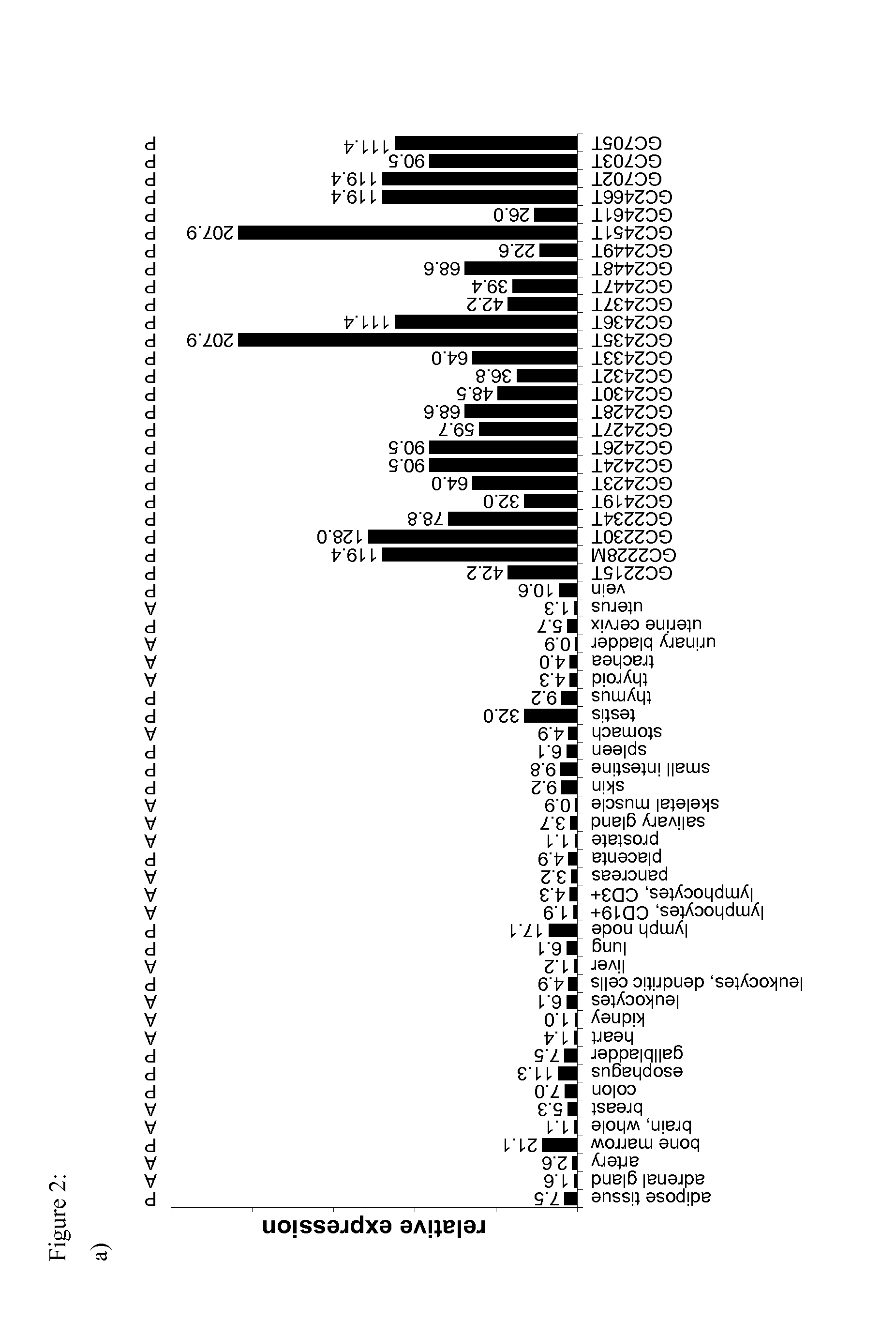
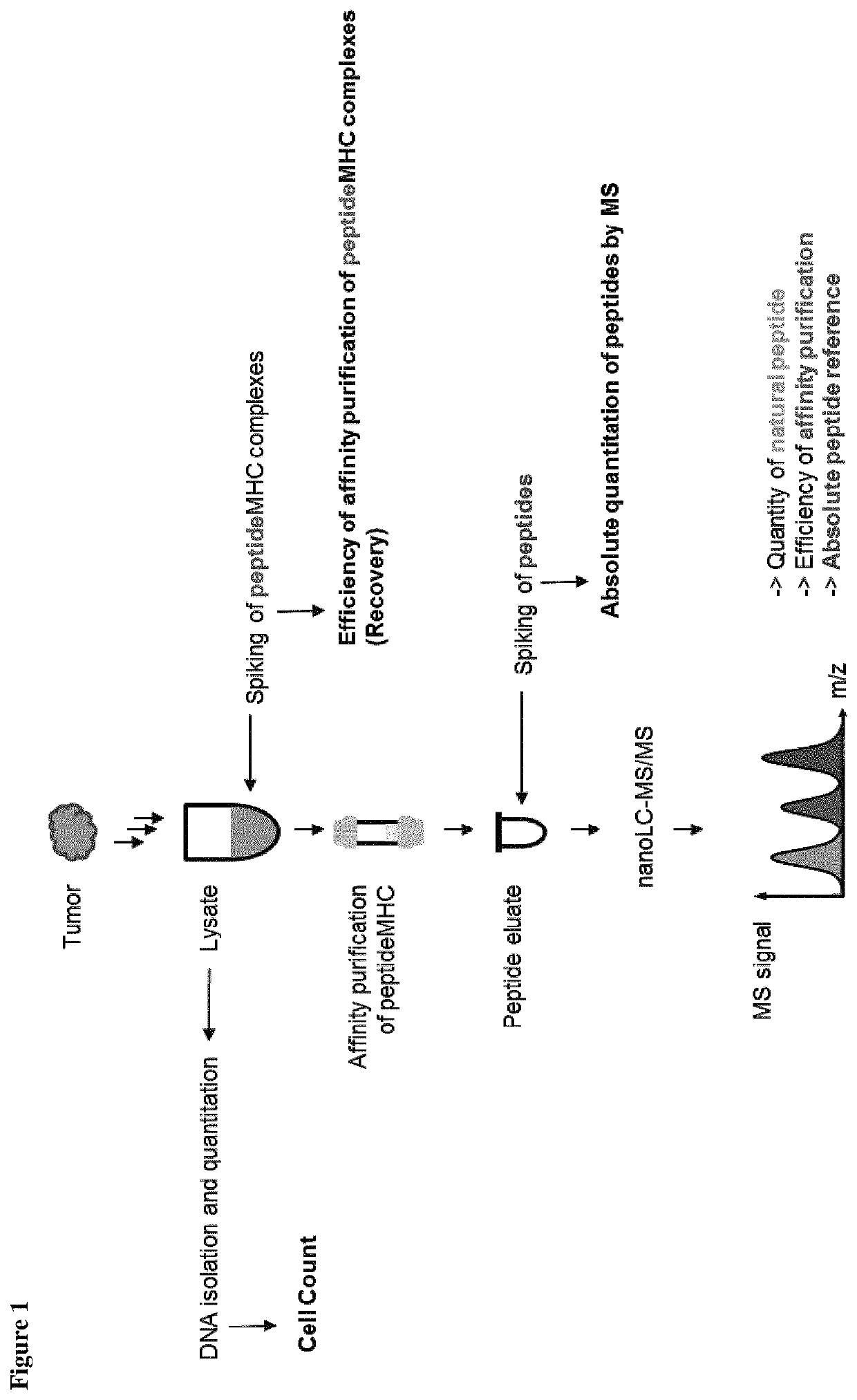
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
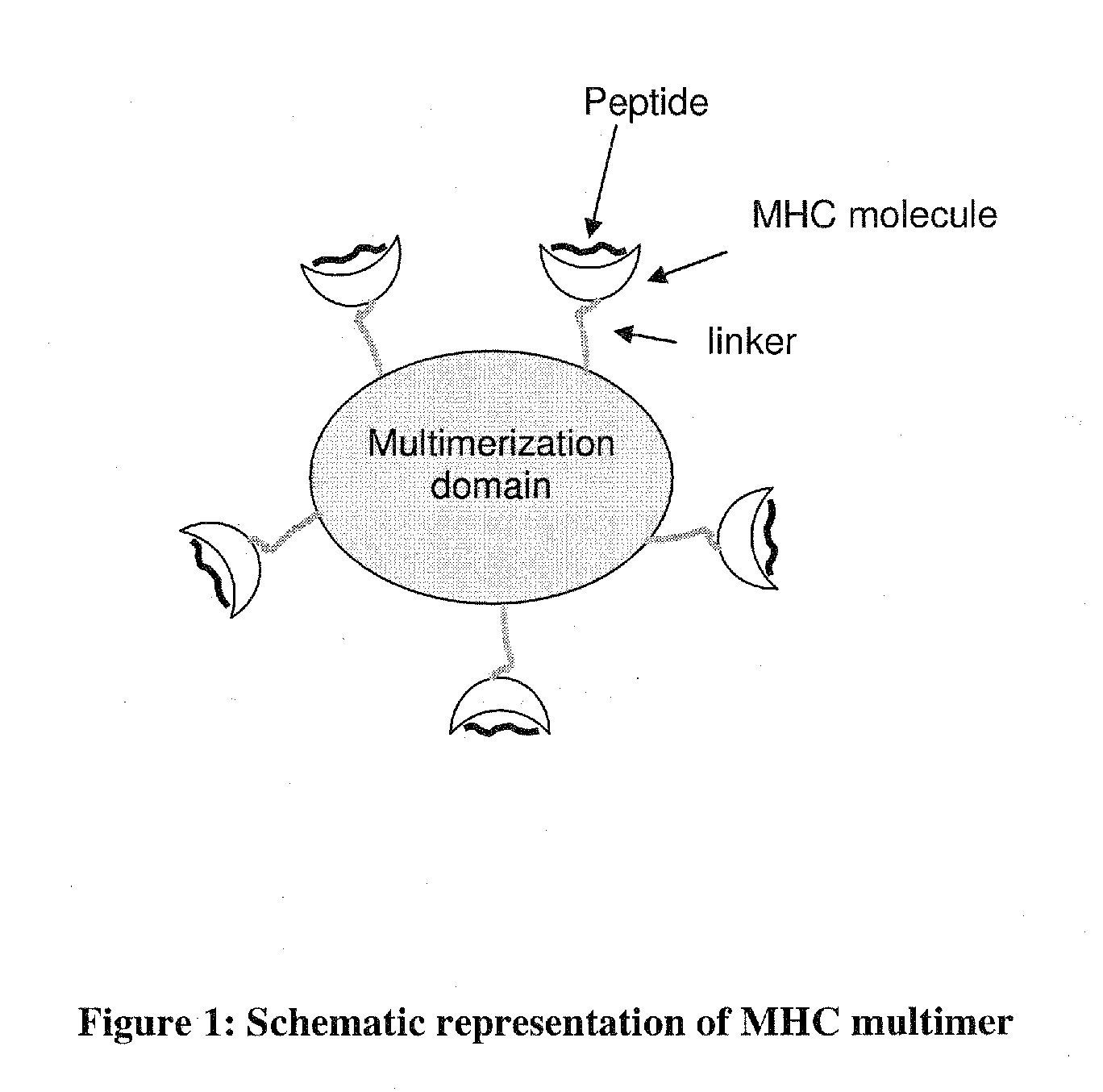
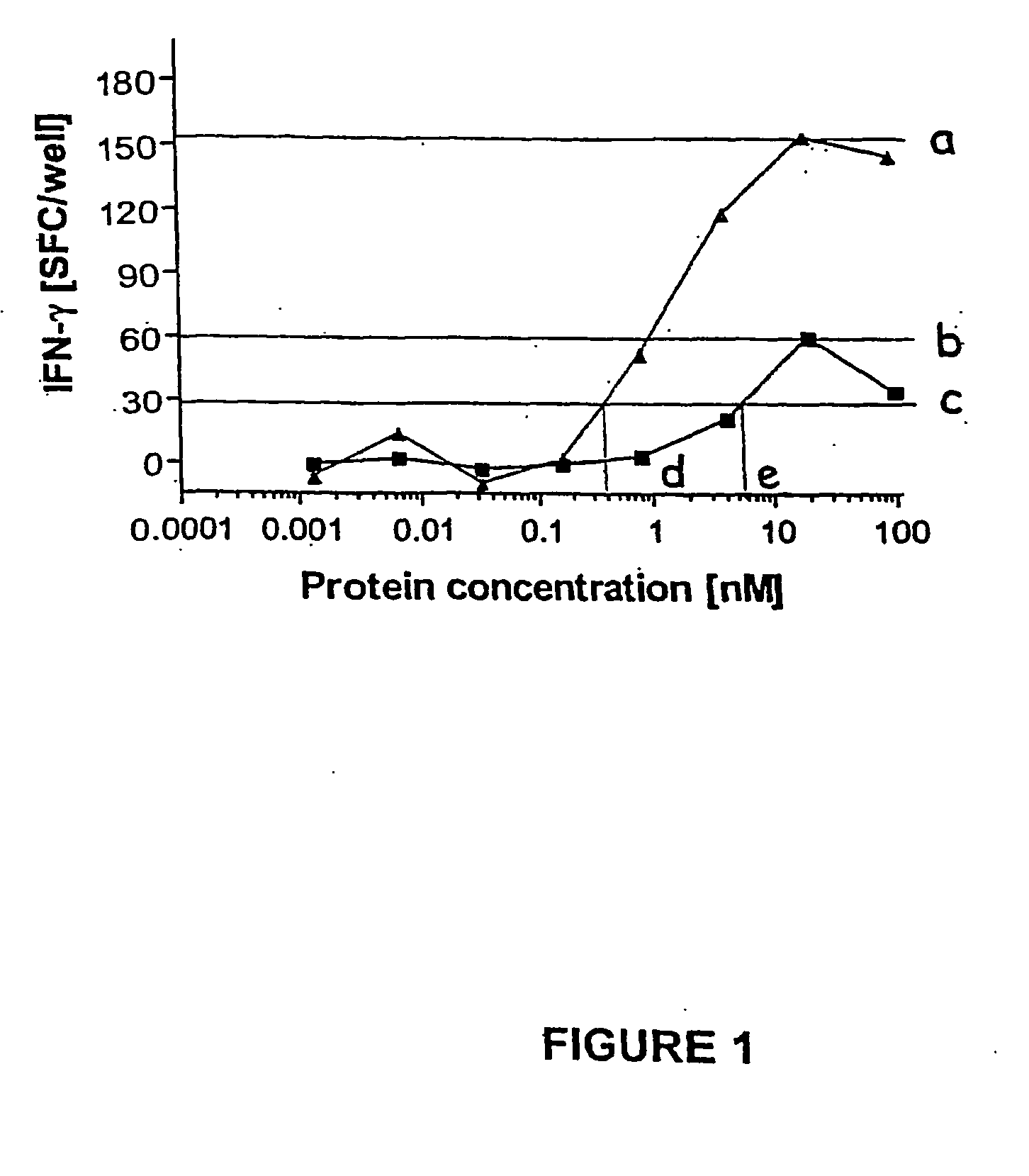
MHC Multimers in Cancer Vaccines and Immune Monitoring
InactiveUS20110318380A1Reduces infectious titerImprove efficacyPeptide/protein ingredientsImmunoglobulinsAntigenDisease
The present invention relates to MHC-peptide complexes and uses thereof in the diagnosis of, treatment of or vaccination against a disease in an individual. More specifically the invention discloses MHC complexes comprising cancer antigenic peptides and uses there of.
Owner:AGILENT TECH INC
Enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody, test method and application thereof
InactiveCN101839917AStrong specificityIncreased sensitivityDepsipeptidesFermentationAntigenDuck hepatitis A virus
The invention relates to an enzyme-linked immunosorbent assay (ELISA) kit for duck hepatitis virus type-I serum antibody and relates to a test method and application of the kit. The kit comprises an enzyme label plate coated by the recombinant VP1 (virus protein) protein, a rabbit anti-duck IgY antibody marked by horseradish peroxidase, a TMB substrate colour reagent, a positive serum, a negative serum and a kit specification. In the invention, by adopting the polymerase chain reaction, the VP1 genes are amplified from the DHV-1genome and the VP1 gene-containing recombinant expression plasmid pET32a-VP1 is constructed; the plasmid is transferred to host cells BL21 (DE3), and the in-vitro expression VP1 protein is purified by a nickel column and then used as the antigen; the enzyme-linked immunosorbent assay kit is established; the positive serum is the standard positive serum of duck hepatitis virus type-I and the negative control is the standard negative serum of duck. The test kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale popularization and application, very important application value in diagnosis of duck hepatitis virus type-I, survey of epidemiology and immunization survey and the like.
Owner:HENAN UNIV OF SCI & TECH
Method of detecting cellular immunity and application thereof to drugs
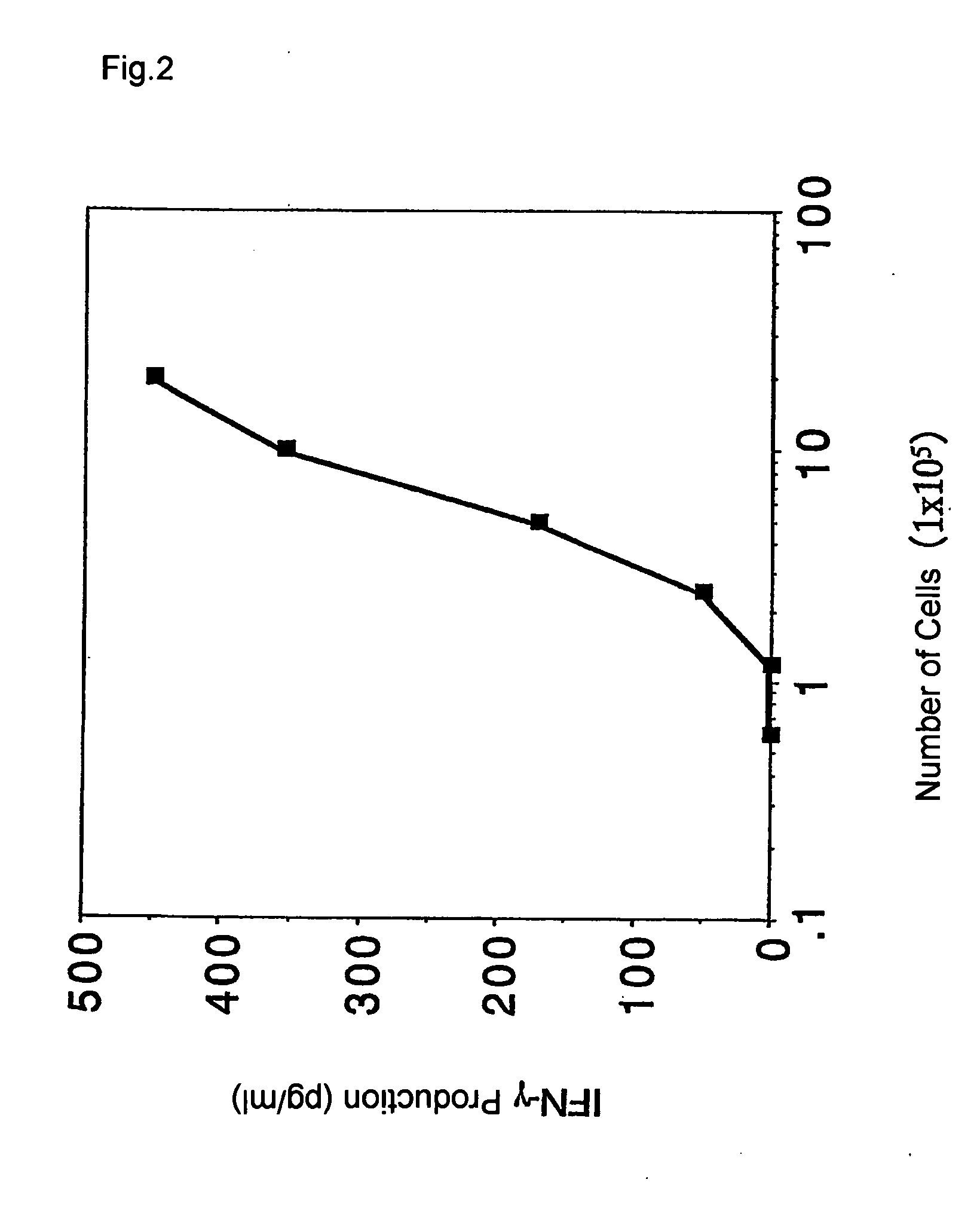
InactiveUS20060035291A1Reduce needAllergen ingredientsCancer antigen ingredientsDiseaseImmune monitoring
It is intended to provide a convenient immunity monitoring system whereby T cell frequencies specific to a plural number of antigen peptides can be assayed by using a relatively small amount of blood. Peripheral monocytes are collected and frequently stimulated with an antigen without directly using any antigen presenting cells. Then T cells specific to the antigen in the thus stimulated peripheral monocytes are detected to thereby detect antigen-specific T cells. thus, diseases such as cancer can be prevented or treated with the use of a peptide having such a function, in particular, cancer tumor-rejection antigen peptide.
Owner:GREEN PEPTIDE CO LTD +1
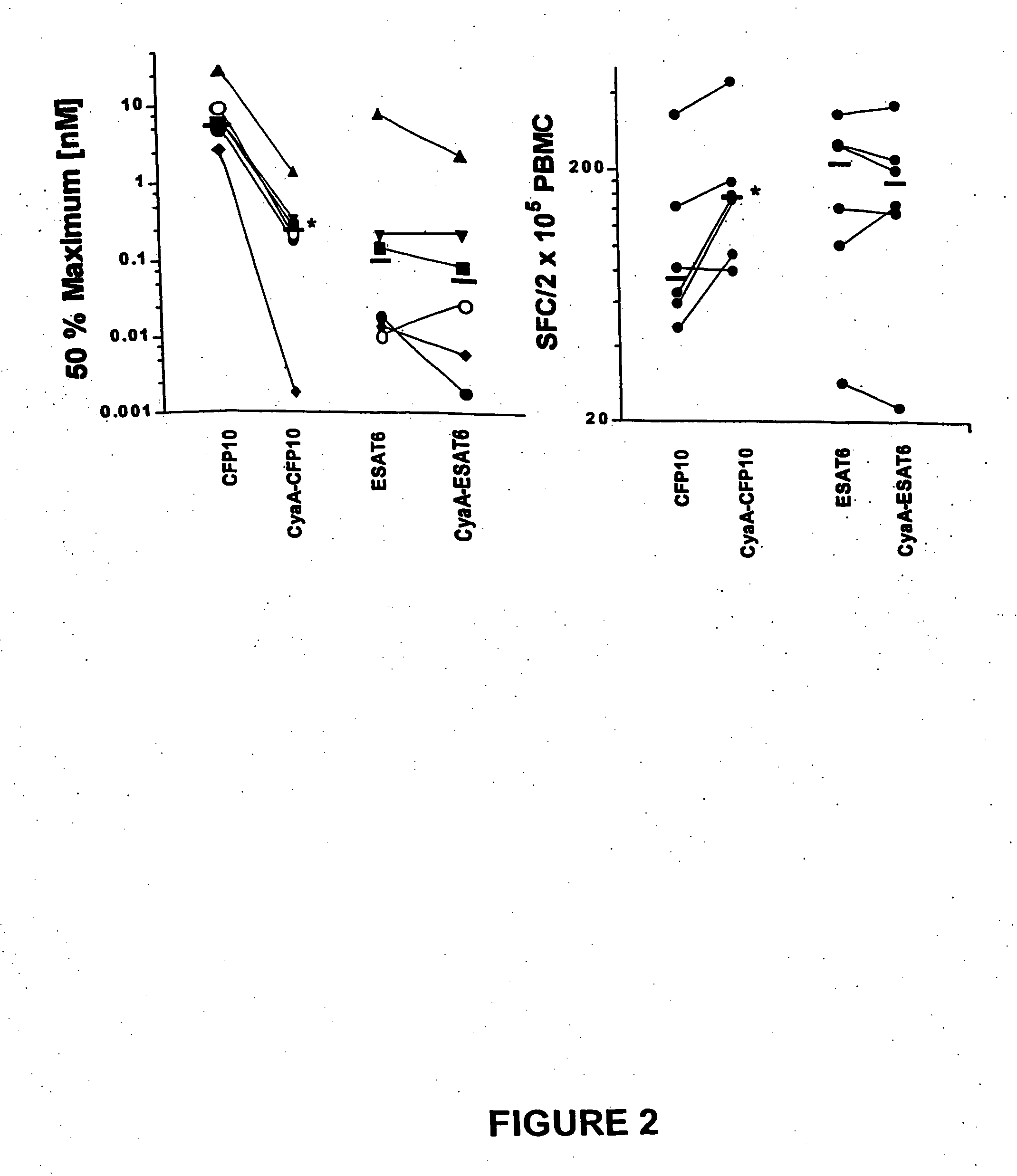
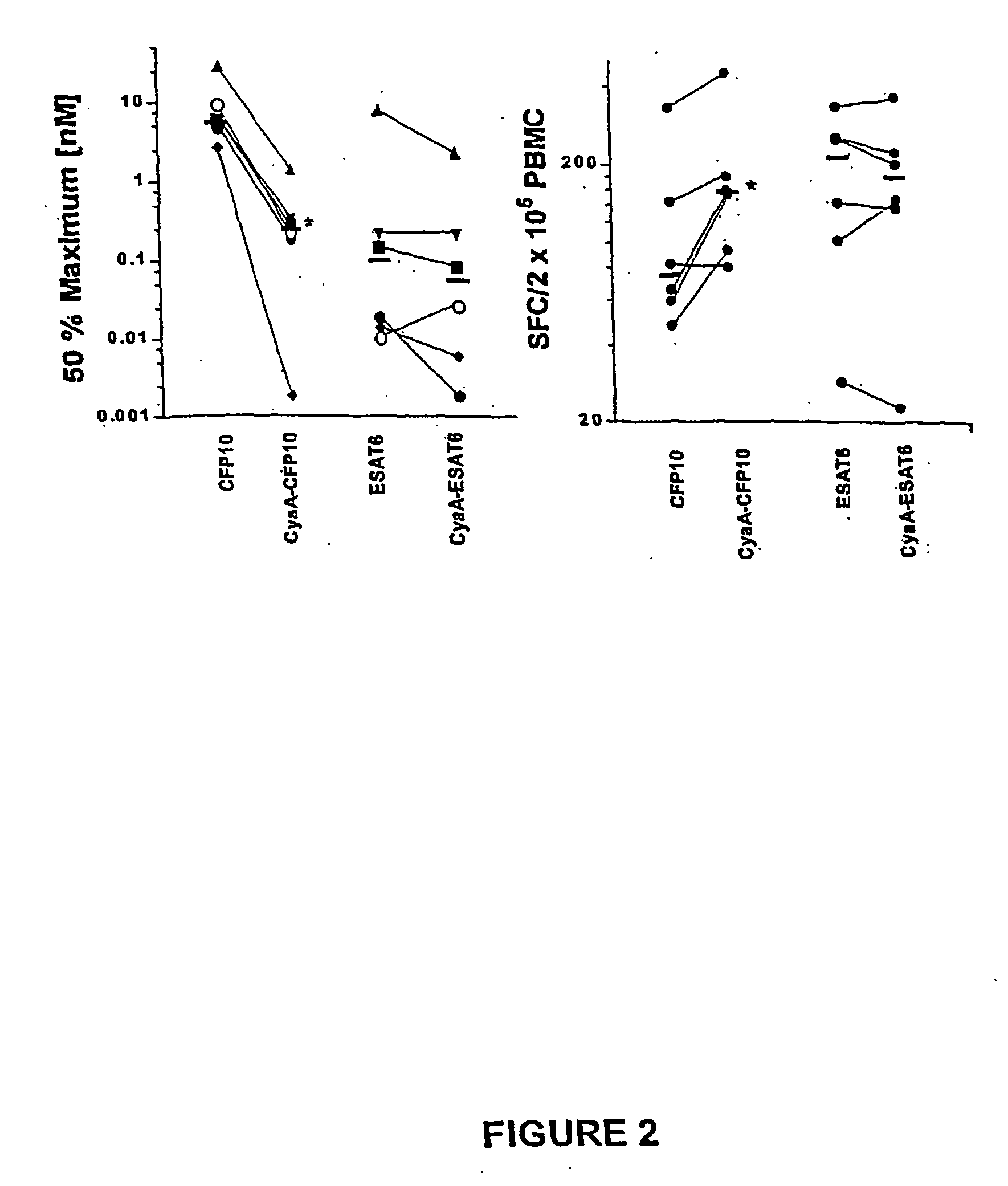
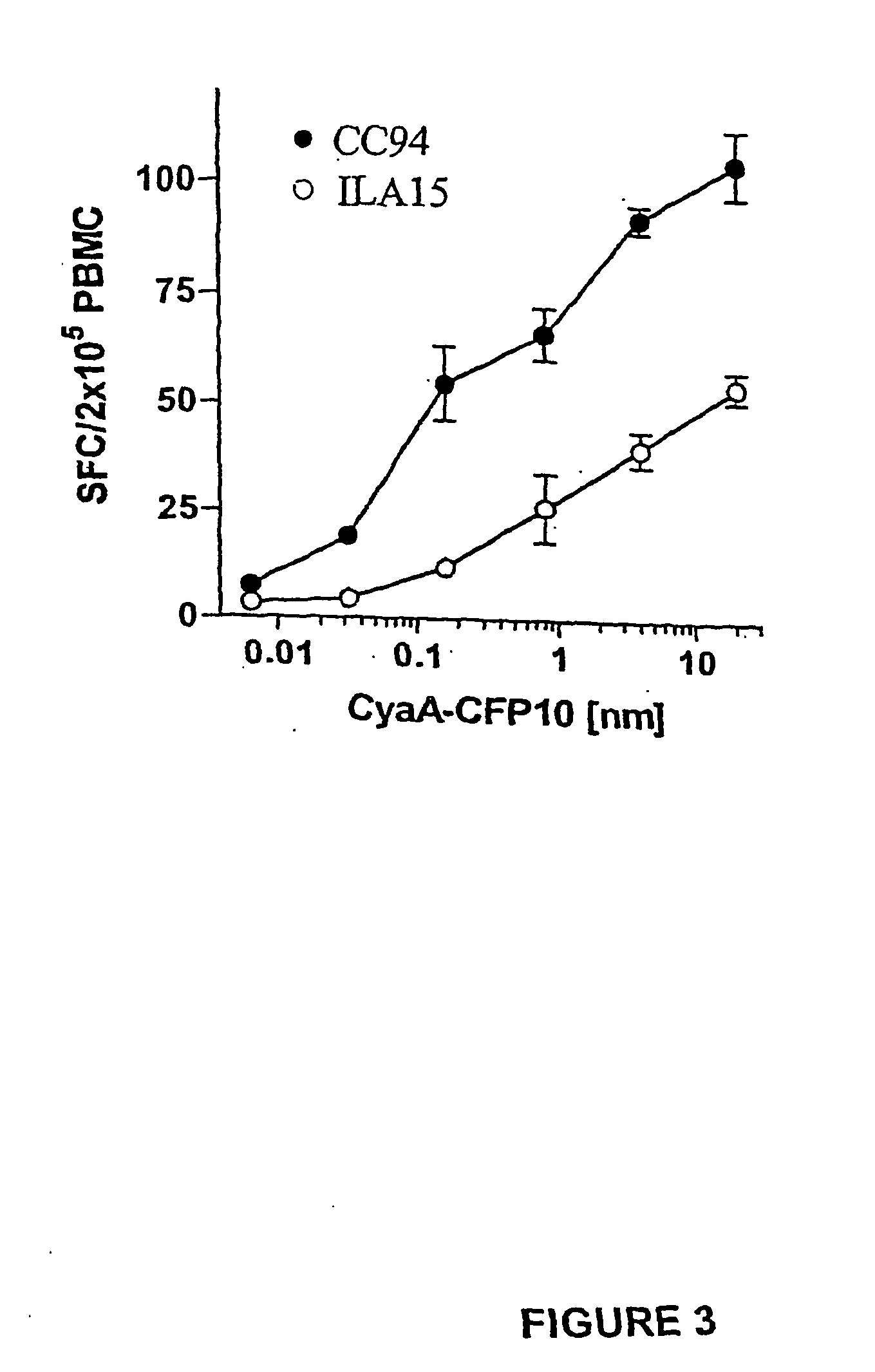
Recombinant adenylate cyclase of Bordetella sp. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase
InactiveUS20060019323A1Enhanced T cell responseImprove responseMicrobiological testing/measurementBiological material analysisAntigenCyaA
Diagnostic testing and immunomonitoring that uses genetically detoxified Bordetella pertussis CyaA as a delivery system are effective in tracking any immune responses, such as those generated by infectious and non-infectious diseases, or vaccinations, for example. T cells previously stimulated by a given antigen can be restimulated in vitro by the same antigen fused or chemically coupled to CyaA or a fragment thereof. The invention includes diagnostic tests and immunomonitoring for tuberculosis by providing a delivery system, which can deliver the M. tuberculosis immunodominant proteins ESAT-6 and CFP-10, to human cells and non-human animal cells, such as cattle. In addition, fusion proteins between CyaA and cancer antigens are also provided as diagnostic tests and immunomonitoring systems for cancers, such as melanoma.
Owner:INST PASTEUR
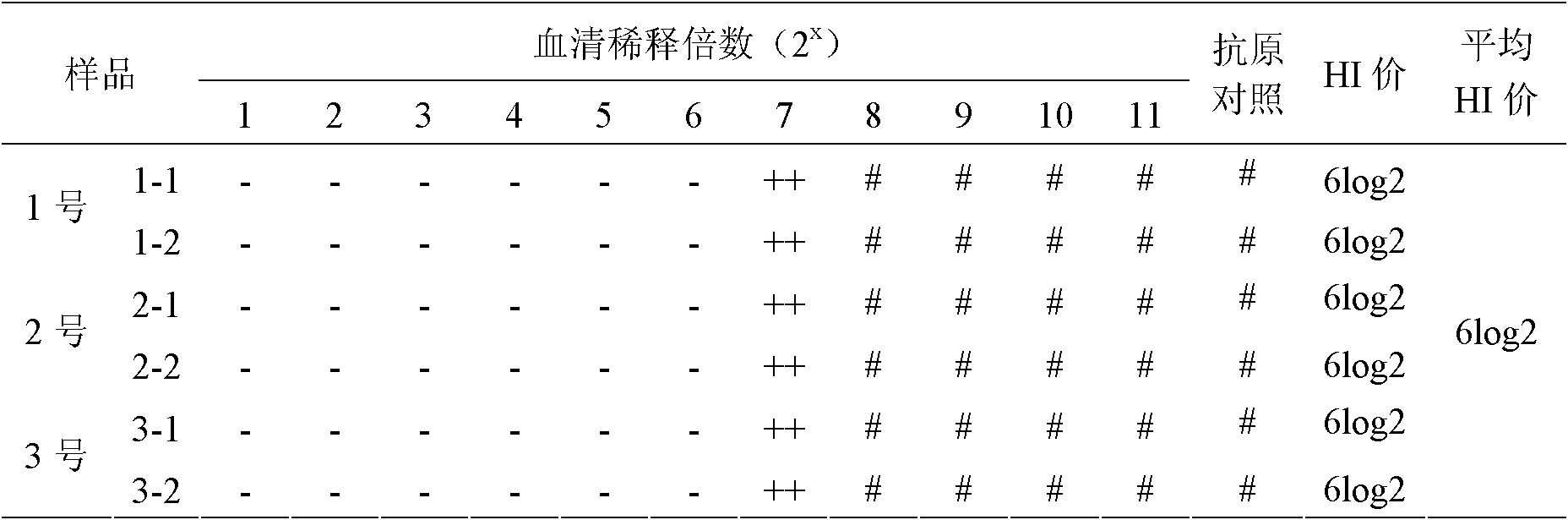
Avian influenza H5N1 subtype Re-5 strain hemagglutination inhibition antigen standard substance and preparation method
InactiveCN102012430AImprove the level of prevention and controlMaterial analysisImmune effectsImmune monitoring
The invention relates to an avian influenza H5N1 subtype Re-5 strain hemagglutination inhibition antigen standard substance and a preparation method thereof. The standard substance is prepared by performing the following series of steps of: preparing and inspecting liquid of avian influenza H5N1 subtype Re-5 strain viruses; inactivating the liquid of the viruses and inspecting a semi-finished product; freeze-drying, inspecting a finished product, homogeneity and stability, demarcating the standard substance, valuing and the like. The standard substance is fundamental guarantee of accurately diagnosing avian influenza H5N1 subtype, performing immune monitoring of an H5N1 subtype Re-5 strain hemagglutination inhibition antibody and accurately evaluating the immune effect of vaccine and improves the prevention and control level of the avian influenza.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Selenium cyclic peptide and fatty acid mixture and preparation method thereof
The invention discloses a selenium cyclic peptide and fatty acid mixture which is prepared from the traditional Chinese medicines of ginseng, radix rubiae, fructus lycii, fructus schisandrae chinensis, semen coicis, poria cocos, radix pseudostellariae, fructus gardeniae, selenium and the like by the modern microbial fermentation technology. The histone deacetylase all-natural synergic combined inhibitor with a brand-new chemical structure comprises the combined compounds such as selenium peptide, cyclic peptide, short-chain fatty acid and the like, and belongs to the tumor epigenetic regulation medicine of a novel anti-tumor action mechanism; and different from the traditional tumor treatment medicine, the selenium cyclic peptide and fatty acid mixture disclosed by the invention performs saturation attack by taking the abnormal epigenetic inheritance of tumor cells as a target spot, exerts an overall synergic anti-tumor effect by inducing the immunologic surveillance and immunity killing function of a patient, and is anti-oxidant and all-natural without chemical pollution.
Owner:北京港生药业科技有限公司
Positive-serum and negative-serum standard substance of avian influenza virus H5N1 subtype Re-5 strain and preparation method thereof
The invention relates to a positive-serum and negative-serum standard substance of avian influenza virus H5N1 subtype Re-5 strain and a preparation method thereof. The preparation method comprises the following steps of: preparing a standard substance positive serum (strongly positive serum and weakly positive serum): intramuscularly injecting an avian influenza virus H5N1 subtype Re-5 strain oil-emulsion inactivated vaccine in a young or adult SPF (Specific Pathogen Free) chicken, and then collecting the serum of the SPF chicken and carrying out semi-finished product inspection, adding a proper stabilizing agent and then freeze-drying; preparing negative serum: taking the blood of the SPF chicken, separating serum, carrying out semi-finished product inspection, and freeze-drying; and carrying out a series of technical processes, such as finished-product inspection, uniformity inspection, stability inspection, calibration and value determination of the standard substance and the like to obtain the positive-serum and negative-serum standard substance. The standard substance is the fundamental guarantee for the accurate diagnosis of avian influenza virus H5N1 subtype, the immune monitoring of a H5N1 subtype Re-5 strain and the accurate evaluation on the vaccine immune effect of the H5N1 subtype Re-5 strain, thereby improving the prevention and control level of avian influenza. The standard substance is the basic guarantee for the diagnosis of avian influenza virus H5N1 subtype and the evaluation and the quality control of the inspection working of related products.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Expression vector, preparation method thereof, PEDV-S1 (Porcine Epidemic Diarrhea Virus-S1) protein, and PEDV-S1 protein-containing indirect ELISA detection kit
The invention provides an expression vector, a preparation method thereof, PEDV-S1 (Porcine Epidemic Diarrhea Virus-S1) protein, and a PEDV-S1 protein-containing indirect ELISA (Enzyme-Linked Immuno-Sorbent Assay) detection kit, and relates to the technical field of molecular biology. The expression vector for expressing the PEDV-S1 protein provided by the invention is constructed by a eukaryoticexpression vector or an insect expression vector. The PEDV-S1 which is constructed by applying the eukaryotic expression vector for expressing the PEDV-S1 provided by the invention is high in expression quantity, easy to purify and good in antigenicity. Moreover, the ELISA detection kit which is constructed by taking the S1 protein provided by the invention as a coating antigen can accurately detect an anti-porcine epidemic diarrhea virus antibody in a clinical sample. Meanwhile, the kit disclosed by the invention is high in specificity, high in sensitivity, simple, quick, simple in preparation method and low in cost, provides a new choice for the diagnosis, general survey and immune monitoring of the PEDV, and has a good clinical application prospect.
Owner:ZHEJIANG UNIV +1
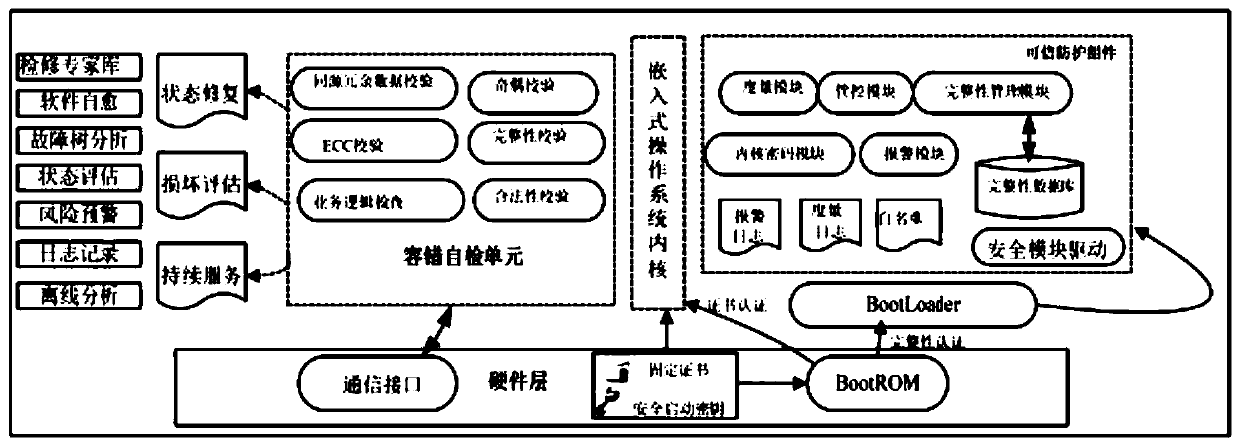
Power terminal and information security protection method and system of embedded system thereof
ActiveCN109992963AImprove information security protection capabilitiesReal-timePlatform integrity maintainanceInformation technology support systemImmune monitoringComputer terminal
The invention discloses a power terminal and an information safety protection method and system of an embedded system of the power terminal, information safety protection is actively carried out through immune self-stabilization, immune monitoring and immune defense modes according to an artificial immune principle, and the method comprises the following steps: S1, establishing an embedded systemendogenous credible operation environment for immune self-stabilization; S2, loading an embedded system abnormality detection logic inspection program for immunomonitoring; S3, selecting whether to load a fault emergency processing and event recording program for immune defense or not according to the result checked in the step S2: if the result checked in the step S2 is abnormal, loading the fault emergency processing and event recording program for immune defense; otherwise, not loading. According to the invention, three major functions of immune self-stabilization, immune monitoring and immune defense of human immunity are simulated, the power terminal is endowed with an anthropomorphic active immune safety protection capability, the information safety of the power industrial control terminal is maintained to a certain extent, and the safety risk of the power terminal is reduced.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Goat corynebacterium pseudotuberculosis PLD recombinant protein as well as preparation method and application thereof
The invention relates to goat corynebacterium pseudotuberculosis PLD recombinant protein as well as a preparation method and an application thereof, and belongs to the technical field of gene engineering. According to the recombinant protein provided by the invention, a sequence of a goat corynebacterium pseudotuberculosis phospholipase D (PLD) that signal peptide is removed is obtained by virtueof a whole-gene synthesis method; the sequence is sub-cloned into a prokaryotic expression vector pET28a; IPTG induction is conducted, so that soluble expression protein is obtained; and two times ofpurification are implemented, so that the expression protein that purity is above 95% is obtained. Based upon western-blot analysis, it is indicated that the protein has bio-activity; and through a series of optimization, a complete set of ELISA detection kits are assembled. Through sensitivity and specificity analysis as well as serological testing and immunological prevention monitoring on goat,testing and monitoring results show that the kits provided by the invention are relatively good in accuracy, sensitivity and repeatability; and the kits are applicable to diagnosis and immunologicalmonitoring of infection caused by goat corynebacterium pseudotuberculosis.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST +1
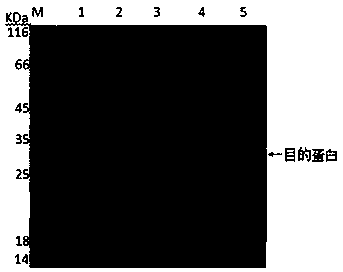
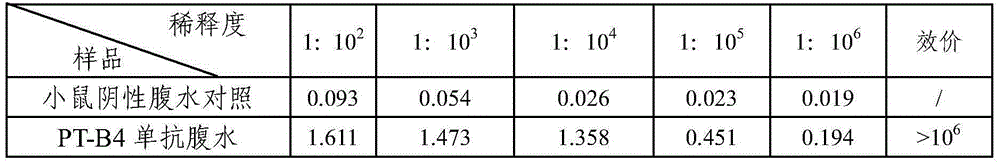
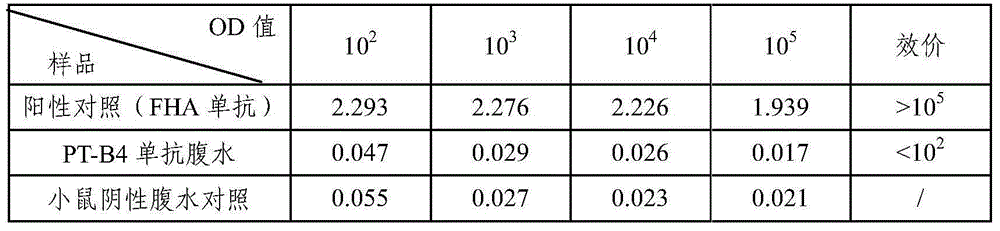
Bordetella pertussis PT (pertussis toxin) antigen monoclonal antibody and application thereof
InactiveCN105039259AIntegrity guaranteedImproving immunogenicityAntibacterial agentsImmunoglobulins against bacteriaAntigenImmune monitoring
The invention relates to a bordetella pertussis PT (pertussis toxin) antigen monoclonal antibody and application thereof, and belongs to the field of preparation of biological products. The bordetella pertussis PT antigen monoclonal antibody is secreted by hybridoma cell strains capable of stably secreting bordetella pertussis FT antigen monoclonal antibodies, and a preservation number of the hybridoma cell strains is CGMCC No.10588. The bordetella pertussis PT antigen monoclonal antibody and the application have the advantages that the monoclonal antibody is high in potency and good in specificity, and can be applied to monitoring the quality in bordetella pertussis vaccine production procedures, carrying out enzyme-linked immunosorbent assay on contents of PT components in finished vaccine and preparing bordetella pertussis PT antigen enzyme-linked immune monitoring reagents or enzyme-linked immune monitoring reagent kits.
Owner:SINOVAC RES & DEV
Preparation method of anti-neweastle disease virus specific transfer factor and oral liquid, and use thereof
InactiveCN103599130AImprove specific immune effectFree from infectionViral antigen ingredientsPharmaceutical delivery mechanismDiseaseSpecific immunity
The invention provides a preparation method of an anti-neweastle disease virus specific transfer factor. The method comprises the following steps: selecting a healthy chicken as an experiment chicken to carry out supplementary immunization of a newcastle disease inactivated vaccine; monitoring the level of an immune antibody of the newcastle disease vaccine inside the experiment chicken body; taking splenic organs of the experiment chicken in a sterile condition when the level of the antibody achieves 28-29; and processing the splenic organs of the chicken, so as to obtain the anti-neweastle disease virus specific transfer factor. By adopting the anti-neweastle disease virus specific transfer factor disclosed by the invention, the specific immune effect of the chicken body on a neweastle disease virus antigen can be improved; normal cells of the body can be prevented from being infected by the virus. Thus, the transfer factor disclosed by the invention can play the roles in preventing the neweastle disease virus infection and protecting normal cells of the body from being damaged by the neweastle disease virus, so as to reduce the morbidity; meanwhile, the chicken infected by the neweastle disease virus can be treated by using the transfer factor. Therefore, the clinical symptoms can be effectively improved; the recovery rate is increased; the mortality is reduced.
Owner:SHANDONG SINDER TECH
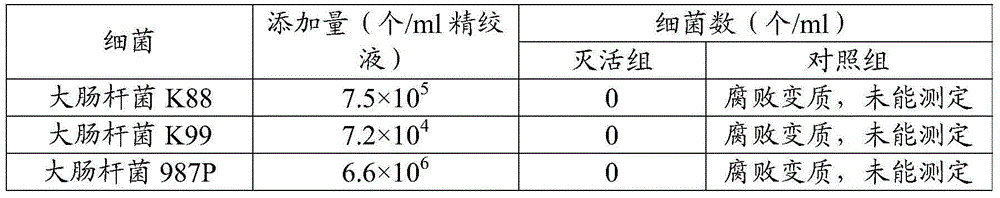
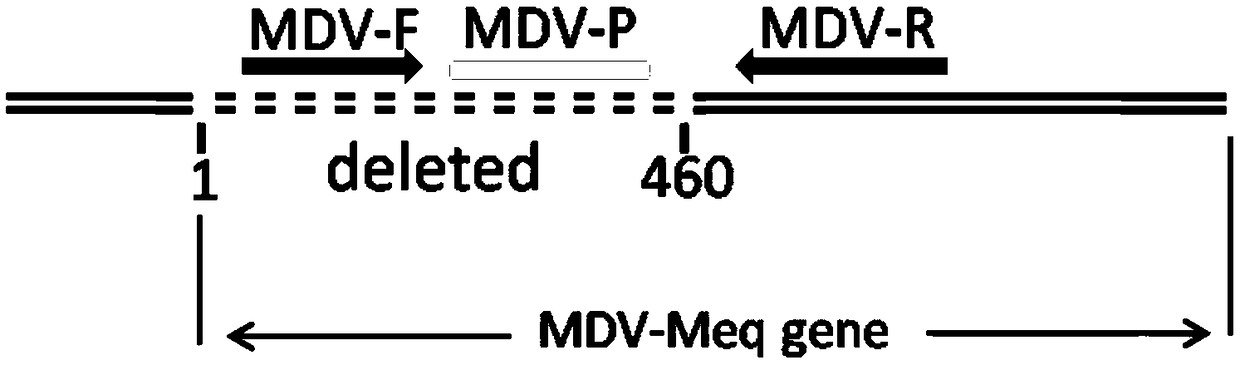
Fluorescence quantitative PCR kit for quantitatively detecting chicken Marek's virus Meq gene deletion vaccine strain and wild strain and application thereof
ActiveCN109355434AAvoid emissionsQuantitatively accurateMicrobiological testing/measurementMicroorganism based processesImmune monitoringFluorescence
The invention discloses a fluorescence quantitative PCR kit for quantitatively detecting chicken Marek's virus Meq gene deletion vaccine strain and wild strain and an application thereof. The kit comprises two sets of fluorescent quantitative PCR primers and probes respectively used for detecting the chicken Marek's virus Meq gene deletion vaccine strain and wild strain, wherein a sequence of thefluorescent quantitative PCR primer used for detecting the chicken Marek's virus Meq gene deletion vaccine strain is shown in SEQ ID NO. 1 and SEQ ID NO.2, the sequence of the probe is shown in SEQ IDNO. 3; the sequence of the fluorescent quantitative PCR primer used for detecting the wild strain of the chicken Marek's virus is shown in SEQ ID NO. 4 and SEQ ID NO.5, and the sequence of the probeis shown in SEQ ID NO. 6. Vitro experiments prove that the kit has high sensitivity, good specificity and good repeatability, can respectively and accurately quantify the chicken Marek's virus Meq gene deletion vaccine strain and MDV wild strain, and has important significance for diagnosis of the chicken Marek's disease, immune monitoring of the vaccine and the like.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Chicken Marek's virus antibody and preparation method thereof
InactiveCN101575374ALow costSafe preparationEgg immunoglobulinsImmunoglobulins against virusesAntigenAdjuvant
The invention discloses a chicken Marek's virus antibody and a preparation method thereof. The preparation method comprises the following steps: adopting at least one of Marek's disease (MD) virus (MDV) vaccine strain, expression vector containing an objective antibody, pathogenic Marek's virus and MDV antigen adjuvant vaccine to inoculate a chicken according to a certain inoculation program, monitoring the objective antibody potency generated by the inoculated laying chicken, and beginning collecting eggs produced by the inoculated laying chicken when the prescribed lowest objective antibodypotency is achieved; and separating egg yolks in the collected eggs, preparing egg yolk supernatant by adopting an acid water method, ultra-filtering the egg yolk supernatant to obtain concentrated solution, and carrying out degreasing and purification treatment for the concentrated solution by adopting chloroform to obtain the objective antibody. Therefore, on the basis of not influencing the specificity and sensibility of the antibody, the preparation of the antibody is safer, simpler, more convenient and feasible, and is convenient for industrialization; and the prepared antibody has high yield and low cost, and can be used for immune monitoring and plague prevention and control.
Owner:张训海
Preparation of bispecific antibody targeting human BCMA and activating NK cells and application of bispecific antibody
ActiveCN111333732AMaintain biological activityReshape immune surveillance functionHybrid immunoglobulinsAntibody ingredientsAntiendomysial antibodiesSingle-Chain Antibodies
Owner:CHINA PHARM UNIV
Method for detecting bastard halibut LITAF gene expression by applying reverse transcription-polymerase chain reaction (RT-PCR)
InactiveCN103276084AHigh degree of automationSolve pollutionMicrobiological testing/measurementDNA/RNA fragmentationDiseaseImmunologic preparation
The invention discloses a method for detecting bastard halibut LITAF gene expression by applying reverse transcription-polymerase chain reaction (RT-PCR). The method lays a foundation for studying bastard halibut LITAF gene expression regulation mechanism and immunology function. TNF-alpha plays an important role in the process of immune response and foreign pathogenic bacteria killing, and the LITAF is a transcription factor of TNF-alpha gene and directly controls the expression of the TNF-alpha gene. Therefore, the expression abundance of the LITAF gene reflects the activity of a bastard halibut immune system to a certain extent and can be used as an immune monitoring index and immune agent quality evaluation index in prevention and treatment of bastard halibut diseases. By detecting the variation of the bastard halibut LITAF gene expression quantity, whether the bastard halibut is infected with a disease can be judged in advance and a measure of prevention and treatment can be taken timely so as to avoid unavoidable loss resulting from serious situation; and besides, the advantages and disadvantages of a fish immune agent can be evaluated so as to judge which immune agent has better effect during prevention and treatment of flounder diseases. The invention provides a technology platform for relative quantitative analysis of LITAF gene at the mRNA level.
Owner:TIANJIN NORMAL UNIVERSITY
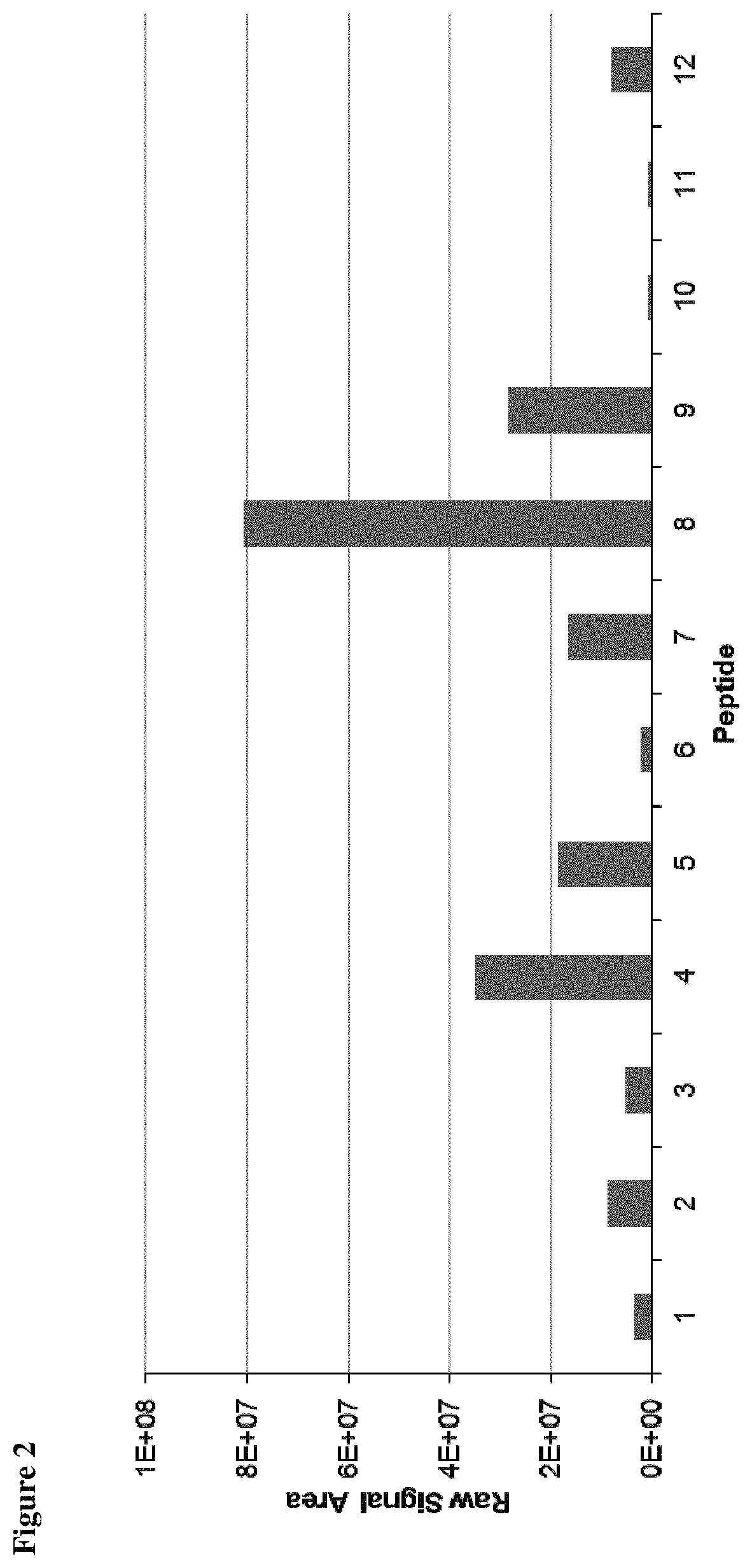
Method for the absolute quantification of naturally processed HLA-restricted cancer peptides
ActiveUS10545154B2Efficiency and precisionEasy to handleMass spectrometric analysisOmicsPeptide antigenAutoimmune condition
The present invention relates to a method for the absolute quantification of naturally processed HLA-restricted cancer peptides, i.e. the determination of the copy number of peptide(s) as presented per cell. The present invention can not only be used for the development of antibody therapies or peptide vaccines, but is also highly valuable for a molecularly defined immuno-monitoring, and useful in the processes of identifying of new peptide antigens for immunotherapeutic strategies, such as respective vaccines, antibody-based therapies or adoptive T-cell transfer approaches in cancer, infectious and / or autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Selenium butyric acid inclusion agent and preparation method thereof
InactiveCN103446492AGood curative effectModerate tastePharmaceutical non-active ingredientsSulfur/selenium/tellurium inorganic active ingredientsChemical structureCancer cell
The invention discloses a selenium butyric acid inclusion agent. The selenium butyric acid inclusion agent is based on the traditional Chinese medicines including ginseng, rubia cordifolia, fructus lycii, schisandra chinensis, coix seed, wolfiporia extensa, pseudostellaria heterophylla, selenium and the like, prepared by using a modern microorganism fermentation technology, processed by using a cyclodextrin inclusion technology, comprises histone deacetylase in a brand-new chemical structure to function as a pure natural synergistic inhibitor, comprises the composite compounds of selenium, butyric acid, flavonoid, saponin and the like, and belongs to the tumor epigenetic regulation medicine using the novel anti-tumor action mechanism; different from a traditional tumor treatment medicine, the selenium butyric acid inclusion agent carries out saturation attack by taking the abnormal epigenetic inheritance of the cancer cell as the target, exerts the whole anti-tumor effect by inducing the immune monitoring and immune killing actions of the patient, and is capable of resisting oxidation, appropriate in taste and free from chemical pollution.
Owner:杨吉星
Recombinant adenylate cyclase of Bordetella sp. for diagnostic and immunomonitoring uses, method of diagnosing or immunomonitoring using said recombinant adenylate cyclase, and kit for diagnosing or immunomonitoring comprising said recombinant adenylate cyclase
Diagnostic testing and immunomonitoring that uses genetically detoxified Bordetella pertussis CyaA as a delivery system are effective in tracking any immune responses, such as those generated by infectious and non-infectious diseases, or vaccinations, for example. T cells previously stimulated by a given antigen can be restimulated in vitro by the same antigen fused or chemically coupled to CyaA or a fragment thereof. The invention includes diagnostic tests and immunomonitoring for tuberculosis by providing a delivery system, which can deliver the M. tuberculosis immunodominant proteins ESAT-6 and CFP-10, to human cells and non-human animal cells, such as cattle. In addition, fusion proteins between CyaA and cancer antigens are also provided as diagnostic tests and immunomonitoring systems for cancers, such as melanoma.
Owner:INST PASTEUR +4
Colloidal gold test paper for detecting IBV, and preparation method thereof
The invention discloses colloidal gold test paper for detecting IBV. The colloidal gold test paper comprises a support plate, a coating film, a sample pad, a gold-labeled antibody pad, water absorbingpaper, a detection line and a quality control line, and is characterized in that the gold-labeled antibody pad is labeled with an anti-IBV IgG antibody, the detection line is coated with an anti-IBVN protein monoclonal antibody, and the quality control line is coated with a goat anti-mouse IgG antibody. The invention also discloses an index method of the colloidal gold test paper for detecting IBV. The test paper prepared by the invention is based on the principle of a double-antibody sandwich ELISA antigen detection method, is used for detecting IBV antigens so as to intuitively, quickly, simply and conveniently detect virus infection and achieve good specificity and the purpose of accurate and quick detection, can provide technical support for immune monitoring and epidemiological monitoring research of avian infectious bronchitis, and has important significance in prevention and control of avian diseases.
Owner:ZHENGZHOU UNIV
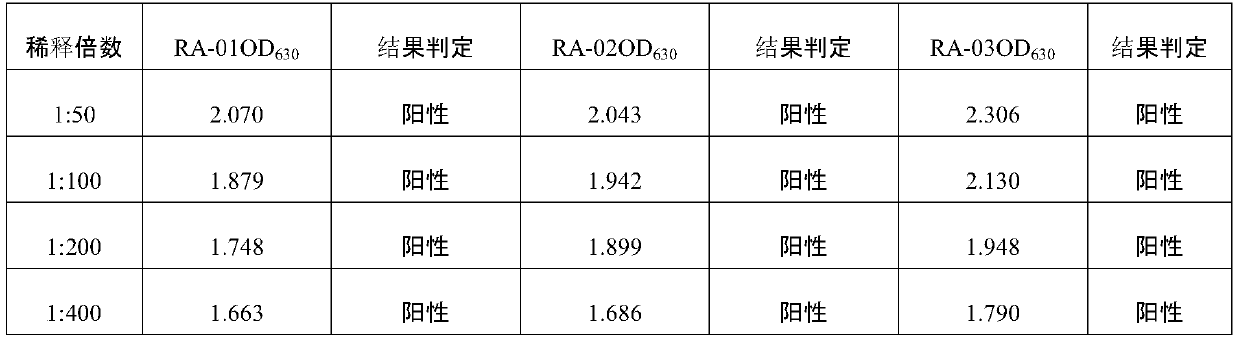
Positive serum for riemerella anatipestifer (RA) as well as preparation method and application of positive serum
ActiveCN110041426AAccurate evaluationAccurate diagnosisSerum immunoglobulinsImmunoglobulins against bacteriaImmune effectsSerum ige
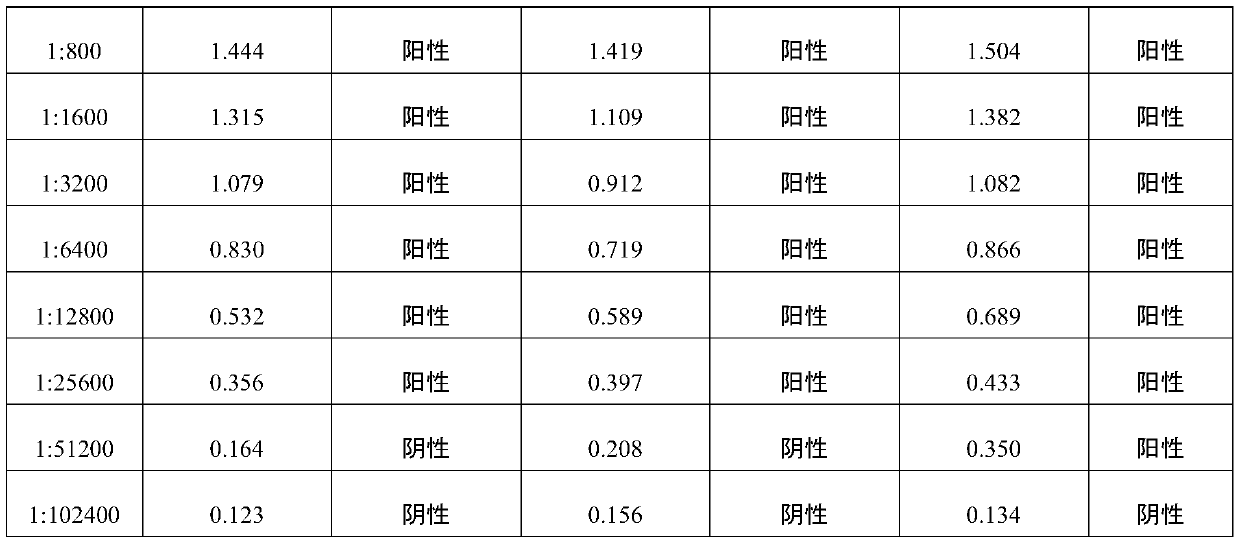
The invention belongs to the technical field of biological products, and particularly relates to positive serum for riemerella anatipestifer (RA) as well as a preparation method and application of thepositive serum. According to the preparation method, 4 times of immunization are performed on each duck, firstly, 3 times of immunization are performed by use of recombinant protein of the RA, the last immunization is finally performed by use of viable bacteria in a bacterial solution of the RA, so that after ducks are immune to the recombinant protein of the RA to produce antibody protection, high RA antibodies are produced under the stimulation of viable bacteria. The titer of the prepared positive serum for the RA is 1:12800 or above; and the positive serum for the RA, prepared by the method provided by the invention provides good technical support for accurate diagnosis and immune monitoring of the RA disease and accurate evaluation on the vaccine immune effect on the RA disease, andhas bright application prospects.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Traditional Chinese medicine composition and applications thereof
InactiveCN107468754AGrowth inhibitionIncrease percentageOrganic active ingredientsAntinoxious agentsSide effectTumor stroma
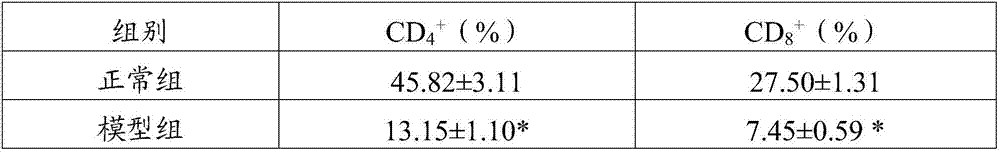
The invention belongs to the field of drugs and health-care food, and discloses a traditional Chinese medicine composition, which contains ganoderma lucidum polysaccharides, grifola frondosa polysaccharides, angelica sinensis oil and cinnamon oil. Experiments show that the prepared traditional Chinese medicine composition is scientifically combined, brings out the best in each other, has no toxic and side effects, not only has nutritional values, but also can inhibit the growth of tumors, remarkably increase the percentage of CD<4+> and CD<8+> cells in tumor stroma lymphocyte compared with single component, enhance the organism immunity function, restore the normal immune monitoring function of the organism, reduce the expression rate of mice tumor tissue VEGF and TGF-beta1 positive cells, help treat tumors, enhance the anti-tumor effect of chemotherapeutic drugs, and improve the organism immunity condition, has the function of restoring the normal immune monitoring of the organism, and can play a role in preventing and / or help treating tumors.
Owner:INFINITUS (CHINA) CO LTD
Method for the absolute quantification of naturally processed hla-restricted cancer peptides
PendingUS20200103408A1Efficiency and precisionEasy to handleMass spectrometric analysisOmicsPeptide antigenAutoimmune condition
The present invention relates to a method for the absolute quantification of naturally processed HLA-restricted cancer peptides, i.e. the determination of the copy number of peptide(s) as presented per cell. The present invention can not only be used for the development of antibody therapies or peptide vaccines, but is also highly valuable for a molecularly defined immuno-monitoring, and useful in the processes of identifying of new peptide antigens for immunotherapeutic strategies, such as respective vaccines, antibody-based therapies or adoptive T-cell transfer approaches in cancer, infectious and / or autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Indirect ELISA (Enzyme-Linked Immunosorbent Assay) detection kit for detecting porcine coronavirus D
The invention discloses an indirect ELISA (Enzyme-Linked Immunosorbent Assay) detection kit for detecting porcine coronavirus D. The ELISA detection kit established by using the NS6 protein of the PDCoV as the coating antigen can accurately detect the porcine coronavirus D antibody in a clinical sample. Meanwhile, as the NS6 protein serves as a specific auxiliary protein of the porcine D-type coronavirus, cross reaction with other coronaviruses does not occur, and the NS6 protein can be used for antibody detection after the PDCoV is infected to human or other animals from pigs. The ELISA kit established by the protein has the characteristics of strong specificity, high sensitivity and good repeatability, provides a new choice for rapid diagnosis and immune monitoring of PDCoV, and has important significance in prevention, control and purification of the disease.
Owner:ZHEJIANG UNIV
Universal cancer peptides derived from telomerase
The invention relates to a peptide of 15 to 20 amino acids deriving from TERT protein, which peptide is capable of (i) binding to HLA class II and (ii) stimulating a CD4 Th response. These universal cancer peptides are especially useful in anti-tumor immunotherapy and immunomonitoring.
Owner:INVECTYS +2
A class of radioactive PET imaging agents targeting interferon gene stimulators
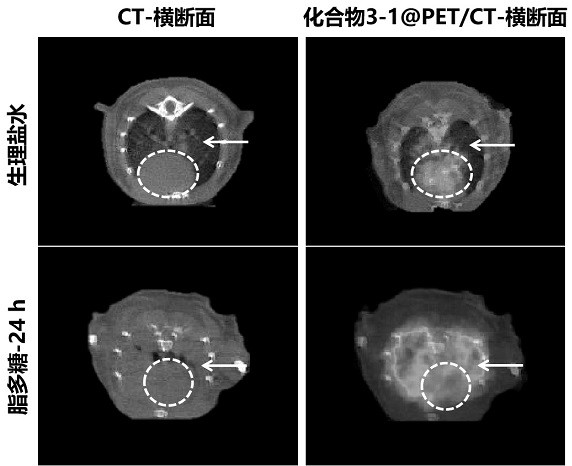
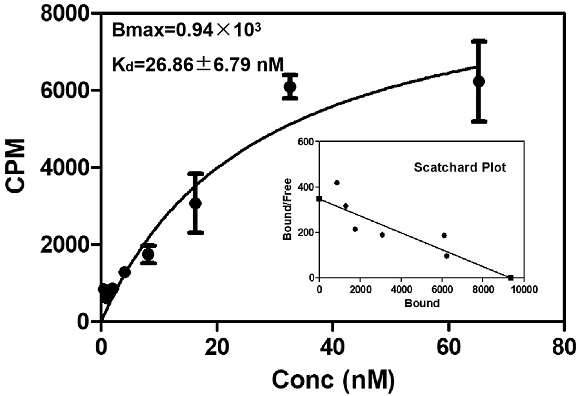
ActiveCN113429384BHigh yieldHigh activityIsotope introduction to heterocyclic compoundsRadioactive preparation carriersImaging agentPolyethylene glycol
The invention discloses a class of interferon gene stimulating factor (STING) targeting imaging agent, which is based on the structure of benzothiophene and composed of radionuclide 18 F marker, which can be used for PET imaging of interferon gene stimulators, its general structural formula is shown in formula A, where n=1‑5: formula A where the benzothiophene structure is used as a targeting group, and polyethylene glycol alcohol as a hydrophilic group, 18 F is a radioactive group. The molecular targeting imaging agent has excellent targeting and specificity to interferon gene stimulators, and can be used for PET imaging to monitor inflammation and immune responses produced by tumors. The molecule can specifically distinguish immune responses of different severities, and meets the conditions of being used as an imaging agent for immune monitoring.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
Interferon gene stimulating factor targeted radioactive PET imaging agent
ActiveCN113429384AHigh yieldHigh activityIsotope introduction to heterocyclic compoundsRadioactive preparation carriersPolyethylene glycolImaging agent
The invention discloses an interferon gene stimulating factor (STING) targeted imaging agent. The imaging agent is based on a benzothiophene structure, is labeled by radionuclide 18F, can be used for PET imaging of interferon gene stimulating factors, and has a structural general formula as shown in a formula A. In the formula, n is equal to 1-5. In the formula A, the benzothiophene structure is used as a targeted group, polyethylene glycol is used as a hydrophilic group, and 18F is a radioactive group. The molecular targeting imaging agent has excellent targeting and specificity on interferon gene stimulating factors, and can be used for monitoring immune response generated by inflammation and tumors through PET imaging. The molecule can specifically distinguish immunoreactions with different severity degrees, and meets the condition of serving as an immune monitoring developer.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
SmcL monoclonal antibody, hybridoma cell strain secreting antibody, application of hybridoma cell strain and competitive ELISA detection method
ActiveCN113845589AStrong specificityHigh affinityImmunoglobulins against bacteriaTissue cultureImmune monitoringBiochemistry
The invention provides an SmcL monoclonal antibody, a hybridoma cell strain secreting the antibody, application of the hybridoma cell strain and a competitive ELISA detection method. The monoclonal antibody is secreted by the hybridoma cell strain preserved in the China Center for Type Culture Collection or a passage cell strain of the hybridoma cell strain, and the preservation number is CCTCC NO:C2021175. The monoclonal antibody secreted by the hybridoma cell strain has the advantages of high titer, good specificity and strong affinity with natural antigens. The listeria ivanovii SmcL monoclonal antibody competitive ELISA detection method established on the basis of the antibody is high in sensitivity and good in stability, has no cross reaction with other pathogenic bacteria, can effectively detect the level of the SmcL antibody in sheep clinical serum, and can be used for listeria ivanovii epidemiological investigation and clinical immune monitoring.
Owner:YANGZHOU UNIV
Traditional Chinese medicine composition and applications thereof
InactiveCN107468730AGrowth inhibitionIncrease percentageOrganic active ingredientsImmunological disordersSide effectTumor stroma
The invention belongs to the field of drugs and health-care food, and discloses a traditional Chinese medicine composition, which contains ganoderma lucidum polysaccharides, morinda officinalis polysaccharides, polygonatum sibiricum polysaccharides, angelica sinensis oil and cinnamon oil. Experiments show that the prepared traditional Chinese medicine composition is scientifically combined, brings out the best in each other, has no toxic and side effects, not only has nutritional values, but also can inhibit the growth of tumors, remarkably increase the percentage of CD<4+> and CD<8+> cells in tumor stroma lymphocyte compared with single component, enhance the organism immunity function, restore the normal immune monitoring function of the organism, reduce the expression rate of mice tumor tissue VEGF and TGF-beta1 positive cells, help treat tumors, enhance the anti-tumor effect of chemotherapeutic drugs, and improve the organism immunity condition, has the function of restoring the normal immune monitoring of the organism, and can play a role in preventing and / or help treating tumors.
Owner:INFINITUS (CHINA) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com