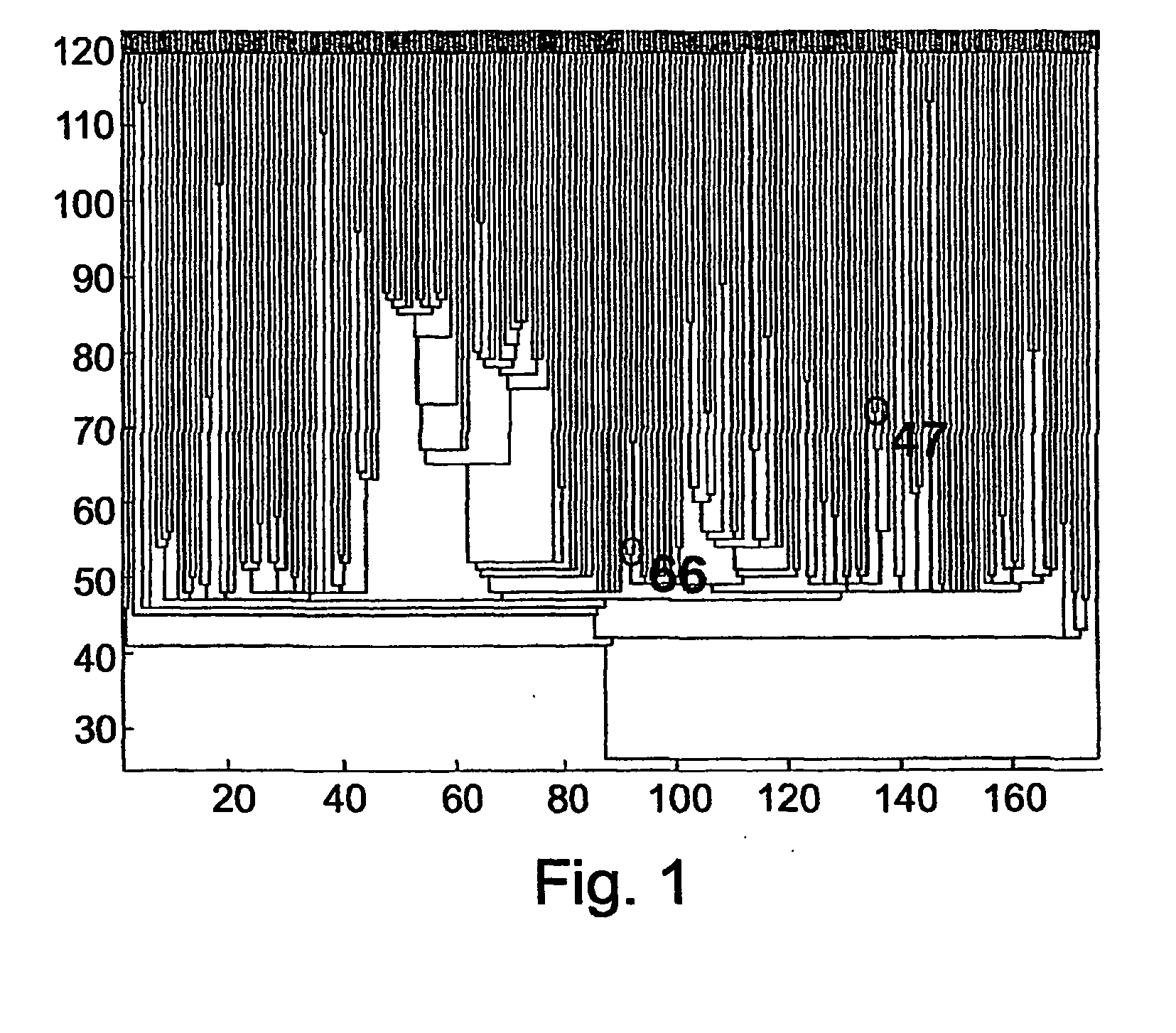
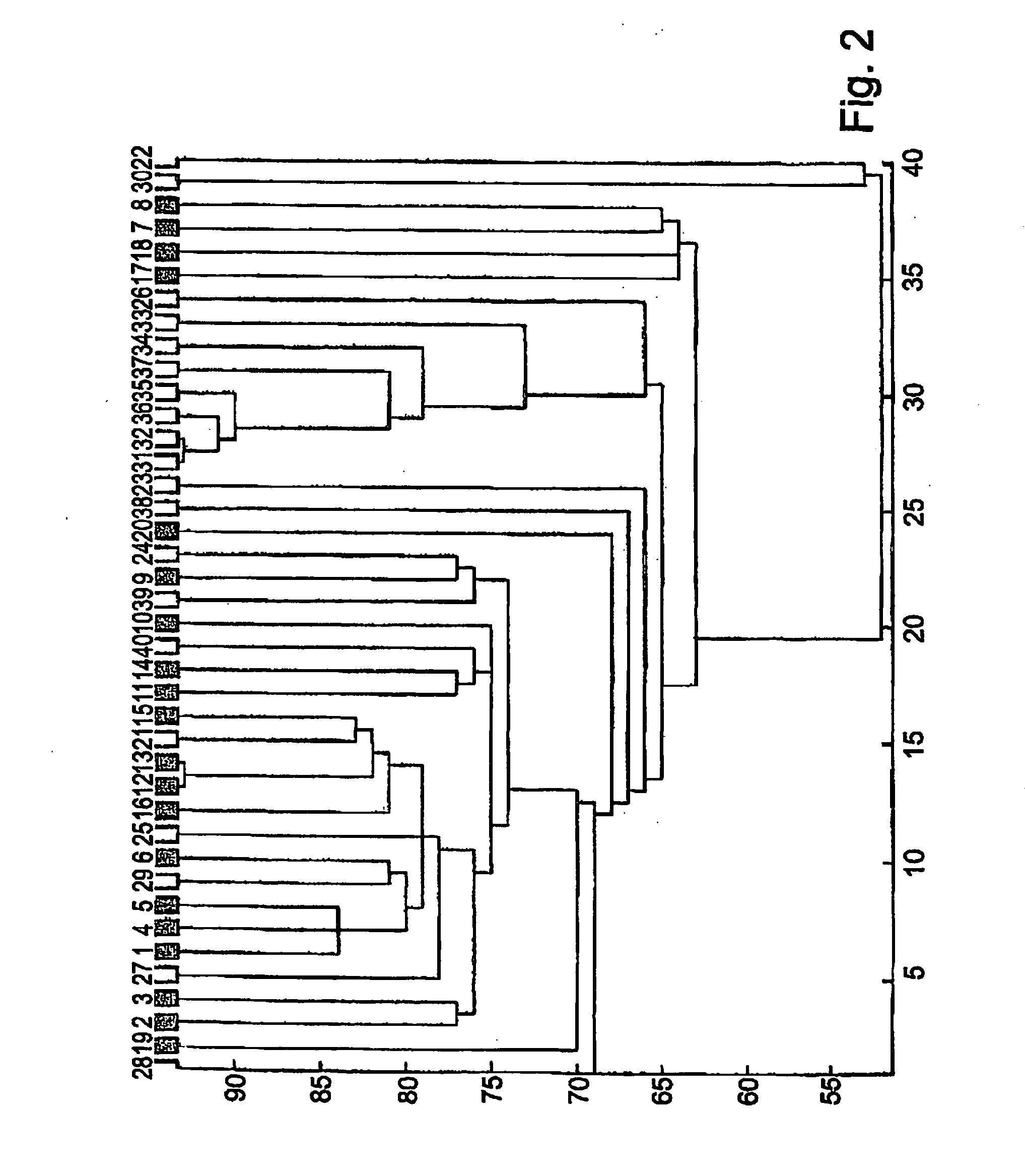
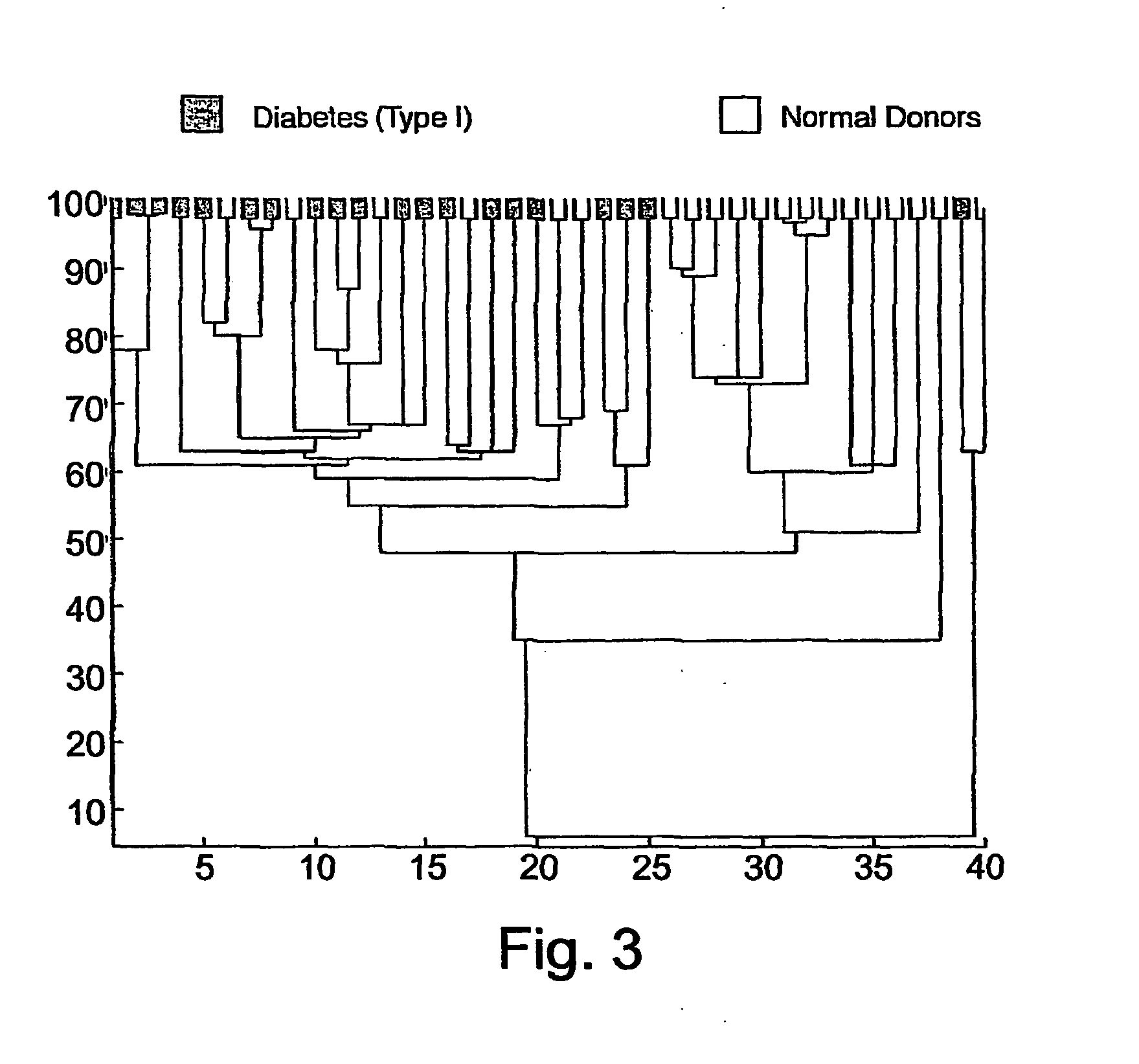
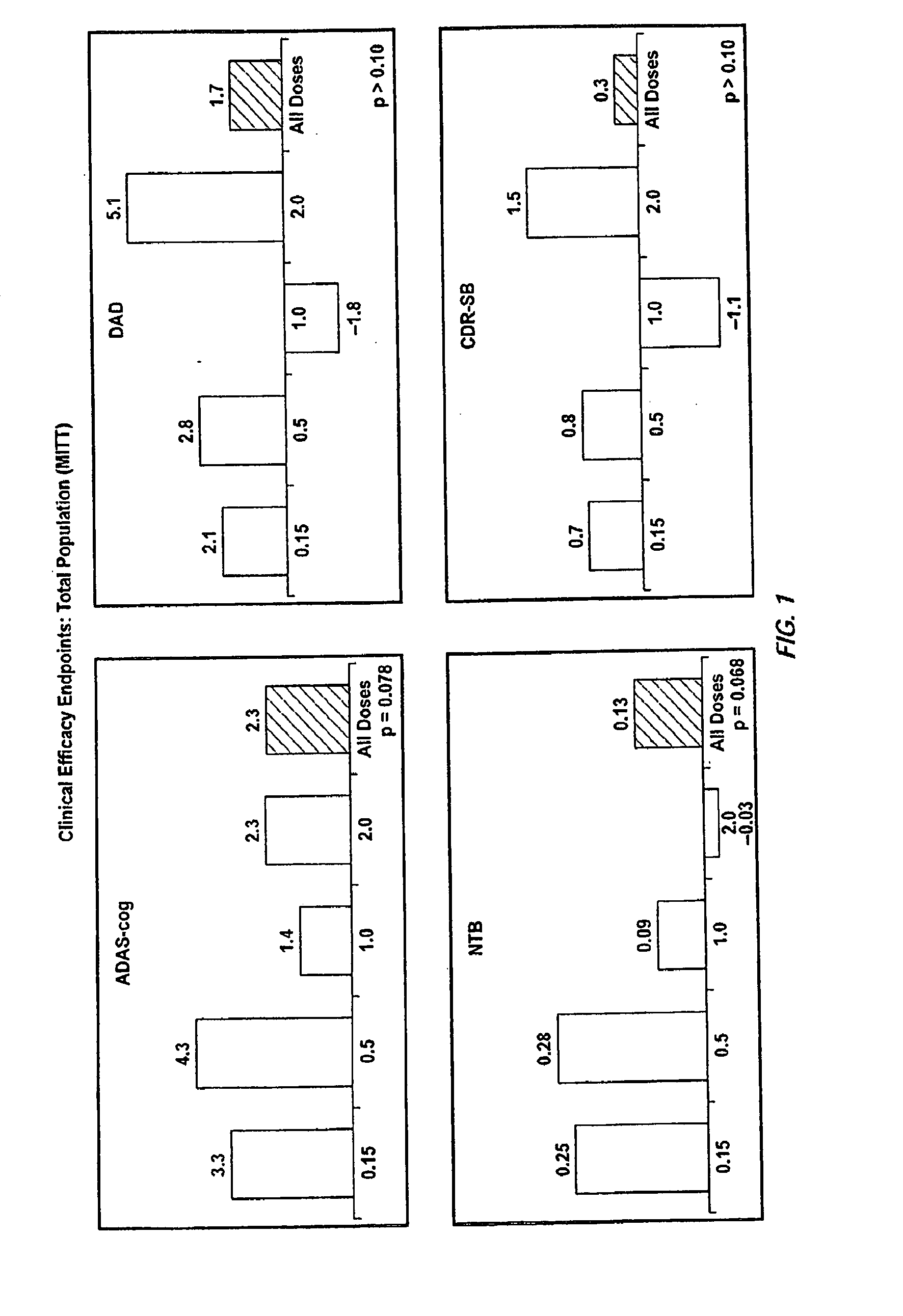
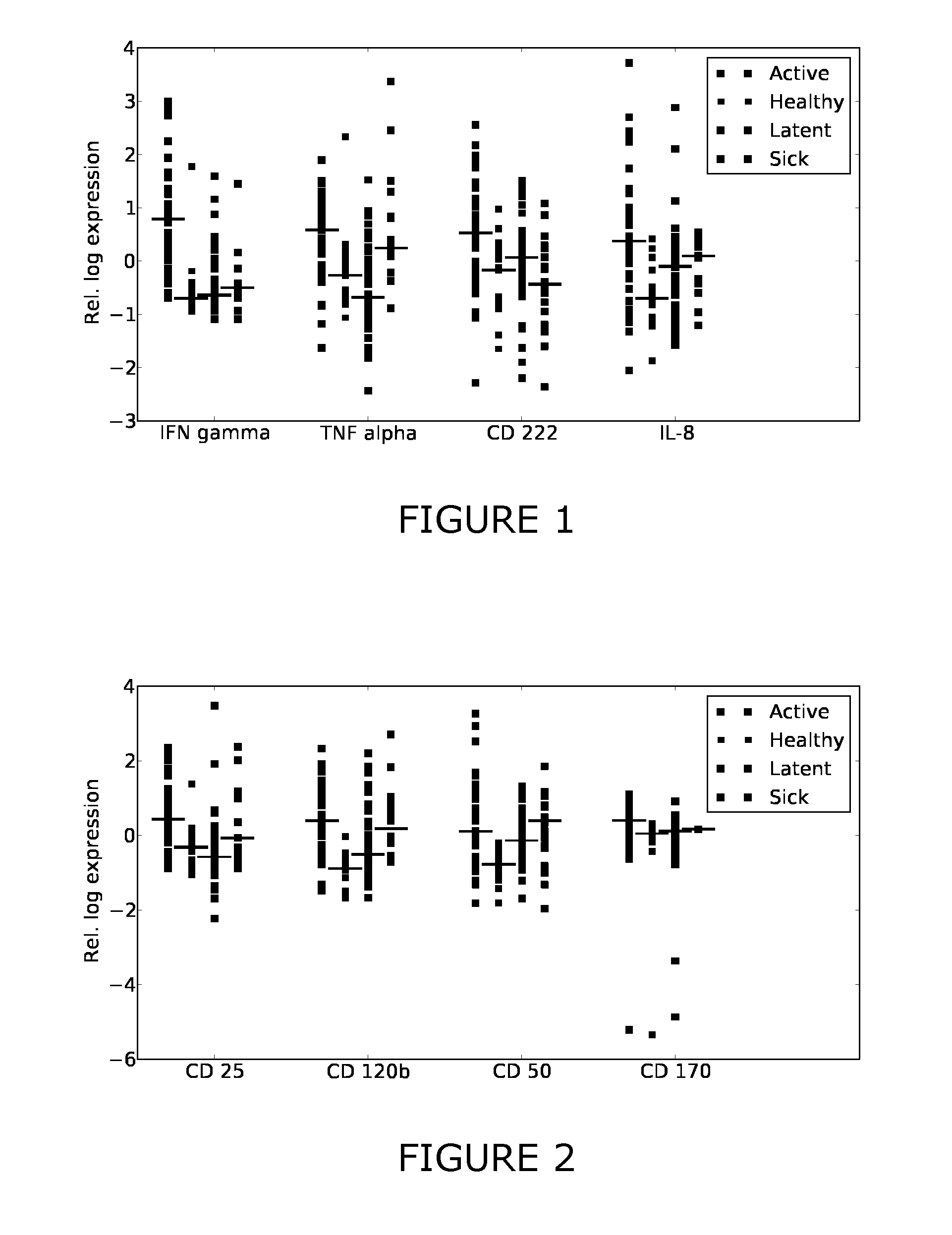
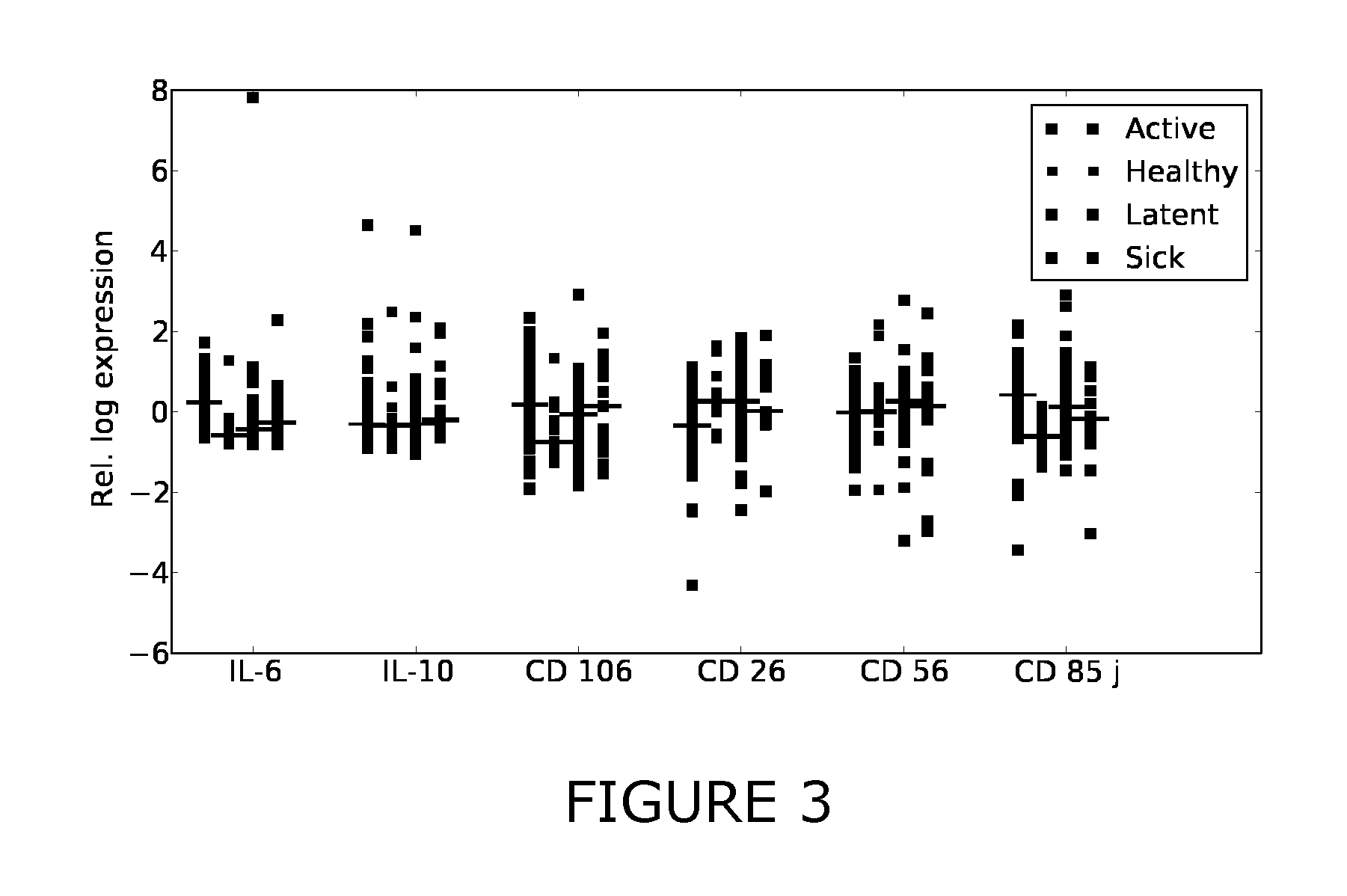
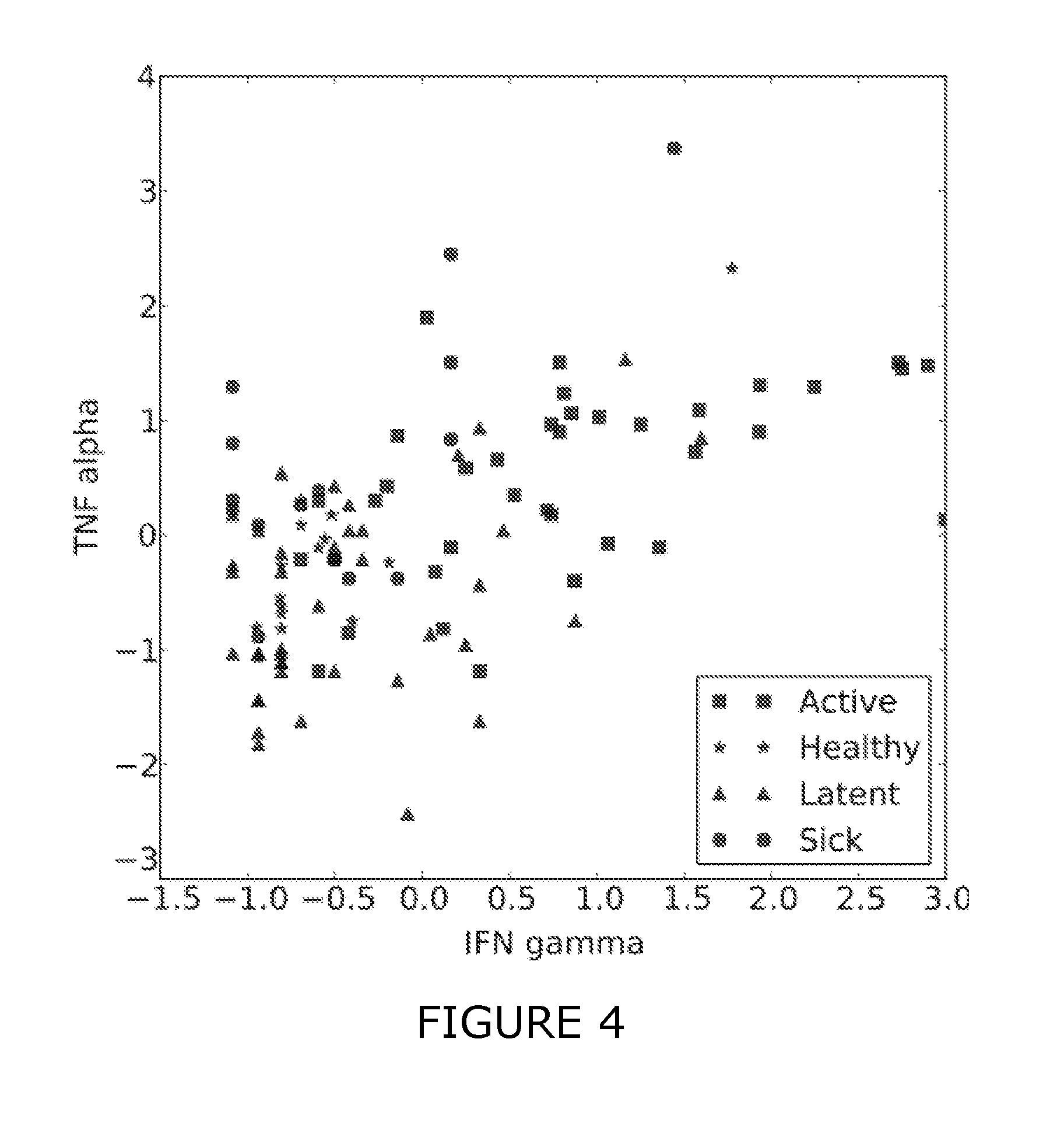
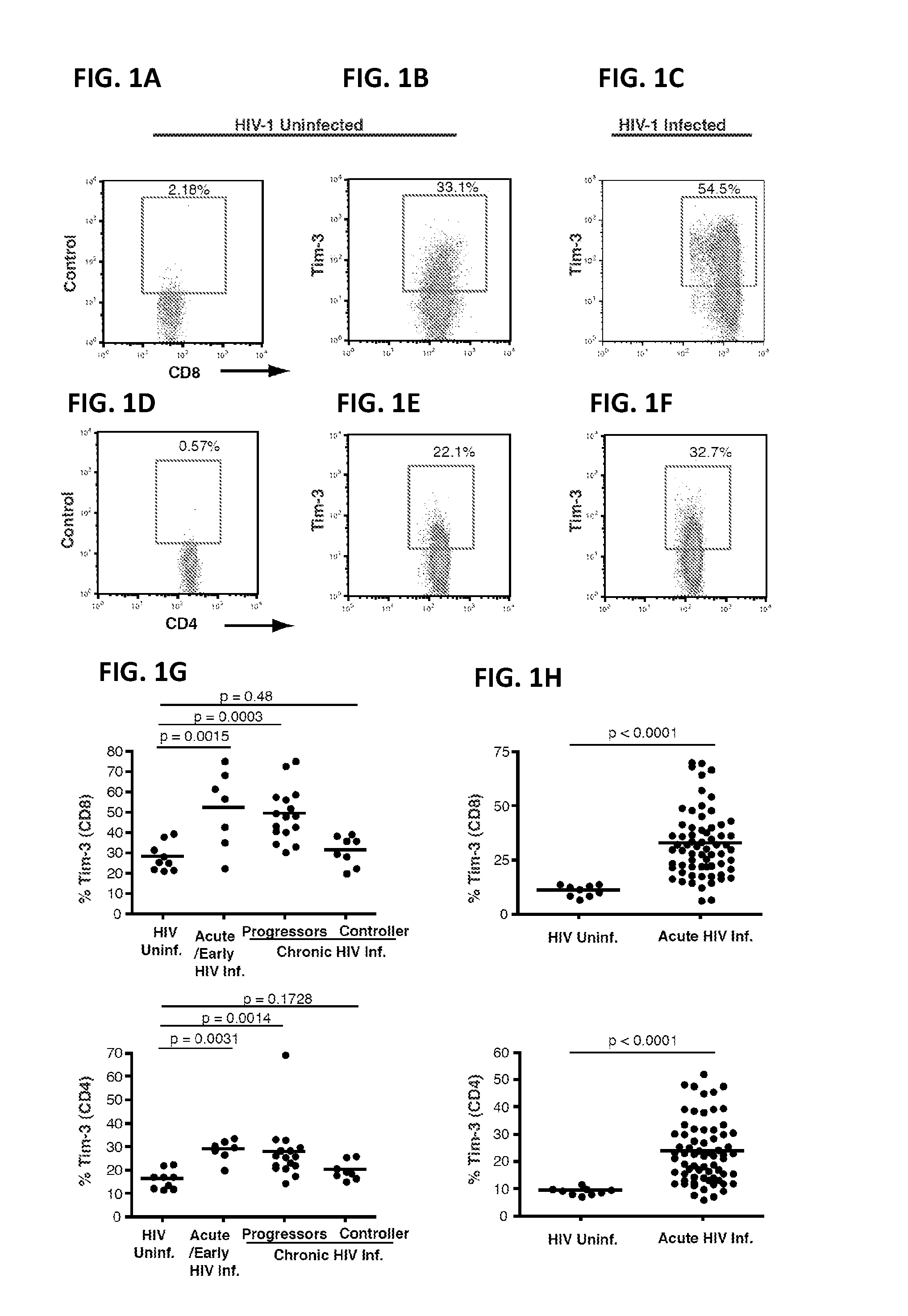
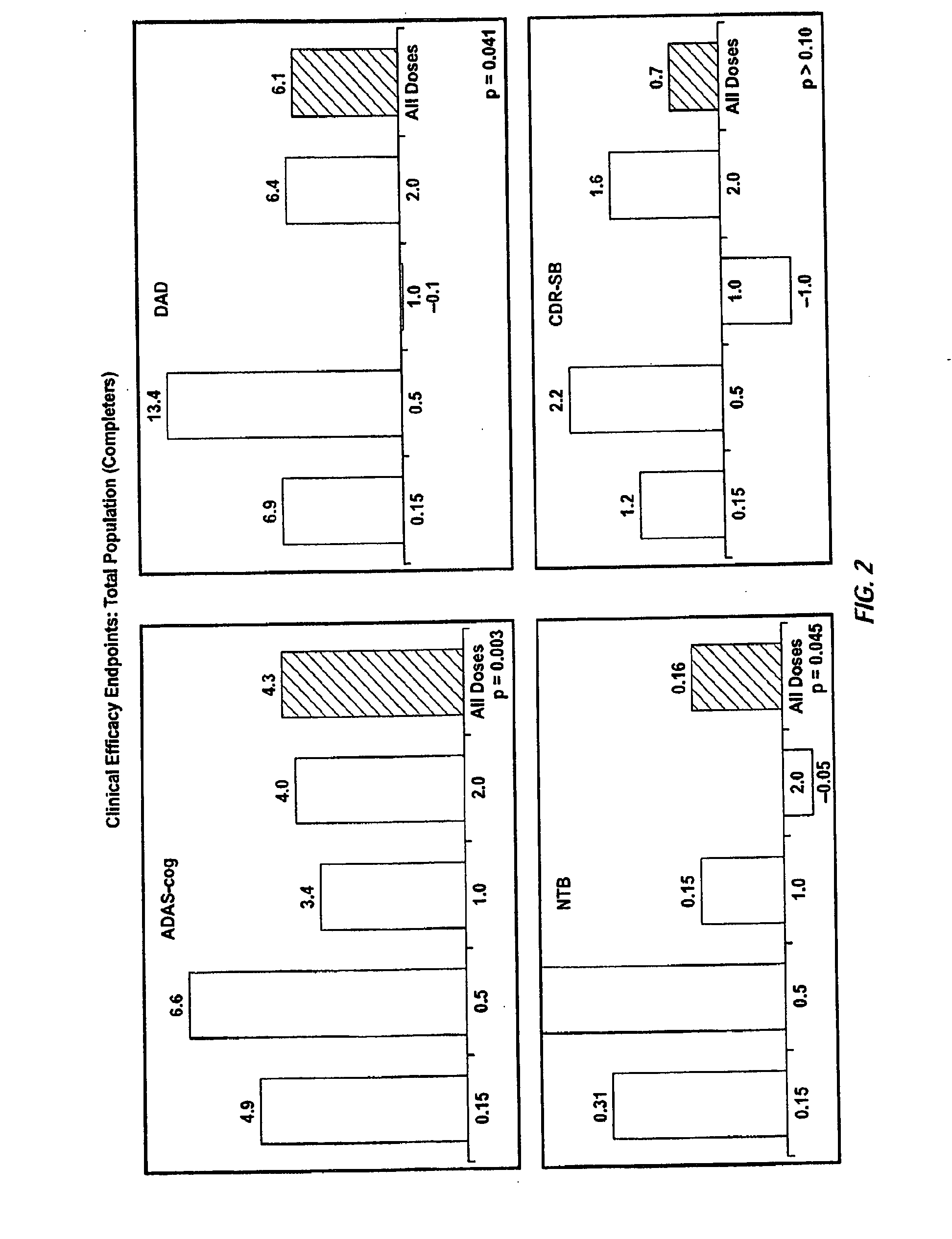
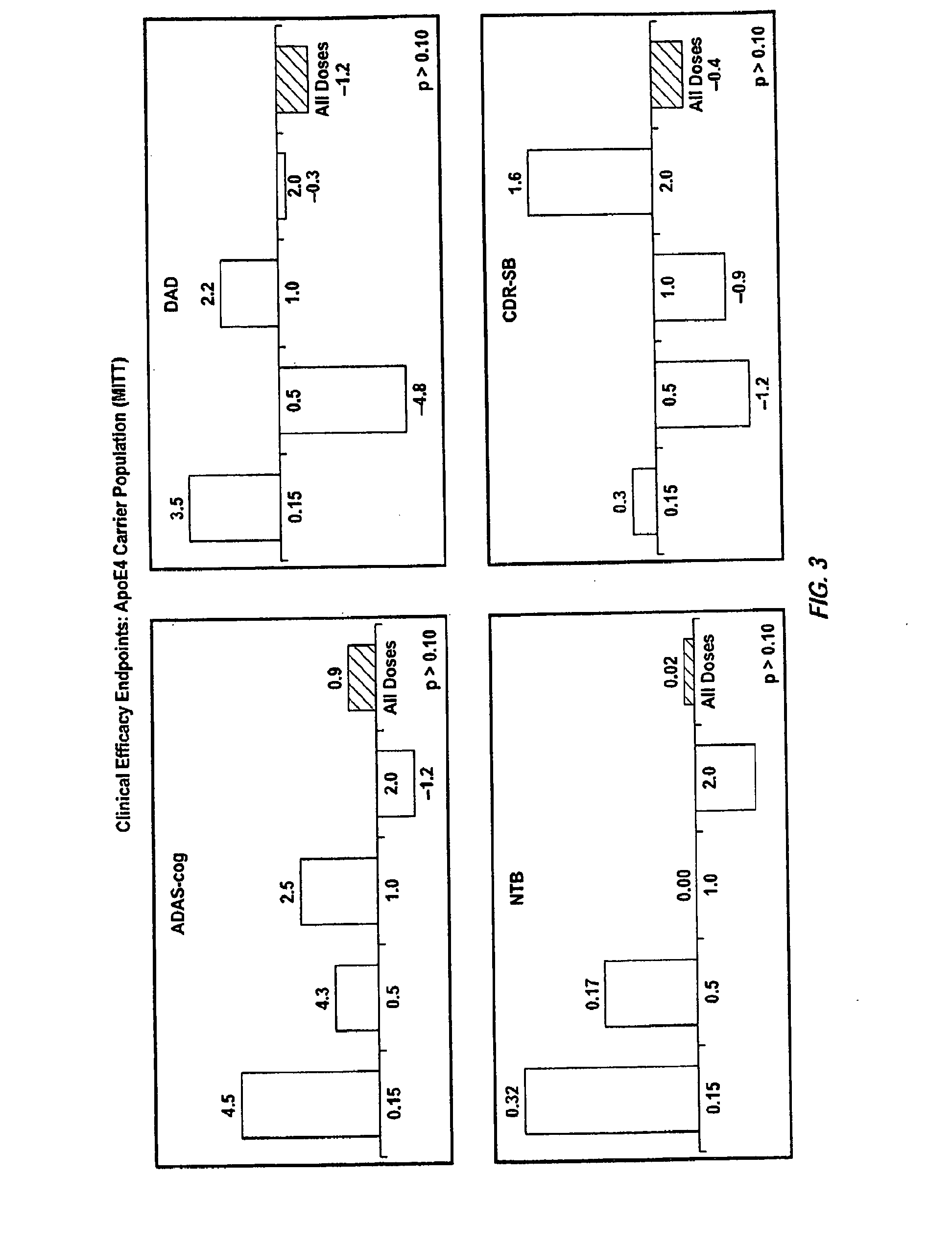
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Monitoring immune" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
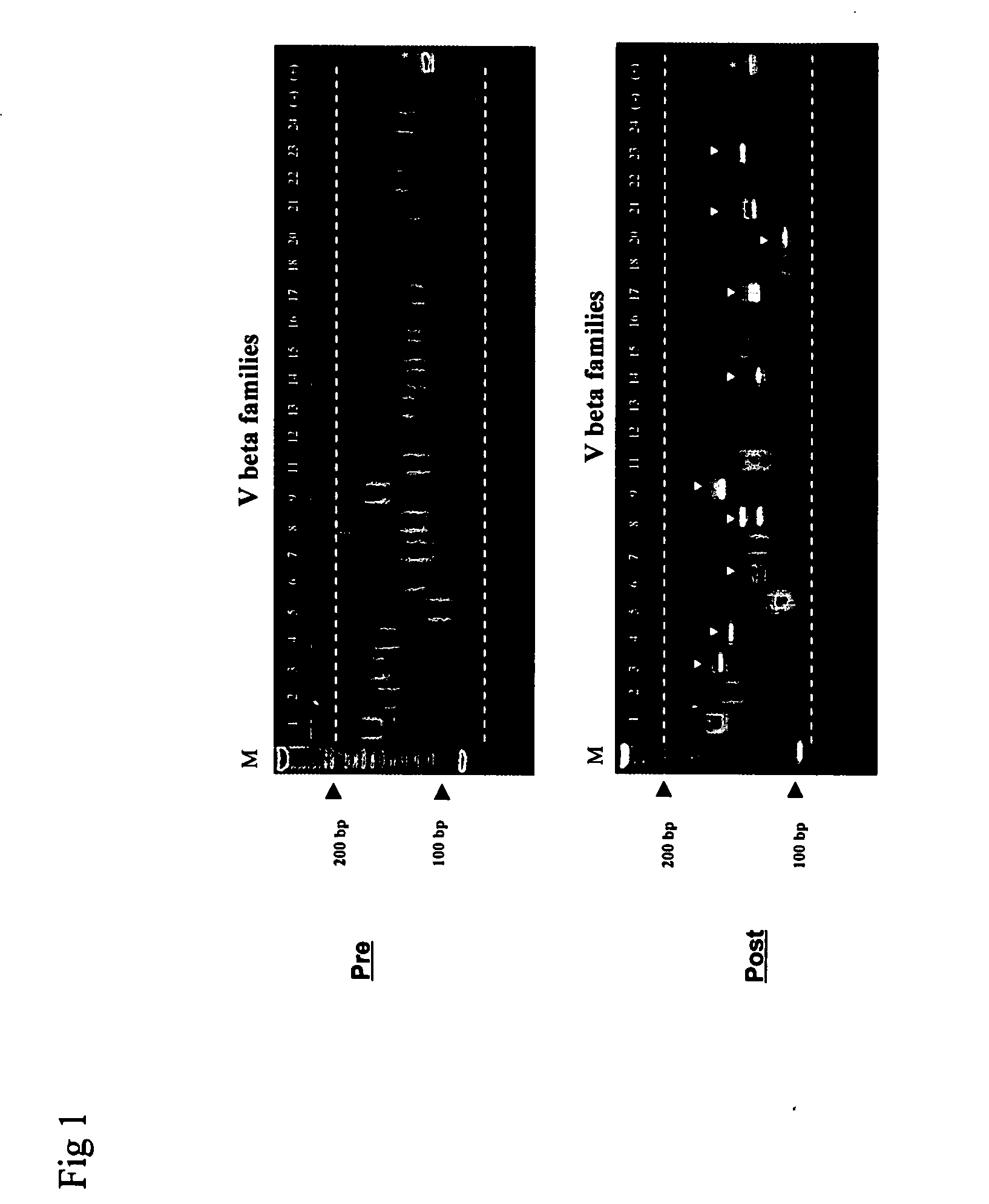
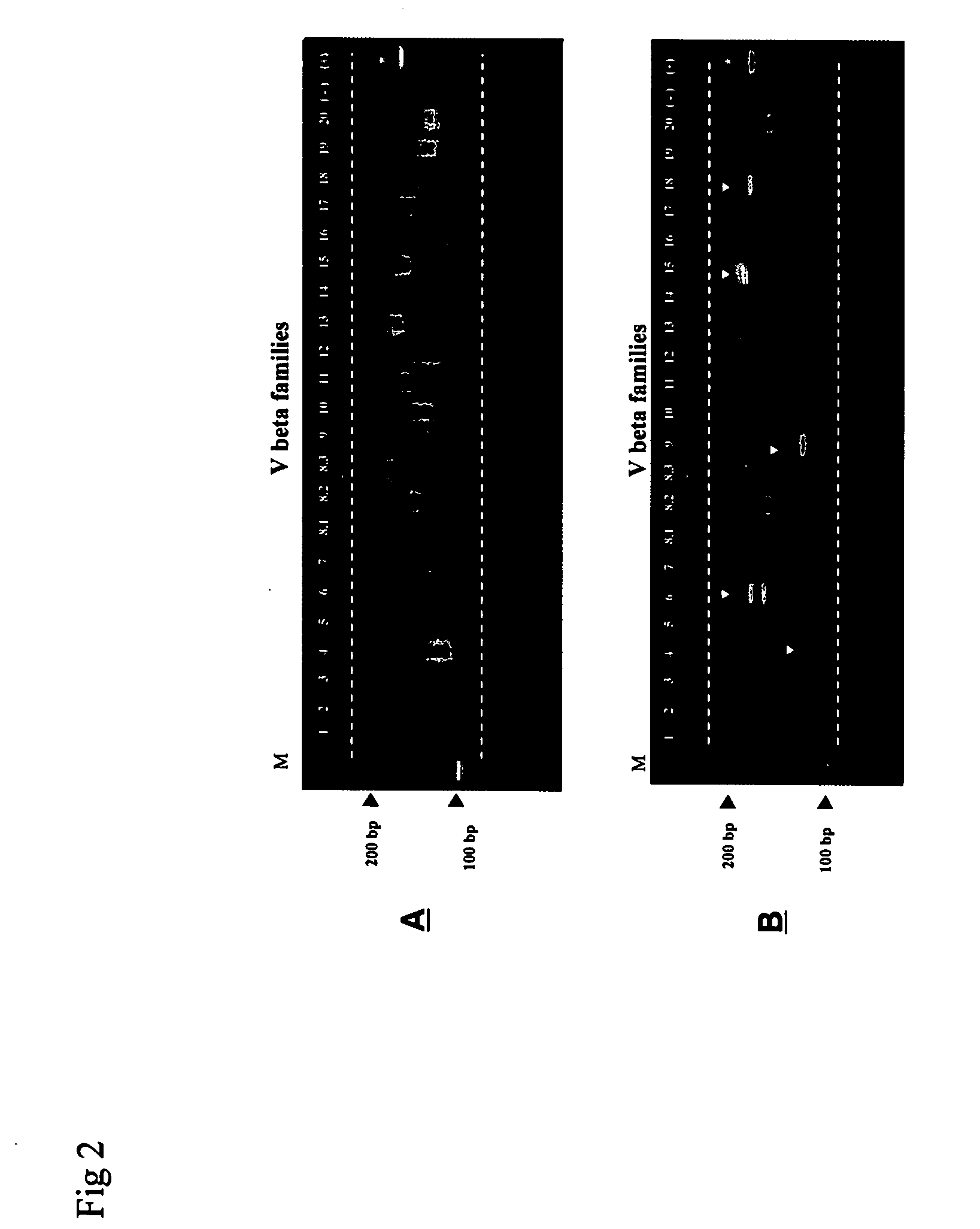
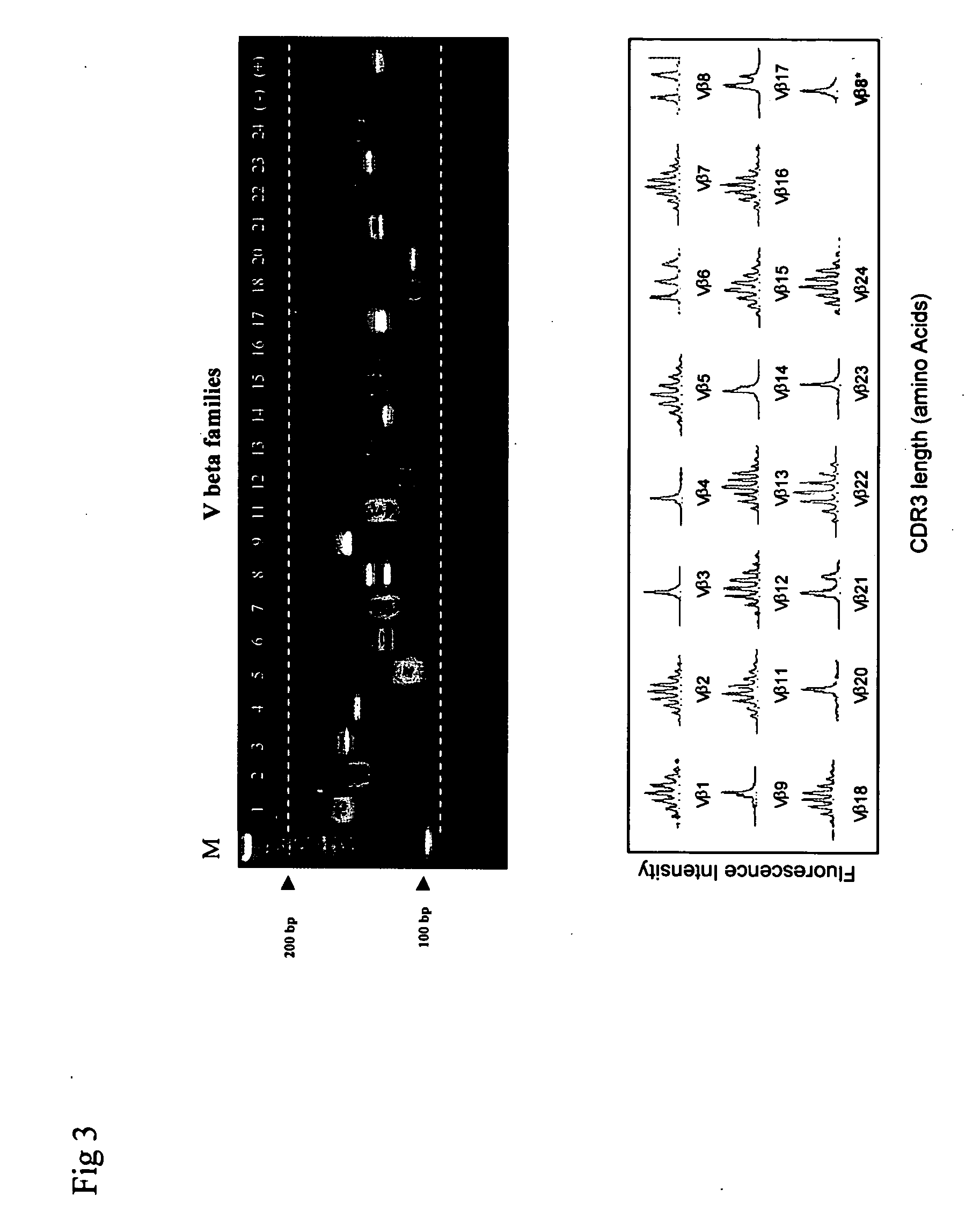
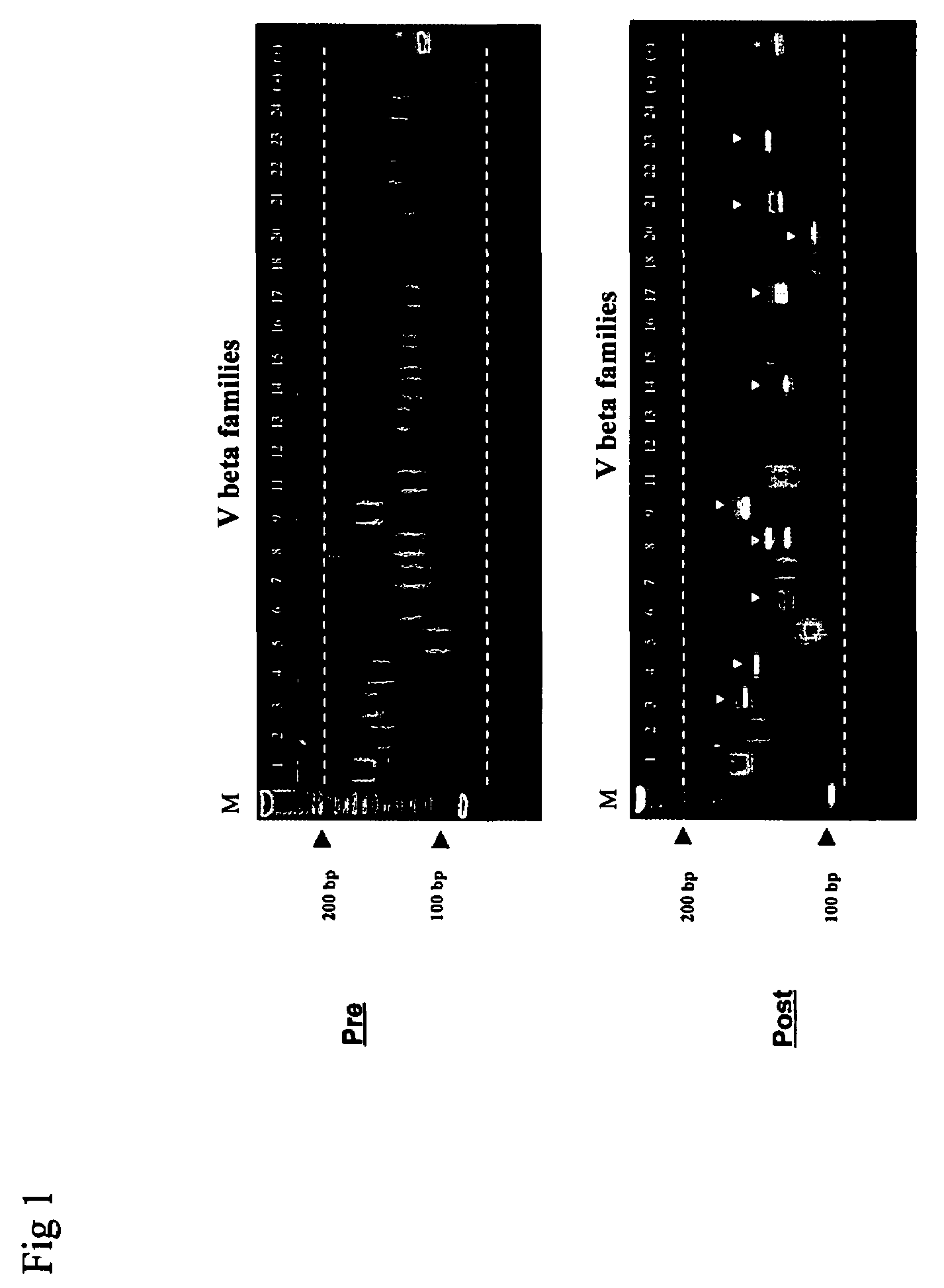
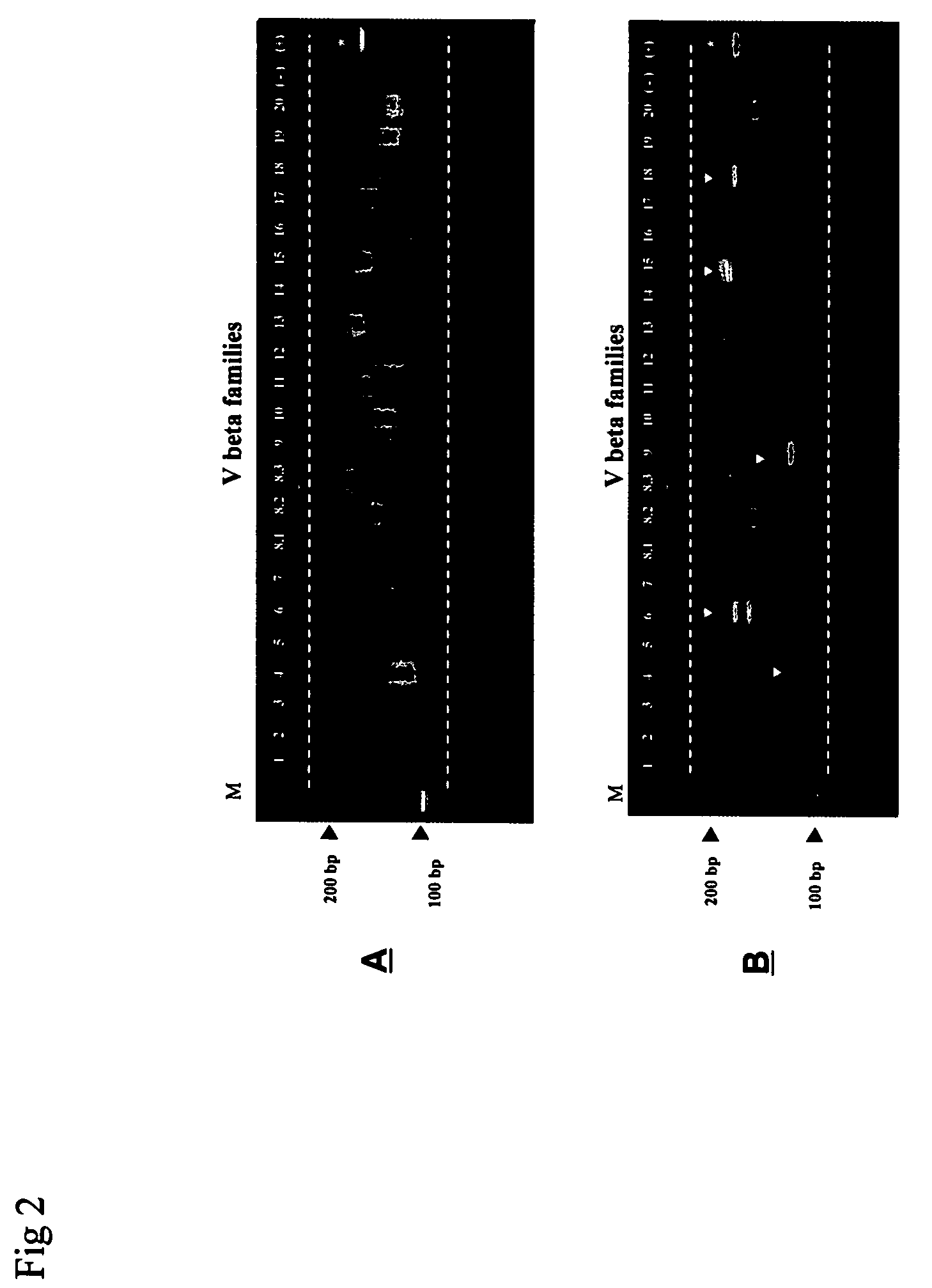
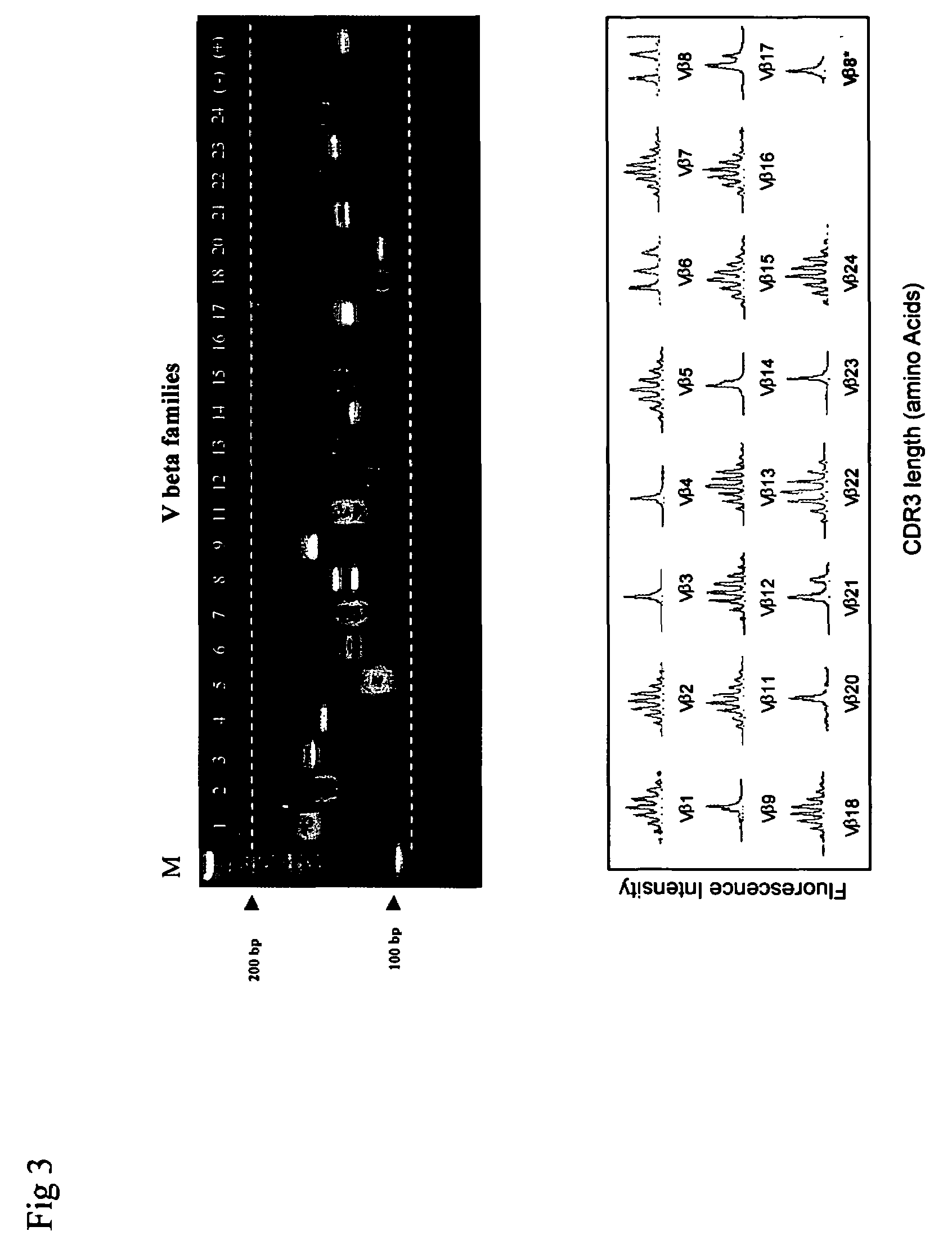
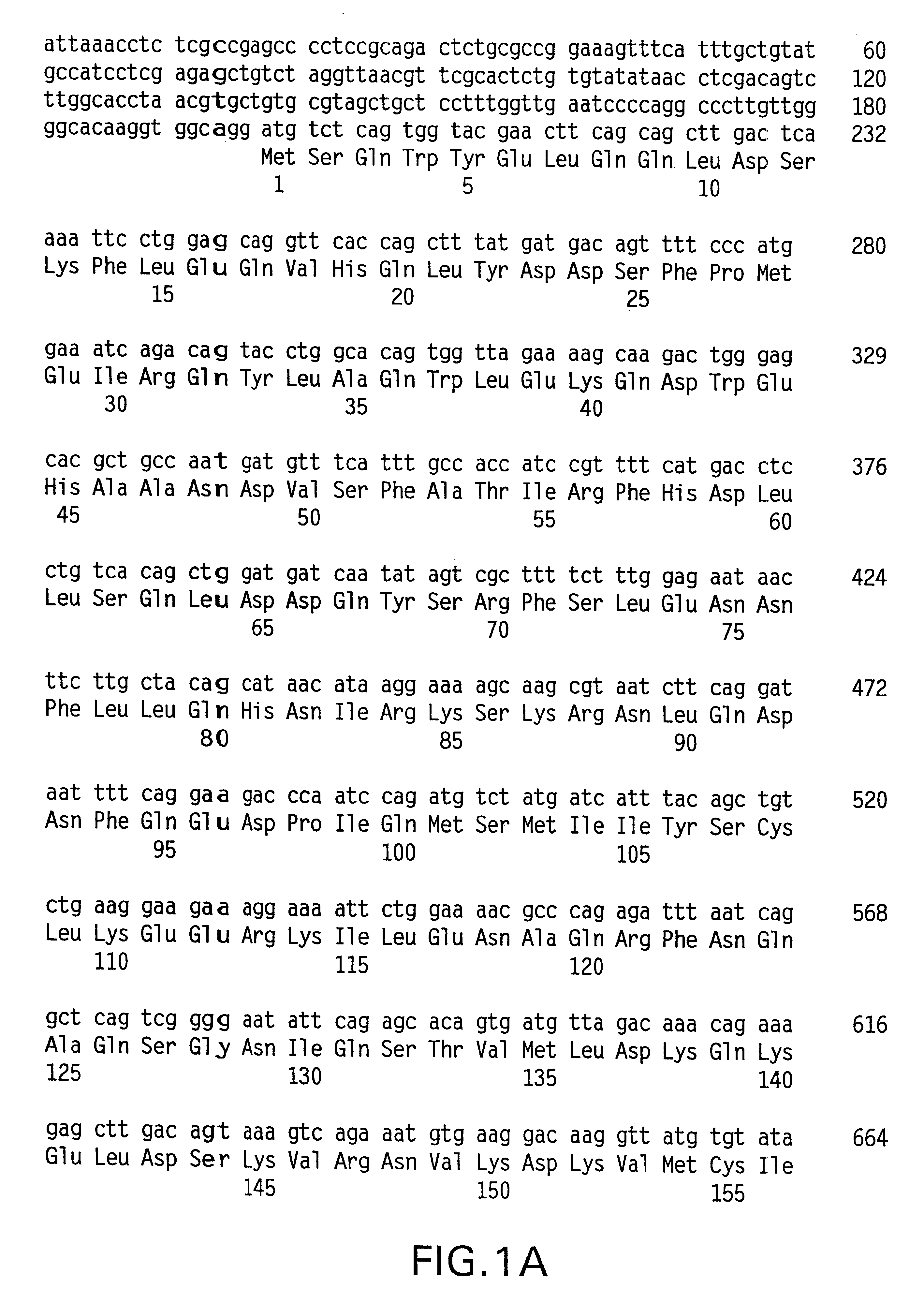
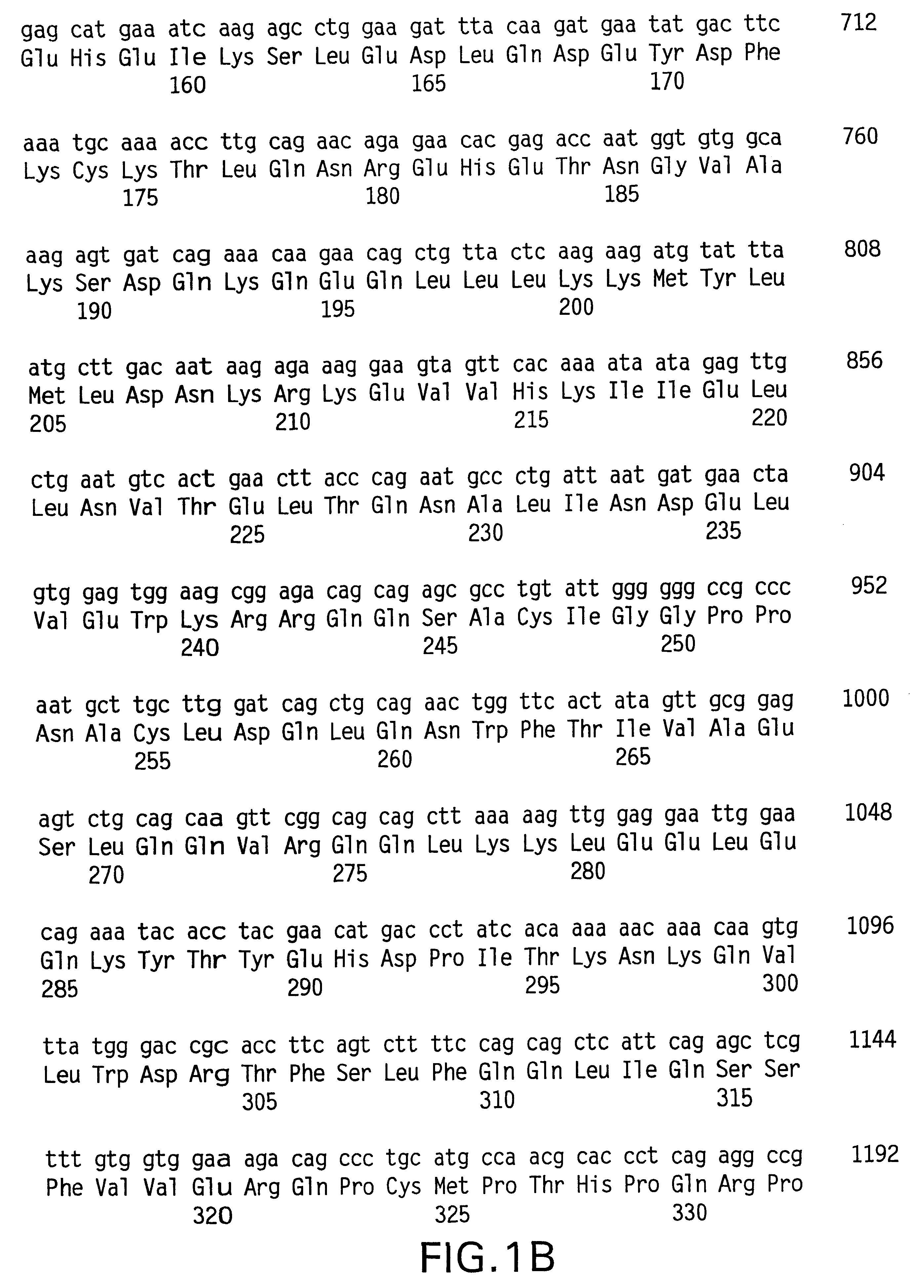
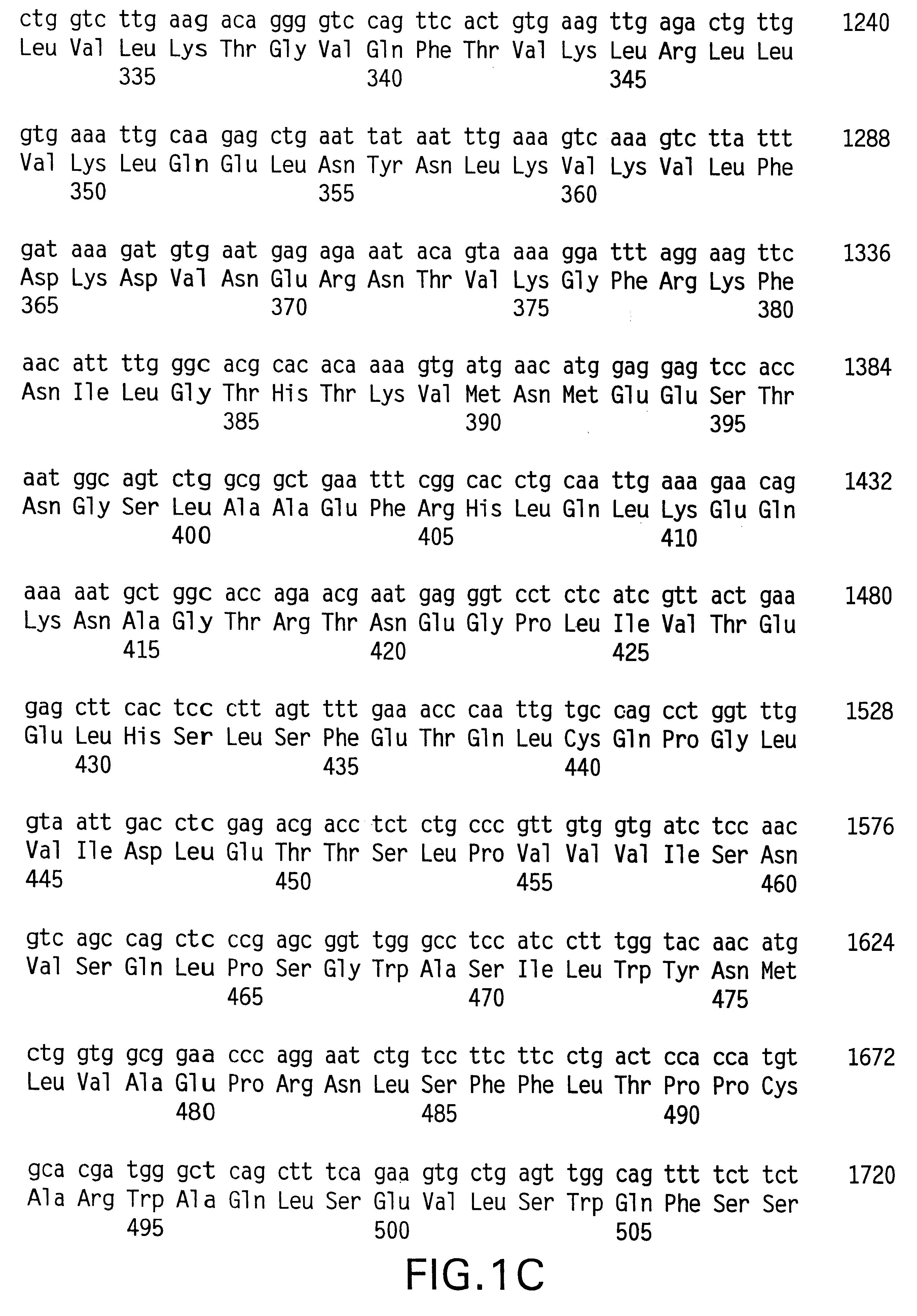
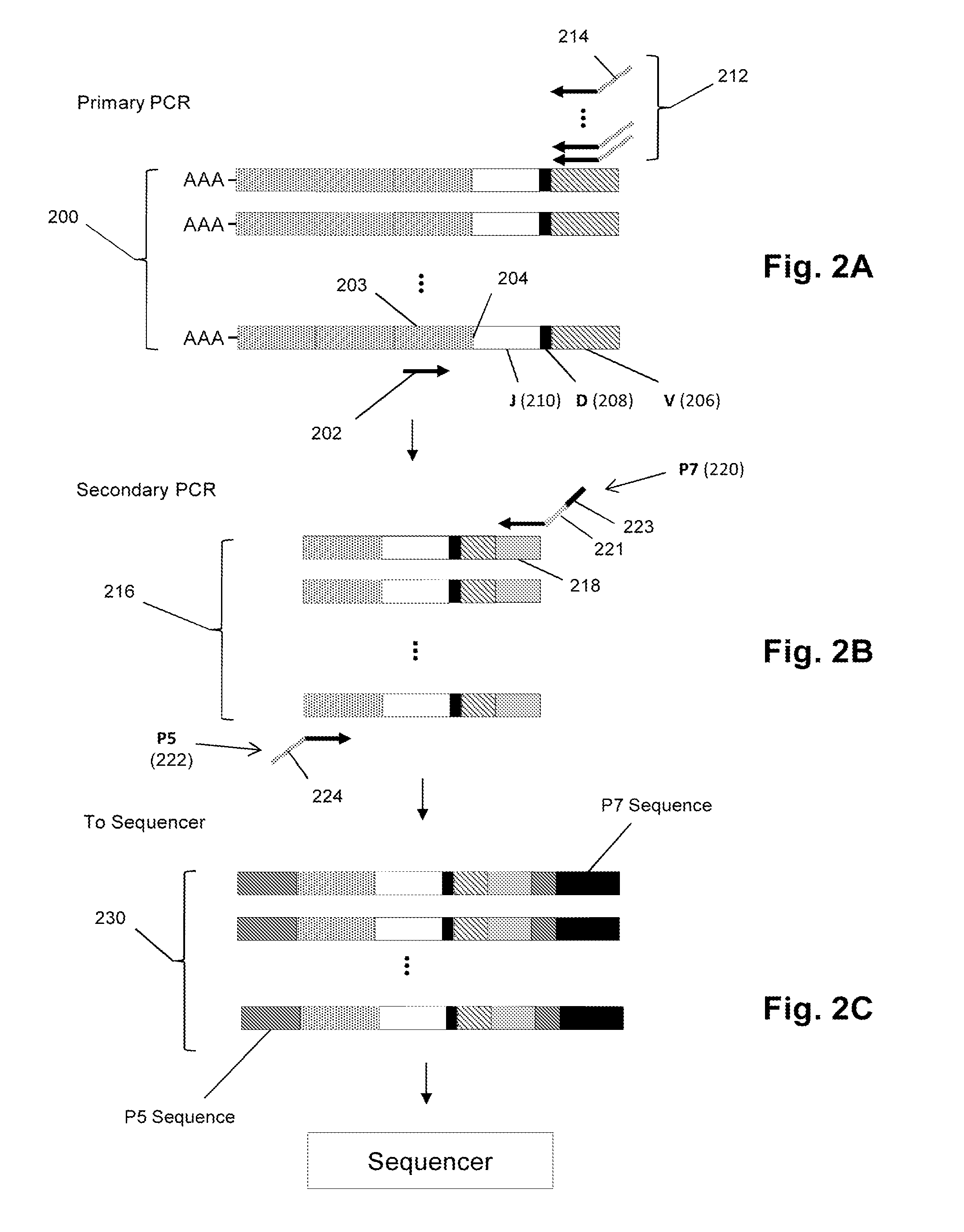
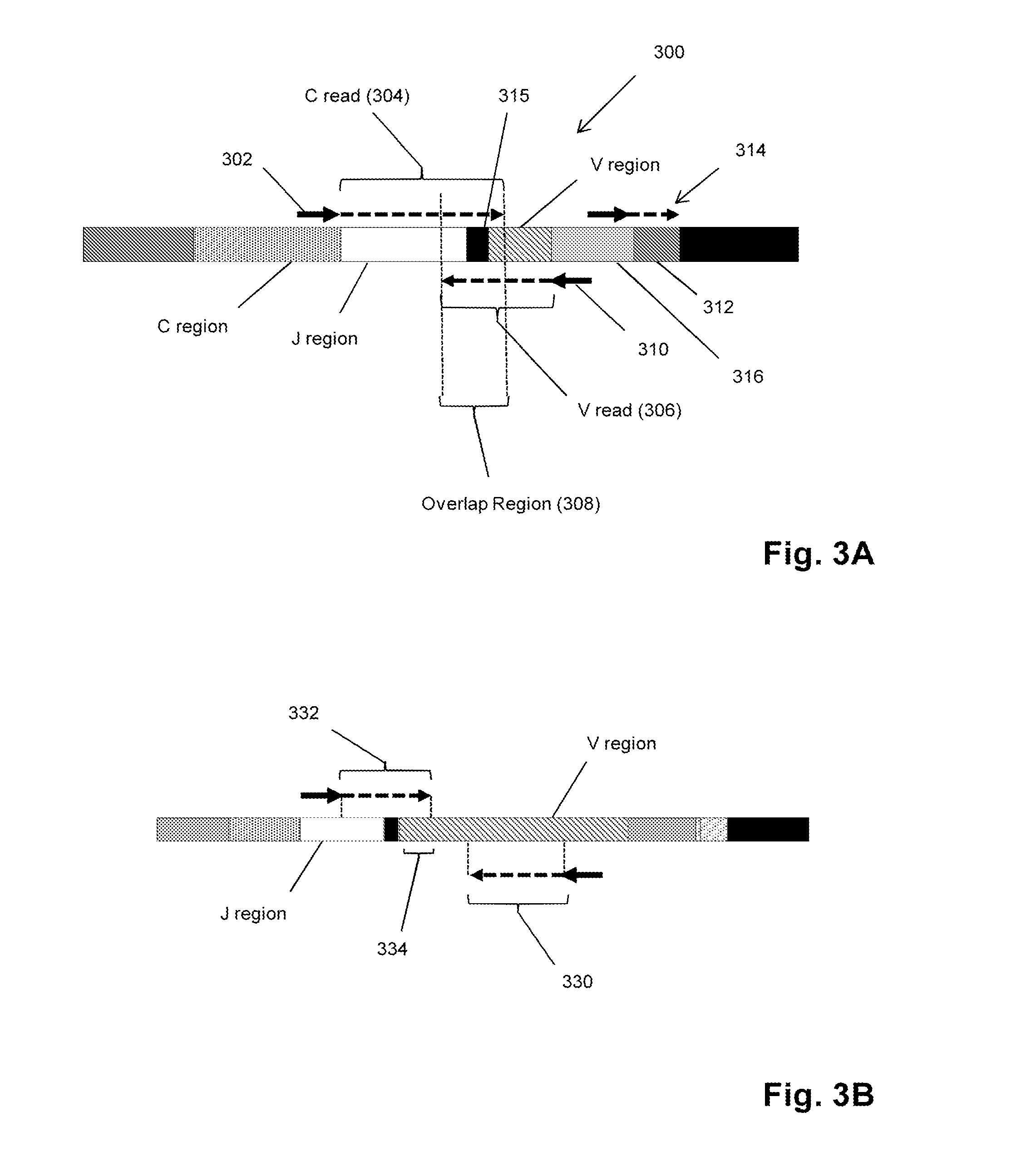
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS20070117134A1Enhances PCR reaction sensitivityRapid determinationAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS7375211B2Rapid determinationEasily detecting clonalityAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Method for identifying a compound to be tested for an ability to reduce immune rejection by determining Stat4 and Stat6 proteins
InactiveUS6534277B1Easy to useReduce immune rejectionMicrobiological testing/measurementLibrary screeningDiseaseStat signaling
The present invention relates to methods for identifying compounds that can reduce immune rejection, for example, transplant- or autoimmune disorder-related immune rejection. The present invention is based, in part, on the discovery, demonstrated herein, that immune rejection can be monitored by determining the amount of particular members of the Jak / Stat signal transduction pathway present within an affected tissue. The present invention is further based, in part, on the discovery, demonstrated herein, that immune rejection can be reduced and tolerance can be induced by modulating the amount of these particular members of the Jak / Stat signal transduction pathway present, expressed or active within an affected tissue. In particular, the results demonstrate that immune rejection can be monitored by determining the amount of mRNA or protein of Stat1, Stat3, Stat4, Stat6, SOCS1, or SOCS3 present, e.g., in an affected tissue.
Owner:MILLENNIUM PHARMA INC
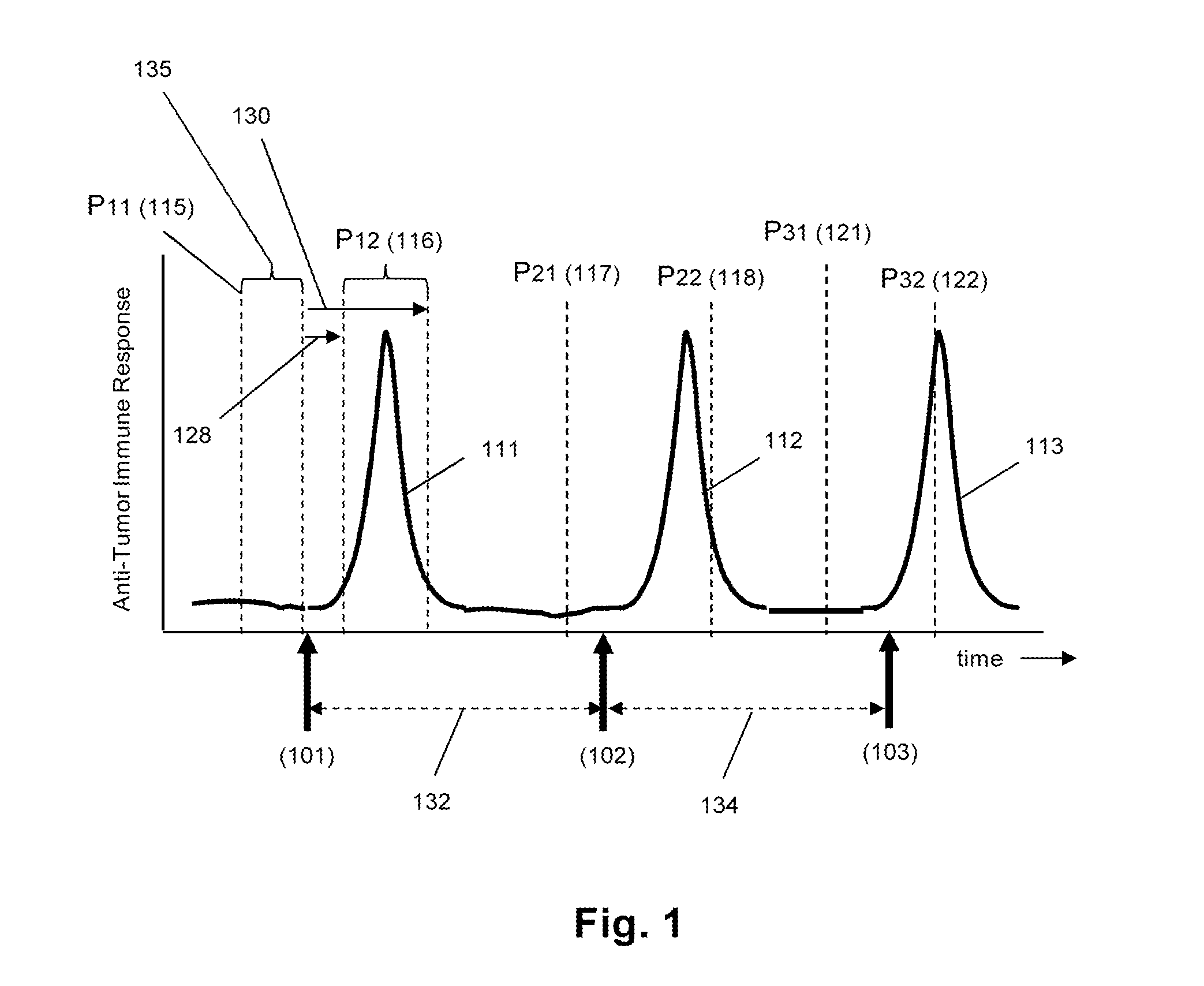
Monitoring immune responsiveness to cancer vaccination
InactiveUS20150038346A1Microbiological testing/measurementDisease diagnosisPost vaccinationMonitoring immune
The invention is direct to a method for determining a cancer patient's immune responsiveness to anti-cancer vaccination. In one aspect, for each of a plurality of vaccinations, pairs of clonotype profiles are obtained, one immediately prior to vaccination and one during the period of peak immune response, usually within two to twenty days after the vaccination. Responsiveness is correlated to successive increases in identical clonotypes within each pair of clonotype profiles in at least two successive vaccinations.
Owner:ADAPTIVE BIOTECH
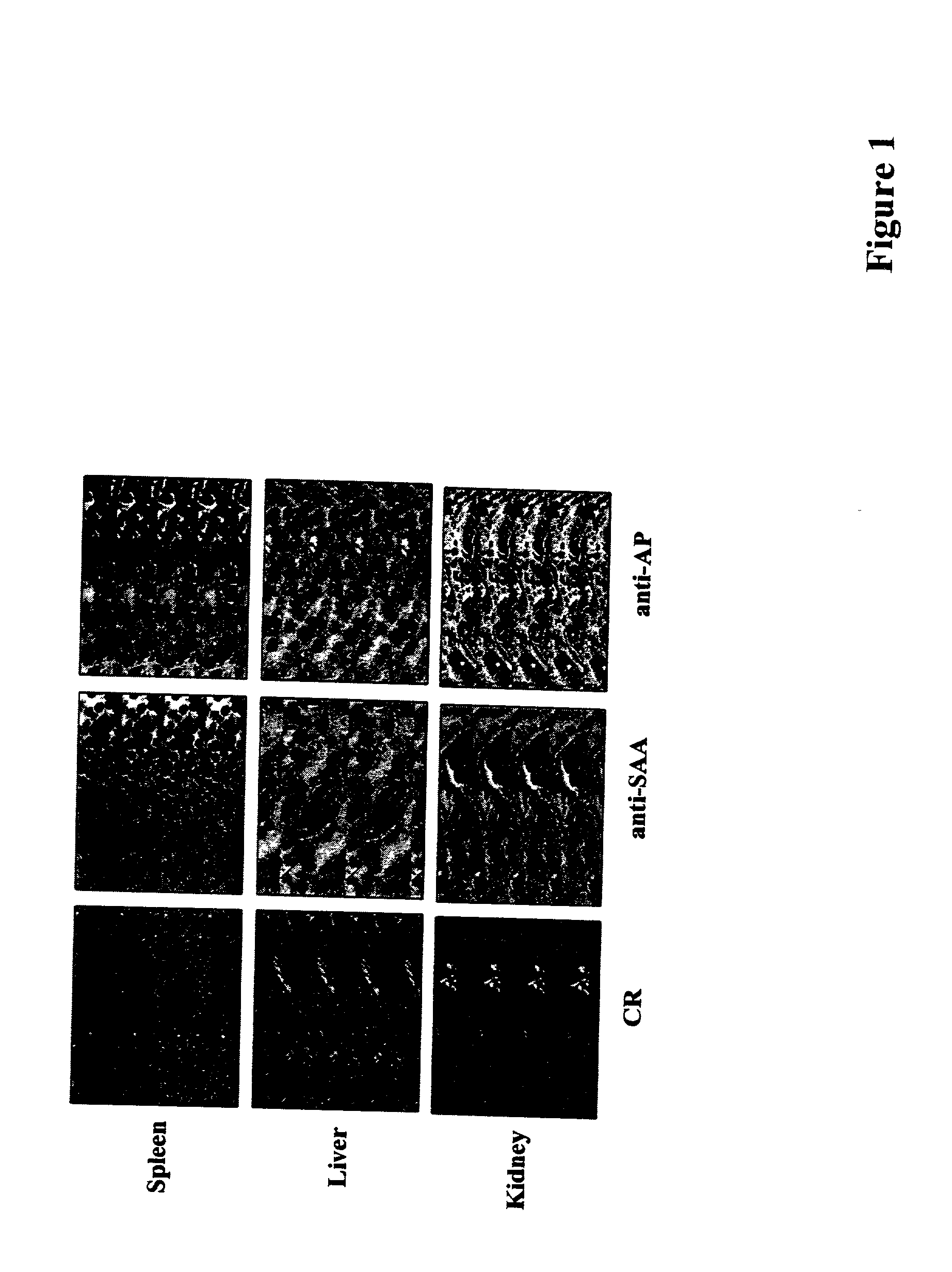
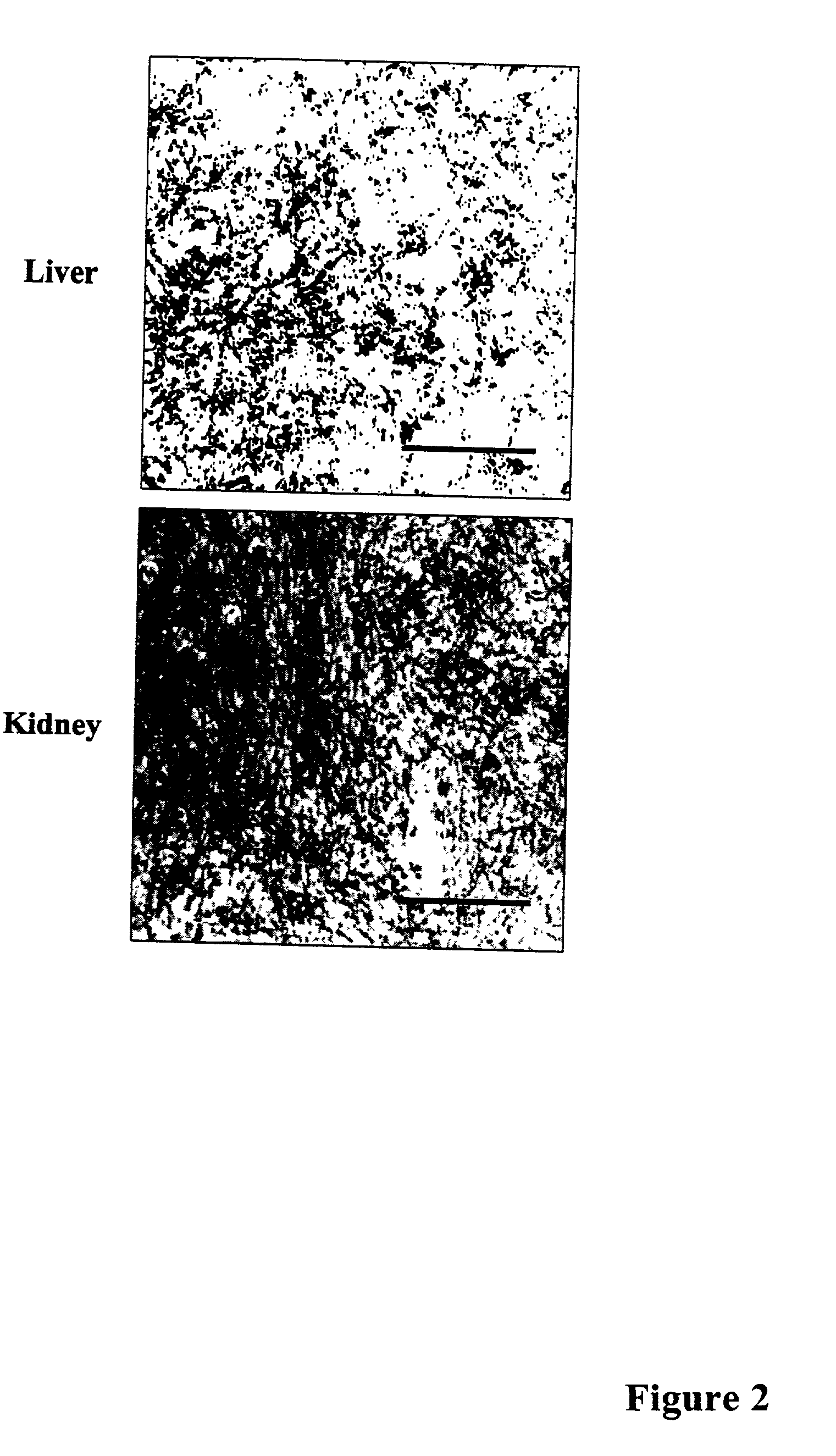
Methods of investigating, diagnosing, and treating amyloidosis
InactiveUS20020019335A1Constant level of amyloid depositsEffective preventionNervous disorderPeptide/protein ingredientsAA amyloidosisAssay
The present invention provides a therapeutic method for removing amyloid fibrils from a patient. The present invention also provides a transgenic animal that develops systemic AA amyloidosis within three weeks for use as a tool to investigate AA amyloidosis and to evaluate agents that may be potentially useful in preventing and treating amyloid-related disorders. Further, the present invention provides diagnostic assays for monitoring immunoglobulin light chain fibrillogenesis in real-time and for identification of the chemical nature of the protein in amyloid deposits which enables the determination of the type of amyloidosis for therapeutic and prognostic purposes.
Owner:UNIV OF TENNESSEE RES FOUND
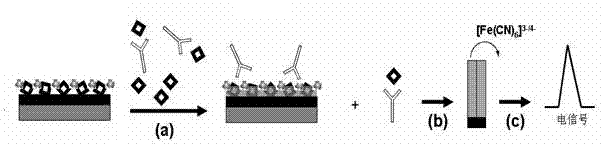
Electrochemical immunosensor for measuring melamine content, preparation method and application
InactiveCN102262115AAchieve qualitativeEasy to detectBiological testingMaterial electrochemical variablesAntigenDifferential pulse voltammetry
The invention discloses a label-free electrochemical immunosensor for melamine content determination, and a preparation method and application thereof. The immunosensor comprises a substrate electrode, wherein the surface of the substrate electrode is modified by a graphene-melamine-chitosan composite, and nonspecific active sites are sealed by bovine serum proteins. The method for preparing the electrochemical immunosensor is implemented by fixing melamine antigens on the surface of a glass carbon electrode with graphene / chitosan composite materials. Based on an immunoreactive competition mode, the electrochemical immunosensor monitors immune reaction through cyclic voltammetry and differential pulse voltammetry by taking K3Fe(CN)6 as a probe,, and can be used for melamine content detection. The immunosensor disclosed by the invention is high in sensitivity and specificity, simple in detection method and wide in application range; the limit of detection can reach 0.2 ng / ml, and the linear range is 5-1500 ng / ml; and the immunosensor has the characteristics of high speed, high efficiency, high sensitivity, good specificity, simple operation, low cost and the like.
Owner:NANJING NORMAL UNIVERSITY
Identifying antigen clusters for monitoring a global state of an immune system
InactiveUS20090258790A1High sensitivityStrong specificityLibrary screeningBiostatisticsAntigenSerum ige
Owner:YEDA RES & DEV CO LTD
Use of tau to monitor immunotherapy
ActiveUS20130209453A1Improve securityReducing brain volume declineImmunoglobulins against animals/humansAntibody ingredientsDiseaseImmunotherapy
The invention provides methods of immunotherapy of Alzheimer's and similar diseases in which the regime administered is monitored by measuring levels of tau.
Owner:WYETH LLC +1
Kit for diagnosis, prognosis, and monitoring the immune status, of patients with chronic inflammatory diseases
ActiveUS9188588B2Disease diagnosisBiological testingNatural Killer Cell Inhibitory ReceptorsNatural killer T cell
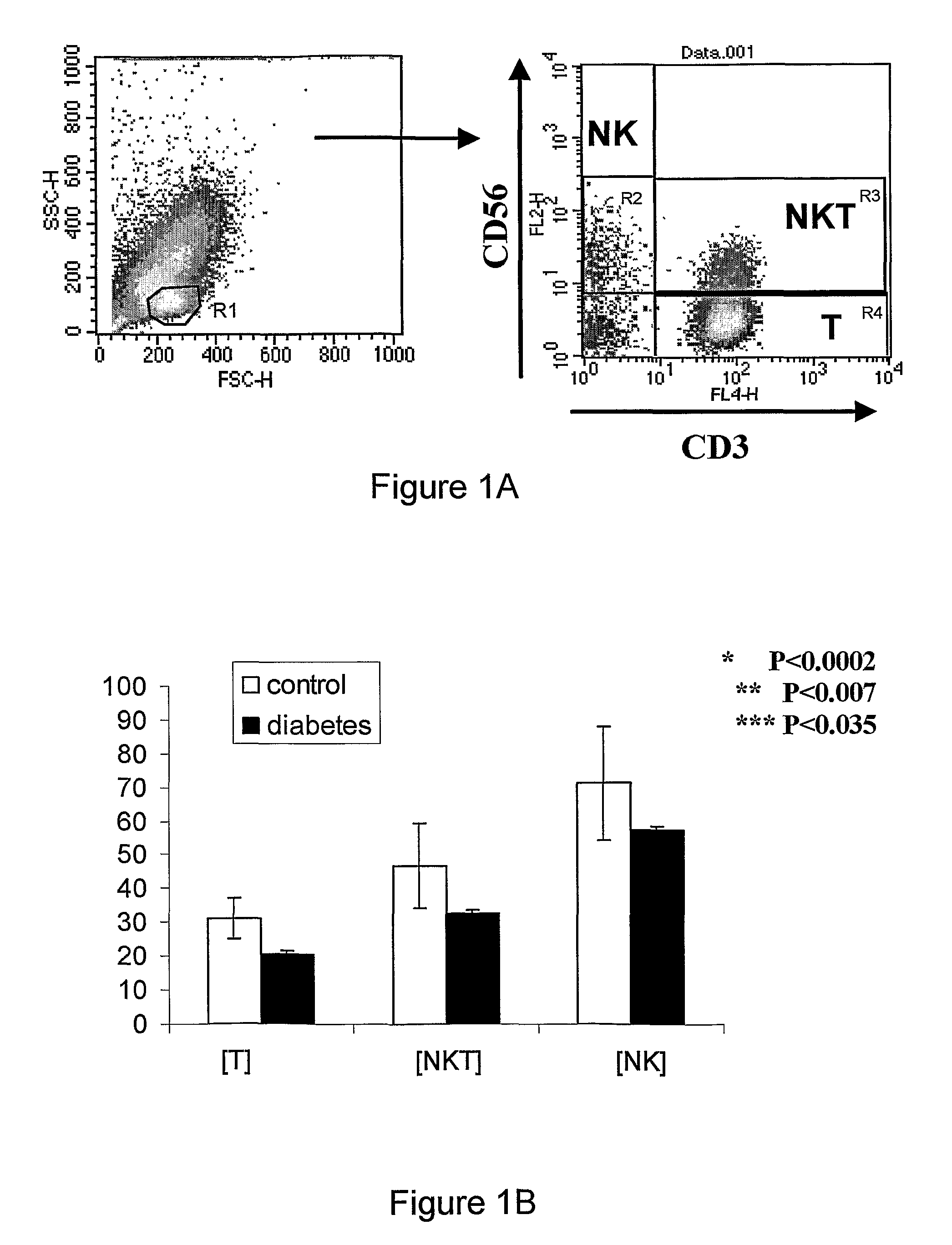
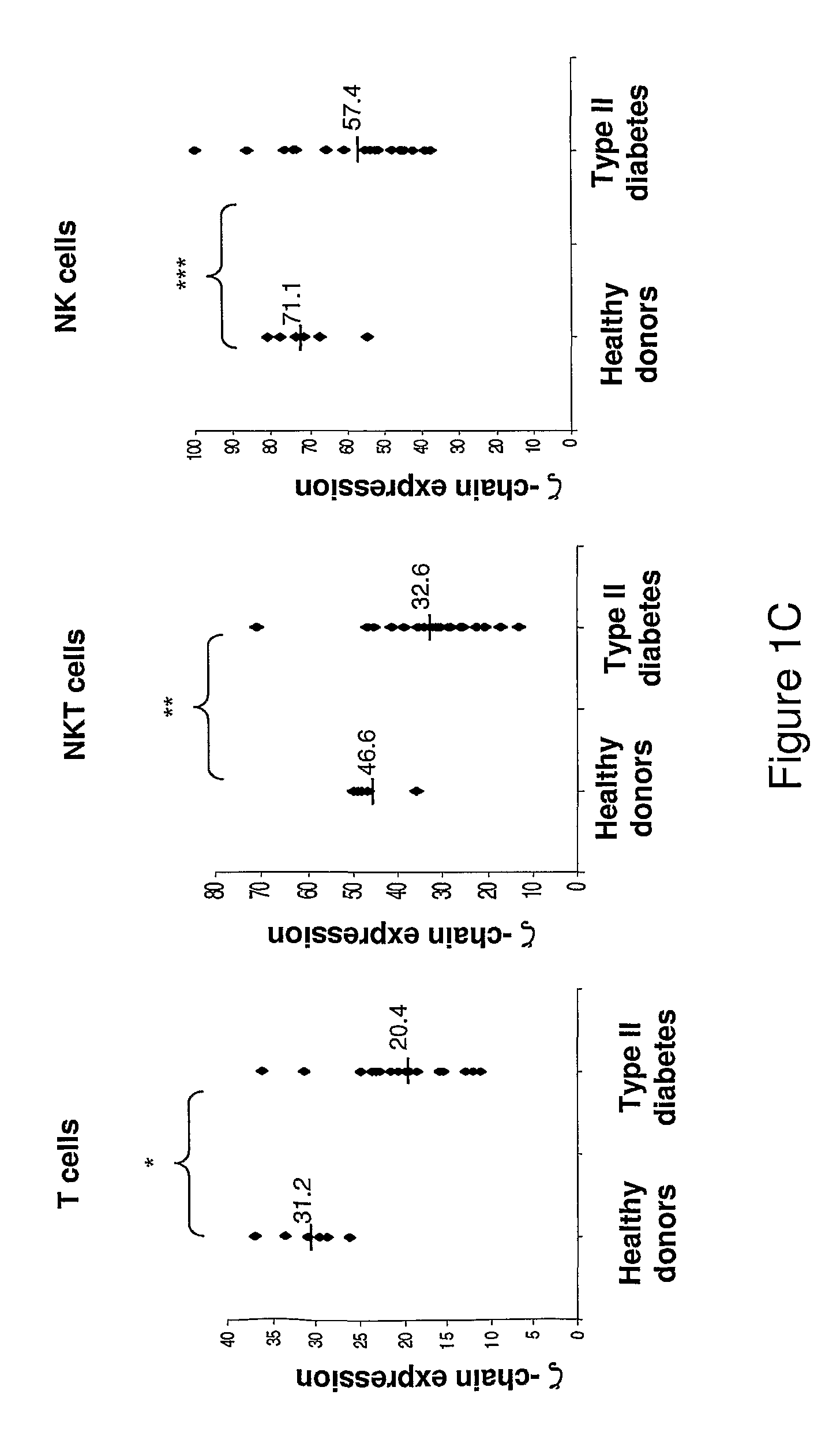
Provided is a method and a kit for testing the immune status of patients with chronic inflammatory diseases by measuring the TCR zeta chain (CD247) expression levels, and in particular a method and a kit for testing the selective downregulation of TCR zeta chain expression in T cells, NK cells, or NKT cells of such patients. Zeta chain expression is measured using antibodies directed against the intracellular zeta chain region, and these levels are compared with the expression levels of other T cell receptor subunits and NK cell markers. Thus, a kit for diagnosis, prognosis, and monitoring the immune status, of patients with chronic inflammatory diseases is presented herein.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Lung cancer diagnosis
InactiveUS20120100558A1High throughput approachReduce tumor burdenBiological testingProteomics methodsAutoantibody
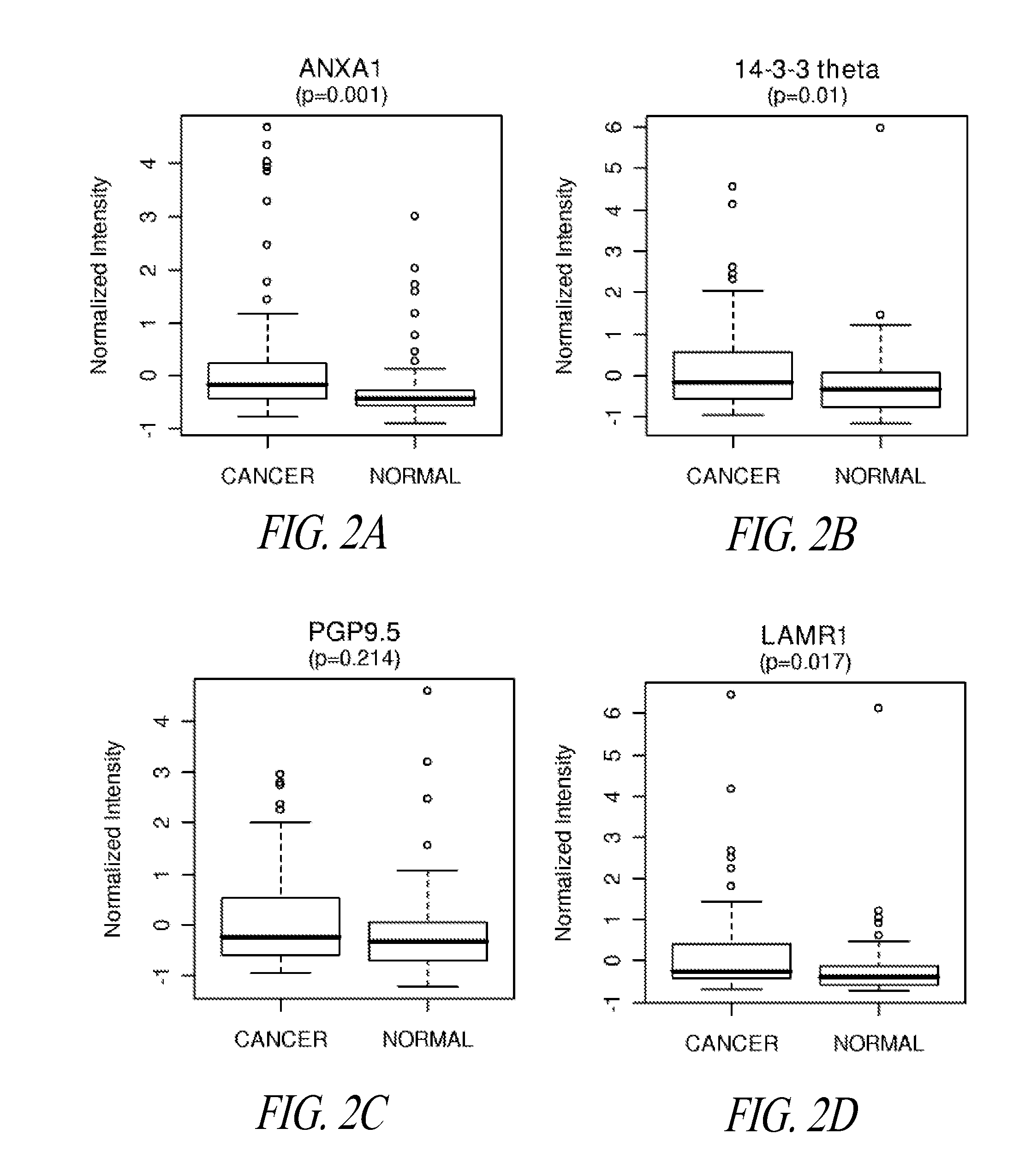
Diagnosis of lung cancer in a subject before onset of symptoms is described herein (i.e., in a pre-diagnostic subject), by screening a biological fluid from the subject for the presence therein of autoantibodies that are specific for one or more pre-diagnostic lung cancer indicator proteins, including LAMR1, and optionally additionally or alternatively including annexin I and / or 14-3-3-theta and / or other pre-diagnostic lung cancer indicator proteins as presently disclosed, as the defined antigens. Related methods, including for monitoring immune reactivity against lung cancer indicator proteins in a lung cancer patient, typing lung cancer subjects or characterizing lung tumors, and application of the described proteomics approach for the identification of additional pre-diagnostic lung cancer indicator proteins, are also contemplated.
Owner:FRED HUTCHINSON CANCER RES CENT
Method for monitoring the immune response and predicting clinical outcomes in transplant recipients
ActiveUS7476514B2Microbiological testing/measurementDisease diagnosisImmunosuppressive drugLymphocyte
Methods for monitoring the immune response and predicting clinical outcomes for patients on immunosuppressive drugs (such as transplant patients) are provided. The methods are based on the measurement of an intracellular metabolic marker in lymphocytes (such as ATP) as an indicator of a patient's immune response.
Owner:EUROFINS VIRACOR INC
Diagnosis of Metastatic Melanoma and Monitoring Indicators of Immunosuppression Through Blood Leukocyte Microarray Analysis
InactiveUS20100076691A1Easy to explainDifficult to interpretNucleotide librariesMicrobiological testing/measurementMetastatic melanomaWhite blood cell
The present invention includes compositions, systems and methods for the early detection and consistent determination of metastatic melanoma and / or immunosuppression using microarrays by calculating one or more expression vectors from the expression of one or more genes.
Owner:BAYLOR RES INST
Indirect ELISA (enzyme-linked immuno-sorbent assay) kit for detecting nephropathogenic avian infectious bronchitis virus and antibody thereof
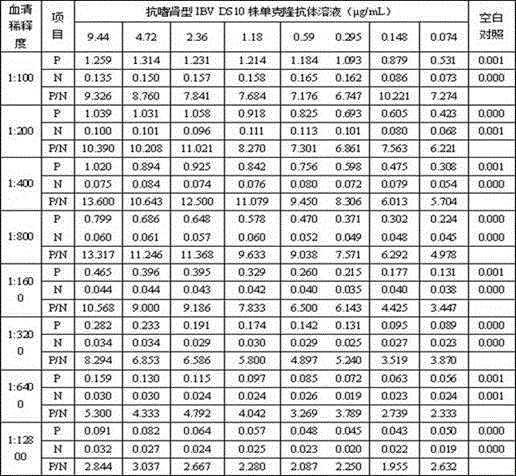
The invention provides an indirect ELISA (enzyme-linked immuno-sorbent assay) kit for detecting a nephropathogenic avian infectious bronchitis virus and an antibody thereof, and belongs to the technical field of biology. The indirect ELISA kit for detecting the nephropathogenic avian infectious bronchitis virus and the antibody thereof comprises an ELISA plate which is coated by DS10 monoclonal antibodies resisting the nephropathogenic avian infectious bronchitis virus and is combined with DS10 inactivated purified antigens resisting the nephropathogenic avian infectious bronchitis virus. The indirect kit for detecting the nephropathogenic avian infectious bronchitis virus and the antibody thereof has the advantages of specificity, sensitivity, quickness and simplicity and convenience in operation, and can be used for quickly diagnosing whether chickens are infected by the nephropathogenic avian infectious bronchitis virus or not and monitoring the immune antibody in actual production.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Diagnosis of metastatic melanoma and monitoring indicators of immunosuppression through blood leukocyte microarray analysis
InactiveCN101601042AMicrobiological testing/measurementData visualisationMetastatic melanomaWhite blood cell
The present invention includes compositions, systems and methods for the early detection and consistent determination of metastatic melanoma and / or immunosuppression using microarrays by calculating one or more expression vectors from the expression of one or more genes.
Owner:BAYLOR RES INST
Moesin fragments associated with immune thrombocytopenia
ActiveUS20130316379A1Polypeptide with localisation/targeting motifPeptide/protein ingredientsMoesinImmune thrombocytopenia
The present application provides compositions and methods useful for detecting and monitoring immune thrombocytopenia.
Owner:SHANGHAI KEXIN BIOTECH
Preparation method and application of autologous tumor vaccine
InactiveCN109876137ATo achieve the goal of individualized tumor immunotherapyImprove immunityCancer antigen ingredientsAntineoplastic agentsAdjuvantAutologous tumor cell
The invention relates to a preparation method and application of an autologous tumor vaccine. The method comprises the following specific steps: radiating and / or heating autologous tumor cells to greatly excite the antigenicity of the autologous tumor cells and enhance the presentation property of the antigen by using heat shock proteins; and inactivating the tumor cells and adding an adjuvant toobtain the vaccine. The vaccine prepared by using the method has the special advantages of high presentation property and high tumor immunogenicity, and can be used for greatly exciting the body fluidand cellular immune response of tumor patients to autologous tumors, monitoring immune response, and timely adjusting a usage method of the novel vaccine and adjuvant treatment, so that the aims of improving the antigenicity and metastatic tumor effect and prolonging the survival time of tumor patients are fulfilled.
Owner:XIAMEN LUJIA BIOTECH
Whole blood type freeze-dried powder immunosuppressant quality control substance as well as preparation method and application thereof
The invention discloses a whole blood type freeze-dried powder immunosuppressant quality control substance as well as a preparation method and application thereof. The substance is a freeze-dried product mainly prepared from human whole blood serving as a matrix and an immunosuppressant. The immunosuppressant comprises one or more of tacrolimus, sirolimus, everolimus and cyclosporin A, or furthercomprises glucocorticoid immunosuppressants: one or more of hydrocortisone, cortisone, prednisolone, prednison and mycophenolic acid ester immunosuppressants for blood detection. The substance has theadvantages that the uniformity and the stability are good, low-temperature storage at -70 DEG C is not needed, and the substance can be used as a calibration product of immunosuppressant kits of different methodologies and can also be used as a quality control product for clinically monitoring an immunosuppressant detection system.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Systems and methods for monitoring immune responses and predicting outcomes in transplant recipients
InactiveUS20070202085A1Reduce the amount requiredBiocideMicrobiological testing/measurementTolerabilityTransplant rejection
The present invention is related to transplant rejection. In particular, the present invention relates to determining the functional status of alloreactive T cells and correlating the functional status to in vivo immune responses (e.g., tolerance, rejection, or absence of rejection mediated by T cells). The present invention finds use in basic research, clinical (e.g., transplant) and therapeutic settings.
Owner:RENOVAR
Methods for diagnosing and for monitoring the treatment of recurrent spontaneous abortion
ActiveUS20070160997A1Decrease in levelAvoid abortionAnalysis using chemical indicatorsMicrobiological testing/measurementAntigenAnti-nuclear antibody
The present invention discloses a method of diagnosis of immunological recurrent spontaneous abortion, comprising in vitro determining the level of antinuclear antibody in a body fluid of the patient and comparing the result with the level of corresponding antinuclear antibody of normal control. Particularly, isolated chromosome No. 2 or fragment thereof containing fibronectin encoding gene derived from male(s) is used as antigen in the method of the present invention for determining the level of corresponding antinuclear antibody in a body fluid sample of the patient. The present invention also discloses a diagnostic kit for immunological recurrent spontaneous abortion, and methods and kits for monitoring the therapeutic effect for immunological recurrent spontaneous abortion.
Owner:BEIJING XINJING ANTAI MEDICAL & TECH SERVICE
F1-protein-based indirect immunofluorescence kit for detecting type 4 fowl adenovirus antibody
The invention belongs to the field of biotechnology detection, in particular to an F1-protein-based indirect immunofluorescence kit for detecting a type 4 fowl adenovirus antibody. The kit comprises 293T cells expressing F1 protein, an FITC-labeled goat-anti-chicken antibody, a sample diluent and a washing liquid. The kit has good specificity and not only can be used for epidemiological investigation of serum type 4 fowl adenovirus infection conditions, but also can be used for monitoring the level of a serum type 4 fowl adenovirus antibody of immune chicken flocks and used for evaluating the immunoprotecive antibody level.
Owner:YANGZHOU UNIV
Detection kit for chicken infectious bronchitis indirect ELISA antibody
InactiveCN105092839ASimple indirect ELISA methodSpecific indirect ELISA methodMaterial analysisMaternal antibodyInfectious bronchitis virus Antibody
The invention discloses a detection kit for chicken infectious bronchitis indirect ELISA antibody, belonging to the field of biotechnology. According to the invention, genetically engineered bacterium capable of steadily expressing partial nucleoprotein of chicken infectious bronchitis virus is utilized for inducible expression of recombined N160 protein which is subjected to affinity chromatographic purification, and the purified recombined N160 protein is taken as a coating antigen, so as to establish a simple and convenient and specific indirect ELISA method. The invention further provides a specific, sensitive, quick and convenient-to-operate kit for detecting the antibody of the chicken infectious bronchitis virus, which can be used for quickly diagnosing whether the chicken flocks are infected by the chicken infectious bronchitis virus and monitoring the immune chicken serum antibody level and the chicken maternal antibody level in actual production, so as to evaluate the degree of risk that the chicken flocks are infected by the infectious bronchitis virus and provide reference for the formulation of an infectious bronchitis immune procedure.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Compositions, Kits, and Methods for the Diagnosis, Prognosis, and Monitoring of Immune Disorders Using Galectin-1
ActiveUS20090176223A1Reduced expression levelHigh expressionMicrobiological testing/measurementAntibody ingredientsAnaplastic CellGalectin-1
The present invention is based, in part, on the discovery that galectin-1 (Gal1) plays a role in immune disorders, including Hodgkin lymphoma, anaplastic large cell lymphoma, or MLL+ pre B-cell ALL. Accordingly, the invention relates to compositions, kits, and methods for diagnosing, prognosing, and monitoring immune disorders, e.g., Hodgkin lymphoma, anaplastic large cell lymphoma, or MLL+ pre B-cell ALL.
Owner:DANA FARBER CANCER INST INC +2
Biomarkers for diagnosing and/or monitoring tuberculosis
InactiveUS20150192593A1Reduce and prevent progressionPromoting and of suppressing generationBiocidePeptide librariesPathogenic microorganismAnti mycobacterial
The invention relates to biomarkers for diagnosing and / or monitoring tuberculosisin both immunocompetent and immunocompromised individuals, monitoring the responses of individuals to anti-mycobacterial chemotherapy, monitoring the progression of latent tuberculosis to active tuberculosis, differentiating active tuberculosis from latent tuberculosis, and from other clinical conditions that mimic tuberculosis (TB). The invention also relates to methods for diagnosing, treating and monitoring tuberculosis using said biomarkers. The above pertain in all aspects both to pulmonary and extrapulmonary Mycobacterium.tuberculosis infections, with Mycobacterium.tuberculosis being the causative organism in tuberculosis.
Owner:PROTEINLOGIC
Therapeutic and Diagnostic Methods Using TIM-3
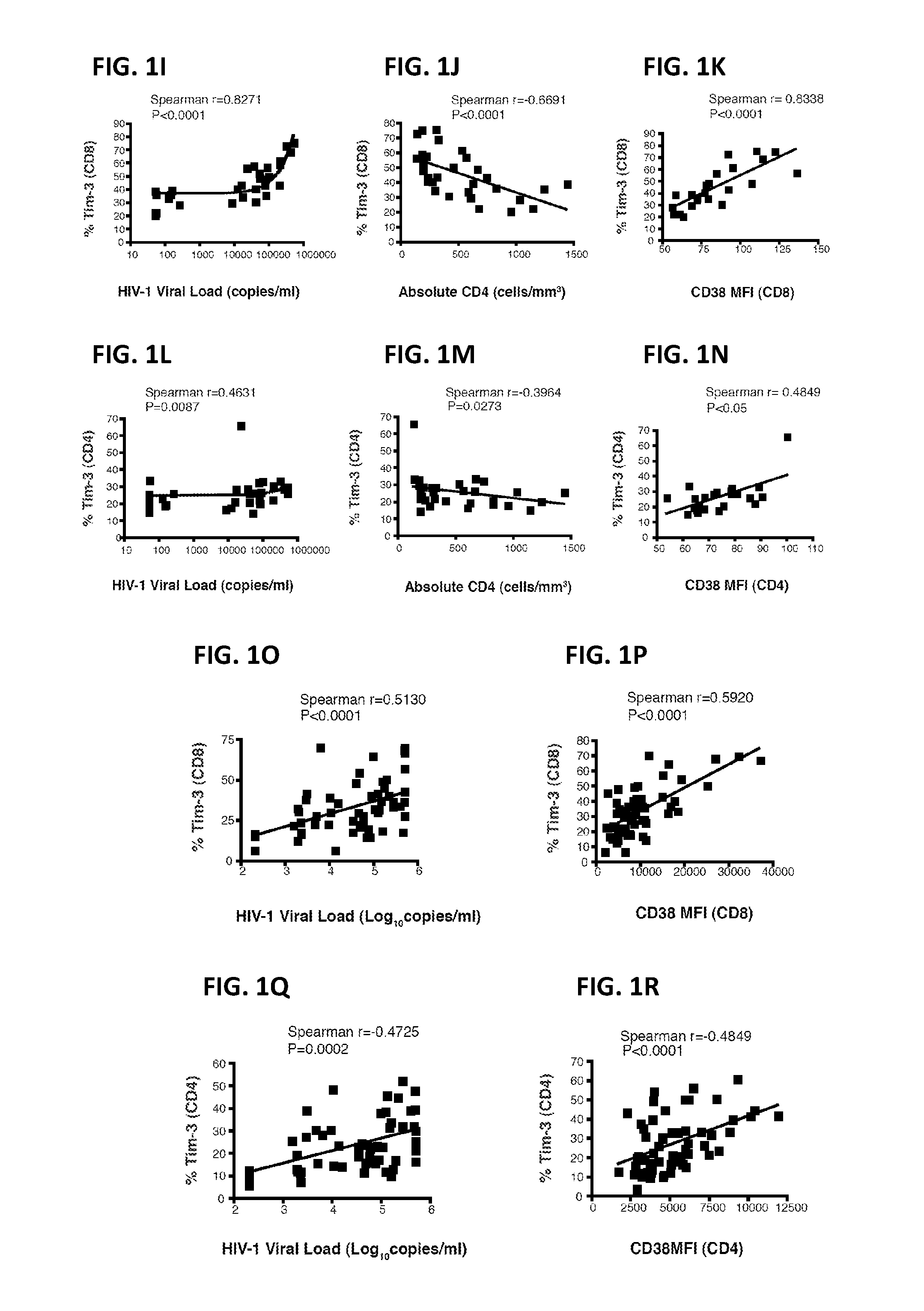
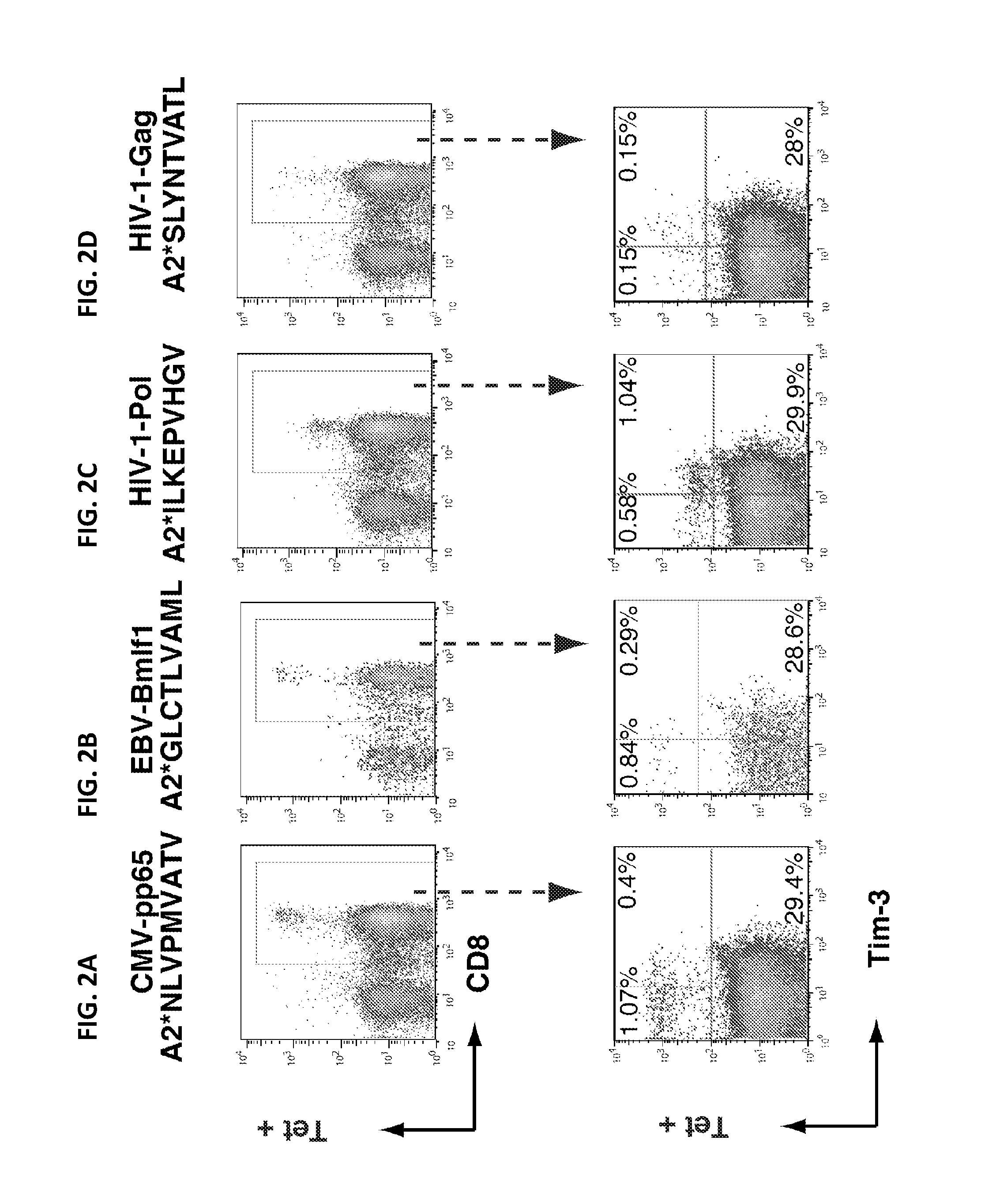
ActiveUS20160311890A1Function increaseImmunoglobulin superfamilySsRNA viruses positive-senseViral infectionDisease cause
The application relates to methods of treating chronic viral infection by modulating Tim-3 activity. In addition, the present application relates to methods of diagnosing or monitoring immune system activity or function, chronic viral infection and inflammatory disease using Tim-3 expression.
Owner:RGT UNIV OF CALIFORNIA
Biomarkers for monitoring immune transformation
PendingUS20190117735A1High activityPeptide/protein ingredientsDisease diagnosisDiseaseRegulatory T cell
The present invention provides novel biomarkers for regulatory T cells (Treg) function and Parkinson's disease.
Owner:BOARD OF RGT UNIV OF NEBRASKA +1
Method and kit for detecting five immunosuppressants in dried blood spot
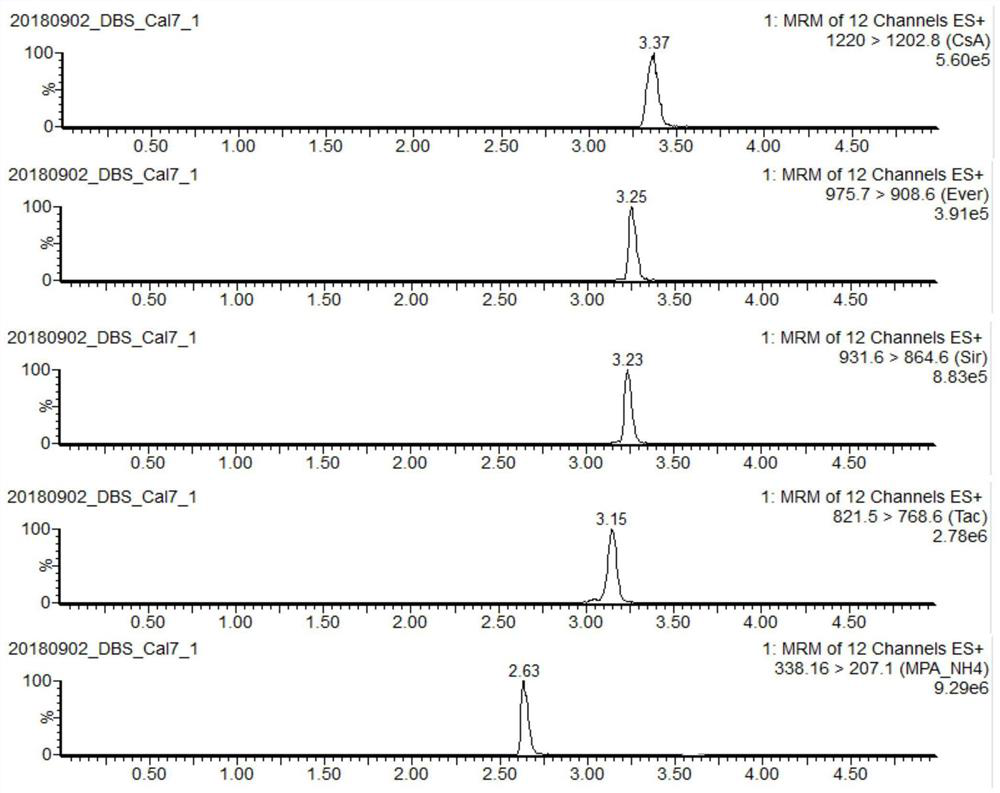
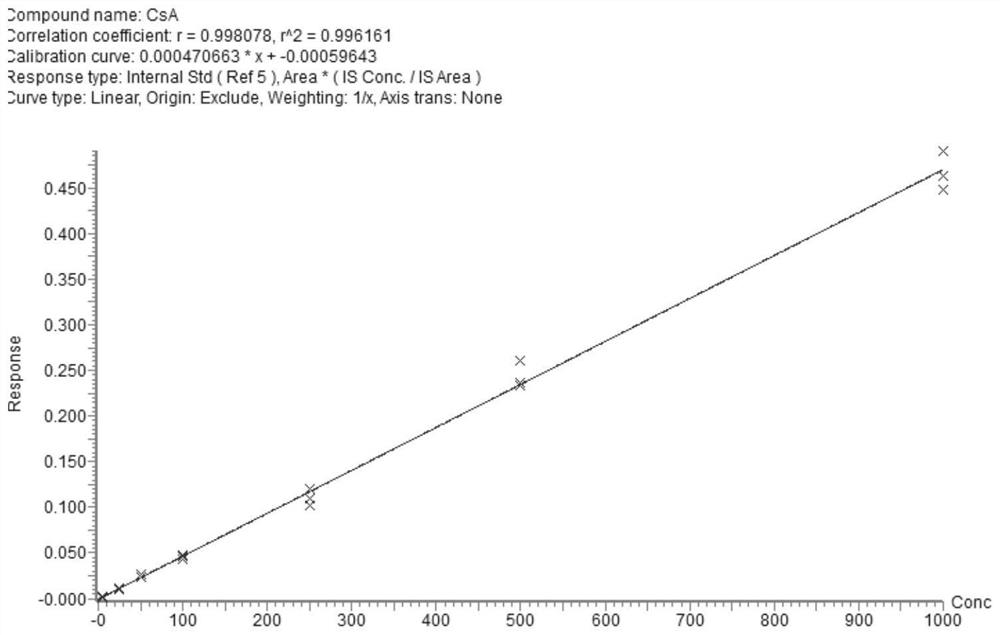
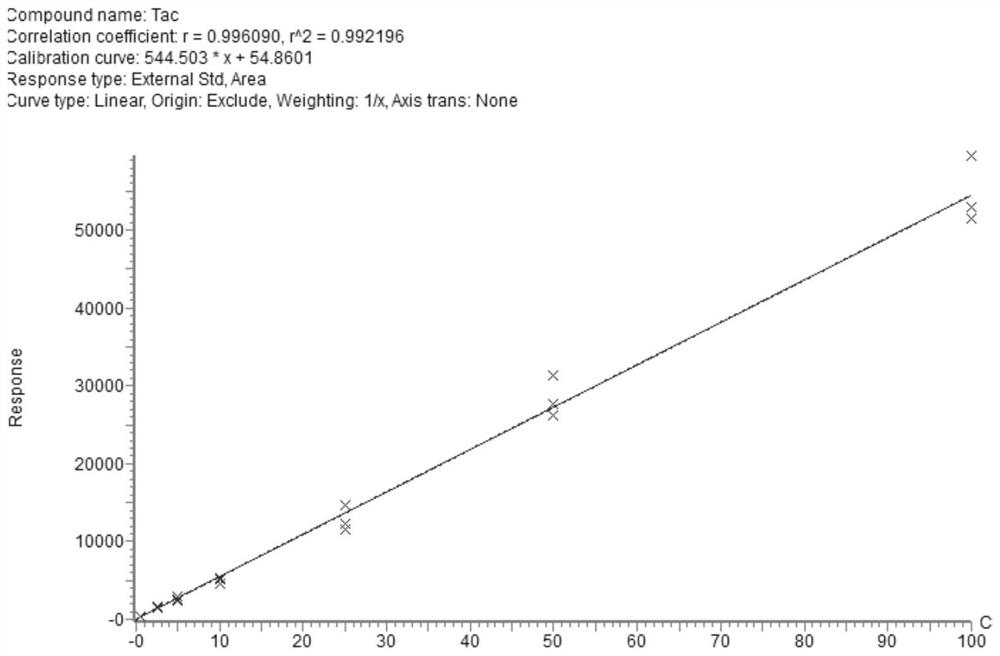
PendingCN112710766AImprove throughputImprove timelinessComponent separationIMMUNE SUPPRESSANTSEverolimus
The invention discloses a liquid chromatography-tandem mass spectrometry method and a kit for accurately determining the content of five immunosuppressants in a dry blood spot. The method comprises the following steps: 1) uniformly mixing an internal standard working solution with an organic solvent to obtain an extracting solution; 2) extracting the dry blood spot of the sample to be detected by using the extracting solution, centrifuging to obtain a supernatant I after the extraction is finished, blow-drying the supernatant I with nitrogen, re-dissolving to obtain a solution II, whirling, centrifuging, and detecting; and 3) detecting the solution II by adopting a liquid chromatography-tandem mass spectrometry method, wherein the internal standard working solution contains a cyclosporin A isotope internal standard substance, an everolimus isotope internal standard substance, a sirolimus isotope internal standard substance and a mycophenolic acid isotope mixed internal standard substance. According to the method, during detection, five immunosuppressants can be quantified at a time only by 6 microliters of a blood sample, the pretreatment method is simple and rapid, the operation time is saved, the immunosuppressive blood concentration can be effectively monitored according to the indexes, and more complete treatment medication information can be obtained in time.
Owner:BGI CLINICAL LAB (SHENZHEN) CO LTD
Immunotherapeutic method
InactiveUS20100124534A1Convenient treatmentRapid determinationVirusesDisease diagnosisAntigenPolynucleotide
The present invention provides a method of monitoring the efficacy of an immunotherapy in a mammalian subject, wherein the subject has been administered an immunotherapy, wherein the immunotherapy comprises a viral vector containing a polynucleotide encoding an antigen, wherein the viral vector is capable of transducing cells in the mammalian subject to cause the cells to express the antigen; the method comprising:(b) measuring, from a biological sample isolated from the subject, an immune response of the subject to the antigen and comparing the immune response of the subject to the antigen to a reference measurement of immune response to the antigen;(c) measuring, from a biological sample isolated from the subject, an immune response of the subject to the viral vector and comparing the immune response of the subject to the viral vector to a reference measurement of immune response to the viral vector; and(d) determining efficacy based on the comparisons of (b) and (c), wherein an elevated immune response to the antigen and a reduced immune response to the viral vector are indicative of an effective immunotherapy.
Owner:OXFORD BIOMEDICA (UK) LTD
Use of tau to monitor immunotherapy
ActiveUS20150118223A9Reduce volumeReduce doseImmunoglobulins against animals/humansAntibody ingredientsDiseaseImmunotherapy
The invention provides methods of immunotherapy of Alzheimer's and similar diseases in which the regime administered is monitored by measuring levels of tau.
Owner:WYETH LLC +1
Moesin fragments associated with immune thrombocytopenia
The present application provides compositions and methods useful for detecting and monitoring immune thrombocytopenia.
Owner:SHANGHAI KEXIN BIOTECH
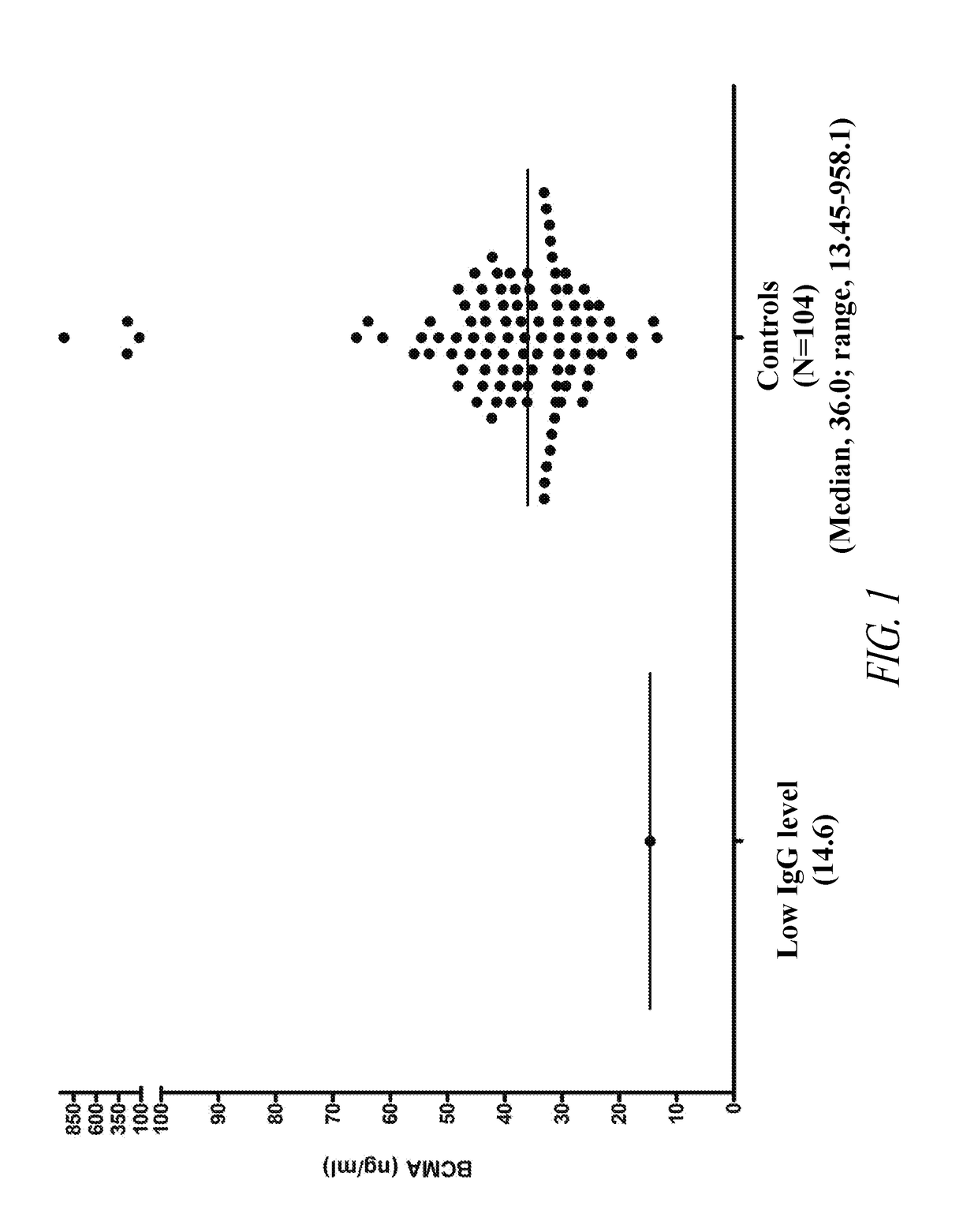
Improved methods for monitoring immune status of a subject
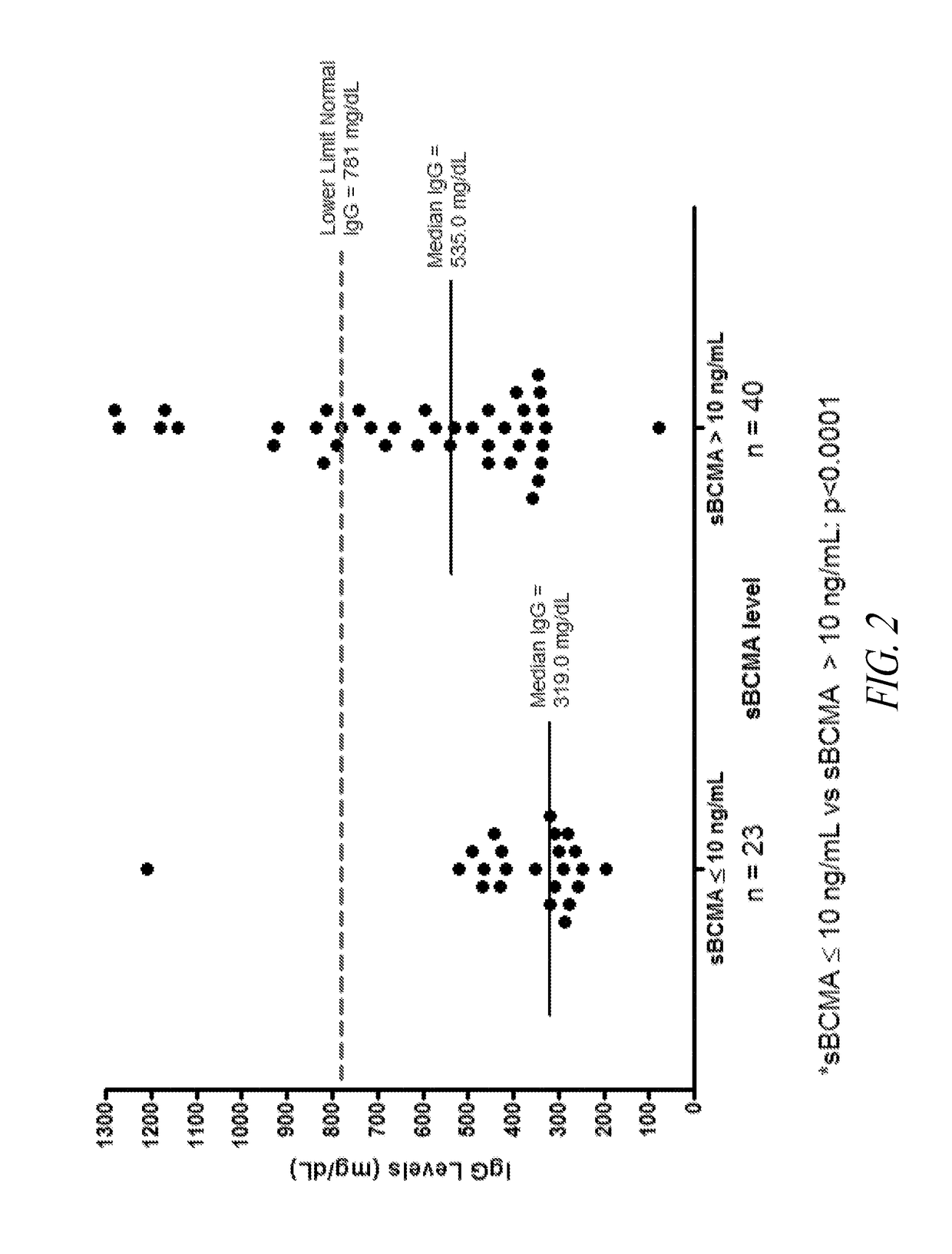
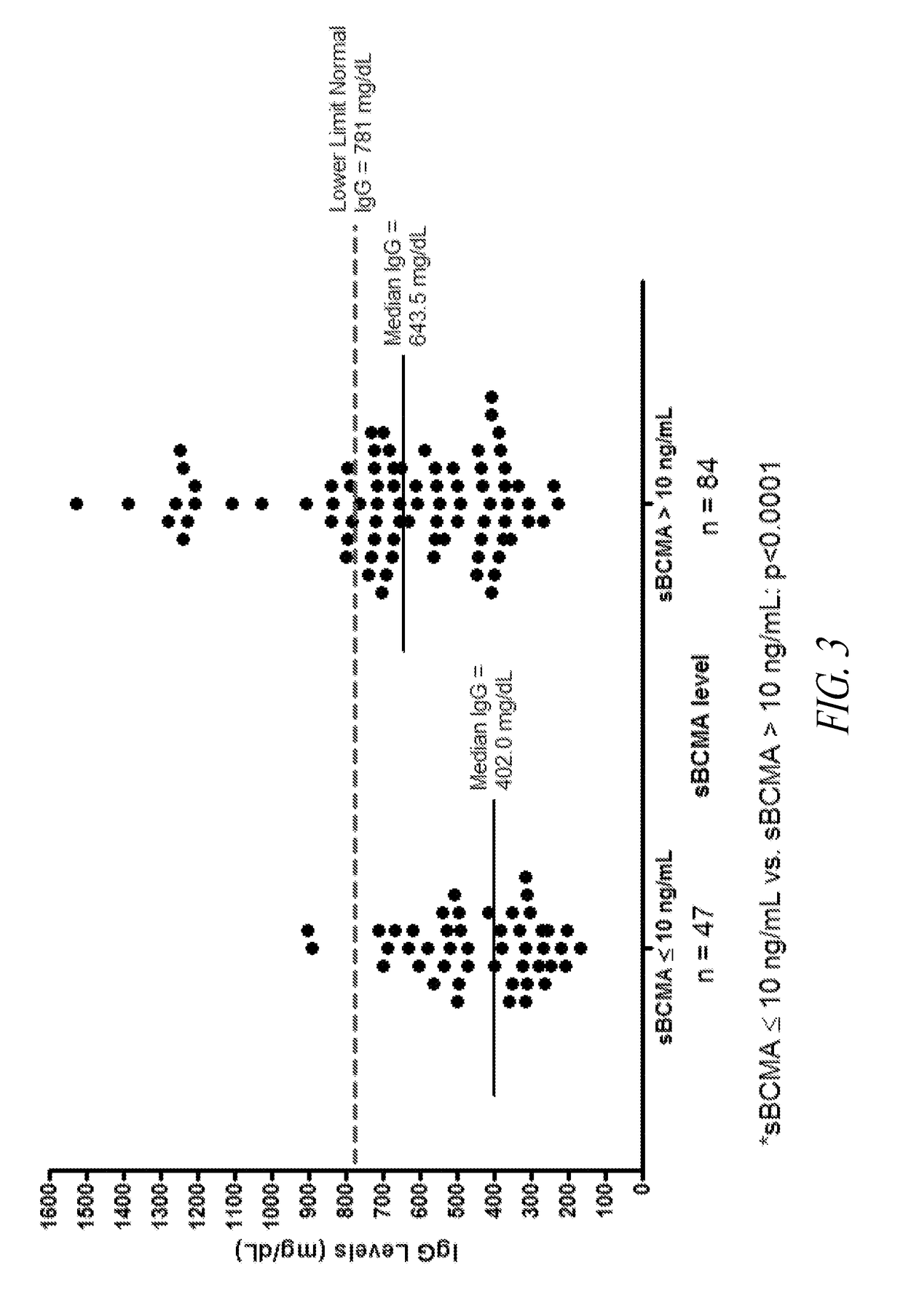
ActiveUS20190049437A1Immunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisBioinformaticsPathology
The invention generally provides improved compositions and methods for monitoring immune status of a subject. In particular, the invention provides methods for detecting BCMA in subjects to reliably monitor immune status of the subject.
Owner:ONCOTRACKER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com