Use of tau to monitor immunotherapy
a technology of immunotherapy and tau, applied in the direction of antibody medical ingredients, instruments, peptides, etc., can solve the problems of neuronal cell death and unroutinely performed genetic screening for e4 , to improve the safety of bapineuzumab
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Phase 1 Trial
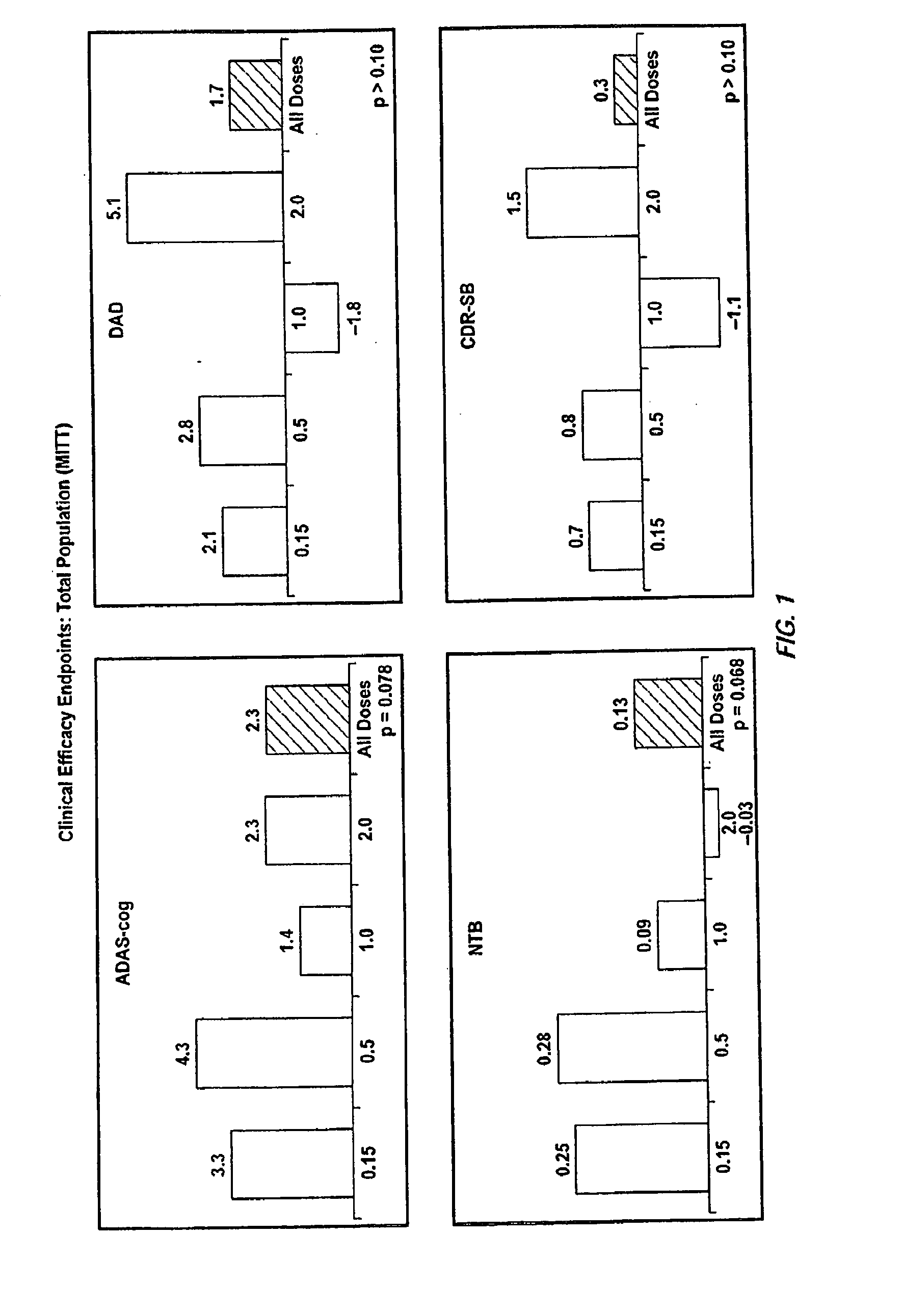
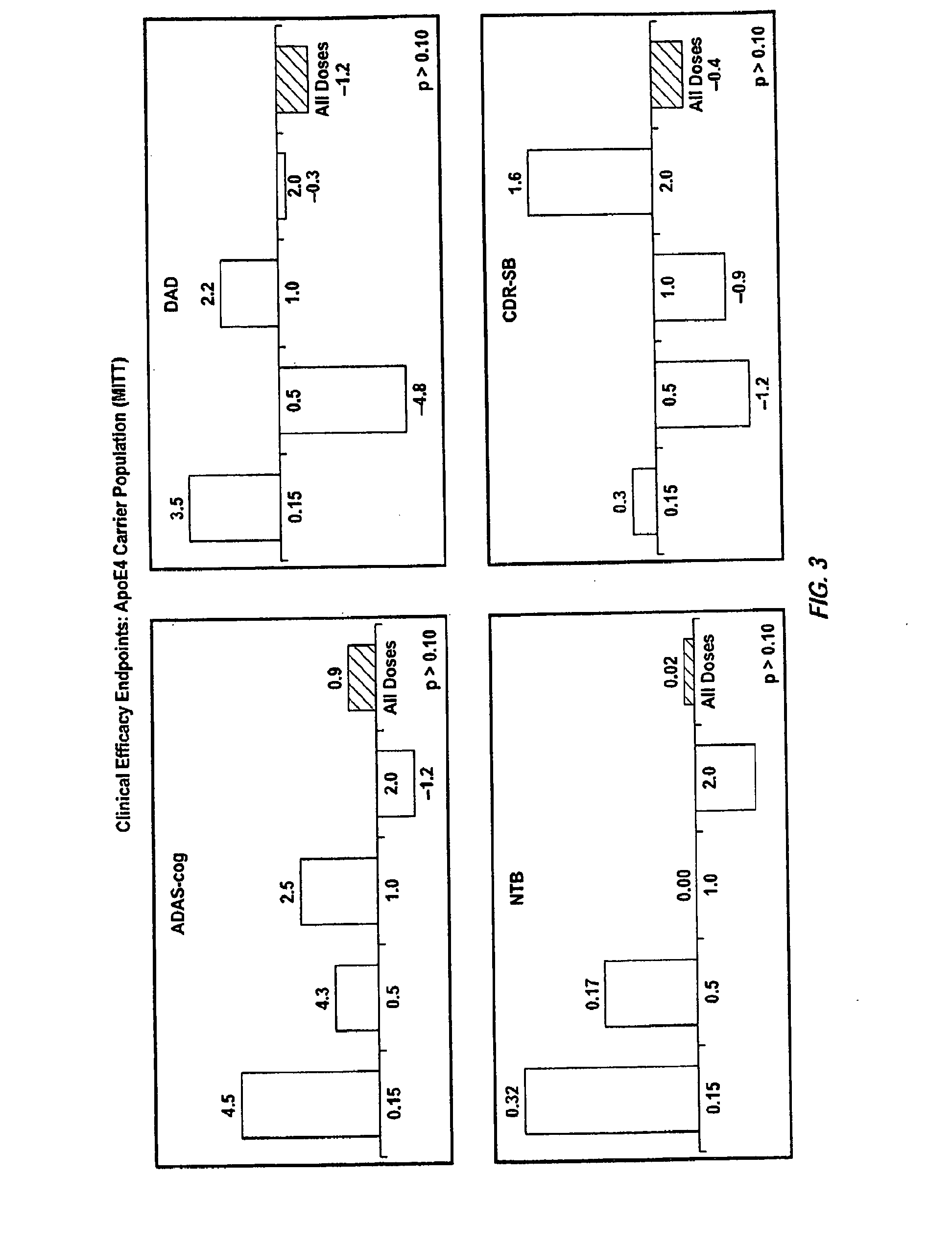
[0348]111 patients with a diagnosis of probable Alzheimer's disease (mild to moderate) were administered the humanized antibody bapineuzumab at doses ranging from 0.15 to 2.0 mg / kg in a multiple ascending dose study (MAD). Antibody was administered by intravenous infusion every thirteen weeks until the dosing regime is complete. Patients were also classified for ApoE4 status. Table 2 shows that eleven patients in the study experienced vasogenic edema detected by MRI. Table 2 also shows symptoms experienced in some of these patients; in other patients the vasogenic edema was asymptomatic. Table 3 shows the risk of vasogenic edema stratified by genotype irrespective of dose. The risk is only 2% in patients lacking an E4 allele but is 35% in patients with two E4 alleles. Table 4 shows the risk of vasogenic edema in only the highest dose group (2 mg / kg). The risk of vasogenic edema for patients with two E4 alleles is 60% and that for patients with one allele is 35%.
[0349]Ta...
example 2
Phase 2 Trial, Study 201
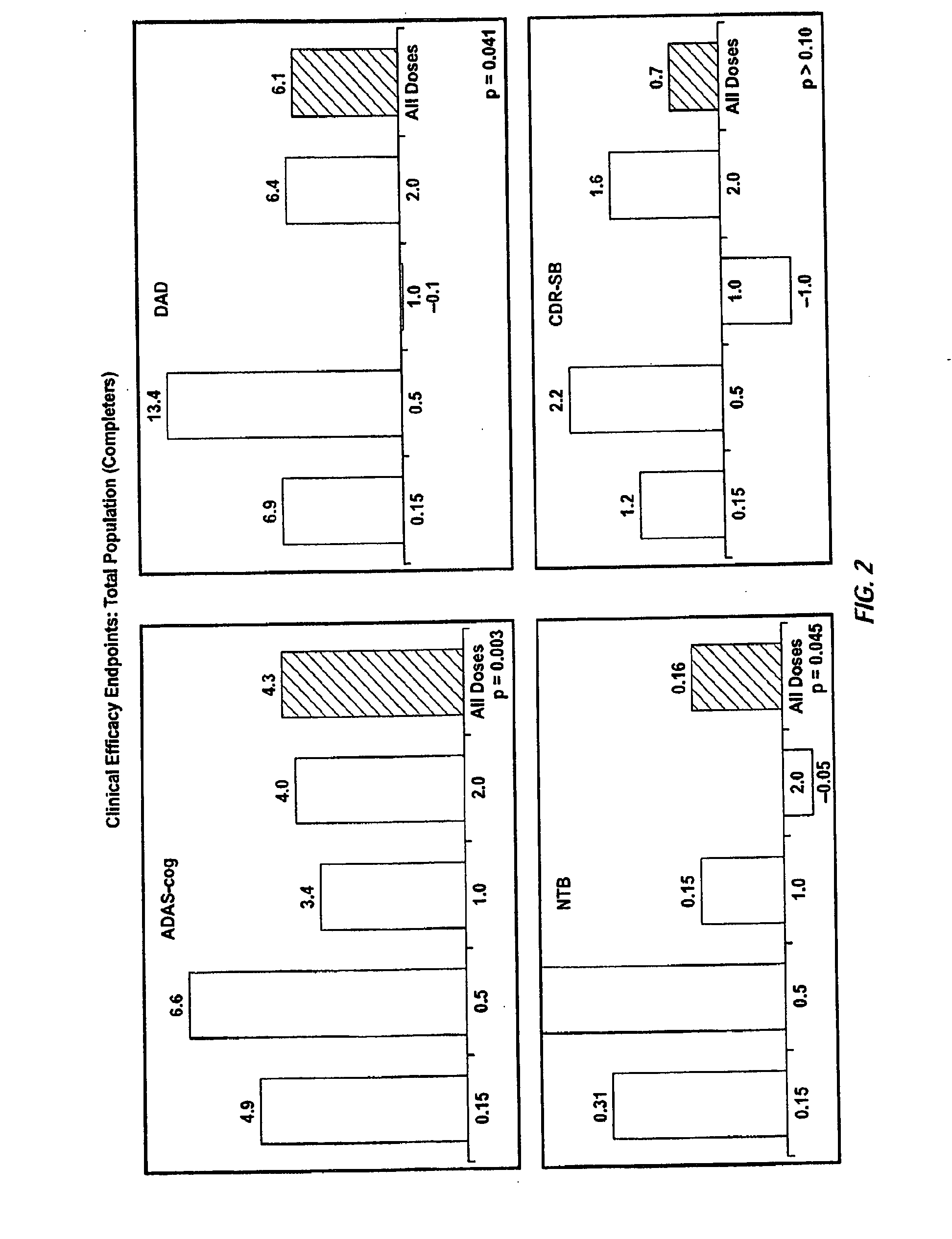
[0350]A randomized double-blind placebo-controlled multiple ascending dose study was conducted on a population of 234 patients randomized from an initial population of 317 screened patients. Patients were assessed for ApoE4 carrier status, but carriers (homozygous and heterozygous) and non-carriers received the same treatment. Inclusion criteria were: probable AD diagnosis; aged 50-85 years; MMSE score 16-26; Rosen Modified Hachinski Ischemic score≦4; Living at home or in a community dwelling with a capable caregiver; MRI consistent with diagnosis of AD; MRI scan of sufficient quality for volumetric analysis; stable doses of medication for treatment of non-excluded conditions; stable doses of AchEIs and / or memantine for 120 days prior to screen. The main exclusion criteria were: current manifestation of a major psychiatric disorder (e.g., major depressive disorder); current systemic illness likely to result in deterioration of the patient's condition; history...
example 2b
Phase II Clinical Trial 202
[0373]The clinical trial was a phase 2, multicenter, randomized, double-blind, placebo-controlled, multiple-ascending dose study. Patients were randomly assigned to receive either intravenous (IV) bapineuzumab or placebo, in one of three dose cohorts (0.5 [A], 1.0 [B], or 2.0 [C] mg / kg). Up to 30 patients were planned for enrollment (10 per dose cohort with patients in each dose cohort [A, B, or C] receiving bapineuzumab or placebo in a 7:3 ratio). Patients who completed the screening phase and met all inclusion criteria were eligible for randomization. 28 patients were enrolled in the study (10 in cohort A, 10 in cohort B and eight in cohort C). The sponsor terminated enrollment in cohort C following the observation of more frequent cerebral vasogenic edema at the 2.0 mg / kg dose in other studies. Randomized patients received study drug as a 1-hour IV infusion every 13 weeks for up to six infusions. Each patient underwent [11C]PiB PET, [18F]FDG PET, clinic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com