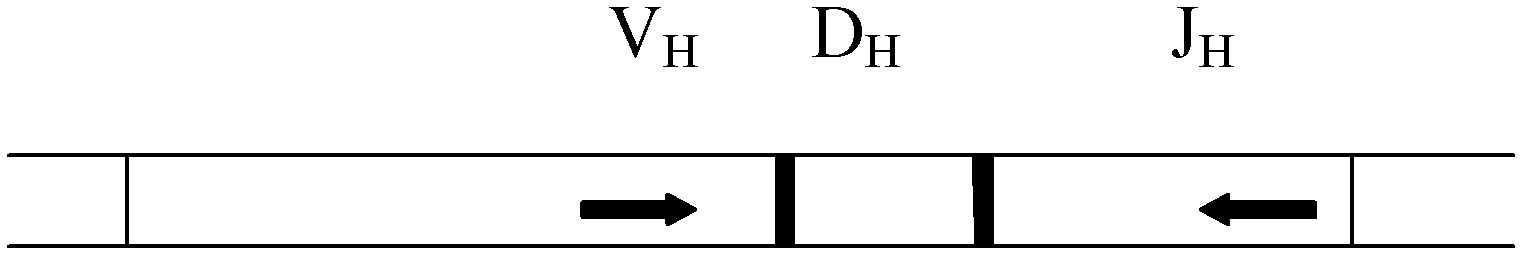Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Immune status" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune status. a nursing outcome from the Nursing Outcomes Classification (NOC) defined as natural and acquired appropriately targeted resistance to internal and external antigens.
Methods of MHC class II epitope mapping, detection of autoimmune T cells and antigens, and autoimmune treatment
The present invention provides of using multimeric MHC class II / peptide complexes. In one aspect, methods provided for identifying MHC class II-restricted immune epitopes of a predetermined polypeptide antigen. Methods for identifying an immunostimulatory epitope for a predetermined polypeptide antigen are provided. In a related aspect, methods for screening a therapeutic polypeptide agent for an MHC class II epitope are provided. In other aspects, methods for modulating T cells and for determining or monitoring an MHC class II-restricted immune status of a patient are also provided.
Owner:BENAROYA RES INST AT VIRGINIA MASON
Autologous immune cell therapy: cell compositions, methods and applications to treatment of human disease
InactiveUS20020182730A1Promote cell-mediated inflammatory reactionActivation functionDead animal preservationMammal material medical ingredientsAutologous immune enhancement therapyEffector cell
Compositions containing clinically relevant numbers of immune cells that have been isolated from a patient differentiated and / or expanded ex vivo. Methods for treating or preventing disease or otherwise altering the immune status of the patient by reinfusing such cells into the donor are also provided. Methods for expanding and / or immune cells, including effector cells, in the absence of exogenous IL-2, and for administering the cells in the absence of co-infused IL-2 are also provided.
Owner:VALEOCYTE THERAPIES
Autologous immune cell therapy: cell compositions, methods and applications to treatment of human disease
InactiveUS20010031253A1Promote cell-mediated inflammatory reactionActivation functionBiocideMammal material medical ingredientsEffector cellCell therapy
Compositions containing clinically relevant numbers of immune cells that have been isolated from a patient differentiated and / or expanded ex vivo. Methods for treating or preventing disease or otherwise altering the immune status of the patient by reinfusing such cells into the donor are also provided. Methods for expanding and / or immune cells, including effector cells, in the absence of exogenous IL-2, and for administering the cells in the absence of co-infused IL-2 are also provided.
Owner:VALEOCYTE THERAPIES
Autologous immune cell therapy: cell compositions, methods and applications to treatment of human disease
InactiveUS20030039650A1BiocideArtificial cell constructsAutologous immune enhancement therapyEffector cell
Compositions containing clinically relevant numbers of immune cells that have been isolated from a patient differentiated and / or expanded ex vivo. Methods for treating or preventing disease or otherwise altering the immune status of the patient by reinfusing such cells into the donor are also provided. Methods for expanding and / or immune cells, including effector cells, in the absence of exogenous IL-2, and for administering the cells in the absence of co-infused IL-2 are also provided.
Owner:VALEOCYTE THERAPIES
Rapid and non-invasive method to evaluate immunization status of a patient
InactiveUS6927068B2Rapid and reliable and non-invasive and safe testingRapidly evaluate immunization status of a patientBioreactor/fermenter combinationsBiological substance pretreatmentsSpecific iggAnthrax protective antigen
An assay method and kit for detecting the presence of a predesignated, target IgG antibody in a sample selected from one or more patient bodily fluids. The method comprises the following steps: (a) contacting the sample of one or more patient bodily fluids with a membrane-bound recombinant protective antigen to bind to the target IgG antibody in the sample; (b) previously, simultaneously or subsequently to step (a), binding the protective antigen (PA) with a conjugated label producing a detectable signal; and (c) detecting the signal whereby the presence of the target IgG antibody is determined in the sample by the intensity of the signal. The method can further comprise the step of evaluating immunization status of the patient from whom the sample came by comparing the signal or lack thereof with immunizations previously received by the patient. In a preferred embodiment, the recombinant protective antigen (PA) specifically binds to anthrax protective antigen-specific IgG antibodies. Preferably, the immunoassay of the present invention comprises a lateral-flow assay comprising a membrane, a conjugated label pad, and a recombinant protective antigen (PA) bound to the membrane.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Method and device for physiotherapy
InactiveUS20060009825A1Reduce inflammationProphylaxis of complicationElectrotherapyMicrowave therapyDamages tissueCell Proliferation Process
This invention relates to biology, veterinary and medicine, and more particularly to methods and devices for physiotherapy with the use of electromagnetic radiation in the ultra-high frequency band. The method consists in that patients are irradiated with electromagnetic microwave field at, at least, one frequency belonging to, at least, one of the 18 frequency ranges. The intensity of radiation at the place of location of the patient does not exceed 3.6 μW / cm2. The device for carrying out the method comprises, at least, one oscillator ensuring the production of electromagnetic radiation in the 18 frequency ranges, both at the main frequencies and at multiple ones. In the result the effect of pain relief is achieved, inflammatory processes are lessened, processes of regeneration of damaged tissues are stimulated, an affected immune status is corrected, the processes of cell proliferation in neoplasms are stabilized, the lysis of necrotic tissue cells occurs.
Owner:CHIRIAEV VIATCHESLAV MIKHAILOVICH +4
Systems and methods for obtaining, storing, processing and utilizing immunologic and other information of individuals and populations
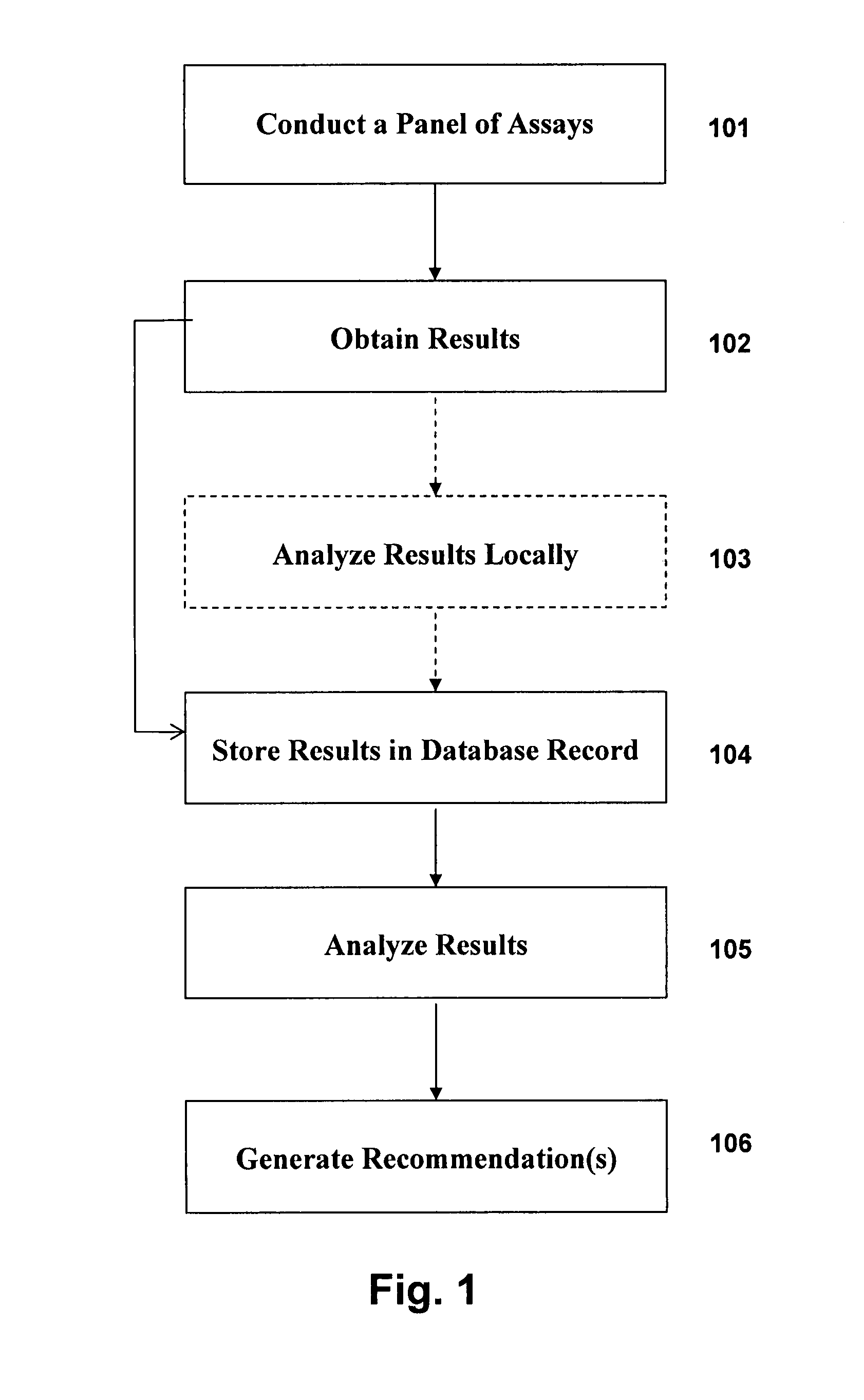
A system and method for assessing immunological status of individuals and populations is presented. The method includes establishing a database containing a plurality of records, each record containing information representative of the immune status of an individual, including (1) bioassay results, and (2) individual specific information such as, medical history, clinical observations and historical, demographic, lifestyle, and familial / genetic information. By processing the information trends and / or patterns are obtained correlating various variables in the records across an individual, population or sub-population. The identified trends and / or patterns can, for example, be used in health care related decision making. In exemplary embodiments such processing can include generating a set of correlations and for each correlation generating a set of explanatory or relevant hypotheses. Then, for example, each hypothesis can be automatically refuted or supported, to the extent possible. The identified correlations, their associated hypotheses and the results of such automated vetting can then be reported to a user.
Owner:32 MOTT STREET ACQUISITION I +1
Rapid Detection of Post-Vaccination Antibody Response
InactiveUS20100322823A1Component separationBiological material analysisAbsorbent materialMechanical engineering
The present inventions are directed to apparatuses for rapidly measuring post-vaccination immune status. In one version, the apparatus has a support platform, with a top side, a bottom side, a first portion, and a second portion. A first void is integrally formed in the first portion. A container is configured to be removably affixed to the top side of the support platform. The container has a housing, a base, and at least one reactant. The container base can be viewed through the first void when the container is removably affixed to the first portion of the top side. An absorbent material is affixed to the second portion, where the base of the container comes into contact with the absorbent material when the container is removably affixed to the second portion of the top side.
Owner:SURAPANENI KRISHNA P +3
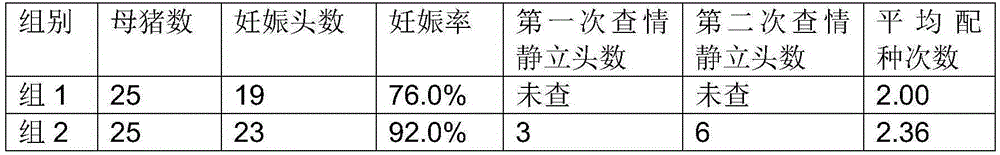
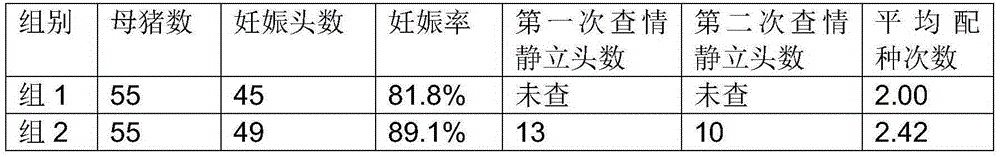
Efficient heat detection and synchronous fertilization method of replacement gilts and multiparous sows
The invention provides an efficient heat detection and synchronous fertilization method of replacement gilts and multiparous sows. The method has the advantages that mating of the multiparous sows and that of the added replacement gilts can be synchronized to allow batch management for sow's production; utilization and mating productivity of sows can also be increased; non-productive days for sows are fewer; work efficiency of mating staff can be improved; the purpose of all in and all out is easily obtainable, and sows can be imparted same reproductive statuses, health statuses and immune statuses.
Owner:NINGBO SANSHENG PHARMA
Enumeration of CD4+ lymphocytes
The invention provides a method of enumerating the number of cells of a cell type in a cell sample by (a) counting the white blood cells in the cell sample to obtain the white blood cell population of the sample; (b) determining the proportion or percentage of the cells of the cell type in the white blood cell population in the sample; and (c) calculating the number of cells of the cell type in the sample. The cell type may be a lymphocyte sub-set selected from the group comprising CD4+ lymphocytes, CD 45 cells, CD19 cells, CD16 and CD56 positive cells, CD8 cells, CD3 cells or any combination thereof. The method is particularly useful in monitoring the immune status of a patient infected with HIV or other immune deficiency state or disease or condition where CD4+ lymphocytes or CD4+ T cells are monitored or counted.
Owner:NAT HEALTH LAB SERVICE
Primer composition for amplifying coding sequence of immunoglobulin heavy chain CDR3 and use thereof
ActiveCN103184216AEfficient enrichmentMicrobiological testing/measurementFermentationImmunoglobulin heavy chainComputational biology
The invention relates to a primer composition for amplifying the coding sequence of immunoglobulin heavy chain CDR3, a method for enriching the coding sequence of immunoglobulin heavy chain CDR3, a method for constructing the sequencing library of the coding sequence of immunoglobulin heavy chain CDR3, a method for determining the sequence information of the coding sequence of immunoglobulin heavy chain CDR3, a method for determining individual immune status, and a system for determining individual immune status. The primer composition comprises a first primer group and a second primer group, wherein the first primer group is composed of at least one V-region primer, and each of the at least one V-region primer comprises a sequence complementary with at least one V-gene segment; and the second primer group is composed of at least one J-region primer, and each of the at least one J-region primer comprises a sequence complementary with at least one J-gene segment. By virtue of the primer composition, the coding sequence of immunoglobulin heavy chain CDR3 can be effectively enriched, thus providing a convenient tool for deep researches on CDR3.
Owner:BGI SHENZHEN CO LTD +1
Methods and compositions for impairing multiplication of HIV-1
InactiveUS20050245454A1Reducing viral levelReduce viral multiplicationFungiVirusesAntigenCarrier protein
A composition which elicits antibodies to multiple known variants of Tat protein of HIV-1 of both the B and non-B clades contains the peptide R1-Asp-Pro-Asn-Leu-Asp-Pro-Trp-Asn-R2 SEQ ID NO: 23, and preferably an additional at least two variants of a peptide or polypeptide of the formula: R1-Asp-Pro-Y7-Leu-X9-Pro-Trp-Z12-R2 (SEQ ID NO: 8). In this composition, at least one of the two variants contains Arg at Y7 and Lys at Z12, and in at least a second of the two variants Y7 is Asn and Z12 is Asn. Vaccinal and pharmaceutical compositions can contain one or more such peptides associated with carrier proteins, associated in multiple antigenic peptides or as part of recombinant proteins. Diagnostic compositions and uses are described for assessing the immune status of vaccinated patients.
Owner:GOLDSTEIN GIDEON
Method and apparatus for discovery, development and clinical application of multiplex assays based on patterns of cellular response
InactiveUS20150025812A1Improve diagnostic capabilitiesHealth-index calculationBiostatisticsVaccine efficacyCell type
A method for the discovery, development and clinical application of multidimensional multiplex synthetic biomarker assays based on patterns of cellular response.After stimulation or inhibition, a selected multiplicity of cell types are assayed for a multiplicity of cellular or molecular responses, and known machine learning techniques are used to synthesize the cellular responses into an optimized clinical biomarker. The computationally derived algorithm includes the relationships within and between the component steps so as to produce an optimized synthetic clinical biomarker. During discover of the assay one or more of the component steps are repeated iteratively until a final clinically optimized algorithm is produced.Such a multidimensional multiplex cell response assay may provide improved diagnostic performance with respect to entities such as immune status, infection, and antibiotic and vaccine efficacy, among others.
Owner:PARADIS NORMAN A
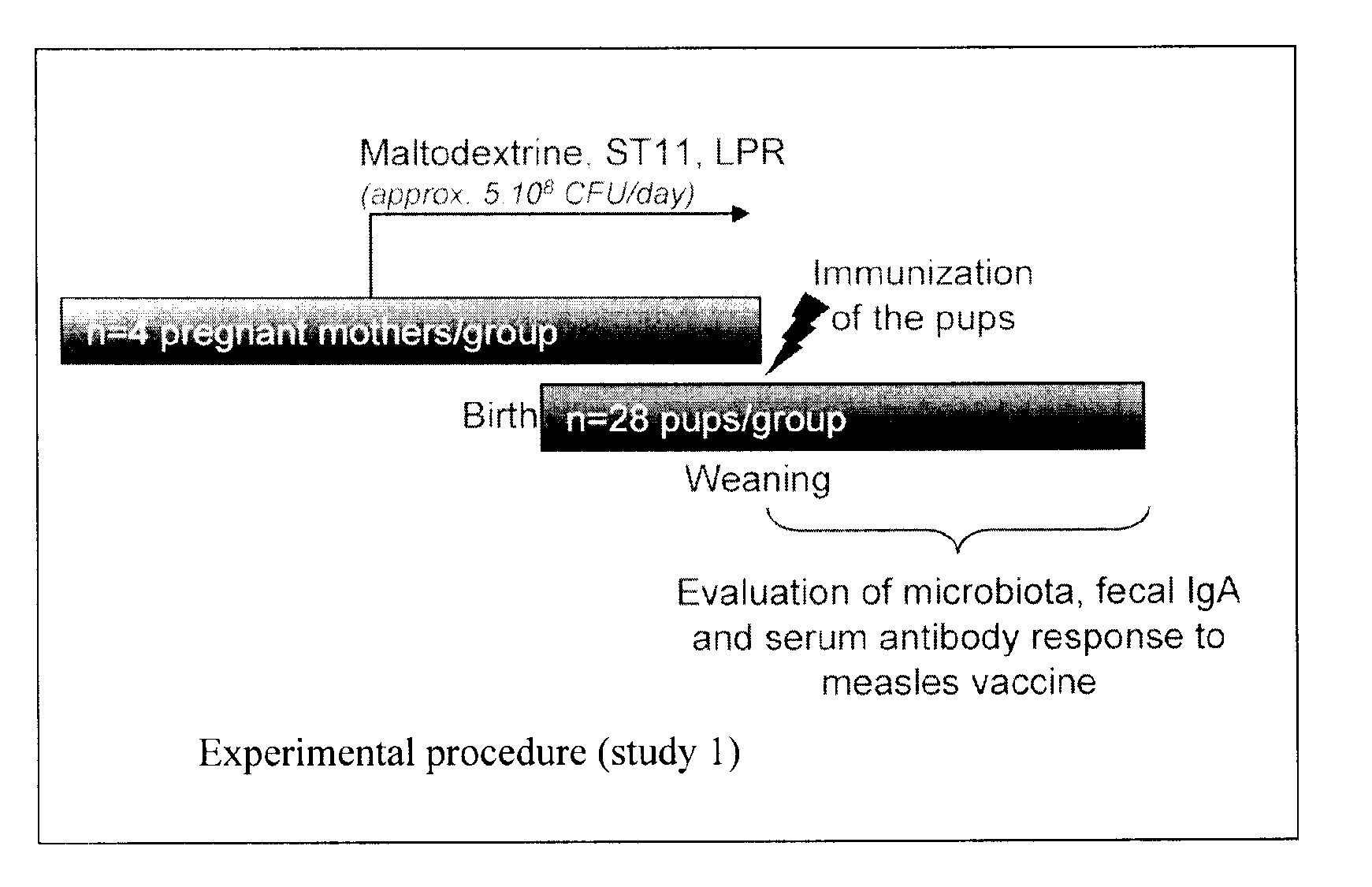
Probiotics for use in expecting female mammals for enhancing the immunity of their offsprings
ActiveUS20110020395A1Bacterial antigen ingredientsProtozoa antigen ingredientsImmunopotencyAnimal science
The present invention relates to the use of probiotic on expecting female mammals to boost the immune status of off-spring. The use can induce an enhanced response of the offspring after birth to an infectious antigenic exposure. Ultimately the use of probiotic in expecting females can induce a better protection of offspring against infectious diseases.
Owner:SOC DES PROD NESTLE SA
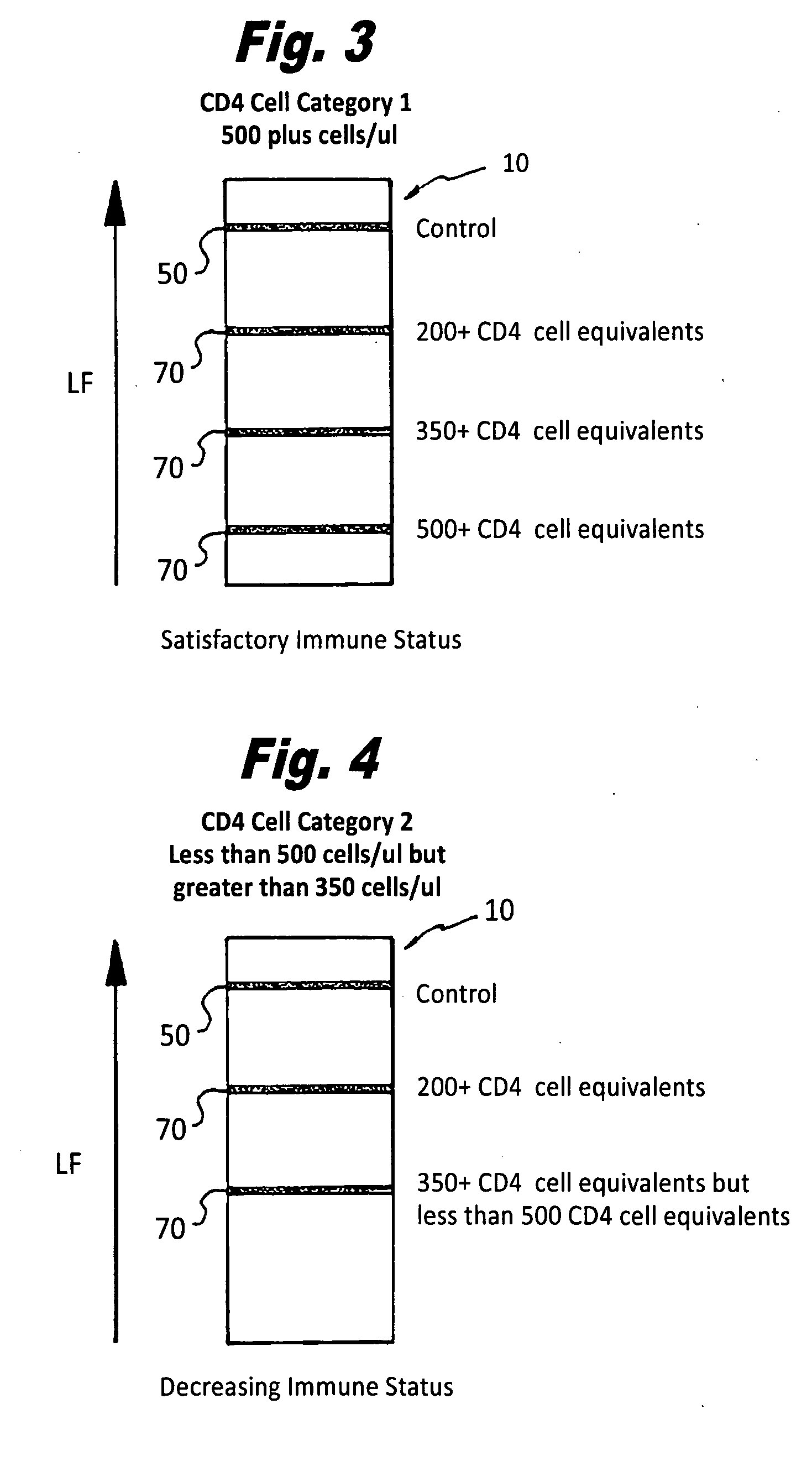
Semi-quantitative immunochromatographic device and method for the determination of HIV/AIDS immune-status via measurement of soluble CD40 ligand/CD 154, A CD4+T cell equivalent
InactiveUS20090104630A1Easy diagnosisEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteCD4 antigen
A semi-quantitative, immunochromatographic device for the detection of HIV / AIDS immune status CD4+ T cell equivalents, such as soluble CD40 ligand / CD 154, includes one or more support materials capable of providing lateral flow. The one or more support materials include a first area for receiving a biological sample containing a target analyte, the analyte being a CD4+ T cell equivalent, such as soluble CD40 ligand / CD 154, a second area having a movably contained detector ligand, wherein the detector ligand is capable of forming a mobile complex with the soluble CD40 ligand / CD 154, and at least one capture area having a predetermined amount of an immobile capture reagent, the immobile capture reagent capable of specifically binding to the mobile complex formed by the soluble CD40 ligand / CD 154 protein and the detector ligand and providing a visible signal.
Owner:REITER PAUL C
Semi-quantitative immunochromatographic device and method for the determination of HIV/AIDS immune-status via measurement of soluble CD40 Ligand/CD154, A CD4+ T cell equivalent and the simultaneous detection of HIV infection via HIV antibody detection
InactiveUS20090258343A1Performed easily and cost-effectivelyEnsure correct executionBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteCD154
A semi-quantitative, immunochromatographic dual test device for the simultaneous detection of HIV / AIDS immune status CD4+ T cell equivalents, such as soluble CD40 ligand / CD 154, and the detection of an HIV antibody, includes one or more support materials capable of providing lateral flow. The one or more support materials include at least one sample receiving area for receiving a biological sample containing a first target analyte, the first target analyte being a CD4+ T cell equivalent, such as soluble CD40 ligand / CD 154, and a second target analyte, the second target analyte being an HIV antibody. A second area, situated on the one or more support materials, has a movably contained detector ligand and or detector antigens, wherein the detector ligand and or detector antigens is capable of forming a mobile complex with the soluble CD40 ligand / CD 154 and or HIV antibodies, and at least a first capture area having a predetermined amount of a first immobile capture reagent, the first immobile capture reagent capable of specifically binding to the mobile complex formed by the soluble CD40 ligand / CD 154 protein and the detector ligand and providing a visible signal. The one or more support materials further have situated thereon at least a second capture area having a predetermined amount of a second immobile capture reagent that is capable of specifically binding to HIV antibodies present in the biological sample and providing a visible signal.
Owner:REITER PAUL C
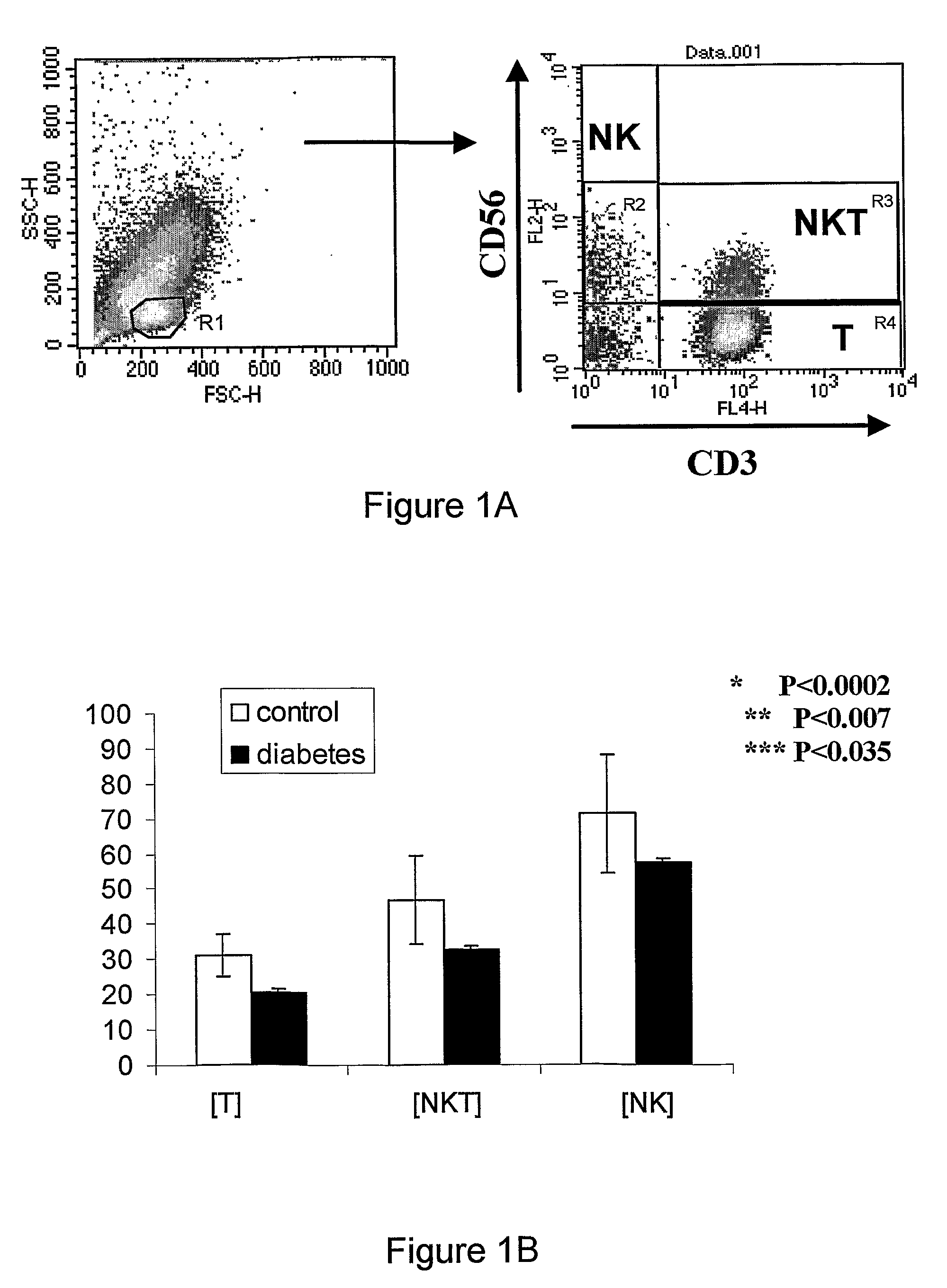
Kit for diagnosis, prognosis, and monitoring the immune status, of patients with chronic inflammatory diseases
ActiveUS9188588B2Disease diagnosisBiological testingNatural Killer Cell Inhibitory ReceptorsNatural killer T cell
Provided is a method and a kit for testing the immune status of patients with chronic inflammatory diseases by measuring the TCR zeta chain (CD247) expression levels, and in particular a method and a kit for testing the selective downregulation of TCR zeta chain expression in T cells, NK cells, or NKT cells of such patients. Zeta chain expression is measured using antibodies directed against the intracellular zeta chain region, and these levels are compared with the expression levels of other T cell receptor subunits and NK cell markers. Thus, a kit for diagnosis, prognosis, and monitoring the immune status, of patients with chronic inflammatory diseases is presented herein.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Compositions and Methods for Determining Immune Status
InactiveUS20120094861A1Low costSample is very smallPeptide librariesLibrary screeningInfectious agentAntibody
Owner:US ARMY MEDICAL RES MATERIEL COMMAND USAMRMC +1
Compositions and methods for determining immune status
InactiveUS20090305899A1Low costSample is very smallPeptide librariesLibrary screeningInfectious agentAntibody
Owner:ARMY UNITEDS STATES
Vaccine Adjuvants
ActiveUS20080131458A1High potencyEasy to testBioreactor/fermenter combinationsBiological substance pretreatmentsMammalCell mediated immunity
The invention relates to a novel adjuvant Mycobacterium w and or its constituents and adjuvant containing composition, which contains antigen (s) with pharmaceutical acceptable carrier and its uses.Mycobacterium w and or its constituents when administered with antigen (s) to mammal results in enhanced immunogenicity of antigen. The enhanced immunogenicity manifests as humoral responses as well as cell mediated immunity. The adjuvant effect is seen with variety of antigens in various mammals irrespective of their immune status at the time of administration of Mycobacterium w and antigen containing composition. e.g. immune naïve or preimmunised status.
Owner:CADILA PHARMA
Kit for diagnosis, prognosis, and monitoring the immune status, of patients with chronic inflammatory diseases
ActiveUS20110124017A1Disease diagnosisBiological testingNatural Killer Cell Inhibitory ReceptorsNatural killer T cell
Provided is a method and a kit for testing the immune status of patients with chronic inflammatory diseases by measuring the TCR zeta chain (CD247) expression levels, and in particular a method and a kit for testing the selective downregulation of TCR zeta chain expression in T cells, NK cells, or NKT cells of such patients. Zeta chain expression is measured using antibodies directed against the intracellular zeta chain region, and these levels are compared with the expression levels of other T cell receptor subunits and NK cell markers. Thus, a kit for diagnosis, prognosis, and monitoring the immune status, of patients with chronic inflammatory diseases is presented herein.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Fermented Chinese herb immunopotentiator for epinephelus
InactiveCN106721487AStimulate nonspecific immune mechanismsImprove digestion and absorption rateFood processingClimate change adaptationSpecific immunityAntibiotic Y
The invention discloses a fermented Chinese herb immunopotentiator for epinephelus. According to the present invention, the fermented Chinese herb immunopotentiator has a variety of effects of disease prevention, disease treatment, immunity enhancing and the like, and is the ideal, safe, green and non-toxic novel feed additive for replacing antibiotics; compound Chinese herbs are subjected to a bacterial mixing fermentation process (aerobic and anaerobic asynchronous fermentation) to prepare the fermented Chinese herb feed additive, wherein various strains can synergistically provide great advantages through the bacterial mixing fermentation so as to overcome the disadvantages of the single strain fermentation; the obtained product has the double advantages of the Chinese herbs and the probiotics so as to effectively regulate the intestinal tract health of epinephelus, maintain the intestinal tract micro-ecological balance and the best immune status, and improve the digestion and absorption rate of nutrients; the non-specific immune mechanism of epinephelus is stimulated through the product so as to enhance the immune response ability; with the product, the farming income is increased, and the farming risk is reduced; and with the application of the fermented Chinese herb immunopotentiator as the antibiotic substitute, drug resistance, drug residue and other problems do not exist, no adverse effect is generated on epinephelus, and the use is safe.
Owner:DINGZHENG ANIMAL PHARMA TIANJIN
Kit capable of quickly and quantitatively detecting hog cholera antibody and preparation method of kit
The invention relates to a kit for rapid quantitative detection of swine fever antibody. The kit includes a colloidal gold immunochromatography test paper card and a standard curve information card. The colloidal gold immunochromatography test paper card is provided with colloidal gold-labeled hog fever E2-E0 The fusion expression antigen, the colloidal gold-labeled classical swine fever E2-E0 fusion expression antigen is synthesized by connecting the classical swine fever E2-E0 fusion expression antigen and a certain amount of colloidal gold particle solution through physical adsorption, and the colloidal gold particle solution contains The average particle size of colloidal gold is 30-35nm. After the sample to be tested is added to the colloidal gold immunochromatography test paper card, use a color analyzer to read the optical density values of the detection zone and the accusation zone of the test paper card, and then calculate through software according to the standard curve recorded on the standard curve information card. The exact content of swine fever antibody. The invention is easy to operate and produces results quickly, and is a very convenient and good tool for accurately evaluating the immune status of swine fever.
Owner:SHENZHEN ZHENRUI BIOTECH CO LTD
ELISA detection method for canine distemper virus and antibody
InactiveCN110108884AGood antigenicityPrevent proliferationSsRNA viruses negative-senseVirus peptidesSerum samplesBasic research
The invention discloses an ELISA detection method for a canine distemper virus and antibody. A canine distemper virus N protein is used to make a canine distemper detection kit, and the amino acid sequence is shown in SEQ ID NO:1. The canine distemper virus N protein is only used as an antibody to build the ELISA detection method, the N protein has good antigenicity as a canine distemper virus conserved protein and can quantify anti-canine distemper virus antibodies in a serum sample on the premise of preventing virus spread, the immune status of a blood-derived organism for the canine distemper virus or the immune level of the canine distemper virus secreted by a cell can be detected, more safety and higher efficiency are achieved compared with other products, and positive meaning is achieved for basic research and clinical detection on the canine distemper virus.
Owner:SOUTH CHINA AGRI UNIV
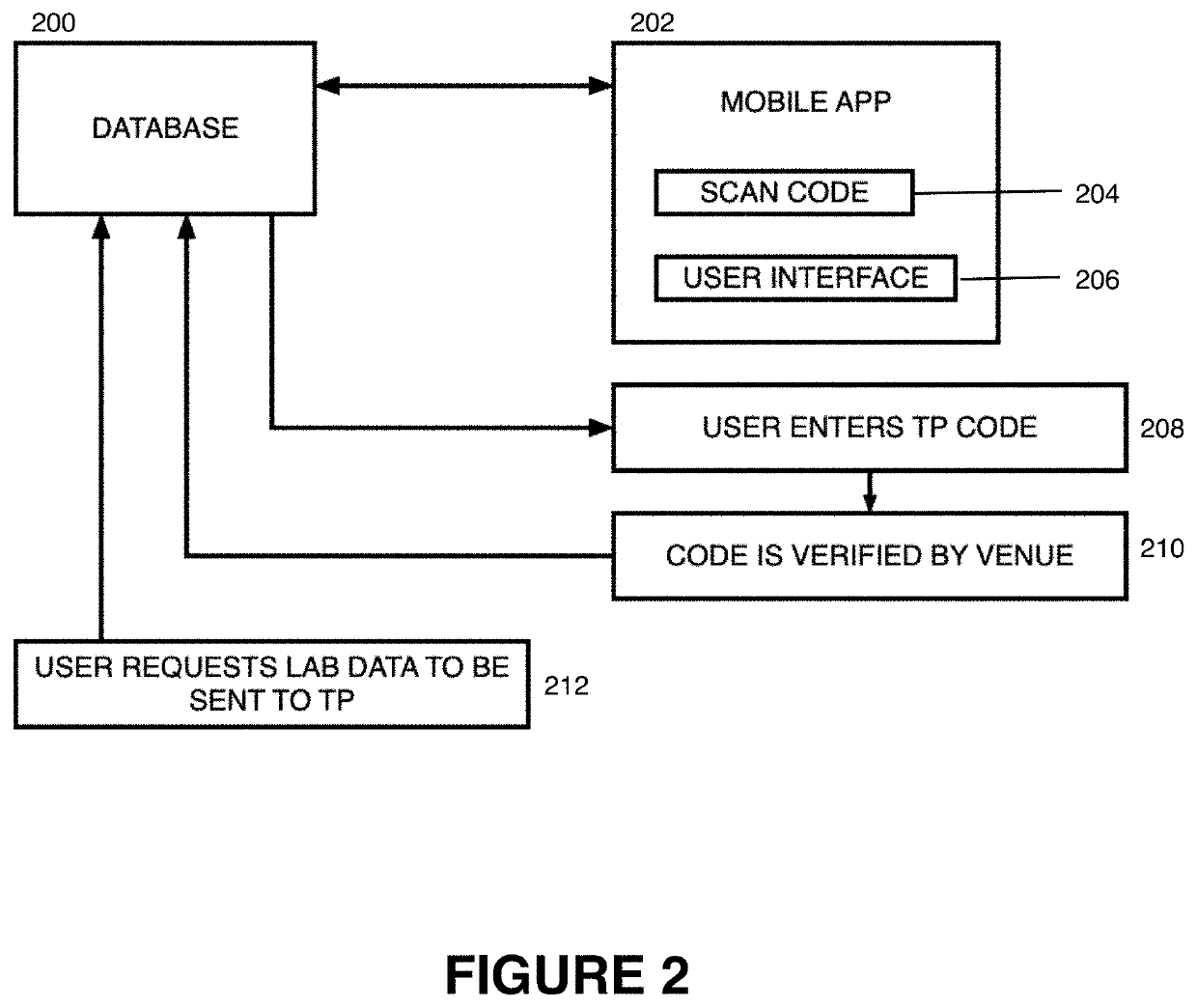
System and method for assessing immune status related to transmissible infectious diseases for mitigating against transmission
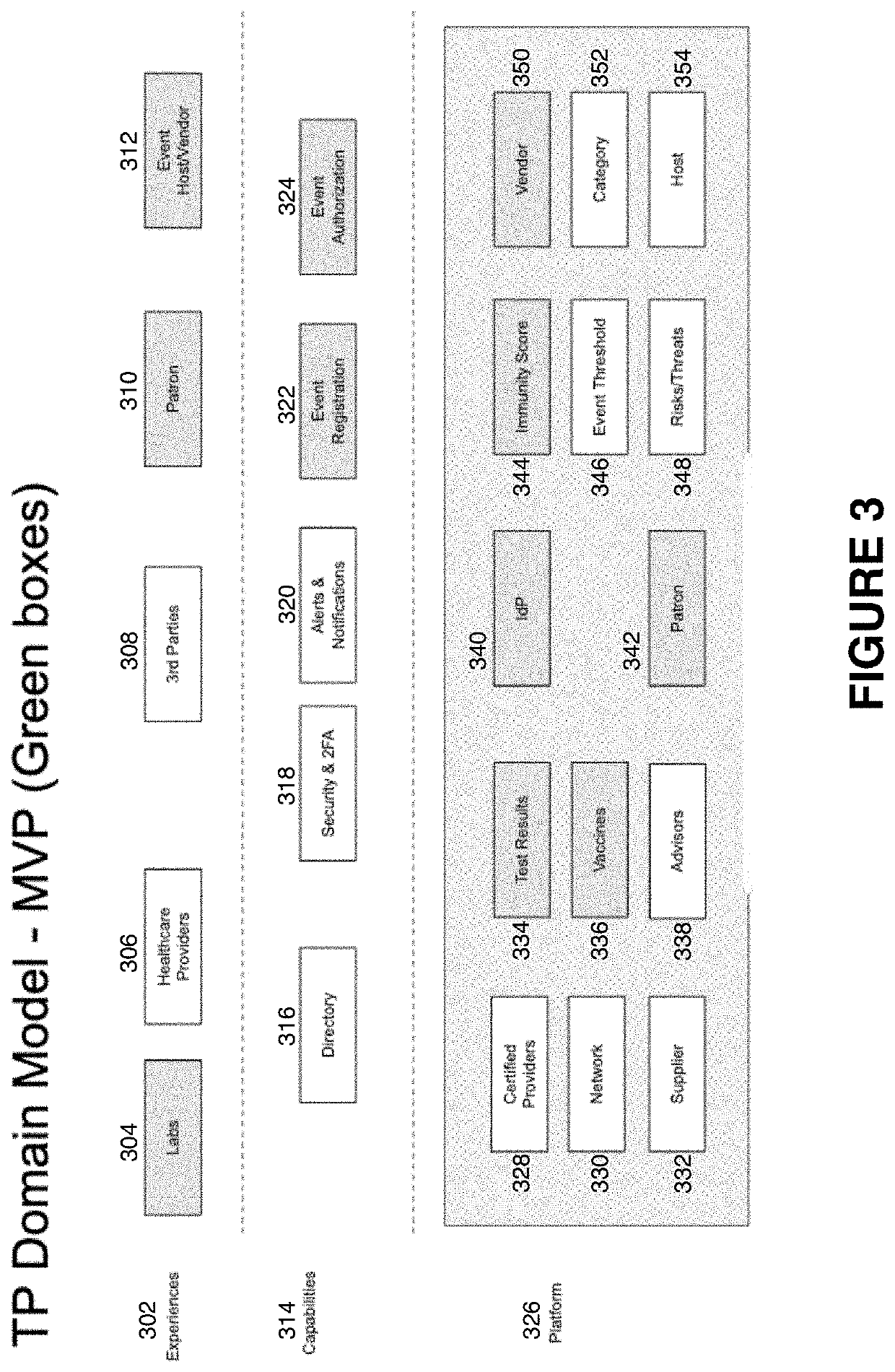
The invention relates to a solution for mitigating against transmitting, receiving or hosting transfers of infectious diseases through access to a user's health record via a mobile, cloud server-based application. The user's heath record data is stored in the application database, and can include immunization records, test results, and pre-existing conditions. This data is accessed using the mobile application interface by scanning a uniquely generated Quick Response (QR) code, and displayed through an application downloaded to a separate device, such as one owned by a venue. The user's code is scanned to display an immunity score and verified by the user's identification data, matched to data on the second device. This data can also be accessed by other users, such as venue owners who want to insure the health and safety of their patrons.
Owner:BROOKS ANDREW
Drug for the treatment of impaired sexual function in middle aged and elderly people and preparation method thereof
InactiveCN107875272AImprove immune statusGood curative effectUnknown materialsSexual disorderSide effectAdemetionine
The invention discloses a medicine for treating hypofunction of middle-aged and elderly people and a preparation method thereof. Zhizhi, Tianxiong, Zhifuzi, Morinda officinalis, Sunburned ephedra, deer glue, turtle glue, seal kidney, epimedium, rehmannia glutinosa and Jiuniuzao. The medicine can significantly improve the immune status of the body, has the effects of nourishing yin and tonifying the kidney, warming the kidney and strengthening yang, benefiting the marrow and replenishing the essence, has a good curative effect on the symptoms of sexual dysfunction in middle-aged and elderly people, and is safe and has no side effects.
Owner:贵州都匀市剑江药业有限公司
Multi-functional aquatic feed additive and preparation method thereof
InactiveCN107028026AEffectively regulate healthImprove immunityFood processingClimate change adaptationAquatic animalMalathion
The invention discloses a multi-functional aquatic feed additive. The multi-functional aquatic feed additive comprises the following raw materials in parts by weight: 5-15 parts of radix astragali, 10-20 parts of scutellaria lateriflora, 10-14 parts of radix codonopsis, 6-8 parts of caulis spatholobi, 3-7 parts of honeysuckle flowers, 3-5 parts of rheum officinale, 2-3 parts of taurine, 2-4 parts of biologically active peptides, 1-2 parts of probiotics, 3-5 parts of prebiotics, 0.1-1 part of malathion, 4-8 parts of rapeseed cakes, 20-30 parts of food processing wastes, and 2-3 parts of a biologically active bacterial agent. The multi-functional aquatic feed additive disclosed by the invention has advantages of both Chinese herbal medicines and the probiotics. The multi-functional aquatic feed additive is capable of efficiently conditioning intestinal health of aquatic animals, maintaining micro-ecological balance and optimal immune status of the intestinal track, increasing digestion and absorption rates to nutrients, enhancing immunity of animal bodies, improving aquaculture yields and reducing aquaculture risks; moreover, the multi-functional aquatic feed additive can be used instead of antibiotics, and is free of adverse effects on the aquatic animals, safe to use, and free of problems, such as drug residues, drug resistance and so on. In addition, a preparation method of the multi-functional aquatic feed additive provided by the invention is relatively low in material cost, easy in raw materials obtaining and simple in processes, and thus, the preparation method thereof has good application prospects.
Owner:安徽帝都农业生态园有限公司
Methods and compositions for impairing multiplication of HIV-1
A composition which elicits antibodies to greater than 95%, and even greater than 99%, of the known variants of HIV-1 Tat protein contains at least one peptide or polypeptide of the formula of Epitope I (based on amino acids 2-10 of HIV-1 Tat consensus sequence) and optionally one or more of a peptide or polypeptide of Epitope II (based on amino acids 41 to 51 of that sequence), of Epitope III (based on amino acids 52-62 of that sequence), or of Epitope IV (based on amino acids 62 through 72 of that sequence with a C-terminal Pro). Vaccinal and pharmaceutical compositions can contain the antibodies induced by the peptide compositions for use in passive therapy. Diagnostic compositions and uses are described for assessing the immune status of vaccinated patients.
Owner:THYMON
Immune mouse model with pulmonary delivery of liquid aerosol of Yersinia pestis F1 vaccine
InactiveCN110141660AAccurate and quantitative study of immune statusStrong targetingCompounds screening/testingBacterial antigen ingredientsYersinia pestisLung
The invention discloses an immune mouse model with pulmonary delivery of liquid aerosol of a Yersinia pestis F1 vaccine. The invention provides a method for preparing an animal model. The method for preparing the animal model comprises the following steps: delivering immunogen to lungs of animals by the means of liquid aerosols; and then, carrying out challenging by using pathogen corresponding tothe immunogen so as to obtain the animal model. According to the method for preparing the animal model, tested substances are directly delivered to lungs of mice by the means of liquid aerosols so asto construct a novel immune mouse model; and the constructed model is highly targeted, good in repeatability, and capable of allowing accurate and quantitative study of immune status of the tested substances in mice.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method of screening
A method of detecting the presence of a functionally inhibitory immunointeractive molecule in a biological sample. Preferably, the immunointeractive molecule is directed to a pathogen derived antigen and, more particularly, a parasite derived antigen and, even more particularly, a Plasmodium drived antigen. The method of the present invention facilitates detection of the presence of functionally inhibitory immunointeractive molecules, both in vitro and in vivo, and is useful for qualitatively and / or quantitatively assessing the immune status of individuals who have been previously infected with a parasite, predicting the immune status of individuals vaccinated with an antigen based vaccine, determining the relative contribution of a specific immunoreactivity of antibody to the total inhibitory antibody elicited by combination vaccines which include two or more antigens, assessing vaccines to determine the efficacy of different forms of an antigen, determining vaccine potency, assessing the protective potential of certain immunoreactivities of antibodies and determining the importance of parasite inhibitory antibodies.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com