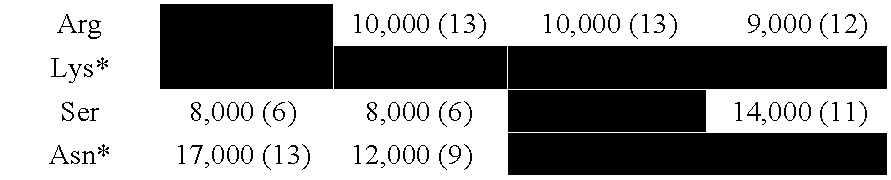Methods and compositions for impairing multiplication of HIV-1
a technology of compositions and compositions, applied in the field of methods and compositions for impairing the multiplication of hiv1, can solve the problems of nullifying the proposed mechanism of action for therapeutic benefit in hiv infection, affecting the clinical effect of the infection, and causing serious side effects in some patients, so as to reduce the viral level of hiv-1, impair the multiplication of the virus, and reduce the viral multiplication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Immunological Studies on Minimal Tat Protein Amino Acid Sequences Necessary for Binding to Antibody for Epitope I in HIV-1 Tat Protein
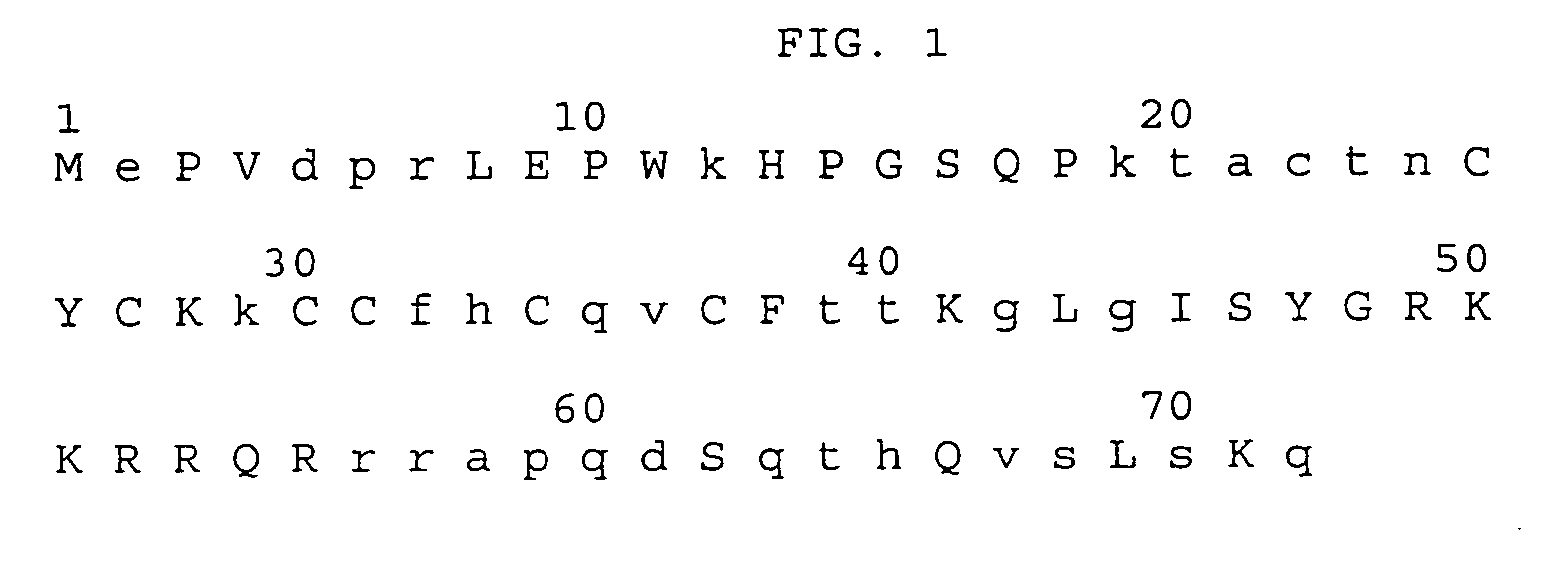
[0151] A peptide corresponding to amino acids 4-16 of SEQ ID NO: 1 illustrated in FIG. 1 was synthesized as described below. This sequence is among the most frequent sequence representation at these positions in 31 Tat protein sequences of the common B subtype reported in the NIAID HIV database. This sequence was chosen as a putative immunogen, named Epitope I.
[0152] A. Peptide Synthesis—Immunizing Peptides
[0153] The amino acid sequence of this immunogen -Val-Asp-Pro-Arg-Leu-Glu-Pro-Trp-Lys-His-Pro-Gly-Ser- (SEQ ID NO: 28) was synthesized by solid phase methodology on polypropylene pegs according to the methods of H. M. Geysen et al., J. Immunol. Meth., 102:259 (1987), with an N-terminal cysteinyl being incorporated to facilitate coupling to a carrier protein. The N-terminus was left as a free amine and the C-terminus was amidated in the immunizing...
example 2
Sequence Variations in Epitope I of HIV-1 Tat Protein and Immunological Cross-Reactivities of Antiserums to These Sequences
[0174] Variations in the sequence of Tat protein AA 5-10 of SEQ ID NO: 1 were analyzed in sequences available in HUMAN RETROVIRUSES and AIDS 1996, published by the Theoretical Biology and Biophysics Group of the Los Alamos National Laboratory, Los Alamos, N. Mex., and additional sequences kindly obtained from GenBank by Esther Guzman of the Los Alamos Laboratory.
[0175] A. Variations in Sequences
[0176] 399 aa 5-10 Tat hexapeptide sequences of the common B subtype of HIV-1 were obtained, as were 18 from the non-B subtypes (6 from subtype A, 2 from subtype C, 7 from subtype D, 2 from subtype F and 1 from subtype U).
[0177] For the B subtype, 386 of the total 399 (97%) hexapeptides had either Arg (289, 74%), or Lys (45, 11%), or Ser (36, 9%) or Asn (16, 4%) in position 3 as the only variation in the hexapeptides. The remaining variations (3%) comprised:
-Gly-Pro...
example 3
Defining an Antibody Binding Amino Acid Sequence (Epitope II) Within the Linear 18 Amino Acid Sequence Following Cys37 of HIV-1 Tat Protein
[0196] A peptide corresponding to amino acids 38-55 of SEQ ID NO: 1 illustrated in FIG. 1 was synthesized as described in Example 1. Using the methods described in Example 1, a low titer antibody response in rabbits was detected and Table 28 summarizes studies defining the sequence involved in this antibody binding. The geometric mean titer (GMT) is reported as percentage of self-titer.
TABLE 13Antiserum to SEQ ID NO:105:Phe-Ile-Thr-Lys-Gly-Leu-Gly-Ile-Ser-Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-ArgGMT (% selfDetector Peptidestiter)SEQ ID NO.Phe-Ile-Thr-Lys-Gly-Leu-Gly-Ile-Ser-Tyr-Gly- 947 (100)105Arg-Lys-Lys-Arg-Arg-Gln-ArgPhe-Ile-Thr-Lys-Gly-Leu-Gly-Ile-Ser-Tyr-Gly-1141 (115)(amino acids 1-15 of SEQ IDArg-Lys-Lys-ArgNO:105)Lys-Gly-Leu-Gly-Ile-Ser-Tyr-Gly- 895 (95)(amino acids 41-55 of SEQArg-Lys-Lys-Arg-Arg-Gln-ArgID NO:1)Leu-Gly-Ile-Ser-Tyr-Gly- 986...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| compositions | aaaaa | aaaaa |
| pharmaceutical composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com