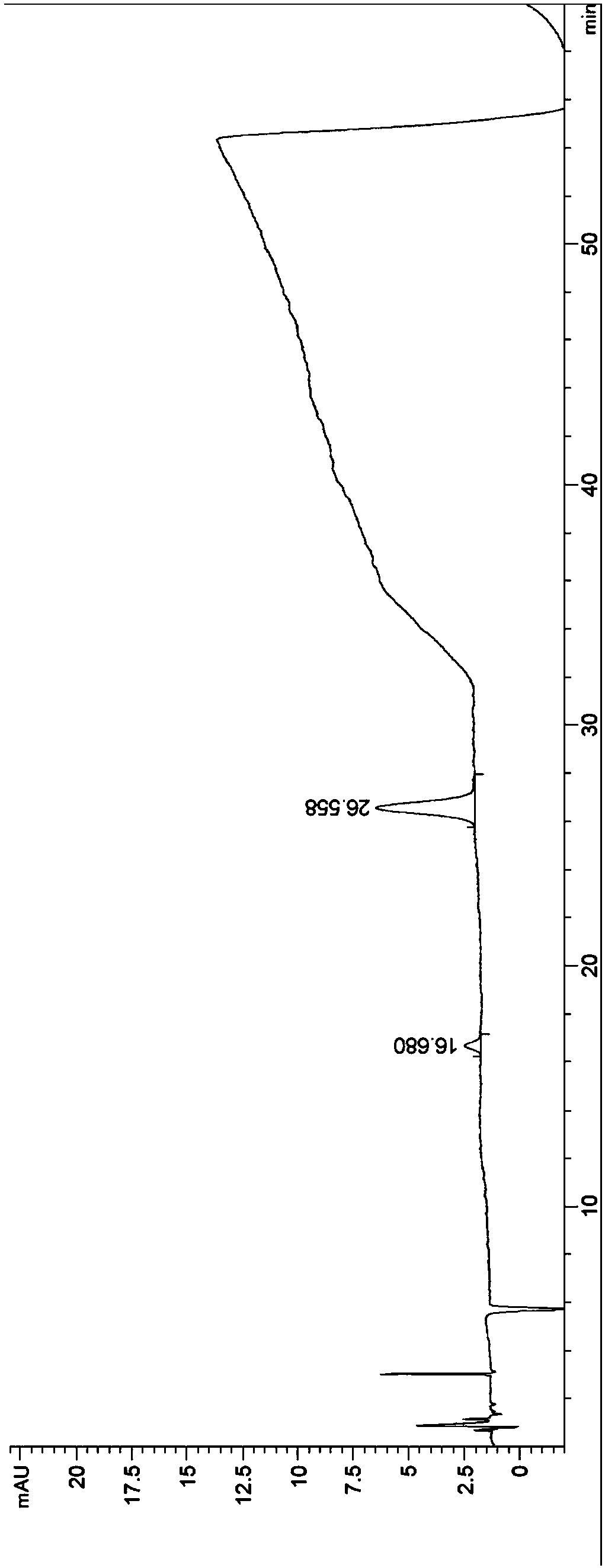
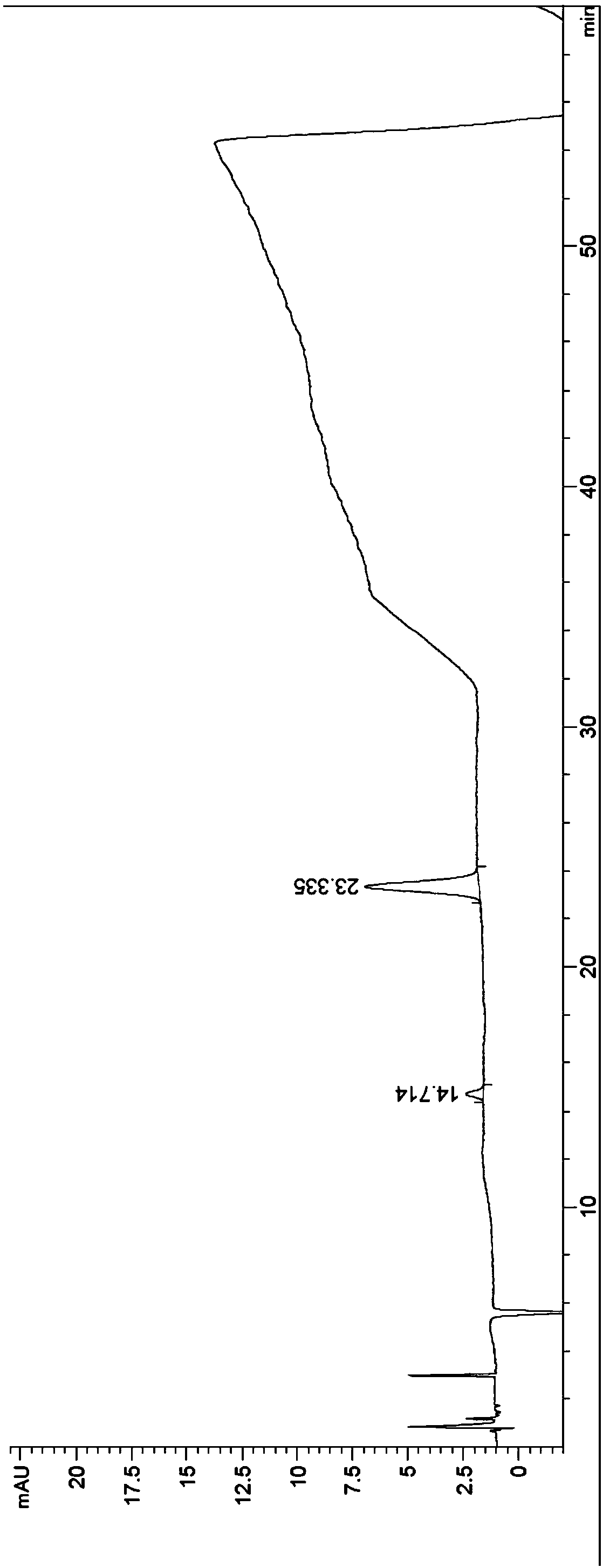
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Immunologic preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunologic preparation information including symptoms, causes, diseases, symptoms, treatments, and other medical and health issues.
Broad spectrum antibody spray for SARS-CoV-2 and SARS-CoV
InactiveCN111228483AFast preparationLow costEgg immunoglobulinsPharmaceutical delivery mechanismAntigenImmunologic preparation
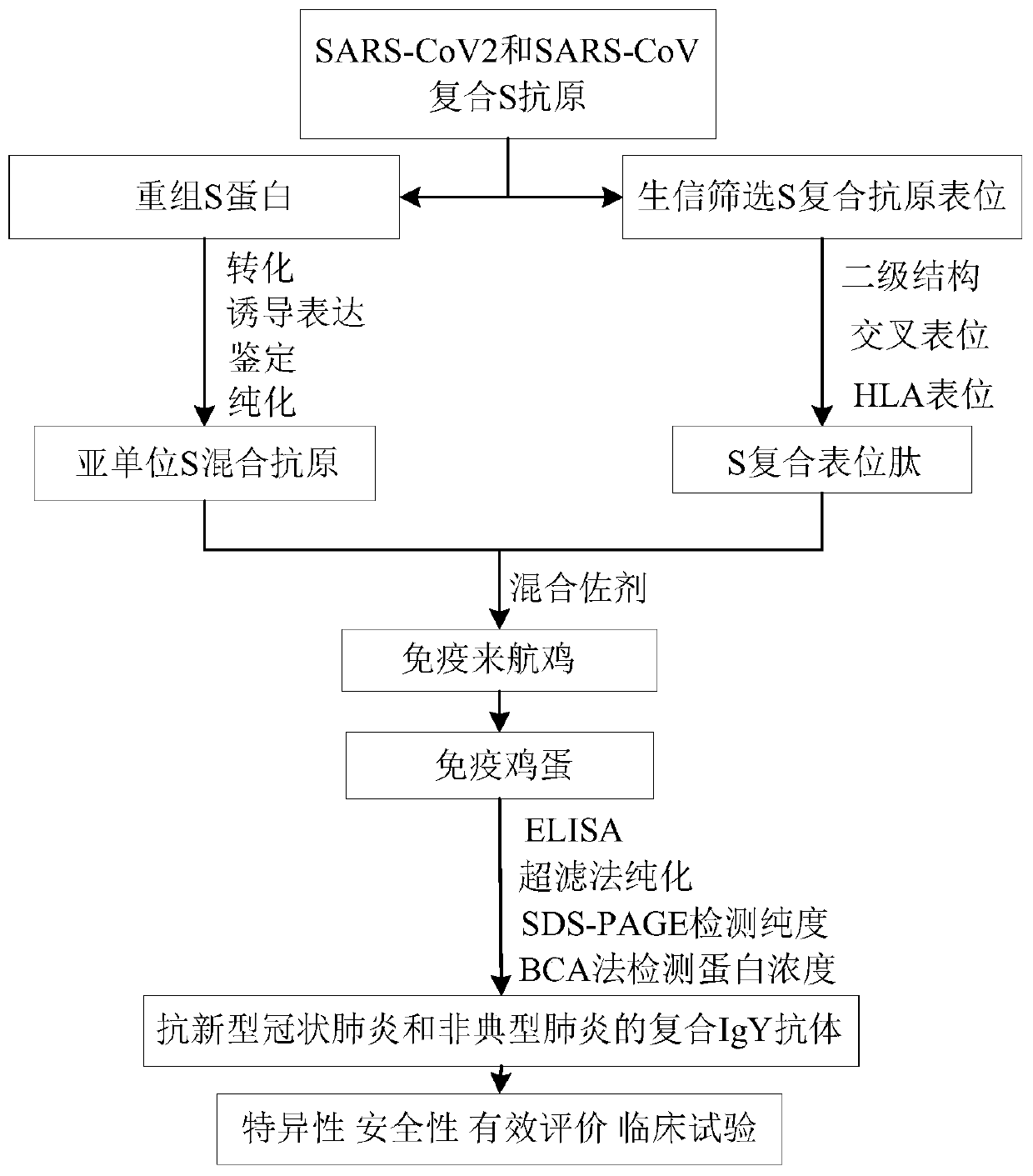
The invention discloses a composite IgY antibody for resisting COVID-19 and SARS. The antibody is prepared by the following method: constructing a recombinant antigen of SARS-CoV-2 and SARS-CoV; preparing an immune preparation with the recombinant antigen to immunize egg-producing birds, and obtaining the specific composite IgY antibody from the immunized eggs. A spray prepared by the antibody canbe used for broad-spectrum killing of SARS-CoV-2 and SARS-CoV, and is suitable for spraying on nasal cavity, oral cavity, skin and other parts. The antibody preparation has a specific virus neutralizing effect, can effectively control infection sources, protect susceptible people, disinfect and protect first-line medical staff and prevent spread of SARS-CoV-2.
Owner:SICHUAN UNIV
Bursopoietin extracting method and its use in disease treating and immune
InactiveCN1528783AImprove immunityIncrease body fluidsAnimal feeding stuffTripeptide ingredientsAdjuvantAntimicrobial drug
The invention relates to a bursin extracting method and its application to curing disease and immunity, having important value in application in the aspects of heightening organismal immunity and acting as immunoenhancer, heightening effect of vaccine, etc., and able to heighten body fluid and cell immune functions of mammal at the same time. It can be used to prevent and cure infectious diseases and young animal diseases singly or together with other drugs such as antivirus and antibacterial drugs or immunomodulators, also be applied to animal vaccine as adjuvant or immunoenhancer to strengthen the disease-resistant ability and immunoresponse ability to peculiar antigens, thus heightening the immune effect.
Owner:王爱华 +1
Saddletail grouper antimicrobial peptide LEAP-2 gene, vector, recombinant strain and protein, and application thereof
ActiveCN103755795AEnriched gene poolReduce manufacturing costAntibacterial agentsFungiAntimikrobielle peptideImmunologic preparation
The invention discloses a Saddletail grouper antimicrobial peptide LEAP-2 gene, vector, recombinant strain and protein, and application thereof. The amino acid sequence of the Saddletail grouper antimicrobial peptide LEAP-2 precursor protein is disclosed as SEQ ID NO.2, or the Saddletail grouper antimicrobial peptide LEAP-2 precursor protein is a protein with identical or higher activity prepared by performing substitution, deletion and / or addition on one or more of amino acids and / or terminal modification on the amino acid sequence disclosed as SEQ ID NO.2. The invention also discloses a Saddletail grouper antimicrobial peptide LEAP-2 gene sequence and a LEAP-2 mature peptide gene mLEAP-2 nucleotide sequence subjected to code modification; and thus, the Saddletail grouper antimicrobial peptide LEAP-2 gene enriches the gene bank of grouper, can be used for preparing a recombinant protein, antimicrobial preparation, fish immune preparation or feed additive, and provides new theoretical and practical basis for physiologic immunity research of grouper.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Multi-epitope artificial antigen of plasmodium falciparum and application thereof
ActiveCN102108355AImproving immunogenicityHigh suppression efficiencyOrganic active ingredientsBacteriaImmunogenicityIn vitro growth
The invention relates to a multi-epitope artificial antigen of plasmodium falciparum and an application thereof. The invention designs a polynucleotide with a sequence as shown in SEQ ID No:1 and a polypeptide with a sequence as shown in SEQ ID No:2, wherein the polynucleotide and the polypeptide are used as artificial antigens to prepare vaccines, and antibodies can be obtained from the antigens and are used to prepare immune preparations against plasmodium falciparum infections and kits for detecting malaria infections. Compared with anti plasmodium falciparum vaccines in the prior art, the multi-epitope artificial antigen of the invention has high immunogenicity and antigen recognition diversity, and the antibody of the invention has high inhibition efficiency on the in vitro growth of plasmodium falciparum.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Staphylococcus aureus enterotoxin B (SEB) immune preparation and its preparation method and use
InactiveCN103160519AImproving immunogenicityImprove development and utilization valueAntibacterial agentsMicroorganism based processesHigh titerImmunogenicity
The invention provides a staphylococcus aureus enterotoxin B (SEB) immune preparation SEB2-HSP65 and its preparation method and use. The preparation method comprises the following steps of modifying a SEB gene into a SEB2 gene, fusing the SEB2 gene and a gene of a heat shock protein HSP65 to obtain a core gene segment SEB2-HSP65, and carrying out expression of the core gene segment SEB2-HSP65 to obtain a recombinant fusion protein SEB2-HSP65. The recombinant protein SEB2 does not have a TCR cell receptor binding capacity thereby solving the problem that the existing SEB immune preparation produces a large amount of inflammatory factors, and the HSP65 enhances immunogenicity of the recombinant fusion protein SEB2. The recombinant fusion protein SEB2-HSP65 as an immune preparation for animal immunization does not need any immunologic adjuvants, can produce high-titer antibodies, can help animals to resist 5*LD50SEB toxin attack and has a protection rate of 100%. The recombinant fusion protein SEB2-HSP65 has large development and use values.
Owner:张婉茹
A tacrolimus separation and purification method
InactiveCN107556327AHigh purityImprove efficacyOrganic chemistryPurification methodsImmunologic preparation
A tacrolimus separation and purification method is provided. Two medium-pressure chromatographic devices are adopted. A tacrolimus fermentation extract liquid having low chromatographic purity is adopted as a raw material. Polysaccharides, protein, and other impurities are removed through a first chromatographic column; then a solvent used for extraction is removed through a rotary evaporation process; an obtained product is dissolved again; a tacrolimus-containing eluate having a low concentration is obtained by separation through a second chromatographic column; the eluates in a plurality ofbatches are combined; and the mixture is concentrated by a nanofiltration membrane to obtain high-purity solid-state tacrolimus. Through the method, direct purification from a tacrolimus-containing fermentation solution can be achieved, the tacrolimus purity is significantly increased, and medicine effects of immunologic preparations prepared from the tacrolimus are indirectly improved.
Owner:WUXI FORTUNE PHARMA
Use of protein TolC in preparing immunological formulation and vaccine
InactiveCN1876180AImproving immunogenicityAntibacterial agentsBiological testingProtective antigenSerum reaction
The invention discloses the usage of TolC protein. The TolC has strong serum reaction, is used to detect and diagnose dysentery, and is used as protective antigens to prepare vaccine.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Recombinant serum amyloid A capable of enhancing immune response of crassostrea gigas, and preparation method thereof
InactiveCN110845594ARaise the level of immune responsePeptide/protein ingredientsPeptide preparation methodsImmunologic preparationOstrea gigas
The invention discloses recombinant serum amyloid A capable of enhancing immune response of crassostrea gigas. The recombinant serum amyloid A is characterized that the amino acid sequence is represented by SEQ ID NO. 1. Gene expression of cytokines CgIL17-1, CgIL17-5, and CgTNF in blood lymphocytes can be remarkably promoted inside and outside bodies of the crassostrea gigas, the immune responselevel of the crassostrea gigas can be enhanced, and the recombinant serum amyloid A has application value in preparation of novel immune preparations and other drugs for shellfishes, especially breeding shellfishes.
Owner:DALIAN OCEAN UNIV
Penaeus vanmamei LvDJ-1 protein as well as coding gene and application thereof
ActiveCN104072597AImprove stress resistanceGreat application potentialMicroorganism based processesAnimal feeding stuffBiotechnologyWAS PROTEIN
The invention discloses a penaeus vanmamei LvDJ-1 protein as well as a coding gene and an application thereof. The LvDJ-1 protein of the penaeus vanmamei is firstly obtained, the amino acid sequence of the LvDJ-1 protein is indicated as SEQ ID NO.2 or the LvDJ-1 protein is protein with one or more amino acid being substituted by the amino acid sequence indicated by the SEQ ID No.2, omitted and / or added and / or derived by the amino acid sequence indicated by the SEQ ID No.2 with end being modified and equal or higher activity. The experiment proves that the penaeus vanmamei LvDJ-1 protein can improve the stress resistance of the penaeus vanmamei and can be used for preparing immune preparation or feed additive related to the aquatic animals, and the application potential is wide.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Method for detecting bastard halibut LITAF gene expression by applying reverse transcription-polymerase chain reaction (RT-PCR)
InactiveCN103276084AHigh degree of automationSolve pollutionMicrobiological testing/measurementDNA/RNA fragmentationDiseaseImmunologic preparation
The invention discloses a method for detecting bastard halibut LITAF gene expression by applying reverse transcription-polymerase chain reaction (RT-PCR). The method lays a foundation for studying bastard halibut LITAF gene expression regulation mechanism and immunology function. TNF-alpha plays an important role in the process of immune response and foreign pathogenic bacteria killing, and the LITAF is a transcription factor of TNF-alpha gene and directly controls the expression of the TNF-alpha gene. Therefore, the expression abundance of the LITAF gene reflects the activity of a bastard halibut immune system to a certain extent and can be used as an immune monitoring index and immune agent quality evaluation index in prevention and treatment of bastard halibut diseases. By detecting the variation of the bastard halibut LITAF gene expression quantity, whether the bastard halibut is infected with a disease can be judged in advance and a measure of prevention and treatment can be taken timely so as to avoid unavoidable loss resulting from serious situation; and besides, the advantages and disadvantages of a fish immune agent can be evaluated so as to judge which immune agent has better effect during prevention and treatment of flounder diseases. The invention provides a technology platform for relative quantitative analysis of LITAF gene at the mRNA level.
Owner:TIANJIN NORMAL UNIVERSITY
Immune preparation for treating tumor and preparation method thereof
InactiveCN105832769APrevent recurrenceInhibit transferTumor rejection antigen precursorsMammal material medical ingredientsDiseaseDendritic cell
The invention discloses an immune preparation for treating tumor; the immune preparation contains dendritic cells (DC) loaded with a tumor antigen and cytokine-induced killer cells (CIK), the concentration of the DC loaded with the tumor antigen in a culture liquid is 1*10<7> to 1*10<8>, and the concentration of the CIK in the culture liquid is 1*10<9> to 1*10<10>. The invention further provides a preparing method of the immune preparation for treating tumor; the preparing method comprises the steps: collection of mononuclear cells of peripheral blood; isolation and culture of the DC and the CIK; extraction of a tumor antigen gene and viral vector packaging; transfection of the DC with a virus mediated tumor antigen gene; and co-culture of the DC loaded with the tumor antigen and the CIK. The accuracy of positioning of re-transported immune cells in a human body can be improved. The objective defect of a conventional cell immunotherapy technology in the same field is not good in solid tumor treatment effect can be effectively improved; adaptation diseases are wide, and the use is safe and effective.
Owner:SHANGHAI PRECISION DIAGNOSTICS CO LTD
Adenitis equorum disease inactivation vaccine and preparation method thereof
InactiveCN104606673AHigh attack protection rateHigh protection rateAntibacterial agentsBacterial antigen ingredientsAntigenDisease
The invention relates to the field of animal immune preparations, in particular to an adenitis equorum disease inactivation vaccine and a preparation method thereof. The adenitis equorum disease inactivation vaccine is prepared by taking a high-generation culture strain ES14-09 of an adenitis equorum streptococ cus Erdos epidemic strain ES14 as specific immunizing antigen through processes of seed batch preparation, culture, concentration, inactivation and adjuvant emulsification. The content of antigen bacteria of the adenitis equorum disease inactivation vaccine is 5*10<8> / ml. The adenitis equorum disease inactivation vaccine is high in protection rate and applicable to epidemic prevention of adenitis equorum diseases of equine animals.
Owner:INNER MONGOLIA AUTONOMOUS REGION ACAD OF AGRI & ANIMAL HUSBANDRY SCI +2
Anti-infection and Anti-tumor mucosal immune preparation
ActiveUS20180360738A1Certain activityCertain characteristicAntibacterial agentsPowder deliveryChemical LinkageAbnormal tissue growth
The present invention relates to an anti-infection and anti-tumor mucosal immune preparation. The mucosal immune preparation includes mucosal immune substances that are mainly formed by organically bonding polyinosinic-polycytidylic acid, non-antibiotic amino compounds and metal cations through chemical bonds. The present invention provides a slow release effect on a local part or the whole body, prevents the degradation of serum ribonucleases of human beings and primates, prolong the half-life period of the mucosal immune preparation, increases the availability and effectiveness of drugs. The mucosal immune preparation can facilitate the mucosal immunity of the body by mucosal immunity and thus facilitate the activation and proliferation of various immune cells, rather than merely acting on diseased local parts, so that the purposes of anti-infection and anti-tumor prevention and treatment with almost no side effects are realized. Mucosal immunity also avoids the pain of repeated injection so that good compliance is achieved.
Owner:LIN HAIXIANG +3
Biphase emulsification adjuvant and the technique for preparing the same
InactiveCN101041077AUniform sizeLow viscosityOrganic non-active ingredientsEmulsion deliveryWaxAdjuvant
The invention relates to a bidirectional oil emulsion agent used in immunity agent, which comprises animal injection white oil, Siban 80, tuwen 80, stabilizer 1.2 propanediol, activator PVP polyvinyl pyrrolidon. And the production comprises that in room temperature, first emulsifying animal injection white oil in an emulsifying pot, to obtain light liquid wax oil, to be added with the lipophilic SIban-80, to be mixed for 3-7min at 75-80r / min speed until foam disappears and transparent, then mixing and adding hydrophilic tuwen-80 to be mixed for 3-7min uniformly, adding 1.2 propanediol, mixing uniformly, adding polyvinyl pyrrolidon until foam disappears and transparent, laying for 25-35min. The invention has low viscidity, injection support, uniform size, and adsorption support, with high stability, long service life, and the color in white or milky white with some pink.
Owner:YIXING ZHONGMU BIOLOGICAL ADJUVANT TECH
Application of tilapia protein peptide to preparation of immune preparation
InactiveCN109350731ASignificant immunomodulatory activityNon-cytotoxicHydrolysed protein ingredientsImmunological disordersTilapiaCell activity
The invention relates to the technical field of bioactive peptides and provides application of a tilapia protein peptide to preparation of an immune preparation. The tilapia protein peptide refers toenzymolysis polypeptide obtained by enzymolysis of tilapia protein. According to researches, the tilapia protein peptide is capable of effectively inducing RAW264.7 cell iNOS expression and increasingNO release amount, and due to dose dependence, the tilapia protein peptide is excellent in immunoregulatory activity and can be used as a raw material for the immune preparation. Further, according to the researches, the tilapia protein peptide has no evident influences on RAW264.7 cell activity under immunoregulatory dosage, namely the tilapia protein peptide has no cytotoxicity when giving playto an immunoregulatory effect. The tilapia protein peptide is preferably derived from enzymatic hydrolysates of tilapia leftovers, and a novel application approach is provided for recycling of tilapia byproducts.
Owner:汕尾市维明生物科技有限公司
Stenotrophomonasmal-tophilia vaccine and its preparing method
InactiveCN1781552AGood treatment effectReduce manufacturing costAntibacterial agentsBacteria material medical ingredientsStenotrophomonas maltophiliaCell immunity
The present invention provides a biological product capable of resisting stenotrophomonas maltophilia and Pseudomonas aeruginosa infection and raising the body fluid immunity and cell immunity of malignant tumor patient. The biological product is prepared with stenotrophomonas maltophilia, and may be prepared into various kinds of immunological preparations and immune regulating preparations. The medicine of the present invention has the special effect of preventing and treating stenotrophomonas maltophilia and Pseudomonas aeruginosa infection and treating ascites carcinoma and leukemia.
Owner:余国华
Nucleic compound and production method thereof, and its use in the preparation of immune formulation
InactiveCN1422888AProtect against tumorProtective therapeuticBacterial antigen ingredientsViral antigen ingredientsDiseaseSolubility
The invention refers to a nucleic acid compound, the producing method and the application at the aspect of making immune preparation, produced by making copolymerization or covalence reaction on nucleic acid segment, multi-peptide and water-solubility multi-polymer under catalysis. It has the common formula; nucleic acid / multi-peptide / water-solubility multi-polymer. It can protect the organism to resist the pathogene infection, resist the tumour and cure the independent immune diseases and effectively inspires the immune response of organism.
Owner:王宾
Biological immune preparation for treating dairy cattle clinical mamitis and its manufacturing method
InactiveCN1589897AReduce resistanceHigh recurrence ratePeptide/protein ingredientsAntipyreticImmunologic preparationMastitis
A bioimmunological medicine for treating the mastitis of milk cow contains hog lymphocyte transfer factor and ox lymphocyte transfer factor. It is prepared from the spleens of hog and ox through mincing, proportionally mixing, mashing, homogenizing, repeated freezing-thawing, freezing, centrifugating, taking supernatant, dialysis, freezing, thawing and filtering.
Owner:NORTHWEST A & F UNIV
Immunological preparation for preventing and treating porcine reproductive and respiratory syndromes
InactiveCN105213918AImprove immunityActivation generationAntiviralsPlant ingredientsEpimediumImmunologic preparation
The invention provides an immunological preparation for preventing and treating porcine reproductive and respiratory syndromes. The immunological preparation comprises epimedium, sealwort, Gorgon fruits, ephedra herbs, centipeda minima, flatstem milkvetch seeds, forsythia, companumoea roots, cape jasmines, yams, tree peony bark, acanthopanax roots, curculigo orchioides, jasminum elongatum, clovers, meadowrue roots and rhizomes, mistletoes, rheum franzenbachii, adenophora tetraphylla and alpinia oxyphylla. The immunological preparation has the advantages that cellular immunity and humoral immunity of organisms can be enhanced through the organism regulation effect, immune cells are activated, interferons are induced, viruses cannot survive and reproduce in porcine body cells, and the effect of resisting the porcine reproductive and respiratory syndromes is achieved.
Owner:QINGDAO HUAREN TECH INCUBATOR
Biological immune preparation for strengthening goatpox vaccine antibody and manufacturing method of biological immune preparation
InactiveCN106552264AImprove immune antibody levelsObvious weight gainPeptide/protein ingredientsViral antigen ingredientsImmunologic preparationFiltration
The invention relates to a biological immune preparation for strengthening a goatpox vaccine antibody and a manufacturing method of the biological immune preparation. The steps adopted in the method include that the fresh spleen of a healthy yak is selected, after fat, the envelope and the internal connective tissue are removed, the spleen is subjected to freezing preservation, the yak spleen obtained after freezing preservation is taken out and melted and is minced through a tissue stamp mill, after sterile tri-distilled water with the same weight is added, the high-speed tissue stamp mill is used for conducting homogenizing at a high speed for two times, and homogenizing is conducted 10 min each time; and prepared homogenate is frozen at the temperature of minus 20 DEG C, freezing and thawing are conducted repeatedly for five times, centrifuging is conducted after the last time of thawing, supernatant is taken and put into a dialysis bag, the supernatant is dialyzed overnight under the environment of 0 DEG C to 4 DEG C through pyrogen-free cold normal saline, dialysate outside the bag is collected and is subjected to filtration sterilization through a filtering film, and the dialysate is subjected to sterile subpackaging and is then subjected to freezing preservation. After the biological immune preparation enters an organism, the effect of the biological immune preparation can be rapidly developed and can last for several months or even longer, the immune antibody level of the vaccine is effectively improved, the biological immune preparation can be rapidly and completely absorbed by the organism without any residue, weight increasing is obvious after the biological immune preparation is injected into the organism, and a goat can be lively in spirit.
Owner:QINGHAI UNIVERSITY
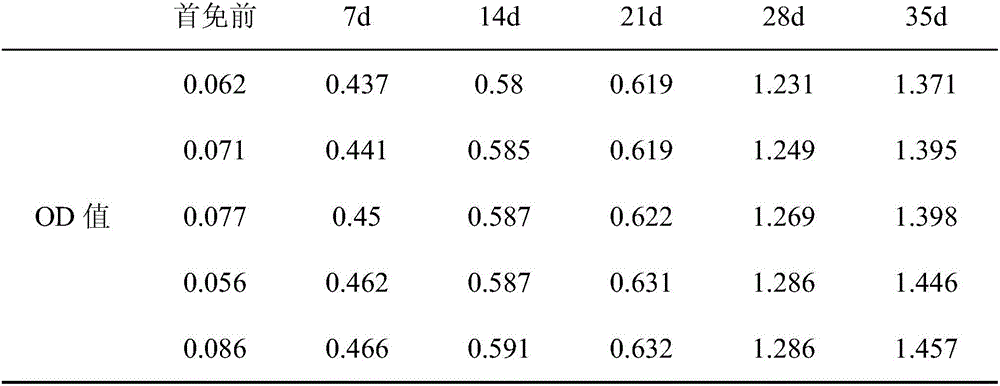
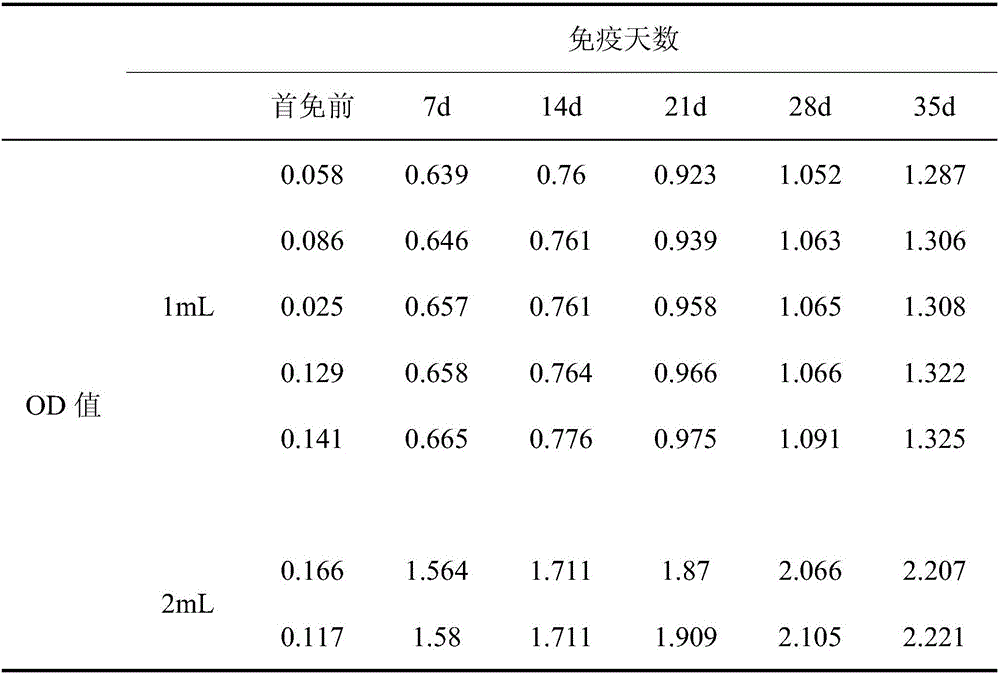
A recombinant lactobacillus that simultaneously expresses Clostridium perfringens α, β2, ε, β1 exotoxin and its construction method and application
ActiveCN106987548BAvoid infectionPreserve immunogenicityAntibacterial agentsBacterial antigen ingredientsImmunologic preparationBacillus perfringens
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Application of immunological stimulant compound in preparing fish immunity preparation
InactiveCN100569285CImprove the ability of proliferation and transformationHigh antibody titerImmunological disordersAntibody medical ingredientsImmunologic preparationImmune complex deposition
The invention discloses an application of an immunostimulating compound in preparing fish immune preparation, and the immune preparation is an oral immune preparation or a bath immune preparation. The advantage of the present invention is that: the oral immune preparation or bath immune preparation made of the immune stimulating compound ISCOMs can not only increase the antibody level, but also improve the proliferation and transformation ability of T lymphocytes and induce immune protection. When immunized with the same dose, the antibody titer of ISCOMs group was significantly higher than that of no adjuvant group and ISM1312 adjuvant group. Similarly, oral immunization with ISCOMs can not only increase the antibody level, but also improve the proliferation and transformation ability of T lymphocytes and induce immune protection.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI +3
Preparation method of high-cleanness pre-filled syringe
ActiveCN102068733BResidue reductionGuaranteed dimensional accuracyInfusion syringesPharmaceutical containersMedicineImmunologic preparation
The invention discloses a high-cleanness pre-filled syringe, which belongs to the field of production of medicinal packing materials. The syringe is characterized in that: a method for manufacturing the syringe comprises the following steps of: 1) producing the syringe; 2) performing rich text format (RTF) treatment; 3) performing ethylene oxide (ETO) sterilization; and 4) accepting, packing and warehousing. Through the syringe, the residue amount of liquid medicine of the prefilled syringe is effectively reduced and the utilization ratio of a medicament is improved while the dimensional accuracy is guaranteed. Particularly, the advantage of the syringe that waste of high-value medicaments is avoided is brought into full play in the fast-developing biological and immunologic preparation industry.
Owner:SHANDONG PHARMA GLASS
External medicine capable of resisting HPV infection and converting HPV positive into HPV negative and preparation method thereof
PendingCN113908218AAvoid first pass effectHigh inhibition ratePharmaceutical delivery mechanismAntiviralsImmunologic preparationRanunculus ternatus
The invention discloses an external medicine capable of resisting HPV infection and converting HPV positive to HPV negative and a preparation method thereof. The external medicine is prepared from the following raw materials in parts by weight: 20 to 50 parts of hedgehog fat, 10 to 30 parts of frankincense, 10 to 20 parts of radix angelicae, 10 to 20 parts of radix ranunculi ternati, 5 to 15 parts of radix et rhizoma rhei, 5 to 10 parts of airpotato yam rhizome, 5 to 10 parts of dried alum and 2 to 5 parts of realgar. Years of clinical experience prove that the medicine disclosed by the invention has no adverse reaction, the medicine can avoid the first-pass effect of the liver when being externally used, has a targeting effect, and has a higher inhibition rate on host cytopathy caused by HPV than that of immune preparations and interferon.
Owner:顾绍亚
Livestock biological immune preparation and preparation method thereof
PendingCN114053404AGood death rateReduce mortalityAntibacterial agentsEgg immunoglobulinsBiotechnologyESCHERICHIA COLI ANTIGEN
The invention discloses a livestock biological immune preparation and a preparation method thereof. The effective component of the immune preparation is a specific yolk immunoglobulin composite antibody. According to the preparation method of the specific yolk immunoglobulin composite antibody, the specific yolk immunoglobulin composite antibody is prepared by taking three pathogenic bacteria k88, k99 and 987P of swine escherichia coli as antigens, and has a relatively good killing effect on three common pathogenic escherichia coli with high morbidity and high mortality in intestinal tracts. The traditional Chinese medicine composition can well regulate the balance of microbial flora in intestinal tracts, has a good prevention and treatment effect on livestock diarrhea, reduces the death rate of livestock, and is suitable for large-scale production and clinical application.
Owner:黑龙江领康生物科技有限公司
Bacillus anthracis capable of showing PA20 protein on surface of spore and application thereof
The invention discloses a bacillus anthracis capable of showing a PA20 protein on the surface of a spore and an application thereof. The bacillus anthracis is obtained by inserting a pagA20 gene into the tail end of a bacillus anthracis spore protein bcIA gene; and particularly the bacillus anthracis can be a bacillus anthracis CGMCC No.3403. The bacillus anthracis can serve as a host and is used for preparing, an immunomodulator which is preferably an inactivated vaccine, an attenuated live vaccine, a subunit vaccine or a gene engineering vaccine.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Application of compound phytonutrients in the preparation of cellular immune preparations
This application relates to the application of a compound phytonutrient in the preparation of cellular immune preparations, the compound phytonutrient includes lycopene oleoresin with a content of 28% to 42% by mass, and grape seed with a content of 19% to 29% by mass The extract, 3%-5% by mass of beeswax and 24%-50% by mass of vegetable oil, and the mass percentage of lycopene in the lycopene oleoresin is 5%-8%. The above compound phytonutrients can be used to prepare cellular immune preparations that not only have good anti-tumor effects, but also can effectively promote cellular immune functions.
Owner:BEIJING DAWN AEROSPACE BIO TECH
Biological immune preparation for treating dairy cattle clinical mamitis and its manufacturing method
InactiveCN100413534CReduce resistanceHigh recurrence ratePeptide/protein ingredientsAntipyreticImmunologic preparationFreezing thawing
A bioimmunological medicine for treating the mastitis of milk cow contains hog lymphocyte transfer factor and ox lymphocyte transfer factor. It is prepared from the spleens of hog and ox through mincing, proportionally mixing, mashing, homogenizing, repeated freezing-thawing, freezing, centrifugating, taking supernatant, dialysis, freezing, thawing and filtering.
Owner:NORTHWEST A & F UNIV
Hefang crucian Ferritin L gene, recombinant protein, preparation method and application and primers of recombinant protein
PendingCN111471687AGrowth inhibitionGrowth inhibitory activityAntibacterial agentsPeptide/protein ingredientsNucleotideAquatic animal
The invention discloses a Hefang crucian(WR) Ferritin L gene and a recombinant protein thereof. The nucleotide sequence of the WR Ferritin L gene is shown as SEQ ID NO: 1, and the amino acid sequenceof the recombinant protein is shown as SEQ ID NO: 2. The discovery of the WR ferritin L gene enriches a gene pool of WR and can be applied to preparation of the recombinant protein. Experiments provethat the recombinant protein can inhibit the growth activity of aeromonas hydrophila, and a new practical basis is provided for physiological immune research of the WR. The invention further disclosesa preparation method of the WR ferritin L recombinant protein. The invention discloses application of the WR Ferritin L recombinant protein in the fields of inhibition of growth of aeromonas hydrophila, immune preparations for aquatic animals or feed additives, and discloses primers for amplifying the WR Ferritin L gene.
Owner:HUNAN NORMAL UNIVERSITY
Preparation method and application of an immune preparation capable of enhancing the immune ability of fish, shrimp and crabs
ActiveCN104012779BIncrease vitalityImprove immunityAccessory food factorsImmunologic preparationMortality rate
The invention relates to a preparation method and application of an immune preparation capable of enhancing immunities of fishes, prawns and crabs, and belongs to the technical field of nutrition immunology. By feeding an immuno-potentiator containing such nutrients as nitric oxide, amino acid, beta-brown shell color, anthocyanin, vitamins and trace elements, oxygen radicals and viruses in the fishes, prawns and crabs are removed or inhibited; moreover, the immune preparation can regulate metabolism of bred animals, and has a certain growth promoting effect; the immune preparation is favorable for reducing a death rate and improving breed output of the bred animals.
Owner:无锡市朗邦生物科技有限公司 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com