Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Bordetella bronchiseptica" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bordetella bronchiseptica is a small, Gram-negative, rod-shaped bacterium of the genus Bordetella. It can cause infectious bronchitis in dogs and other animals, but rarely infects humans. Closely related to B. pertussis—the obligate human pathogen that causes pertussis (whooping cough); B. bronchiseptica can persist in the environment for extended periods.
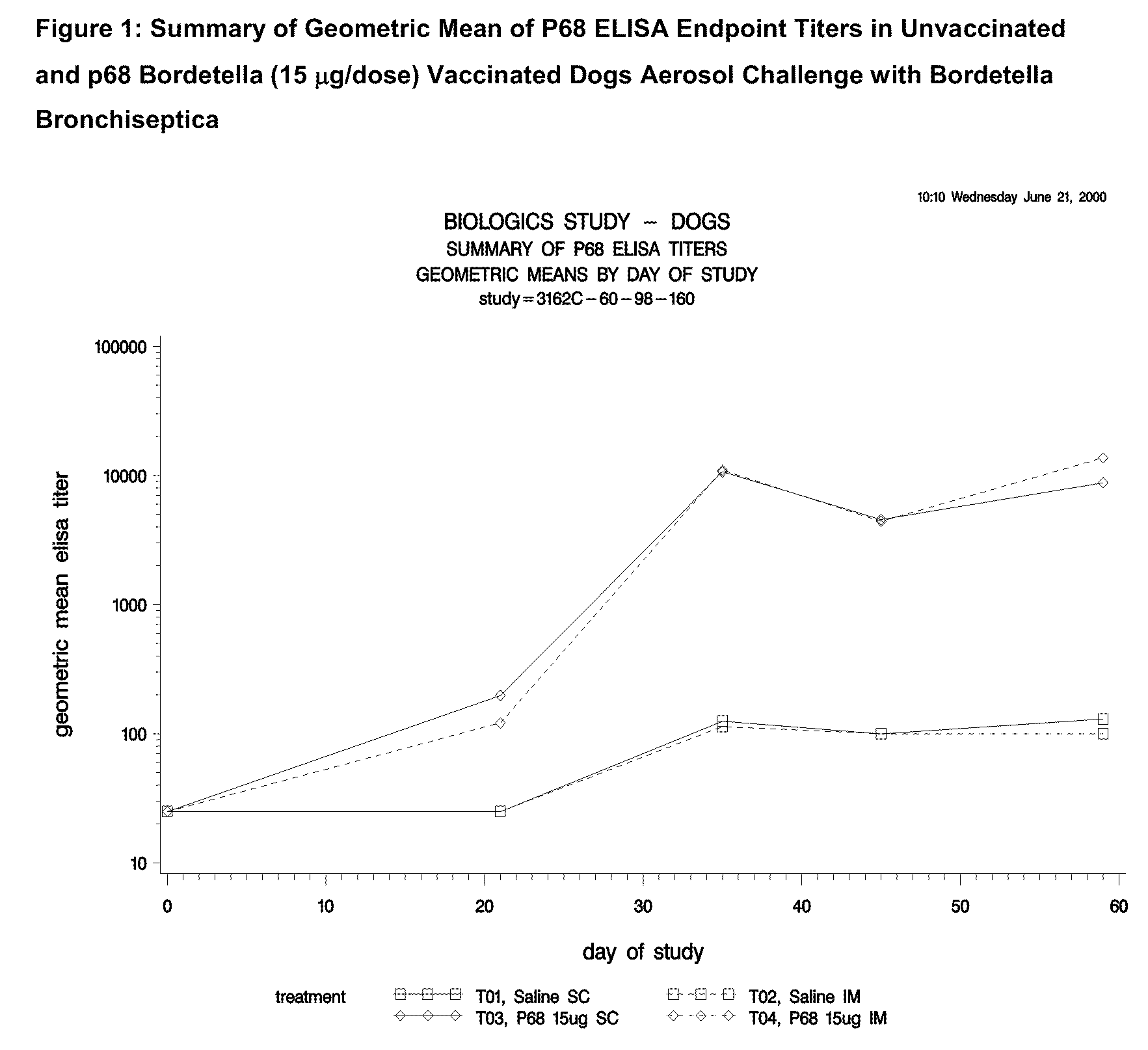
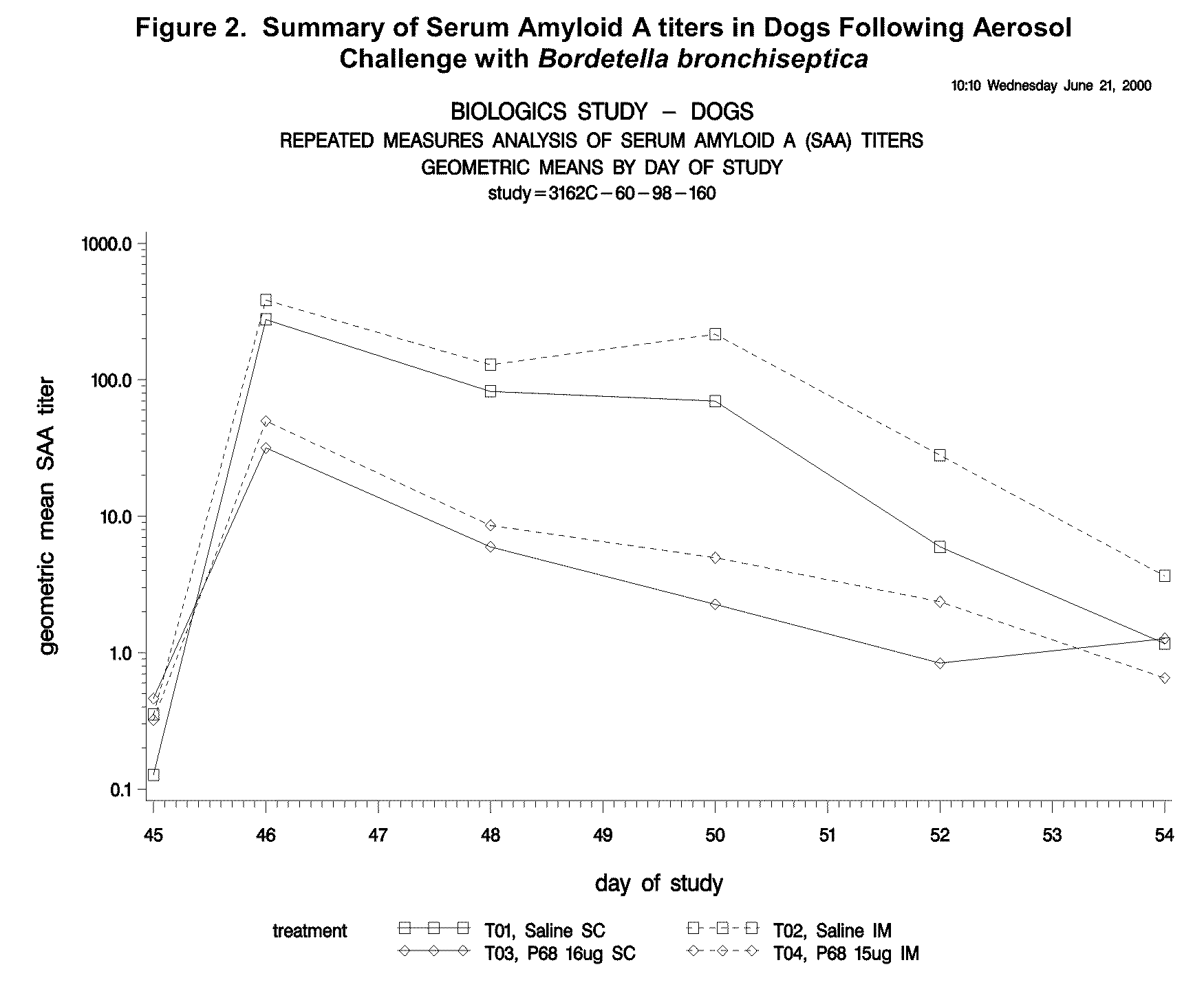
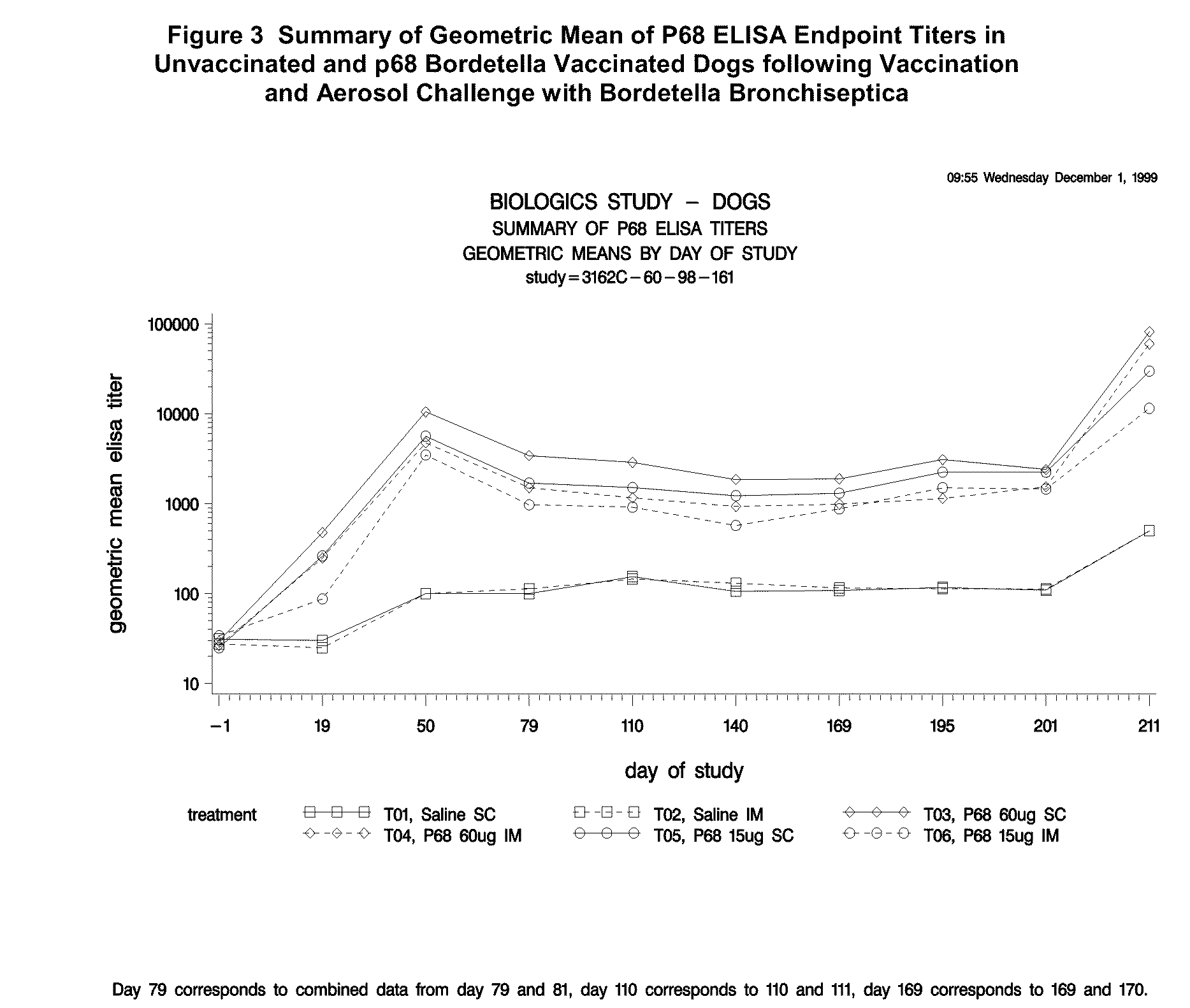
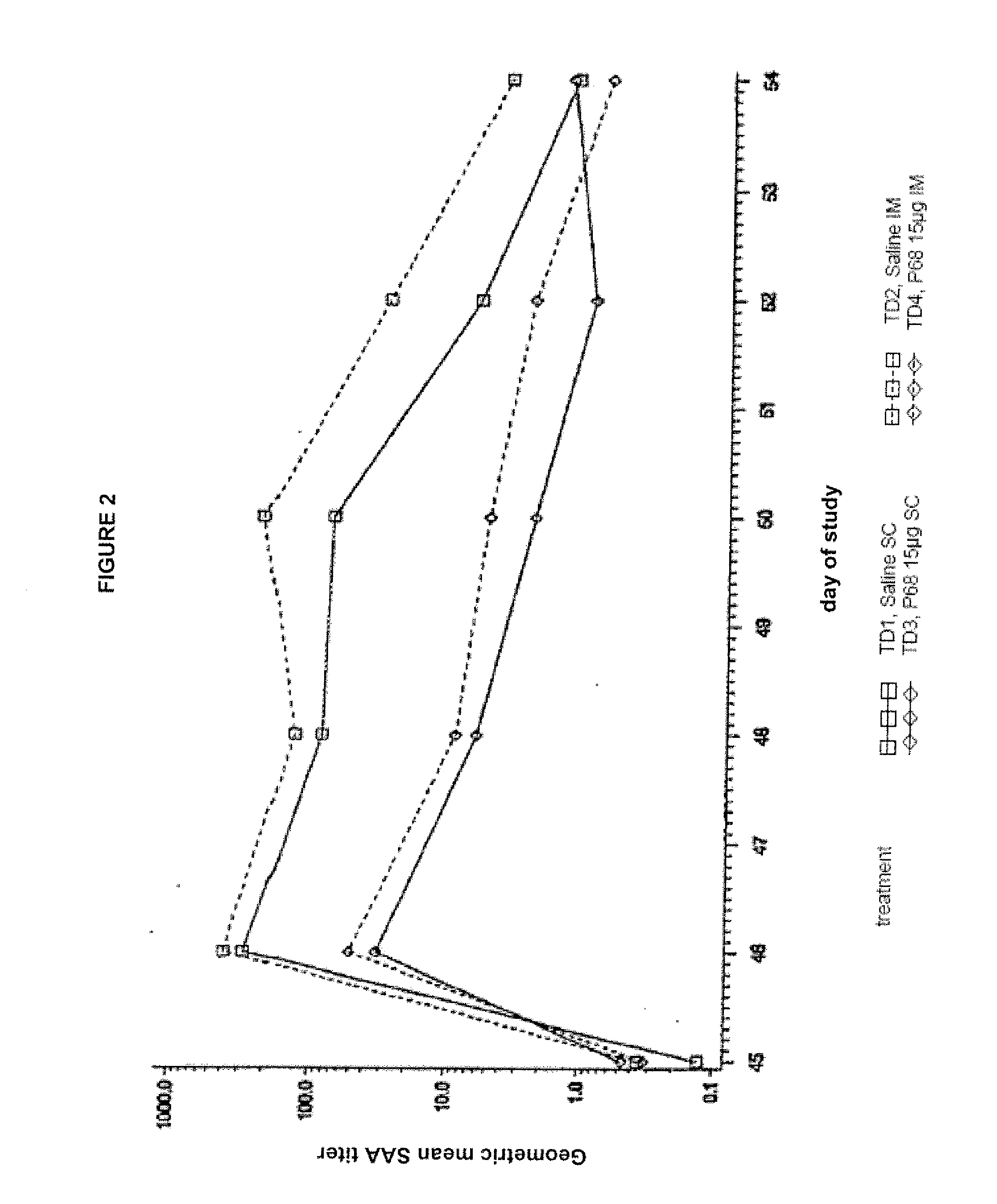
Canine combination vaccines
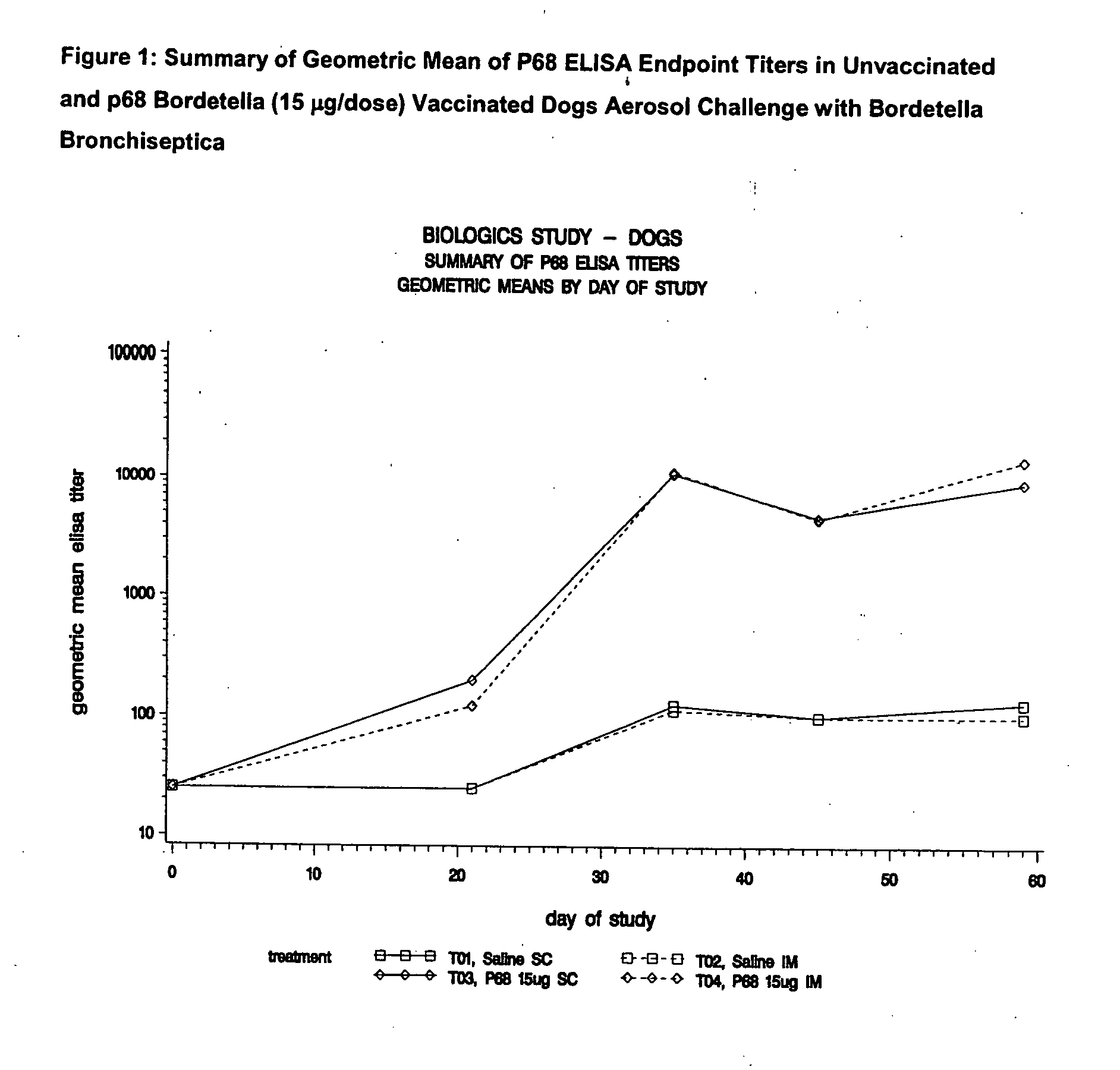
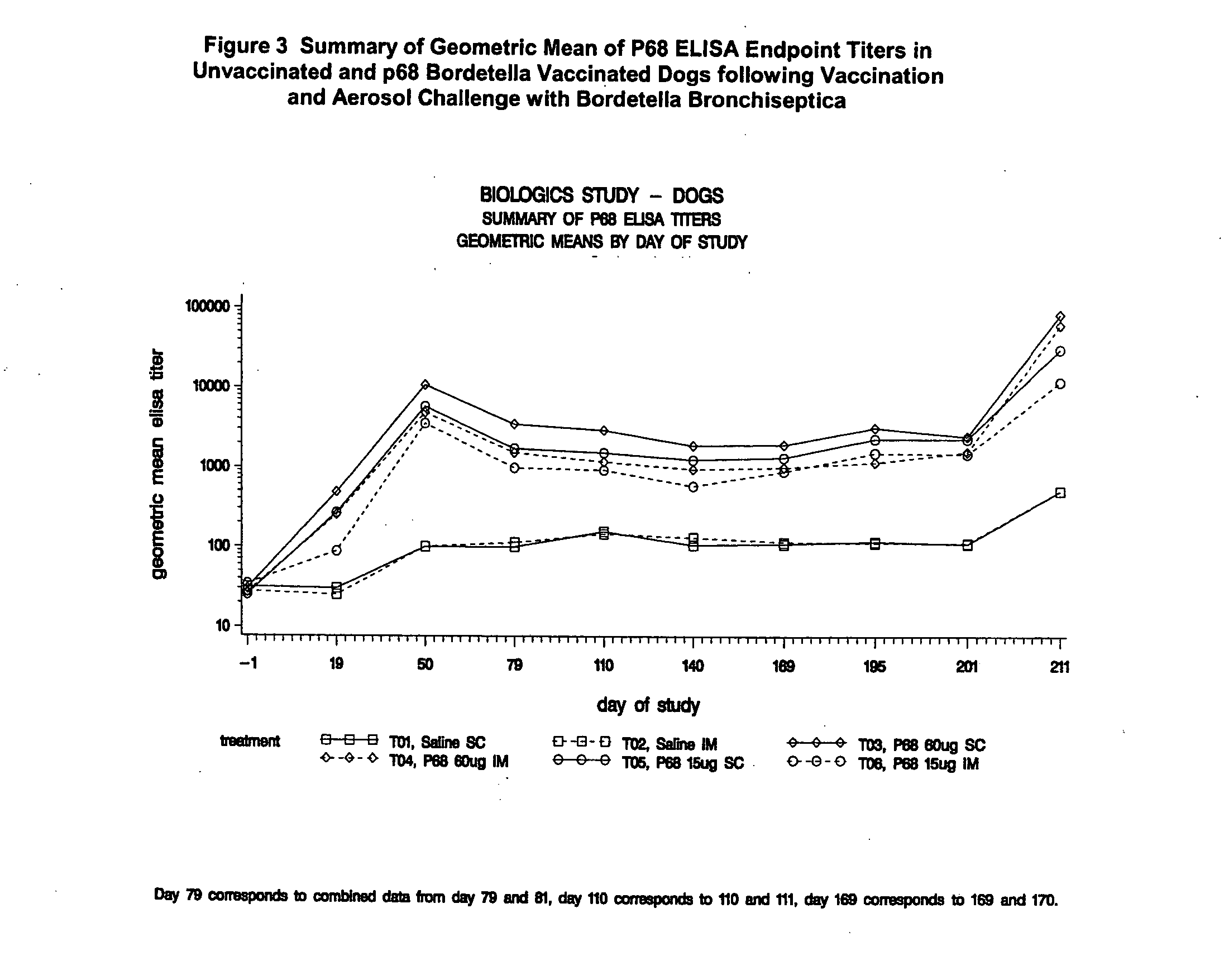
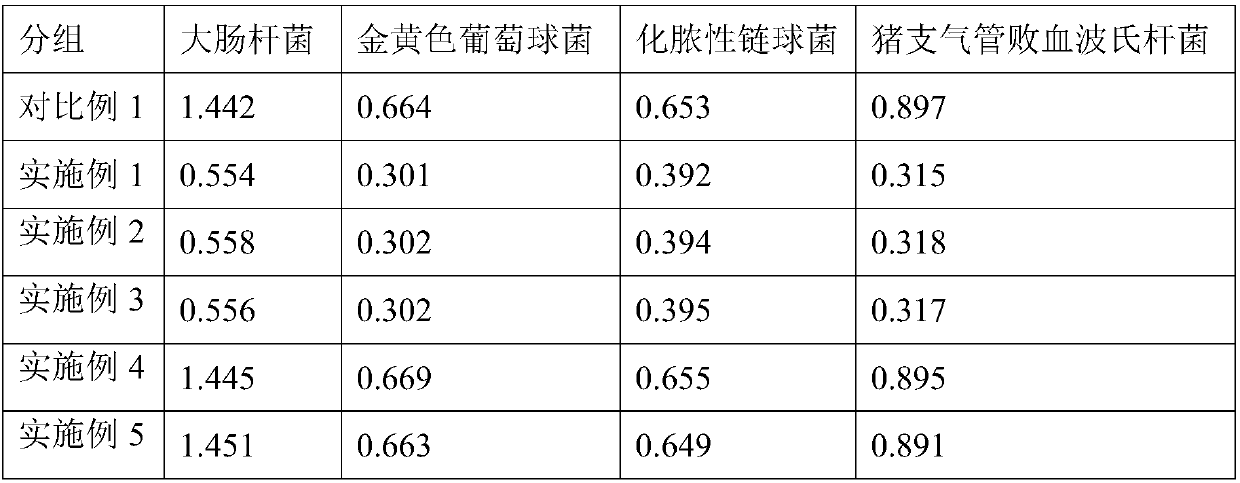
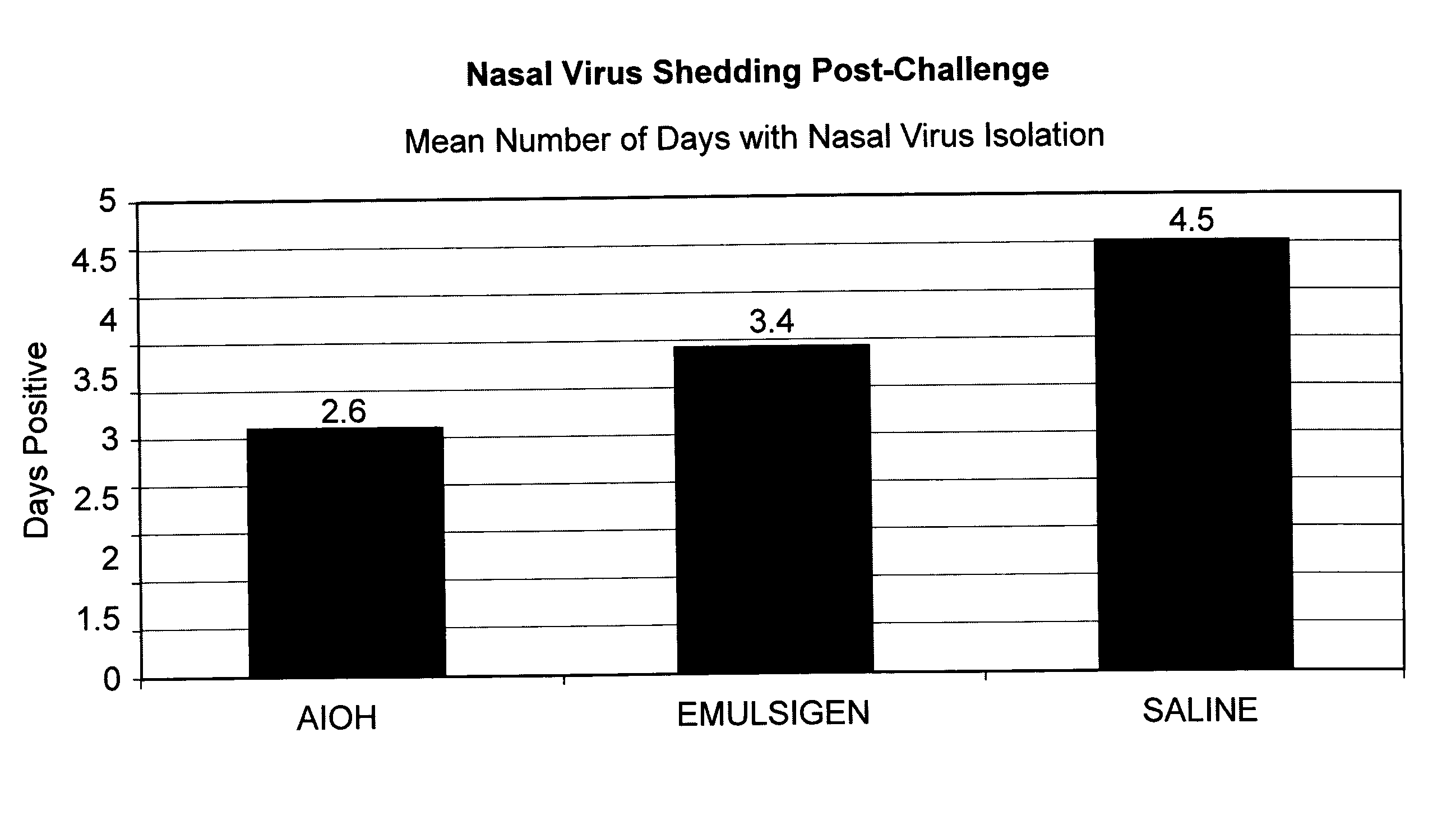
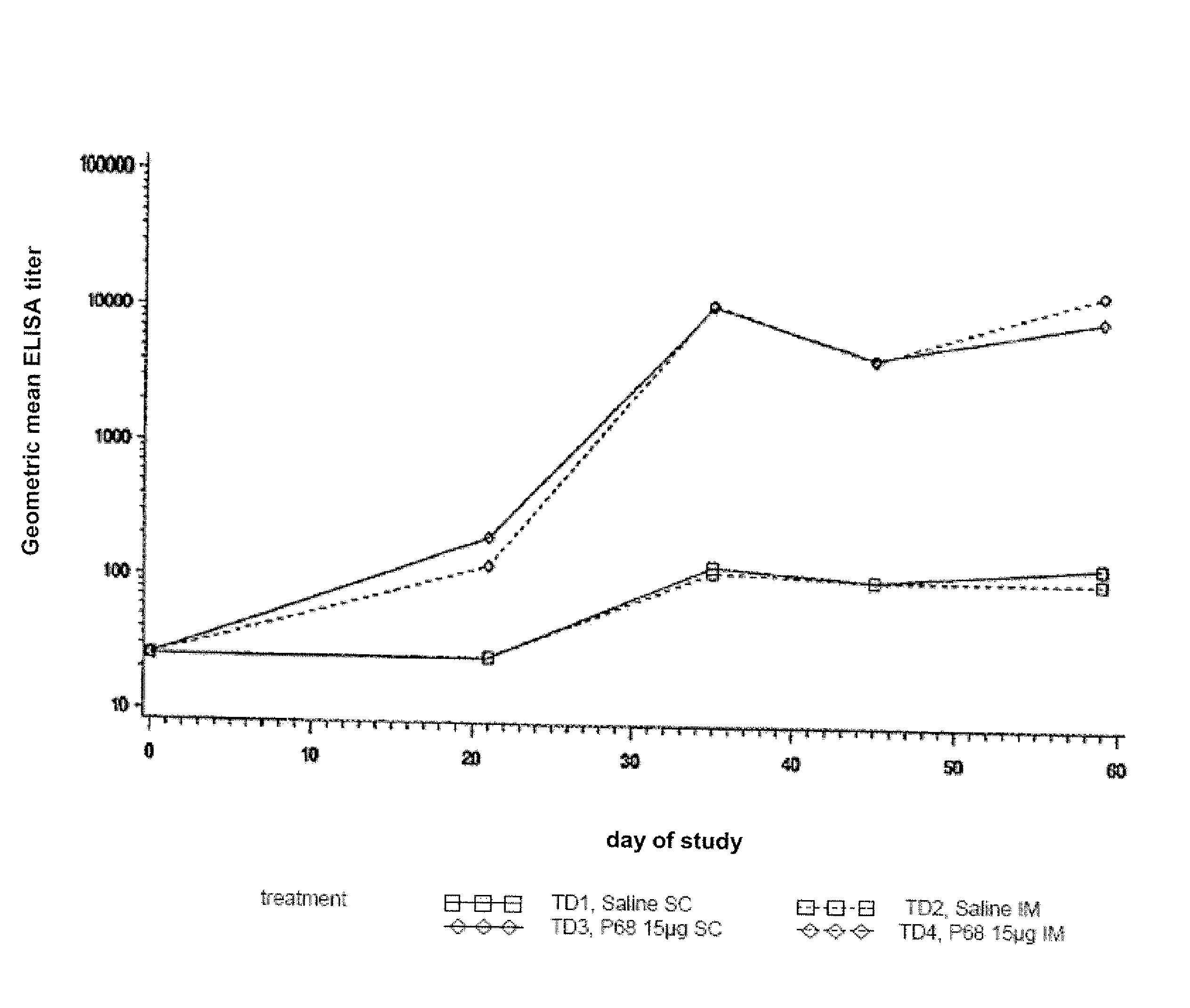
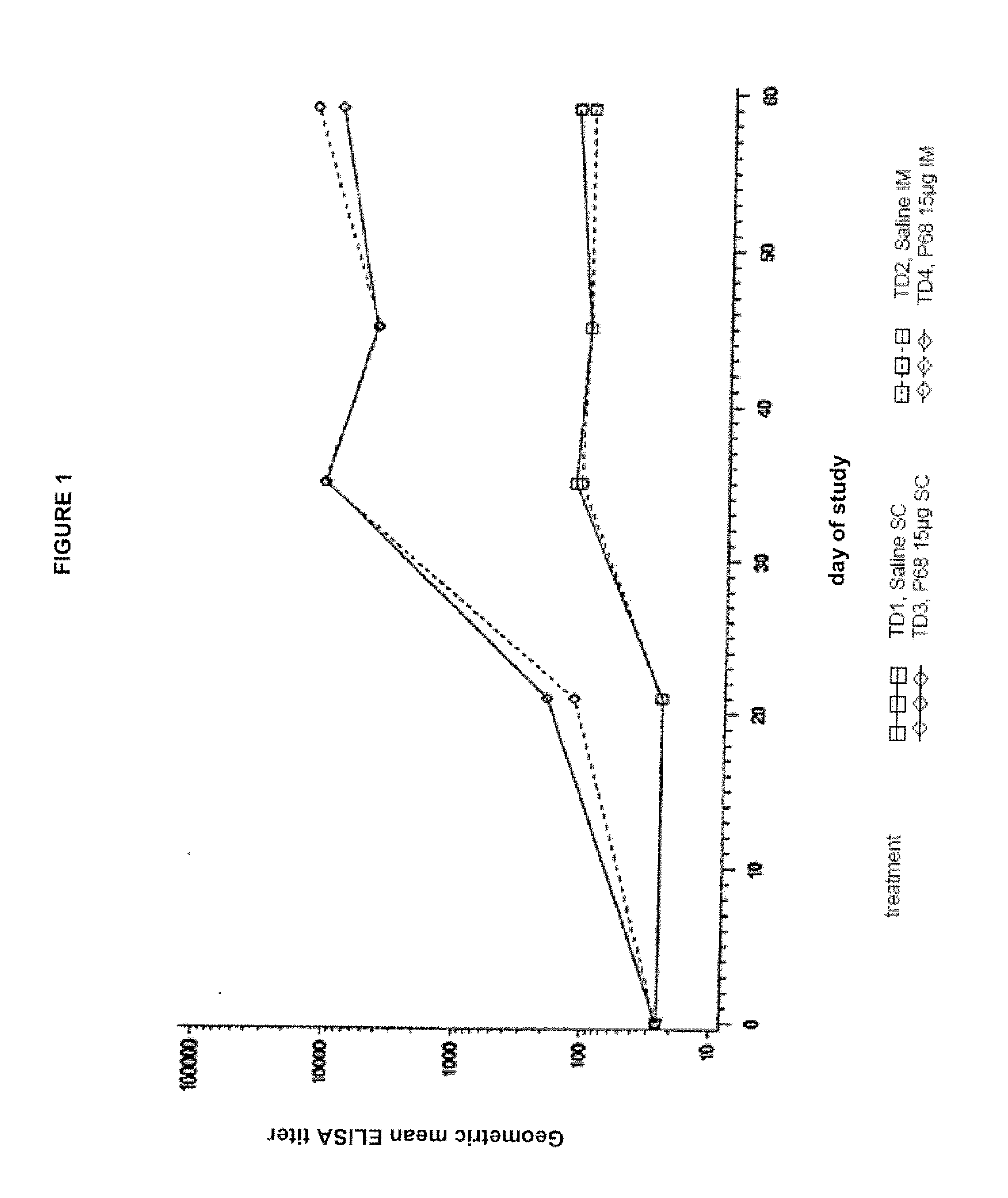
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
Polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of swine
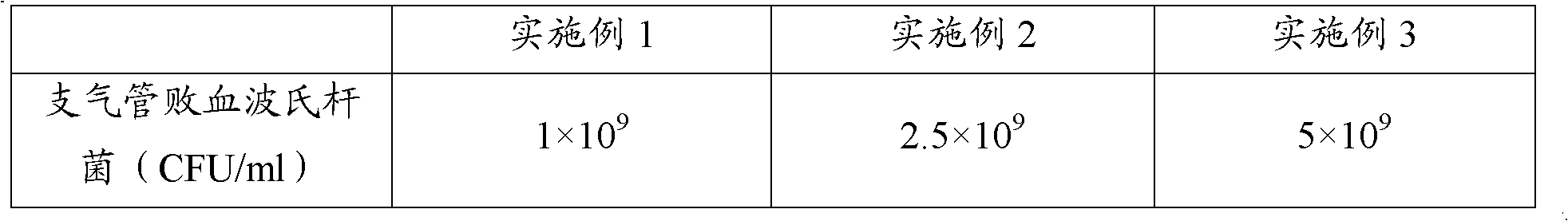
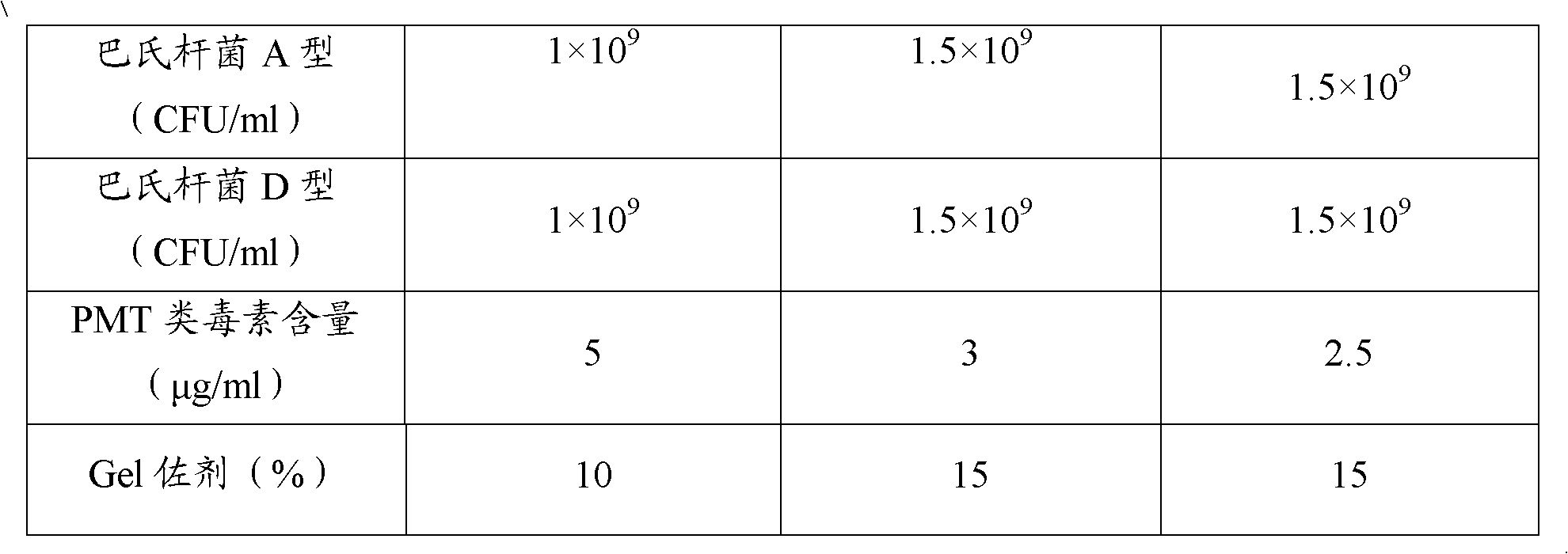
ActiveCN102302771AEffective therapeuticEffective preventionAntibacterial agentsBacterial antigen ingredientsPasteurella multocida toxinImmune effects
The invention provides a polyvalent inactivity vaccine for preventing and treating atrophic rhinitis of a swine and a preparation method thereof. The polyvalent inactivity vaccine contains inactivated Bordetella bronchiseptica, Pasteurella multocida A, Pasteurella multocida D and PMT (Pasteurella Multocida Toxin) anatoxin. The invention further provides a novel method for culturing and extractingPMT. Compared with the traditional atrophic rhinitis of the swine, the polyvalent inactivity vaccine for the atrophic rhinitis of the swine, provided by the invention, can be used for more generally and effectively treating and preventing the atrophic rhinitis of the swine by comprehensive antigen protection. Finally, in the polyvalent vaccine provided by the invention, the vaccine with a plurality of antigens in a reasonable proportion can be used for solving the problem that the plurality of the antigens interfere each other, thereby improving an immune effect. Furthermore, the inventor provides a water adjuvant by which defects such as incomplete absorption, large side reaction and the like after the traditional alumina gel adjuvant, Freund adjuvant and water-in-oil adjuvant are injected into the water adjuvant can be overcome.
Owner:PU LIKE BIO ENG
Canine vaccines against Bordetella bronchiseptica
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention relates to vaccines and methods for protecting dogs against disease caused by Leptospira bratislava. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona.
Owner:PFIZER INC +1
Recombinant salmonella choleraesuis strain for expression of pig origin bordetella bronchisepatica fhaB and prn gene segment, bacterin and uses thereof
InactiveCN101157907AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesBordetella
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Vaccine composition, and preparation method and application thereof
ActiveCN104208666ASmall dose of immunizationGood immune effectAntibacterial agentsBacterial antigen ingredientsBordetella bronchiseptica antigenPasteurella multocida toxoid
The invention provides a vaccine composition, and the vaccine composition comprises immunization amount of a bordetella bronchiseptica antigen, immunization amount of pasteurella multocida toxoid antigen and an adjuvant acceptable in veterinary medicine. The vaccine composition is capable of effectively controlling co-infected swine atrophic rhinitis caused by bordetella bronchiseptica and pasteurella multocida, and further comprises other antigen compositions for controlling respiratory syndrome caused by secondary infection of swine atrophic rhinitis. By reducing the antigen composition and using pasteurella multocida toxoid to replace pasteurella multocida antigens with different serotypes, the purpose of preventing swine atrophic rhinitis is realized. Also by improving the antigen content in per volume of the vaccine composition, the immunization dosage of the vaccine composition is reduced to 1 / 4 of the dosage of a conventional vaccine without reducing the immunization effect. Therefore, the vaccine composition is relatively low in immunization dosage, relatively less in antigen compositions, relatively low in immunization cost and convenient to use.
Owner:PU LIKE BIO ENG
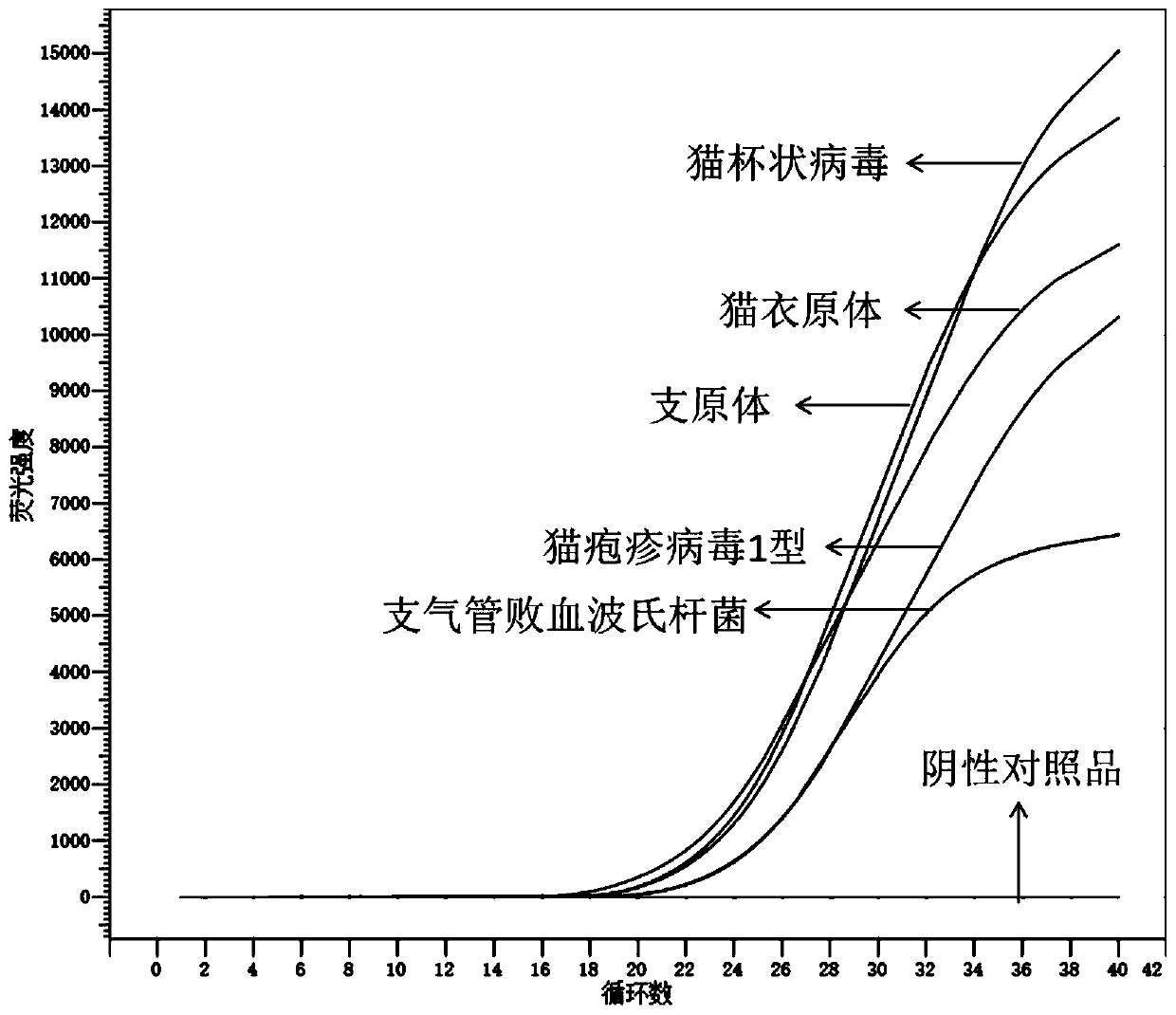
Primer group, product and method for detecting feline respiratory tract pathogens as well as application
InactiveCN111455107ADoes not affect analysisHigh sensitivityMicrobiological testing/measurementMicroorganism based processesFeline calicivirus infectionNucleic acid test
The invention provides a primer, product and method for detecting feline respiratory tract pathogens as well as application, and relates to the technical field of nucleic acid tests. In the primer group for detecting the feline respiratory tract pathogens, a primer for detecting feline herpesvirus type 1 (FHV1), a primer for detecting feline calicivirus (FCV), a primer for detecting mycoplasma, aprimer for detecting feline chlamydia and a primer for detecting bordetella bronchiseptica are primers designed through highly conservative regions of each pathogen, have no cross reaction with otherfeline pathogens, and have the characteristics of high specificity and high sensitivity. By use of the primer group, five feline respiratory tract pathogens including the FHV1, the FCV, the mycoplasma, the feline chlamydia and the bordetella bronchiseptica can be identified in the same reaction system, and the detection cost and time are greatly reduced as compared with a traditional method.
Owner:HANGZHOU BIOER TECH CO LTD
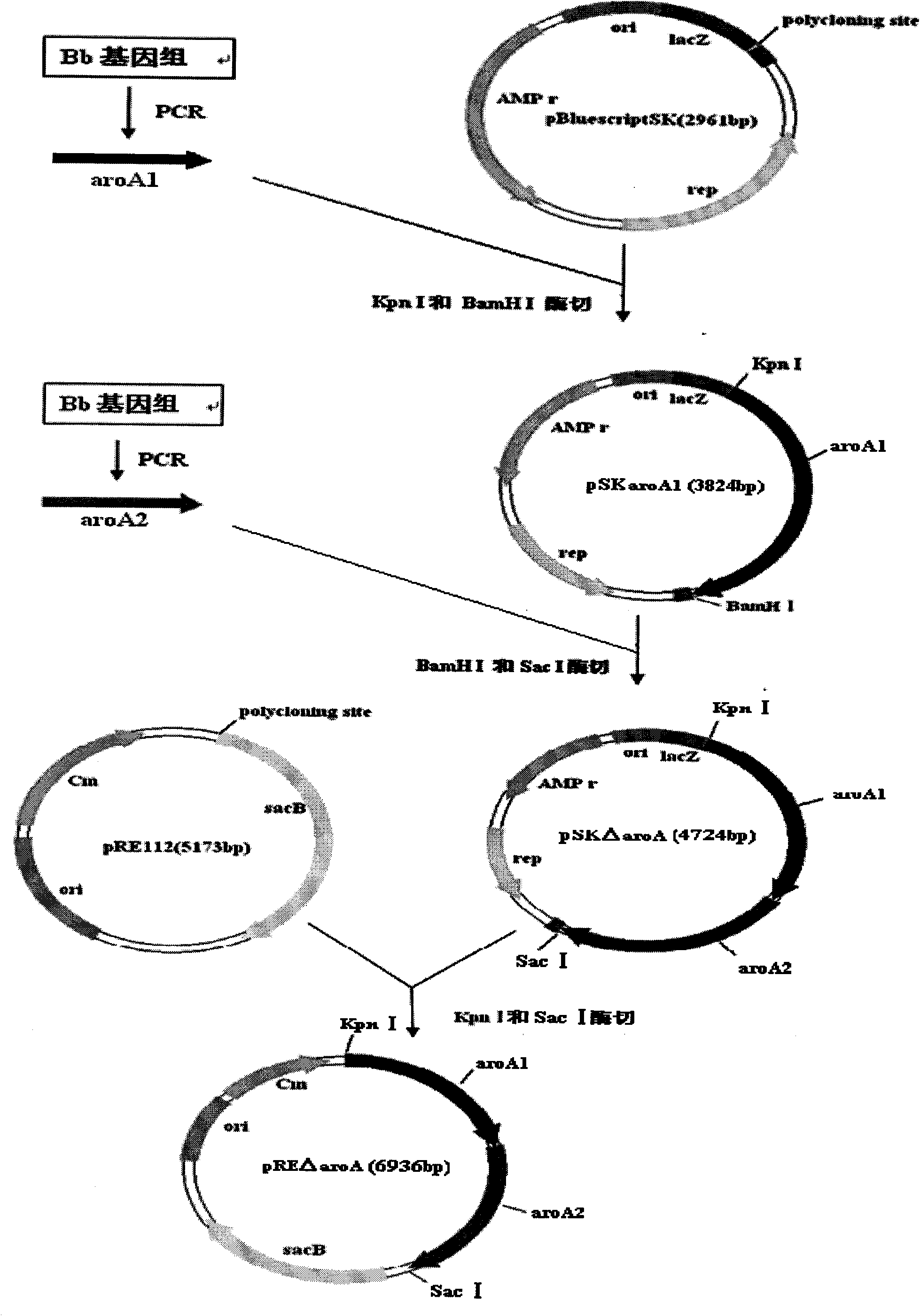
Bordetella bronchiseptica gene deleted vaccine and application
InactiveCN101575586AGood virulence factorGood other immunogenicityAntibacterial agentsBacterial antigen ingredientsBordetella bronchisepticaMicrobiology
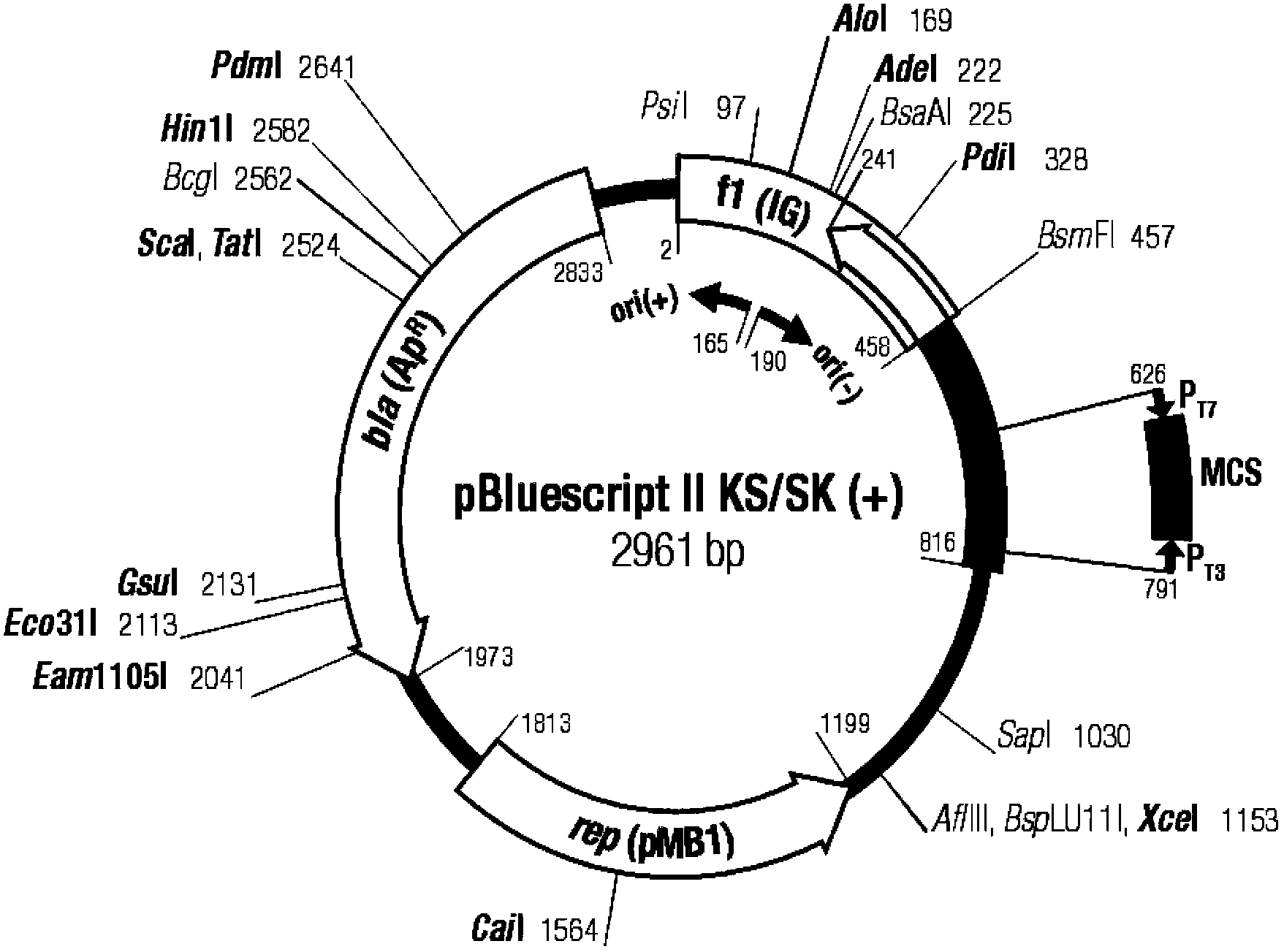
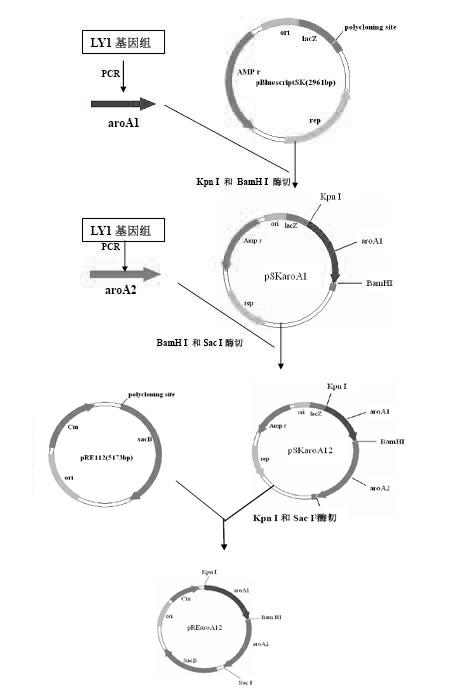
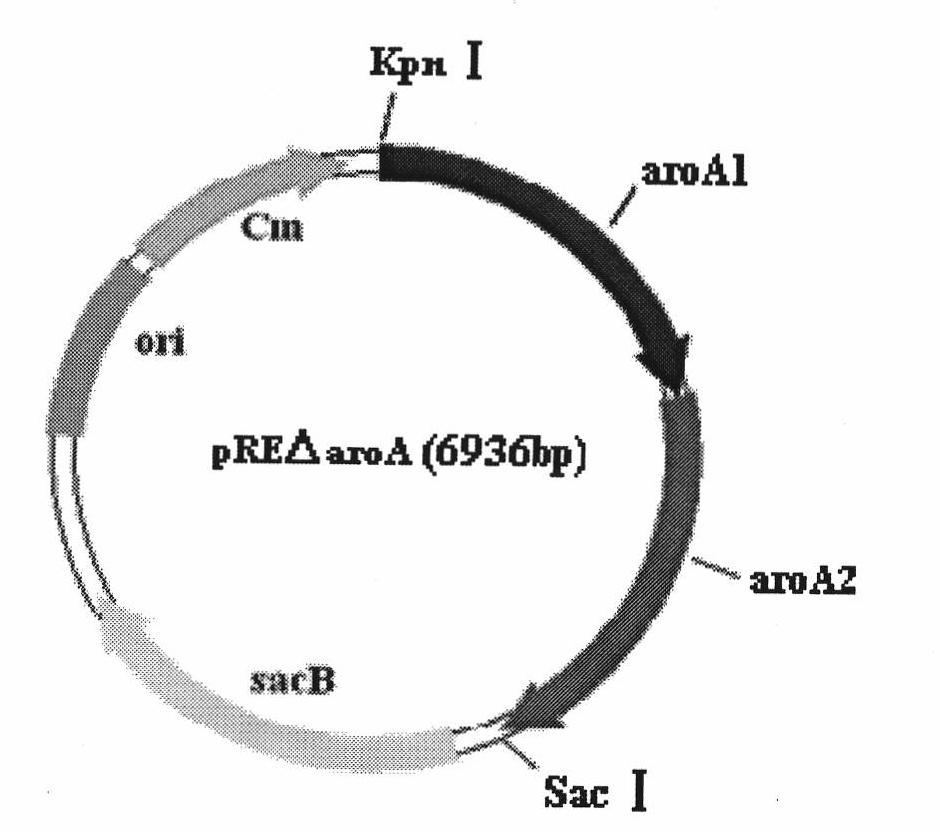
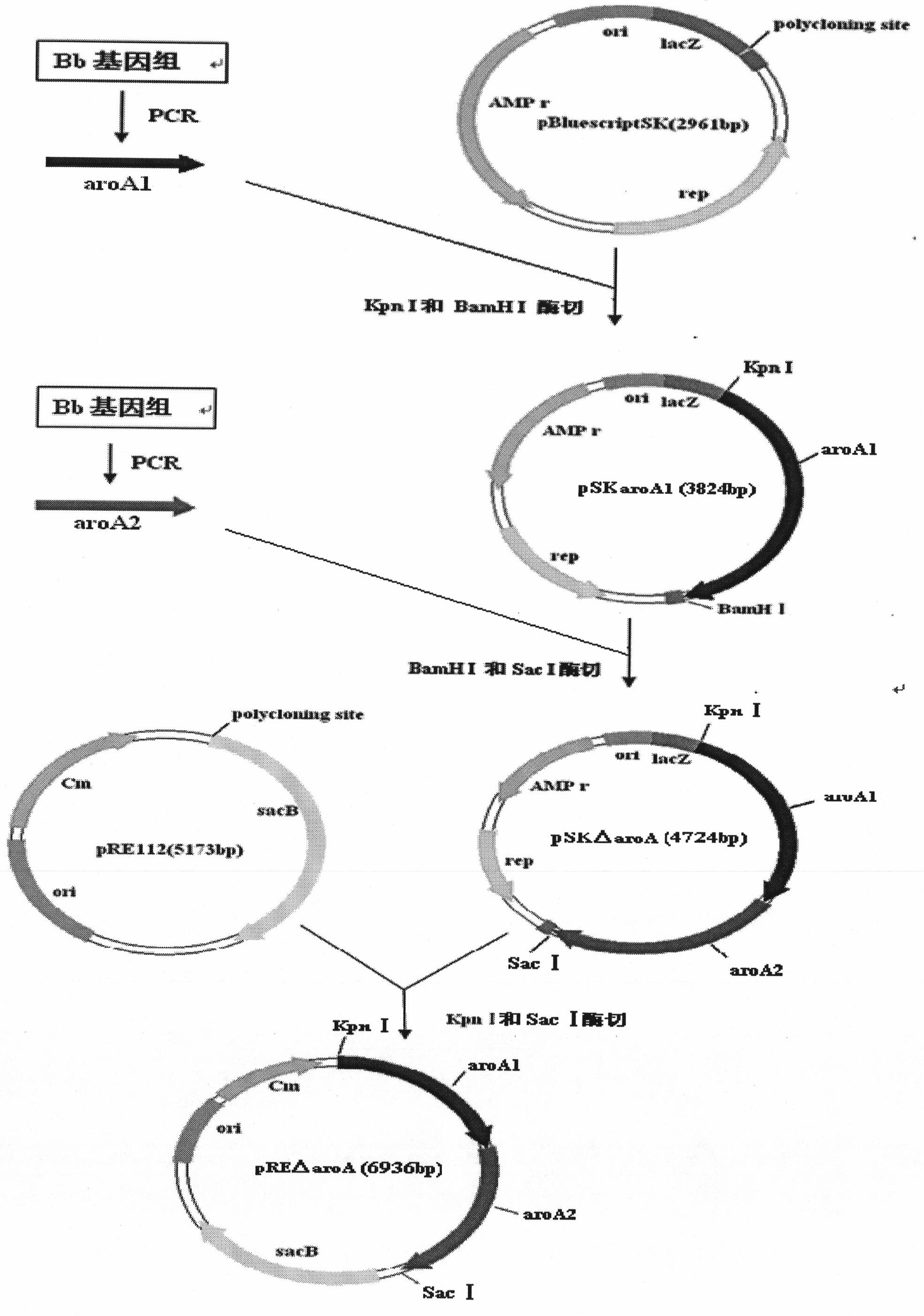
The invention belongs to the technical field of vaccine preparation of animal gene engineering and particularly relates to the construction of a Bordetella bronchiseptica aroA (5-enolpyruvyl-shikimate-3-phosphate synthase) gene deleted bacterial strain, and the preparation and application of a gene deleted vaccine. The Bordetella bronchiseptica gene deleted bacterial strain QH0814DeltaaroA is preserved in the China Center for Type Culture Collection with the collection number of CCTCCNO: M208018. The deletion of 5-enolpyruvyl-shikimate-3-phosphate synthase (aroA) gene with the full length of 1340bp causes an obstacle when the bacterial strain metabolizes aromatic amino acid. The invention prepares the Bordetella bronchiseptica gene deleted vaccine by using the gene deleted bacterial strain. The invention also discloses the application of the gene deleted bacterial strain in preparing the Bordetella bronchiseptica gene deleted vaccine (attenuated live vaccine).
Owner:HUAZHONG AGRI UNIV
Recombinant bordetella bronchiseptica strain, vaccine and use
ActiveCN103421728AGood immune protectionImmunization is easy to operateAntibacterial agentsBacterial antigen ingredientsBiotechnologyBordetella bronchiseptica
The invention belongs to the technical field of animal bacteriology and vaccine genetic engineering preparation, and relates to a resistance mark-free recombinant bordetella bronchiseptica strain for expression of a pig toxaigenic pasteurella multocida toxA gene fragment, and its vaccine containing the resistance mark-free recombinant bordetella bronchiseptica strain, a preparation method and a use. The resistance mark-free recombinant bordetella bronchiseptica strain QH0814 delta aroA / toxA-N for expression of the pig toxaigenic pasteurella multocida toxA gene fragment does not contain a 5-enolpyrul-shikimate-3-phosphate synthase aroA gene of bordetella bronchiseptica, and contains the pig toxaigenic pasteurella multocida toxA gene fragment. The invention also discloses the preparation method of the resistance mark-free recombinant bordetella bronchiseptica strain and the vaccine use. The genetic engineering vaccine can stimulate a pig to produce a protective immune response for resisting pig toxaigenic pasteurella multocida and a wild strain of pig bordetella bronchiseptica, and can effectively prevent the double infection caused by pig toxaigenic pasteurella multocida and pig bordetella bronchiseptica.
Owner:HUAZHONG AGRI UNIV
Canine combination vaccines
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira Bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona. The vaccines of the present invention include a Bordetella bronchiseptica p68 antigen.
Owner:ZOETIS SERVICE LLC
Bordetella bronchiseptica gene deletion strain, vaccine prepared from Bordetella bronchiseptica gene deletion strain and application
ActiveCN102676421AGood immune protectionWeak toxicityAntibacterial agentsBacterial antigen ingredientsBordetella bronchisepticaMicrobiology
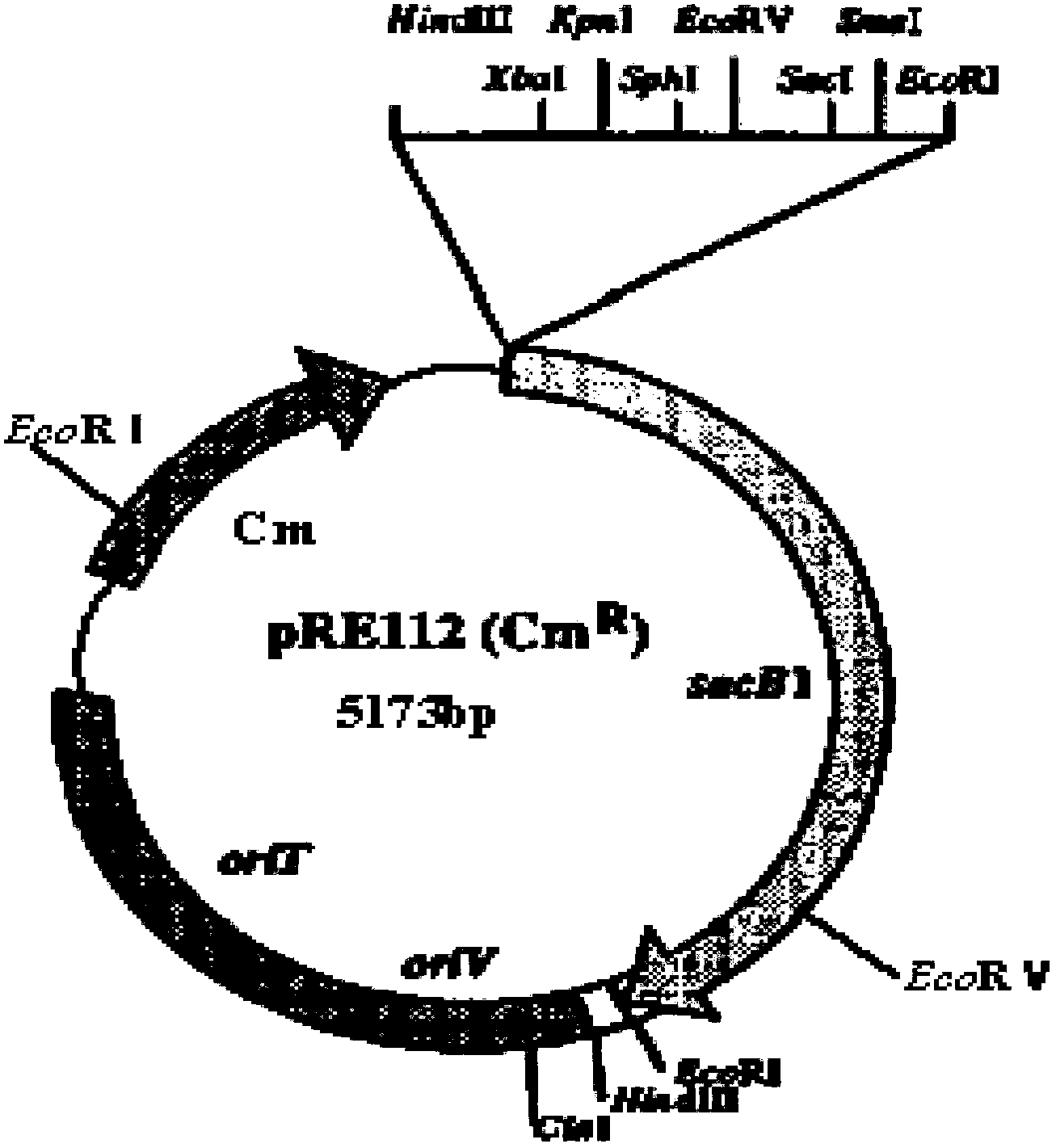
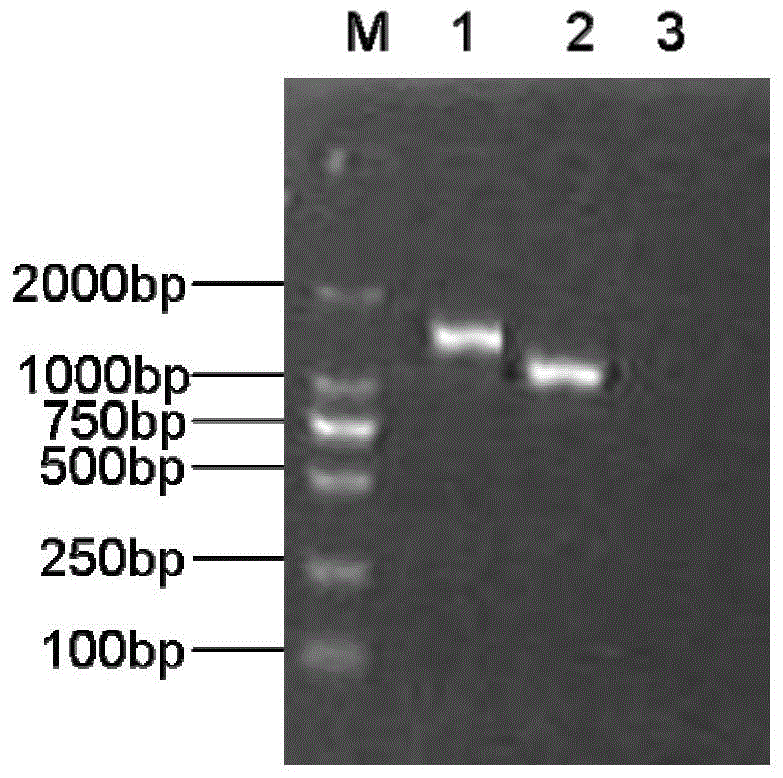
The invention relates to a Bordetella bronchiseptica gene deletion strain, vaccine prepared from the Bordetella bronchiseptica gene deletion strain and application of the strain. The aroA gene deletion strain, namely aroA-LY1 (Bordetella bronchiseptica), is preserved in the China Center for Type Culture Collection (CCTCC) on 23 December 2011, and the preservation number is CCTCCNO: M 2011479. The Bordetella bronchiseptica gene deletion strain disclosed by the invention has excellent immune protection efficacy and weak toxicity without any adverse side effect on immunized pigs and is safer compared with the traditional vaccine. Therefore, the vaccine which is prepared from the gene deletion strain built by a parent strain has wide market application prospect. In addition, the Bordetella bronchiseptica gene deletion strain disclosed by the invention retains the immune protective efficacy aiming at Bordetella bronchiseptica infection, and has clear genetic background and stable characteristics, so that the Bordetella bronchiseptica gene deletion strain has better biosecurity. The Bordetella bronchiseptica gene deletion strain disclosed by the invention does not contain a resistance tag and totally accords with the biological security requirement of vaccine.
Owner:HENAN UNIV OF SCI & TECH
Vaccine composition and its preparation method and use
ActiveCN105797152AImproving immunogenicityHigh expressionCarrier-bound antigen/hapten ingredientsRespiratory disorderBordetella bronchisepticaToxin protein
The invention provides a vaccine composition. The vaccine composition comprises the immune dose of Bordetella bronchiseptica pertactin (PRN) protein end N and end C, toxigenic Pasteurellamultocida Pasteurellamultocidatoxin (PMT) toxin protein end N and end C and pharmaceutically acceptable carriers. The vaccine composition has good immunogenicity and can effectively prevent and / or treat atrophic rhinitis of a pig. The invention provides a preparation method of the vaccine composition. Through gene engineering means, a lot of protein antigens are expressed. The vaccine composition has good safety and is conducive to large scale production.
Owner:PU LIKE BIO ENG
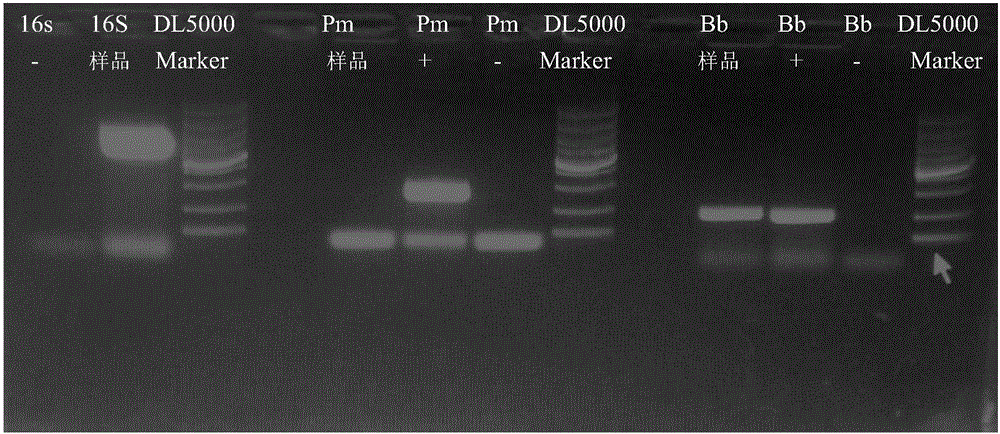
Primer group and kit for multiple PCR detection of rabbit Pasteurella multocida and rabbit Bordetella bronchiseptica and detection method thereof
ActiveCN101979661AEasy to operateStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationDNA SolutionsBacterial disease
The invention relates to the technical field of diagnosis of bacterial diseases of poultry, in particular to a primer group and a kit for multiple PCR detection of rabbit Pasteurella multocida and rabbit Bordetella bronchiseptica and a detection method thereof. The kit comprises 7 freezing tubes, wherein two freezing tubes are respectively filled with a 2*PCR core reagent premix, and the other five freezing tubes are respectively filled with rabbit Pasteurella multocida DNA solution, Bordetella bronchiseptica positive DNA solution, DL2000 molecular weight standard solution, ddH2O and a primermixture used by a PCR system. The detection method for the kit comprises the following steps of: 1, providing DNA of a sample to be detected; and 2, taking the kit, amplifying the DNA of the sample to be detected by adopting the conventional PCR method, detecting an amplification result by adopting an agarose gel electrophoresis method, and judging according to the result. The multiple PCR detection method established by the invention is specific, sensitive, simple, convenient and quick, can be used for quickly detecting clinical samples and investigating molecular epidemiology of the rabbit Pasteurella multocida and Bordetella bronchiseptica.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Triple inactivated vaccine for rabbit viral hemorrhagic disease, pasteurellosis and bordetella disease and preparation method of vaccine
ActiveCN110201153AProduced fastHigh immune attack protection rateAntibacterial agentsBacterial antigen ingredientsDiseaseBordetella
The invention relates to a triple inactivated vaccine for rabbit viral hemorrhagic disease, pasteurellosis and bordetella disease and a preparation method of the vaccine. The effective components of the vaccine comprise VP60 protein antigen of rabbit viral hemorrhagic disease virus, inactivated pasteurella multocida QLT-1 strain and bordetella bronchiseptica JN01 strain antigen. The triple inactivated vaccine has fast generation of protective antibody after immunization and high immune attack protection rate, with the attack protection rate for rabbit viral hemorrhagic disease virus AV-34 strain, pasteurella multocida QLT-1 strain and bordetella bronchiseptica JN01 strain all reaching above 80%. The results show that the vaccine is safe and reliable, and can be used for preventing the occurrence of rabbit viral hemorrhagic disease (rabbit plague), rabbit pasteurella multocida disease (type A) and rabbit bronchisepticaemia Bordetella disease.
Owner:QILU ANIMAL HEALTH PROD
Quintuple PCR (polymerase chain reaction) detection method capable of detecting multiple pathogens simultaneously
InactiveCN106520986AHigh skill requirementsQuick checkMicrobiological testing/measurementConserved sequenceStaphylococcus aureus
The invention discloses a quintuple PCR (polymerase chain reaction) detection method capable of detecting multiple pathogens simultaneously. According to the method, firstly, genes with conservative sequences of staphylococcus aureus, pseudomonas aeruginosa, pasteurella multocida, bordetella bronchiseptica and mycoplasma pulmonis are screened out and taken as targets for PCR detection, specific primers of corresponding conservative sequences are synthesized and amplified respectively, five pairs of primers are placed in one PCR system, and the quintuple PCR detection method capable of detecting five pathogens once from a sample directly is established through optimization of each item. Compared with a conventional PCR detection method, the detection method has the characteristics of rapidness, sensitivity, simplicity, convenience and accuracy, and the requirements for detection people and the detection cost are reduced.
Owner:HEBEI MEDICAL UNIVERSITY
High yield pertussis vaccine production strain and method for making same
InactiveUS20060154354A1Increase chanceOvercomes shortcomingBiocideBacterial antigen ingredientsHemagglutininAntibiotic resistance
The present invention provides a vaccine production strain of Bordetella bronchiseptica that produces a pertussis toxin in high yield. The present invention provides a method for creating a Bordetella bronchiseptica cell line which produces a Bordetella pertussis toxin comprising the steps of introducing a plasmid containing a DNA encoding antibiotic resistance into a Bordetella bronchiseptica strain, selecting for isolates in which the DNA encoding antibiotic resistance is recombinantly incorporated into the chromosome in place of the Bordetella bronchiseptica toxin gene, introducing a plasmid containing DNA encoding subunits of the Bordetella pertussis toxins into the Bordetella bronchiseptica isolates; and, selecting for isolates in which DNA encoding Bordetella pertussis toxin subunit is recombinantly incorporated into the chromosome, the resulting cells producing the Bordetella pertussis toxin. The present invention further provides a method for creating a Bordetella bronchiseptica cell line which produces a Bordetella pertussis toxin and does not express filamentous hemagglutinin.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Bordetella bronchiseptica gene deleted vaccine and application
InactiveCN101575586BGood antigenStrong targetingAntibacterial agentsBacterial antigen ingredientsMicrobiologyBordetella bronchiseptica
The invention belongs to the technical field of vaccine preparation of animal gene engineering and particularly relates to the construction of a Bordetella bronchiseptica aroA (5-enolpyruvyl-shikimate-3-phosphate synthase) gene deleted bacterial strain, and the preparation and application of a gene deleted vaccine. The Bordetella bronchiseptica gene deleted bacterial strain QH0814DeltaaroA is preserved in the China Center for Type Culture Collection with the collection number of CCTCCNO: M208018. The deletion of 5-enolpyruvyl-shikimate-3-phosphate synthase (aroA) gene with the full length of 1340bp causes an obstacle when the bacterial strain metabolizes aromatic amino acid. The invention prepares the Bordetella bronchiseptica gene deleted vaccine by using the gene deleted bacterial strain. The invention also discloses the application of the gene deleted bacterial strain in preparing the Bordetella bronchiseptica gene deleted vaccine (attenuated live vaccine).
Owner:HUAZHONG AGRI UNIV
Original ecology and high survival rate breeding method of Rex Rabbit
An original ecology and high survival rate breeding method of Rex Rabbit comprises the following steps: 1, selecting automatic feeding and defecating breeding devices; 2, selecting breeding rabbits: selecting a female rabbit with slim figure, regular features, no deformity, wide and deep chest, 8 nipples and lotus leaf-shaped genital, and selecting a male rabbit with heavy-boned physique, regular features, no deformity, large and round head, strong legs, and disease-free, pink and bended genitalia; 3, feeding baby rabbits in the suckling period in a breast milk and formula forage combination mode, allowing rabbits in the growing period to stay in a rabbit house, and allowing rabbits with the age of 3 months to grow under certain illumination, reinforced ventilation and fresh air conditions, wherein a forage in the growing period adopts a high-fiber and medium-protein forage, the temperature in the house for rabbits in the fattening period is properly reduced, and the form of the forage in the growing period is a particulate forage sold in the market; and 4, injecting an ivermectin vaccine to the breeding rabbits every March-April and August-September every year, injecting a Bordetella bronchiseptica and Pasteurella bigeminal vaccine 28-30d after the baby rabbits are born, and injecting a rabbit fever vaccine 45-50d after the babies are born.
Owner:金寨县万紫千红农业科技开发有限公司
Immunopotentiator for swine atrophic rhinitis inactivated vaccine and preparation method of immunopotentiator
ActiveCN107261134ASuppress latent infectionAvoid latent infectionAntibacterial agentsBacterial antigen ingredientsPig farmsVitamin C
The invention discloses an immunopotentiator for a swine atrophic rhinitis inactivated vaccine. The immunopotentiator comprises a liquid containing dry powder immunity particles, and a solvent, wherein the liquid containing the dry powder immunity particles mainly comprises an aluminium hydroxide aqueous solution, ginsenoside, cholesterol, a vitamin C, diethylaminoethyl glucan, potassium iodide and polyethylene glycol according to an appropriate ratio by compatibility. Compared with the prior art, the prepared immunopotentiator for the swine atrophic rhinitis inactivated vaccine can effectively inhibit establishment and reactivation of latent infection of bordetella bronchiseptica and pasteurella multocida, can shorten a window phase of antibody production, improves an immunity effect of the swine atrophic rhinitis vaccine, prolongs the immunity duration, effectively avoids latent infection of swine atrophic rhinitis, provides guarantee for prevention and elimination of the swine atrophic rhinitis, can be widely applied to a pig farm for prevention and treatment of the swine atrophic rhinitis, and increases an economic benefit of the pig farm.
Owner:SHANGHAI CHUANG HONG BIOTECH
Radix isatidis and folium isatidis granules with good curative effect and preparation method of radix isatidis and folium isatidis granules
InactiveCN107898876AHigh antibacterial activityGood curative effectAntibacterial agentsGranular deliveryEscherichia coliBetaine
The invention provides a banqing granule with good curative effect and a preparation method thereof. The antibacterial ability of the banqing granule to pathogenic strains such as Streptococcus suis and Escherichia coli is greatly improved. The present invention has added betaine, chitosan of small molecular weight, sodium tripolyphosphate in the process of decocting and extracting, and compared with the conventional technique recorded on the Chinese Pharmacopoeia, the Banqing granules obtained after these treatments are less harmful to livestock and poultry. The antibacterial activity of pathogens was significantly improved. In addition, we found that adjusting the order of adding betaine, chitosan and sodium tripolyphosphate, that is, adding sodium tripolyphosphate first, then adding chitosan, and finally adding betaine, can speed up the solubility of Banqing granules. It can be completely dissolved after stirring for 1 minute; at the same time, the antibacterial effect of Banqing granules on porcine bronchiseptica Bordetella and Escherichia coli can be further improved.
Owner:江西纵横生物科技有限公司
Wide host perforating plasmid carrier, construction method thereof and applications in bacterial ghost preparation
InactiveCN101182536AImprove cracking efficiencyBacteriaVector-based foreign material introductionDecompositionPlasmid Vector
The invention discloses a broad-host perforated plasmid vector p BBR-E and a construction method thereof; the invention also discloses the purpose of the broad-host perforated plasmid vector p BBR-E in the process of preparing the ghost of Bordetella bronchiseptica, which belongs to the gene engineering field. The broad-host perforated plasmid vector p BBR-E of the invention consists of a temperature sensitive controlling gene cI857, a phage bacteriolysis gene E and a broad-host plasmid p BBR4-MCS. The perforated plasmid vector p BBR-E of the invention is constructed based on the plasmid vector p BBR4-MCS which is provided with a broad host range and the vector can be duplicated in mostgram negativebacteria and express the bacteriolysis gene E with a high efficiency; the decomposition rate is high in the process of preparing the ghost of Bordetella bronchiseptica which can reach 99.9997 percent and is about 4 magnitudes high than the prior art.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Compositions for Canine Respiratory Disease Complex
ActiveUS20140079733A1Avoid infectionSsRNA viruses negative-senseSsRNA viruses positive-senseDiseaseSerotype
Provided herein are compositions comprising a canine influenza virus and a canine respiratory coronavirus. They can further comprise Bordetella bronchiseptica, pertactin, canine parainfluenza virus, and canine adenovirus serotype 2. The compositions are effective for treating or preventing canine respiratory diseases, including canine infectious respiratory disease complex.
Owner:ZOETIS SERVICE LLC
Canine vaccines
This invention relates to vaccines and methods for protecting dogs against disease caused by Bordetella bronchiseptica. This invention relates to vaccines and methods for protecting dogs against disease caused by Leptospira bratislava. This invention also relates to combination vaccines and methods for protecting dogs against disease or disorder caused by canine pathogens, for example, infectious tracheobronchitis caused by Bordetella bronchiseptica, canine distemper caused by canine distemper (CD) virus, infectious canine hepatitis (ICH) caused by canine adenovirus type 1 (CAV-1), respiratory disease caused by canine adenovirus type 2 (CAV-2), canine parainfluenza caused by canine parainfluenza (CPI) virus, enteritis caused by canine coronavirus (CCV) and canine parvovirus (CPV), and leptospirosis caused by Leptospira bratislava, Leptospira canicola, Leptospira grippotyphosa, Leptospira icterohaemorrhagiae or Leptospira pomona.
Owner:PFIZER INC +1
Tiamulin hydrogen famarate premixing agent and preparing method thereof
InactiveCN109771408ASolve the problem of bad smellSolve the problem of strong hygroscopicityAntibacterial agentsTetracycline active ingredientsTreatment effectMycoplasmal pneumonia
The invention provides a tiamulin hydrogen famarate premixing agent. The tiamulin hydrogen famarate premixing agent is prepared from, by mass, 75-85% of tiamulin hydrogen famarate and 15-25% of lacticacid. The invention further provides a preparing method of the tiamulin hydrogen famarate premixing agent. The tiamulin hydrogen famarate premixing agent can treat pig bacillary enteritis, bacterialpneumonia and treponema swine dysentery and has obvious therapy effects on mycoplasmal pneumonia and pneumonia which is caused by mixed infection of bordetella bronchiseptica and pasteurella multocida, during preparation, conventional equipment is adopted, the preparing method is simple, the substance cost is low, and the preparing method is suitable for industrial large-batch production of the livestock antibiotic premixing agent.
Owner:江西九信药业有限公司
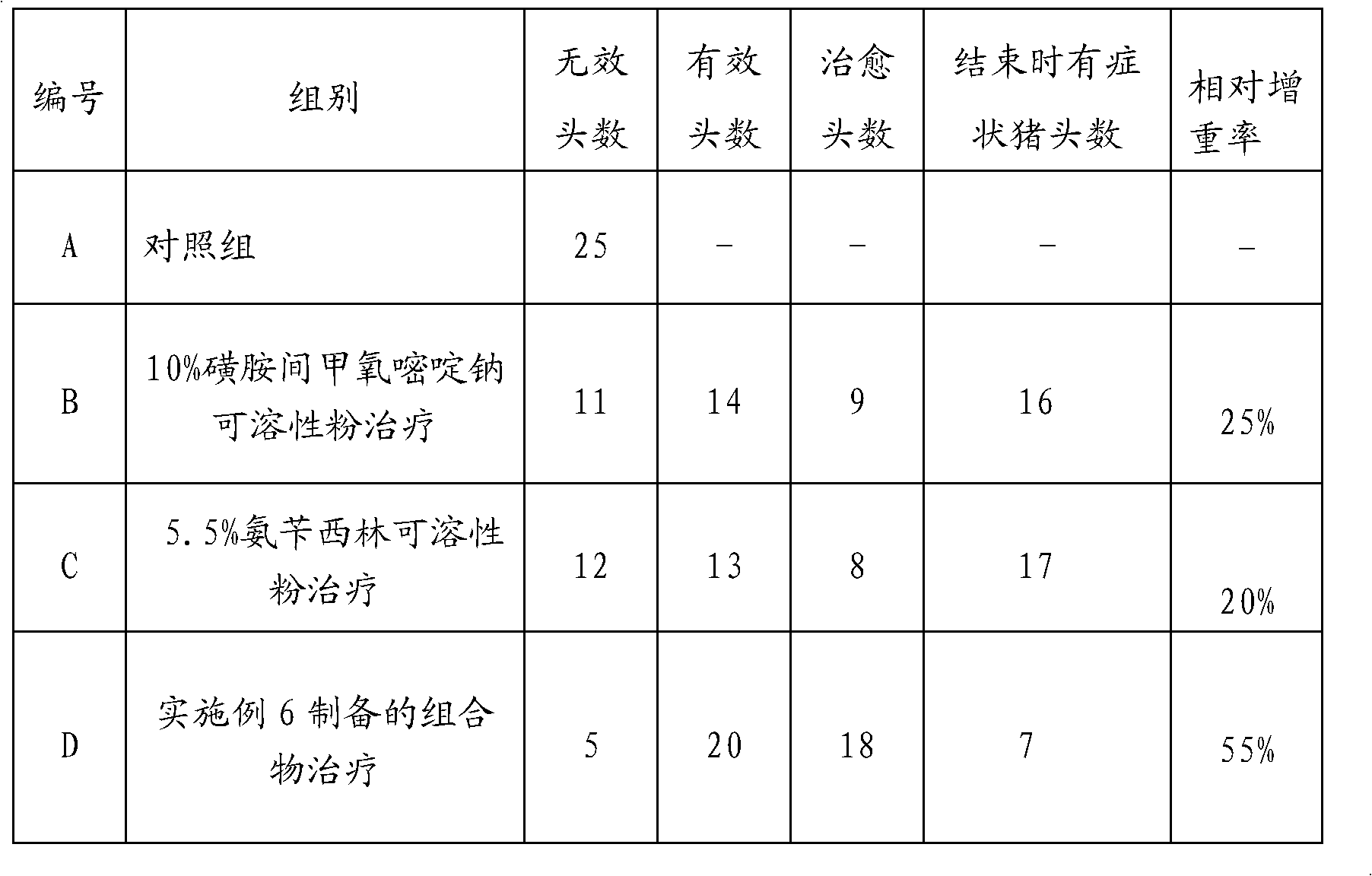
Composition for treating swine infectious atrophic rhinitis and preparation method thereof
InactiveCN101972266AImprove side effectsEliminate side effectsAntibacterial agentsInorganic non-active ingredientsSodium bicarbonateSide effect
The invention discloses a composition for treating swine infectious atrophic rhinitis and a preparation method thereof, and aims to provide the composition for treating the swine infectious atrophic rhinitis caused by infection of swine bordetella bronchiseptica and toxigenic pasteurella multocida, which reduces pathogenic medicament resistance and is convenient to use, and the preparation method thereof. The composition comprises the following components in percentage by weight: 5 to 15 percent of sulfamonomethoxine sodium, 1 to 3 percent of lactic acid trimethoprim (TMP1), 3 to 8 percent of ampicillin sodium, 5 percent of sodium carbonate, 5 to 15 percent of sodium bicarbonate and the balance of anhydrous dextrose. By adopting the sulfamonomethoxine sodium and the ampicillin sodium as anti-pathogenic components, the pathogenic microorganism can be effectively killed, the probability of medicament resistance can be reduced, the composition has an effect on secondary bacterial infection, the lactic acid TMP has obvious synergistic effect on the sulfamonomethoxine sodium and the ampicillin sodium, the sodium carbonate can contribute to dissolving the sulfamonomethoxine sodium and the ampicillin sodium, and the sodium bicarbonate relieves the side effect of the sulfamonomethoxine sodium and prevents crystalluria.
Owner:TIANJIN SHENGJI GRP CO LTD
Rabbit bordetella bronchiseptica subunit vaccine
ActiveCN106620685APrevention of Bordetella septicemiaAntibacterial agentsBacterial antigen ingredientsAdjuvantBordetella bronchiseptica
The invention provides a rabbit bordetella bronchiseptica subunit vaccine, particularly relates to rabbit bordetella bronchiseptica (QDBb01 strain with the preservation number of CCTCC M 2016607) identified through isolated culture, Bb gene specific primer PCR amplification and sequencing. Rabbit bordetella bronchiseptica OMP (osteoblast milk protein) is extracted, antigenic protein with the protein molecular weight larger than 40 kDa is purified, then the protein concentration is measured with a Bradford protein content measuring method and diluted to be 150 ug / ml, the subunit vaccine ( about 30 ug / ml for finished products) is prepared by emulsifying the protein and a aluminum hydroxide adjuvant in a volume ratio being 1:4, the vaccine is injected subcutaneously into a rabbit with the immunity weight being about 1.5 kg in the dosage of 1 ml for each rabbit, after 14 days, ear intravenous infusion challenge is performed with the rabbit bordetella bronchiseptica QDBb01 strain (LD50 being 4.8*105 CFU) according to 100 LD50, the rabbit is observed for 7 days after challenge, and the challenge protection rate of the rabbit is 10 / 10 through statistics.
Owner:YEBIO BIOENG OF QINGDAO
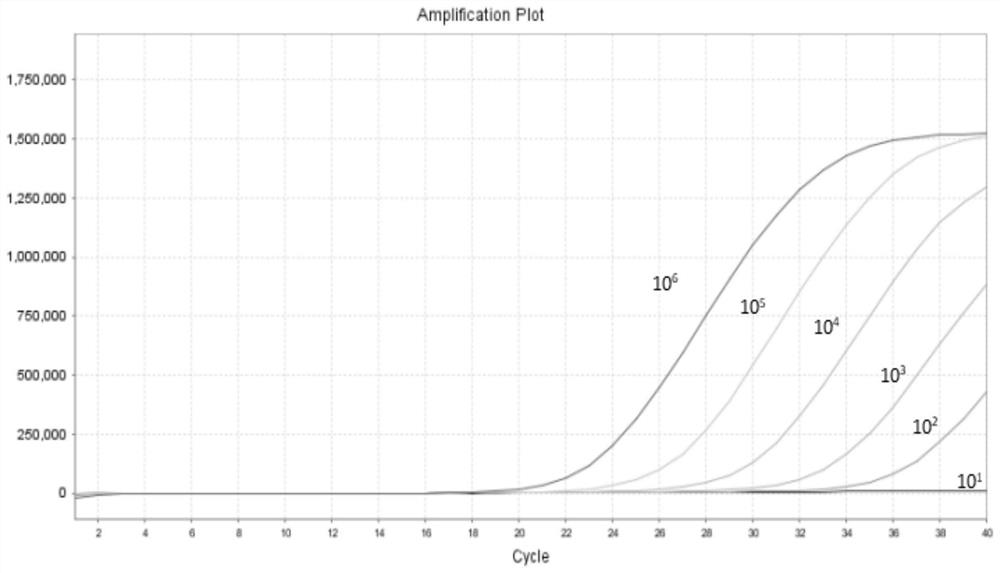
DNA fragment, primer, probe and kit for detecting four kinds of abalone bacteria and specifically detecting pertussis abalone bacteria and application
ActiveCN113718045ARealize typing detectionAccurate typingMicrobiological testing/measurementMicroorganism based processesBiotechnologyMicroorganism
The invention relates to the technical field of microbiological detection, and particularly relates to a DNA fragment, primer, probe and kit for detecting four kinds of abalone bacteria and specifically detecting pertussis abalone bacteria and application. The DNA fragment and specific primer for detecting the pertussis abalone bacteria, the abalone bacteria and the bordetella bronchiseptica can achieve sequencing and typing detection of the four kinds of abalone bacteria and have high specificity and accuracy. The invention further provides a primer probe set for specifically detecting the pertussis abalone bacteria. The pertussis abalone bacteria can be specifically detected through a multiple fluorescence PCR method, high sensitivity, specificity and repeatability are achieved, and an effective tool and method are provided for detecting the abalone bacteria.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD BEIJING BRANCH +1
Preparation of bordetella bronchiseptica selective medium
The invention relates to preparation of a bordetella bronchiseptica selective medium. The preparation comprises the following steps: taking 120g of NaCl, 20g of peptones, 18g of agar powder, 10g of glucose, 10g of lactose, 1.5g of No.3 cholate, 3ml of a 1% neutral red aqueous solution and 100ml of neonatal bovine serum; adding distilled water to a fixed volume of 1000ml; after dissolving and uniformly mixing, autoclaving for 15 minutes at 115 DEG C; cooling to 55 DEG C; adding a proper amount of a 1% furazolidone dimethylformamide solution, ampicillin, actidione and spectinomycin, taking 20ml of the solution, uniformly mixing and pouring into a plate with the diameter of 90mm; drying, collecting cotton swabs of naval cavities of pigs with atrophic rhinitis clinical symptoms; placing the cotton swabs into 2ml of a sterile PBS (phosphate buffer solution) solution; after fully and uniformly mixing, coating 50 microlitres on the selective medium; culturing for 48 hours at 37 DEG C; and picking up suspicious colonies and identifying, wherein the separation rate of the selective medium is remarkably higher than that of existing separating mediums.
Owner:TECON BIOLOGY CO LTD
Rabbit bordetella bronchiseptidca detection method
The present invention provides a rabbit bordetella bronchiseptidca suspension fluorescence immunoassay method including a rabbit bordetella bronchiseptidca suspension fluorescence immunoassay kit and a rabbit bordetella bronchiseptidca indirect suspension fluorescence immunoassay method. The kit includes components of a single factor serum antibody used for rabbit bordetella bronchiseptidca detection, FITC labeled goat anti mouse IgG, a positive control sample, a fixed solution and a PBS washing solution; and the single factor serum antibody used for rabbit bordetella bronchiseptidca detection is prepared by immunizing mice with outer membrane protein antigen of rabbit Bordetella bronchiseptica strain QDBb01. The rabbit bordetella bronchiseptidca suspension fluorescence immunoassay method based on the rabbit bordetella bronchiseptidca suspension fluorescence immunoassay kit can be used for clinical detection of antigens in rabbit bordetella bronchiseptidca infected sample tissue to determine whether the tissue is infected by rabbit bordetella bronchiseptidca, and the method has strong specificity, high sensitivity, good repeatability and fast speed of detection.
Owner:YEBIO BIOENG OF QINGDAO
High yield pertussis vaccine production strain and method for making same
InactiveUS7101558B2Increase chanceLess expensiveBiocideBacterial antigen ingredientsHemagglutininVaccine Production
The present invention provides a vaccine production strain of Bordetella bronchiseptica that produces a pertussis toxin in high yield. The present invention provides a method for creating a Bordetella bronchiseptica cell line which produces a Bordetella pertussis toxin comprising the steps of introducing a plasmid containing a DNA encoding antibiotic resistance into a Bordetella bronchiseptica strain, selecting for isolates in which the DNA encoding antibiotic resistance is recombinantly incorporated into the chromosome in place of the Bordetella bronchiseptica toxin gene, introducing a plasmid containing DNA encoding subunits of the Bordetella pertussis toxins into the Bordetella bronchiseptica isolates; and, selecting for isolates in which DNA encoding Bordetella pertussis toxin subunit is recombinantly incorporated into the chromosome, the resulting cells producing the Bordetella pertussis toxin. The present invention further provides a method for creating a Bordetella bronchiseptica cell line which produces a Bordetella pertussis toxin and does not express filamentous hemagglutinin.
Owner:UNITED STATES OF AMERICA
A detection method for Bordetella bronchiseptica in rabbits
The present invention provides a rabbit bordetella bronchiseptidca suspension fluorescence immunoassay method including a rabbit bordetella bronchiseptidca suspension fluorescence immunoassay kit and a rabbit bordetella bronchiseptidca indirect suspension fluorescence immunoassay method. The kit includes components of a single factor serum antibody used for rabbit bordetella bronchiseptidca detection, FITC labeled goat anti mouse IgG, a positive control sample, a fixed solution and a PBS washing solution; and the single factor serum antibody used for rabbit bordetella bronchiseptidca detection is prepared by immunizing mice with outer membrane protein antigen of rabbit Bordetella bronchiseptica strain QDBb01. The rabbit bordetella bronchiseptidca suspension fluorescence immunoassay method based on the rabbit bordetella bronchiseptidca suspension fluorescence immunoassay kit can be used for clinical detection of antigens in rabbit bordetella bronchiseptidca infected sample tissue to determine whether the tissue is infected by rabbit bordetella bronchiseptidca, and the method has strong specificity, high sensitivity, good repeatability and fast speed of detection.
Owner:YEBIO BIOENG OF QINGDAO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com