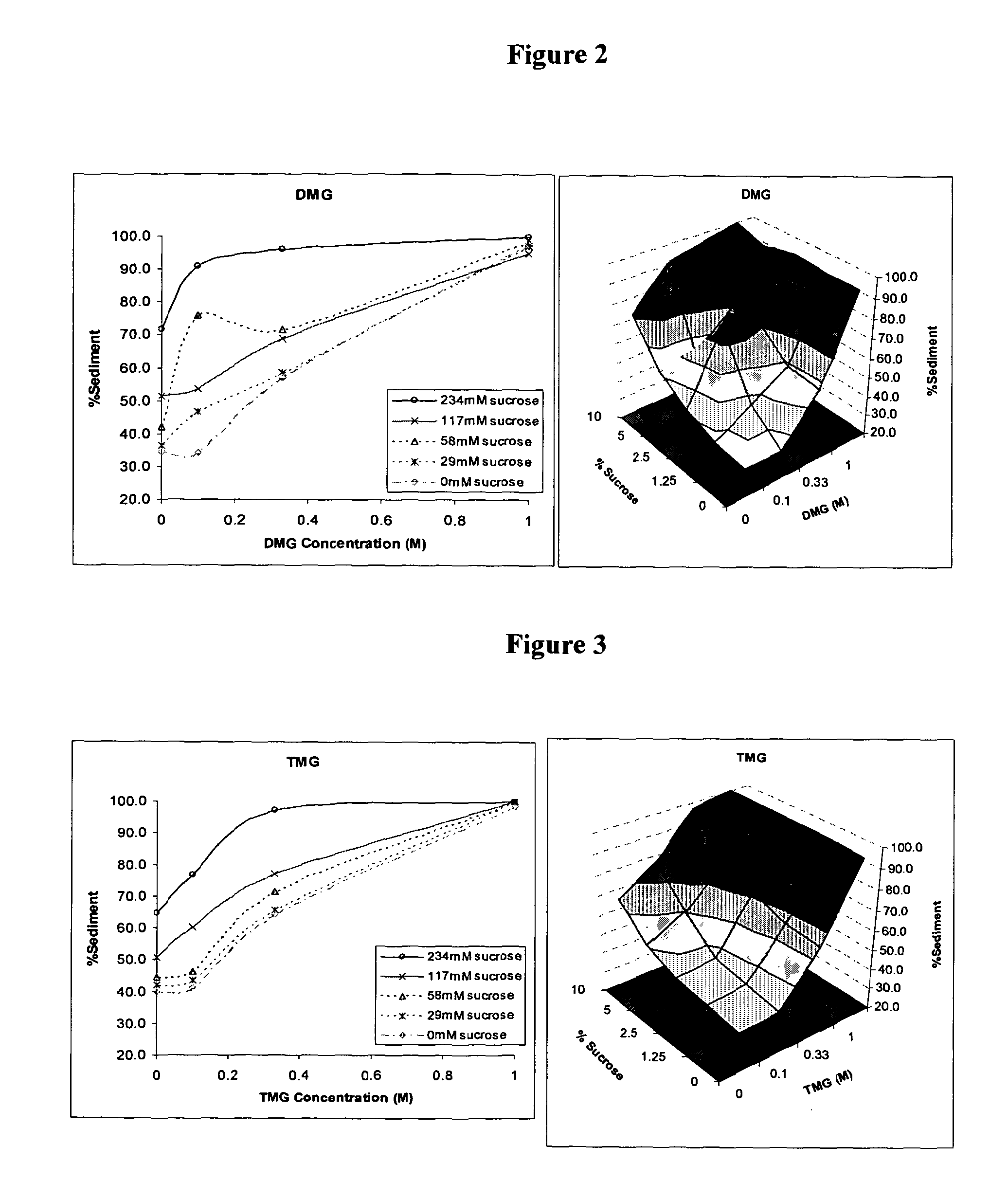
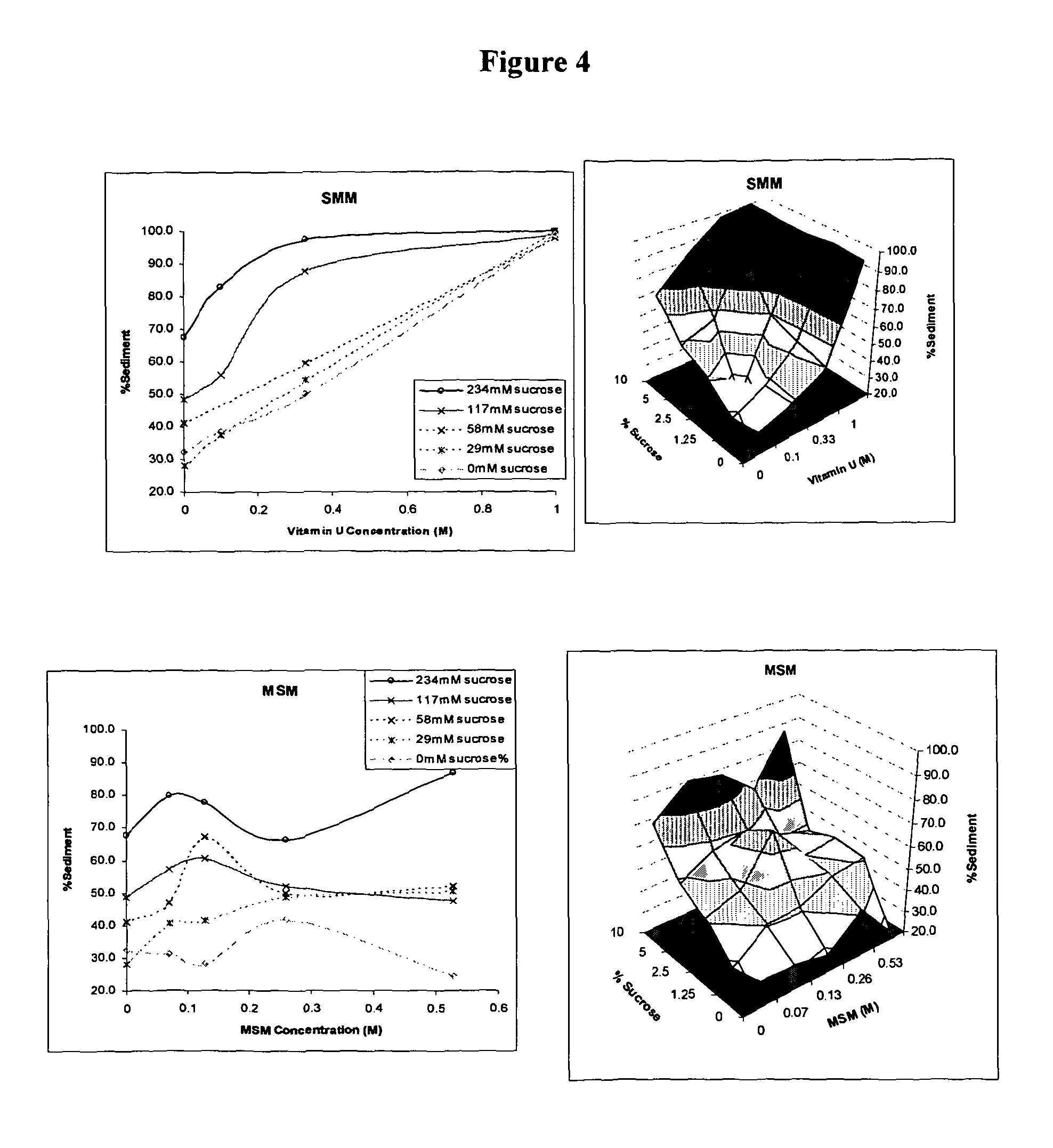
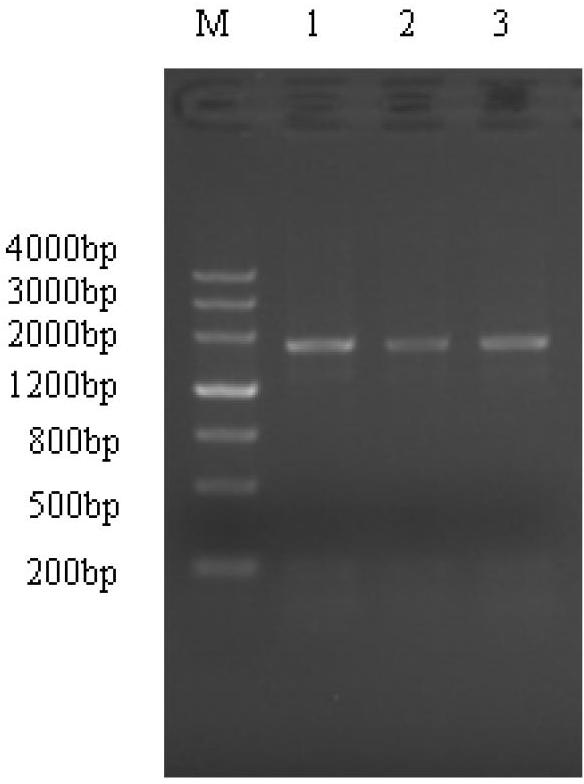
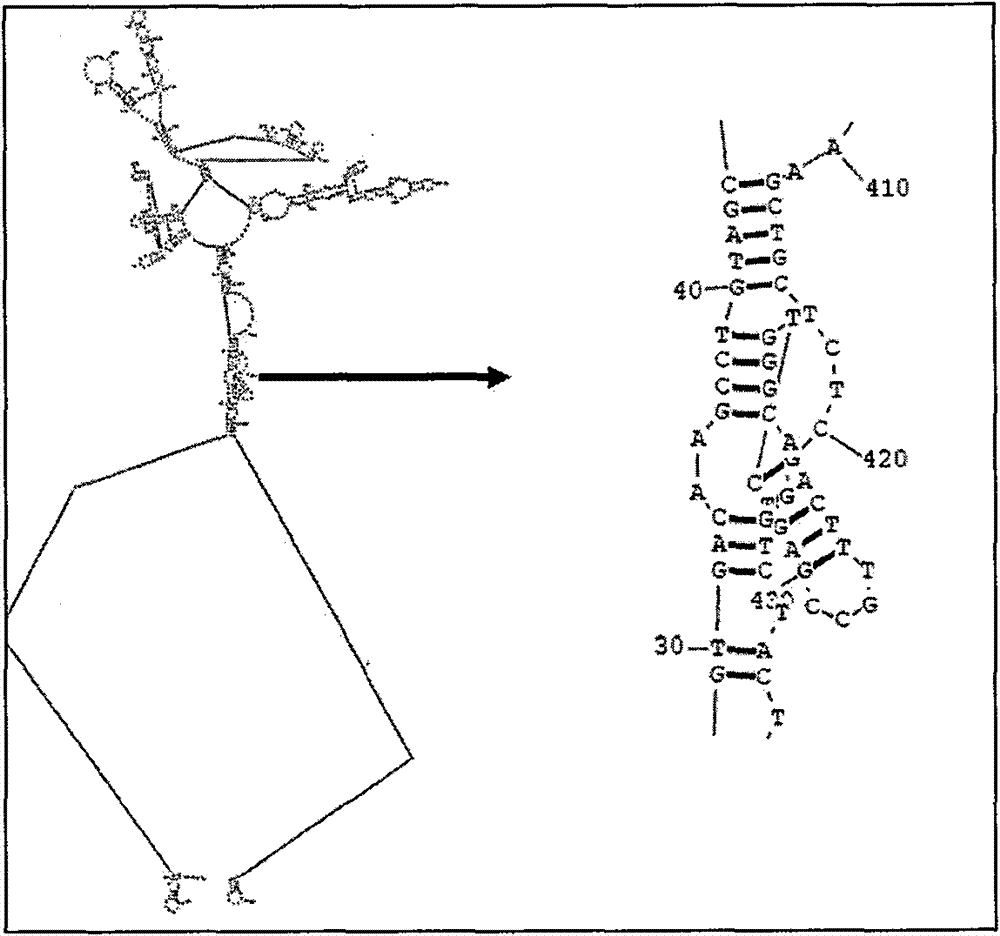
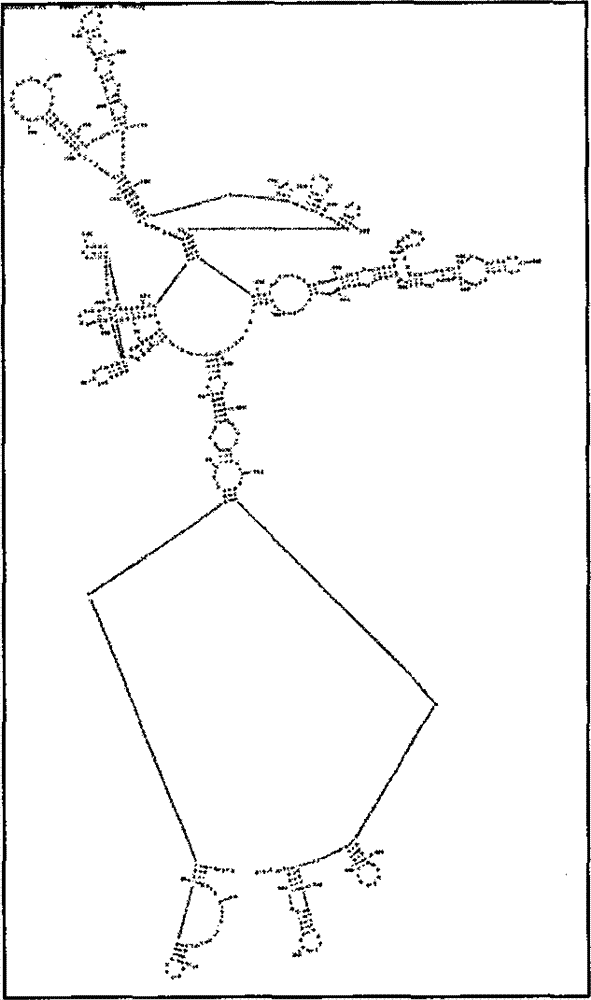
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
112results about How to "Maintain immunogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Small intestinal submucosa (SIS) soft tissue repair patch and preparation method thereof
InactiveCN102462561AImprove surface porosityImprove biological activityProsthesisTissue repairSoft tissue.FNA
The invention relates to a method for preparing a small intestinal submucosa (SIS) soft tissue repair patch, and the soft tissue repair patch prepared by the method. The invention also relates to application of the soft tissue repair path to tissue repair.
Owner:BEIJING MED ZENITH MEDICAL SCI CORP LTD
Live attenuated rotavirus vaccine for oral administration
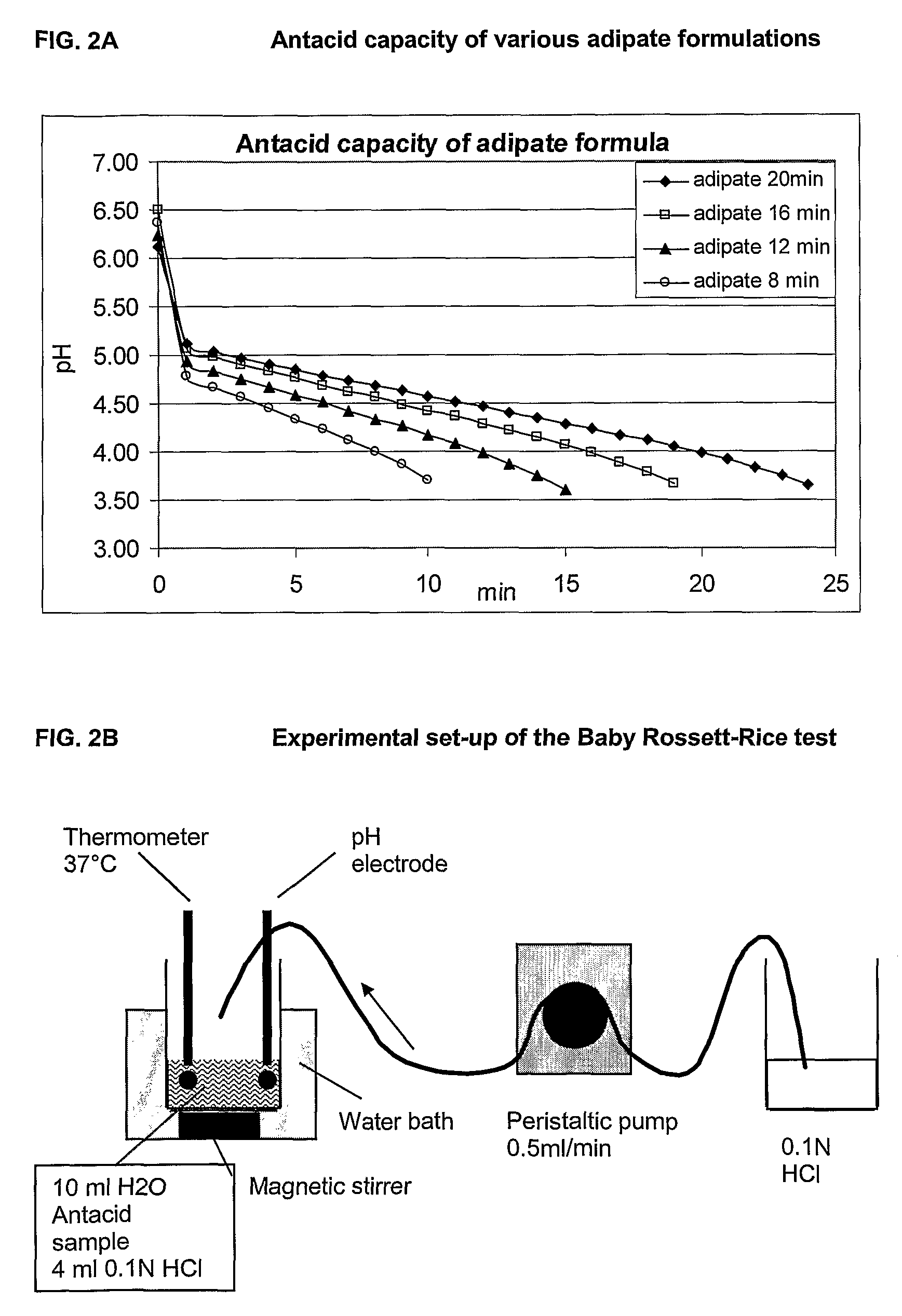
ActiveUS8192747B2Maintain immunogenicityStable over a long shelf-lifeBacterial antigen ingredientsViral antigen ingredientsDiseaseOral medication
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
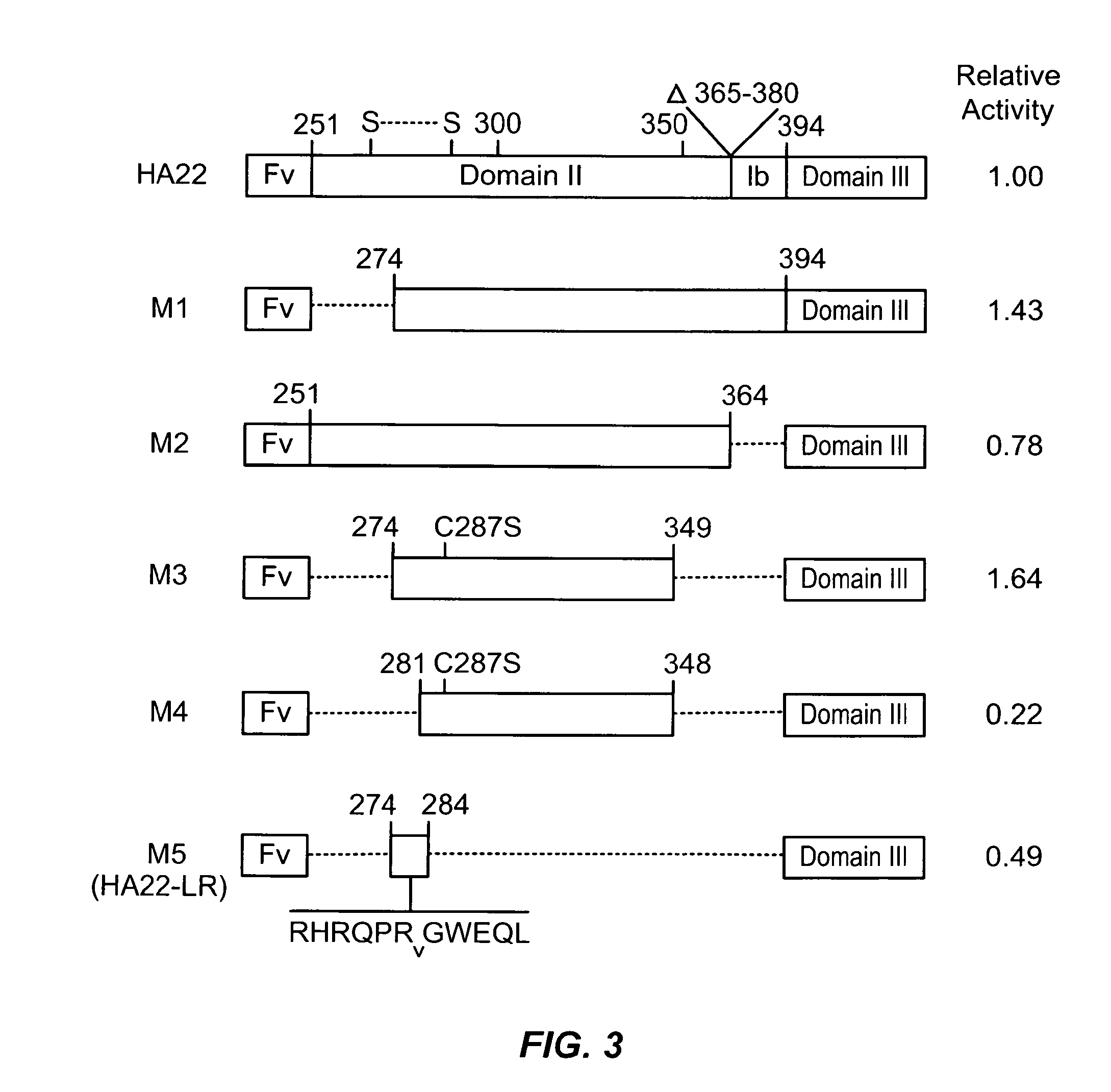
Deletions in domain ii of pseudomonas exotoxin a that remove immunogenic epitopes
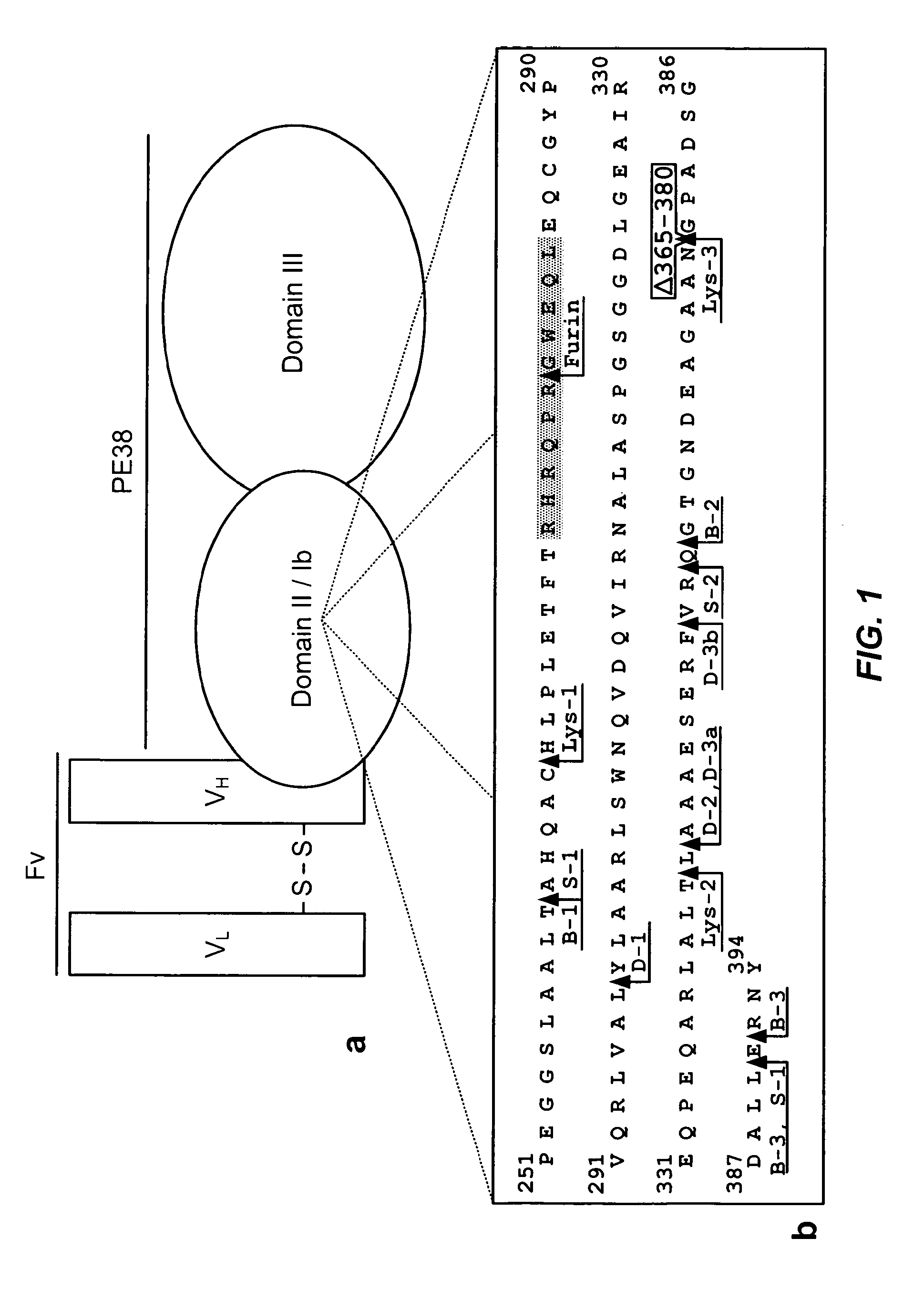
ActiveUS20100215656A1Maintain immunogenicitySugar derivativesPeptide/protein ingredientsPseudomonas aeruginosa exotoxin AEpitope
The invention provides mutated, cytotoxic forms of Pseudomonas exotoxin A (PE) comprising a furin cleavage sequence conjugated or fused directly to residues 395-613 of PE or variants of that sequence. These minimal forms of PE are smaller than previous cytotoxic forms of PE, reduce non-specific toxicity, and reduce immunogenicity due to domain II or domain Ib of PE. The invention further provides nucleic acids encoding said PEs, chimeric molecules containing them, and methods of use thereof.
Owner:UNITED STATES OF AMERICA
Mink distemper-canine parvovirus enteritis bivalent vaccine and its preparation method and use
InactiveCN105031639AGuaranteed loss of activityMaintain immunogenicityAntiviralsAntibody medical ingredientsCanine parvovirusProtective Agents
The invention discloses a mink distemper-canine parvovirus enteritis bivalent vaccine and its preparation method and use and belongs to the technical field of bioengineering. The mink distemper-canine parvovirus enteritis bivalent vaccine mainly comprises a mink distemper live vaccine, a mink canine parvovirus enteritis inactivated vaccine, a vaccine adjuvant and a protective agent. Aiming at one object, a ratio of the mink distemper live vaccine to the mink canine parvovirus enteritis inactivated vaccine is 1: 1. The invention also provides a preparation method and a use method of the mink distemper-canine parvovirus enteritis bivalent vaccine. The mink distemper-canine parvovirus enteritis bivalent vaccine is safe, has high efficiency, can save labor and can reduce a culture cost.
Owner:JL TEYAN BIOLOGICAL TECH LIMITED LIABILITY
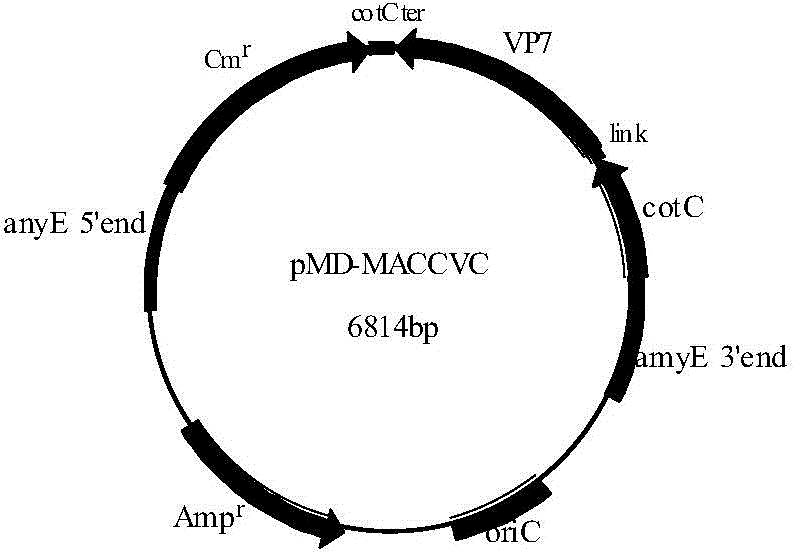
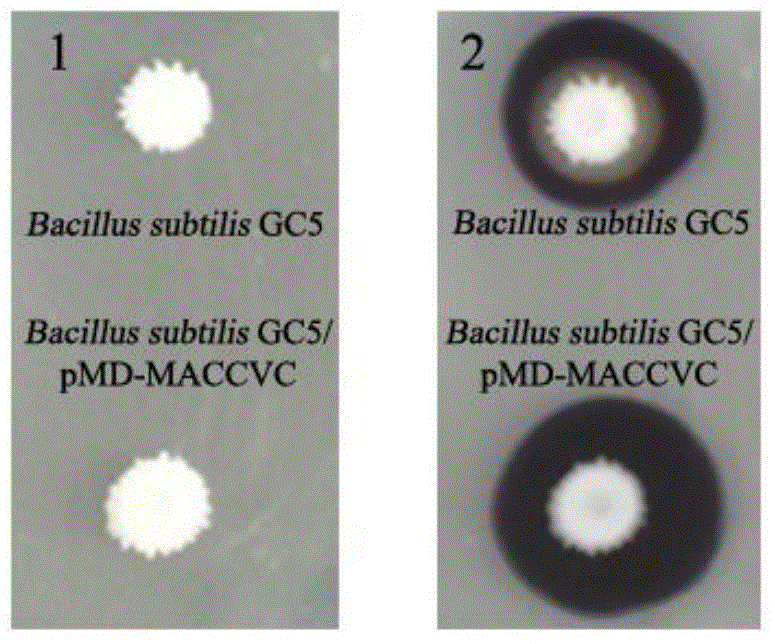
Recombinant bacillus displaying GCRV VP7 proteins on surface of bacillus subtilis GC5 and preparation method
ActiveCN104560860AAvoid immunogenicityAvoid security issuesBacteriaVirus peptidesBiotechnologyAntigen
The invention discloses a recombinant bacillus displaying GCRV VP7 proteins on the surface of bacillus subtilis GC5 and a preparation method. The preparation method comprises the following steps: (1) obtaining a present epidemic strain type-II GCRV VP7 nucleotide sequence; (2) separating wide bacillus from the body of a grass carp, determining the wide bacillus to be bacillus subtilis, and naming the wide bacillus as Bacillus subtilis GC5, CCTCC NO: M2014654; (3) constructing a fusion expression recombinant integrated carrier, wherein a vp7 sequence in recombinant plasmids is the nucleotide sequence shown in SEQ ID No. 1; (4) preparing and authenticating recombinant bacillus subtilis, CCTCC NO: M2014655; (5) inducing and authenticating the recombinant bacillus displaying VP7 on the surface. The generation rate of spores is up to 100%, and the recombinant bacillus is simple and convenient to produce, low in cost, and capable of being used as a feed additive; the recombinant bacillus has an intestinal customization capacity, as well as is beneficial to regulating the intestinal bacterial colony balances of animals, improving the body immunocompetence of the animals, and enhancing the nutrition metabolism functions of the animals. The spores are high in stress resistance and easy for large-scale production, as well as solve the problem of instability of GCRV VP7 proteins used as immunizing antigens in extreme environments.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979AMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticL929 cellEscherichia coli
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Acinetobacter baumannii zinc dependent oligopeptide A1S-1610 recombinant protein and preparation method and application thereof
ActiveCN104861050AHigh expressionQuality and safety controllableAntibacterial agentsBacterial antigen ingredientsProtective antigenGenetic engineering
The invention relates to an acinetobacter baumannii A1S-1610 protein, a carrier, engineering bacteria, a composition or a kit which comprises the acinetobacter baumannii 1 A1S-1610 protein, and a preparation method and application of the acinetobacter baumannii 1 A1S-1610 protein, the acinetobacter baumannii 1 A1S-1610 protein is never used in the field of recombinant subunit vaccines, the acinetobacter baumannii 1 A1S-1610 protein can effectively stimulate the body to cause a protective immune response so as to resist acinetobacter baumannii lethal infection. The present invention also discloses a method for preparation of the recombinant protein by construction of an expression vector of the recombinant protein, and transformation of host bacteria, and use of the recombinant protein in the preparation of an acinetobacter baumannii resistant subunit vaccine and a related detection kit. The protective antigen composition A1S_1610 protein is expressed by use genetic engineering technology to clone, and the A1S_1610 protein is high in expression amount, easy to separate and purify, efficient and safe, and the genetic engineering recombinant subunit vaccine has good acinetobacter baumannii infection resistant immune protection effect.
Owner:ARMY MEDICAL UNIV
Preparation method of triple inactivated vaccine for pigs
InactiveCN104998256AImprove immunityHighlight immune functionAntibacterial agentsBacterial antigen ingredientsAntigenProtective antigen
The invention provides a preparation method of a triple inactivated vaccine for pigs. The method determines an antigen composition with excellent immunization effects by selection of the antigen. The prepared polyvalent vaccine has outstanding immunization effects. The prepared vaccine contains a PCP immunization protective antigen exotoxin (Aps), has cross immunization protection effects better than those of a whole cell inactivated vaccine, greatly reduces side reaction, and can simultaneously prevent haemophilus parasuis, swine streptococcosis and actinobacillus pleuropneumonia by combined immunization with inactivated haemophilus parasuis and streptococcus suis. Compared with haemophilus parasuis and streptococcus suis inactivated vaccines sold on the market, the triple inactivated vaccine has the same corresponding pathogen immune protection force. Compared with the actinobacillus pleuropneumonia inactivated vaccine sold on the market, the triple inactivated vaccine has a cross immunization protecting force on diseased pigs with different serotypes and realizes multiple protection purposes.
Owner:TIANJIN RINGPU BIO TECH
Subunit vaccine for preventing novel coronavirus infection based on bur protein S1 region of novel coronavirus
PendingCN112043825AAvoid ADE RiskADE low riskSsRNA viruses positive-senseViral antigen ingredientsViral antigensPost immunization
The invention provides a subunit vaccine for preventing novel coronavirus infection based on a bur protein S1 region of novel coronavirus and belongs to the field of vaccines. The subunit vaccine comprises a bur protein S1 region antigen of the novel coronavirus and an adjuvant and is used for preventing the novel coronavirus through subcutaneous or intramuscular injection for two or three times.According to the subunit vaccine provided by the invention, potential ADE risk caused by taking a full-length S protein as a novel coronavirus vaccine can be avoided, immunogenicity of RBD can also bereserved without introducing other viral antigens, and an antibody generated after immunization is sufficient to neutralize the novel coronavirus.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Bovini Asia 1/O type foot-and-mouth disease bivalent multi-epitope vaccine and preparation method and application thereof
ActiveCN103897065ARealize prevention and controlOvercome limitationsGenetic material ingredientsAntiviralsIgG.heavy chainImmunogenicity
The invention discloses a bovini Asia 1 / O type foot-and-mouth disease bivalent multi-epitope vaccine and a preparation method and an application thereof and belongs to the field of veterinary biological vaccines. The bovini Asia 1 / O type foot and mouth disease virus compound multi-epitope recombinant antigen is obtained by coupling dominant epitope of epidemic bovini O and Asia 1 type foot-and-mouth disease virus representative strains connected in series in China with a bovini IgG heavy chain constant region. Immune efficacy experiments verify that the bovini Asia 1 / O type foot-and-mouth disease bivalent multi-epitope vaccine prepared by the invention has good immunogenicity, and high level protective antibodies can be generated by the body induced by immune guinea pig or immune bovine. The inoculated vaccine is safe and harmless to immune animals. The vaccine is a novel vaccine which is abroad in prospect, supports matter storage and technical support to prevention and control of bovini Asia 1 / O type foot-and-mouth disease in China, and is of great importance.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
African swine fever virus recombinant protein containing intramolecular adjuvant, expression vector and application
ActiveCN113150171AFunction has no effectMaintain immunogenicityAntibody mimetics/scaffoldsVirus peptidesDendritic cellAdjuvant
The invention relates to an African swine fever virus recombinant protein containing an intramolecular adjuvant, an expression vector and application, and belongs to the field of biotechnology pharmacy. The recombinant protein is formed by fusing African swine fever virus p30 and mp54 (modified p54) and an outer membrane protein I of pseudomonas aeruginosa. The recombinant protein has a general formula of OprI-(Linker)n-p30- (Linker)n-mp54, wherein a Linker sequence of a connecting peptide is selected from GGGGS or GGSGG, and is preferably GGGGS; and n is 1, 2, 3 or 4, and is preferably 3. Experiments prove that the recombinant protein can significantly promote maturation and differentiation of dendritic cells and secretion of cytokines. A vaccine immune animal prepared from the recombinant protein can stimulate an organism to generate a high-level neutralizing antibody and a cellular immune response, and can be used for preparation of an African swine fever virus diagnostic reagent and a preventive or therapeutic drug.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Respiratory syncytial virus F expressing protein and preparation method thereof
InactiveCN109694400AIntegrity guaranteedMaintain immunogenicitySsRNA viruses negative-senseVirus peptidesSequence designF protein
The invention provides a respiratory syncytial virus F expressing protein and a preparation method thereof, wherein the F protein has the amino acid sequence of SEQ ID NO:1. Amino acid point mutationis carried out at a Furin recognition site of the soluble F protein; at the same time, a GCN4 fragment is added at a C end to prepare the F protein; through the sequence design, the F protein expressed in an eukaryotic system is guaranteed to avoid being enzymatically hydrolyzed by Furin so as to maintain the integrity of an extracellular fragment and the Pre-fusion state and complete immunogenicity of the F protein are maintained. The F protein is necessary to induce a body to produce antibodies with immunoprotective effect against RSV, and effectively improves the probability to screen positive antibodies in the later stage.
Owner:苏州高泓利康生物科技有限公司
Clostridium perfringens Beta toxin recombination subunit vaccine and production method thereof
ActiveCN109078178AEfficient expressionEfficient soluble expressionAntibacterial agentsBacterial antigen ingredientsClostridium perfringens beta toxinVaccine Production
The invention relates to a clostridium perfringens Beta toxin recombination subunit vaccine and a production method thereof. The prepared clostridium perfringens Beta toxin recombination subunit vaccine is produced by using a recombination clostridium perfringens Beta toxin protein which is processed by codon optimization and contains 4 amino acid mutations, namely integrity and spatial conformation of a natural toxin protein are reserved in the greatest degree, so immunogenicity of the natural toxin protein is kept, and a biological potential safety hazard caused by the single amino acid mutation is avoided. The vaccine further has the advantages of simple preparation process, low immunizing dose, good vaccine efficacy and the like. Compared with a current commercial clostridium perfringens natural toxin inactivated vaccine in China, a bio-safety risk in a vaccine production process is greatly reduced. The vaccine is a perfect candidate vaccine for updating a current C-type clostridium perfringens toxin vaccine in China. In addition, while a mixed vaccine is prepared by the vaccine with other antigens, the mixed vaccine can be prepared without increasing a using dose of the mixedvaccine.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Desorption agent for desorbing antigen in aluminum salt adsorption type vaccine and antigen content detection method
ActiveCN111308071AFacilitate desorptionStructure is not affectedMaterial analysis by electric/magnetic meansActive agentSurface-active agents
The invention discloses a desorption agent for desorbing an antigen in an aluminum salt adsorption type vaccine and an antigen content detection method. The desorption agent is a sodium citrate solution and comprises a nonionic surfactant which stabilizes protein conformation and increases detection specificity. The desorption agent for desorbing an antigen in an aluminum salt adsorption type vaccine can well desorb, meanwhile, a virus antigen structure is not affected or almost not affected, and the immunogenicity of the virus antigen structure is completely kept.
Owner:长春生物制品研究所有限责任公司
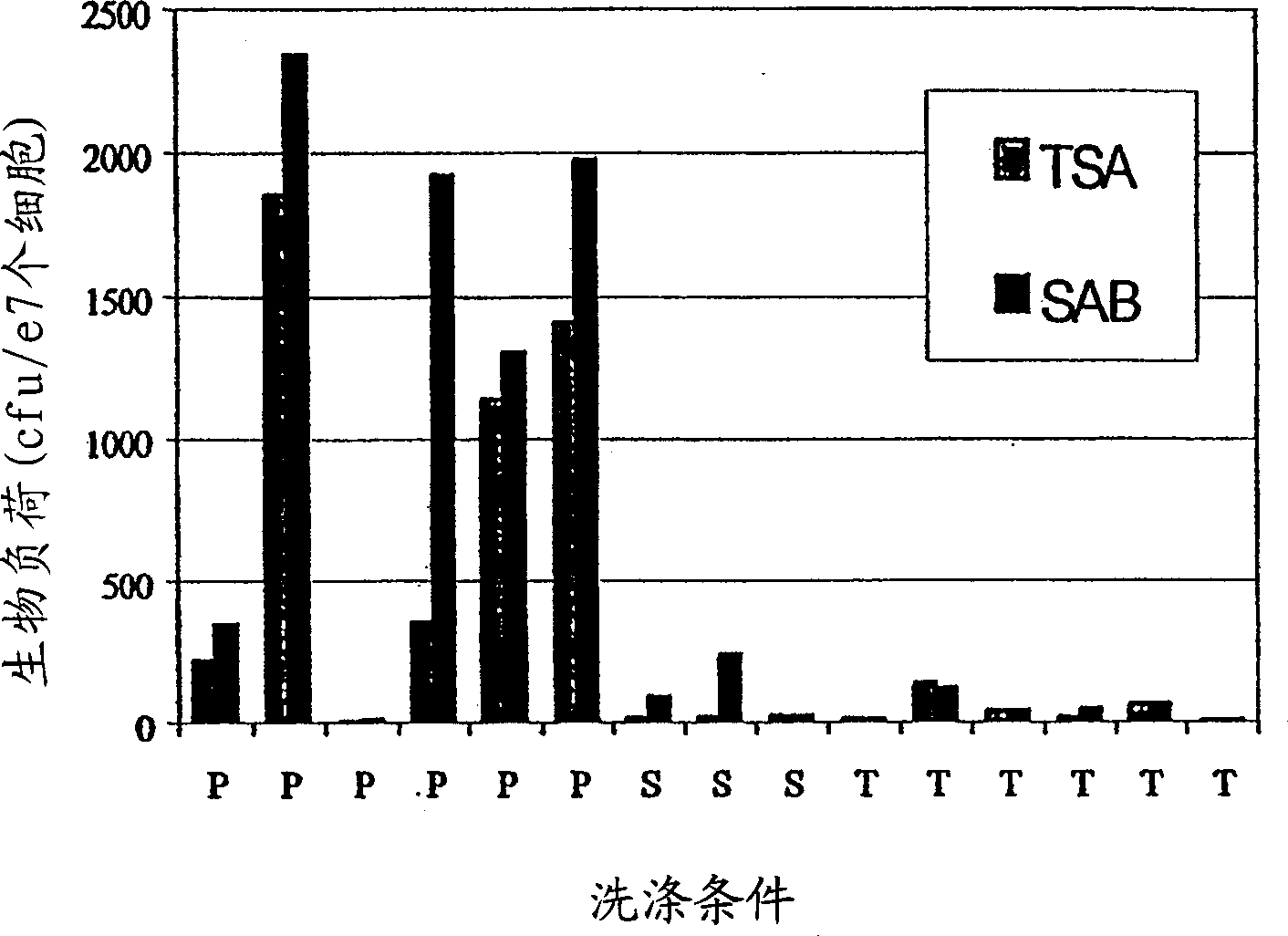
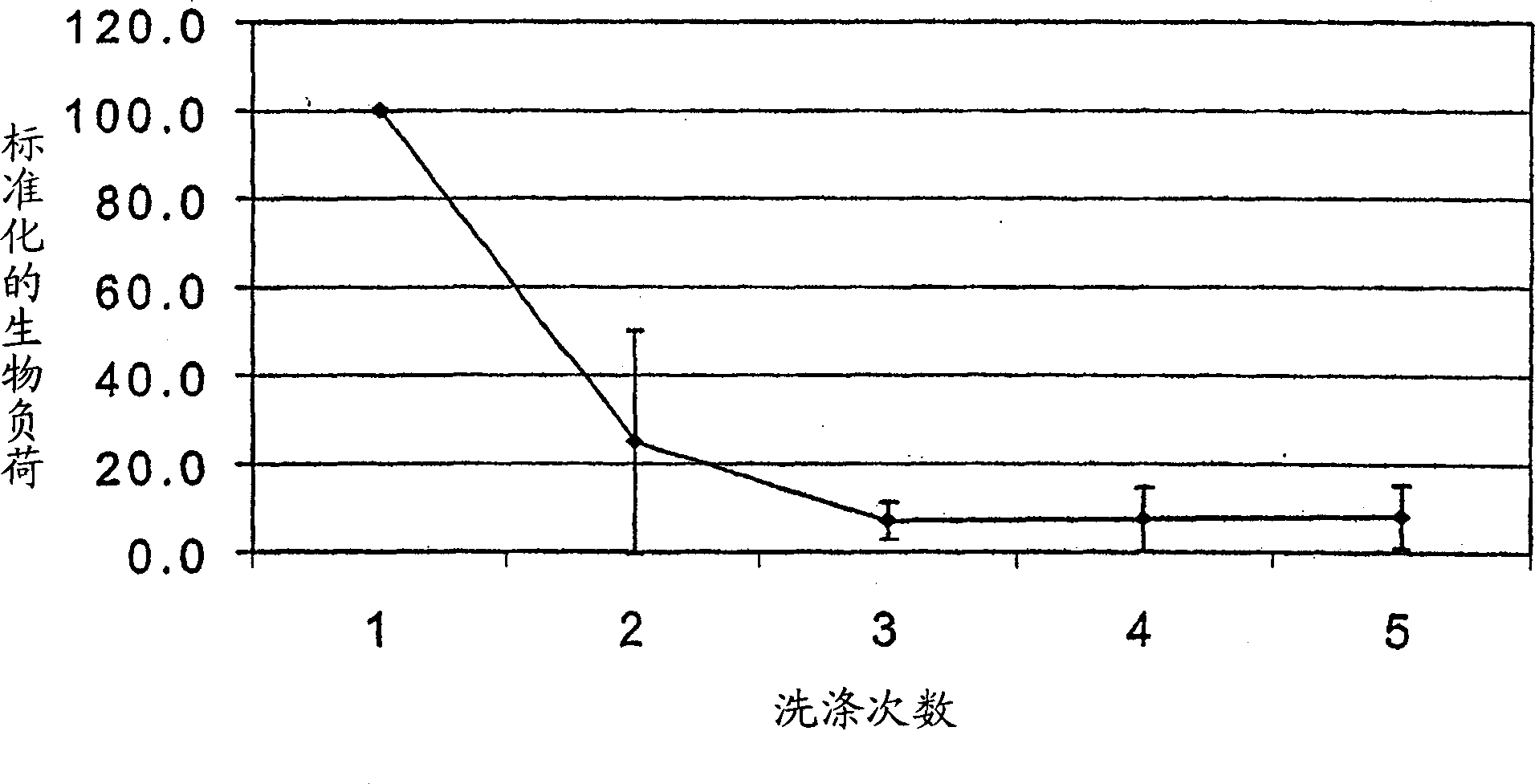
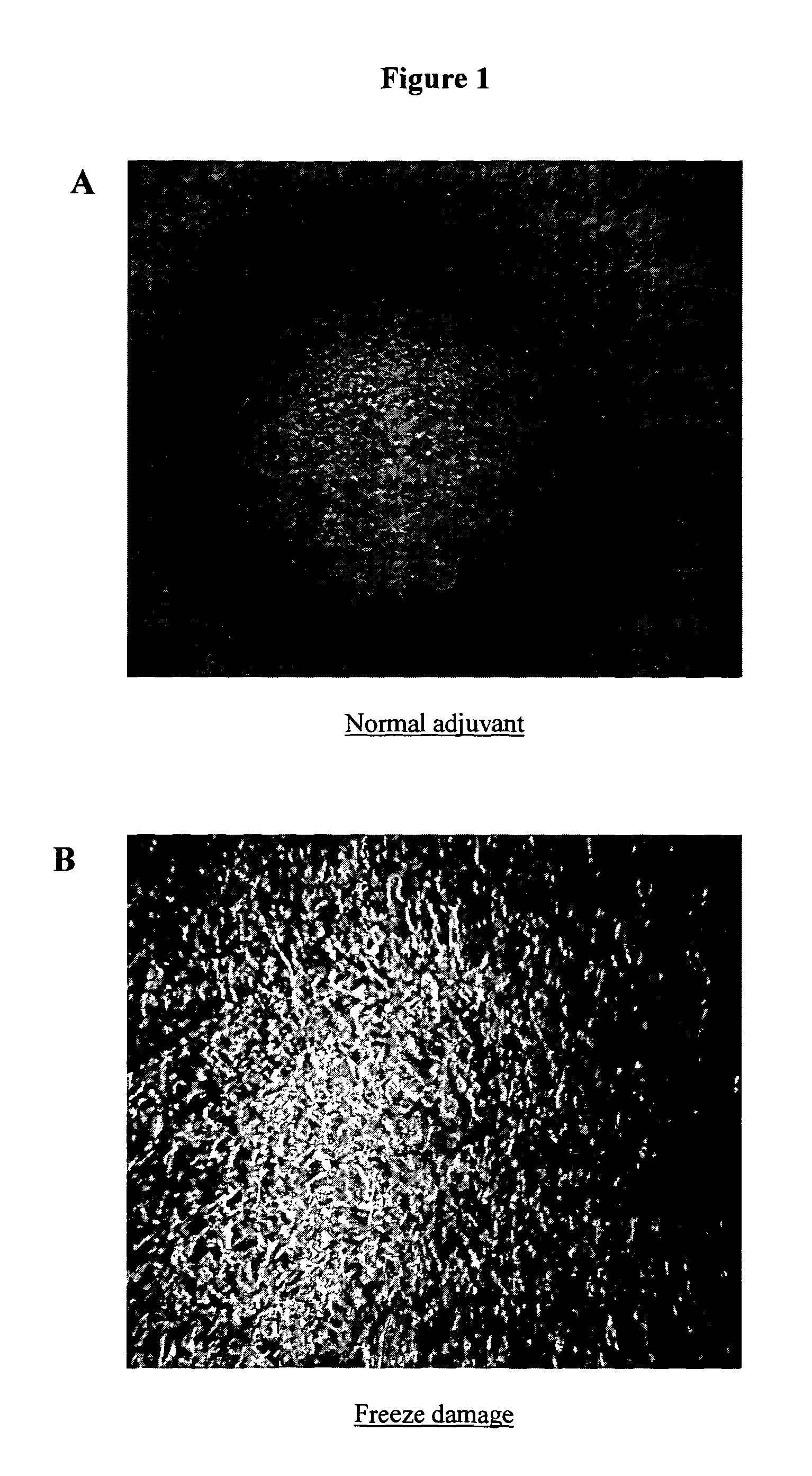
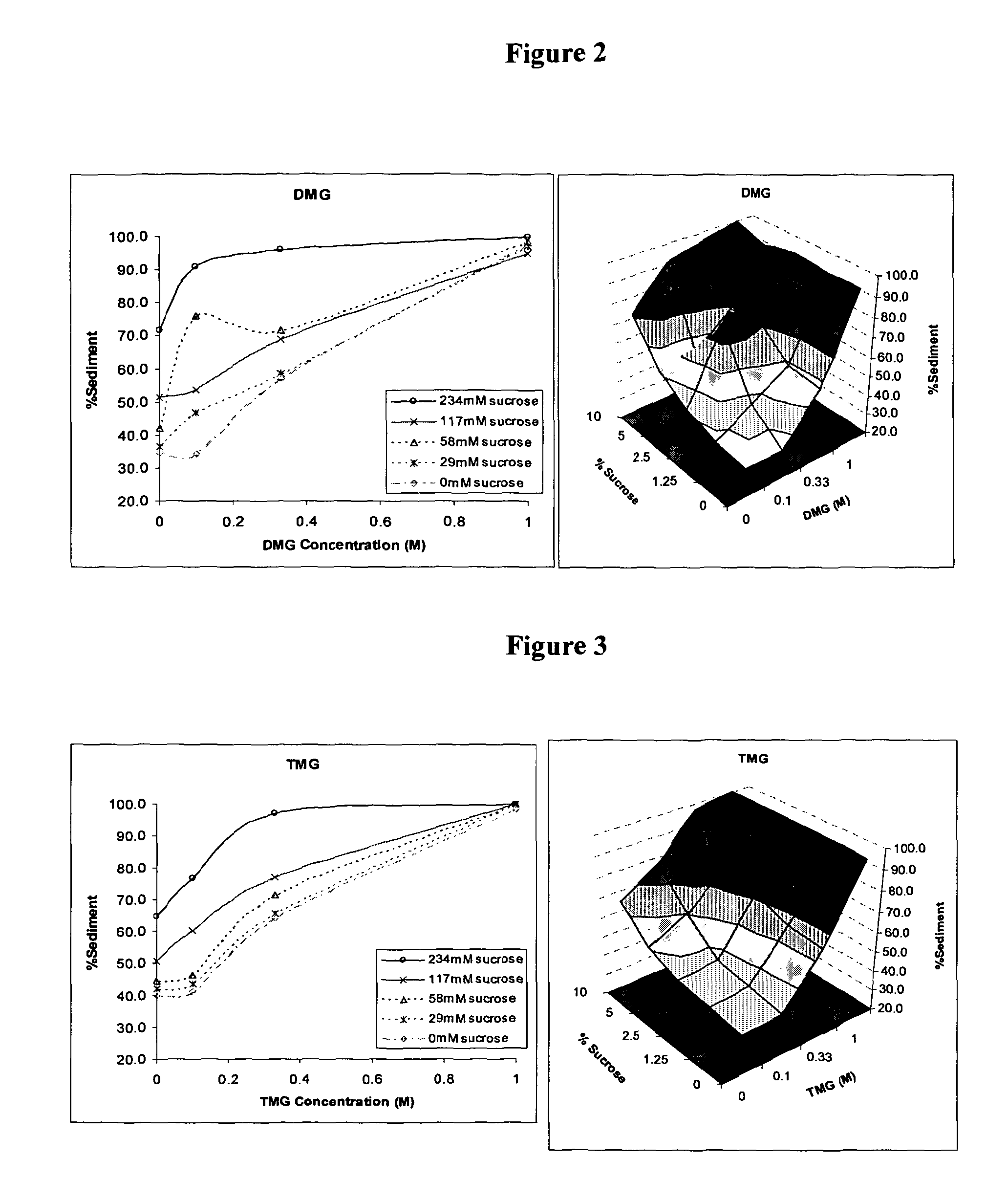
Method for Preserving Alum Adjuvants and Alum-Adjuvanted Vaccines
ActiveUS20130156797A1Simple structureReduce structural damageSnake antigen ingredientsSulfur/selenium/tellurium active ingredientsAdjuvantAlum adjuvant
A method for preserving an aluminium-salt adjuvant during freezing or drying comprising freezing or drying an aqueous suspension or solution comprising: (a) an aluminium salt adjuvant; (b) a compound of formula (I) or a physiologically acceptable salt or ester thereof or a compound of formula (II) or a physiologically acceptable salt or ester thereof; and (c) optionally, one or more sugars.
Owner:IOSBIO LTD
Preparation method of duck 2 type adenovirus inactivated vaccine
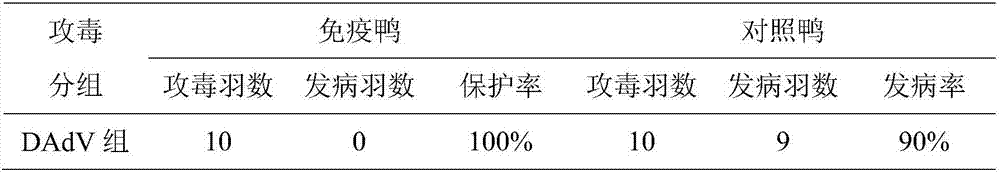
InactiveCN107982529AImprove securityHigh protection rateMicroorganism based processesAntiviralsAdjuvantGamma interferon
The invention provides a preparation method of a duck type 2 adenovirus inactivated vaccine. According to the technical scheme, duck adenovirus is proliferated by utilizing chicken liver cancer cells;after the duck adenovirus is inactivated, gamma-interferon is added; an imported white oil adjuvant is utilized to prepare the safe and effective duck type 2 adenovirus inactivated vaccine. The ducktype 2 adenovirus inactivated vaccine prepared by the preparation method has good safety, can be used for stimulating organisms to generate antibodies earlier and has a high protection rate. The vaccine provided by the invention can be used for effectively ensuring immunogenicity; the defect that the vaccine is lacked in the prior art is overcome; meanwhile, the duck type 2 adenovirus inactivatedvaccine can have an exact effect on prevention of the duck type 2 adenovirus.
Owner:TIANJIN RINGPU BIO TECH
Controlled-release immunogenic formulations to modulate immune response
ActiveUS7858093B1Effective contributionBalanced immune responseBiocideBacterial antigen ingredientsControl releasePolyanhydrides
Single-dose controlled-release immunogenic formulations, such as vaccines, based on bioerodible polyanhydride copolymer or homopolymer microparticles for the control of immune response mechanisms are provided. The copolymer or homopolymer microparticles degrade by surface-erosion from in vivo hydrolysis of anhydride linkages at the surface of the microparticle, which results in controlled release of immunogen(s) to a patient.
Owner:IOWA STATE UNIV RES FOUND
Sterile immunogenic non-tumorigenic tumor cell compositions and methods
InactiveCN1774255AStay aliveMaintain metabolic activityMammal material medical ingredientsCancer antigen ingredientsCell AggregationsMetastasis tumor
This invention relates to methods of removing bioburden from an aggregate of cells to obtain sterile cells that remain viable and immunogenic for the production of vaccines. This invention further relates to a method of eliciting an immune response to prevent a recurrence of metastases that involves preparing and administering a sterile vaccine derived from solid tumors. The vaccine is prepared by excising a solid tumor from a cancer patient, digesting the tumor cells with an enzyme to obtain dissociated cells, irradiating the dissociated cells to render the cells non-tumorigenic, and sterilizing the cells.
Owner:INTRACEL RESOURCES
Method for preserving alum adjuvants and alum-adjuvanted vaccines
ActiveUS9101607B2Simple structureReduce structural damagePowder deliverySnake antigen ingredientsAdjuvantAlum adjuvant
A method for preserving an aluminium-salt adjuvant during freezing or drying comprising freezing or drying an aqueous suspension or solution comprising: (a) an aluminium salt adjuvant; (b) a compound of formula (I) or a physiologically acceptable salt or ester thereof or a compound of formula (II) or a physiologically acceptable salt or ester thereof; and (c) optionally, one or more sugars.
Owner:IOSBIO LTD
Recombinant protein of methicillin-resistant staphylococcus aureus IsdB protein active segment, preparation method thereof and application thereof
InactiveCN102675433AHigh expressionEasy to separate and purifyAntibacterial agentsBacteriaProtective antigenAnti mrsa
The invention discloses a recombinant protein of active segment IsdB2 of decision protein IsdB on the surface of methicillin-resistant staphylococcus aureus iron ion, wherein the amino acid sequence of the recombinant protein is SEQ ID No: 3 or the sequence which has the same or similar function as the SEQ ID No: 3 obtained by adding or deleting a plurality of amino acids at the amino terminal and / or the carboxyl terminal of the SEQ ID No: 3. The invention further discloses a method for preparing the recombinant protein by building the expression vector of the recombinant protein and transforming the host bacteria, and the use of the recombinant protein in the aspect of preparing the subunit vaccine and the related assay kits resisting the methicillin-resistant staphylococcus aureus. By adopting the gene engineering technology, in the invention, the truncated protective antigens component IsdB2 is expressed by cloning through the protein expressing, thereby being high in expression index, convenient to separate and purify, and high-efficiency and safe. Due to the gene engineering, the recombinant polyvaccine has good immune protective effect on resisting the MRSA (methicillin-resistant staphylococcus aureus) infection.
Owner:CHONGQING YUANLUN BIOTECH +1
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979BMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticEscherichia coliL929 cell
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method of chicken infectious bronchitis N-2-hydroxypropyl trimethyl ammonium chloride chitosan/carboxymethyl chitosan nano living vaccine
InactiveCN103212068AAvoid damageReduce pollutionAntiviralsPharmaceutical non-active ingredientsHydroxypropyltrimethyl ammonium chloride chitosanVeterinary Drugs
The invention provides a preparation method of a chicken infectious bronchitis N-2-hydroxypropyl trimethyl ammonium chloride chitosan / carboxymethyl chitosan nano living vaccine and relates to a preparation method of a chicken infectious bronchitis nano living vaccine. The invention aims at providing the preparation method of chicken infectious bronchitis N-2-hydroxypropyl trimethyl ammonium chloride chitosan / carboxymethyl chitosan nano living vaccine. The preparation method comprises the following steps of: firstly, preparing a virus solution and adding the virus solution into an N-2-hydroxypropyl trimethyl ammonium chloride chitosan solution after filtration sterilization, and stirring to obtain a mixed solution A; secondly, dropwise adding a carboxymethyl chitosan solution into the mixed solution A after filtration sterilization to obtain a mixed solution B; thirdly, centrifuging the mixed solution B, then washing sediments with sterile deionized water at the temperature of 4 DEG C for three times, and collecting solid-phase materials; and fourthly, adding the solid-phase materials into the sterile deionized water at the temperature of 4 DEG C, then adding 5% of cane sugar skimmed milk solution, and then carrying out vacuum freeze drying, thus the preparation method is completed. The preparation method provided by the invention is applied to the field of veterinary drugs.
Owner:HEILONGJIANG UNIV
PAL recombination protein of acinetobacter baumannii and coding gene and application of PAL recombination protein
The invention relates to the field of genetic engineering, and discloses PAL recombination protein of acinetobacter baumannii and a coding gene and application of the PAL recombination protein. The PAL recombination protein is (a) recombination protein as shown in SEQID NO:1 or SEQID NO:3; and (b) protein which is obtained by substituting, deleting and adding one or more amino acids for the aminoacid sequence as shown in SEQ ID NO:1 or SEQID NO:3, and has the same function as the recombination protein as shown in SEQID NO:1 or SEQID NO:3 and derived from (a) or protein which is prepared by connecting labels to the terminal of amino terminal and / or carboxyl terminal of the SEQID NO:1 or the SEQID NO:3 and shown in the amino acid sequence. The recombination protein is high in expression level, convenient to separate and purify, and high in efficacy and safe, can directly cooperate with an adjuvant for preparing a subunit vaccine and a relevant detection product, for resisting infectionof the acinetobacter baumannii.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Pseudomonas aeruginosa vaccine recombinant protein SBP, and preparation method and applications thereof
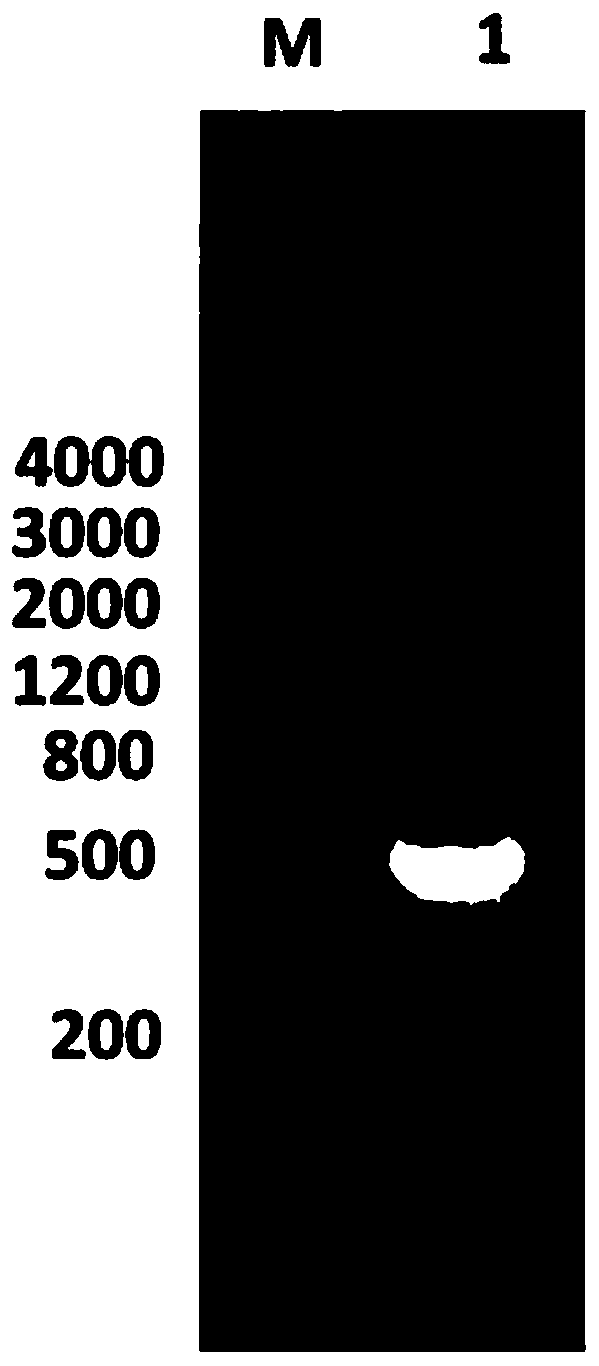
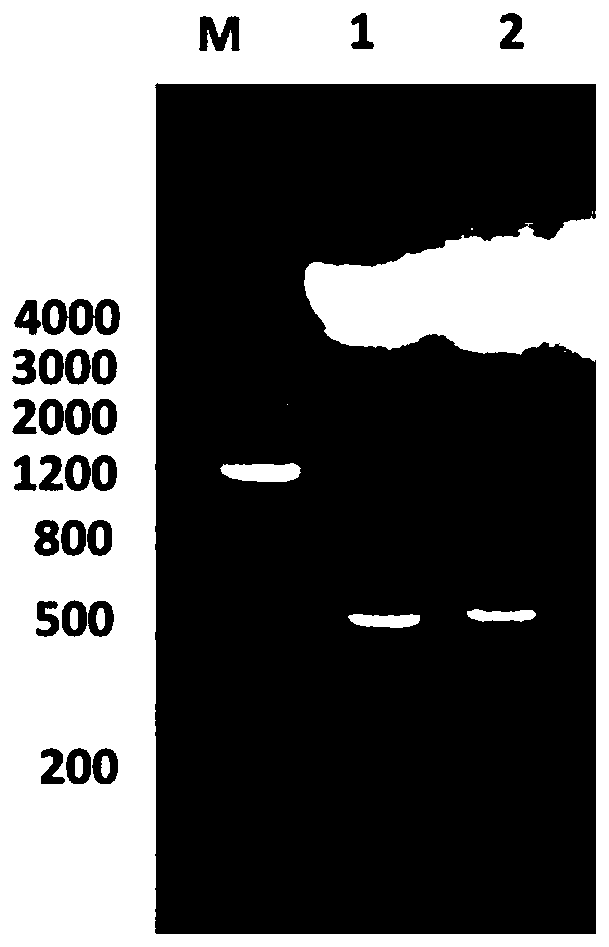
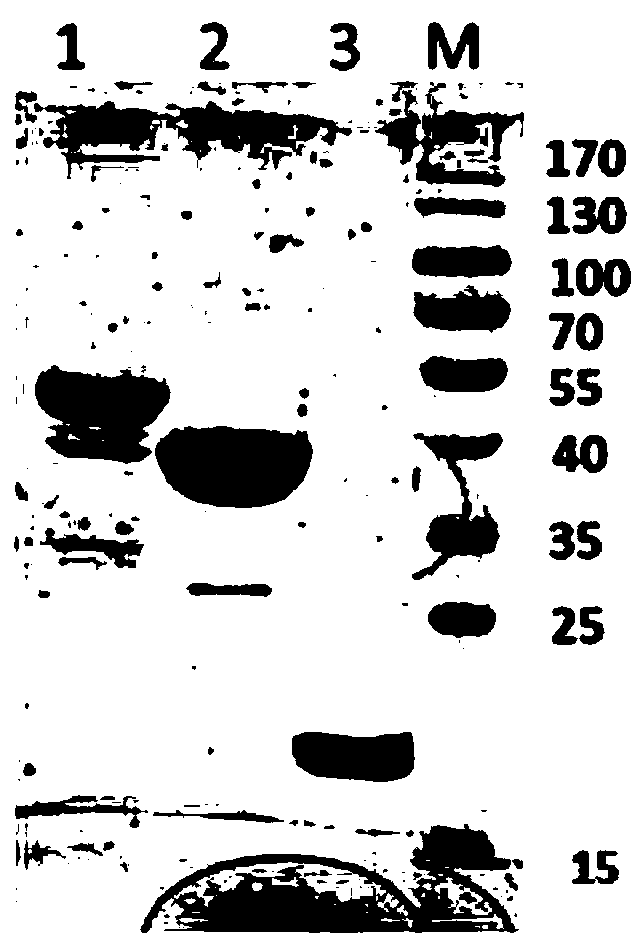
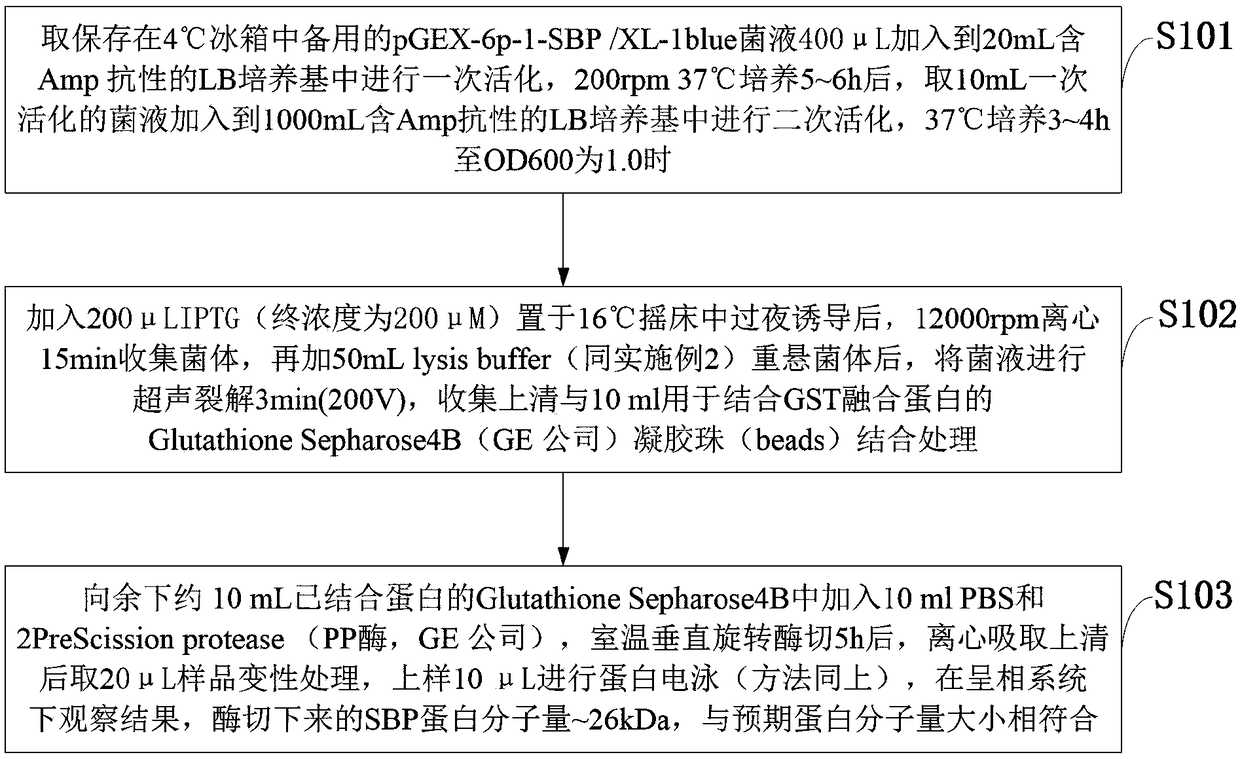
ActiveCN109293750AHas immune protectionGood immune protectionAntibacterial agentsDepsipeptidesImmunogenicityTGE VACCINE
The invention belongs to the technical field of bacterial antigen, and discloses a pseudomonas aeruginosa vaccine recombinant protein SBP, and a preparation method and applications thereof. The nucleotide sequence is SEQ ID NO:1; the amino acid sequence is SEQ ID NO:2; a recombinant expression carrier contains a nucleotide sequence SEQ ID NO:1. According to the preparation method, pGEX-6p-1carrieris adopted in construction of a recombinant expression plasmid for expression of recombinant protein SBP; pGEX is an expression fusion protein carrier constructed by Smith and Johnson in 1987, and the main characteristic is that the carrier is grafted with glutathione-S-transferase (GST) with a molecular weight of 26kDa; the expressed fusion protein contains a GST label; and the label is a protein purification label. Compared with other fusion carriers, the pGEX carrier is capable of maintaining the space conformation and the immunogenicity of the purified protein as far as possible.
Owner:重庆艾力彼生物科技有限公司
High fidelity porcine reproductive and respiratory syndrome virus attenuated strain and application thereof
ActiveCN107058247AChange tropismDecreased tropismSsRNA viruses positive-senseViral antigen ingredientsMicroorganismHighly pathogenic
Belonging to the field of biotechnology, the invention provides a high fidelity porcine reproductive and respiratory syndrome (PRRS) virus attenuated strain and application thereof. The attenuated strain is porcine reproductive and respiratory syndrome virus PRRSV-NJRb strain with a microbial preservation number of CGMCC No.13800. The invention also provides a preparation method of an attenuated strain virus solution, and the method includes: using Marc-145 cell to proliferate the high fidelity porcine reproductive and respiratory syndrome virus attenuated strain, performing cell lysis, conducting centrifugation and taking the supernatant liquid, thus obtaining the virus solution. The invention also provides a porcine reproductive and respiratory syndrome attenuated vaccine, the active component of which is the porcine reproductive and respiratory syndrome virus attenuated strain. The attenuated strain has low mutation rate and high genetic stability, can still maintain the original immunogenicity and virulence after 100 passages, and has good protection on high pathogenic PRRS. The attenuated vaccine provided by the invention has good protection on high pathogenic PRRS, and has higher safety.
Owner:YICHUN UNIVERSITY +1
Novel tumor vaccine preparation method
PendingCN110124021AGood biosafety and biocompatibilityAvoid tumor inductionCancer antigen ingredientsAntineoplastic agentsTumour-associated antigenCulture fluid
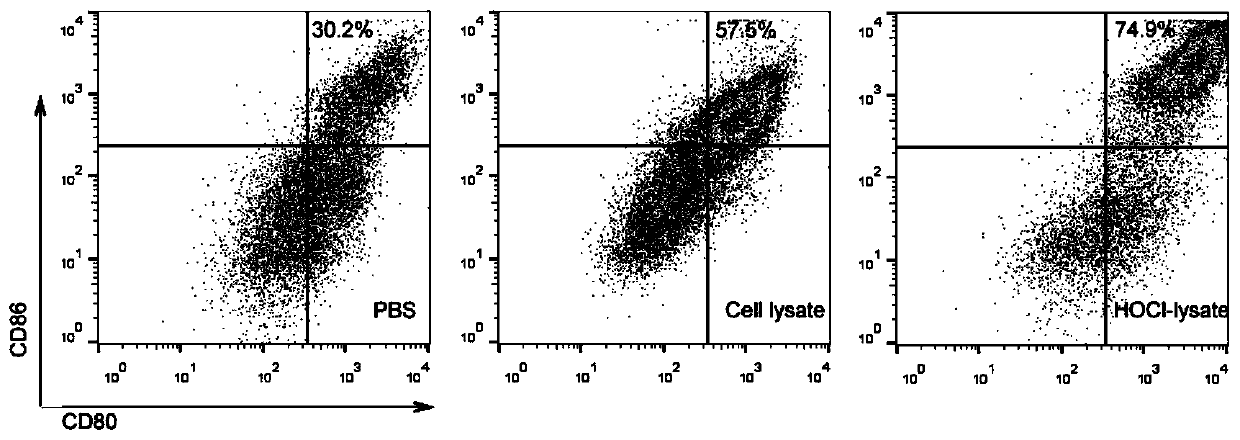
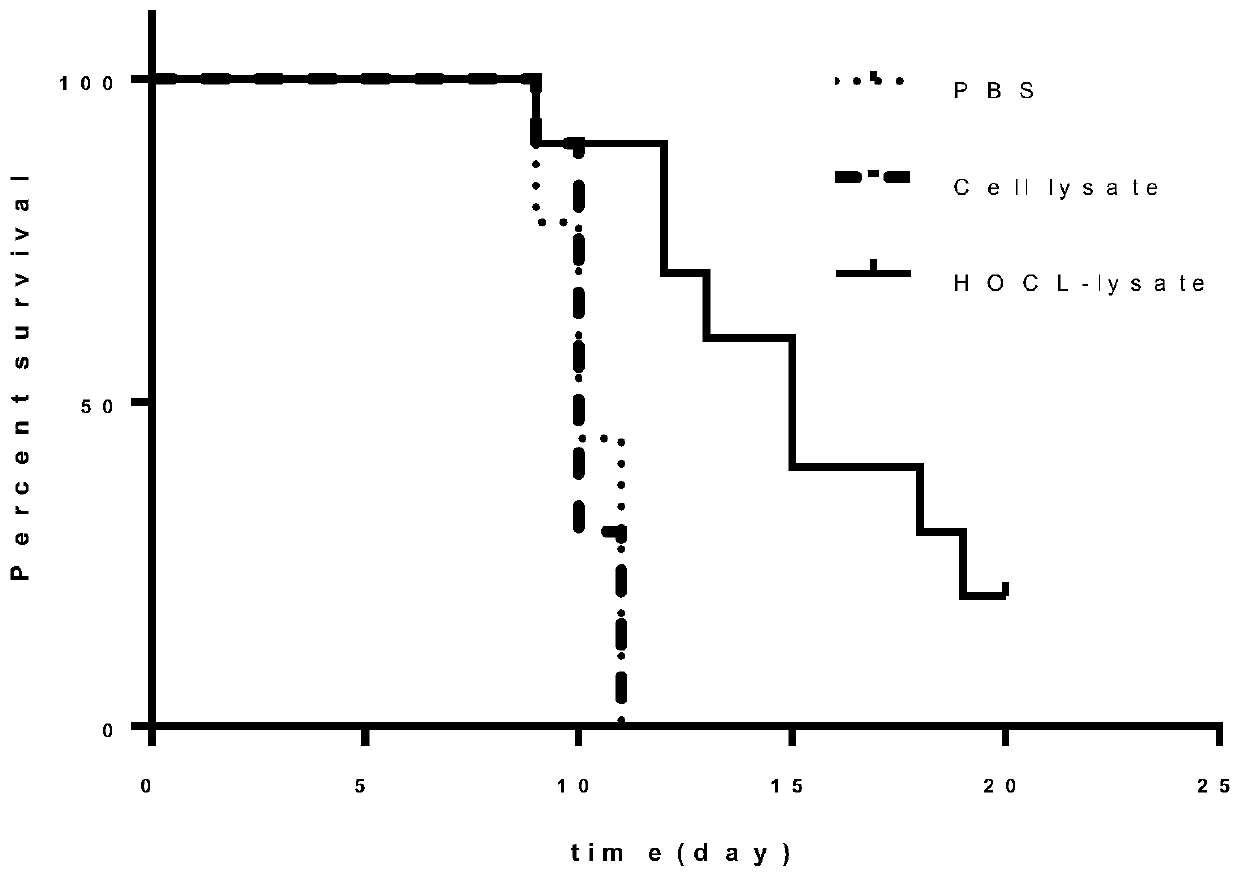
The invention relates to the antitumor technical field, in particular to a novel tumor vaccine preparation method. Tumor cell lysis buffer subjected to treatment with hypochlorous acid is mixed with an immune activation agent to form a novel tumor vaccine. The method includes steps: 1) after fresh tumor tissues are obtained, performing primary tumor cell culture to obtain primary tumor cells; 2) subjecting the primary tumor cells obtained in the step 1) and culture solution to treatment with hypochlorous acid, collecting mixed solution obtained after cell treatment with hypochlorous acid to obtain required lysis buffer and apoptotic tumor cell debris mixture; 3) centrifuging the mixed solution obtained in the step 2), and repeatedly concentrating and purifying to obtain concentrated solution containing cell lysis buffer; 4) performing radiation inactivation on the cell lysis buffer purified and concentrated in the step 3). Problems of low immunogenicity, inaccuracy and low immunoreaction activation efficiency of common tumor antigen vaccines can be solved.
Owner:迪亚思生命科技(武汉)有限公司
Recombinant bacteria obtained from Brucella abortus 104-M vaccine strain with Omp25 gene knockout and application
ActiveCN107267430ALow immunogenicityMaintain immunogenicityAntibacterial agentsBacterial antigen ingredientsProtein codingImmunogenicity
The invention discloses recombinant bacteria obtained from brucella 104M with Omp25 gene knockout for a vaccine strain and an application. The recombinant bacteria are obtained for reducing and / or inhibiting protein activity of Omp25 in brucella 104M. For the recombinant bacteria, reduction and / or inhibition of protein activity of Omp25 in brucella 104M refer / refers to inhibition or silencing of expression of an Omp25 protein coding gene in brucella 104M. Experiments prove that the recombinant bacteria are obtained by knocking out virulence gene Omp25 from brucella 104M, and delta Omp25 as a brucella attenuated vaccine candidate for human is screened out by means of research in virulence and immunogenicity of the recombinant bacteria.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Acinetobacter baumanniihy pothetical protein A1S-1462 protein and preparation method and application thereof
ActiveCN104861048AHigh expressionQuality and safety controllableAntibacterial agentsBacterial antigen ingredientsAdjuvantGenetic engineering
The invention relates to an A1S-1462 recombinant protein and a preparation method and application thereof. The recombinant protein comprises A1S-1462 mature peptide, and the amino acid sequence of the recombinant protein is shown as SEQ ID NO. 3. The recombinant protein is high in expression amount, easy to separate and purify, efficient and safe, can be directly used with an adjuvant, and is used for the preparation of an acinetobacter baumannii infection resistant subunit vaccine and a related detection kit. Confirmed by animal experiments, the genetic engineering recombinant subunit vaccine has good acinetobacter baumannii infection resistant immune protection effect, lays a foundation for the further study on combined vaccines and multicomponent fusion vaccines, and plays an important role for development and application of prevention and control vaccines and diagnostic kits.
Owner:ARMY MEDICAL UNIV
Preparation method of Burkholderia pseudomallei recombined BLF1 protein, product prepared through preparation method and application of Burkholderia pseudomallei recombined BLF1 protein
InactiveCN105505973AMaintain immunogenicityThe purification conditions matchPeptide/protein ingredientsBacteria peptidesImmunogenicityWilms' tumor
The invention discloses a preparation method of Burkholderia pseudomallei recombined BLF1 protein, the product prepared through the preparation method and an application of the Burkholderia pseudomallei recombined BLF1 protein. The preparation method includes the steps that a primer for amplifying a Burkholderia pseudomallei BLF1 gene is designed firstly, a sequence shown in SEQ ID NO.3 of a coding area sequence is obtained through amplification, then the acquired sequence is connected to an expression vector to construct a recombinant expression vector, the recombinant expression vector is converted into host bacteria, and through inducible expression, the Burkholderia pseudomallei recombined BLF1 protein is obtained. The preparation method is simple, the space design conception and immunogenicity of the purified protein can be kept to the maximum degree, the purity of the protein is larger than 95%, it is proved through a mouse experiment that the purified protein has animal toxicity, it is shown that the purified protein has biological activity, the purified protein acts on A549 cells, cell growth can be restrained, and thus the Burkholderia pseudomallei recombined BLF1 protein can be used for preparing anti-tumor medicine and has wide application prospects.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Gene of coded recombined helicobacter pylori serine protease, egg yolk antibody and application
ActiveCN108531468AMaintain immunogenicityEasy to operateEgg immunoglobulinsBacteriaFood additiveNucleotide
The invention belongs to the technical field of biology, and specifically relates to a gene of coded recombined helicobacter pylori serine protease, an egg yolk antibody and application. The nucleotide sequence of the gene of the coded recombined helicobacter pylori serine protease is shown in SEQ ID NO.1. The invention further provides an egg yolk antibody inhibiting helicobacter pylori serine protease. The antibody is prepared through the following steps of performing gene synthesis to obtain the gene of the coded recombined serine protease; performing prokaryotic expression on the gene, purifying, acquiring recombined serine protease, using the recombined serine protease as an immunogen to immunize hens, collecting eggs laid by immunized hens, extracting the egg yolk antibody in the egg, purifying, and obtaining the egg yolk antibody inhibiting helicobacter pylori serine protease. The egg yolk antibody can be used as a food additive or added into healthcare products, or can be prepared into drugs preventing or treating gastric ulcer and gastric cancer.
Owner:北京宇诚健康科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com