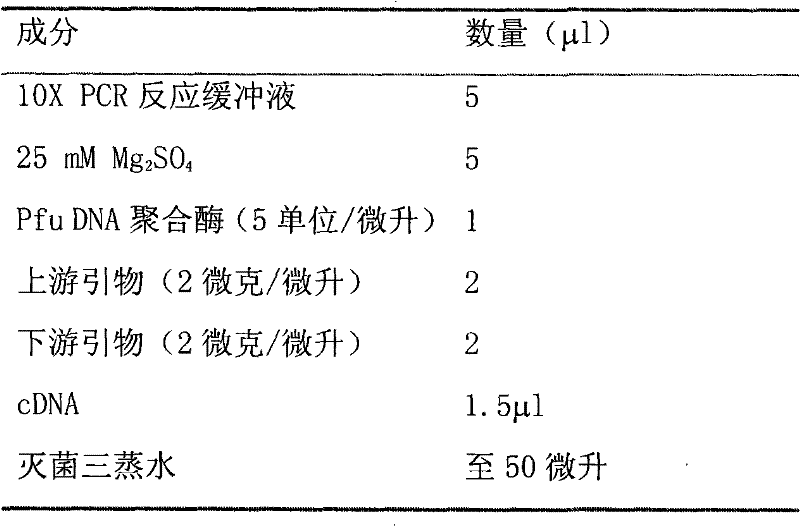
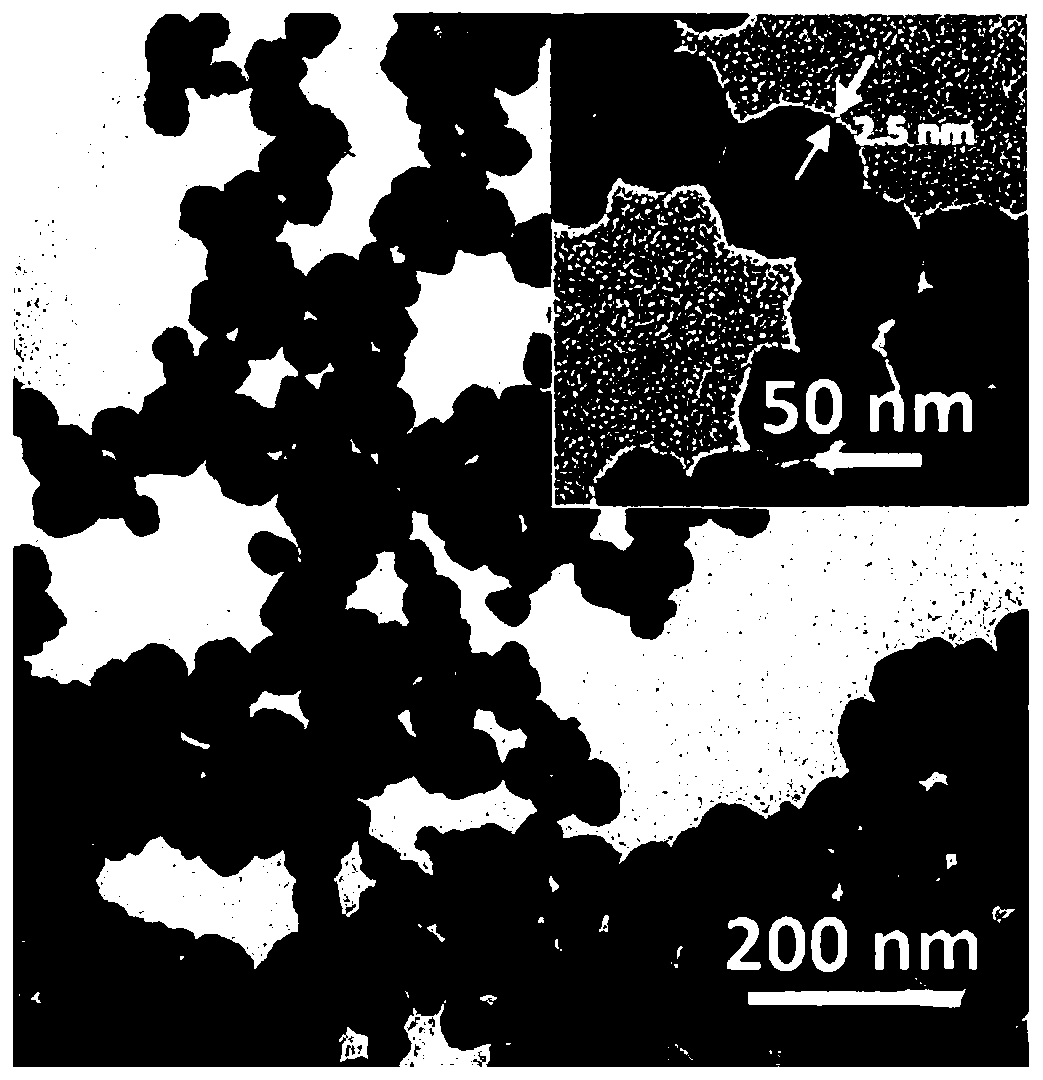
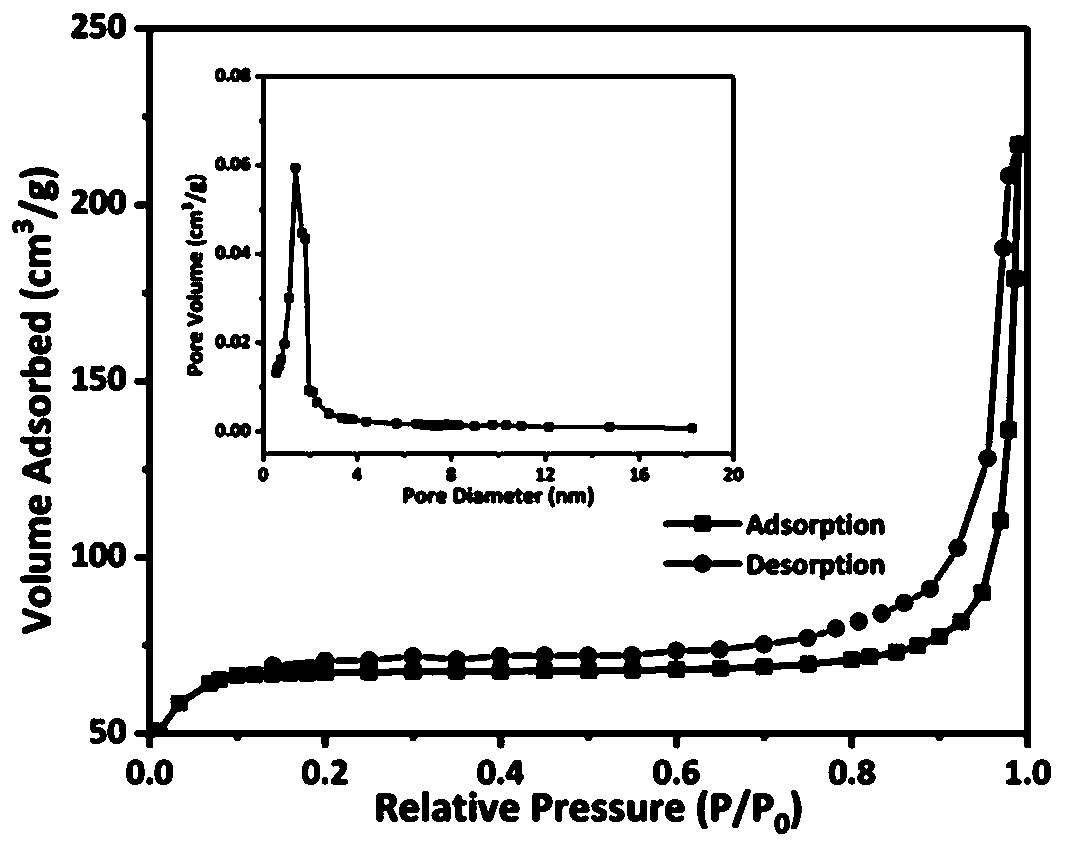
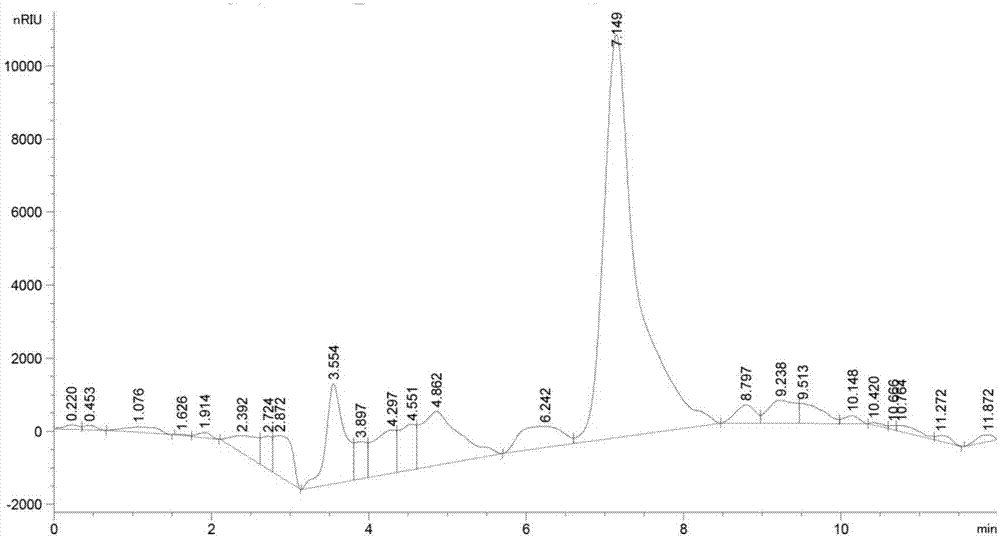
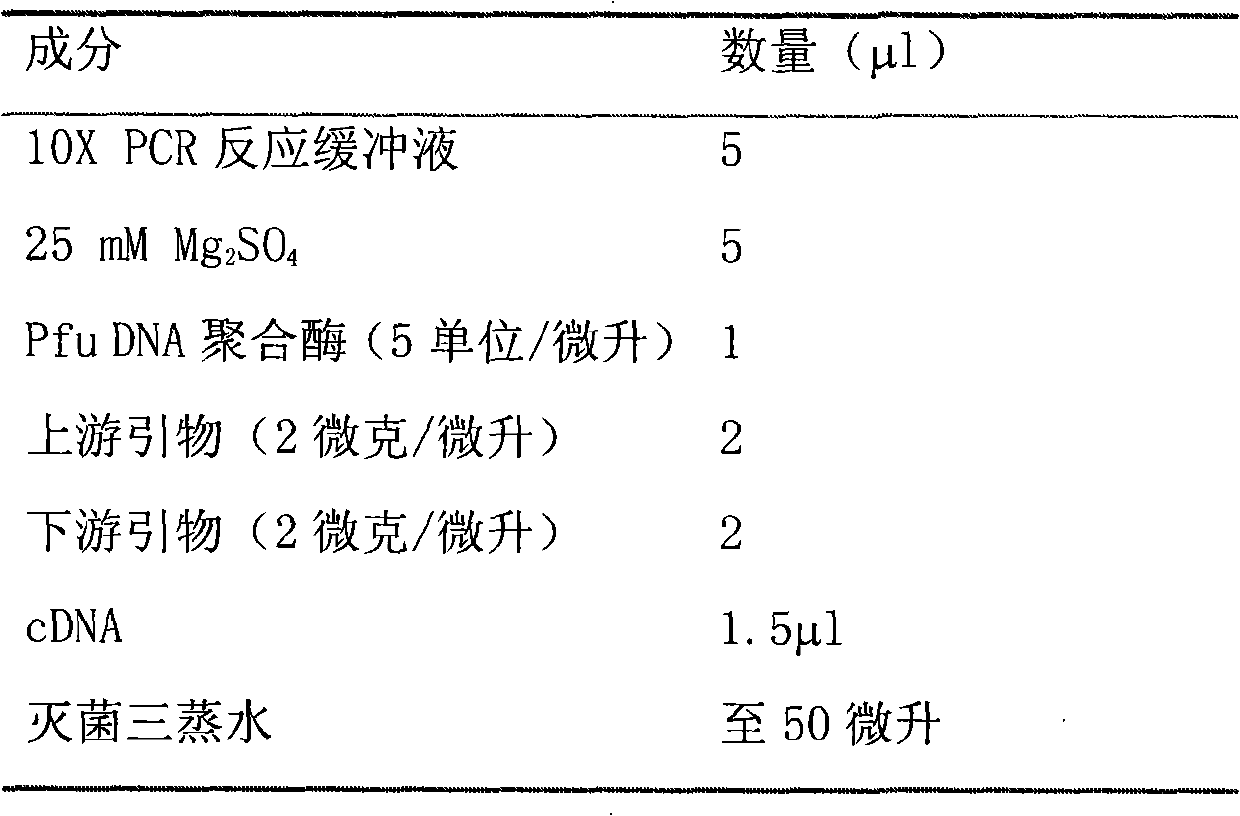
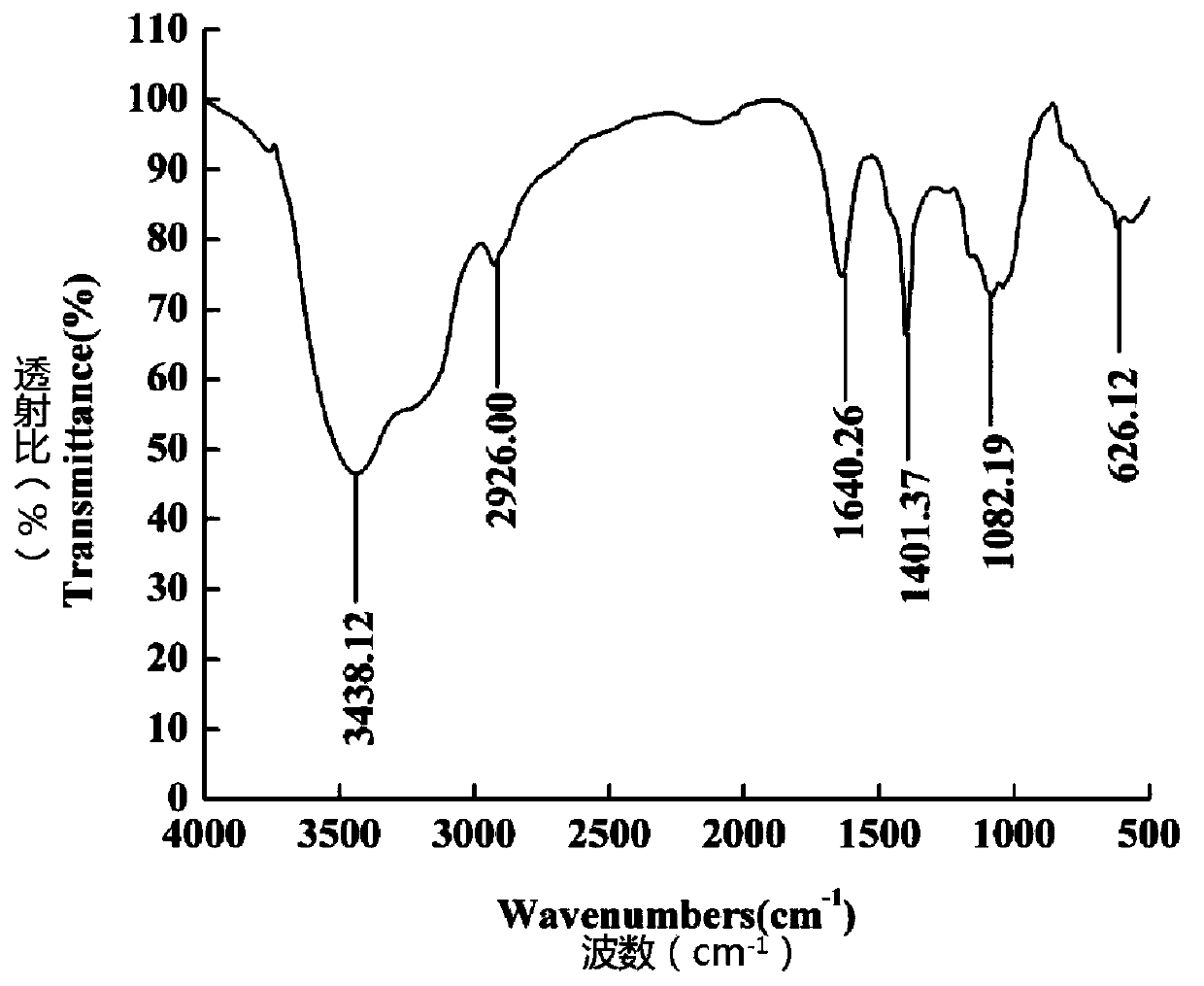
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "L929 cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
L929/A cells can be used in the development of novel anti-cancer treatments. Resistance can be circumvented by modulating agents such as verapamil and quinine. The parent cell line L929 was derived from normal subcutaneous areolar adipose tissue.
Oligopolynucleotide of inhibiting activity of necrosin in human tumor
InactiveCN1550501AInhibition of killingInhibit bindingOrganic active ingredientsSugar derivativesDiseaseL929 cell
Oligonucleotide sequence i.e. adaptor including DNA sequence and RNA sequence can combine with TNF alpha in specificity so as to restrain damaging effect on L929 cells from TNF alpha. The invented adaptor can be utilized to test TNF alpha and cure disease caused by raised level of TNF alpha. Comparing with current antagon of TNF alpha, the invented adaptor possesses advantages of high specificity, high affinity, fast reaching target tissue and quick plasma clearance etc. The oligonucleotide sequence possesses affinity and specificity of monoclonal antibody as well as permeability and pharmacokinetics characters of small molecule polypeptide. The invention is also related to derivation sequence and modified sequence of oligonucleotide, as well as its usage, preparation and diagnosis in curing disease relevant to TNF alpha.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Oligonucleotide antagonist for human tumor necrosis factor alpha (TNF-alpha)
InactiveUS7309786B2Strong specificityHigh affinitySugar derivativesPeptide/protein ingredientsL929 cellHigh concentration
The present invention relates to a group of new oligonucleotides sequences with human tumor necrosis factor α (TNF-α) inhibiting activity, which includes DNA sequences and RNA sequences. These oligonucleotides or aptamer can specifically be bound to TNF-α and inhibit the cytoxicity of TNF-α to L929 cells. Therefore, the aptamer of the present invention may be used to detect TNF-α and provide a therapeutic method for diseases related to the increasing level of TNF-α. Compared with other TNF antagonists such as monoclonal antibody and soluble receptor, the present invention has high specificity, high affinity, quick penetration to target tissue, rapid plasma clearance, and lower immunogenecity. Turthermore, it can be used repeatedly and keeps high concentration in target tissue and the like. It has the advantages of affinity and specificity similar to monoclonal antibodies and also has permeability and pharmacokinetics characteristics similar to small molecular polypeptide. The present invention also refers to derivative of the oligonucleotides sequence, including modified sequence. The present invention may further be manufactured as medicine for therapy and diagnosis of TNF-α related diseases.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
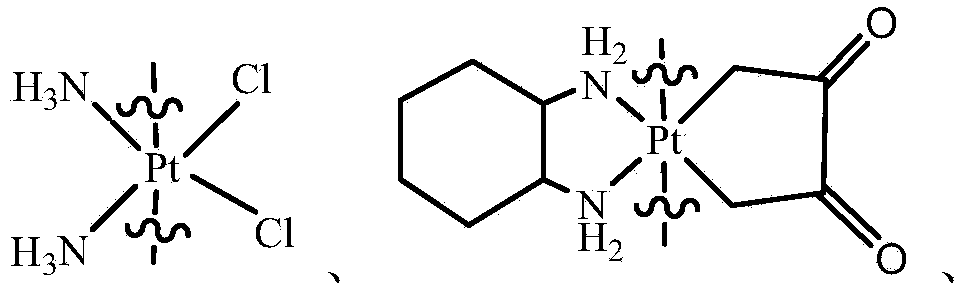
Compound having anticancer activity and preparation method
ActiveCN103588818ALow cytotoxicityImprove anti-cancer effectGroup 8/9/10/18 element organic compoundsAntineoplastic agentsL929 cellCantharidin
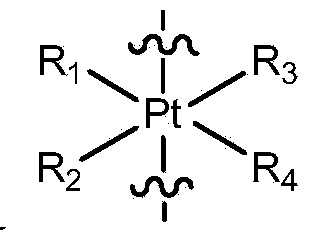
The invention provides a compound having anticancer activity, which is obtained by cooperation of Norabieta cantharidin and platinum (IV) and modification of a piperazine derivative. The compound is characterized in that a Norabieta cantharidin piperazine derivative ligand is introduced, thereby cytotoxicity of a platinum (IV) compound is reduced, and high antineoplastic activity is provided. The result of the cytotoxicity experiments shows that the provided platinum (IV) compound has less destruction to normal mice fibroblast L929, but has good killing effect to cancer cells such as human breast cancer MCF-7 cell, human cervical carcinoma HeLa cell and human lung adenocarcinoma A549 cell, so that the compound having anticancer activity is capable of reducing the system toxicity and having equal anticancer activity with cisplatin. The compound having anticancer activity has good killing effect by aiming at cisplatin resistance human lung adenocarcinoma A549 / DDP cell. Therefore, the toxicity is reduced, the cisplatin resistance can be reversed, and anticancer effect of the platinum medicines can be increased.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
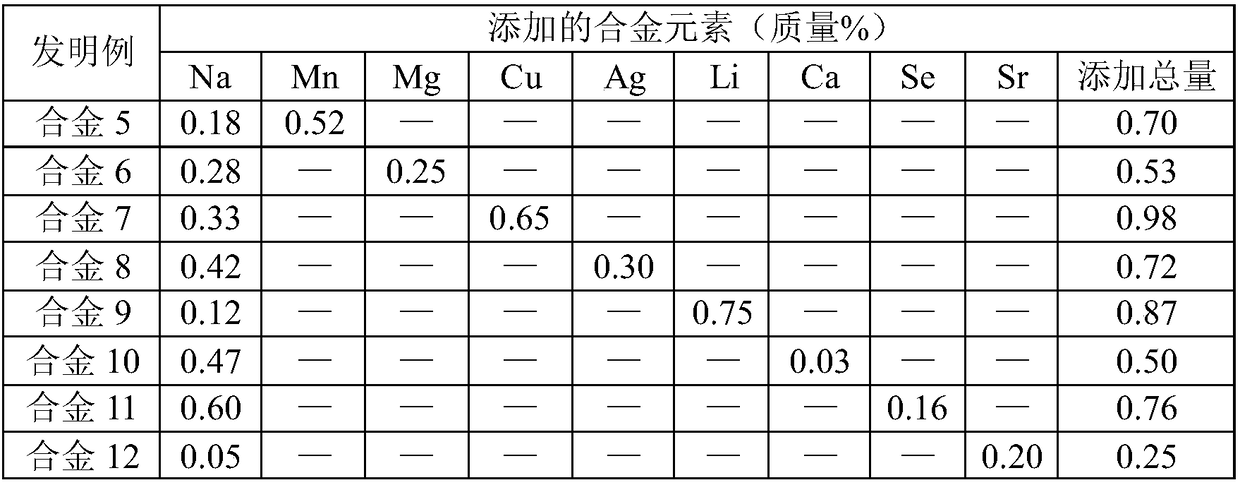
Biodegradable Zn-Na-series zinc alloy and preparation method thereof
The invention discloses a biodegradable Zn-Na-series zinc alloy, and belongs to the field of medical implant materials. The biodegradable Zn-Na-series zinc alloy comprises 0.01-0.97% of Na, one or more of 27 elements which are harmless or beneficial to a human body, and the balance Zn, wherein in order to lower the cost and obtain excellent comprehensive performance, the total amount of the addedvarious alloy elements is optimally controlled to be no more than 2.0%. A preparation processing process of the zinc alloy comprises the steps of continuous casting, hot extrusion and rolling; or continuous casting, hot extrusion, solidification heat treatment and drawing; or continuous casting, uniform heat treatment, hot extrusion and rolling. According to the biodegradable Zn-Na-series zinc alloy, the yield strength is 100-500 MPa, the tensile strength is 150-700 MPa, and the elongation is 1.5-100%; the degradation rate in simulated body fluid is no more than 0.8 mm / year; and the cytotoxicity to L929 cells and human mesenchymal stem cells is at the zero stage or the one stage, good biocompatibility is shown, and the biodegradable Zn-Na-series zinc alloy can be used for various medical implants.
Owner:北京尚宁科智医疗器械有限公司
Embryo cattle serum substitute without serum for animal cell culture
InactiveCN1800368ALow costTo achieve the purpose of replacing serumAnimal cellsL929 cellHuman platelet
The invention relates to a non-serum animal cell culture used fetus cattle serum displace agent, which is formed by recombination human insulin growth factor-1:0.1g-0.5g / l, recombination human platelet sourced growth factor-B:0.1g-0.6g / l, recombination human alkali desmocyte growth factor: 0.05-0.4g / l, cattle transferring: 1g-5g / l, cattle serum protein (V component): 2g-15g / l, and mercaptoethanol 0.3-0.5mol / l.
Owner:上海尚优生物科技有限公司
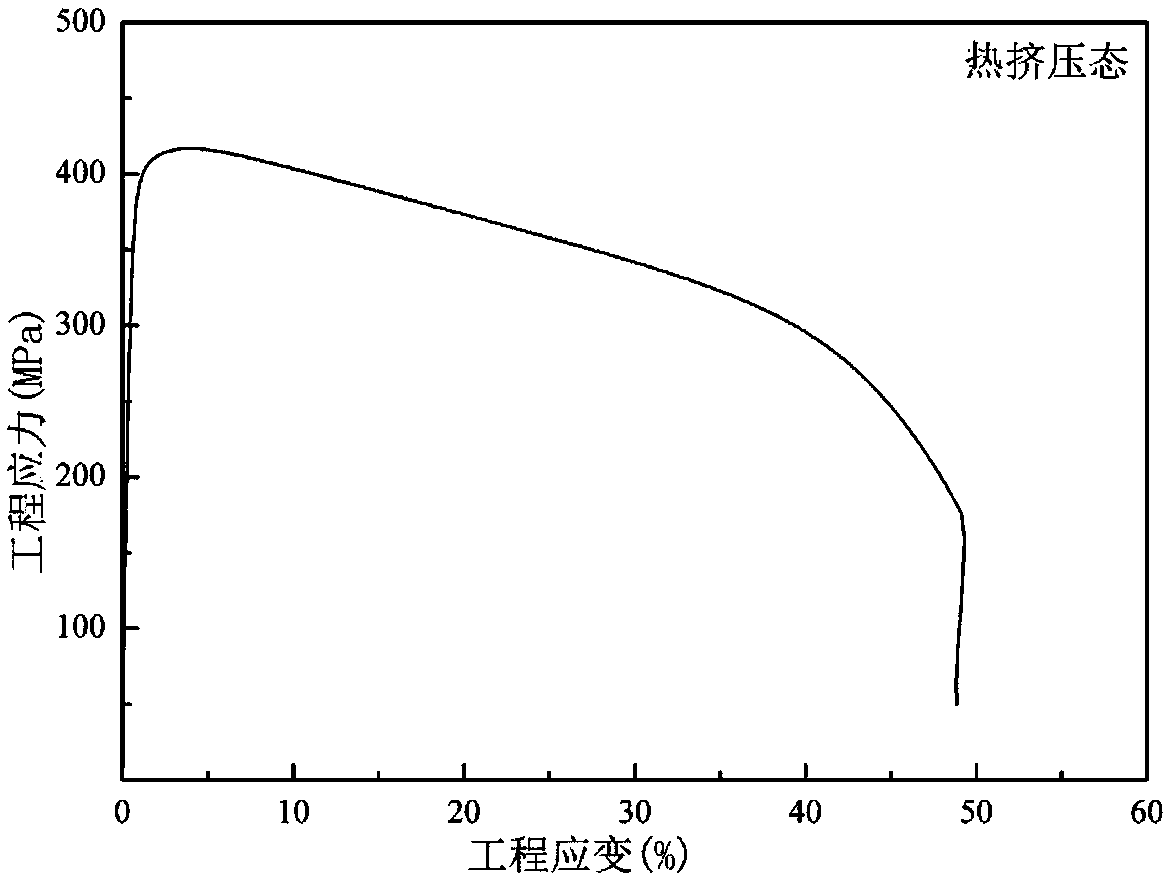
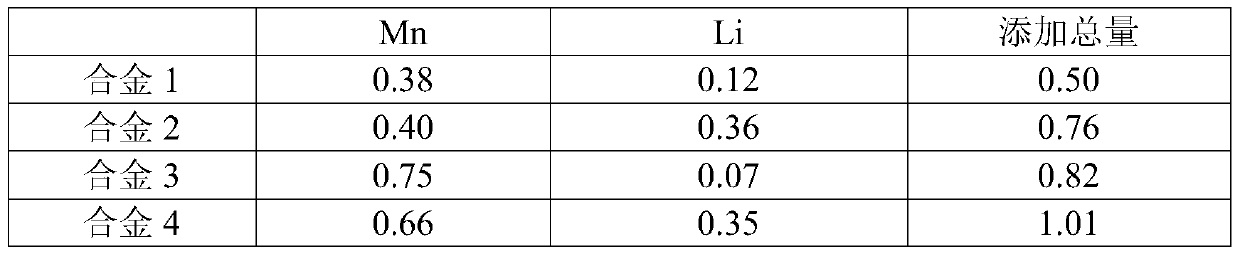
High-strength high-plasticity biodegradable Zn-Mn-Li zinc alloy and application thereof
ActiveCN108754232AGood cell compatibilityLow costSurgeryTissue regenerationL929 cellSimulated body fluid
Provided is high-strength high-plasticity biodegradable Zn-Mn-Li zinc alloy. The alloy comprises 0.01-0.8% of Mn and 0.005-0.4% of Li, wherein Mn is the main alloying element, Li is the minor alloyingelement, and the content of Mn is not lower than the content of Li in the alloy; at least one of Na, K, Ca, Sr, Ti, Mg, Fe, Cu and Ag is selected, wherein the content of Na and the content of K are both not higher than 0.1%, and the total content of Na, K and Li is not higher than 0.4%; the content of Ca, the content of Sr, the content of Ti and the content of Mg are all not higher than 0.2%, andthe total content of Ca, Sr, Ti and Mg is not higher than 0.2%; the content of Fe is not higher than 0.05%; the adding amount of Cu and the adding amount of Ag are not higher than 0.4%, and the totalcontent of Cu and Ag is not higher than 0.4%; and the total content of alloy elements added into the Zn-Mn-Li zinc alloy is not higher than 1.8%, and the balance Zn. The yield strength of the zinc alloy is 250-450 MPa, the tensile strength of the zinc alloy is 350-600 MPa, and the ductility is 20-60%; the degrading rate in simulated body fluid is not higher than 0.15 mm / year; the cytotoxicity tothe L929 cell is at the level 0 or level 1, and good cytocompatibility is presented. The zinc alloy is used for a degradable bracket or other medical implants.
Owner:北京尚宁科智医疗器械有限公司
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979AMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticL929 cellEscherichia coli
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Embryo cattle serum substitute without serum or animal protein for animal cell culture
The invention relates to a non-serum non-animal origin protein animal cell culture used fetus cattle serum displace agent, which is formed by recombination human insulin growth factor-1:0.1g-0.5g / l, recombination human platelet sourced growth factor-B:0.1g-0.6g / l, recombination human alkali desmocyte growth factor: 0.05-0.4g / l, polyethylene glycol (MW7000-20000), 0.04-0.15%(w / v), polyvinyl alcohol (density 1.27-1.31): 0.001-0.05%(w / v), mercaptoethanol: 0.3-0.5mol / l.
Owner:上海席诺生物技术有限公司
Building method for autovaccine by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule
InactiveCN102370979BMaintain immunogenicityRemove natural biological activityBacteriaAntipyreticEscherichia coliL929 cell
The invention discloses a building method for autovaccine in-vivo induced by aiming at human TNF(Tumor Necrosis Factor)-alpha molecule. With a step-by-step cloning method, a fusion gene of hTNF-TT830-844, hTNF-HEL46-61 and hTNF-PADRE is built; point mutation (T439-A,C440-G) is introduced into a natural human TNF gene to optimize a mRNA (Ribonucleic Acid) secondary structure; the fusion gene is cloned into a pET22b prokaryotic expression vector, and efficient expression is achieved in the bacterial strain of escherichia coli; three T accessory cell epitope peptides are introduced between the epitope peptide structure domains of hTNF by the computer-aided analysis and is fused with the hTNF-alpha to overcome the immunological tolerance of an organism for the autologous protein, and therefore the organism generates high-level humoral immune response; the generated high-level hTNF-alpha neutralizing polyclone antibody can neutralize killing activity of the hTNF-alpha on L929 cells in vitro; the hTNF-PADRE has the strongest immunogenicity; the high-level antibody can be induced under the condition of using no immunological adjuvant; and the vaccine has favorable protection and curing action on mouse models suffering from rheumatoid arthritis induced by the II-type collagen, cachexia and the like induced by LPS (lipopolysaccharide).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
A kind of high-strength and high-plastic biodegradable zn-mn-li series zinc alloy and its application
Provided is high-strength high-plasticity biodegradable Zn-Mn-Li zinc alloy. The alloy comprises 0.01-0.8% of Mn and 0.005-0.4% of Li, wherein Mn is the main alloying element, Li is the minor alloyingelement, and the content of Mn is not lower than the content of Li in the alloy; at least one of Na, K, Ca, Sr, Ti, Mg, Fe, Cu and Ag is selected, wherein the content of Na and the content of K are both not higher than 0.1%, and the total content of Na, K and Li is not higher than 0.4%; the content of Ca, the content of Sr, the content of Ti and the content of Mg are all not higher than 0.2%, andthe total content of Ca, Sr, Ti and Mg is not higher than 0.2%; the content of Fe is not higher than 0.05%; the adding amount of Cu and the adding amount of Ag are not higher than 0.4%, and the totalcontent of Cu and Ag is not higher than 0.4%; and the total content of alloy elements added into the Zn-Mn-Li zinc alloy is not higher than 1.8%, and the balance Zn. The yield strength of the zinc alloy is 250-450 MPa, the tensile strength of the zinc alloy is 350-600 MPa, and the ductility is 20-60%; the degrading rate in simulated body fluid is not higher than 0.15 mm / year; the cytotoxicity tothe L929 cell is at the level 0 or level 1, and good cytocompatibility is presented. The zinc alloy is used for a degradable bracket or other medical implants.
Owner:北京尚宁科智医疗器械有限公司
Application of anti-human immunodeficiency virus (HIV) drug amprenavir in preparation of antitumor drug
InactiveCN103536605AShorten the development cycleReduce R&D investmentOrganic active ingredientsAntineoplastic agentsL929 cellFibroblastic Tumor
The invention discloses application of anti-human immunodeficiency virus (HIV) drug amprenavir in preparation of an antitumor drug. The anti-HIV drug amprenavir identified by food and drug administration (FDA) is taken as a material; the killing effect of the amprenavir on a tumor cell is detected by adopting a microwave theory and techniques (MTT) method. An experiment proves that the amprenavir has a significant inhibiting effect on multiplication of a plurality of tumor cells, such as a breast cancer michigan cancer foundation (MCF-7) cell, a non-small cell lung cancer A549 cell, a mouse embryonic fibroblast glioblastoma multiforme L929 cell, a colon cancer HT29 cell, a brain glioma U87 cell and the like, so as to clarify new use of an old drug. Therefore, foundation is provided for development of a novel anti-tumor drug.
Owner:SICHUAN UNIV
Boletus speciosus Frost polysaccharide BSF-X as well as preparation method and application thereof
ActiveCN109232753AGood immunomodulatory activityPromote proliferationOrganic active ingredientsImmunological disordersL929 cellSide chain
The invention discloses boletus speciosus Frost polysaccharide BSF-X as well as a preparation method and an application thereof. The boletus speciosus Frost polysaccharide mainly comprises beta-D-glucose and alpha-D-galactose in the ratio being 2:1, has the average molecular weight of about 141309 Da, has a main chain of (1 arrow 4)-beta-D-glucose, a side chain of arrow 1,6)-alpha-D-galactose connected on 6-O and a arrow 4)-beta-D-glucose connected on galactose 2-O of the side chain. The boletus speciosus Frost polysaccharide has higher immunoregulation activity and can significantly or very significantly promote proliferation of three immune cells including B, T and Raw264.7; BSF-X can promote proliferation of cells, promote macrophage to release immune factors IL-23 and TNF-alpha, has aquite significant inhibition function on L929 in vitro and can inhibit growth of S180 tumor in vivo.
Owner:西充星河生物科技有限公司 +1
Whole human TNF alpha (tumor necrosis factor alpha) monoclonal antibody and preparation and application thereof
The invention relates to the technical field of biology, and discloses a whole human TNF alpha (tumor necrosis factor alpha) monoclonal antibody and preparation and application thereof. The invention adopts a whole human antibody technology and acquires an anti-human TNF alpha whole human antibody. Preliminary analyses on the physical, chemical and biological activities of the antibody indicate that the antibody has better affinity with TNF, and can effectively neutralize the killing effect of the TNF on L929 cells in vitro. The whole human TNF alpha monoclonal antibody disclosed by the invention minimizes the immunogenicity of a human body. A PEG (polyethylene glycol) surface modification technology is adopted, thus avoiding the defect of short half life of small molecule antibody, and the whole human TNF alpha monoclonal antibody is more suitable for application in vivo while keeping the anti-TNF alpha activity.
Owner:优锐生物医药科技(深圳)有限公司
(S,R)-3-phenyl-4,5 dihydro-5-isoxazole acetic acid-nitric oxide and use thereof as anti-cancer and antiviral agent
Owner:ONCONOX
Hybridoma fluid nutrient medium free of feeder cells
The invention provides a hybridoma fluid nutrient medium free of feeder cells. The hybridoma fluid nutrient medium free of feeder cells contains a, 20-30% in volume of a culture fluid culturing L929 cells; b, 20-30% in volume of a culture fluid culturing L6 / 36 cells; and c, 8-20% in volume of fetal calf serum. Compared with the prior art, the tedious process of preparing feed cells is cancelled, and the synthesized hybridoma are more easily observed and can grow and breed normally.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Construction of domestic porcine tumor necrosis factor mutant and protein expression purification method
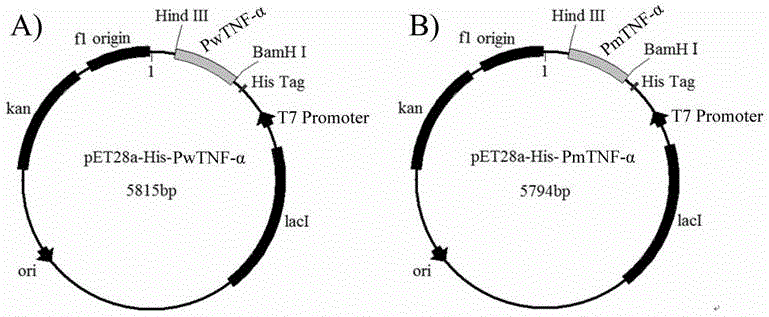
InactiveCN104561022ALow cytotoxicityHigh cytotoxic activityPeptide/protein ingredientsTumor necrosis factorEscherichia coliL929 cell
The invention relates to site-directed mutagenesis of a domestic porcine tumor necrosis factor (TNF-alpha) gene, and a protein preparation method and application. One mutant gene of the porcine tumor necrosis factor (TNF-alpha) has the sequence disclosed as SEQ ID NO.2. The mutant gene translation protein amino acid sequence is disclosed as SEQ ID NO.3; the mutant can be connected into a pET28a prokaryotic expression plasmid and transformed into Escherichia coli BL21 (DE3) to induce expression, and Ni<+>- / NTA column purification is performed to obtain the recombinant His6-PmTNF-alpha; and the CCK-8, LDH detection and other experiments prove that the mutant protein can obviously enhance the cytotoxicity for L929 cells and lower the toxic or side effects on normal cells L02 as compared with the TNF-alpha wild type protein.
Owner:NANJING NORMAL UNIVERSITY
A kind of biodegradable Zn-Na series zinc alloy and preparation method thereof
The invention discloses a biodegradable Zn-Na-series zinc alloy, and belongs to the field of medical implant materials. The biodegradable Zn-Na-series zinc alloy comprises 0.01-0.97% of Na, one or more of 27 elements which are harmless or beneficial to a human body, and the balance Zn, wherein in order to lower the cost and obtain excellent comprehensive performance, the total amount of the addedvarious alloy elements is optimally controlled to be no more than 2.0%. A preparation processing process of the zinc alloy comprises the steps of continuous casting, hot extrusion and rolling; or continuous casting, hot extrusion, solidification heat treatment and drawing; or continuous casting, uniform heat treatment, hot extrusion and rolling. According to the biodegradable Zn-Na-series zinc alloy, the yield strength is 100-500 MPa, the tensile strength is 150-700 MPa, and the elongation is 1.5-100%; the degradation rate in simulated body fluid is no more than 0.8 mm / year; and the cytotoxicity to L929 cells and human mesenchymal stem cells is at the zero stage or the one stage, good biocompatibility is shown, and the biodegradable Zn-Na-series zinc alloy can be used for various medical implants.
Owner:北京尚宁科智医疗器械有限公司
Porous cuprous oxide nano crystals, and preparation method and application thereof
InactiveCN110526274AEasy to prepareLow priceMaterial nanotechnologyInorganic active ingredientsFiberL929 cell
The invention relates to porous cuprous oxide nano crystals, and a preparation method and application thereof. The preparation method comprises following steps: ultrasonically dissolving polyethyleneglycol in a copper acetate monohydrate aqueous solution, adding a sodium hydroxide aqueous solution, adding an ascorbic acid aqueous solution after the reaction is finished, and continuously reactingan obtained mixed solution under the protection of nitrogen after the addition is finished; and then centrifuging the mixed solution, washing with deionized water and ethanol respectively, drying a solid obtained by centrifugation in a vacuum drying oven, and storing in nitrogen. The preparation method of the porous cuprous oxide nano crystals is simple; the raw materials are widely available andcheap. The prepared cuprous oxide nano crystals have an obvious pore channel structures. The prepared porous cuprous oxide nano crystals have a remarkable inhibition effect on tumor cells A549, and have low cytotoxicity on normal cell mouse fibroblasts L929.
Owner:SOUTHEAST UNIV
(s,r.)-3-phenyl-4,5 dihydro-5-isoxazole acetic acid-nitric oxide and use thereof as Anti-cancer and antiviral agent
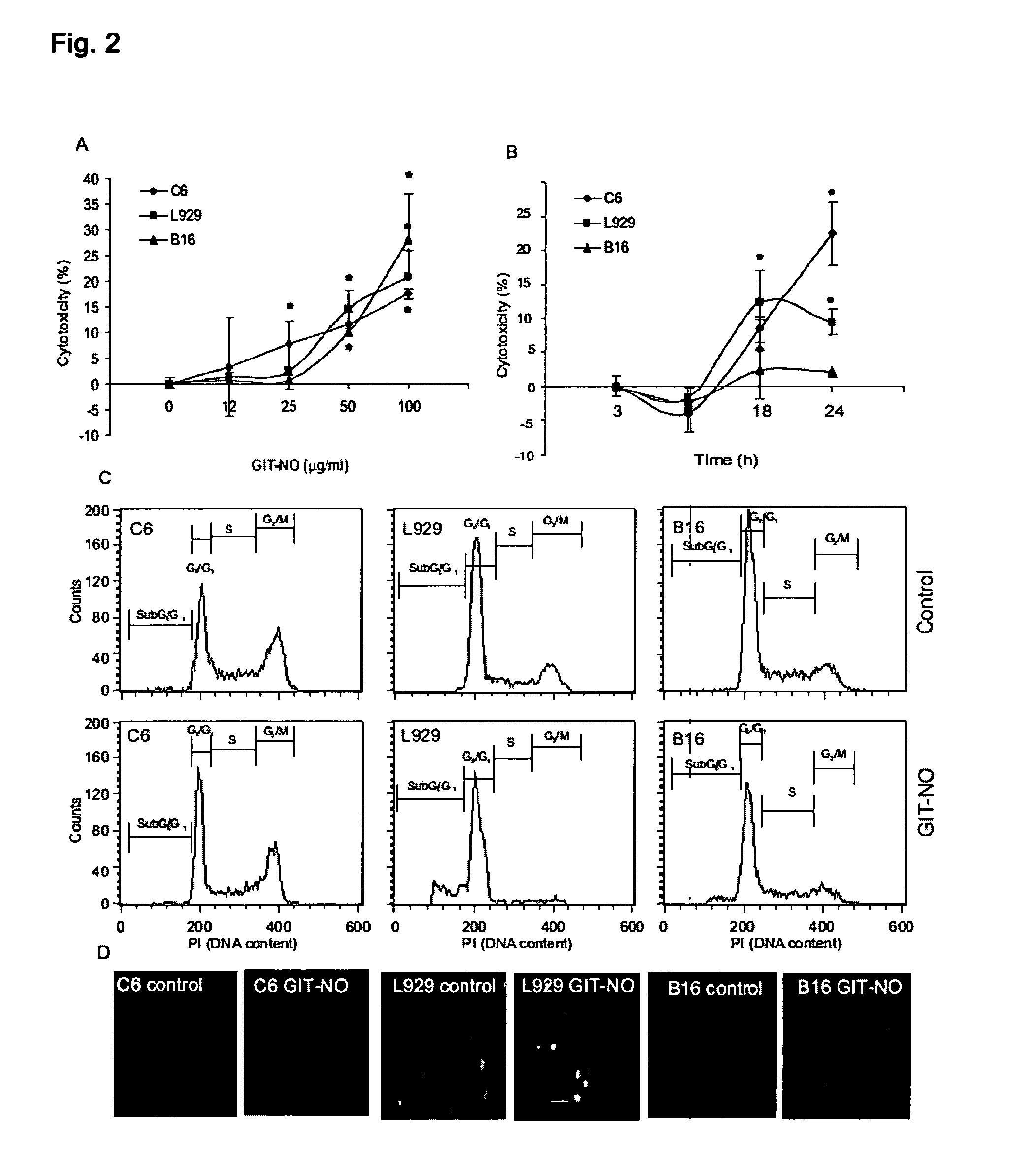
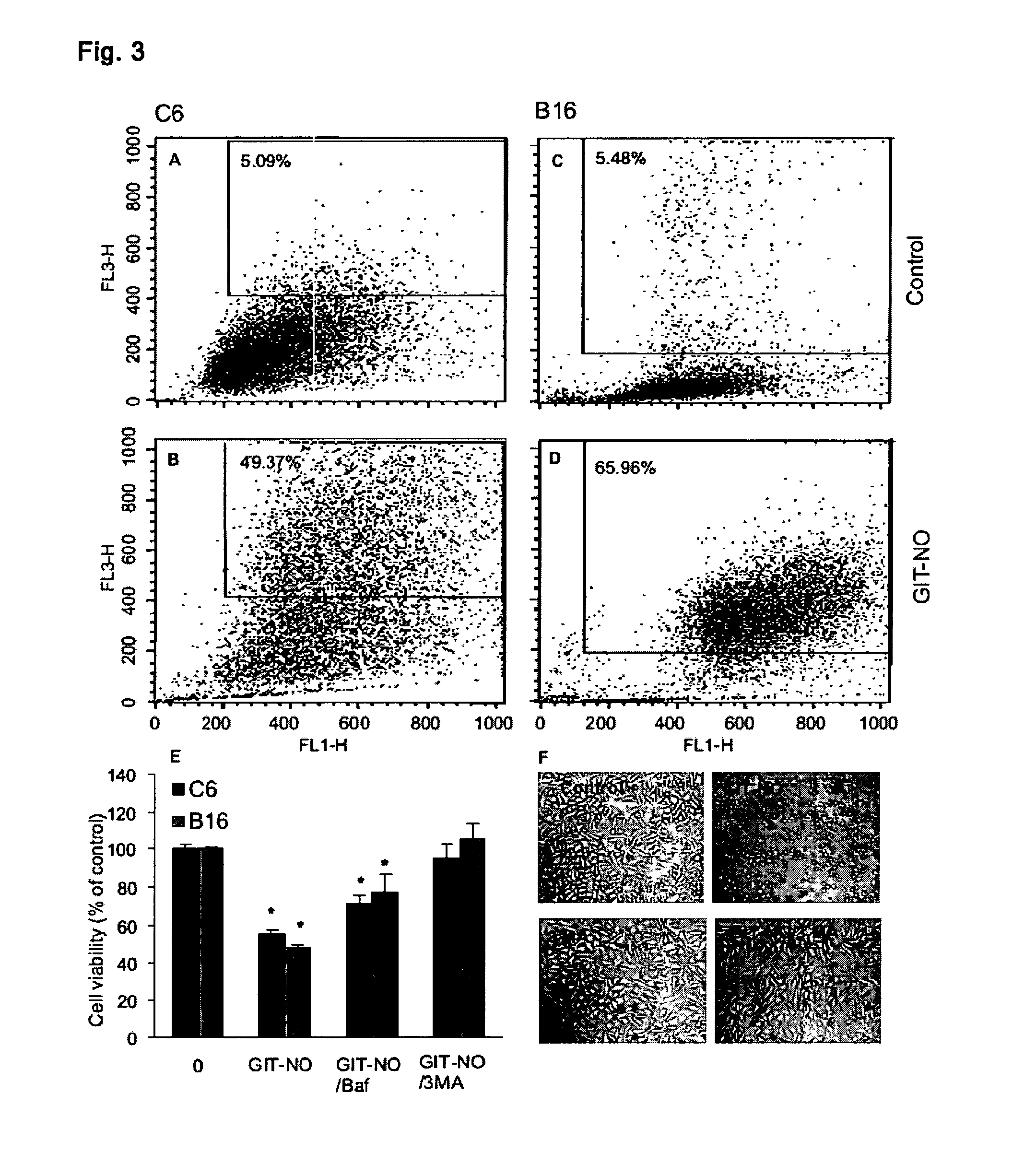
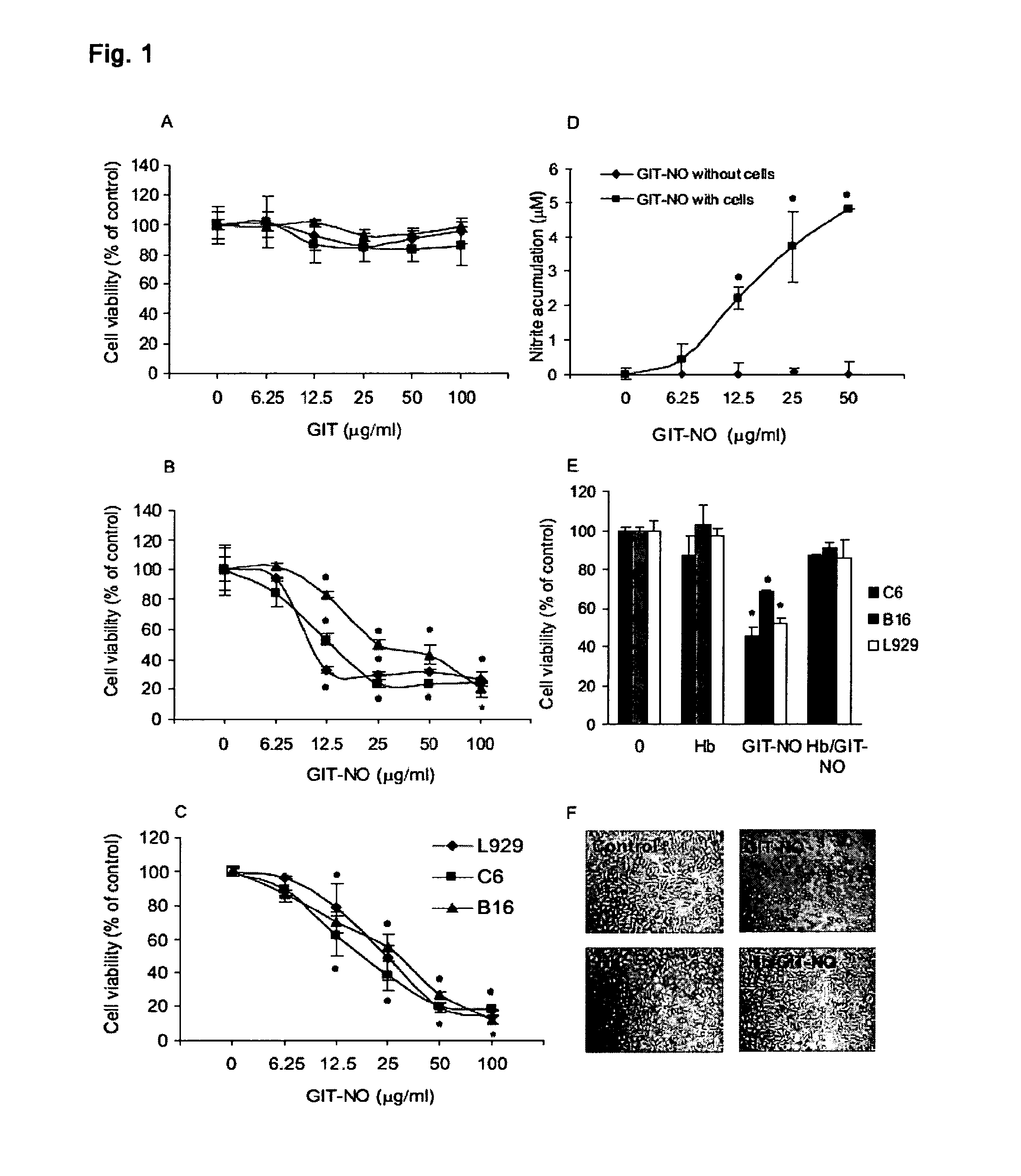
The present invention relates to an isoxazole derivative, the compound of formula (I)herein after referred to as GIT27-NO, which is the NO-donating structurally modified form of (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid, herein after referred to as VGX-1027.Treatment of three tumor cell lines, rat astrocytoma C6, mouse fibrosarcoma L929, and mouse melanoma B16 cells with GIT27-NO resulted in a significant reduction of cell respiration and of number of viable cells, while VGX-1027 was completely ineffective. Hemoglobin, which act as NO-scavenger, restored cell viability, thus indicating the NO-mediated tumoricidal effect of compound (I). GIT27-NO triggered apoptotic cell death in L929 cell cultures, while autophagic cell death is mainly responsible for the diminished viability of C6 and B16 cells. Moreover, GIT27-NO induced the production of reactive oxygen species which can be neutralized by antioxidant N-acetyl cysteine (NAC), indicating that reactive oxygen species (ROS) are at least partly involved in the reduction of cell viability. The anti-tumor activity of GIT27-NO is mediated through activation of MAP kinases (ERK1 / 2, p38 and JNK) in cell-specific manner. The role of MAP kinases was further confirmed by specific inhibitors of these molecules, PD98059, SB202190, and SP600125. Finally, in vivo treatment with GIT27-NO significantly reduced tumor growth in syngeneic C57BL / 6 mice implanted with B16 melanoma.
Owner:ONCONOX
Extracting method for plant composition containing valeriana officinalis and application
The invention provides an extracting method for a plant composition containing valeriana officinalis. The plant composition is prepared with 100 g of the valeriana officinalis, 60 g of cissus javana, 50 g of coprinopsis atramentaria, 70 g of salix cheilophila roots and 150 g of callicarpa macrophylla leaves as raw medicinal materials. Preparing is carried out with a microwave extraction method, so that the content is greatly increased, and use amount is reduced. The invention further provides an application of the plant composition containing the valeriana officinalis in preparing medicine for inhibiting mice fibroblast L929 cell proliferation.
Owner:南京多宝生物科技有限公司
A kind of feeder-free hybridoma cell liquid culture medium
ActiveCN108239624BSolving the challenge of adding feeder cells to growAvoid pollutionFused cellsL929 cellCulture fluid
The application provides a feeder-free hybridoma liquid culture medium, wherein the hybridoma liquid culture medium contains: a, 20%-30% of the total volume of cultured L929 cells; b, accounting for 20%-30% of the total volume; 20%-30% of the total volume of culture medium for culturing C6 / 36 cells; c, 8%-20% of the total volume of fetal bovine serum. Compared with the prior art, this application omits the cumbersome process of preparing feeder cells, making the synthetic hybridoma cells easier to observe and able to grow and reproduce normally.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
New natural product catathelasma imperiale sing polysaccharide CIS-A and application thereof
ActiveCN107325199ASignificant immunomodulatory activityPromote proliferationImmunological disordersAntineoplastic agentsL929 cellSide chain
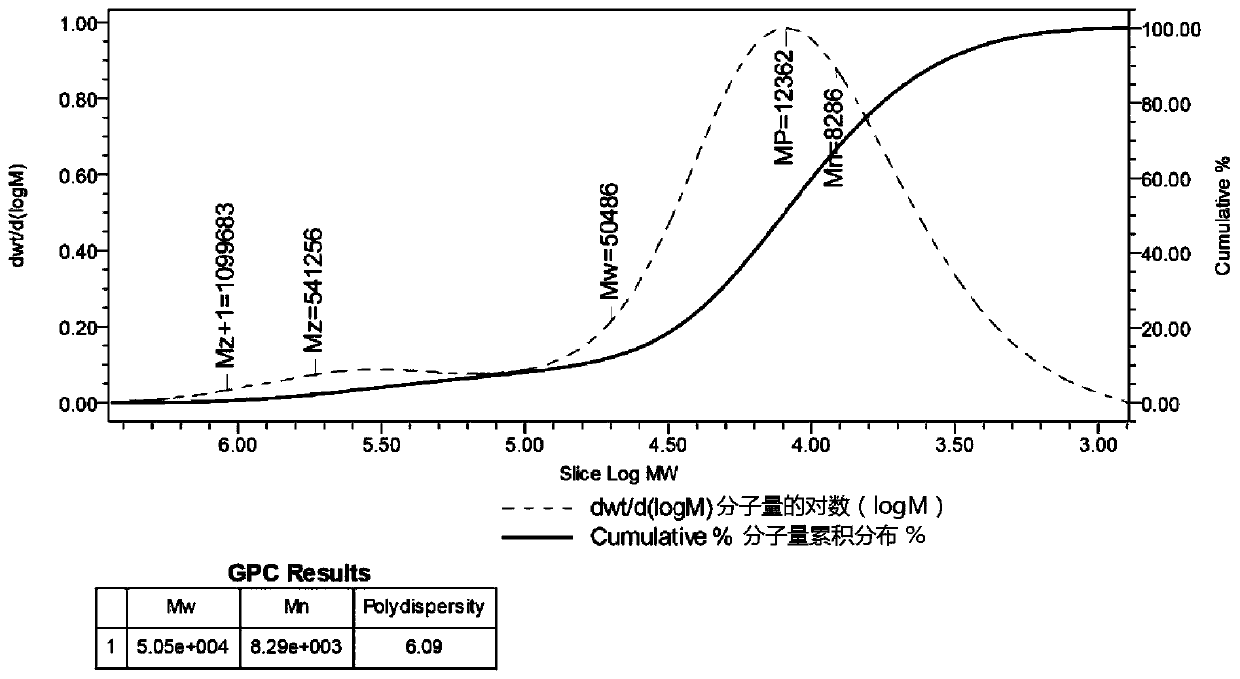
The invention discloses a new natural product catathelasma imperiale sing polysaccharide CIS-A and an application thereof. The catathelasma imperiale sing polysaccharide CIS-A is heteropolysaccharide composed of two monosaccharides, namely alpha-D-glucose and beta-L-galactose, in a ratio of 3:2, the average molecular weight is about 50486Da, the alpha-D-glucose connected by 1,3- and the alpha-D-glucose connected by 1,2,3- are main chains, and a side chain is the beta-L-galactose connected by two 2,3-. The catathelasma imperiale sing polysaccharide CIS-A laetiporus sulphureus polysacharride has obvious immunoregulatory activity, heteropolysaccharide CIS-A can have an obvious inhibiting effect on L929 cells cultured in vitro, and the survival rate of the cells is 68.9% when the concentration of CIS-A is 5 mu g / mL; and growth of S180 tumor is inhibited in vivo, and the inhibition ratio reaches up to 64.4%.
Owner:CHINA WEST NORMAL UNIVERSITY
Cytotoxicity test method for medical device
A new two-layer modified agar overlay cytotoxicity method for medical device safety assessment are developed. The new two layer modified agar overlay cytotoxicity method has equivalent sensitivity, and greater efficiency compared to the ISO / USP direct contact and agar overlay assays. Assays were carried out in 6-well microplates. Nutrient agar medium with a 0.5% agar concentration was used to provide a base nutrient layer to support cell growth, L929 cells mixed nutrient agar (0.33% agar) were seeded on top of the base agar and cytotoxicity was evaluated after 24 hours per USP. Results demonstrated the two layer modified agar overlay cytotoxicity assay performs as well as the ISO / USP direct contact method with distinct advantages. Results showed that this two layer modified agar overlay method is more promising as compared to the traditional agar overlay and direct contact methods due to the diluted soft agar, avoided potential mechanical damage from test materials, and represents a valuable tool to evaluate medical device cytotoxicity.
Owner:ALCON INC
Whole human TNF alpha (tumor necrosis factor alpha) monoclonal antibody and preparation and application thereof
The present invention discloses anti-human TNF-alpha monoclonal antibodies which have light chain amino acid sequence (SEQ ID NO: 3) and heavy chain amino acid sequence (SEQ ID NO: 4 or SEQ ID NO: 6). The anti-human TNF-alpha monoclonal antibodies have complete human antibody sequences to ensure low immunogenicity. The manufacturing methods and uses of the antibodies are also provided.
Owner:优锐生物医药科技(深圳)有限公司
A new natural product polysaccharide cis-a from Mushroom magnificence and its application
ActiveCN107325199BSignificant immunomodulatory activityPromote proliferationImmunological disordersAntineoplastic agentsL929 cellSide chain
The invention discloses a new natural product catathelasma imperiale sing polysaccharide CIS-A and an application thereof. The catathelasma imperiale sing polysaccharide CIS-A is heteropolysaccharide composed of two monosaccharides, namely alpha-D-glucose and beta-L-galactose, in a ratio of 3:2, the average molecular weight is about 50486Da, the alpha-D-glucose connected by 1,3- and the alpha-D-glucose connected by 1,2,3- are main chains, and a side chain is the beta-L-galactose connected by two 2,3-. The catathelasma imperiale sing polysaccharide CIS-A laetiporus sulphureus polysacharride has obvious immunoregulatory activity, heteropolysaccharide CIS-A can have an obvious inhibiting effect on L929 cells cultured in vitro, and the survival rate of the cells is 68.9% when the concentration of CIS-A is 5 mu g / mL; and growth of S180 tumor is inhibited in vivo, and the inhibition ratio reaches up to 64.4%.
Owner:CHINA WEST NORMAL UNIVERSITY
Embryo cattle serum substitute without serum for animal cell culture
InactiveCN100432218CLow costTo achieve the purpose of replacing serumAnimal cellsL929 cellHuman platelet
The invention relates to a non-serum animal cell culture used fetus cattle serum displace agent, which is formed by recombination human insulin growth factor-1:0.1g-0.5g / l, recombination human platelet sourced growth factor-B:0.1g-0.6g / l, recombination human alkali desmocyte growth factor: 0.05-0.4g / l, cattle transferring: 1g-5g / l, cattle serum protein (V component): 2g-15g / l, and mercaptoethanol 0.3-0.5mol / l.
Owner:上海尚优生物科技有限公司
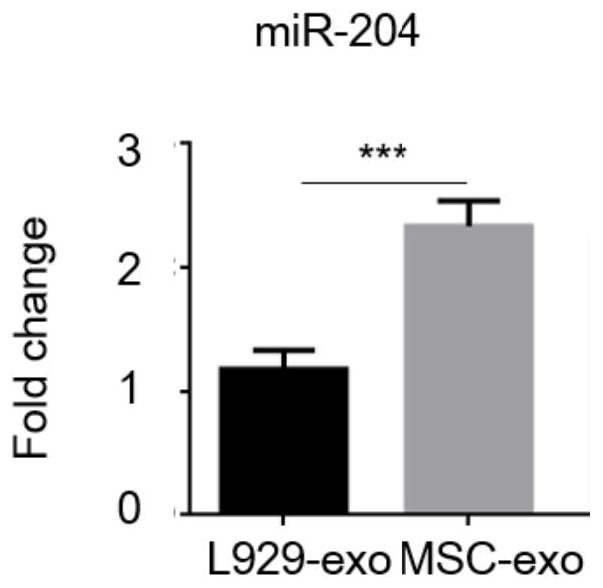
A kind of exosome containing mir-204 and its preparation method and application
ActiveCN112094808BImprove dry eye symptomsSymptoms improvedOrganic active ingredientsSenses disorderLipofectamineL929 cell
The present invention provides an exosome loaded with miR-204, which can significantly improve the symptoms and signs of dry eye disease. By highly expressing miR-204 in L929 cells, the present invention obtains exosomes loaded with miR-204 that are safe, high-yield, and stable in source, which solves many limitations such as difficult cultivation and poor stability of MSC-derived exosomes in practical applications, At the same time, it solves the problems of unstable transfection efficiency of liposome transfection, large differences between different cells, high toxicity of liposome transfection reagents, short action time, and poor stability, and promotes clinical transformation.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Application of hance brandisia herb tablets to preparing medicine for suppressing proliferation of fibroblast L929
The invention belongs to the technical field of traditional Chinese medicine, and particularly relates to an application of hance brandisia herb tablets to preparing medicine for suppressing proliferation of fibroblast L929 and a preparing method of the hance brandisia herb tablets. The hance brandisia herb tablets are prepared with 1,700 g of hance brandisia herbs as raw medicinal materials in a supercritical-extraction mode, and the content of acteoside is greatly increased accordingly.
Owner:JINAN XINSHIDAI MEDICINE SCI & TECH
Exosome containing miR-204 and preparation method and application of exosome
ActiveCN112094808AImprove dry eye symptomsSymptoms improvedOrganic active ingredientsSenses disorderL929 cellLipofectamine
The present invention provides an exosome loaded with miR-204. The exosome is capable of remarkably relieving symptoms and signs of xerophthalmia. The miR-204 is highly expressed in L929 cells, the obtained exosome loaded with miR-204 is safe, high in yield and stable in source, various problems that the MSC-derived exosome is difficult in culture, poor stability and the like in practical applications are solved, meanwhile, the problems of unstable liposome transfection efficiency, large differences between different cells, relatively high toxicity of a liposome transfection reagent, short action time and poor stability are solved, and clinical transformation is promoted.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
A compound with anticancer activity and its preparation method
ActiveCN103588818BLow cytotoxicityImprove anti-cancer effectGroup 8/9/10/18 element organic compoundsAntineoplastic agentsL929 cellCantharidin
The invention provides a compound having anticancer activity, which is obtained by cooperation of Norabieta cantharidin and platinum (IV) and modification of a piperazine derivative. The compound is characterized in that a Norabieta cantharidin piperazine derivative ligand is introduced, thereby cytotoxicity of a platinum (IV) compound is reduced, and high antineoplastic activity is provided. The result of the cytotoxicity experiments shows that the provided platinum (IV) compound has less destruction to normal mice fibroblast L929, but has good killing effect to cancer cells such as human breast cancer MCF-7 cell, human cervical carcinoma HeLa cell and human lung adenocarcinoma A549 cell, so that the compound having anticancer activity is capable of reducing the system toxicity and having equal anticancer activity with cisplatin. The compound having anticancer activity has good killing effect by aiming at cisplatin resistance human lung adenocarcinoma A549 / DDP cell. Therefore, the toxicity is reduced, the cisplatin resistance can be reversed, and anticancer effect of the platinum medicines can be increased.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com