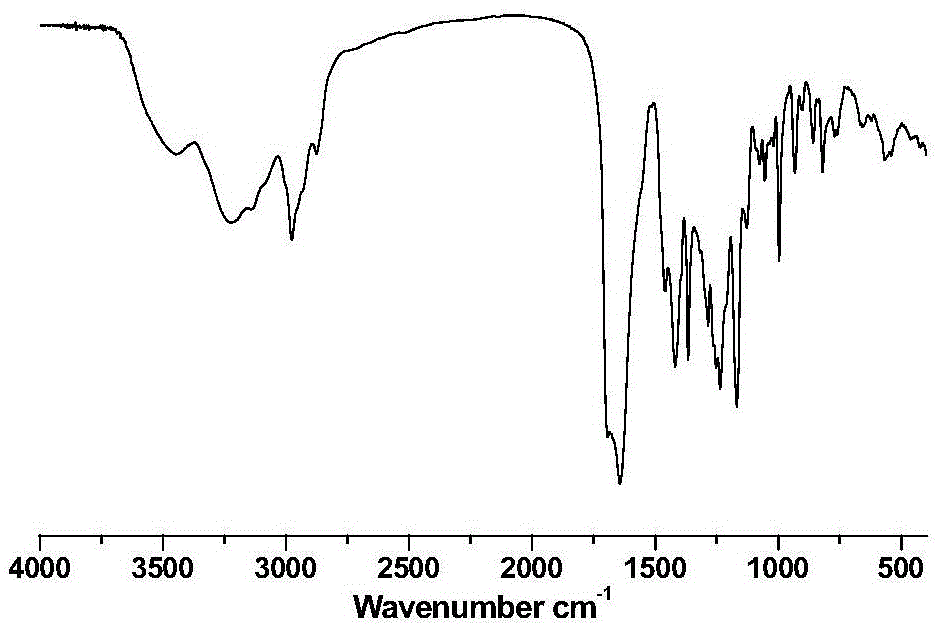
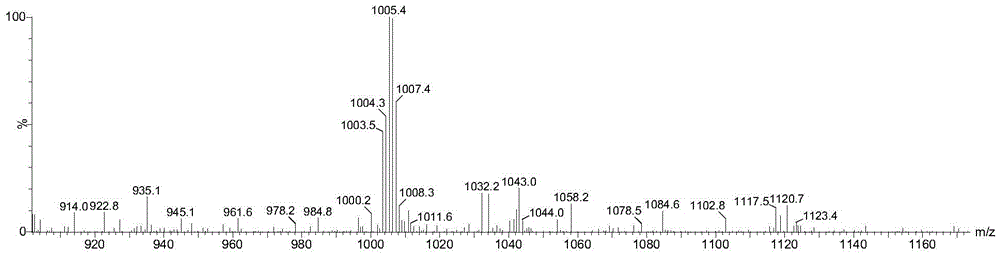
A compound with anticancer activity and its preparation method
A technology of anti-cancer activity and compound, applied in the field of compounds with anti-cancer activity and its preparation, can solve the problems of Satraplatin’s lack of outstanding advantages and further improvement of anti-tumor activity, so as to improve anti-cancer effect and reverse cisplatin resistance sex, reduce cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention also provides a preparation method of a compound with anticancer activity, comprising:
[0035] A) reacting platinum (II) compound with hydrogen peroxide to obtain platinum (IV) compound;
[0036] B) reacting the platinum (IV) compound obtained in step A) with demethylcantharidin to obtain a platinum (IV) complex with demethylcantharidin as a ligand;
[0037] C) react the platinum (IV) complex obtained in step B) with N-tert-butoxycarbonylpiperazine in the presence of a condensing agent to obtain the compound shown in the structure of formula (I); wherein, the R5 is tert-butoxycarbonyl;
[0038] Or react the complex of platinum (IV) and demethylcantharidin obtained in step B) with N-methylpiperazine in the presence of a condensing agent to obtain the compound shown in the structure of formula (I); wherein , the R5 is a methyl group.
[0039] The compound represented by the formula (I) provided by the present invention is synthesized in three ste...
Embodiment 1
[0061] Put cisplatin (600mg, 2mmol) in a flask, then add 10mL of 30% hydrogen peroxide, stir at room temperature in the dark for 8 hours, remove the hydrogen peroxide by filtration to obtain Cisplatin(IV)-(OH) 2 yellow powder.
[0062] The resulting Cisplatin(IV)-(OH) 2 (334mg, 1mmol) and demethylcantharidin (336mg, 2mmol) were dissolved in dry DMF, and stirred at 70°C for 12h. Then drain the DMF in the system, dissolve the remaining substance with acetone, and filter off the insoluble matter, concentrate the filtrate and settle the ether, filter to obtain a solid and vacuum dry it to obtain a white solid powder Cisplatin (IV)-(DMC) 2 .
[0063] The resulting Cisplatin(IV)-(DMC) 2 (335mg, 0.5mmol) and N,N'-carbonyldiimidazole (162mg, 1mmol) were dissolved in dry DMF and stirred at 40°C for 2h. Then N-tert-butoxycarbonylpiperazine (186mg, 1mmol) was added, and the stirring reaction was continued for 24h. After the reaction, the DMF in the system was vacuum-dried, and then ...
Embodiment 2
[0069] Put cisplatin (600mg, 2mmol) in a flask, then add 10mL of 30% hydrogen peroxide, stir at room temperature in the dark for 8 hours, remove the hydrogen peroxide by filtration to obtain Cisplatin(IV)-(OH) 2 yellow powder.
[0070] The resulting Cisplatin(IV)-(OH) 2 (334mg, 1mmol) and demethylcantharidin (1009mg, 6mmol) were dissolved in dry DMF, and stirred at 70°C for 24h. Then drain the DMF in the system, dissolve the remaining substance with acetone, and filter off the insoluble matter, concentrate the filtrate and settle the ether, filter to obtain a solid and vacuum dry it to obtain a white solid powder Cisplatin (IV)-(DMC) 2 .
[0071] The resulting Cisplatin(IV)-(DMC) 2 (335mg, 0.5mmol) and N,N'-carbonyldiimidazole (203mg, 1.25mmol) were dissolved in dry DMF and stirred at 60°C for 12h. Then N-tert-butoxycarbonylpiperazine (372.5mg, 2mmol) was added, and the stirring reaction was continued for 72h. After the reaction, the DMF in the system was vacuum-dried, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com