Construction of domestic porcine tumor necrosis factor mutant and protein expression purification method
A technology of tumor necrosis factor and mutants, applied in the direction of tumor necrosis factor and peptide preparation methods, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The construction of porcine tumor necrosis factor mutant (PmTNF-α) gene, the pig spleen was obtained from Nanjing Qixia Yaohua pig slaughterhouse, and it was Landrace pig:
[0032] (1) Total RNA extraction: RNA extraction kit (TIANGEN) was used to extract total RNA from porcine spleen tissue according to its operation manual, and its quality and purity were identified by formaldehyde-denatured agarose gel electrophoresis, and its concentration was determined by an ultraviolet spectrophotometer. SMART TM RACE kit (TaKaRa) was reverse transcribed into first-strand cDNA.
[0033] (2) Design large fragment primers containing mutation sites, and construct tumor necrosis factor mutant genes by PCR method. Primer sequences such as SEQ ID NO.4 and SEQ ID NO.5, using the cDNA template obtained by reverse transcription for PCR:
[0034]①The reaction system is 50 μl, 10 μmol / L F1, 2 μl each of R1, 2.5 mmol / L dNTP 8 μl, 2×GC buffer II 25 μl, cDNA template 3 μl, DreamTaq enzyme 0...
Embodiment 2
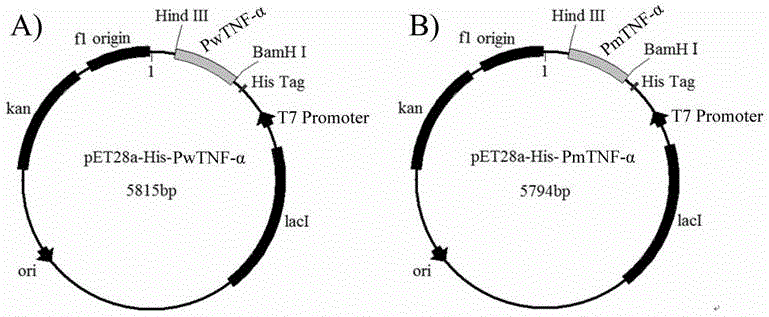
[0037] Construction of recombinant expression vector of porcine tumor necrosis factor (TNF-α) mutant, its induced expression in Escherichia coli, purification and identification;
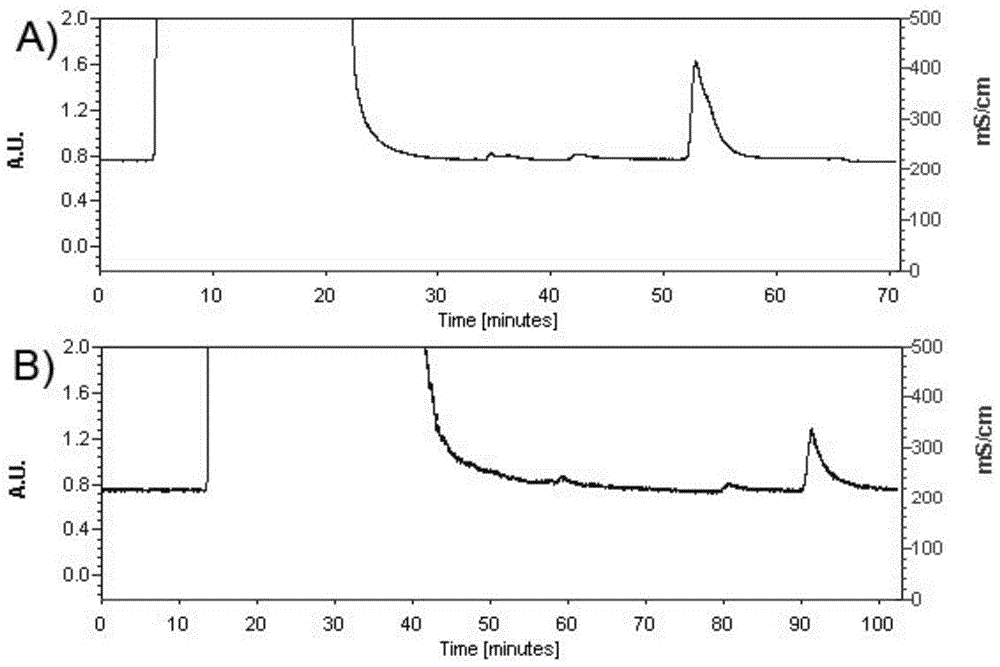
[0038] According to the constructed TNF-α mutant sequence (gene sequence such as SEQ ID NO.2), design primers F2 and R2. The 5' end of F2 has a BamHI restriction site, and the 5' end of R2 has a HindⅢ restriction site. The first-strand cDNA of porcine is used as a template, and F2 and R2 are used as primers (F2 5'–CGCGGATCCAAGCGCAAGCCCGTCGCCCACGTTGT-3' (SEQ ID NO.6), R25'-CCCAAGCTTTCAGAAGGCAATGATCCCAAAATAGT-3' (SEQ ID NO.7), the gene was amplified by PCR (10×pfu Buffer 5 μl, dNTP (2.5mM) 4 μl, F2 (10 μmol / L) 2 μl, R2 (10 μmol / L) 2μl, H 2 O 33.5 μl, cDNA 3 μl, pfu Taq 0.5 μl) The reaction conditions are as follows: 94 ° C for 5 min, (94 ° C for 30 s, 59 ° C for 30 s, 72 ° C for 1 min), 35 cycles, and finally 72 ° C for 7 min, and then the PCR product was tapped and recovered , the recovered produc...
Embodiment 3
[0044] Analysis of His by CCK-8 method 6 - Cytotoxic activity of PmTNF-α on L929 cells;
[0045] Culture L929 cells in 7ml 1640 (10% FBS) medium for 24 hours. After removing the medium, digest 1ml of trypsin for 30s, add 1ml of medium to stop the digestion, resuspend the cells, pipette into a 15ml centrifuge tube, 1000g, 20°C, 3min. After removing the supernatant, add 2ml of medium (1640, 10% FBS), resuspend the cells, pipette 1ml into a 50ml centrifuge tube, add 10% FBS 1640 medium to dilute, and count on a counting plate to make the number of cells 3×10 5 pieces / ml. Pipette the above-mentioned diluted cell suspension into a 96-well plate, 100 μl per well, 37°C, 5% CO 2 , cultivated for 24h. Remove culture medium. Use 10% FBS 1640 medium to configure different concentrations of TNF-α dilutions (divided into two groups of wild-type and mutant types), and then add 100 μl to each well to make the final concentration of TNF-α reach 10 5 、10 4 、10 3 、10 2 , 10, 10 0 、10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com