Novel tumor vaccine preparation method
A tumor vaccine and a new technology, applied in the field of anti-tumor, can solve the problems of low vaccine activation efficiency, limited large-scale use, poor clinical efficacy, etc., and achieve easy large-scale production, good biosafety and biocompatibility, Addressing the effect of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The invention provides a preparation method of a novel tumor vaccine, which is prepared by mixing tumor cell lysates treated with hypochlorous acid (HClO) and an immune activator, and the immune activator is specifically CpG.
[0030] The preparation method of the tumor cell lysate after the hypochlorous acid (HClO) treatment, the specific steps are as follows:
[0031] 1): Primary tumor cells are cultured after fresh tumor tissue is obtained, and primary tumor cells are obtained, and the tumor tissue is an autologous isolated tumor tissue;
[0032] 2): Treat primary tumor cells with hypochlorous acid (HClO). Before treatment with hypochlorous acid (HClO), the tumor cells need to be washed with HBSS balanced salt solution for 2-3 times. The concentration of hypochlorous acid (HClO) is 60-80μM, the supernatant was collected 2 hours after the treatment with hypochlorous acid (HClO), and the supernatant obtained after the cells were treated with hypochlorous acid (HClO) wa...
Embodiment 2
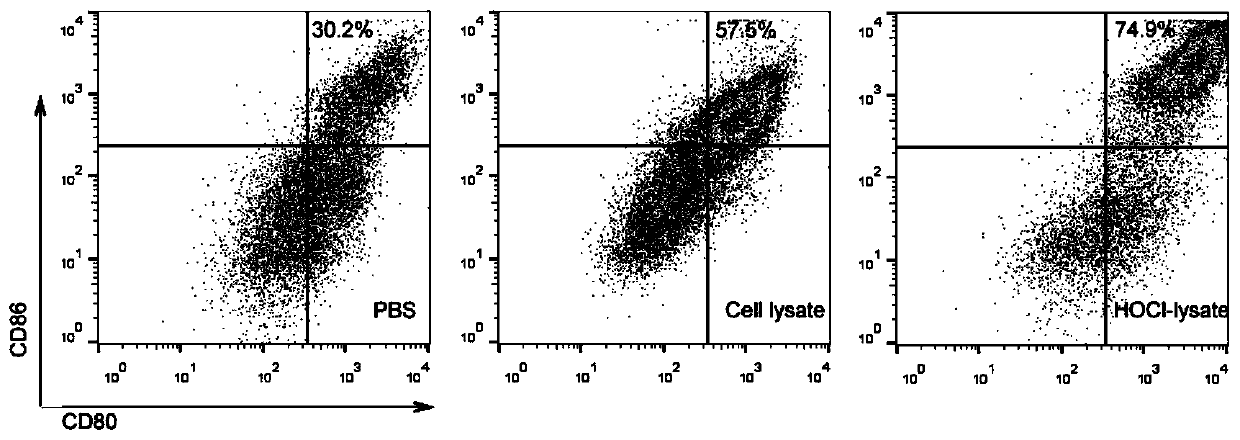
[0039] refer to Figure 1-2 , assessing the activation of DC cells by novel tumors:
[0040] Bone marrow stem cells were obtained from the femur and tibia of male C57 mice aged 6-8 weeks, cultured in 1640 medium containing M-CSF for 5 days to obtain immature DC cells, and then planted in 24-well plates, divided into PBS group, Cell lysate group group (obtained by repeated freezing and thawing), HClO-lysate group, each group took 40 μl of the corresponding solution and incubated with immature DC cells to stimulate the maturation of DC cells. After 24 hours, CD11c, CD80, and CD86 cells were detected by flow cytometry Activation (such as figure 1 shown), the experimental results showed that having a novel tumor vaccine can promote the maturation of DC cells.
Embodiment 3
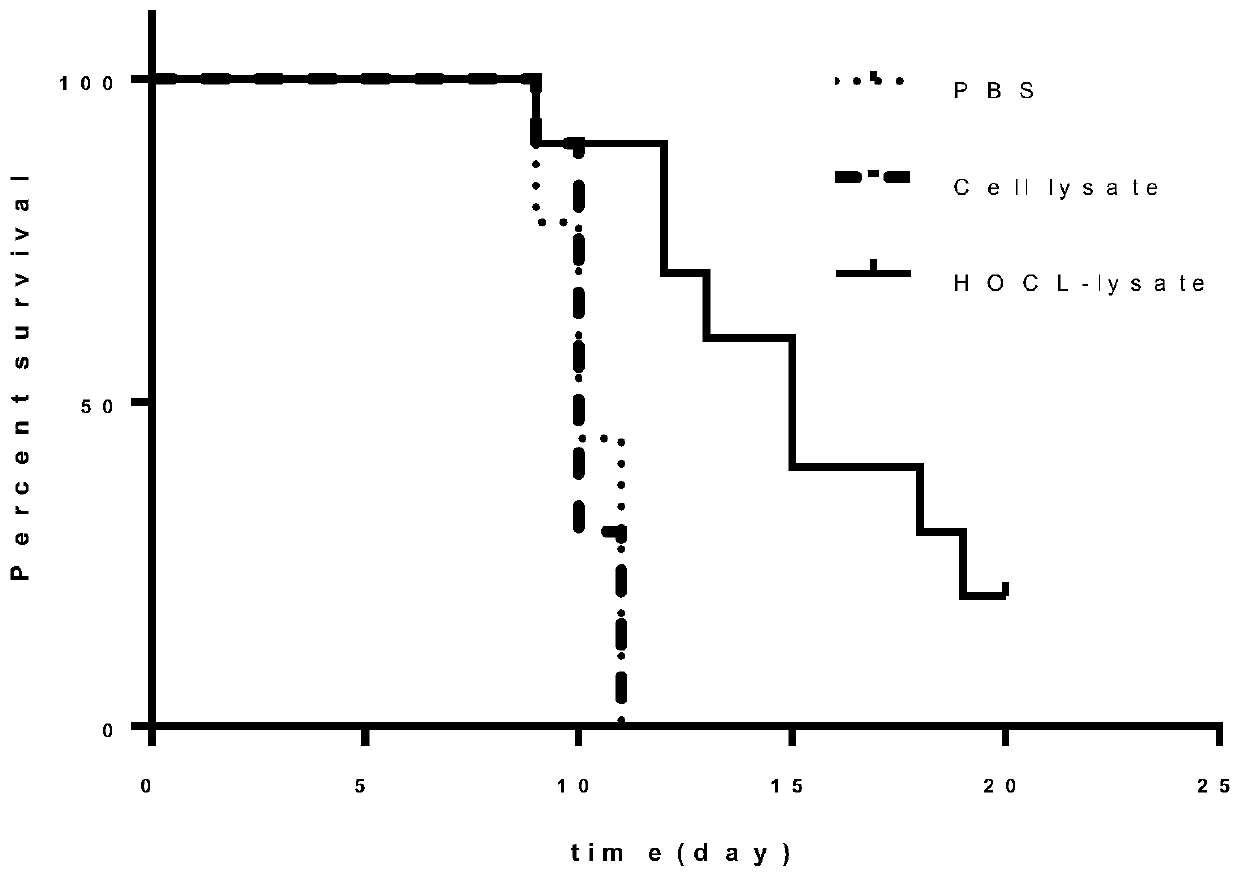
[0041] Example 3: Research on anti-tumor effect of novel tumor vaccine preventive therapy
[0042] The C57 mice were randomly divided into 3 groups, the first group of mice were injected with 50 μl PBS at the tail base + foot pads, the second group were injected with 50 μl Cell lysate at the tail base + foot pads, the third group were injected with 50 μl HClO-lysate at the tail base + foot pads, At this time, it was recorded as the first day, and the drug was given once every other day, for a total of 3 times. On the seventh day, malignant melanoma cells B16 (2*10 5 / 100μl / only), the survival situation was monitored regularly, experiments showed that the new tumor vaccine can significantly inhibit tumor growth.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com