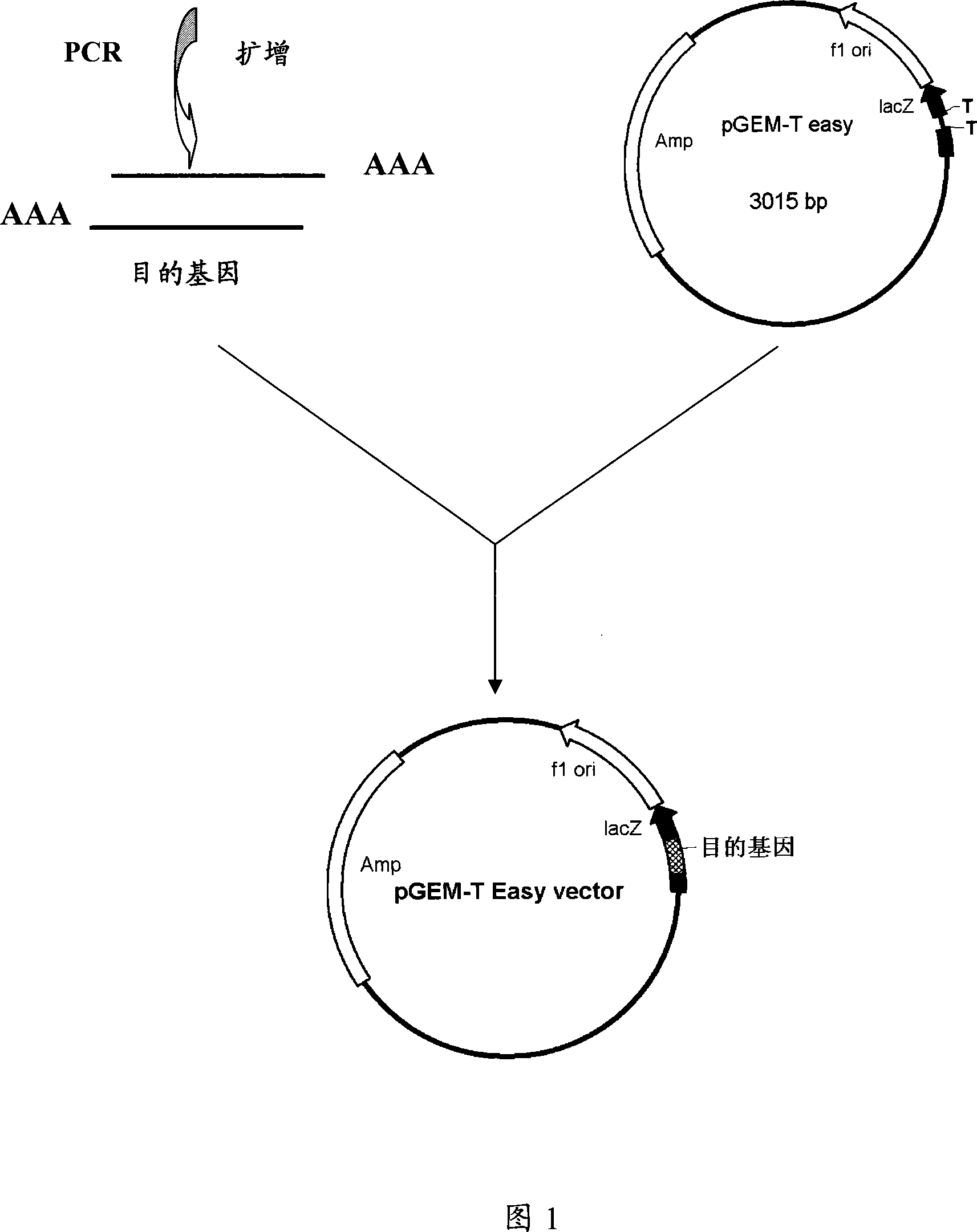
Recombinant expression and use for pertussis vaccine protective antigen
A pertussis and vaccine technology, applied in the field of bioengineering, can solve the problems of limited yield of active ingredients, antigenic variation, complex purification process, etc., and achieve the effect of improving immune protection effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Cloning of embodiment 1., PRN, FIM2 and FIM3 genes
[0074] 1.1 Amplification of PRN, FIM2 and FIM3 genes
[0075] Pertussis genomic DNA was extracted from B. pertussis CS strain (preserved in the serum laboratory of China National Institute for the Control of Pharmaceutical and Biological Products) with a whole genome DNA extraction kit (Promega). Primers were designed using PrimerPremier 5.0 software, and according to the pertussis PRN, FIM2 and FIM3 gene sequences reported by GeneBank (GenBank accession numbers are J04560, Y00527, X51543): respectively design 3 pairs of primers as shown in Table 1:
[0076] Gene
Primer sequence
(5'-3')
Location
(nt)
length
(bp)
PRN
FIM2
701
FIM3
Upstream 5'-ATGGCGGGCGTCTGCTCTCCACCTG-3'
Downstream 5-ACCTTGAAGTCATTCTCCAGGCGGC-3′
Upstream 5′-ACCCATGCAAATCCCTTTTCCAACGC-3′
Downstream 5′-GTATGTTGGCGATTTCCAGTTTCTC-3′
Upstre...
Embodiment 3
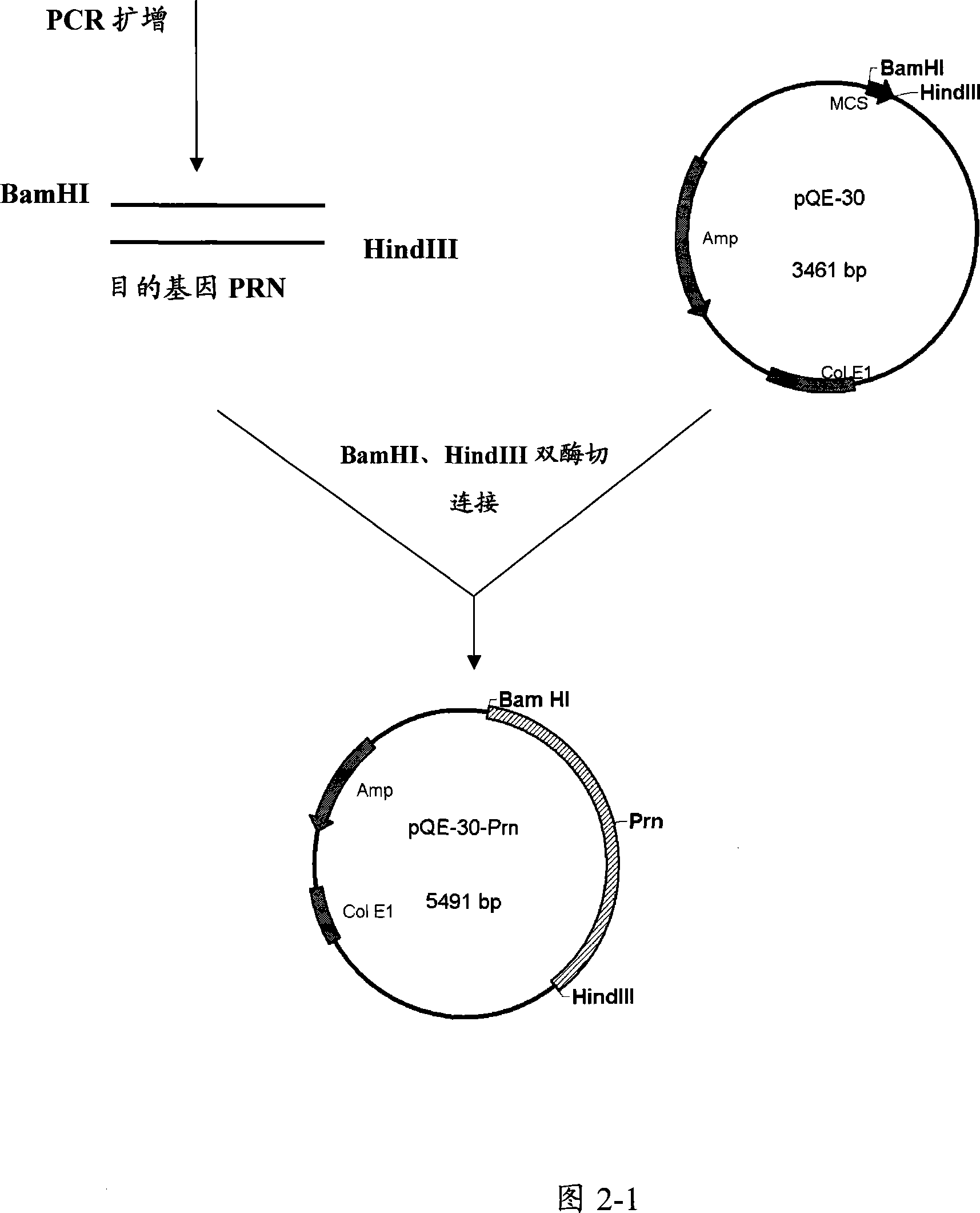
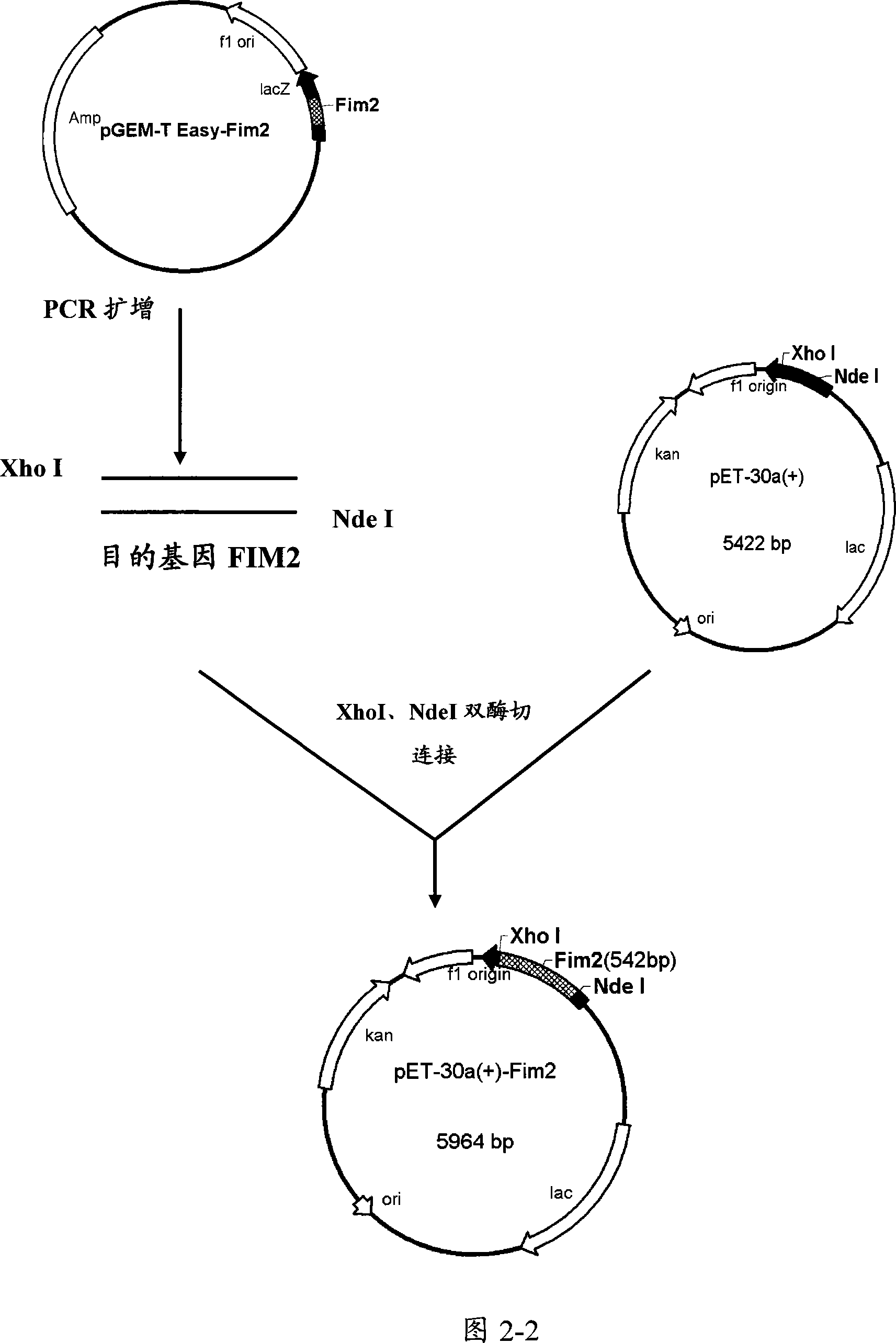
[0086] Example 3. Construction of recombinant expression plasmids
[0087] Target genes were amplified by PCR, and corresponding primers were designed according to the pertussis PRN, FIM2 and FIM3 gene sequences reported by GeneBank (GenBank accession numbers are J04560, Y00527, and X51543, respectively). The designed primers were used to amplify PRN, FIM2 and FIM3 genes respectively, and construct the corresponding expression plasmids of PRN, FIM2, FIM3 and FIM2-FIM3 fusion genes. Referring to Figures 2-1, 2-2, 2-3, and 2-4, the constructed prokaryotic expression plasmids are PRN-PQE30, FIM3-PQE30, FIM2-PET30a and FIM2-FIM3-PET30a.
Embodiment 4
[0088] Example 4. Expression of recombinant protein in Escherichia coli
[0089] The constructed recombinant plasmids were introduced into Escherichia coli to induce expression, that is, the recombinant plasmids PRN-PQE30 and FIM3-PQE30 were transferred into E. coli M15, FIM2-PET30a and FIM2-FIM3-PET30a were transferred into E. coli BL21(DE3), IPTG induced the expression of the target protein PRN, FIM2, FIM3 and FIM2-FIM3 fusion protein, and then purified the target protein; then analyzed by SDS-PAGE.
[0090] 4.1 Expression levels of recombinant proteins FIM2, FIM2-FIM3, FIM3 and PRN
[0091] SDS-PAGE gel scanning analysis was performed on the expression products, and ImageMaster TotalLab software analyzed and determined the protein expression level. The expression levels of the target proteins PRN, FIM2 and FIM2-FIM3 accounted for more than 40% of the total bacterial protein, and the expression level of FIM3 was 25%. See Figures 3, 4, 5, and 6.
[0092] 4.2 Existing forms...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com