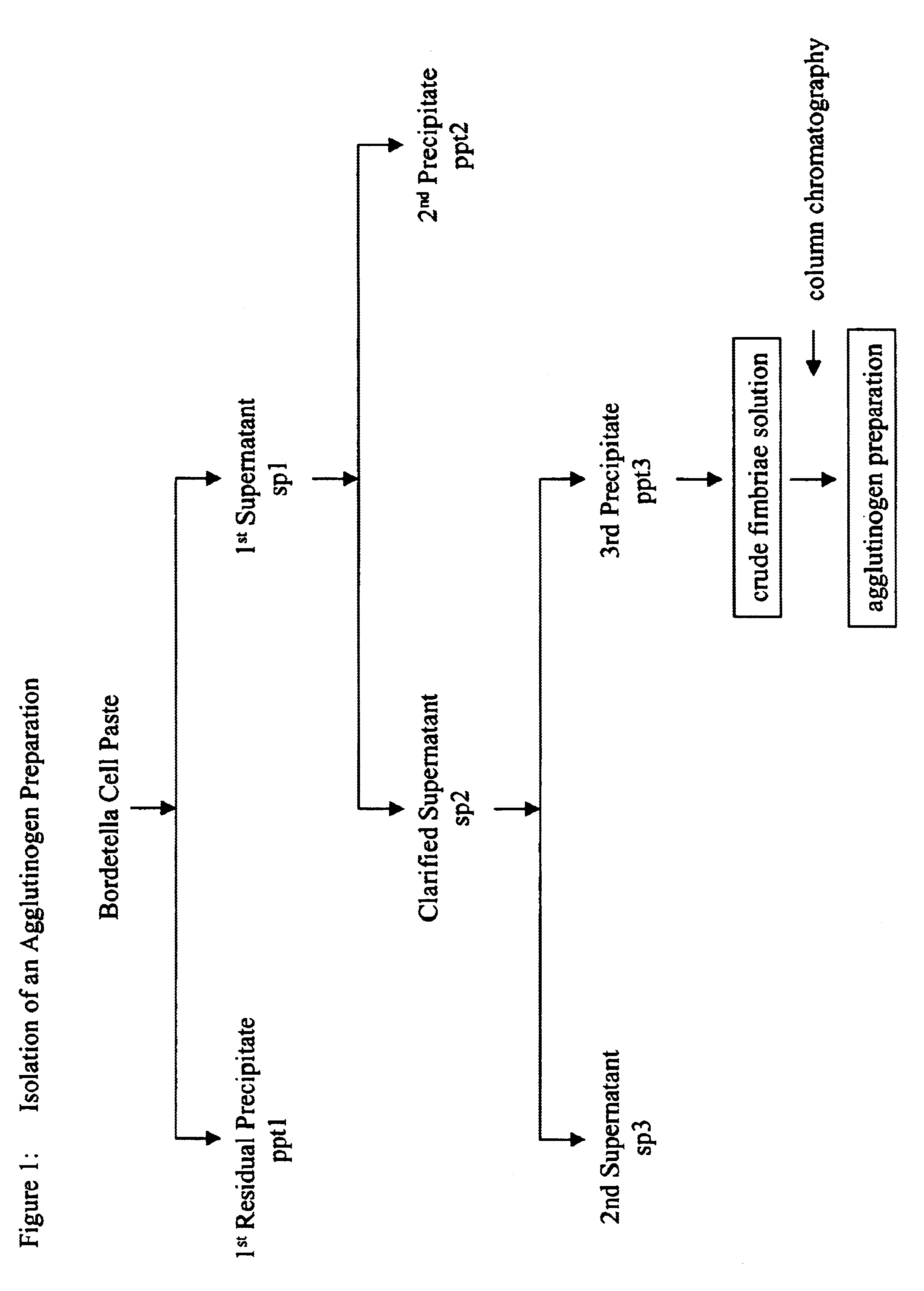
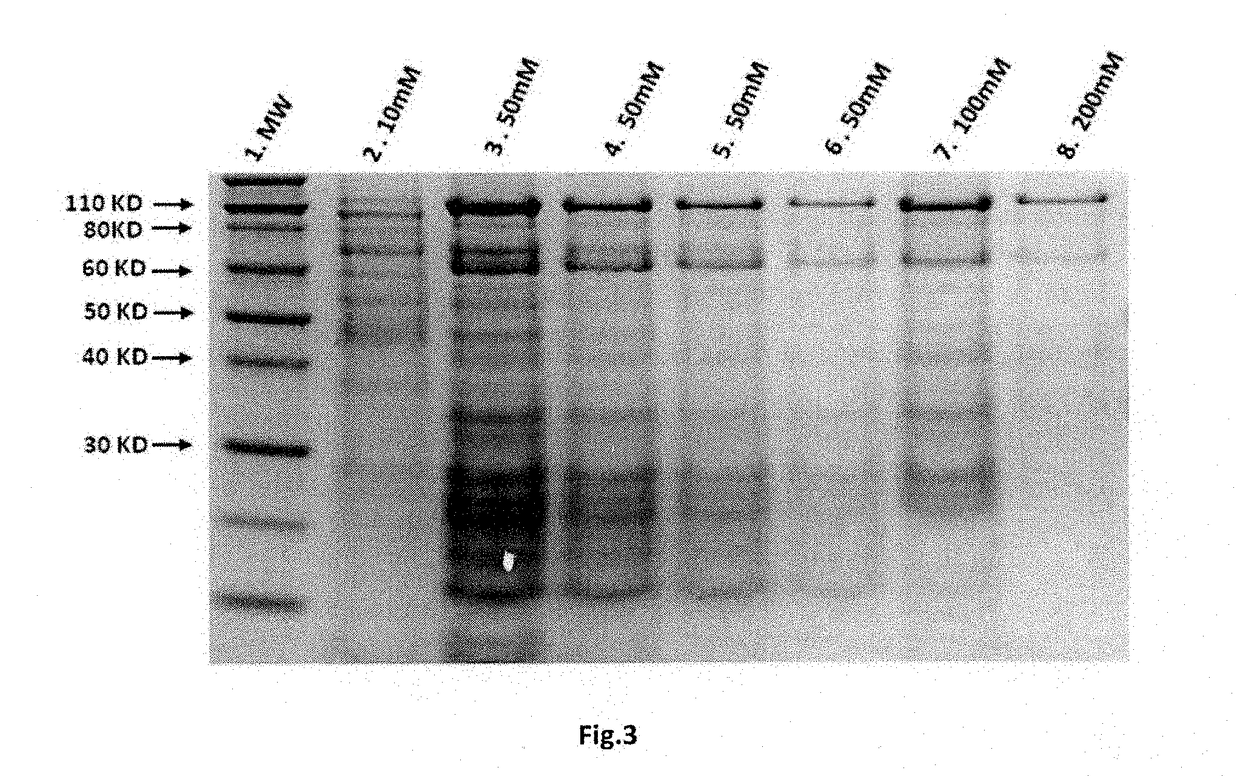
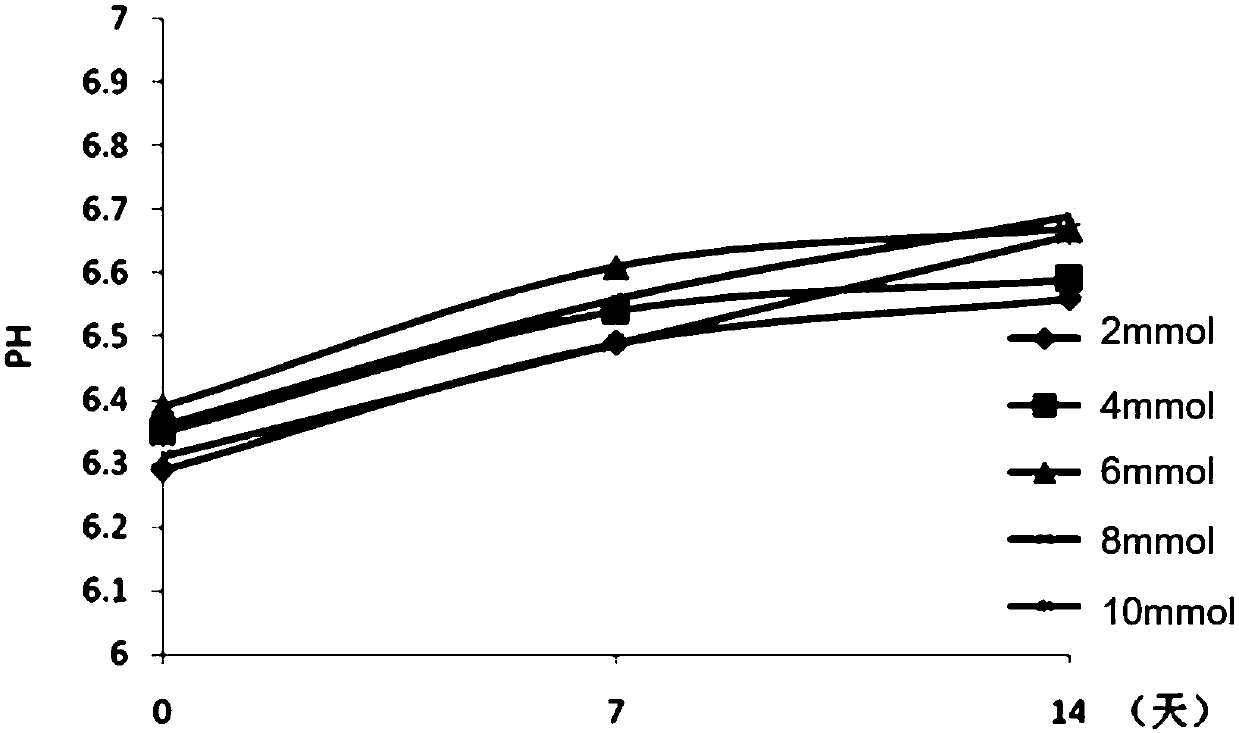
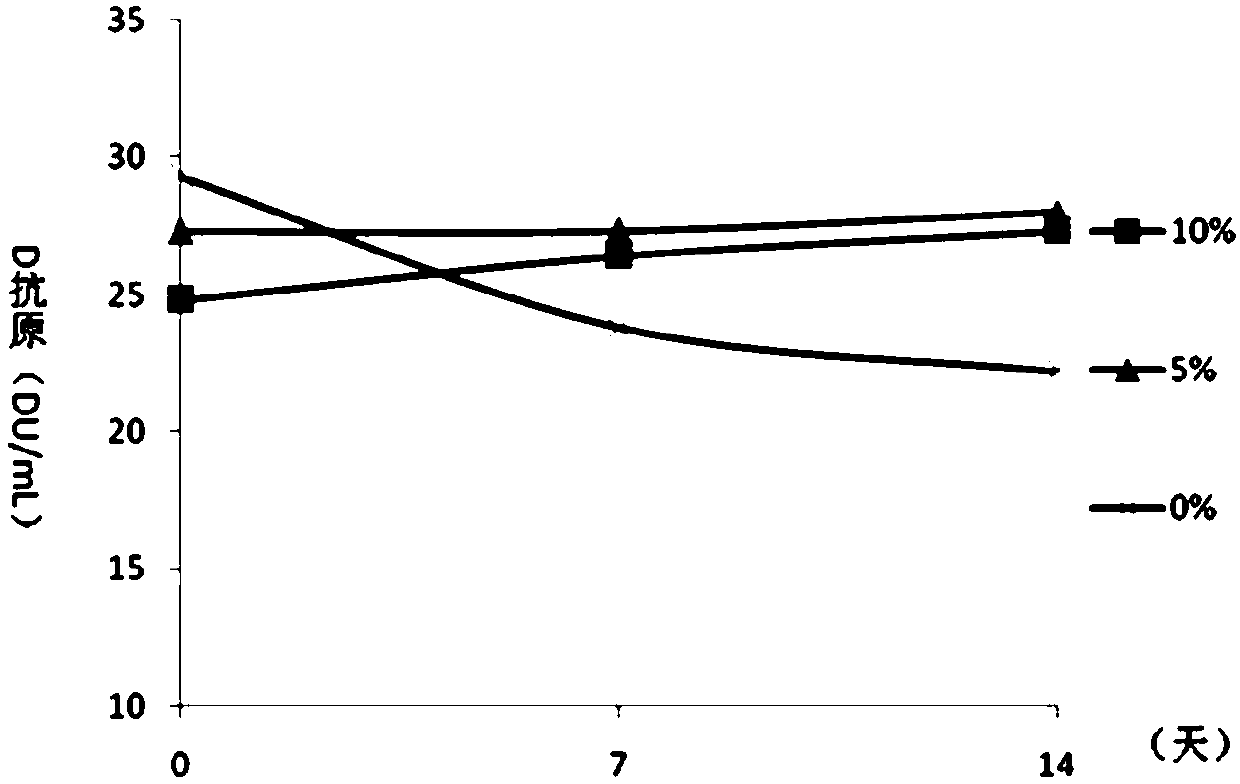
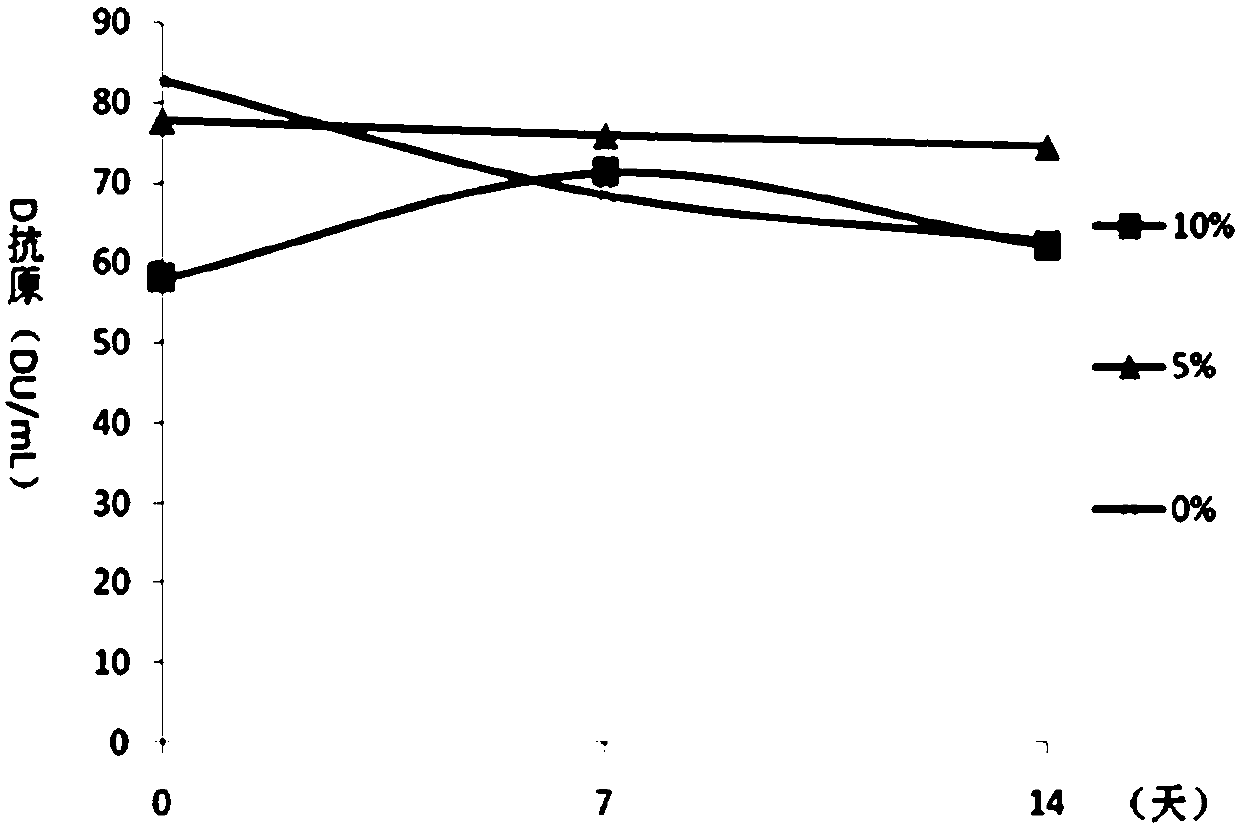
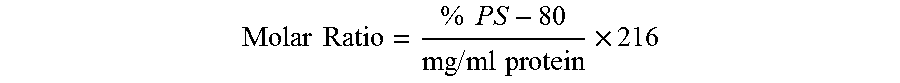Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74results about "Diphtheria-tetanus-pertussis combination vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
Vaccine
InactiveUS20100074918A1Lower immune responseAntibacterial agentsSsRNA viruses positive-senseCo administrationCombination vaccines
The present invention relates to the field of vaccines and in particular to combination vaccines and co-administration schedules. The present inventor discloses that overuse of CRM in paediatric vaccines can result in bystander immune interference to certain antigens and provide solutions to this problem.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Novel glycan conjugates and use thereof
ActiveUS20170275389A1Shrink tumorElimination of malignant cellBiological material analysisDepsipeptidesAdjuvantMedicine
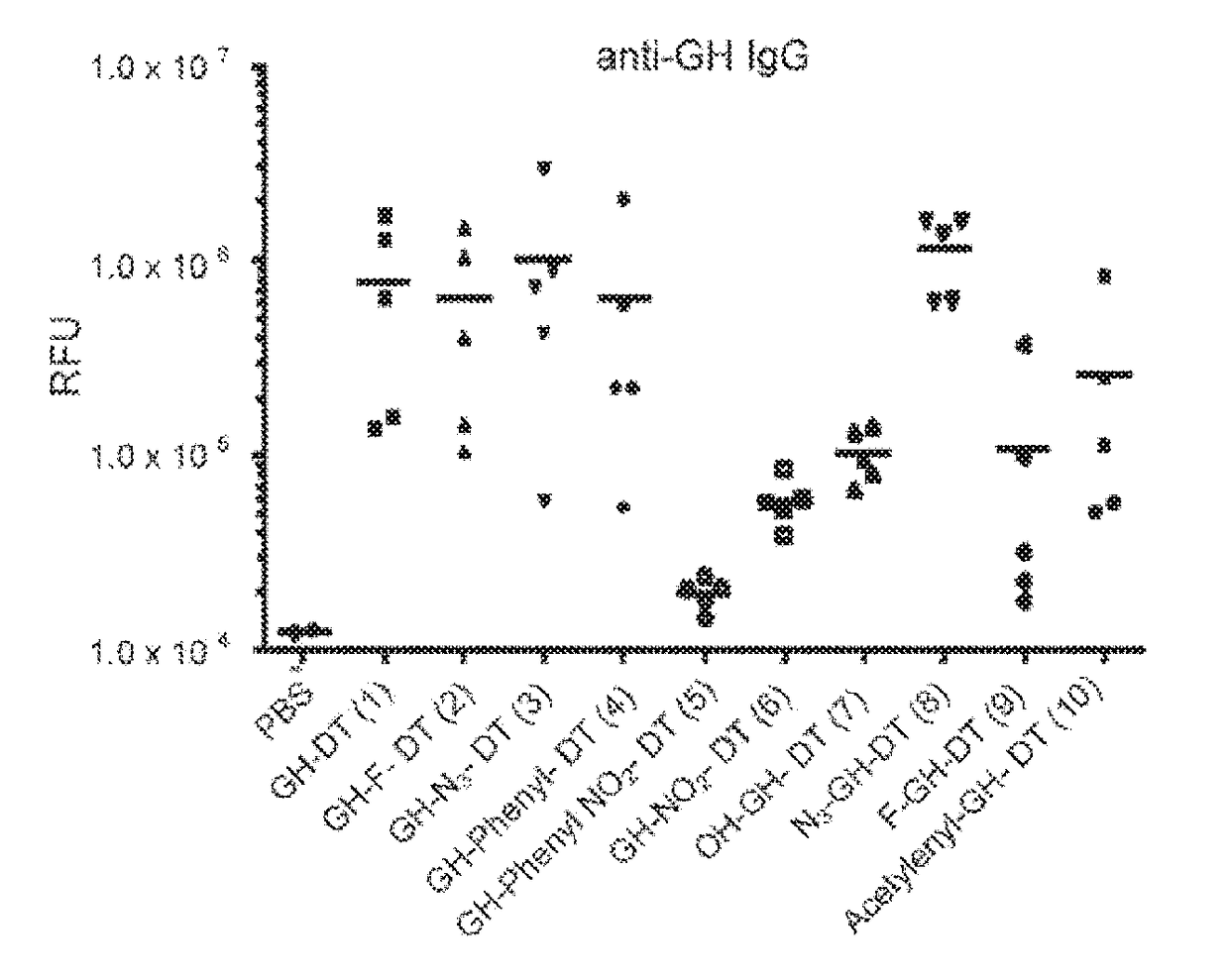
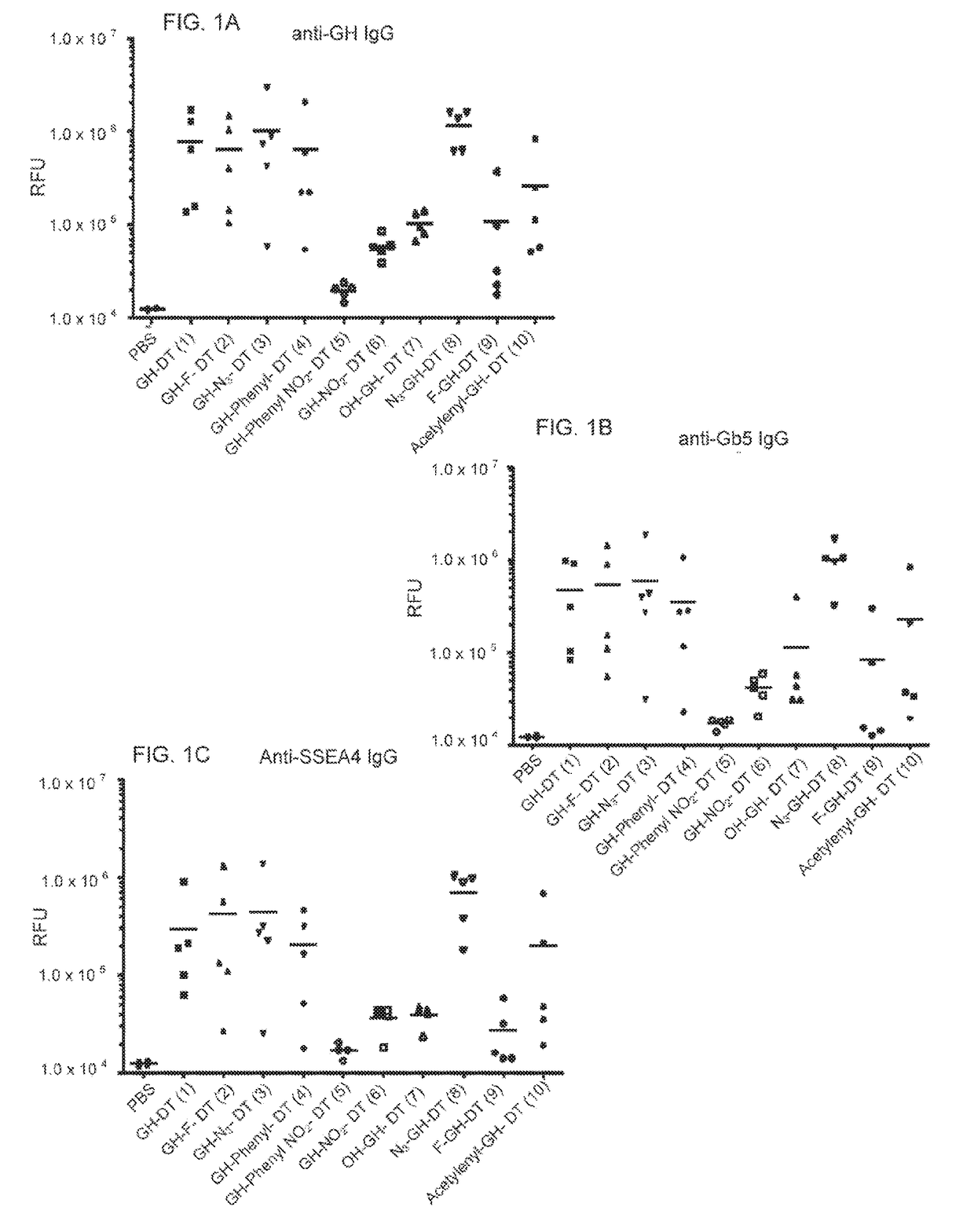
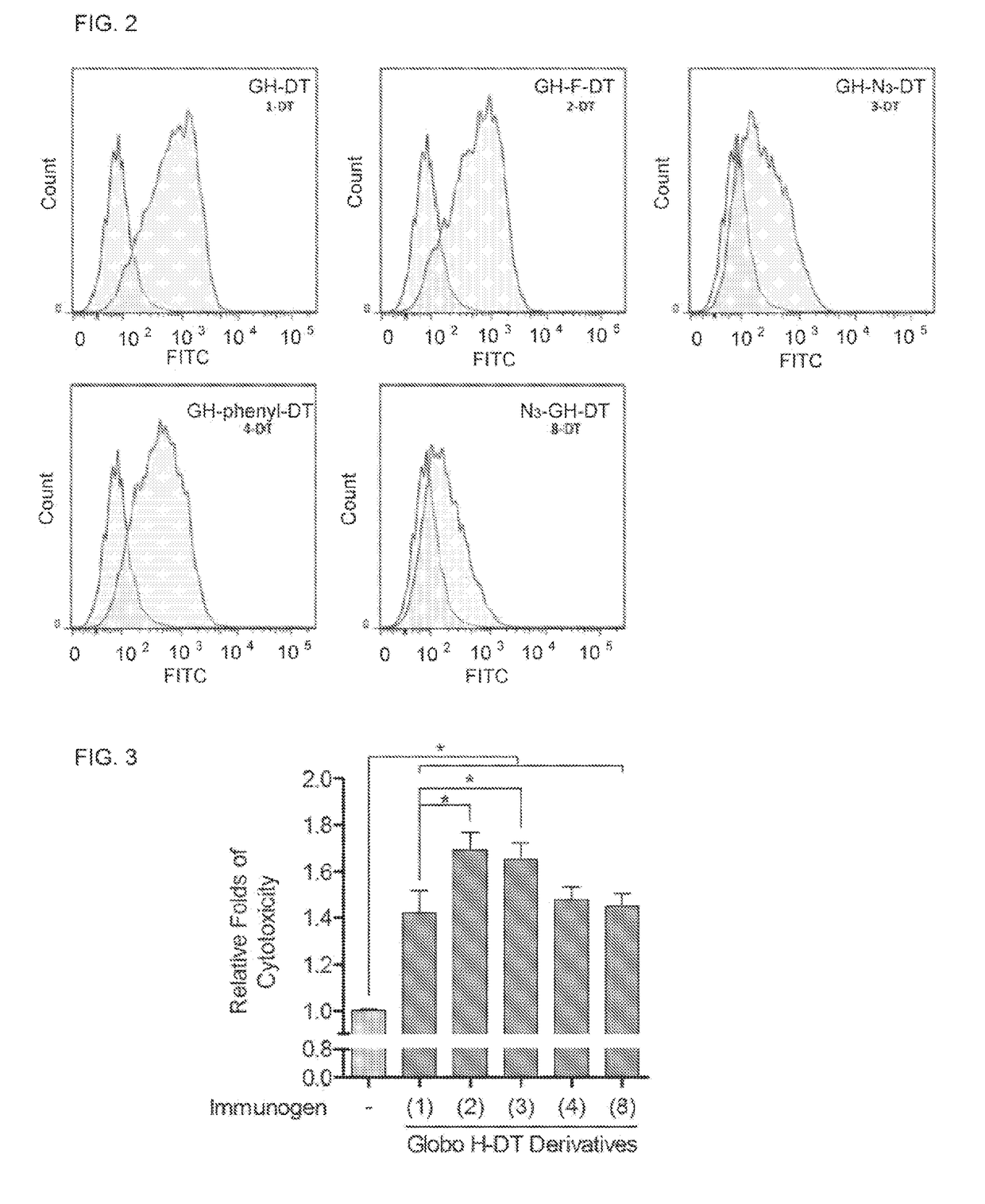
This disclosure includes an immunogenic composition containing (a) a glycan conjugate including a carrier and one or more glycans, wherein each of the one or more glycans is conjugated with the carrier through a linker, and optionally (b) an adjuvant. The one or more glycan is each a Globo H derivative.
Owner:ACAD SINIC
Vaccine
ActiveUS20100034850A1Adequate and improved level of protectionAntibacterial agentsSsRNA viruses positive-senseReduced doseAntigen Unit
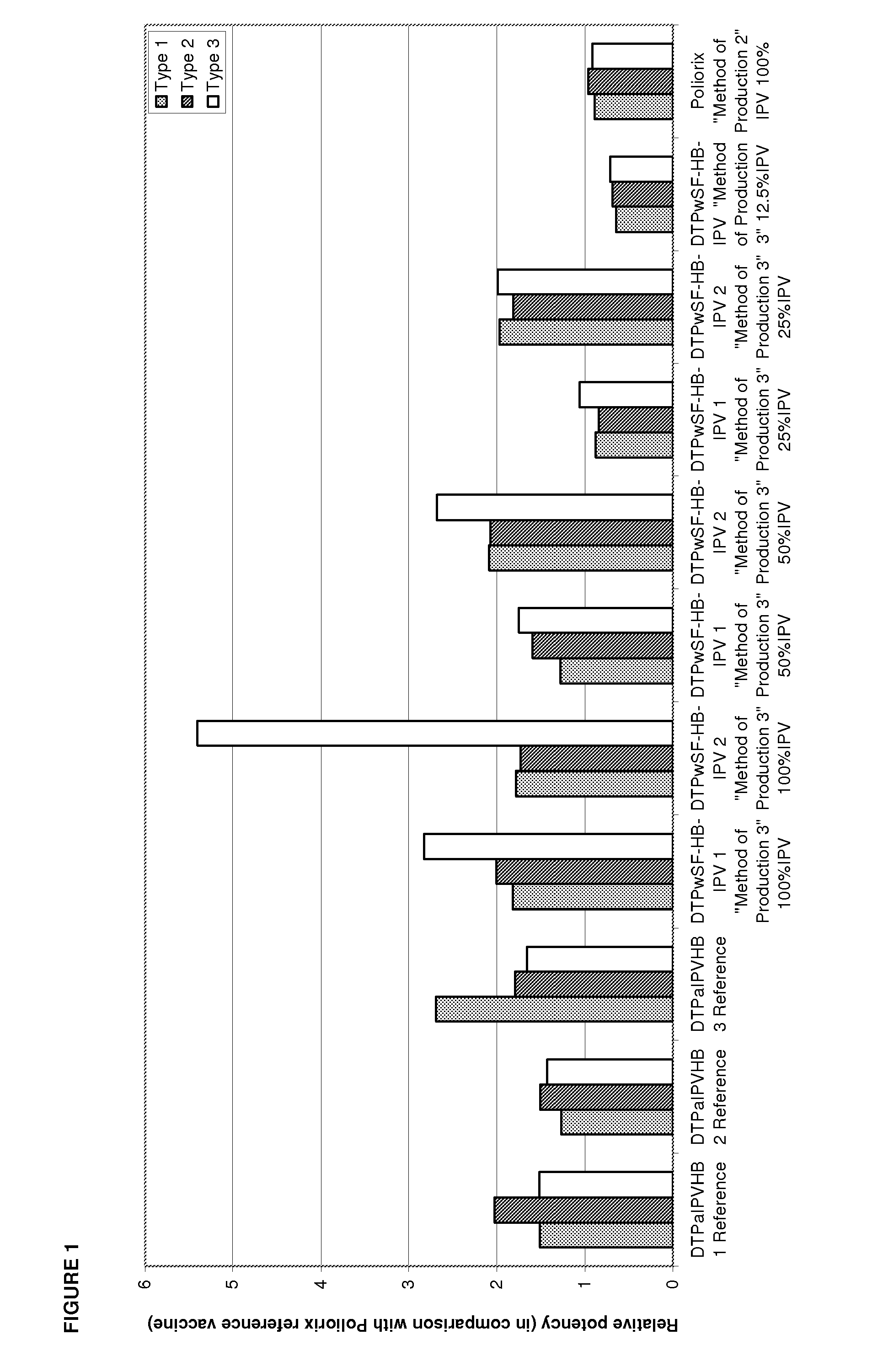
The standard dose of polio vaccines contains 40 D-antigen units of inactivated poliovirus type 1 (Mahoney), 8 D-antigen units of inactivated poliovirus type 2 (MEF-1), and 32 D-antigens units of inactivated poliovirus type 3 (Saukett). The present invention teaches that reduced doses of inactivated poliovirus can maintain adequate or improved level of protection against polio.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multiple vaccination including serogroup C meningococcus
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminum phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Combination vaccines with 1-hydroxy-2-phenoxyethane preservative
Processes for preparing combination vaccines that include diphtheria and tetanus toxoids, where these two toxoids are used in the processes as a single component containing both toxoids, and also containing 1-hydroxy-2-phenoxyethane.
Owner:NOVARTIS AG
Combination vaccines with lower doses of antigen and/or adjuvant
InactiveUS20140112950A1Without loss of immunoprotective effectMany solutionsAntibacterial agentsBacterial antigen ingredientsAdjuvantImmunogenicity
Combination vaccine compositions as well as methods for their manufacture have a relatively low amount of antigen and / or a relatively low amount of aluminium, but they can nevertheless have immunogenicity which is comparable to combination vaccines with a relatively high amount of antigen and / or a relatively high amount of aluminium. Aluminium-free combination vaccine compositions are also provided e.g. compositions which are adjuvanted with an oil-in-water emulsion adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Neisseria meningitidis compositions and methods thereof
In one aspect, the invention relates to a composition including a first polypeptide having the sequence set forth in SEQ ID NO: 1 and a second polypeptide having the sequence set forth in SEQ ID NO: 2. In one embodiment, the composition includes about 120 μg / ml of a first polypeptide including the amino acid sequence set forth in SEQ ID NO: 1, 120 μg / ml of a second polypeptide including the amino acid sequence set forth in SEQ ID NO: 2, about 2.8 molar ratio polysorbate-80 to the first polypeptide, about 2.8 molar ratio polysorbate-80 to the second polypeptide, about 0.5 mg / ml aluminum, about 10 mM histidine, and about 150 mM sodium chloride. In one embodiment, a dose of the composition is about 0.5 ml in total volume. In one embodiment, two-doses of the composition induce a bactericidal titer against diverse heterologous subfamily A and subfamily B strains in a human.
Owner:PFIZER INC
Immunization Compositions and Methods
ActiveUS20110189226A1SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Multiple vaccination including Serogroup C Meningococcus
ActiveUS20100034847A1Accurate measurementAvoid potential suppression effectAntibacterial agentsBacterial antigen ingredientsVaccinationAdjuvant
Various improvements to vaccines that include a serogroup C meningococcal conjugate antigen, including: (a) co-administration with acellular B. pertussis antigen; (b) co-administration with an inactivated poliovirus antigen; (c) supply in a kit together with a separate pneumococcal conjugate component, which may be in a liquid form; and (d) use in combination with a pneumococcal conjugate antigen but without an aluminium phosphate adjuvant. A kit may have: (a) a first immunogenic component that comprises an aqueous formulation of a conjugated capsular saccharide from Streptococcus pneumoniae; (b) a second immunogenic component that comprises a conjugated capsular saccharide from Neisseria meningitidis serogroup C.
Owner:GSK VACCINES GMBH
Combination vaccine with acellular pertussis
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, acellular pertussis, and infections caused by Haemophilus influenzae and polio viruses. The present invention also relates to inclusion of antigens for protection against infections caused Hepatitis virus and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
Combination Vaccines With Whole Cell Pertussis Antigen
InactiveUS20090214586A1Speeding drying processImproving immunogenicityAntibacterial agentsViral antigen ingredientsMeningococcal carriageTime of use
Vaccines have been studied that comprise (a) D-T-Pw-HepB-Hib antigens and (b) one or more meningococcal conjugate antigens. A number of improvements and variations of these vaccines have been discovered. The vaccines can be prepared extemporaneously at the time of use by mixing together two components: (a) a first component comprising D, T, wP and HBsAg antigens; and (b) a second component comprising a Hib conjugate and one or more meningococcal conjugates.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent DTP-POLIO vaccines
InactiveCN101310769AEffective preventionSsRNA viruses negative-senseAntibacterial agentsTetanus toxoidsHaemophilus influenzae type
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide of Haemophilus influenzae type b and tetanus toxoid or diphteria toxoid, which may be reconstituted from a lyophilized state by the other components of the vaccine. The administration of the multiple component vaccine results in no diminution in the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:CONNAUGHT LAB
Neisseria meningitidis compositions and methods thereof
ActiveUS20180000923A1Antibacterial agentsBacterial antigen ingredientsHeterologousNeisseria meningitidis
In one aspect, the invention relates to a composition including a first polypeptide having the sequence set forth in SEQ ID NO: 1 and a second polypeptide having the sequence set forth in SEQ ID NO: 2. In one embodiment, the composition includes about 120 μg / ml of a first polypeptide including the amino acid sequence set forth in SEQ ID NO: 1, 120 μg / ml of a second polypeptide including the amino acid sequence set forth in SEQ ID NO: 2, about 2.8 molar ratio polysorbate-80 to the first polypeptide, about 2.8 molar ratio polysorbate-80 to the second polypeptide, about 0.5 mg / ml aluminum, about 10 mM histidine, and about 150 mM sodium chloride. In one embodiment, a dose of the composition is about 0.5 ml in total volume. In one embodiment, two-doses of the composition induce a bactericidal titer against diverse heterologous subfamily A and subfamily B strains in a human.
Owner:PFIZER INC
Combination vaccines with whole cell pertussis antigen
InactiveUS8883166B2Improving immunogenicityAntibacterial agentsViral antigen ingredientsMeningococcal carriageTGE VACCINE
Vaccines have been studied that comprise (a) D-T-Pw-HepB-Hib antigens and (b) one or more meningococcal conjugate antigens. A number of improvements and variations of these vaccines have been discovered. The vaccines can be prepared extemporaneously at the time of use by mixing together two components: (a) a first component comprising D, T, wP and HBsAg antigens; and (b) a second component comprising a Hib conjugate and one or more meningococcal conjugates.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine
InactiveUS20100040647A1Adequate and improved level of protectionAntibacterial agentsSsRNA viruses positive-senseDiseaseTetanus
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus are provided
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
IPV-DPT vaccine
InactiveUS8753646B2Efficient productionAntibacterial agentsBacterial antigen ingredientsProtective antigenTetanus toxoids
The invention provides a process for producing a combined vaccine containing an inactivated Sabin strain of poliovirus, a Bordetella pertussis protective antigen, a diphtheria toxoid and a tetanus toxoid, the process including a step of producing a high-titer Sabin strain poliovirus. The inventive process for producing a combined vaccine, including a step of culturing, in the presence of from about 4 g / L to about 6 g / L of a microcarrier, Vero cells to be inoculated with a Sabin strain of poliovirus, is useful as a process for efficiently producing a combined vaccine containing an inactivated Sabin strain of poliovirus.
Owner:TAKEDA PHARMA CO LTD +1
Composition for Oral or Nasal Delivery of Tetanus, Diphtheria, and Pertussis Vaccine alone or in combination using Neurotoxin Associated Proteins
The present invention describes a neurotoxin associated protein from botulinum neurotoxin complex used as an oral or nasal delivery system for a vaccine. The vaccine is selected from tetanus, diphtheria and pertussis alone or in combination. Further the oral or nasal delivery of tetanus vaccine in combination with other drug molecules.
Owner:PRIME BIO INC
Adsorbed DTaP-IPV/Hib combined vaccine and preparation method thereof
ActiveCN109550046AImprove securityHigh biosecurityAntibacterial agentsBacterial antigen ingredientsMedicineHaemophilus influenzae type B antigen
The invention discloses an adsorbed DTaP-IPV / Hib combined vaccine and a preparation method thereof. The combination vaccine provided by the present invention includes an acellear pertussis antigen, adiphtheria antigen, a tetanus antigen, inactivated poliovirus and a haemophilus influenzae type b antigen, wherein the poliovirus is a Sabin strain. The method adopts the attenuated strain of the poliovirus Sabin strain to prepare the combined vaccine, and the combined vaccine has higher biosafety, less adverse reaction, and low production cost. By optimizing the antigen composition, adjuvants andstabilizers of the combined vaccine, the immunogenicity of various antigens in the combined vaccine provided by the invention are fully exerted, and the combined vaccine has high stability, and is suitable for popularization and application.
Owner:BEIJING MINHAI BIOTECH
Mixing lyophilised meningococcal vaccines with D-T-Pa vaccines
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Purification of HBV antigens for use in vaccines
InactiveUS20060159705A1Suitable for useAntibacterial agentsSsRNA viruses positive-senseAntigenCysteine thiolate
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Manufacturing Method of Combined Vaccine
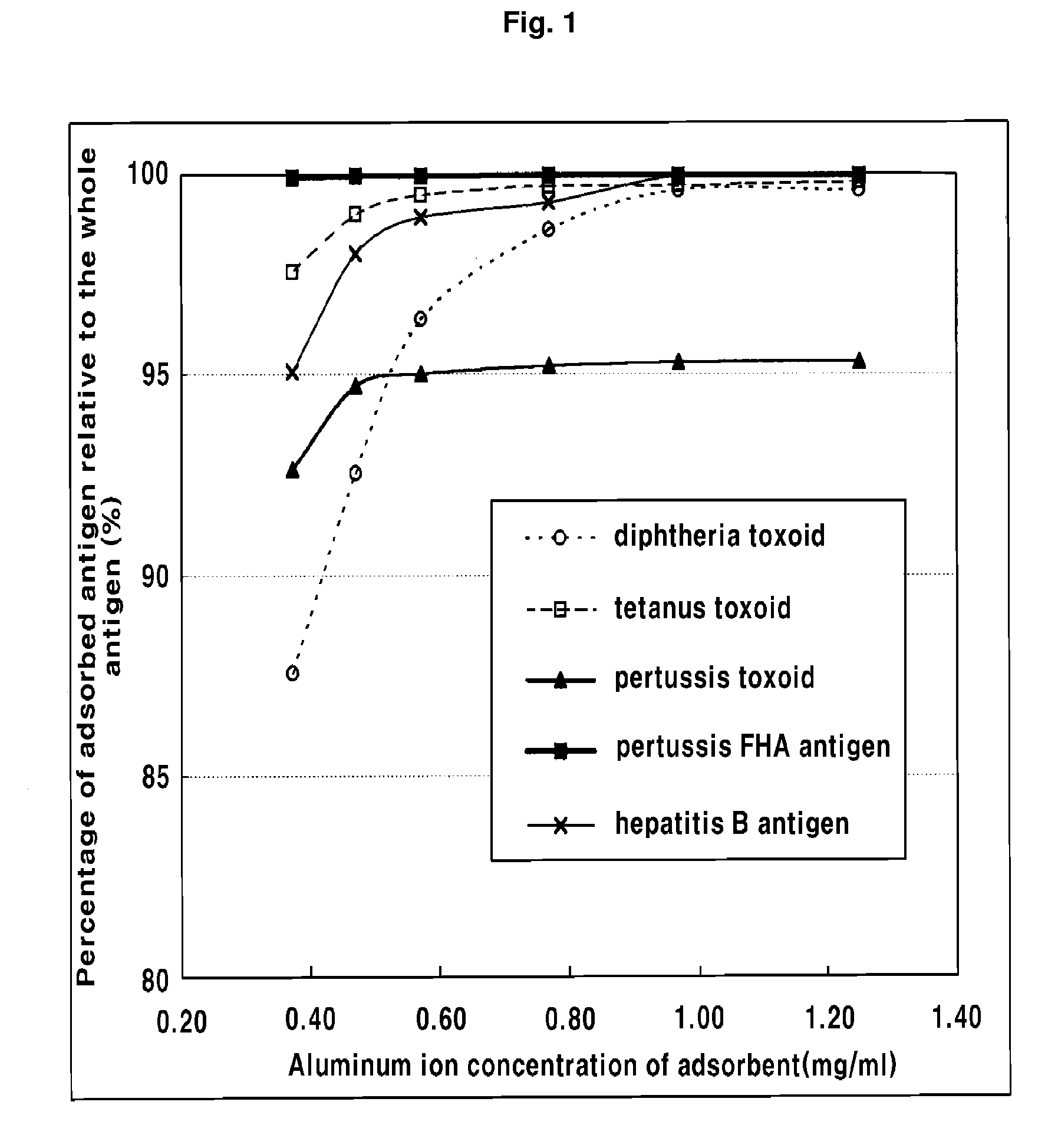
The present invention relates to a method for manufacturing a combined vaccine capable of concurrently preventing multiple diseases such as diphtheria, tetanus, pertussis, and hepatitis B which should be prevented in an infant. The method for manufacturing a combined vaccine according to the present invention includes the steps of independently adsorbing each protective antigen to an adsorbent of a aluminum hydroxide gel with respect to various diseases such as diphtheria, tetanus, pertussis, and hepatitis B which should be prevented in the infants, and combining each protective antigen adsorbed to the adsorbent after the adsorption. In the present invention, it is possible to concurrently prevent multiple diseases such as diphtheria, tetanus, pertussis, and hepatitis B which should be prevented in the infant using a combined vaccine manufactured according to the present invention.
Owner:LG LIFE SCI LTD
Vaccine for inducing an improved immune reaction
ActiveUS20130273101A1Efficient preparationGood effectAntibacterial agentsSsRNA viruses negative-senseHepatitis B virusNeisseria meningitidis
The present invention relates to a pharmaceutical vaccine composition comprising: (a) a pathogen-derived antigen selected from the group consisting of Mycobacterium tuberculosis antigen, Bacillus anthracis antigen, HAV (hepatitis A virus) antigen, HBV (hepatitis B virus) antigen, HCV (hepatitis C virus) antigen, HIV (human immunodeficiency virus) antigen, influenza virus antigen, HSV (herpes simplex virus) antigen, Hib (Haemophilus influenzae type b) antigen, Neisseria meningitidis antigen, Corynebacterium diphtheriae antigen, Bordetella pertussis antigen, Clostridium tetani antigen and Varicella virus antigen; (b) a deacylated non-toxic LOS (lipooligosaccharide); and (c) a pharmaceutically acceptable carrier.
Owner:EYEGENE INC
Non-cross-linked acellular pertussis antigens for use in combination vaccines
InactiveUS20150273036A1Bacterial antigen ingredientsSsRNA viruses positive-senseCross-linkAcellular pertussis antigen
The present invention relates to stable compositions comprising acellular pertussis antigens that have not been cross-linked with a cross-linking agent such as formaldehyde or glutaraldehyde and their use as acellular pertussis components in combination vaccines. Processes for preparing these antigens and compositions are also disclosed.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Bioactive vitamin combinations
InactiveUS20210338678A1Improve responseImprove efficiencyBacterial antigen ingredientsTetracycline active ingredientsAdenosineMethylfolic acid
The present invention describes bioactive vitamin combinations that can be used in combination with other bioactive compounds, such as active drug ingredients or active vaccine components, to increase their therapeutic effects. The bioactive vitamin combinations comprise therapeutically effective amounts of L-Methylfolate, Adenosylcobalamin and Methylcobalamin.
Owner:BIOVIT INC
Combination vaccine with whole cell pertussis
InactiveUS20110195087A1Safe wayLow costAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus
The present invention relates to a combination vaccine comprising a mixture of antigens for protection against diseases such as diphtheria, tetanus, whole cell pertussis and polio. The present invention also relates to inclusion of one or more antigens in the said combination vaccine, for protection against infections caused by Haemophilus influenzae. Hepatitis virus, and other pathogens, such that administration of the vaccine can simultaneously immunize a subject against more than one pathogen. The invention in particular relates to a fully liquid stable combination vaccine comprising the antigens as indicated above and the methods for manufacturing the same.
Owner:PANACEA BIOTEC
Immunization compositions and methods
ActiveUS8697353B2SsRNA viruses negative-senseBacterial antigen ingredientsSerotypeNeutralizing antibody
The present invention provides methods and compositions to induce neutralizing antibodies in mammals to serotypes of dengue virus, measles virus, mumps virus, rubella and / or VZV virus.
Owner:SANOFI PASTEUR SA
Neisseria meningitidis compositions and methods thereof
ActiveUS10888611B2Antibacterial agentsSsRNA viruses positive-senseHeterologousNeisseria meningitidis
In one aspect, the invention relates to a composition including a first polypeptide having the sequence set forth in SEQ ID NO: 1 and a second polypeptide having the sequence set forth in SEQ ID NO: 2. In one embodiment, the composition includes about 120 μg / ml of a first polypeptide including the amino acid sequence set forth in SEQ ID NO: 1, 120 μg / ml of a second polypeptide including the amino acid sequence set forth in SEQ ID NO: 2, about 2.8 molar ratio polysorbate-80 to the first polypeptide, about 2.8 molar ratio polysorbate-80 to the second polypeptide, about 0.5 mg / ml aluminum, about 10 mM histidine, and about 150 mM sodium chloride. In one embodiment, a dose of the composition is about 0.5 ml in total volume. In one embodiment, two-doses of the composition induce a bactericidal titer against diverse heterologous subfamily A and subfamily B strains in a human.
Owner:PFIZER INC
Injectable vaccines against multiple meningococcal serogroups
ActiveUS20160354457A1Antibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeSalmonella serotype typhi
An injectable immunogenic composition comprising capsular saccharides from at least two of serogroups A, C, W135 and Y of Neisseria meningitidis, wherein said capsular saccharides are conjugated to carrier protein(s) and / or are oligosaccharides, and wherein (i) the composition comprises <50 μg meningococcal saccharide per dose, and / or (ii) the composition further comprises an antigen from one or more of (a) serogroup B N. meningitidis; (b) Haemophilus influenzae type B; and / or (c) Streptococcus pneumoniae. Saccharide antigens in the compositions are generally conjugated to a carrier.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com