Adsorbed DTaP-IPV/Hib combined vaccine and preparation method thereof
A technology for Haemophilus influenzae and cell-free DTP, which is applied in the fields of medical biotechnology and biological products, can solve problems such as strong toxicity, and achieve the effects of low production cost, optimized composition and content, and less adverse reactions to vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The IPV neutralizing antibody level analysis of the combined vaccine of embodiment 1 different Sabin strain antigen content
[0055] In order to analyze the influence of different Sabin strain antigen content on the IPV immune effect of combined vaccine, prepare three kinds of DTaP-IPV / Hib combined vaccine test articles (test articles 1, 2) containing different concentrations of I, II, and III type D antigens. , 3), the antigenic content of three kinds of combination vaccines for testing is as shown in table 1, is reference substance with commercially available IPV vaccine. Wistar rats were immunized with three kinds of test products and control products, 10 rats in each group, each rat was injected with 0.5 mL, blood was collected 21 days after immunization to separate serum, and the titer of poliomyelitis neutralizing antibody in rats was detected by cell method, calculated ED50 value.
[0056] Table 1 Antigen content of three kinds of combination vaccines for testin...
Embodiment 2
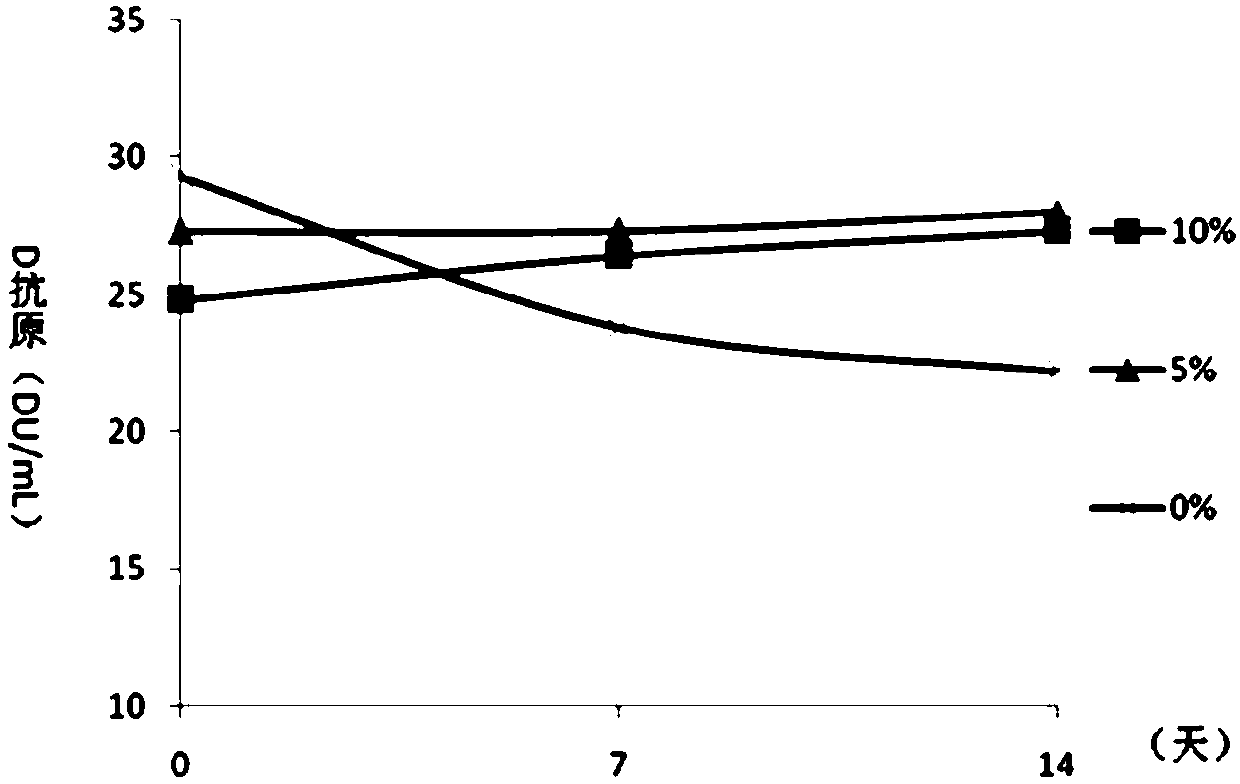
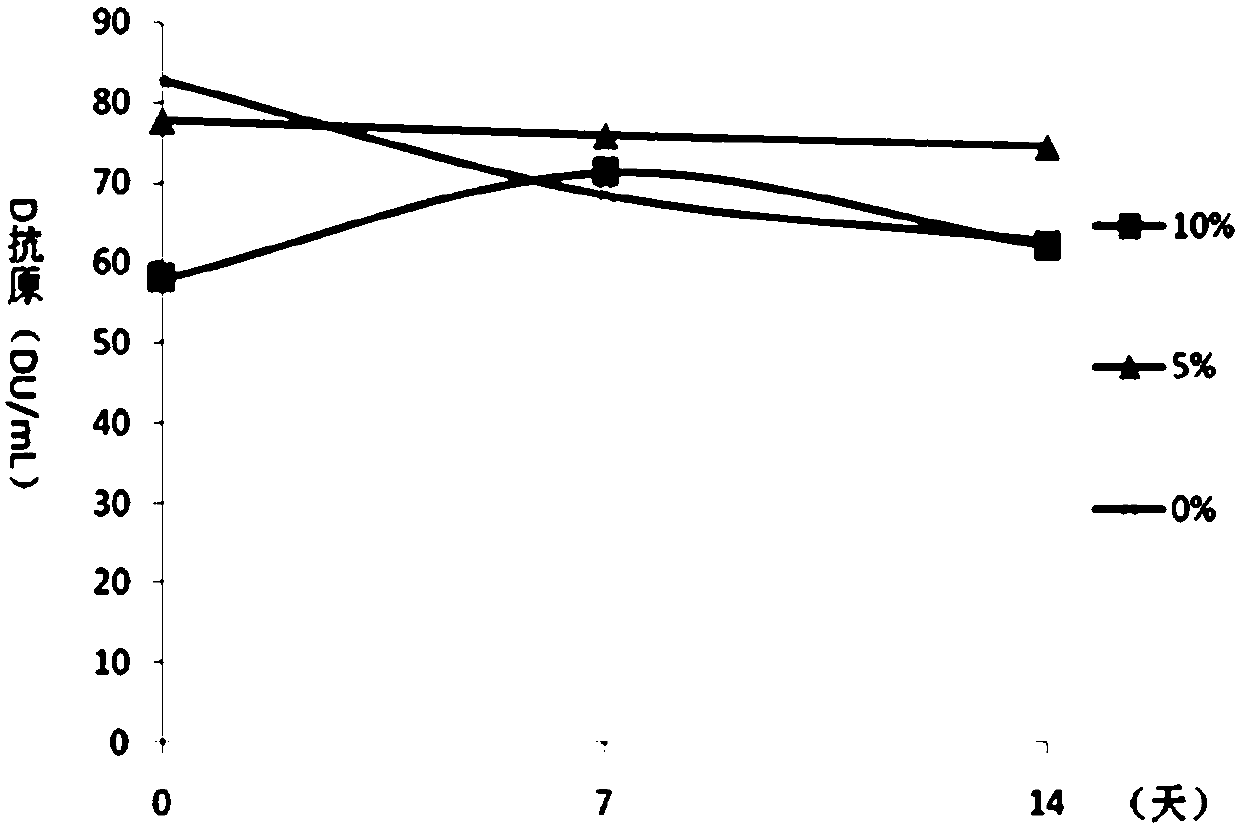
[0065] Embodiment 2 Contains the protective effect analysis of the DTaP-IPV / Hib combination vaccine of different concentrations of aluminum hydroxide
[0066] On the basis of the test product 1 in Example 1, the adjuvant of the DTaP-IPV / Hib combination vaccine was optimized, and the DTaP-IPV / Hib combination vaccine containing different concentrations of aluminum hydroxide adjuvant was prepared respectively, wherein each 0.5mL The aluminum hydroxide content in the preparation is respectively 0.5mg / mL, 1.0mg / mL, 1.5mg / mL, 2.0mg / mL and 2.5mg / mL, carry out pertussis, diphtheria, tetanus potency, Hib antibody test respectively. The conversion rate and the in vivo immune efficacy experiment of IPV were used to analyze the immune effect of the combined vaccine. The diphtheria, tetanus, pertussis and Hib potency tests were carried out according to the third part of the "Chinese Pharmacopoeia" 2015 edition. IPV was used to immunize wistar rats with 4 dilutions of original times, 1 / 3 t...
Embodiment 3
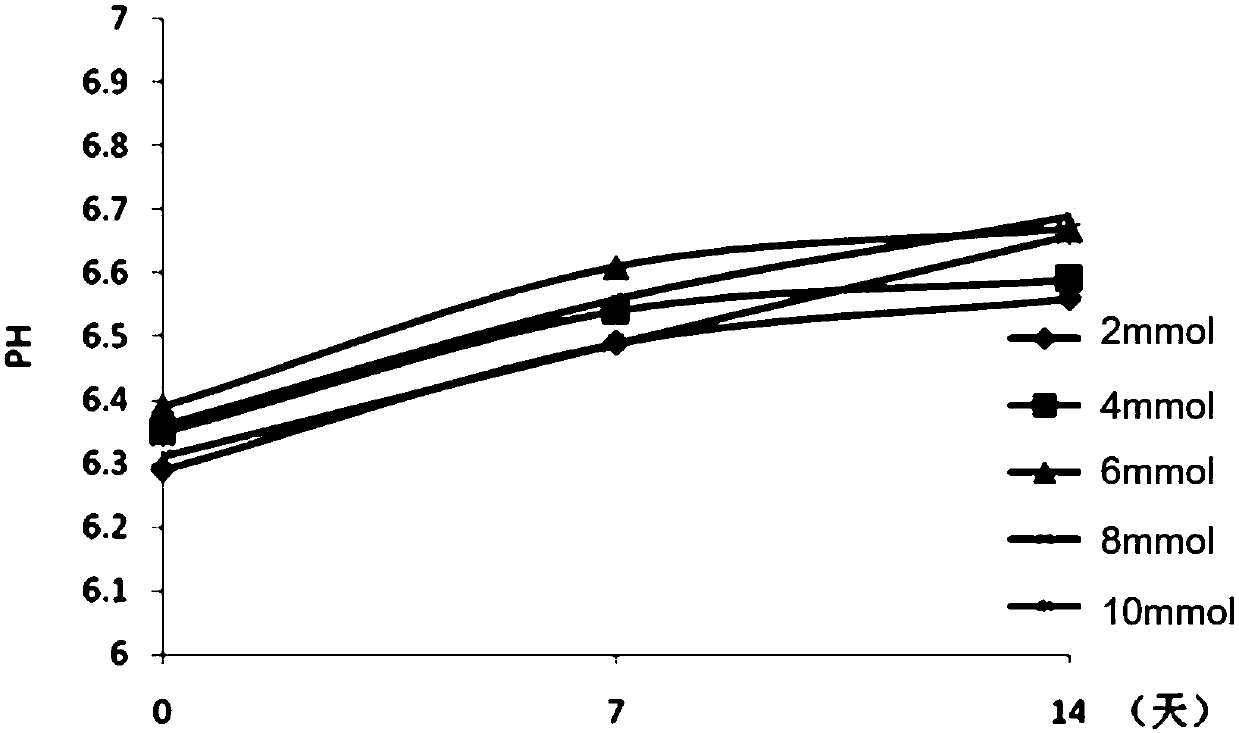
[0078] The effect of embodiment 3 phosphate buffer saline on the antigenic protein adsorption rate and pH stability of DTaP-IPV / Hib combination vaccine
[0079] On the basis of the DTaP-IPV / Hib combined vaccine that the test product 1 of Example 1 contains 1.5mg / mL aluminum hydroxide adjuvant, investigate adding 2mmol, 4mmol, 6mmol, 8mmol, 10mmol sodium dihydrogen phosphate-disodium hydrogen phosphate phosphate buffer (pH 6.0-7.0), the influence of aluminum hydroxide adjuvant on antigenic protein adsorption rate and pH stability. The protein adsorption rate analysis is shown in Table 10. After adding phosphate buffer, the protein adsorption rate decreased gradually with the increase of the concentration of phosphate buffer. When the concentration of phosphate buffer was 8mmol / mL, the protein adsorption rate dropped to below 90%. When the concentration is 2-6mmol / mL, the adsorption rate is above 90%, so the concentration of the phosphate buffer in the combined vaccine should n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com