Mycoplasma bovis alcohol dehydrogenase gene and coded protein and application thereof
A technology of alcohol dehydrogenase and Mycoplasma bovis, which is applied in the field of Mycoplasma bovis alcohol dehydrogenase gene and its encoded protein and its application, and can solve problems such as economic loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
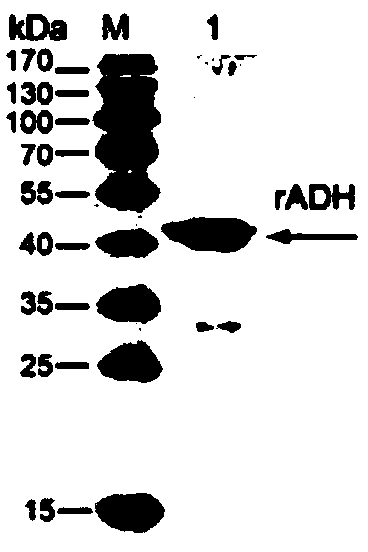
[0041] Embodiment 1: Expression and purification of Mycoplasma bovis ADH recombinant protein (rADH)
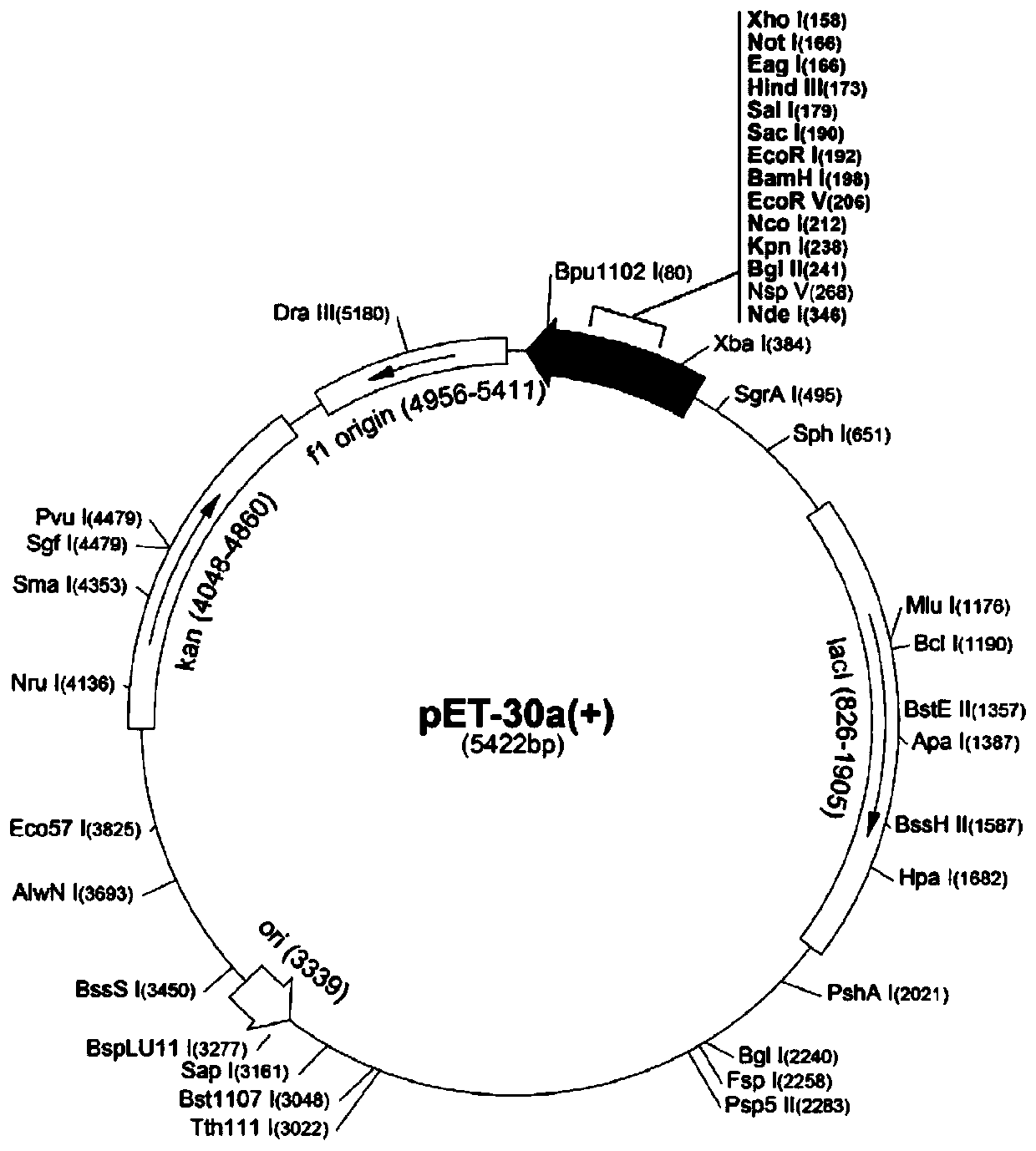
[0042] 1.1 Cloning and expression of Mycoplasma bovis Mbov_0338 gene
[0043] Because E. coli is to codon preference, in the present invention, the codon UGA of the tryptophan of encoding tryptophan in the Mycoplasma bovis Mbov_0338 gene is used as terminator in Escherichia coli, therefore, when expressing Mycoplasma bovis gene with Escherichia coli, need to mycoplasma The Mbov_0338 gene was mutated, and the codon UGA was mutated to the codon UGG that could express tryptophan in Escherichia coli. The specific steps are: using the Mbov_0338 gene in Mycoplasma bovis HB0801 (genome GenBank accession number is WP_038582875.1) as a template, using 4 pairs of primers designed as follows (the numbers are respectively: 0338a1 / 0338a2, 0338b1 / 0338b2, 0338c1 / 0338c2, 0338d1 / 0338d2) to amplify 4 fragments of the mutated Mbov_0338 gene respectively, and then use the 4 mutated fragments as...
Embodiment 2
[0077] Embodiment 2: Alcohol dehydrogenase activity test of rADH protein
[0078] Use the Alcohol Dehydrogenase Activity Assay Kit (Alcohol Dehydrogenase Activity AssayKit, Sigma) detection, operate according to the product instructions, briefly as follows: add 22.5ng rADH to the 96-well plate, and then add ethanol, NAD+, alcohol dehydrogenase The detection solution and the chromogenic solution were thoroughly mixed and incubated at 37°C for 3 minutes. Then, the absorbance was measured every 5 min at 37° C. with a wavelength of 450 nm using a microlabel detector. The test results confirmed that the rADH protein can catalyze the substrate ethanol to form acetaldehyde, and at the same time reduce NAD+ to NADH. The amount of NADH produced is proportional to the absorbance value of the substrate. At this time, the absorbance at 450nm wavelength increases with the prolongation of the enzyme catalysis time ( Figure 4 ).
Embodiment 3
[0079] Example 3: Preparation of rADH protein polyclonal antibody
[0080] The purified rADH protein was immunized into male Japanese white rabbits with an immunization dose of about 1 mg / rat. Calculate the volume of the recombinant Mbov_338 protein used according to the immunization dose, mix it with an equal volume of Freund’s complete adjuvant to emulsify completely, and inject it at multiple points under the skin After immunization, immunize once every two weeks. From the second immunization, Freund’s incomplete adjuvant was used for emulsification, and one week after the third immunization, blood was collected from the ear vein for indirect ELISA to detect the antibody level of white rabbits. The required titer can be achieved after 4-5 times of immunization. When the antibody level of the white rabbit no longer increases, the heart blood can be collected to purify the polyclonal antibody. The results show that the antibody titer that rADH protein can produce is 2 11 ×10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com