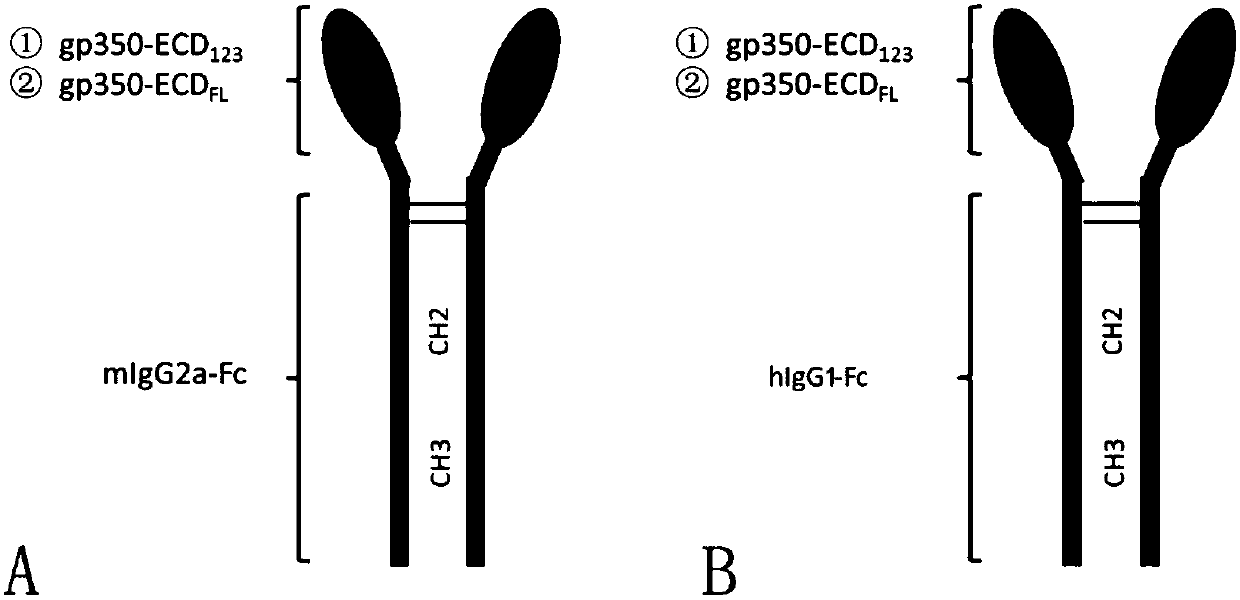
Fusion protein comprising Fc domain of IgG and extracellular domain of EB virus envelope glycoprotein
A technology of envelope glycoprotein and fusion protein, applied in antiviral agents, medical preparations containing active ingredients, hybrid peptides, etc., can solve the problem of lack of animal models, restrictions on the prevention and treatment of EB virus malignant diseases, and inability to infect Rodents and other problems, to achieve the effect of improving immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of fusion protein
[0041] 1. Materials
[0042] (1) Vector and strain: shuttle vector pFastBac I, E. coli competent DH10B strain carrying bacmid;
[0043] (2) Virus packaging and protein expression cell lines: insect Sf9 cell line and H5 cell line;
[0044](3) Reagents: Cellfectin transfection reagent and reagents related to plaque analysis purchased from Invitrogen, serum-free insect cell culture medium, HisTrap and MabSelect protein purification prepacked columns purchased from GE, and other commercially available chemical reagents.
[0045] 2. Steps
[0046] (1) Link the coding sequence of the Fc domain of human IgG1 or mouse IgG2a to the ECD of the Epstein-Barr virus envelope glycoprotein gp350 by enzyme digestion 123 or ECD FL domain coding sequence (ECD 123 It is the first three amino domains at the N-terminal of the extracellular domain of gp350; ECD FL It is the 3' end of the gp350 ectodomain full length), and the secretion signal pe...
Embodiment 2
[0054] Example 2 Detection of the conformation of the neutralizing epitope in the extracellular domain of gp350 in the fusion protein
[0055] 1. Materials
[0056] (1) Reagents and consumables: commercially available chemical reagents and ELISA plates;
[0057] (2) Antibody: commercially available mouse-derived HRP-labeled secondary antibody, affinity-purified gp350 neutralizing antibody mAb72A1. Among them, mAb72A1 is secreted by the HB168 hybridoma cell line in the ATCC depository, ATCC number: 72A1 (ATCC® HB-168™).
[0058] 2. Grouping
[0059] (1) Control group: bovine serum albumin fraction IV (BSA) as the ELISA negative control group, purified non-Fc fusion gp350-ECD 123 -6His recombinant protein (6His expressed in gp350-ECD 123 fused with 6 histidine tags at the C-terminus) as a positive control group;
[0060] (2) Experimental group: four purified fusion proteins gp350-ECD 123 -Fc hIgG1 , gp350-ECD FL -Fc hIgG1 , gp350-ECD 123 -Fc mIgG2a with gp350-ECD FL ...
Embodiment 3
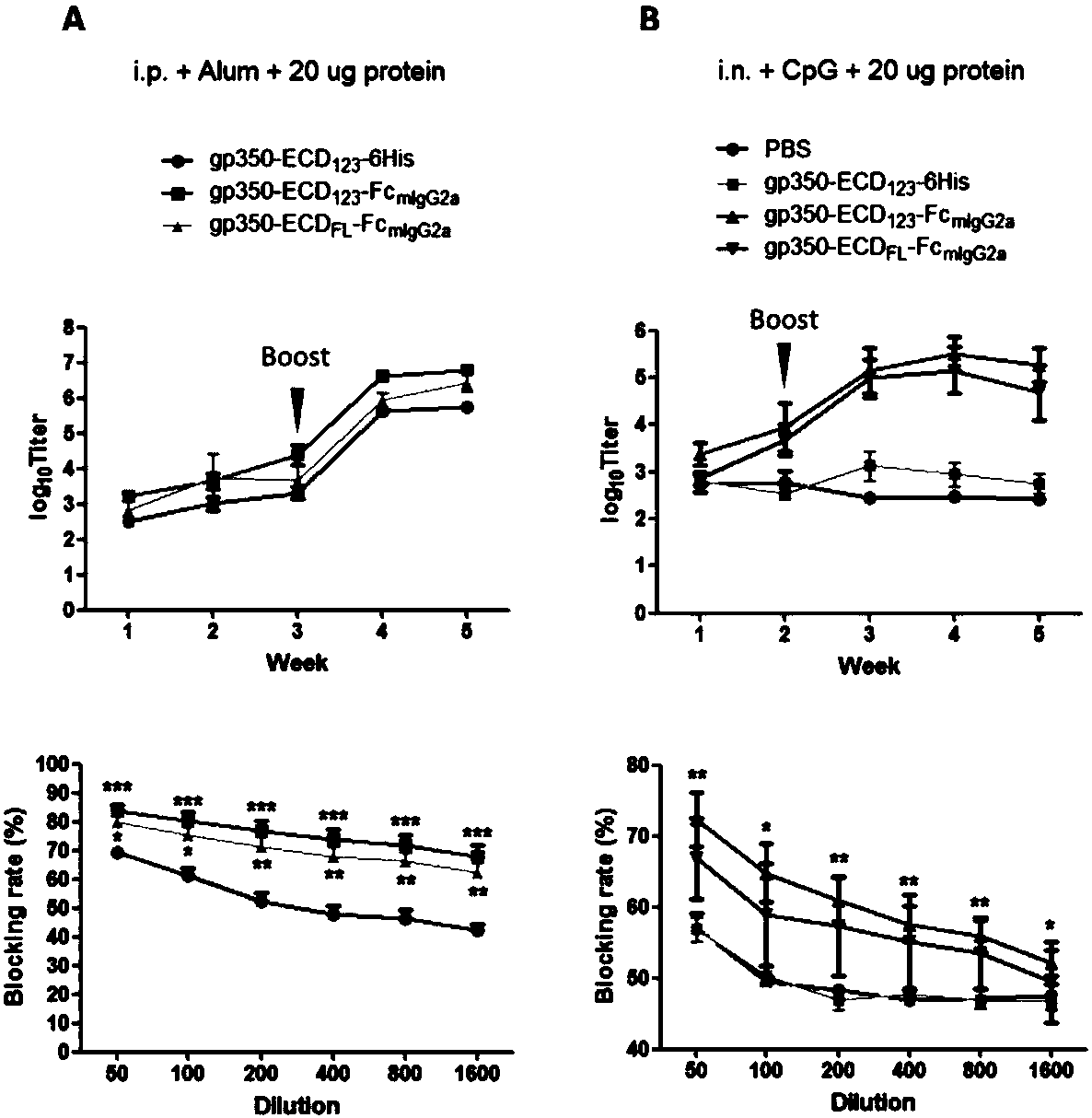
[0066] Example 3 Detection of specific antibody total titer and neutralizing antibody titer in immune serum of fusion protein
[0067] 1. Materials
[0068] Reagents: commercially available chemical reagents and ELISA plates, commercially available aluminum adjuvants;
[0069] Animals: Commercially purchased BALB / c mice aged 6-8 weeks.
[0070] 2. Grouping
[0071] Control group: use of purified non-Fc fusion gp350-ECD 123 -6His recombinant protein immunized mouse serum was used as a control group;
[0072] Experimental group: using purified fusion protein gp350-ECD 123 -Fc hIgG1 , gp350-ECD FL -Fc hIgG1 , gp350-ECD 123 -Fc mIgG2a with gp350-ECD FL -Fc mIgG2a The sera of immunized mice were used as the experimental group.
[0073] 3. Steps
[0074] (1) Mix the recombinant protein of the control group and the experimental group with aluminum adjuvant in equal volumes, and immunize each mouse at a dose of 20 μg. The immunization methods are divided into two types: ①...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com