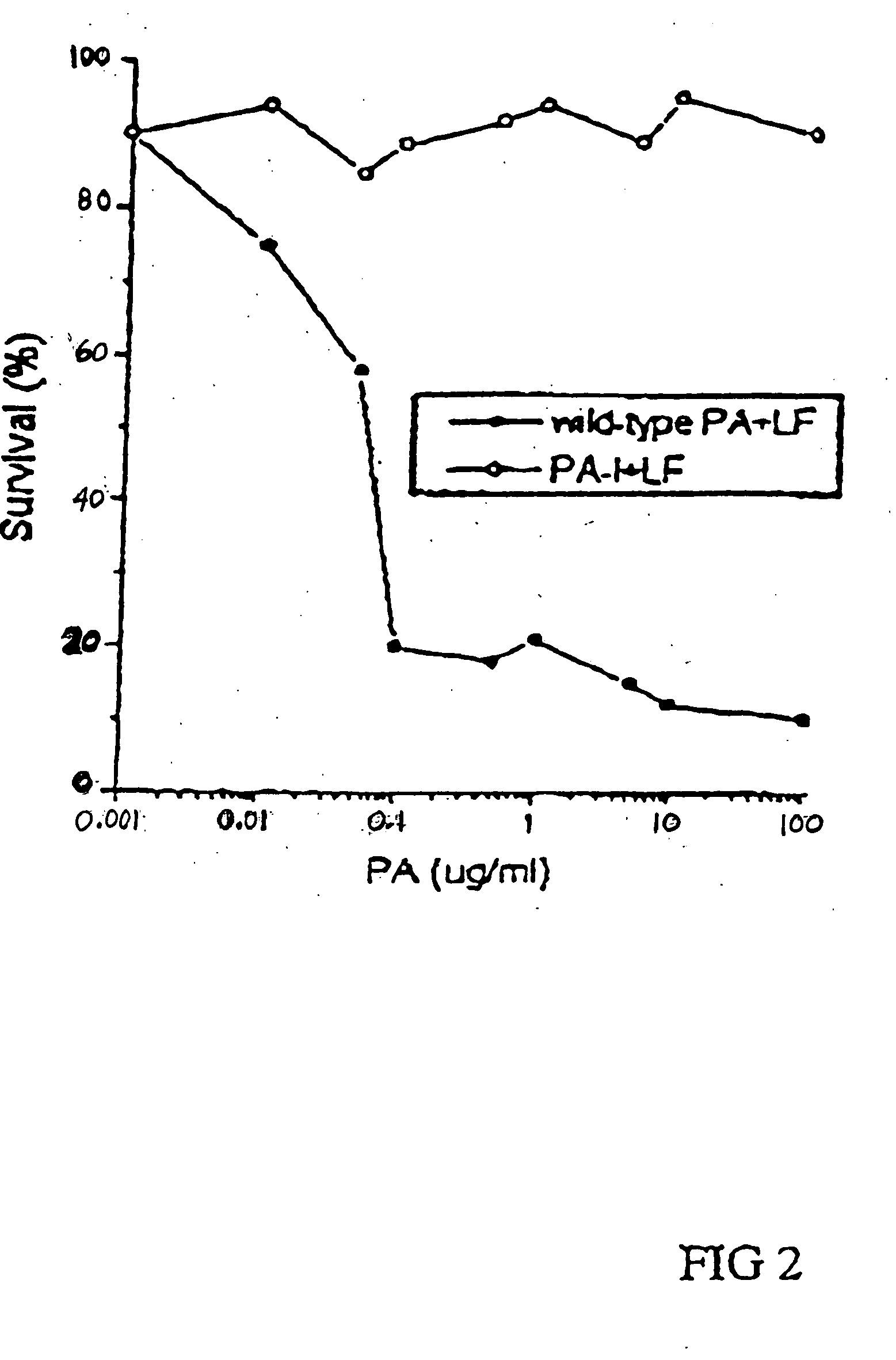
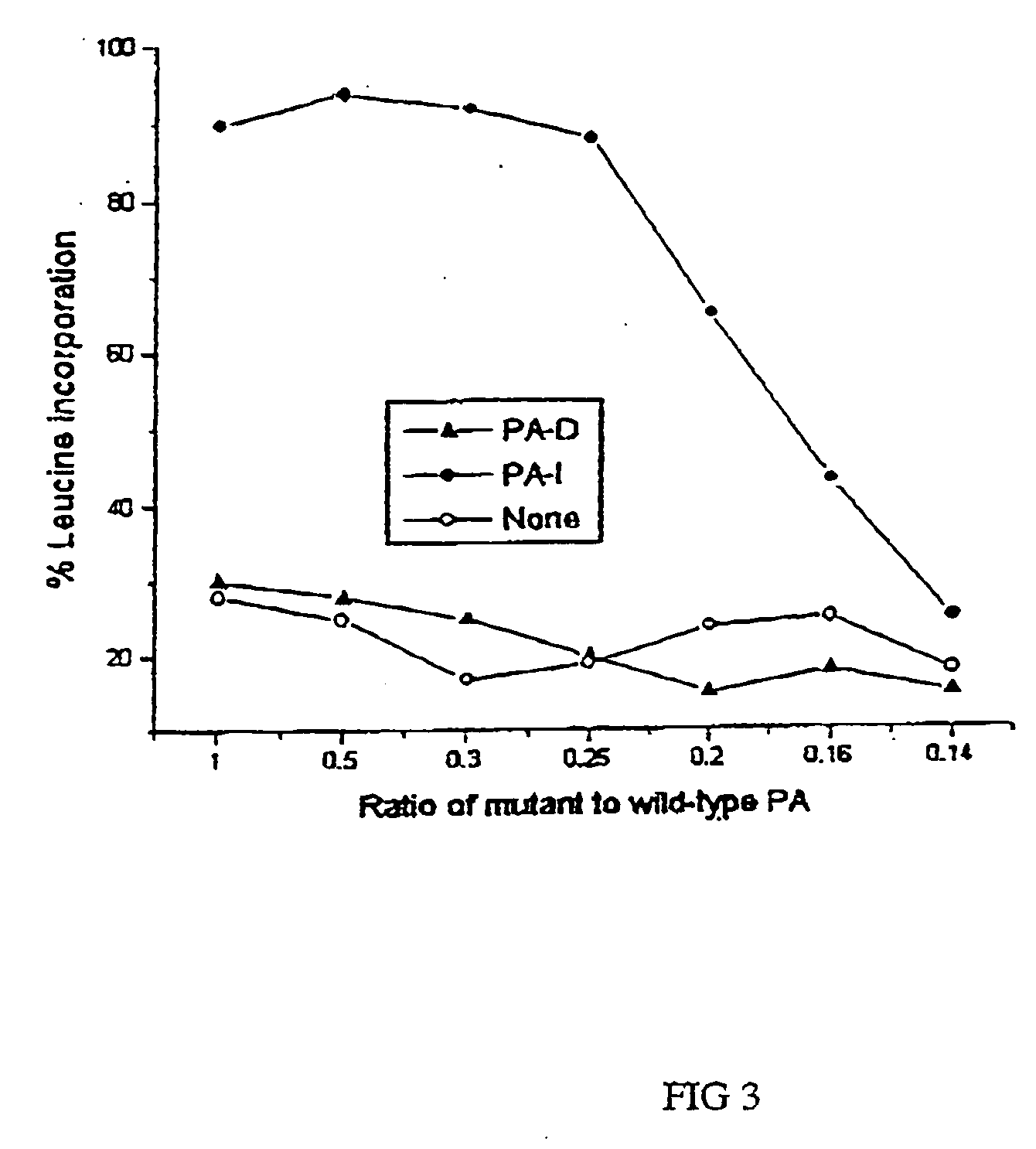
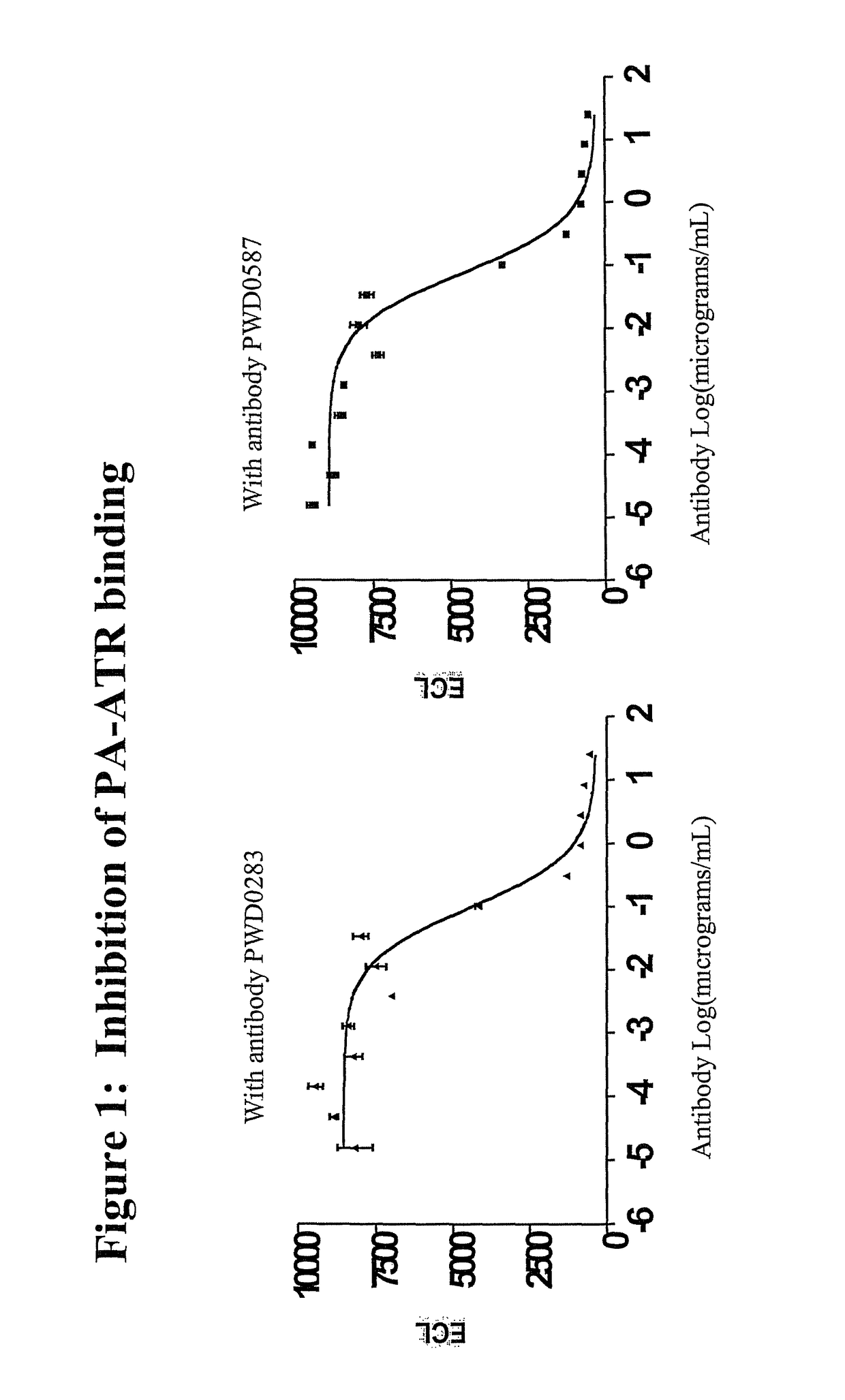
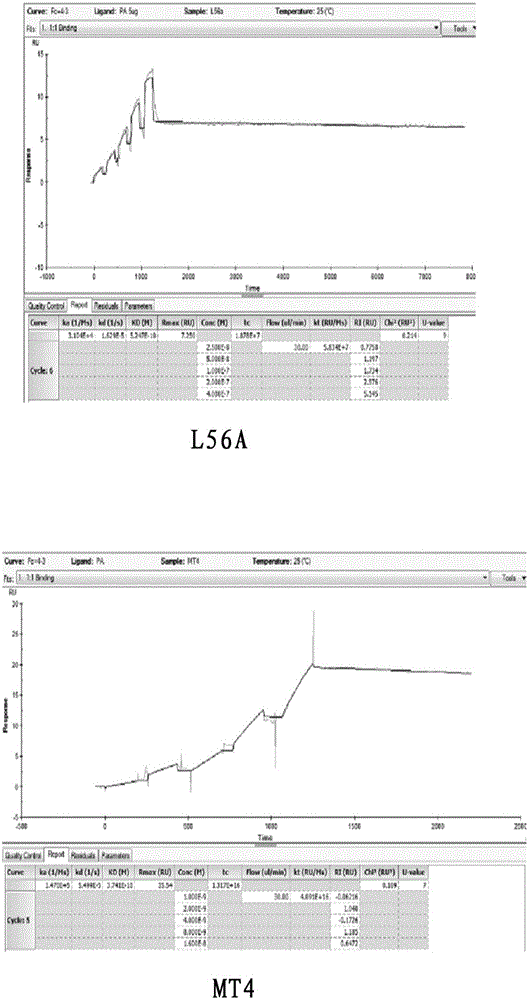
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Anthrax toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
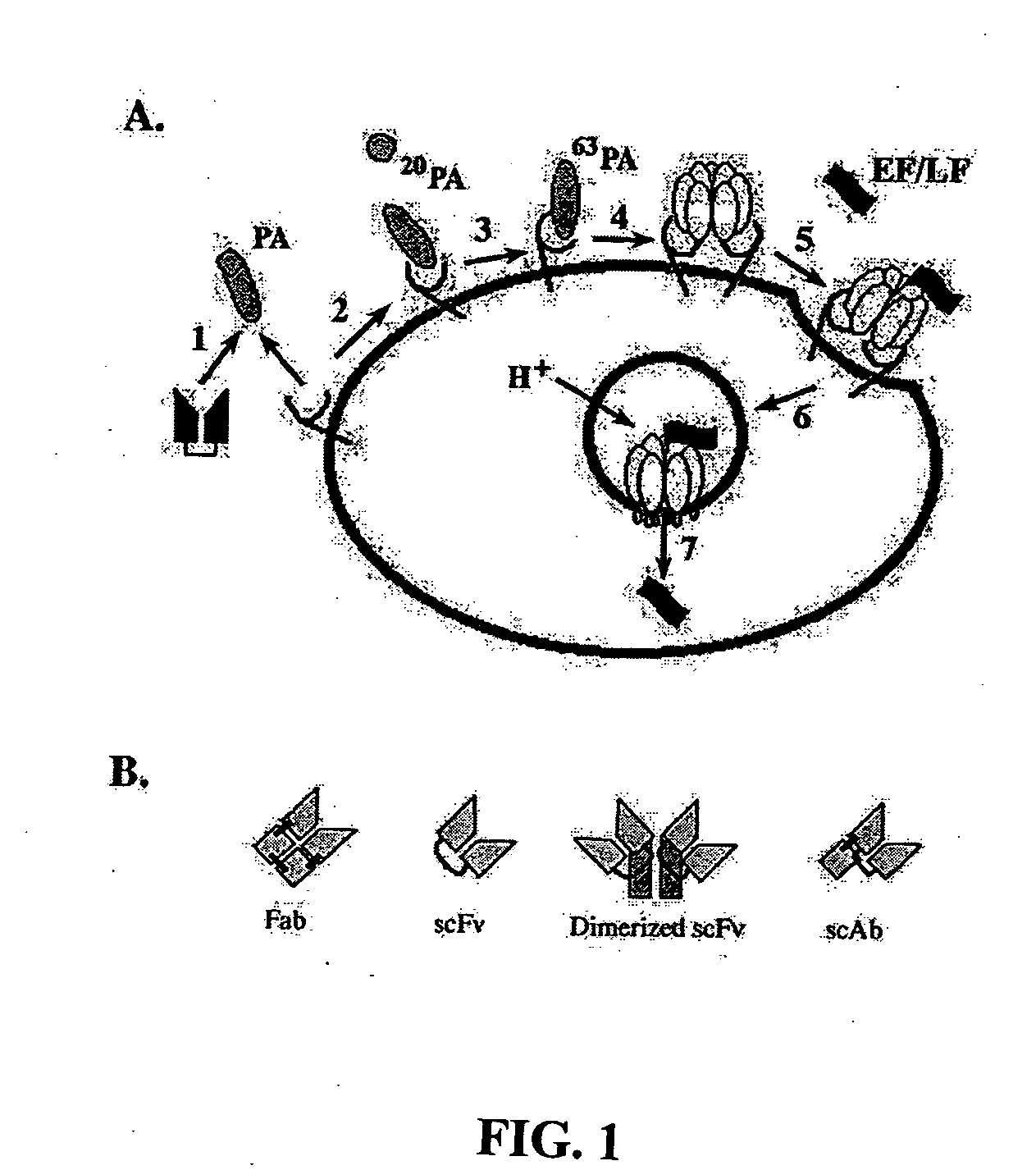
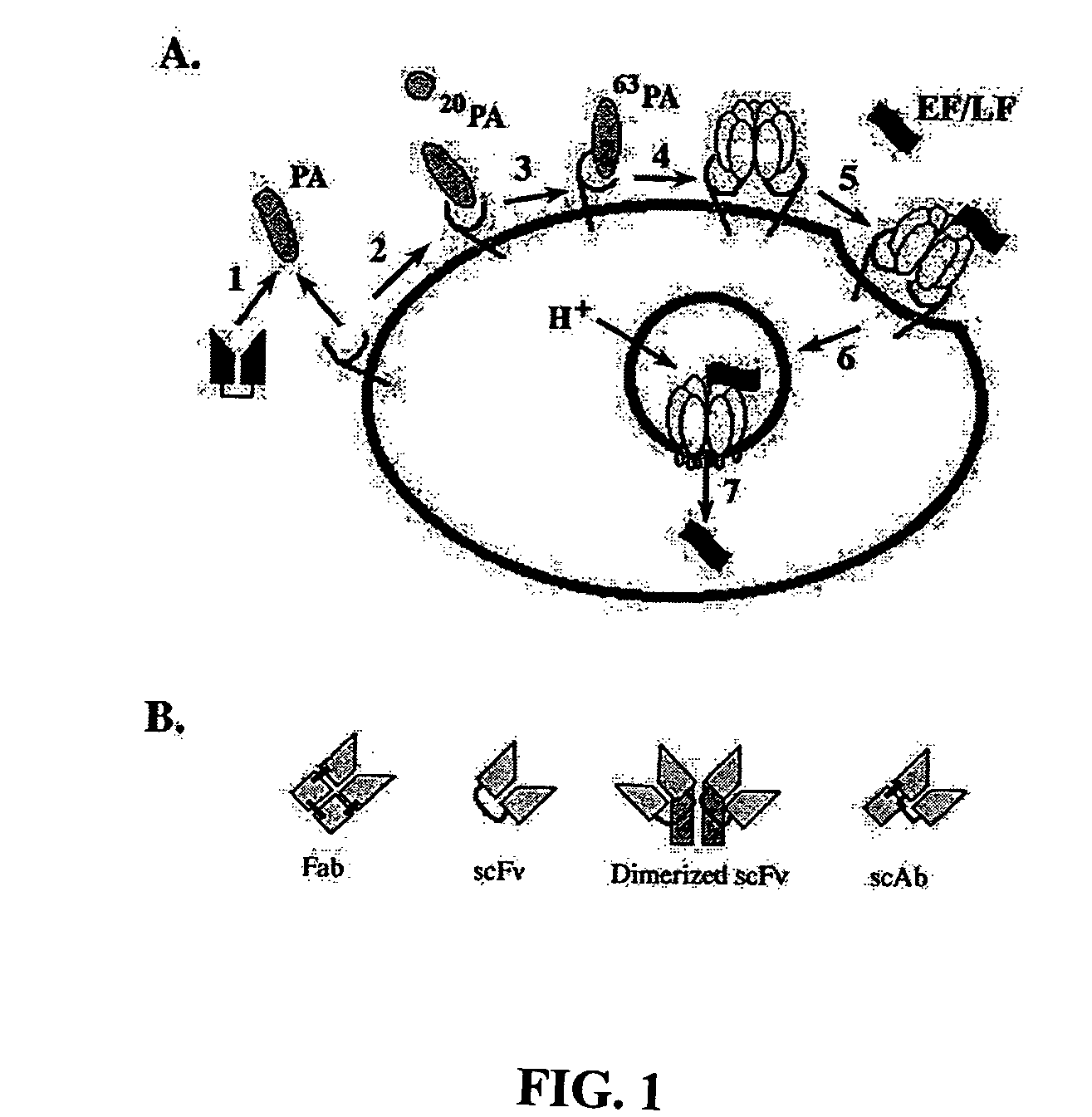
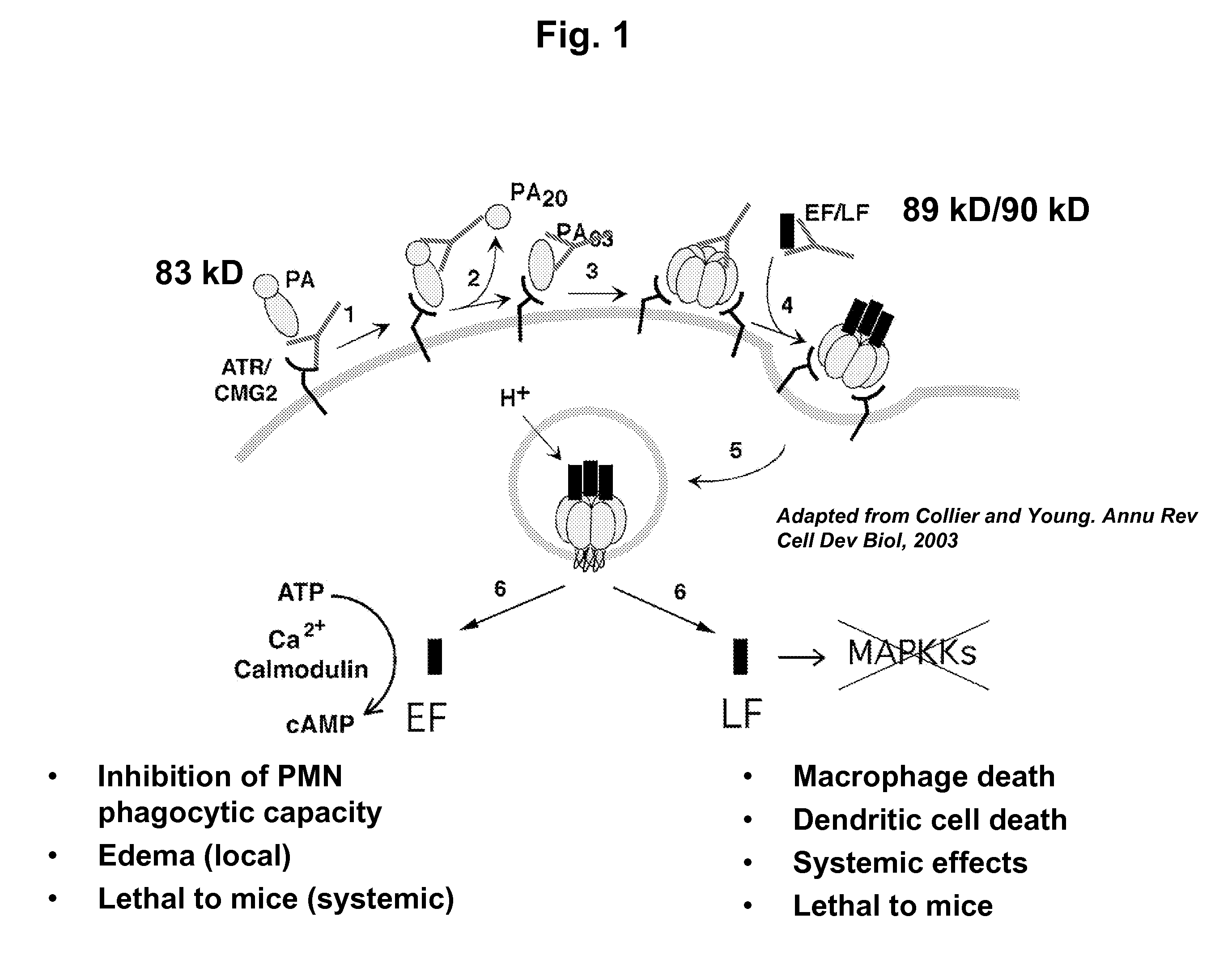
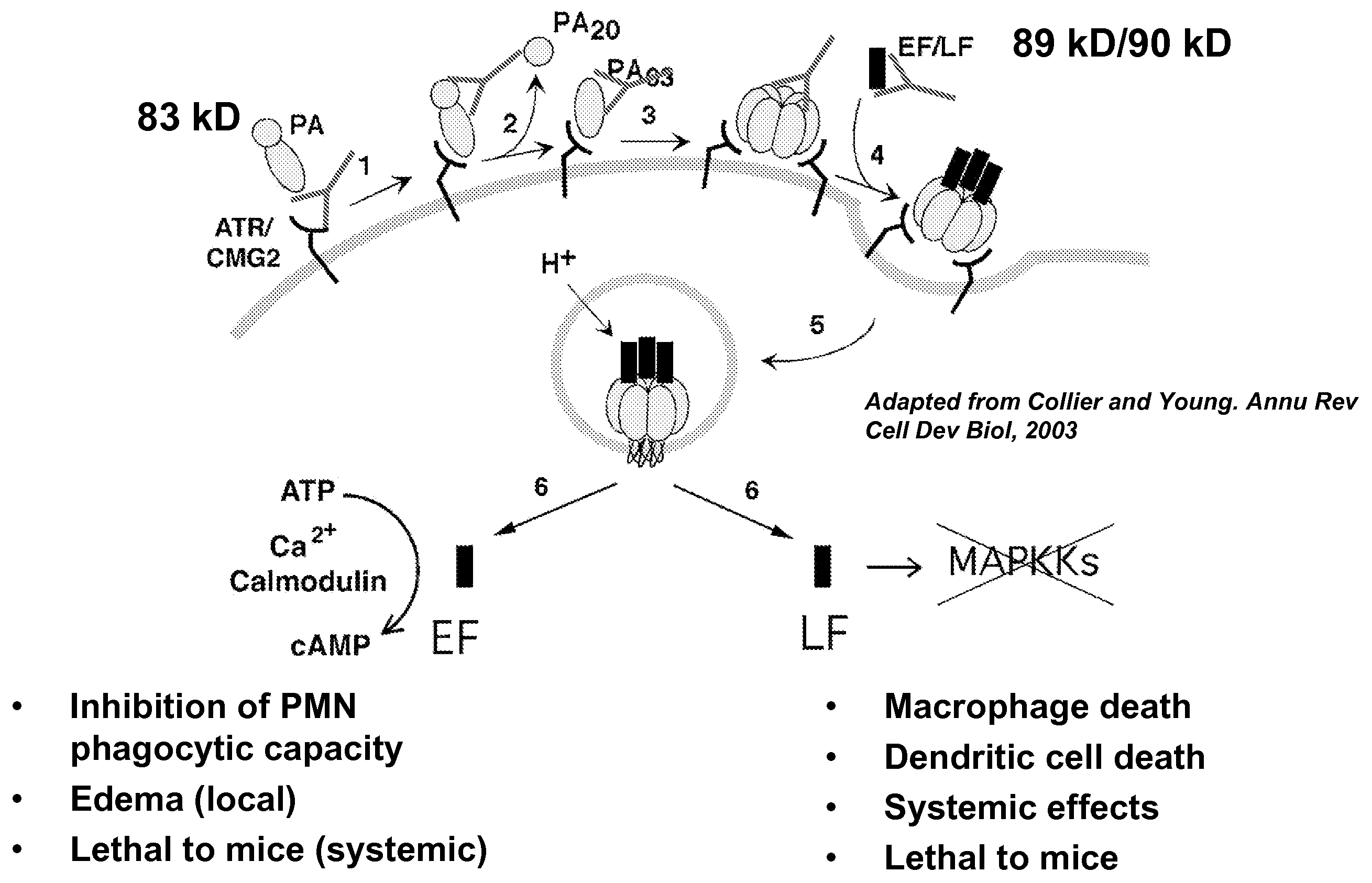
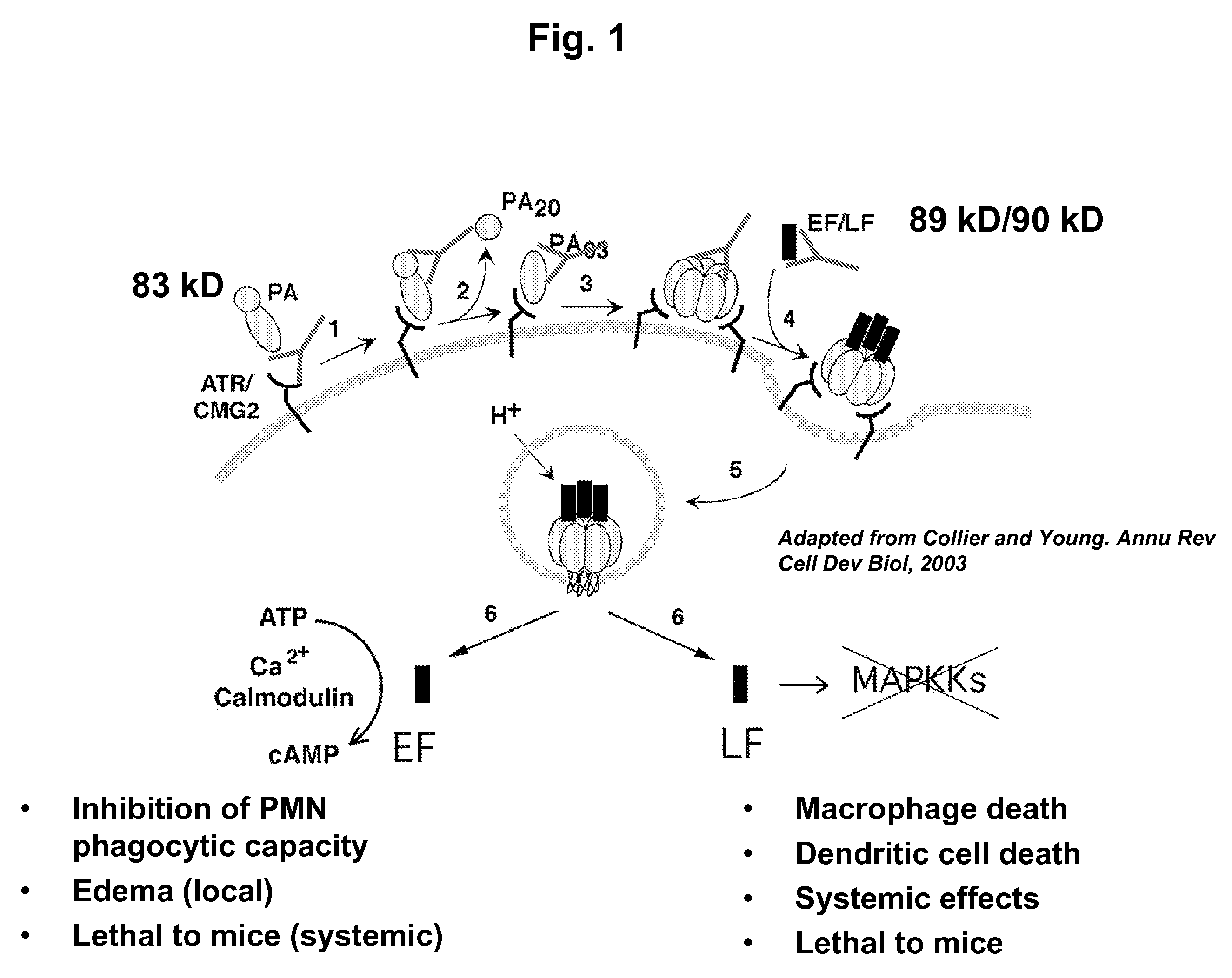
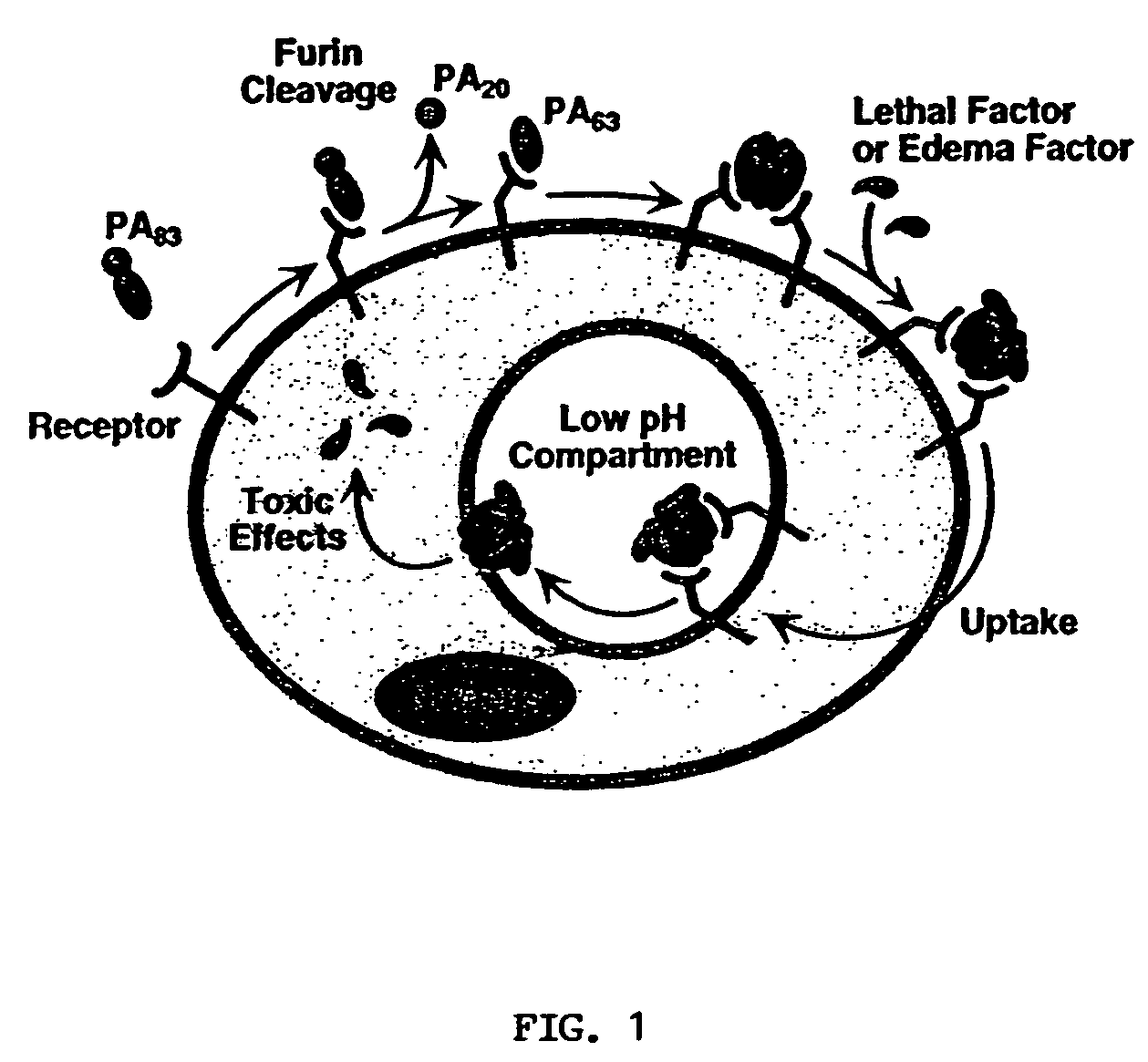
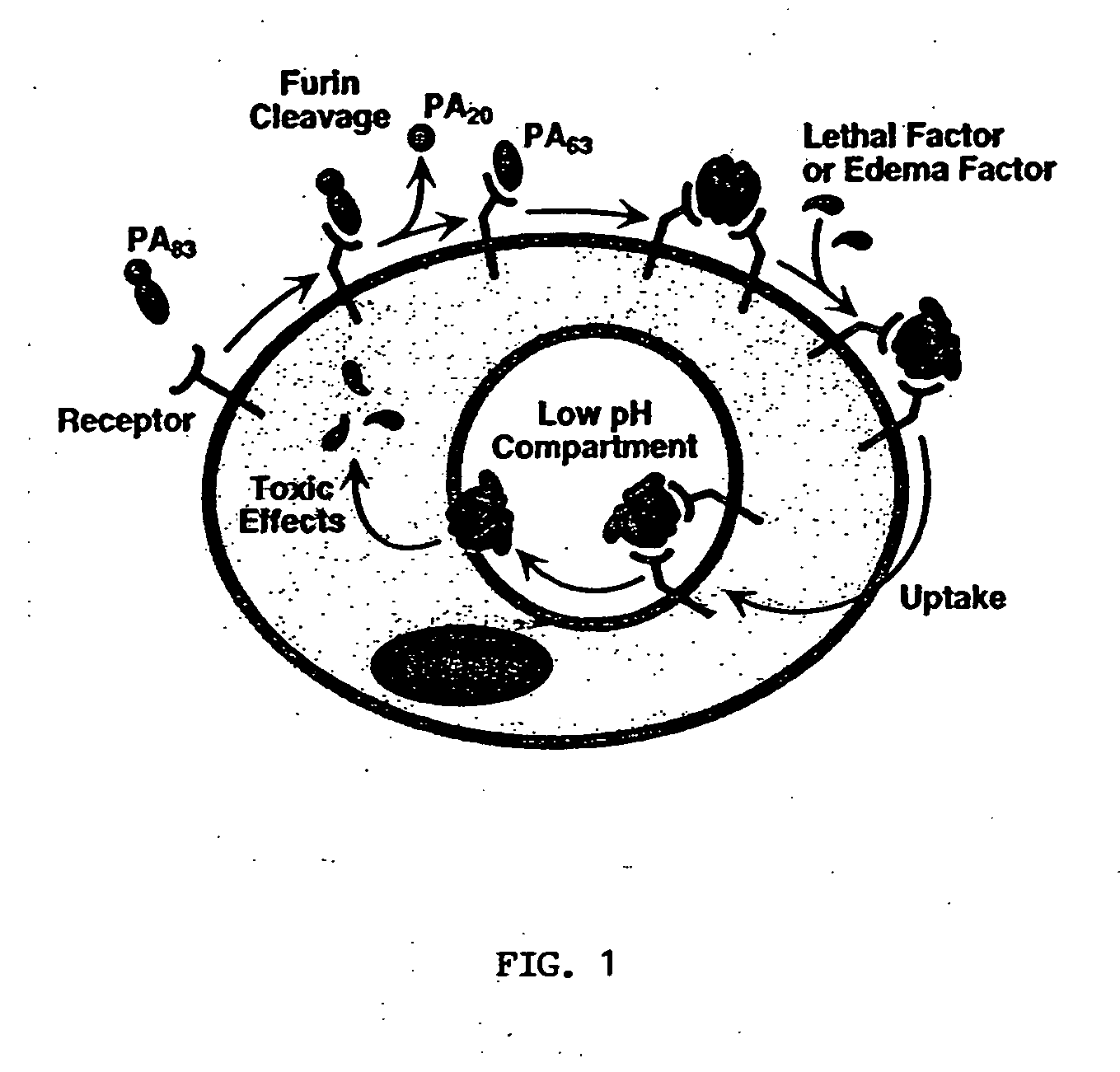
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis—the causative agent of anthrax. The toxin was first discovered by Harry Smith in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen (PA), and two enzyme components, called edema factor (EF) and lethal factor (LF). These three protein components act together to impart their physiological effects. Assembled complexes containing the toxin components are endocytosed. In the endosome, the enzymatic components of the toxin translocate into the cytoplasm of a target cell. Once in the cytosol, the enzymatic components of the toxin disrupts various immune cell functions, namely cellular signaling and cell migration. The toxin may even induce cell lysis, as is observed for macrophage cells. Anthrax toxin allows the bacteria to evade the immune system, proliferate, and ultimately kill the host animal. Research on anthrax toxin also provides insight into the generation of macromolecular assemblies, and on protein translocation, pore formation, endocytosis, and other biochemical processes.
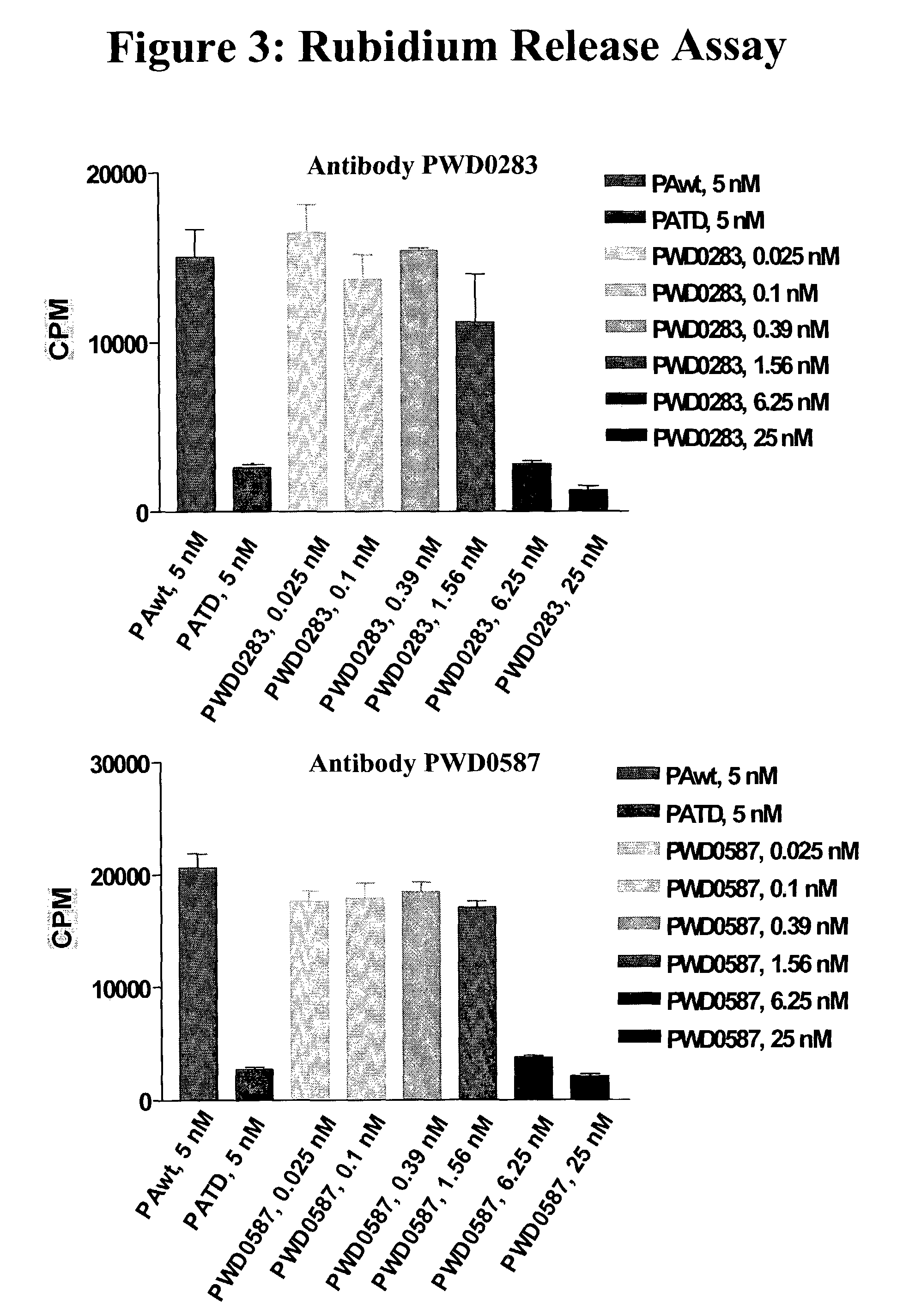
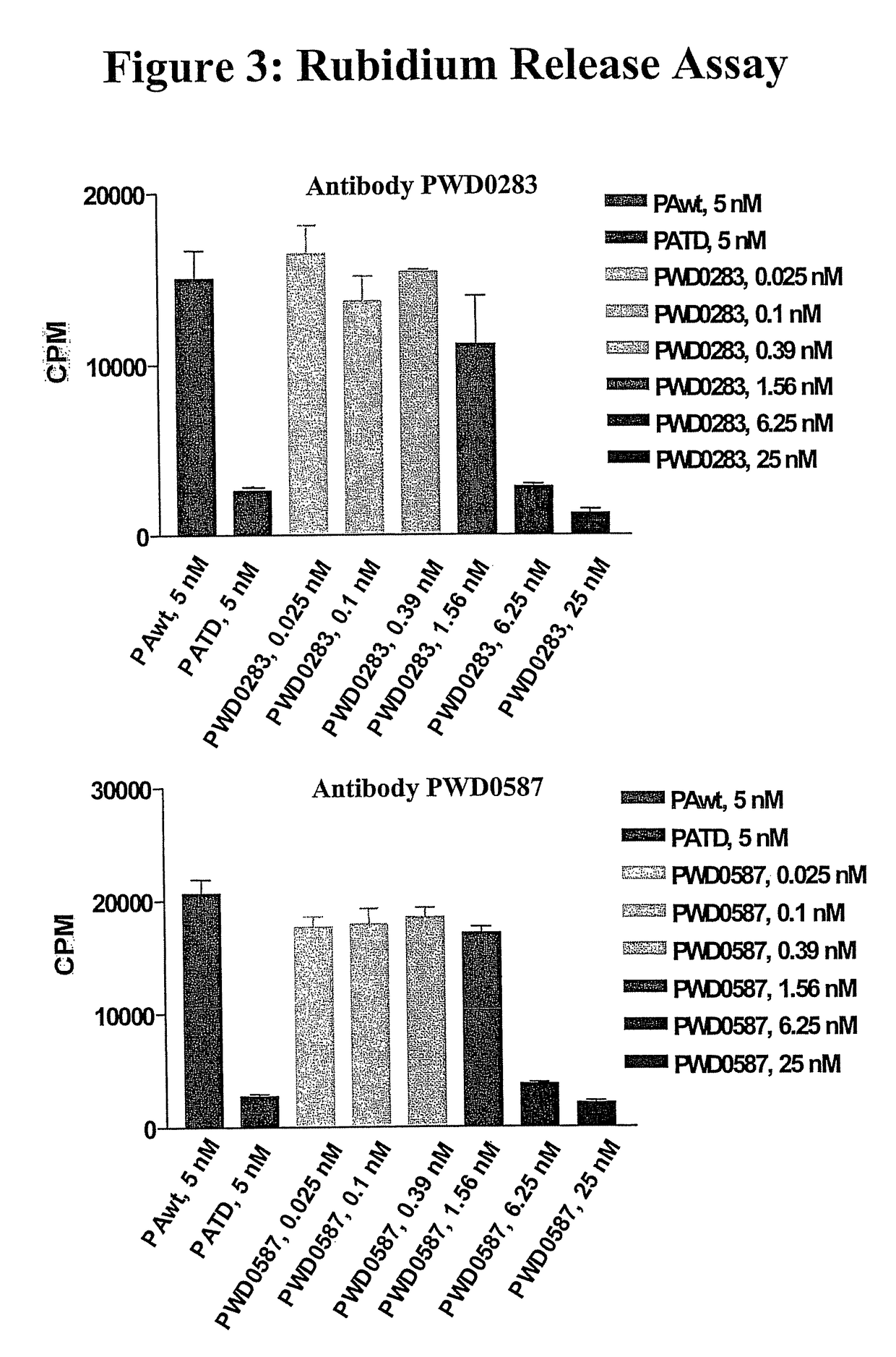
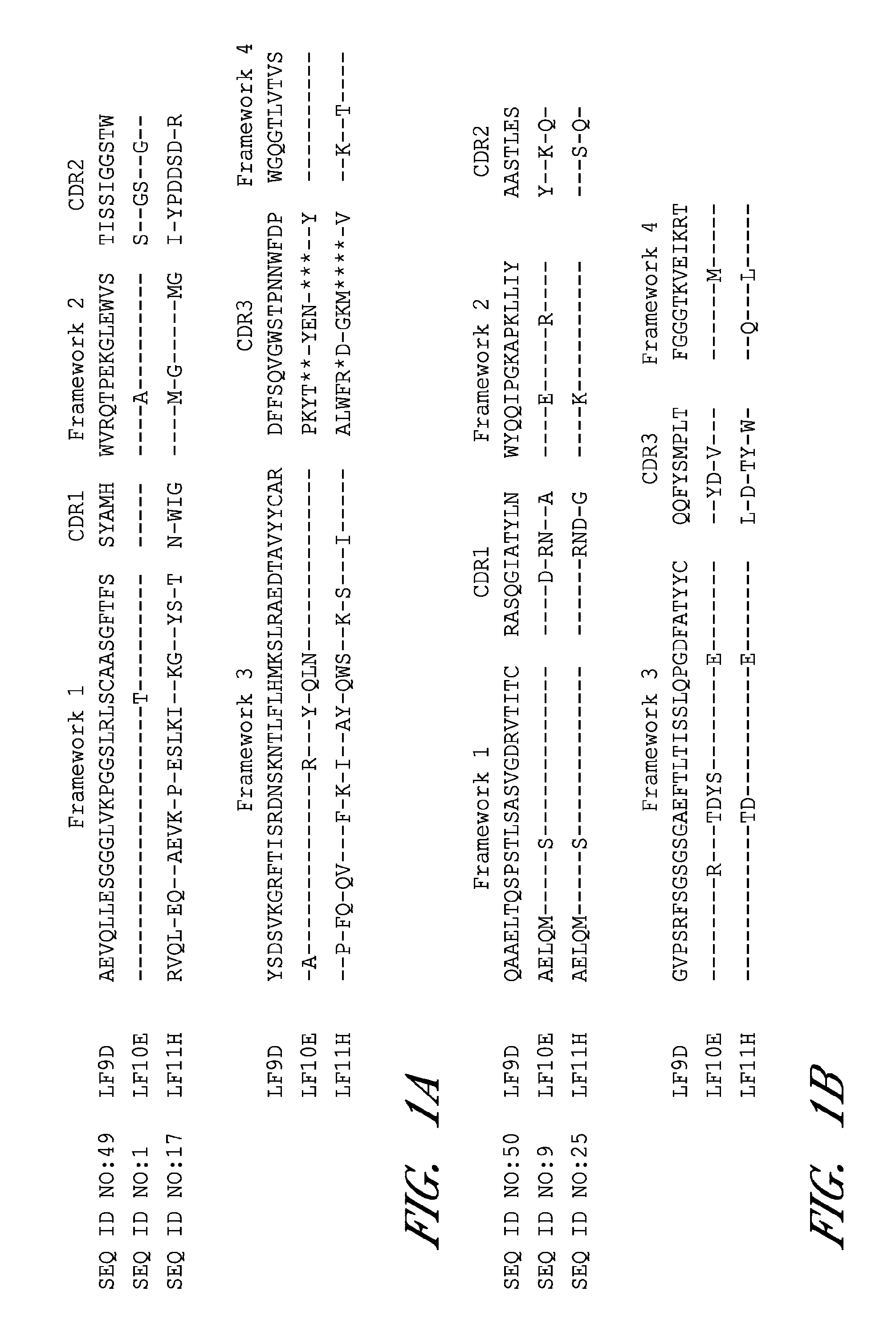
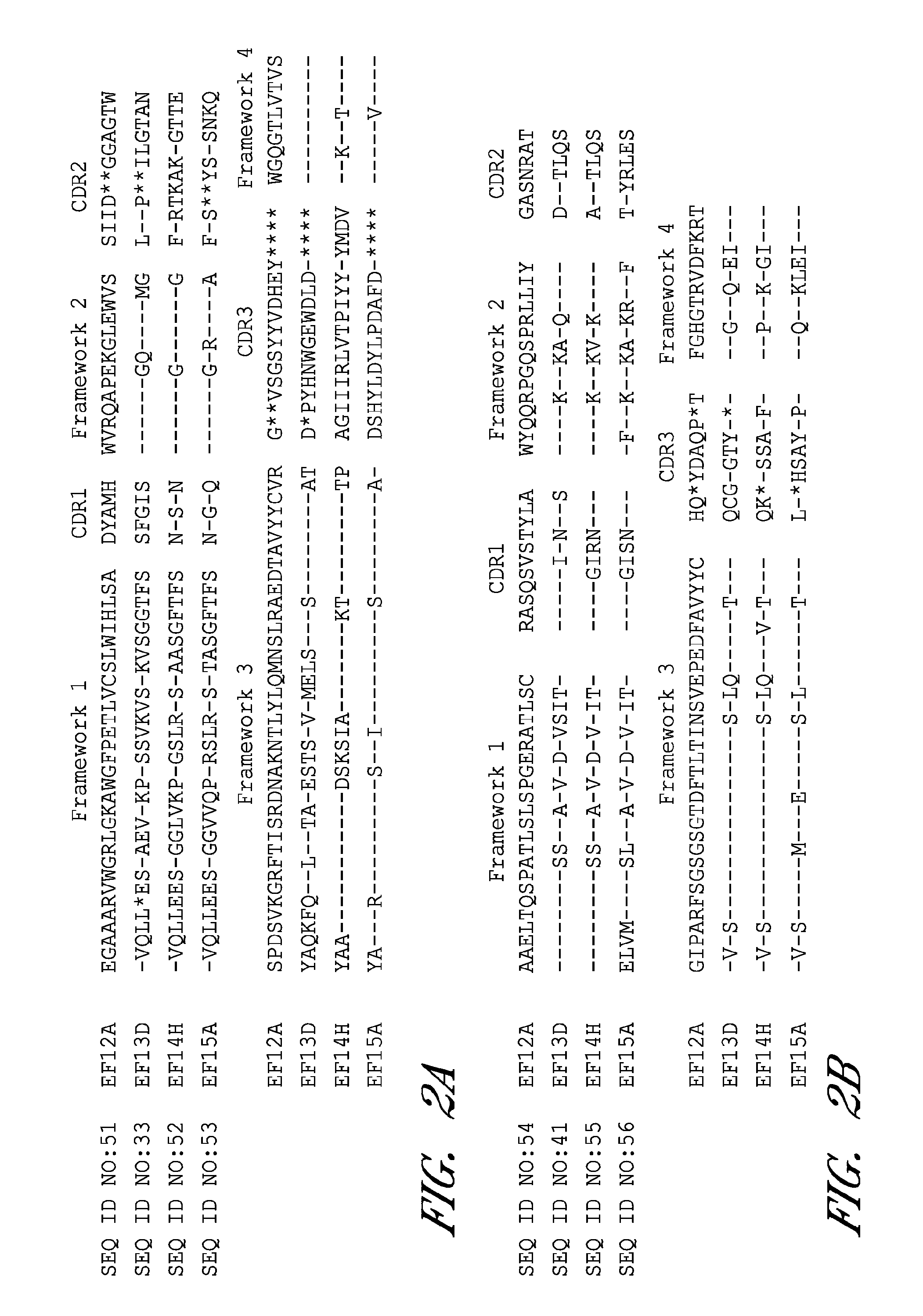
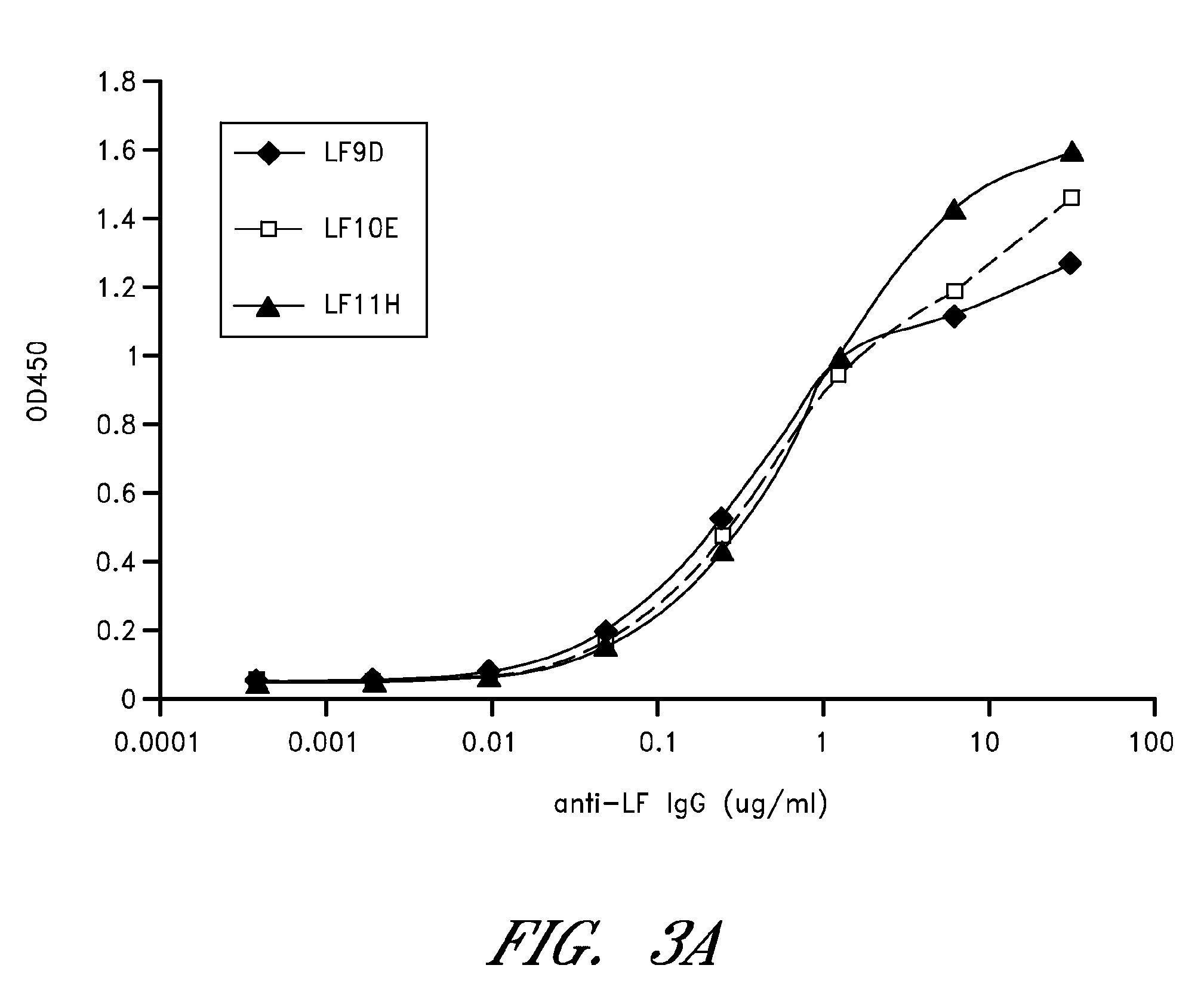
Antibodies against protective antigen
ActiveUS7601351B1Efficient killingIncreased activationAntibacterial agentsAntibody ingredientsAntigenProtective antigen
The present invention relates to antibodies and related molecules that specifically bind to protective antigen of Bacillus anthracis (PA). Such antibodies have uses, for example, in the prevention and treatment of anthrax and anthrax toxin poisoning. The invention also relates to nucleic acid molecules encoding anti-PA antibodies, vectors and host cells containing these nucleic acids, and methods for producing the same.
Owner:EMERGENT MFG OPERATIONS BALTIMORE LLC
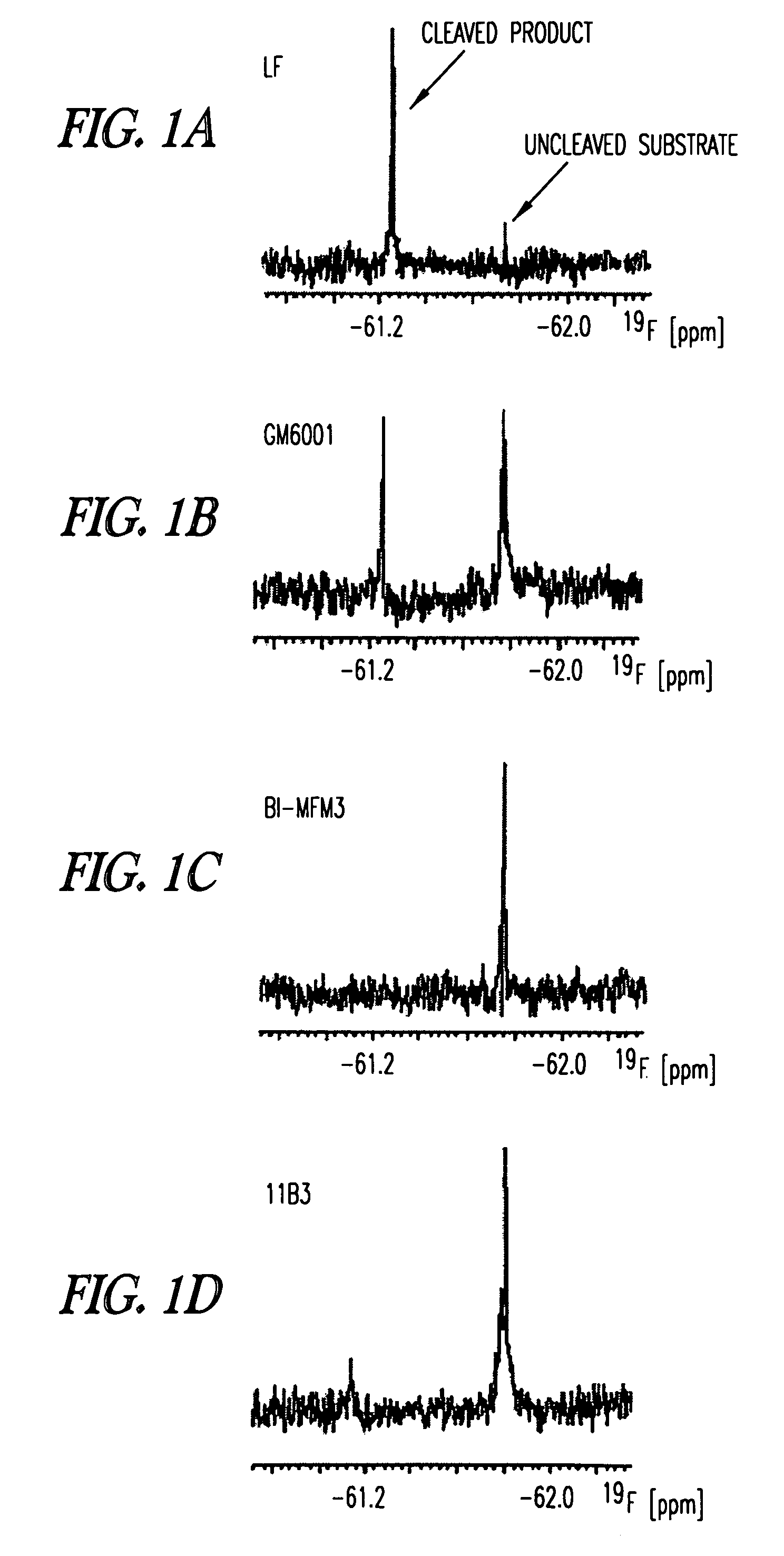
Inhibition of lethal factor protease activity from anthrax toxin
The present invention provides compounds that efficiently and specifically inhibit lethal factor (LF) protease activity of anthrax toxin.
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
Detection, prevention, and treatment systems for anthrax
InactiveUS20050281830A1Avoid toxicityReduce severityBiological material analysisAntibody ingredientsAntigenMammal
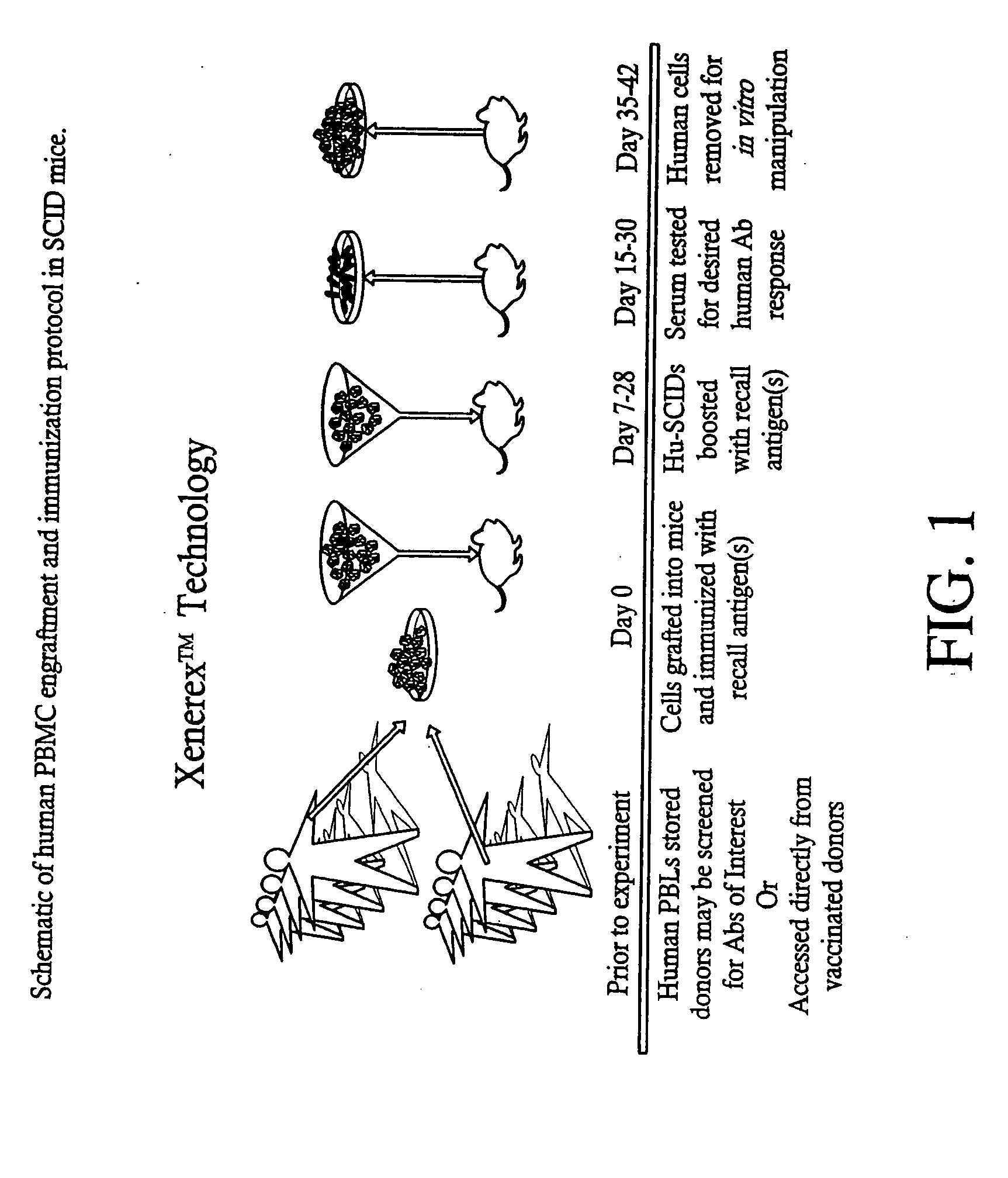
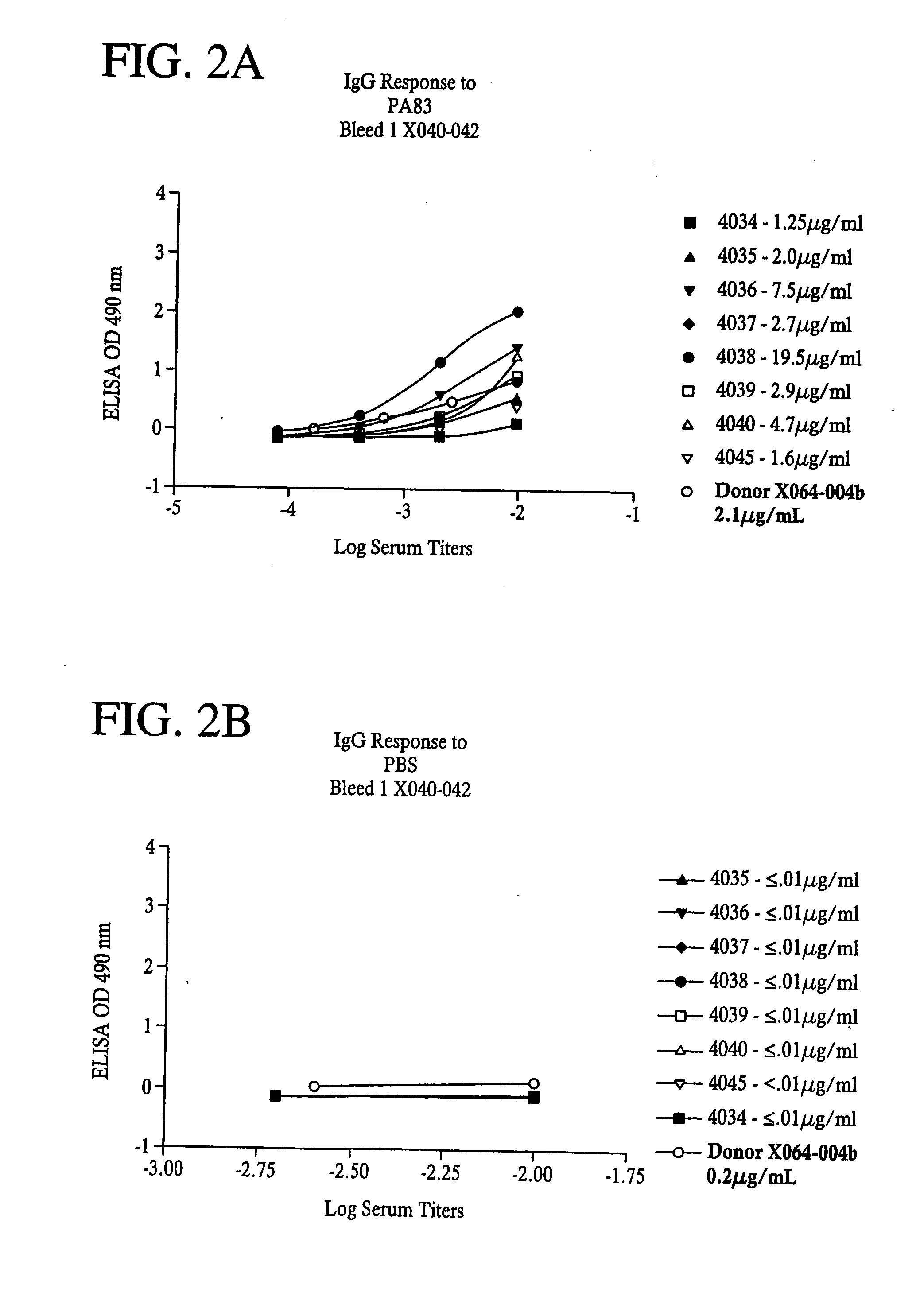
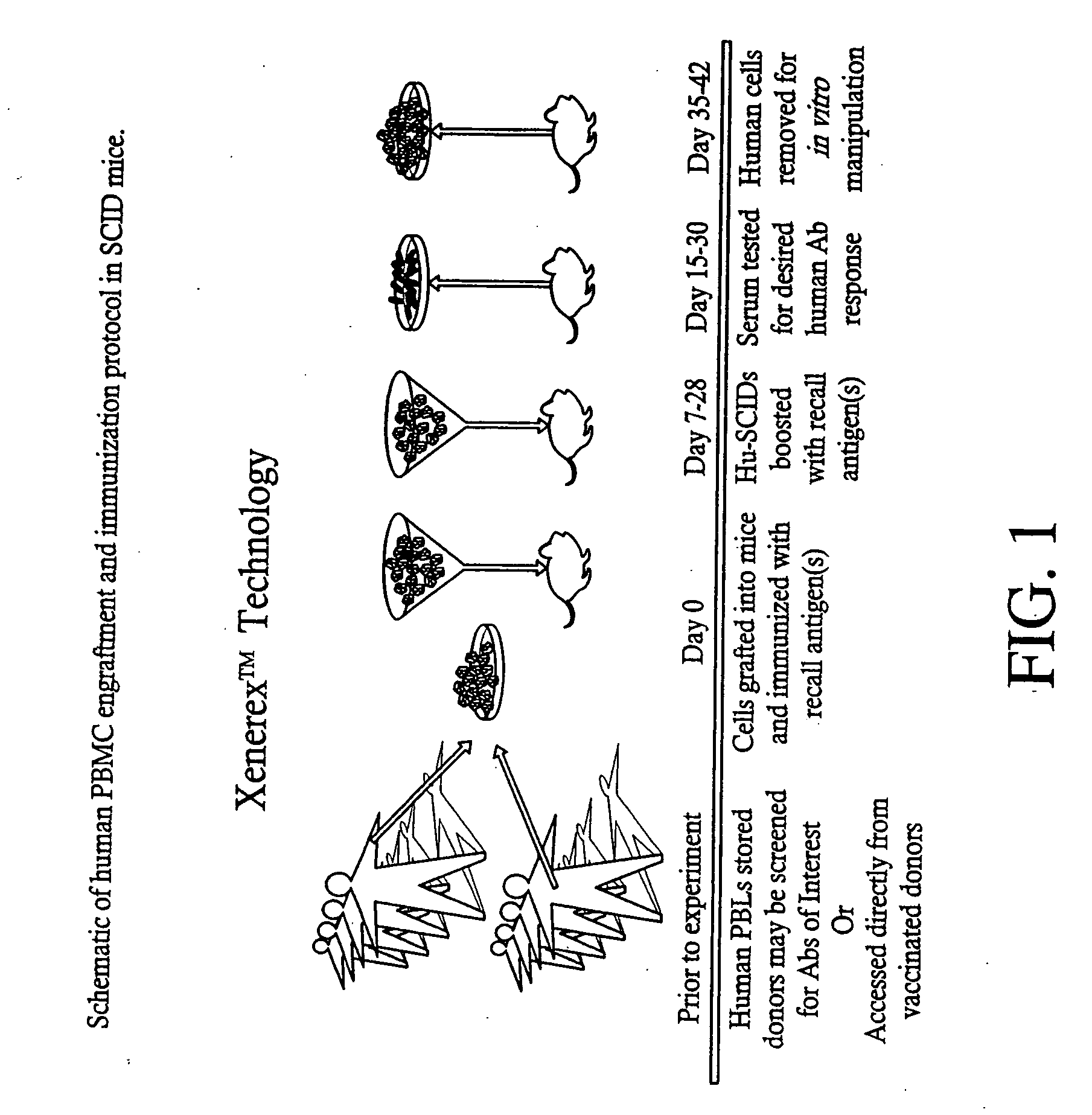
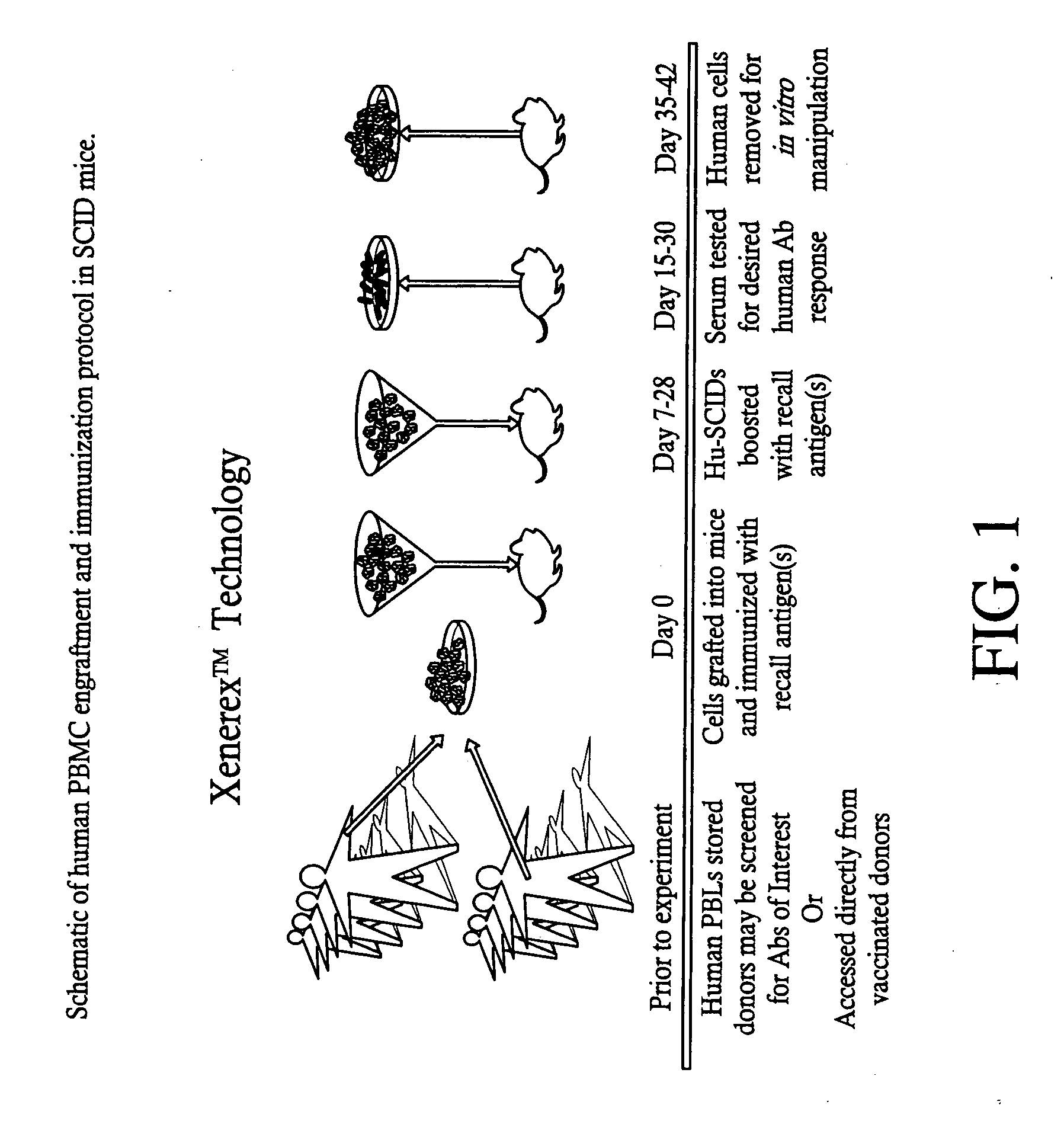
A highly efficient method for generating human antibodies using recall technology is provided. In one aspect, human antibodies which are specific to the anthrax toxin are provided. In one aspect, human peripheral blood cells that have been pre-exposed to anthrax toxin are used in the SCID mouse model. This method results in high human antibody titers which are primarily of the IgG isotype and which contain antibodies of high specificity and affinity to desired antigens. The antibodies generated by this method can be used therapeutically and prophylactically for preventing or treating mammals exposed to anthrax. Thus, in one embodiment, a prophylactic or therapeutic agent used to counter the effects of anthrax toxin, released as a mechanism of bioterrorism, is provided. In one embodiment, a formulation and method for preventing and / or treating anthrax infection comprising a binding agent that prevents the assembly of the PA63 heptamer is also provided. Methods for diagnosis and methods to determine anthrax contamination are also described.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Inhibition of lethal factor protease activity from anthrax toxin
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
Inhibitors of lethal factor protease
The invention provides compounds that can efficiently and specifically inhibit bacterial toxins, such as inhibit the lethal factor (LF) protease activity of anthrax toxin and / or botulinum neurotoxin type A. The invention also provides methods for inhibiting proteases, such as lethal factor protease, as well as methods for treating bacterial infections, such as anthrax and botulinum.
Owner:BURNHAM INST FOR MEDICAL RES
Novel protein molecule useful for inhibition of anthrax toxin
The present invention relates to a novel molecule useful for anthrax toxin inhibition in vivo and also provides a method for in vivo inhibition of anthrax toxin action using the new molecule.
Owner:COUNCIL OF SCI & IND RES
Antibodies against protective antigen
ActiveUS7906119B1Efficient killingIncreased activationAntibacterial agentsAntibody ingredientsProtective antigenAnthrax toxin
The present invention relates to antibodies and related molecules that specifically bind to protective antigen of Bacillus anthracis (PA). Such antibodies have uses, for example, in the prevention and treatment of anthrax and anthrax toxin poisoning. The invention also relates to nucleic acid molecules encoding anti-PA antibodies, vectors and host cells containing these nucleic acids, and methods for producing the same.
Owner:EMERGENT MFG OPERATIONS BALTIMORE LLC
Monoclonal antibodies that neutralize anthrax toxins
ActiveUS20100119520A1Antibacterial agentsImmunoglobulins against bacteriaProtective antigenAnthrax toxin
The present invention relates to monoclonal antibodies that bind or neutralize anthrax lethal factor (LF), edema factor (EF), and / or protective antigen (PA). The invention provides such antibodies, fragments of such antibodies retaining anthrax toxin-binding ability, fully human or humanized antibodies retaining anthrax toxin-binding ability, and pharmaceutical compositions including such antibodies. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. Additionally, the invention provides for prophylactic, therapeutic, and diagnostic methods employing the antibodies and nucleic acids of the invention.
Owner:UNITED STATES OF AMERICA
Recombinant antibodies for the detection and neutralization of anthrax toxin
A composition and method for treating a host having or at risk of infection by Bacillus anthracis using an affinity matured antibody or portion thereof derived from a monoclonal antibody.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Antibodies against protective antigen and methods of use for passive immunization and treatment of anthrax
InactiveUS20080063647A1Avoid toxicityReduce severityAntibacterial agentsImmunoglobulins against bacteriaProtective antigenMammal
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Recombinant antibodies for the detection and neutralization of anthrax toxin
A composition and method for treating a host having or at risk of infection by Bacillus anthracis using an affinity matured antibody or portion thereof derived from a monoclonal antibody.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
CMG 2mutant and Fc fusion protein, its encoding gene and its application
InactiveCN102516394AFree from attackDon't worry about mutationsAntibacterial agentsGenetic material ingredientsAntigenADAMTS Proteins
The invention discloses a CMG 2mutant and Fc fusion protein, its encoding gene and its application. The provided protein is any one of the protein in the following 1) or 2) 1) protein composed of an amino acid residue sequence of a sequence 2 in a sequence table; and 2 ) protein derived from 1) by that the amino acid residue sequence of the sequence 2 in the sequence table is subjected to one or more amino acid residue replacement andor deletion andor addition and possesses the same function. The experiment proves that the provided protein can prevent cells from attacking of anthrax toxin, can be taken as anthrax vaccine, the protein and antigen enables high affinity combination, are equivalent to a Fc fragment of the antibody, and the Fc fragment provides the biological activity of the antibody.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Anthrax compositions and methods of use and production
InactiveUS7794732B2Reducing debilitating effectEliminate the effects ofAntibacterial agentsPeptide/protein ingredientsProtective antigenImmunogenicity
Compositions and methods effective for eliciting an immune response for preventing or reducing infection or improving clinical outcomes caused by Bacillus anthracis are provided. The compositions include a naturally occurring or synthetic protein, peptide, or protein fragment containing all or an active portion of an antigenic epitope associated with anthrax toxin proteins optionally combined with a pharmaceutically acceptable carrier. The preferred antigenic epitopes correspond to immunogenic regions of protective antigen, lethal factor or edema factor, either individually or in combination. In addition, methods and compositions containing antibodies for reducing the effects of anthrax toxins are described. The methods involve administering to a human or animal the compositions described herein in a dosage sufficient to elicit an immune response or treat the anthrax infection.
Owner:OKLAHOMA MEDICAL RES FOUND
Neutralizing human antibodies to anthrax toxin
InactiveUS20060246079A1Avoid toxicityReduce severityAntibacterial agentsImmunoglobulins against bacteriaAntigenMammal
A highly efficient method for generating human antibodies using recall technology is provided. In one aspect, human antibodies which are specific to the anthrax toxin are provided. In one aspect, human peripheral blood cells that have been pre-exposed to anthrax toxin are used in the SCID mouse model. This method results in high human antibody titers which are primarily of the IgG isotype and which contain antibodies of high specificity and affinity to desired antigens. The antibodies generated by this method can be used therapeutically and prophylactically for preventing or treating mammals exposed to anthrax. Thus, in one embodiment, a prophylactic or therapeutic agent used to counter the effects of anthrax toxin, released as a mechanism of bioterrorism, is provided. In one embodiment, a formulation and method for preventing and / or treating anthrax infection comprising a binding agent that prevents the assembly of the PA63 heptamer is also provided. Methods for diagnosis and methods to determine anthrax contamination are also described.
Owner:EMERGENT PROD DEV GAITHERSBURG INC
Novel protein capable of inhibiting anthrax toxin activity
The invention particularly relates to inhibition of the cleavage of protective antigen (PA) of Bacillus anthracis, which subsequently leads to inhibition of activity of anthrax toxin.
Owner:COUNCIL OF SCI & IND RES
Inhibitors of lethal factor protease
The invention provides compounds that can efficiently and specifically inhibit bacterial toxins, such as inhibit the lethal factor (LF) protease activity of anthrax toxin and / or botulinum neurotoxin type A. The invention also provides methods for inhibiting proteases, such as lethal factor protease, as well as methods for treating bacterial infections, such as anthrax and botulinum.
Owner:BURNHAM INST FOR MEDICAL RES
Protein molecule useful for inhibition of anthrax toxin
The present invention relates to a novel molecule useful for anthrax toxin inhibition in vivo and also provides a method for in vivo inhibition of anthrax toxin action using the new molecule.
Owner:COUNCIL OF SCI & IND RES
Compositions and methods for treatment of pain
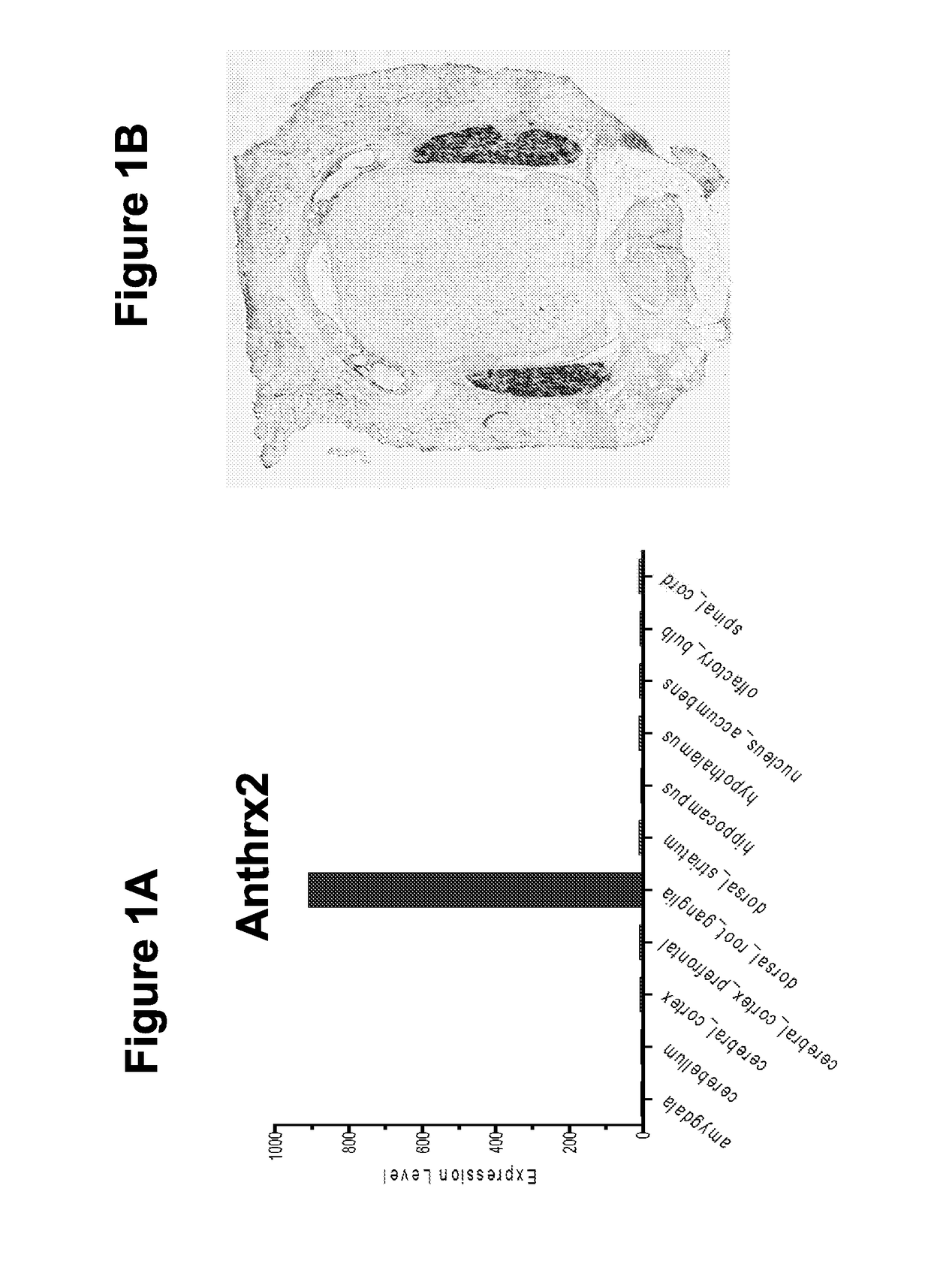
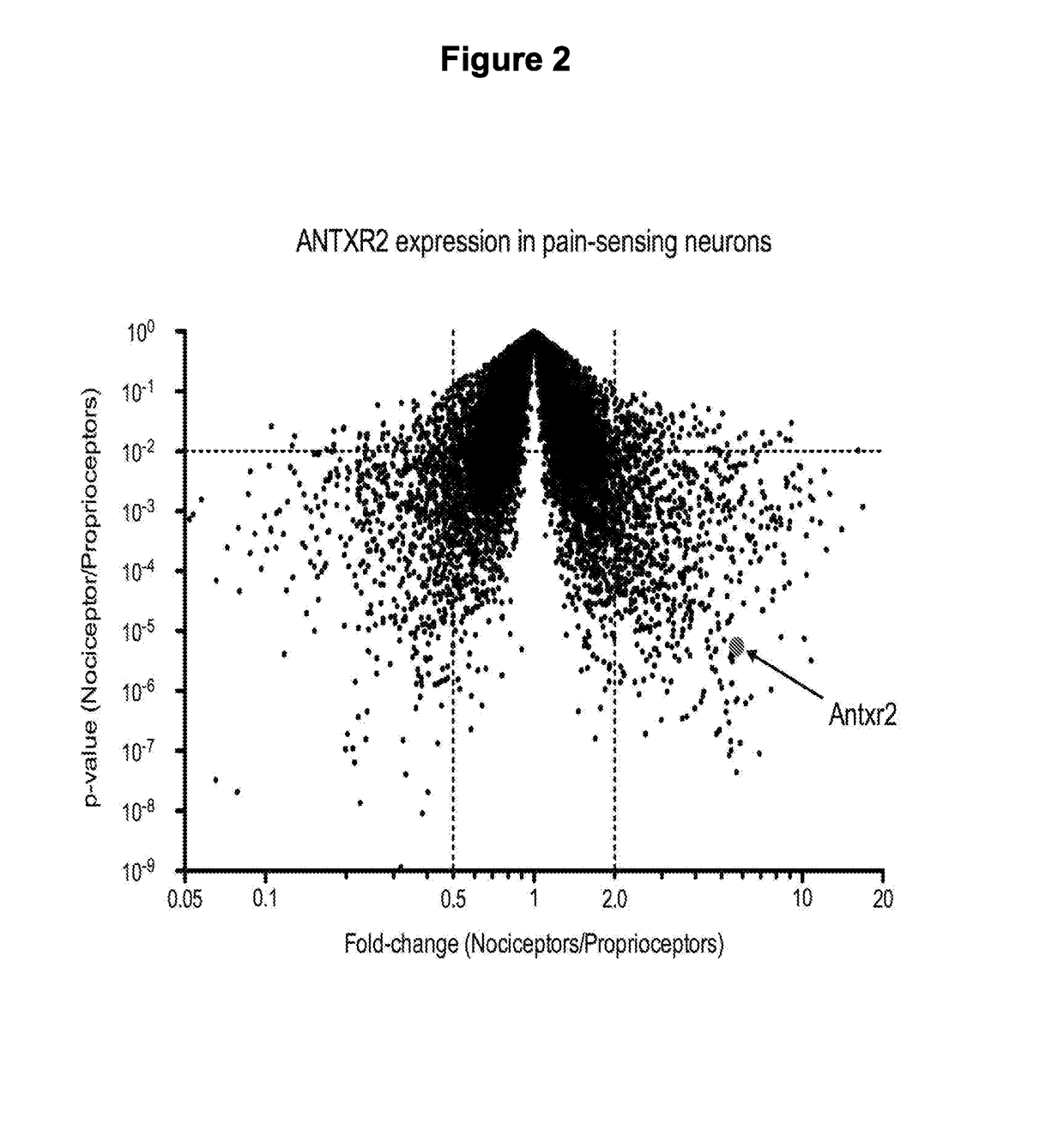
PendingUS20180244731A1Improve bindingReduce releaseHydrolasesPeptide/protein ingredientsAnthrax toxinNociceptor
Embodied herein are engineered fusion proteins that bind and target nociceptor neurons, compositions comprising these engineered fusion proteins, and methods for treatment of pain using these engineered fusion proteins or compositions containing the engineered fusion proteins. The engineered fusion proteins contain domains derived from protein toxins such as the anthrax toxin, clostridial botulinum family of toxins, disulphide-containing toxins, and AB component type toxins.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
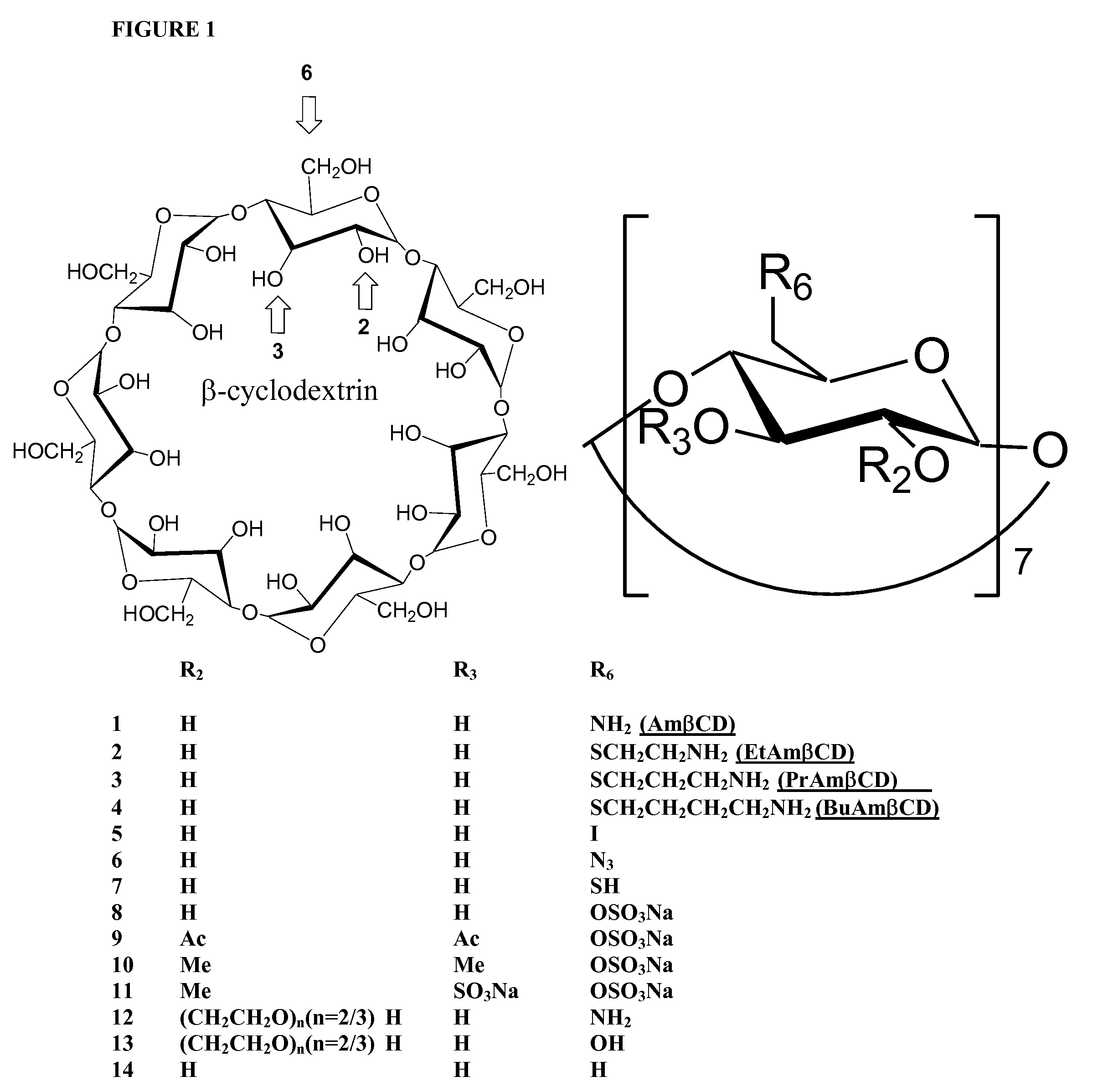
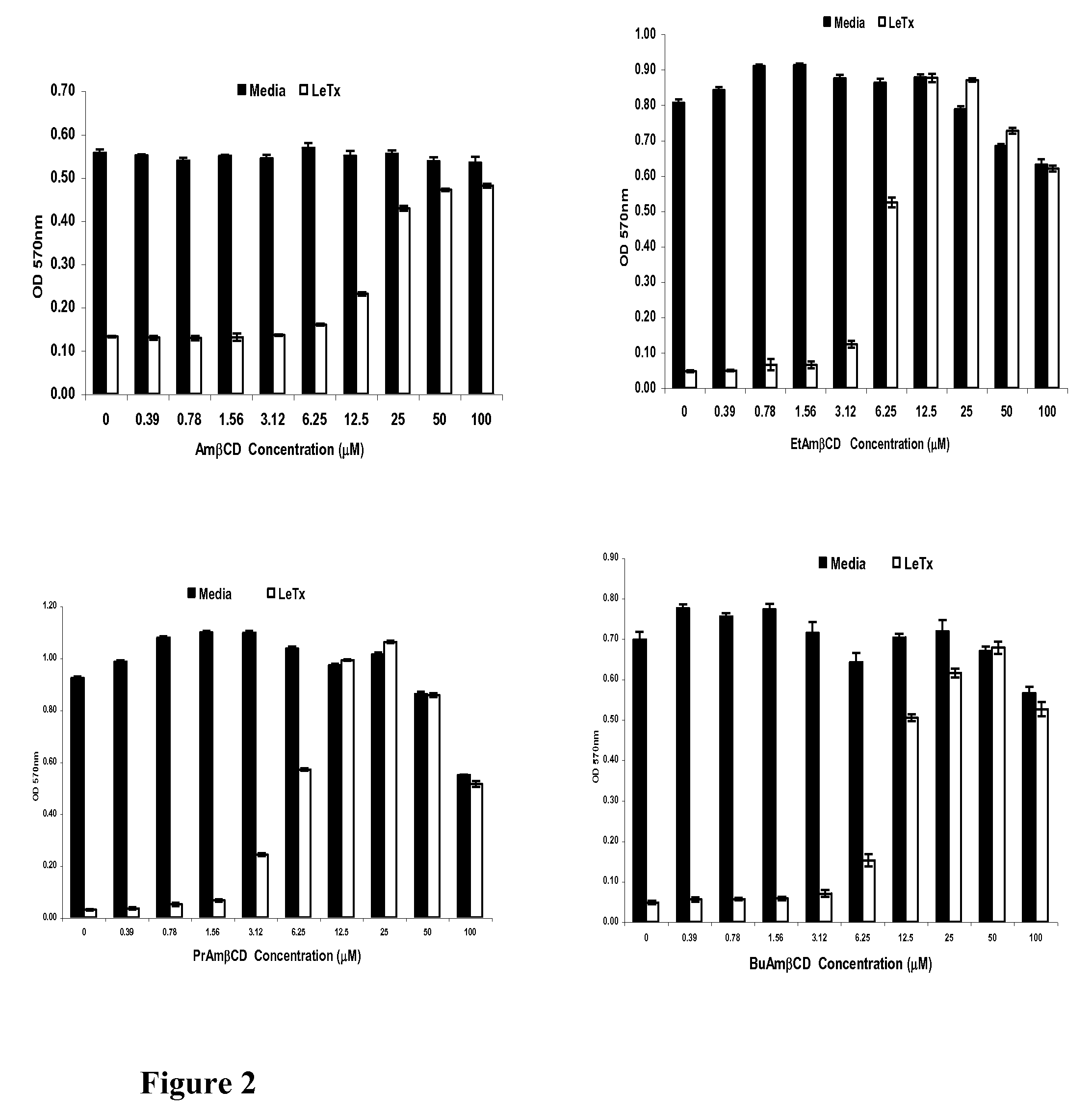
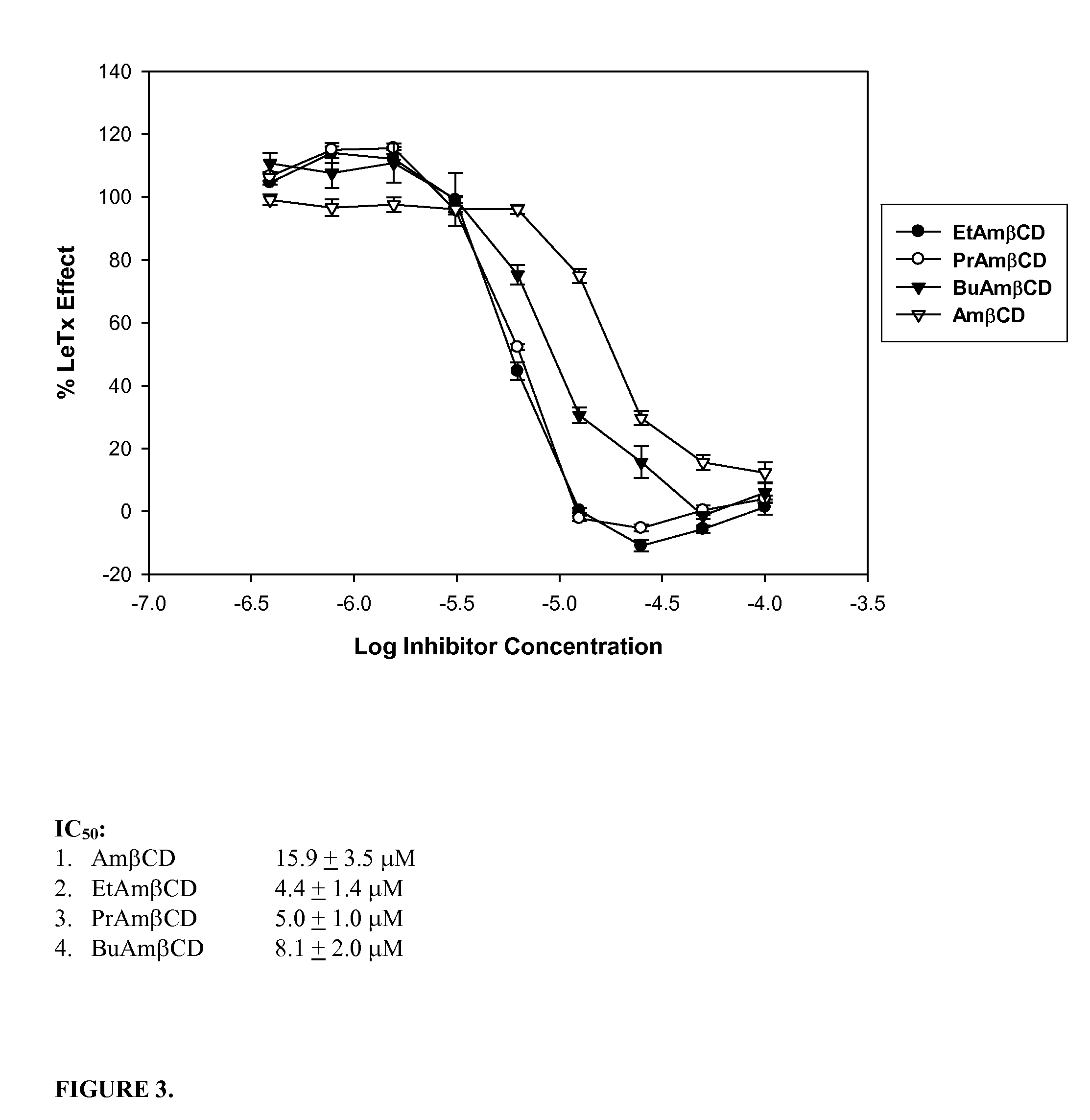
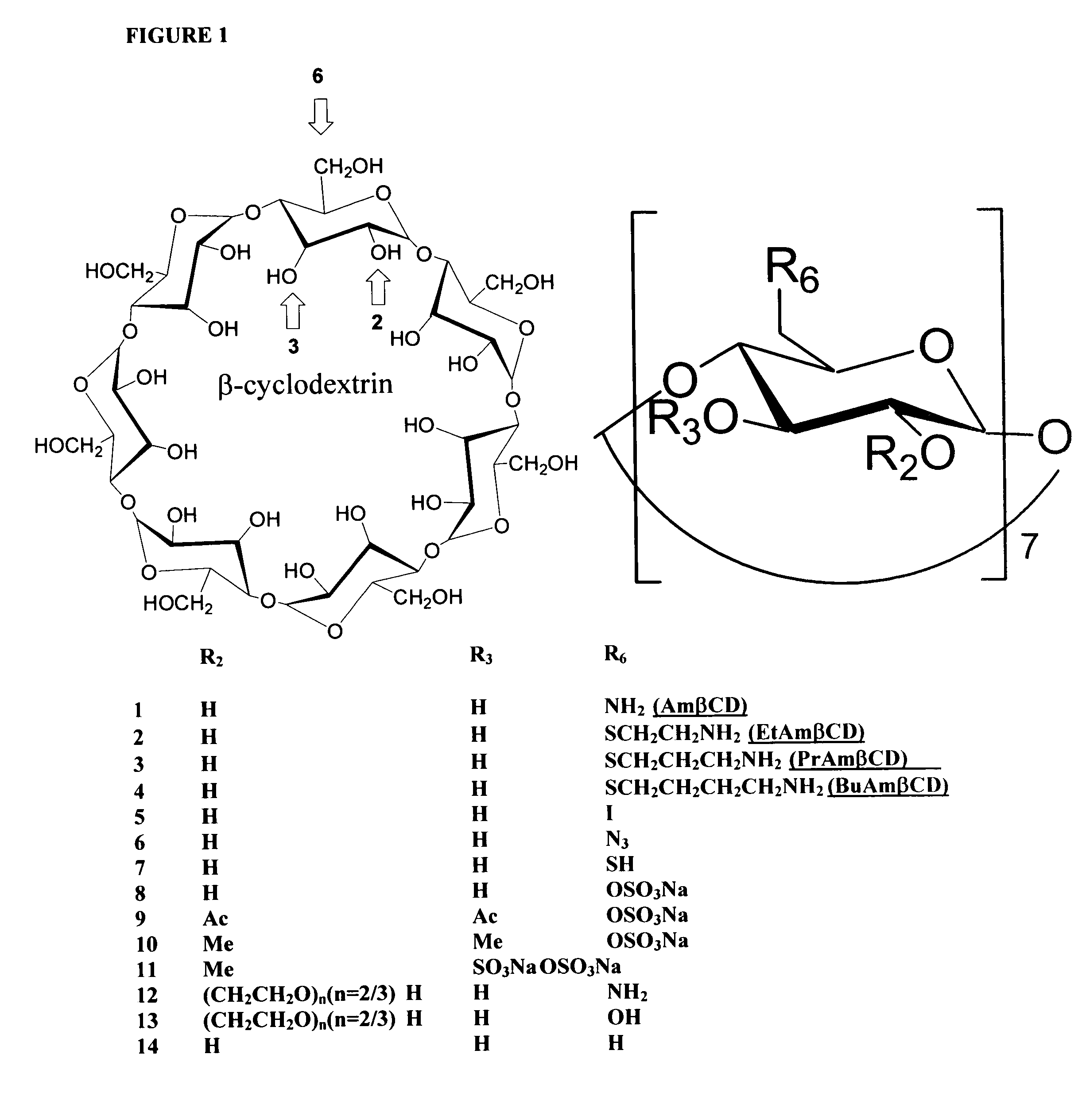
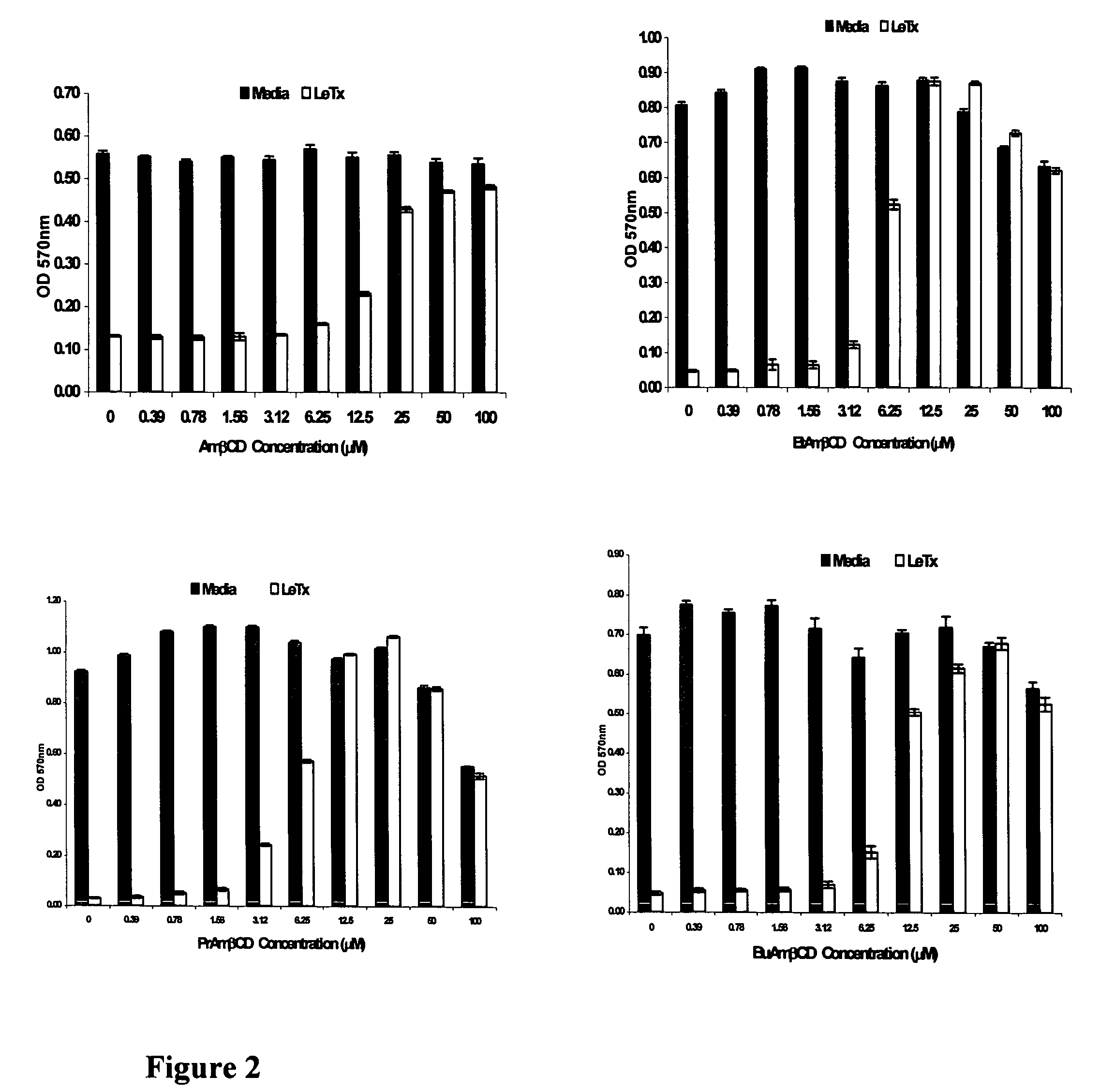
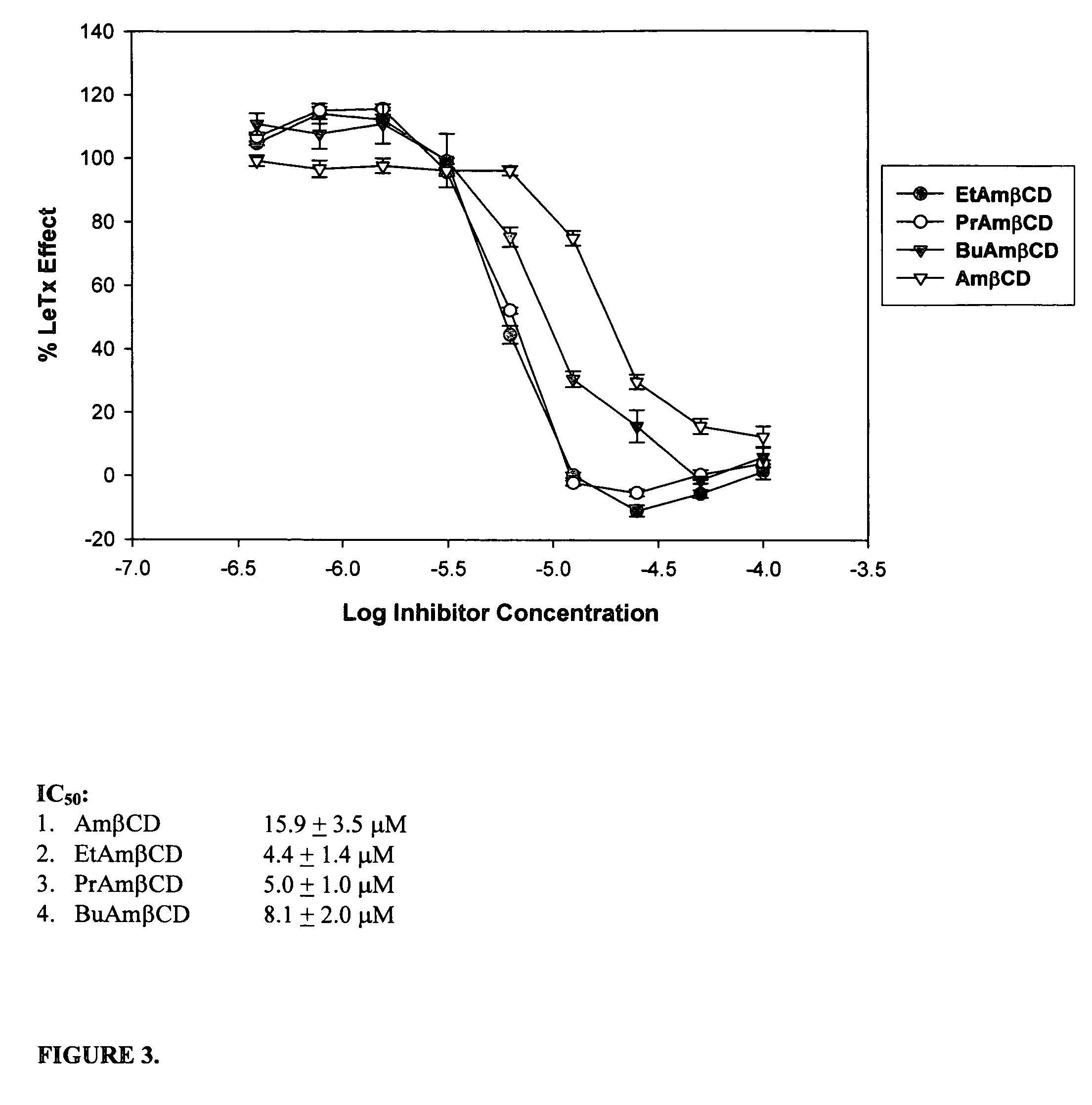
B-cyclodextrin derivatives and their use against anthrax lethal toxin
The invention provides low molecular weight compounds that block the pore formed by protective antigen and inhibit anthrax toxin action. Structures of the compounds are derivatives of β-cyclodextrin. Per-substituted alkylamino derivatives displayed inhibitory activity, and they were protective against anthrax lethal toxin action at low micromolar concentrations. Also, the addition of one of the alkylamino derivatives to the bilayer lipid membrane with multiple PA channels caused a significant decrease in membrane conductance. Thus, the invention also provides methods for protection against anthrax toxicity.
Owner:INNOVATIVE BIOLOGICS
Anthrax compositions and methods of use and production
InactiveUS20100172926A1Reducing and preventing effectReducing debilitating effectAntibacterial agentsPeptide/protein ingredientsProtective antigenADAMTS Proteins
Compositions and methods effective for eliciting an immune response for preventing or reducing infection or improving clinical outcomes caused by Bacillus anthracis are provided. The compositions include a naturally occurring or synthetic protein, peptide, or protein fragment containing all or an active portion of an antigenic epitope associated with anthrax toxin proteins optionally combined with a pharmaceutically acceptable carrier. The preferred antigenic epitopes correspond to immunogenic regions of protective antigen, lethal factor or edema factor, either individually or in combination. In addition, methods and compositions containing antibodies for reducing the effects of anthrax toxins are described. The methods involve administering to a human or animal the compositions described herein in a dosage sufficient to elicit an immune response or treat the anthrax infection.
Owner:OKLAHOMA MEDICAL RES FOUND
Compositions and methods for treatment of pain
ActiveUS20180251740A1Improve bindingReduce releaseHydrolasesPeptide/protein ingredientsAnthrax toxinNociceptor
Embodied herein are engineered fusion proteins that bind and target nociceptor neurons, compositions comprising these engineered fusion proteins, and methods for treatment of pain using these engineered fusion proteins or compositions containing the engineered fusion proteins. The engineered fusion proteins contain domains derived from protein toxins such as the anthrax toxin, clostridial botulinum family of toxins, disulphide-containing toxins, and AB component type toxins.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
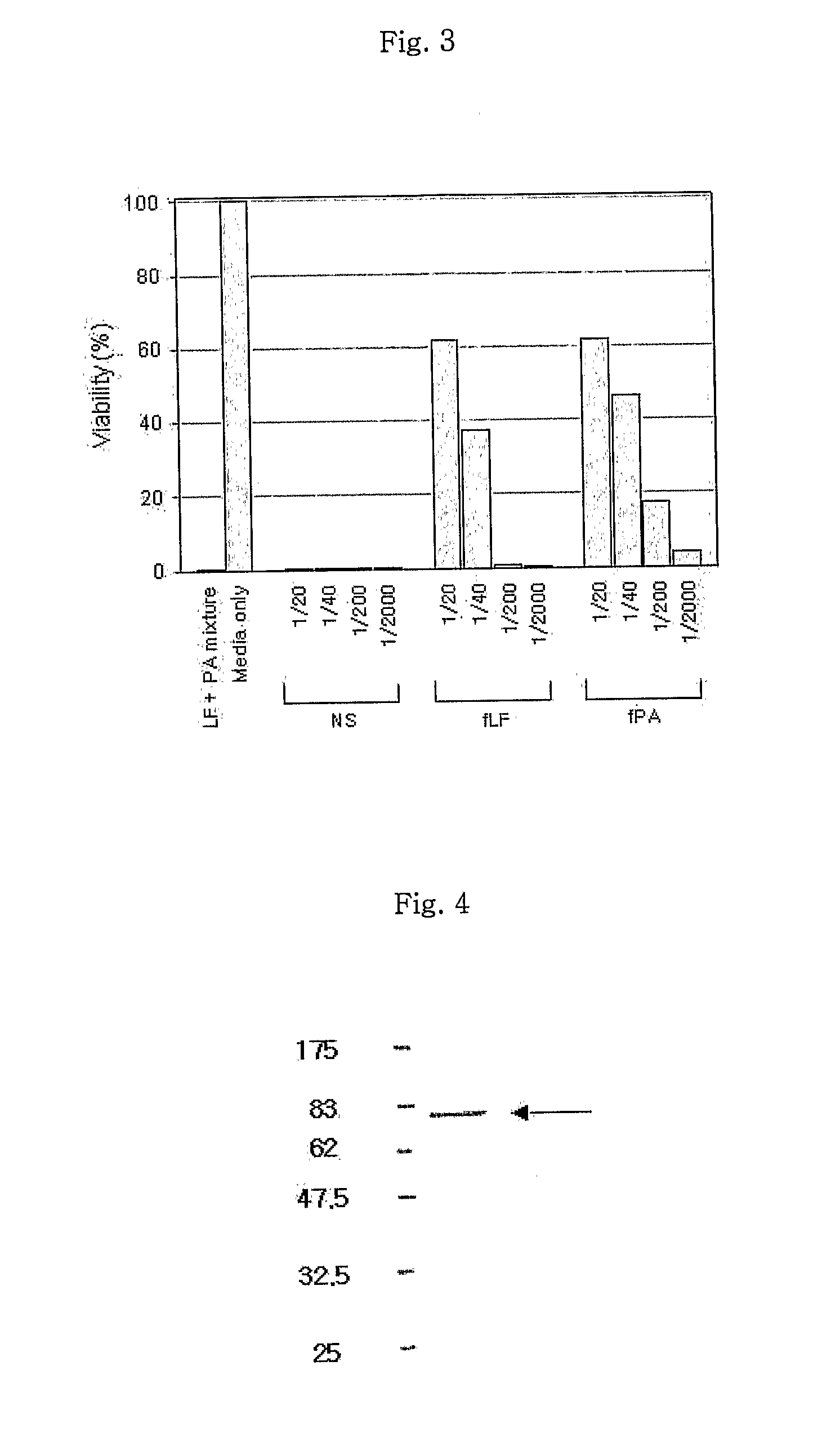
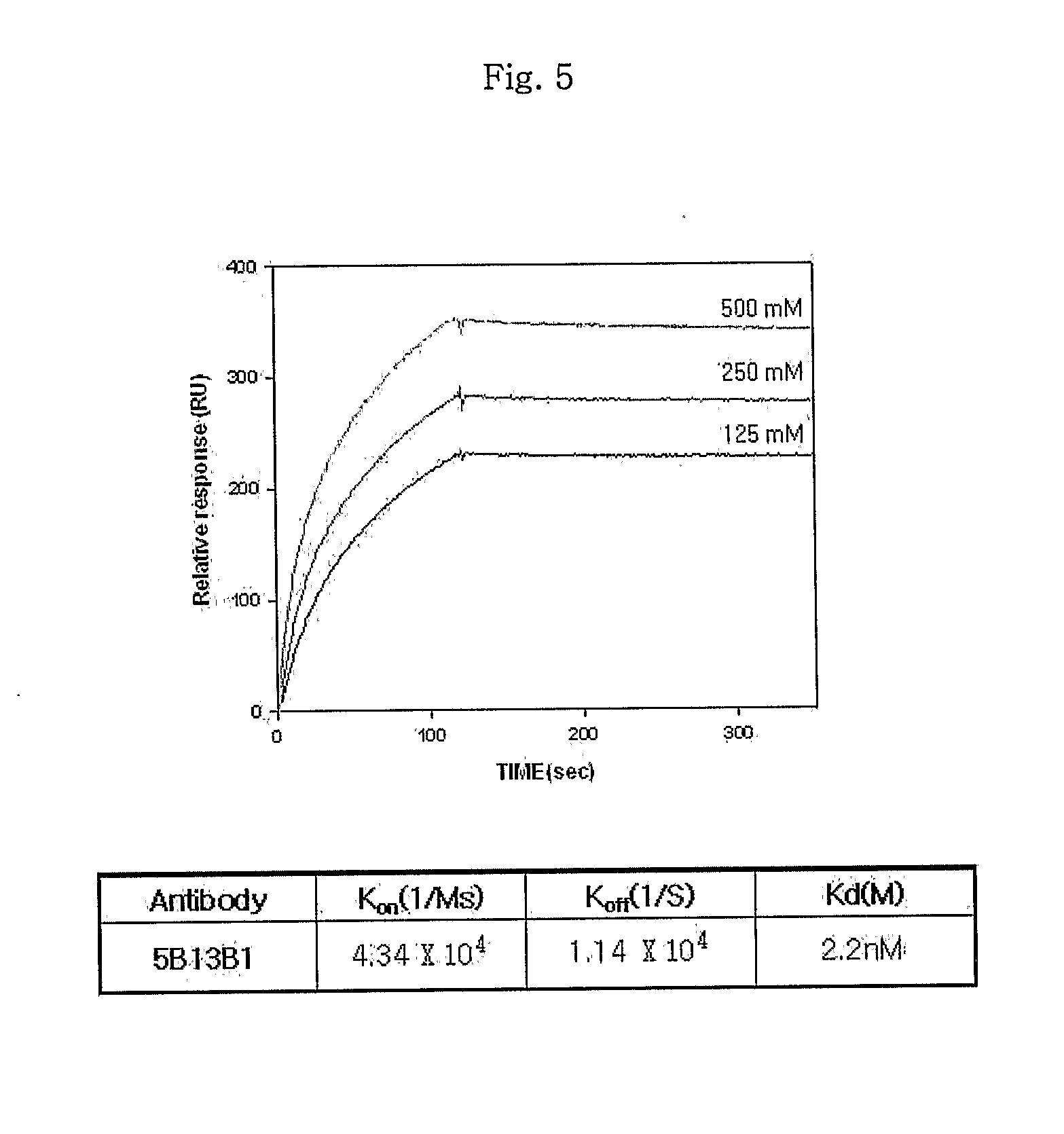
Monoclonal antibody specific to anthrax toxin
InactiveUS20110117104A1Preventing and treating anthraxAntibacterial agentsAnimal cellsMonoclonal antibodyAnthrax toxin
Disclosed is a monoclonal antibody having very high affinity to anthrax toxin and potent toxin-neutralizing activity. Also disclosed are a composition for neutralizing anthrax toxin comprising the antibody and a kit for detecting anthrax toxin.
Owner:APROGEN INC
Anthrax antitoxins
Owner:WISCONSIN ALUMNI RES FOUND
Monoclonal antibodies that neutralize anthrax toxins
The present invention relates to monoclonal antibodies that bind or neutralize anthrax lethal factor (LF), edema factor (EF), and / or protective antigen (PA). The invention provides such antibodies, fragments of such antibodies retaining anthrax toxin-binding ability, fully human or humanized antibodies retaining anthrax toxin-binding ability, and pharmaceutical compositions including such antibodies. The invention further provides for isolated nucleic acids encoding the antibodies of the invention and host cells transformed therewith. Additionally, the invention provides for prophylactic, therapeutic, and diagnostic methods employing the antibodies and nucleic acids of the invention.
Owner:UNITED STATES OF AMERICA
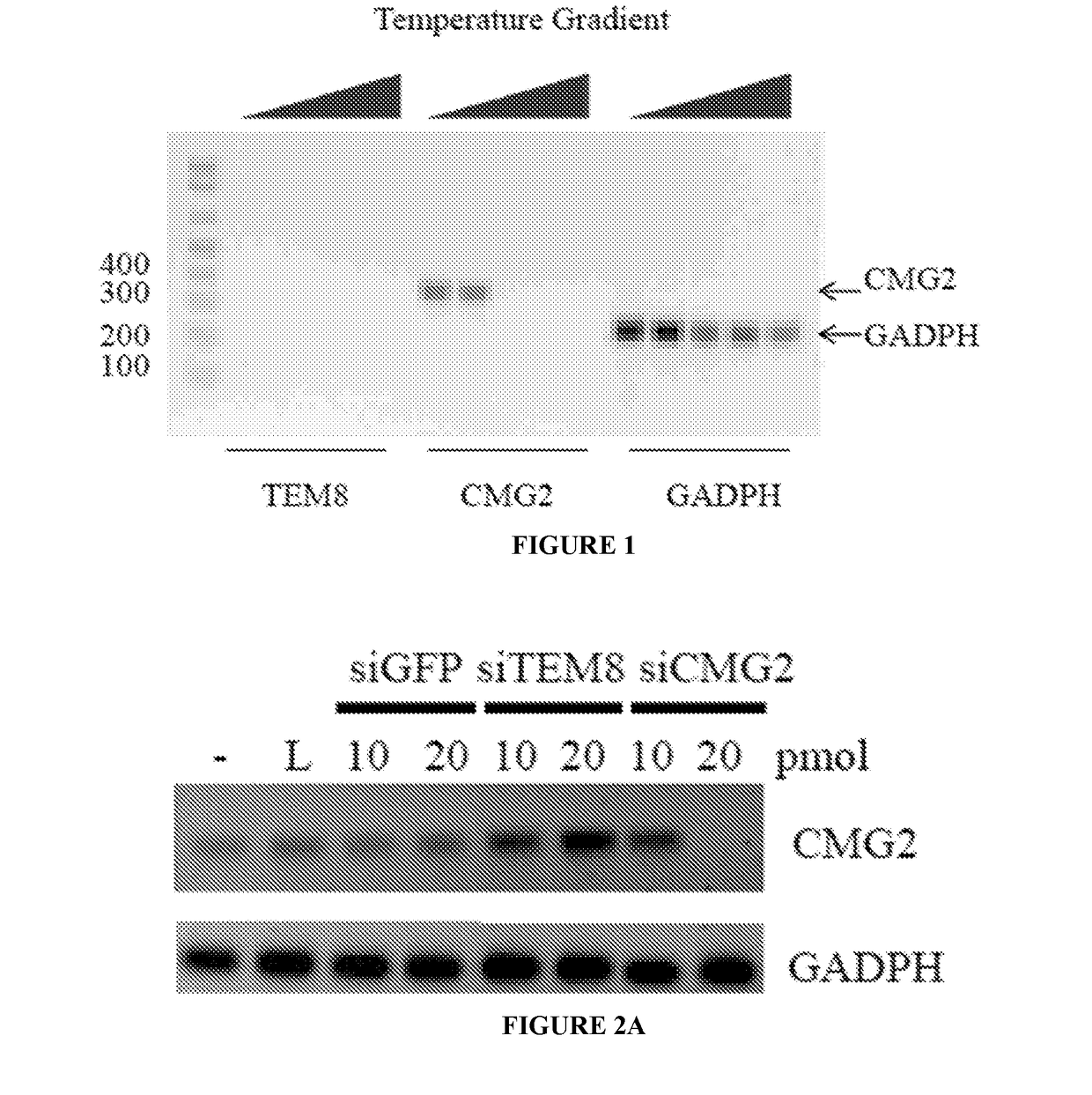
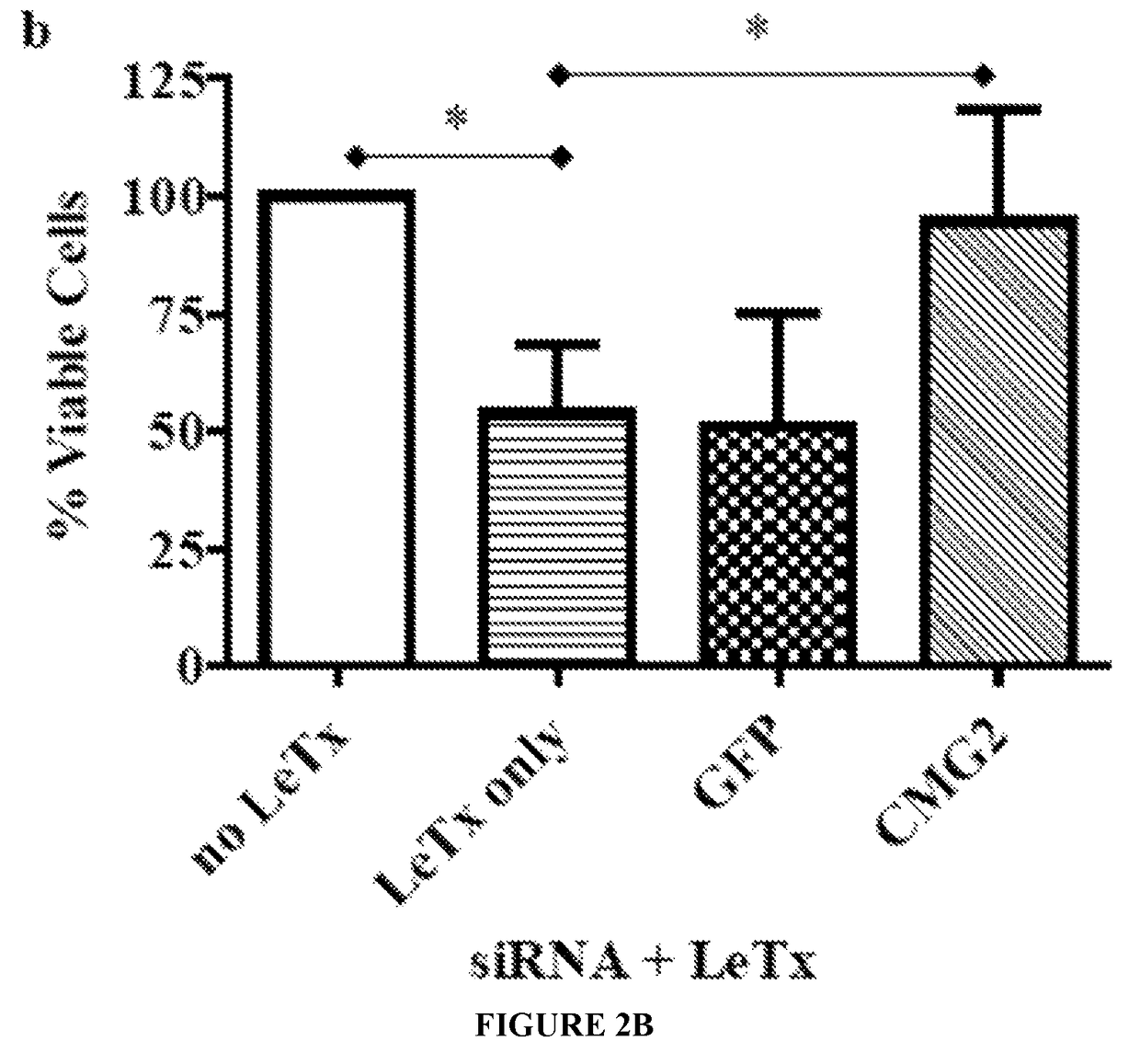
RNA interference-based therapeutic against anthrax
ActiveUS9862951B2Antibacterial agentsImmunoglobulins against cell receptors/antigens/surface-determinantsCell Surface ProteinsAnthrax toxin
The present invention includes siRNAs and antibodies that block the interaction between TEM8 and / or CMG2 cell surface proteins and anthrax toxin and methods of treating anthrax exposure with the same.
Owner:TEXAS TECH UNIV SYST
Monoclonal antibody specific to anthrax toxin
Disclosed is a monoclonal antibody having very high affinity to anthrax toxin and potent toxin-neutralizing activity. Also disclosed are a composition for neutralizing anthrax toxin comprising the antibody and a kit for detecting anthrax toxin.
Owner:APROGEN INC
B-cyclodextrin derivatives and their use against anthrax lethal toxin
InactiveUS20060247208A1Reduced effectivenessEffective treatmentAntibacterial agentsBiocideAntigenLipid formation
The invention provides low molecular weight compounds that block the pore formed by protective antigen and inhibit anthrax toxin action. Structures of the compounds are derivatives of β-cyclodextrin. Per-substituted alkylamino derivates displayed inhibitory activity, and they were protective against anthrax lethal toxin action at low micromolar concentrations. Also, the addition of one of the alkylamino derivatives to the bilayer lipid membrane with multiple PA channels caused a significant decrease in membrane conductance. Thus, the invention also provides methods for protection against anthrax toxicity.
Owner:INNOVATIVE BIOLOGICS
Anthrax antitoxins
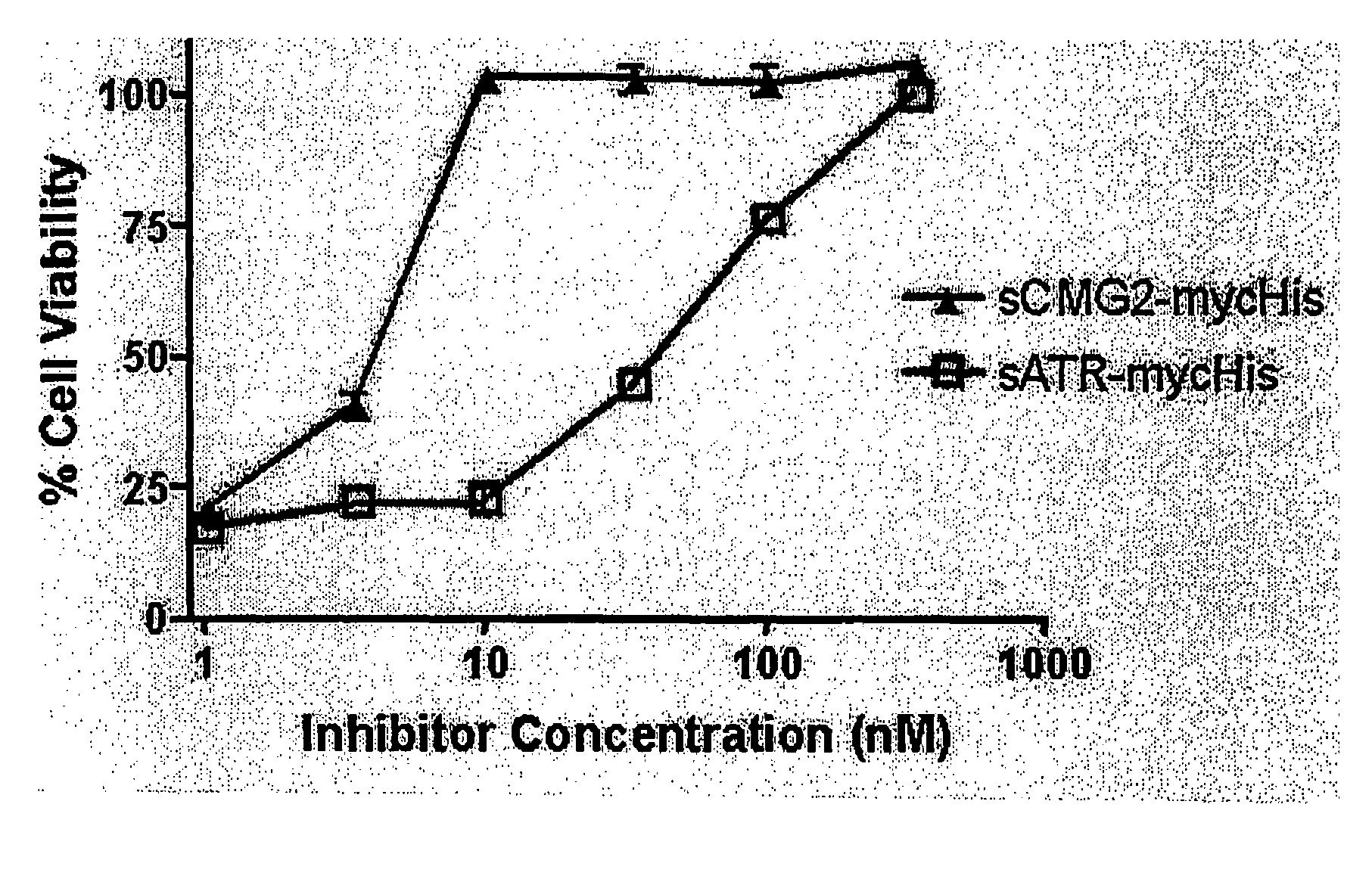
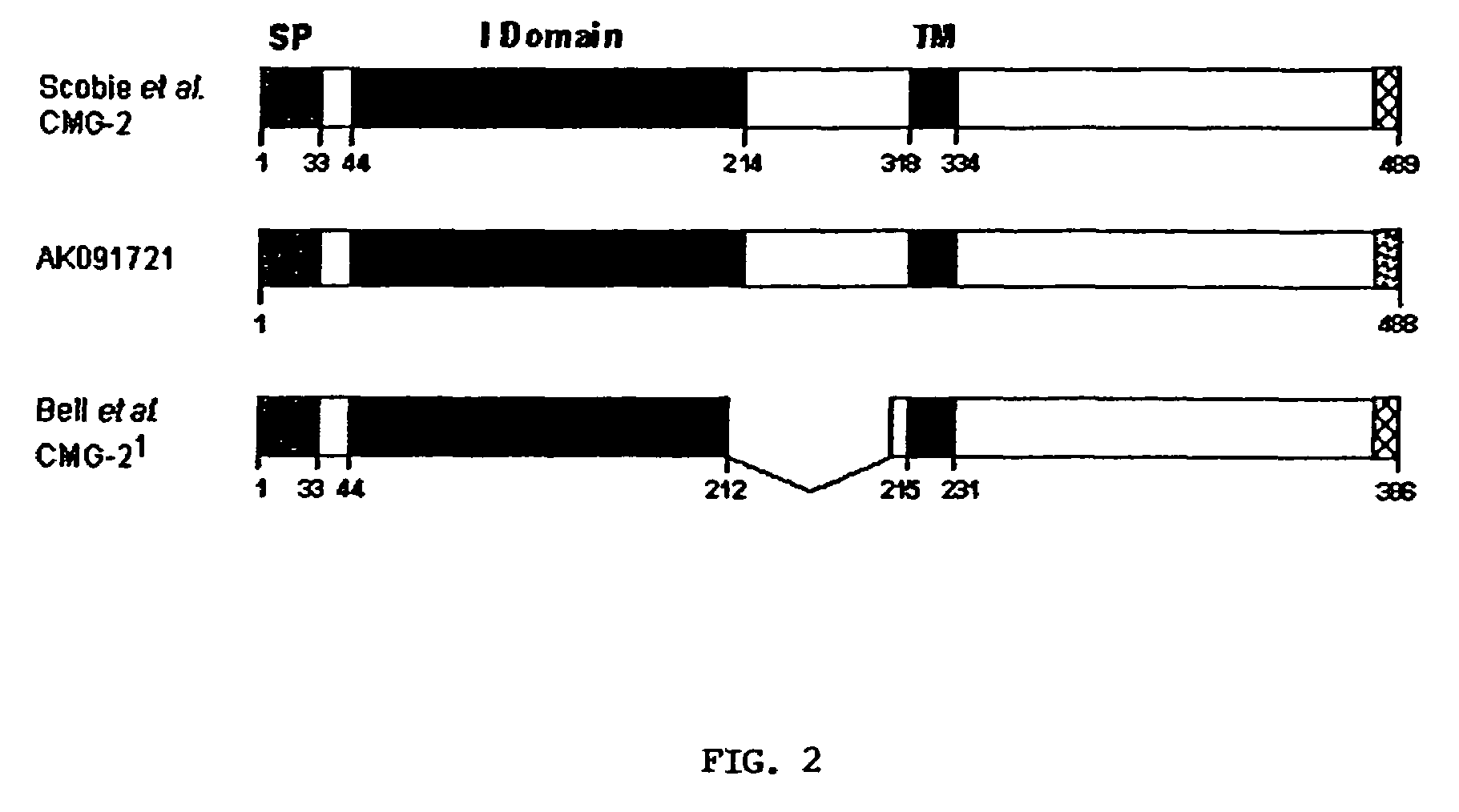
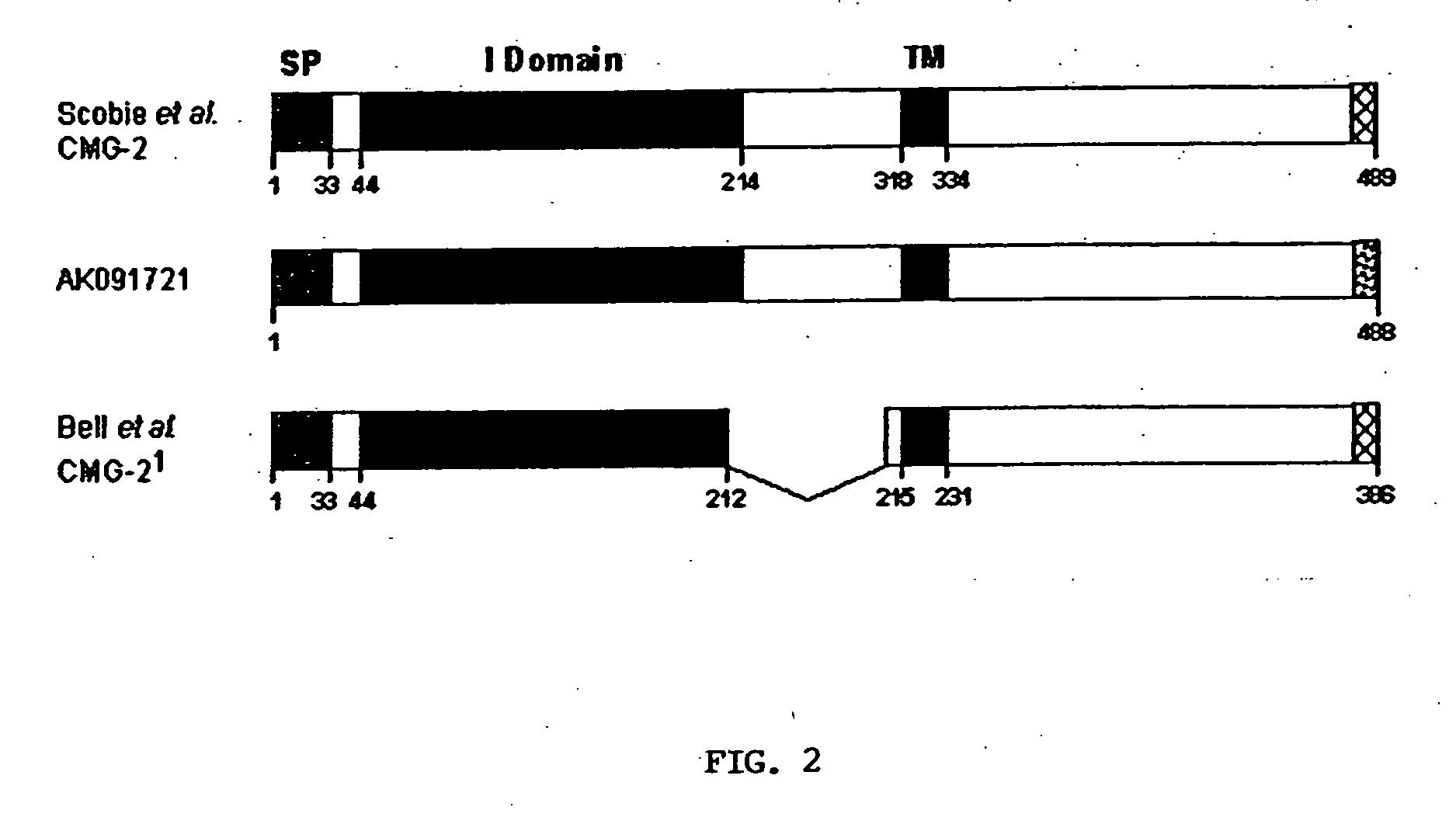
The present invention provides compositions useful in preparing and / or serving as antitoxins against Bacillus anthracis, the causative agent of anthrax. The present invention also provides polypeptides and polynucleotides relating to the capillary morphogenisis gene 2 (CMG-2), vectors containing the polynucleotides and polypeptides, and host cells containing related polynucleotide molecules, all used in association with the treatment of, or the research and development of treatments for anthrax. The present invention also relates to methods for identifying molecules that bind CMG-2 and molecules that reduce the toxicity of anthrax toxin. Finally, the present invention provides methods for treating human and non-human animals suffering from anthrax.
Owner:WISCONSIN ALUMNI RES FOUND
Tumor vascular endothelial cell marker 8 mutant, fusion protein and application thereof
The invention discloses a protein mutant of a tumor vascular endothelial cell marker 8, a fusion protein of the protein mutant and human serum albumin and application thereof in preparation of drugs for treatment and / or prevention of anthrax infection. The protein mutant disclosed by the invention significantly increases the affinity with an anthrax PA antigen, has similar inhibitory activity to anthrax toxin with sCMG2, has 100% protection rate on rats attached by anthrax toxin LeTx. When the protein mutant is fused with human serum albumin, the half-life in vivo is extended by nearly 10 times, and the fusion protein can completely protect test animals attacked by anthrax toxin LeTx in 14 days.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Anthrax rPA dry powder inhalant and application thereof
PendingCN111000989AImprove performanceCompliantAntibacterial agentsPowder deliveryAntiendomysial antibodiesAnthrax toxin
The invention discloses an anthrax rPA dry powder inhalant and application thereof. The anthrax rPA vaccine dry powder inhalant prepared according to the invention achieves immunization by a lung delivery inhalation route, so that mice can generate better immune response and generate very high IgG antibody titer, thus reaching certain effect of resisting the infection and progress of bacillus anthracis, and effectively resisting the killing effect of anthrax toxin.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com