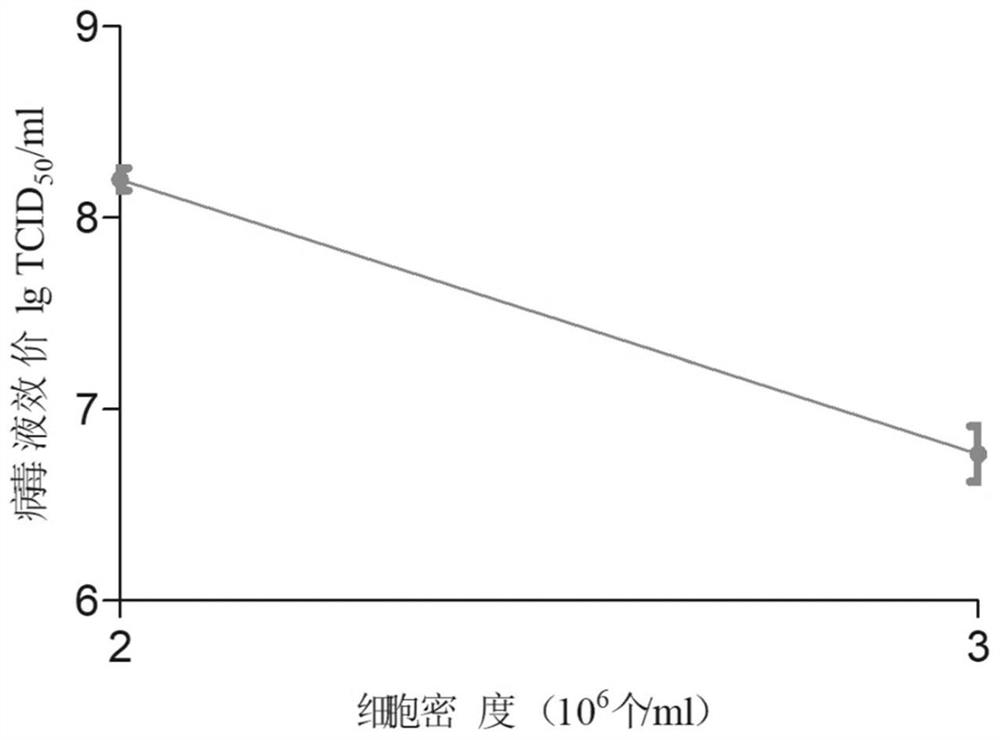
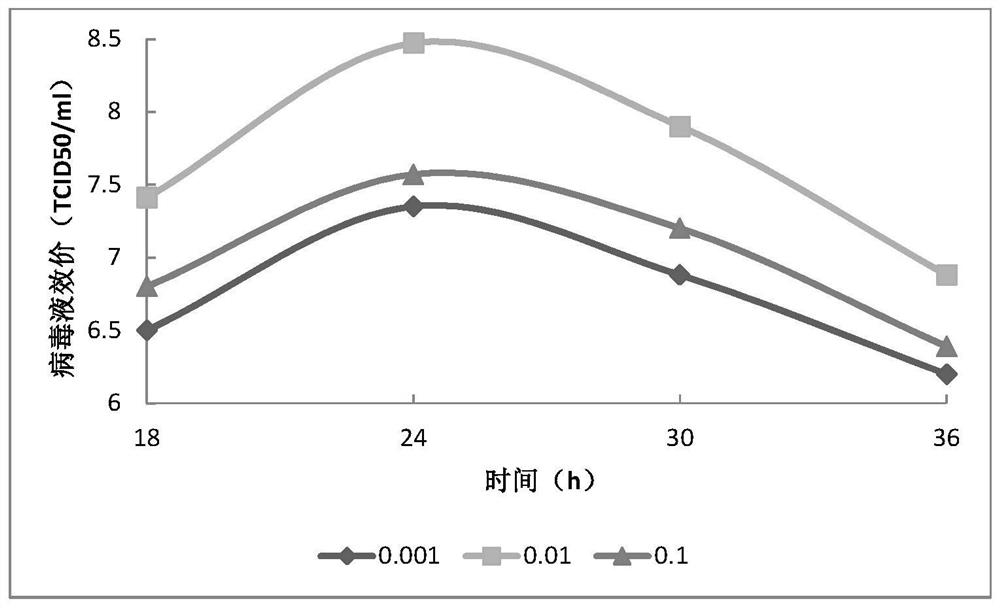
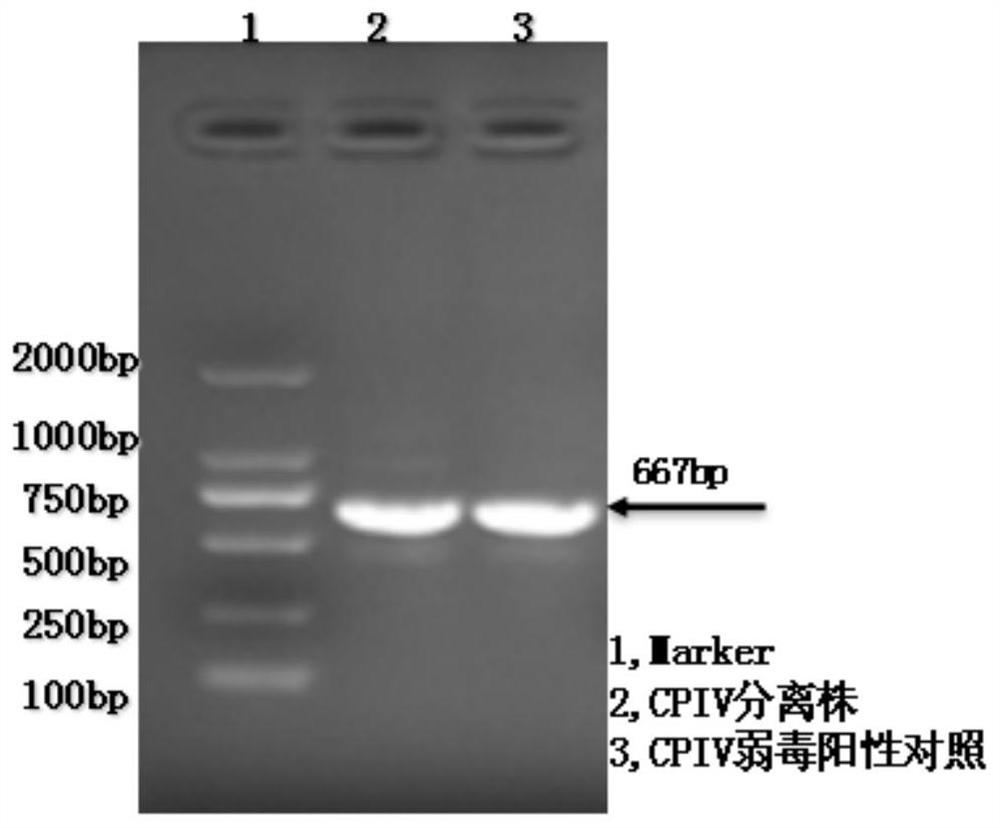
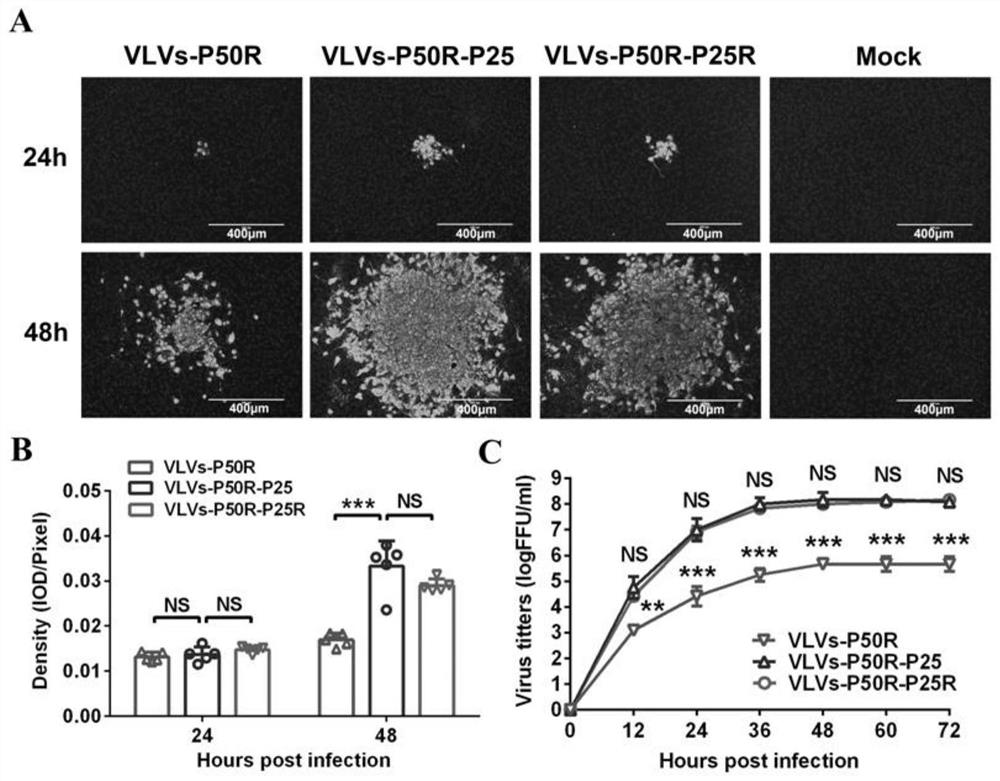
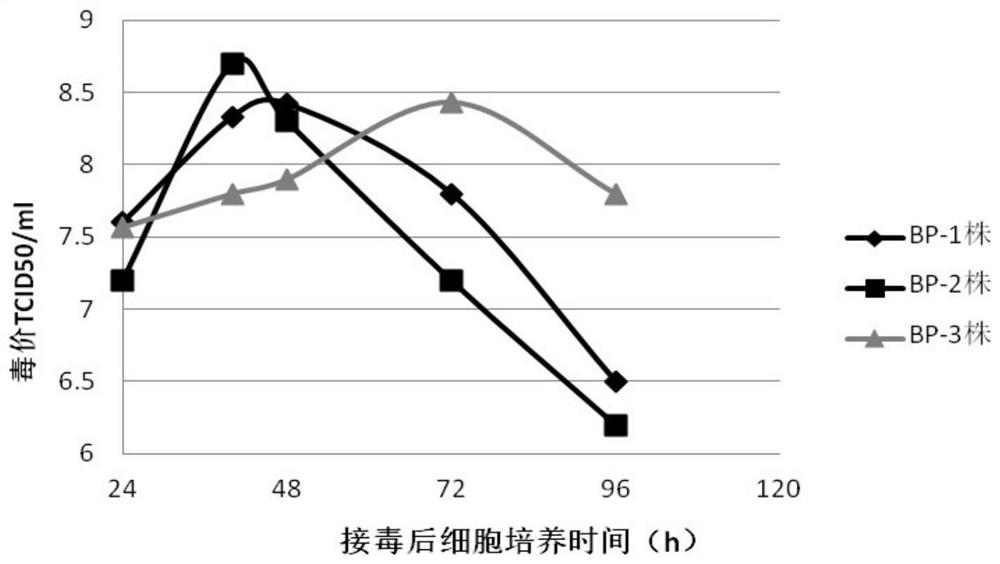
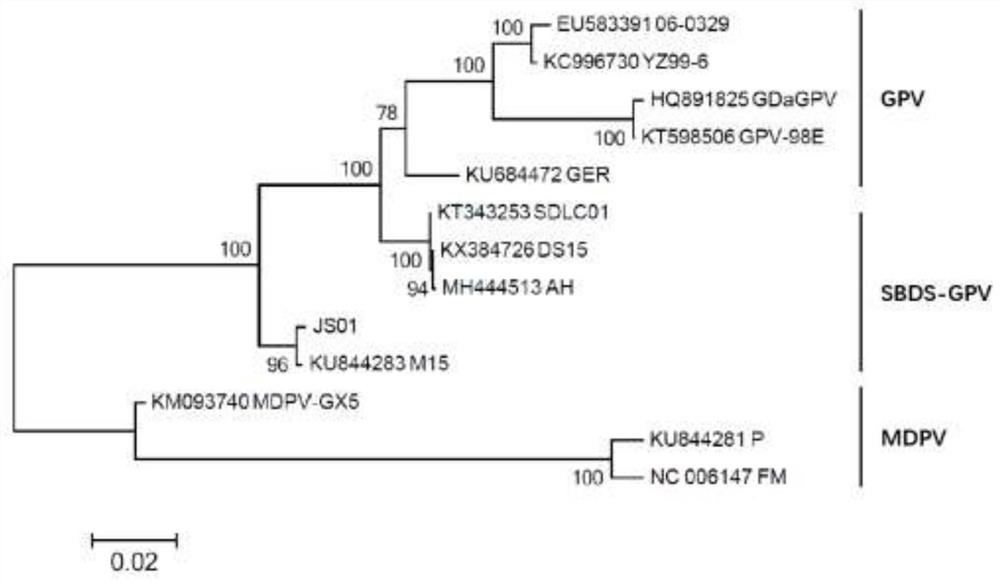
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40results about How to "High poison price" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
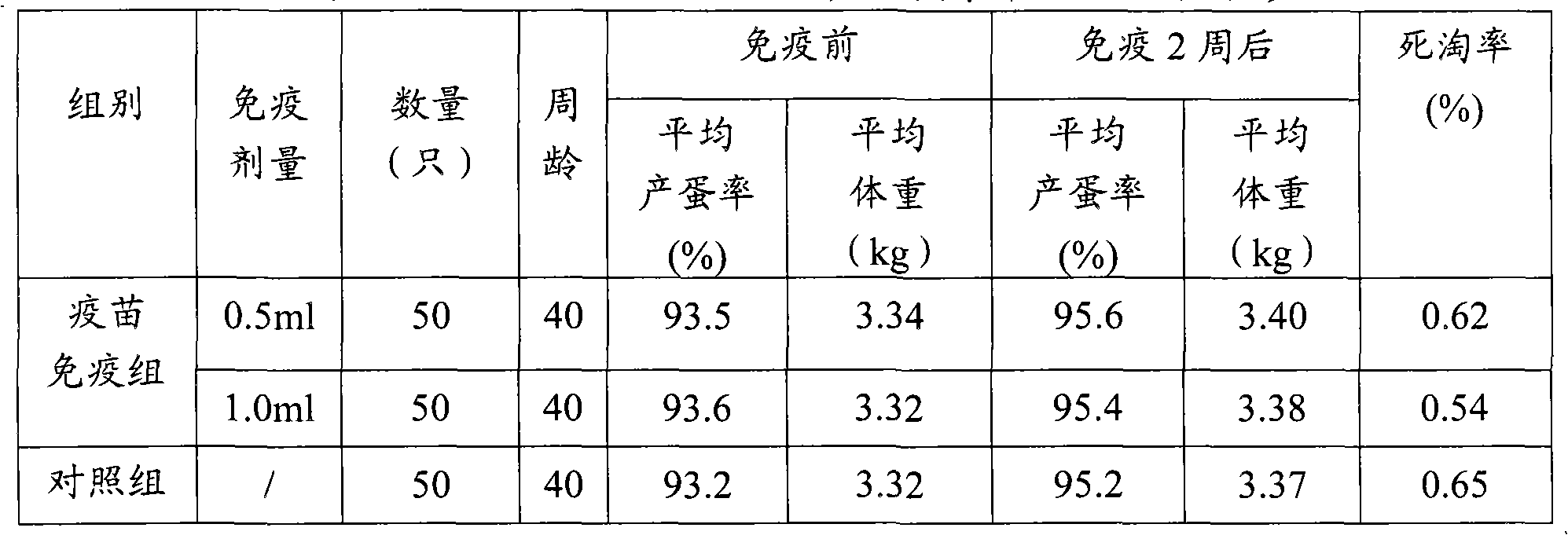
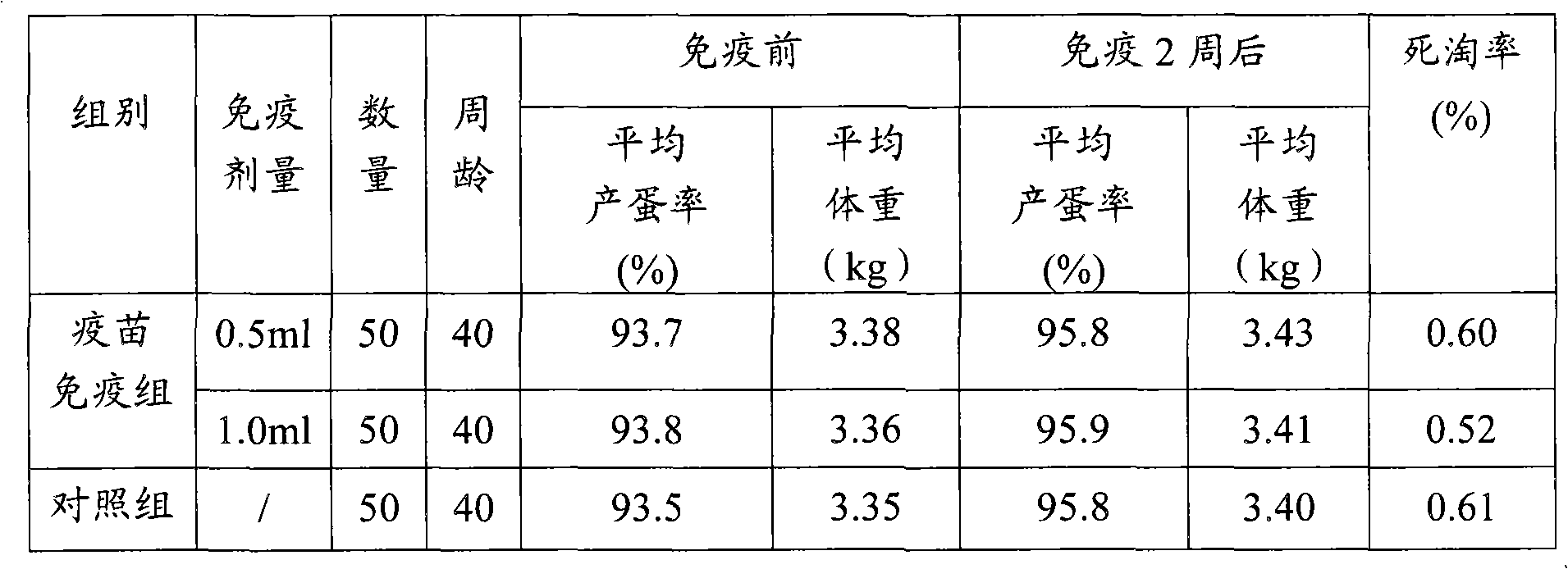
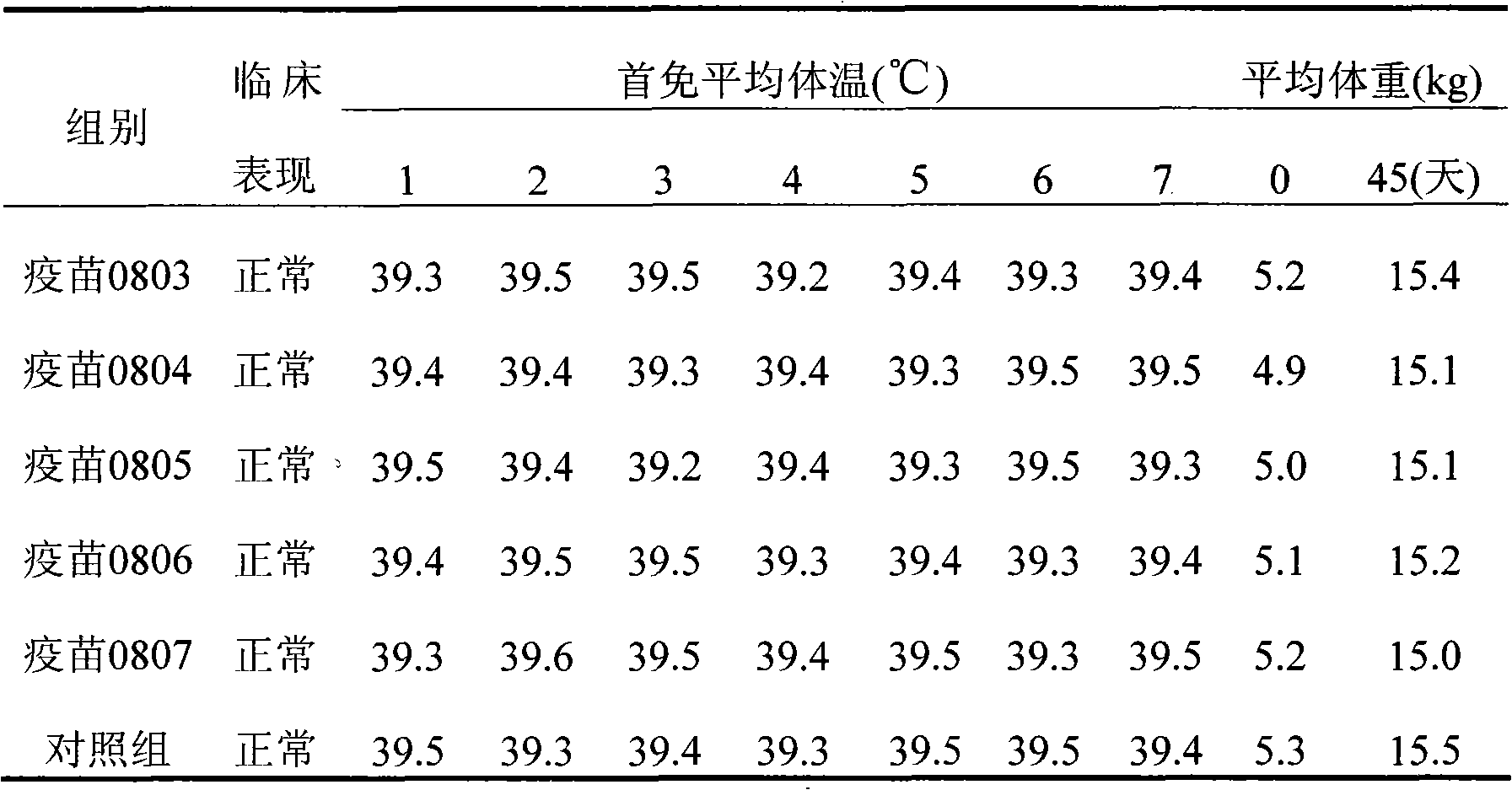
Duck virus hepatitis strains and inactivated vaccine
ActiveCN102086447AHigh poison priceRaise antibody levelsDigestive systemMicroorganism based processesDuck hepatitis A virusFreeze thawing
The invention discloses methods for preparing a duck virus hepatitis strain and an inactivated vaccine. The strain is obtained with the method comprising the following steps of: collecting duck hepatitis material with obvious pathological changes in a duck farm where duck virus hepatitis prevails, washing with bi-anti-sterilization PBS, diluting and grinding, freeze thawing twice and performing centrifugal separation; diluting virus separated by sterilization PBS, vaccinating chick embryo, selecting chick embryo with classic pathological changes that is dead 48-96h after vaccination, respectively gaining chick embryo liquid, transferring for 18-25 generations continuously to obtain a duck virus hepatitis virus chick embryo suitable strain. The strain can be in production, vaccinated in the chick embryo to gain chick embryo liquid, and is emulsified after vaccination to prepare the inactivated vaccine. The inactivated vaccine is high in safety, has good immune efficacy to young ducks, long immune time for breeding ducks and high level of antibody of breeding ducks, and the young ducks bred by the breeding ducks can resist duck virus hepatitis strains.
Owner:PU LIKE BIO ENG
Preparation method for vaccine of porcine circovirus II
InactiveCN101773667AHigh poison priceExpand production scaleViral antigen ingredientsBiological material analysisAdjuvantHigh density
The invention discloses mass production of inactivated vaccine of porcine circovirus II (PCV2) and a preparation method thereof. The preparation method comprises the following technical steps: (1) high-density culture of cells for vaccine preparation; (2) reproduction of venom for vaccine preparation; and (3) addition of adjuvant to prepare the inactivated vaccine. Compared with the prior art, the invention has the advantages that the virus yield is high, the virus titer is high, the production scale is large, the yield of a single batch is high, the production cost is relatively low, the product quality is high and stable, the operation is convenient, the operation space is small, the technological parameters are controlled accurately and the like. The inactivated vaccine has high safety, can induce pig bodies to generate immune protection and fully satisfies the national biological product standards.
Owner:PU LIKE BIO ENG
Method for preparing inactivated vaccine of porcine reproductive and respiratory syndrome
InactiveCN101607082AResist attackImproving immunogenicityViral antigen ingredientsAntiviralsOil phaseMixing ratio
The invention discloses a method for preparing an inactivated vaccine of porcine reproductive and respiratory syndrome, which comprises the following steps: (1) preparation of virus seed for production; (2) preparation of virus liquid for preparing vaccine; and (3) preparation of the inactivated vaccine which comprises: inactivating the virus liquid with the virus content more than or equal to 10TCID50 / ml at 4 DEG C for 48 hours; adding 94 to 98 volume portions of inactivated virus liquid into 2 to 6 volume portions of tween-80 and mixing to prepare aqueous phase; mixing 94 volume portions of white oil for injection and 6 volume portions of span-80 to prepare oil phase; mixing the aqueous phase and the oil phase in a mixing ratio of 1:1.5-1:2.5, emulsifying the mixture to prepare the inactivated vaccine; and sub-packaging and entering warehouse. The method has the advantages of simple production process, good effect of the prepared inactivated vaccine of the porcine reproductive and the respiratory syndrome and the like.
Owner:PU LIKE BIO ENG
Culture method of porcine pseudorabies virus
InactiveCN103865886AComplete controlHigh poison priceMicroorganism based processesViruses/bacteriophagesSocial benefitsPseudorabies
The invention discloses a culture method of porcine pseudorabies virus; hamster kidney cells (BHK-21) are used as a cell source for culturing the porcine pseudorabies virus, and while cell passage, the virus is inoculated, so that multiplication culture of the virus is realized. The method has the characteristics of simple technology, high increment, high yield and low cost, and a vaccine prepared by use of the porcine pseudorabies virus cultured by the method has a complete preventive effect on the porcine pseudorabies virus, and has good social benefits and application prospects.
Owner:QINGDAO ZHONGREN PHARMA
Classical swine fever virus vaccine and production method thereof
InactiveCN101926991AHigh poison priceExpand production scaleInactivation/attenuationMicroorganism based processesCulture fluidFreeze-drying
The invention discloses a method for preparing a classical swine fever (CSF) vaccine by using a cell microcarrier suspension culture system, which comprises the following steps of: (1) inoculating cells for preparing the vaccine to a carrier tank containing culture solution and a microcarrier, and uniformly mixing the cells and the microcarrier to make the cells attached to the microcarrier; (2) when the concentration after cell proliferation is 5 to 40 times of the initial inoculation concentration, inoculating CSF virus (lapinized virus) to the cells according to multiplicity of infection (M.O.I.) of the virus of 0.01-1 and reproducing the virus; and (3) mixing prepared virus liquid, adding an appropriate freeze-drying protective agent, fully and uniformly mixing, quantitatively packaging, and freeze-drying to obtain the CSF vaccine. The CSF vaccine produced by the method has the advantages of high density of cultured cells, continuous culture, high yield of the virus, high immune effect, high safety, complete immune protection on attack of violent CSF, and the like.
Owner:PU LIKE BIO ENG
Virus and vaccine of porcine reproductive and respiratory syndrome and preparation method of same
ActiveCN101979514AHigh viral titerHigh poison priceViral antigen ingredientsAntiviralsFreeze-dryingCells/microL
The invention discloses a method for preparing virus of porcine reproductive and respiratory syndrome on a large scale. In the method, the virus of the porcine reproductive and respiratory syndrome is prepared in a cell microcarrier suspension culture system by a bioreactor. The method comprises the following steps of: inoculating host cells for preparing the virus to a carrier tank containing culture solution and a microcarrier, and mixing the cells and the microcarrier uniformly to ensure that the cells are attached to the microcarrier; providing sufficient nutrients and appropriate gas environment for the cells under the appropriate culture environment to ensure that the cells are grown until the cells are in an amount which are 10 to 20 times of the inoculation concentration on the microcarrier; preparing virus suspension from the virus of the porcine reproductive and respiratory syndrome by using cell maintenance culture solution to ensure that the suspension is adsorbed to the cells; culturing the virus under the appropriate culture environment; culturing continuously for 2 to 3 days to obtain virus solution; and after the virus solution passes inspection, performing freeze thawing on the virus solution twice at the temperature of -20 DEG C, and inactivating and purifying to prepare an inactivated vaccine of the porcine reproductive and respiratory syndrome or adding a freeze-drying protective agent for freeze drying to prepare a live vaccine of the porcine reproductive and respiratory syndrome. The method has large production scale, high yield of single batch and low production cost.
Owner:PU LIKE BIO ENG
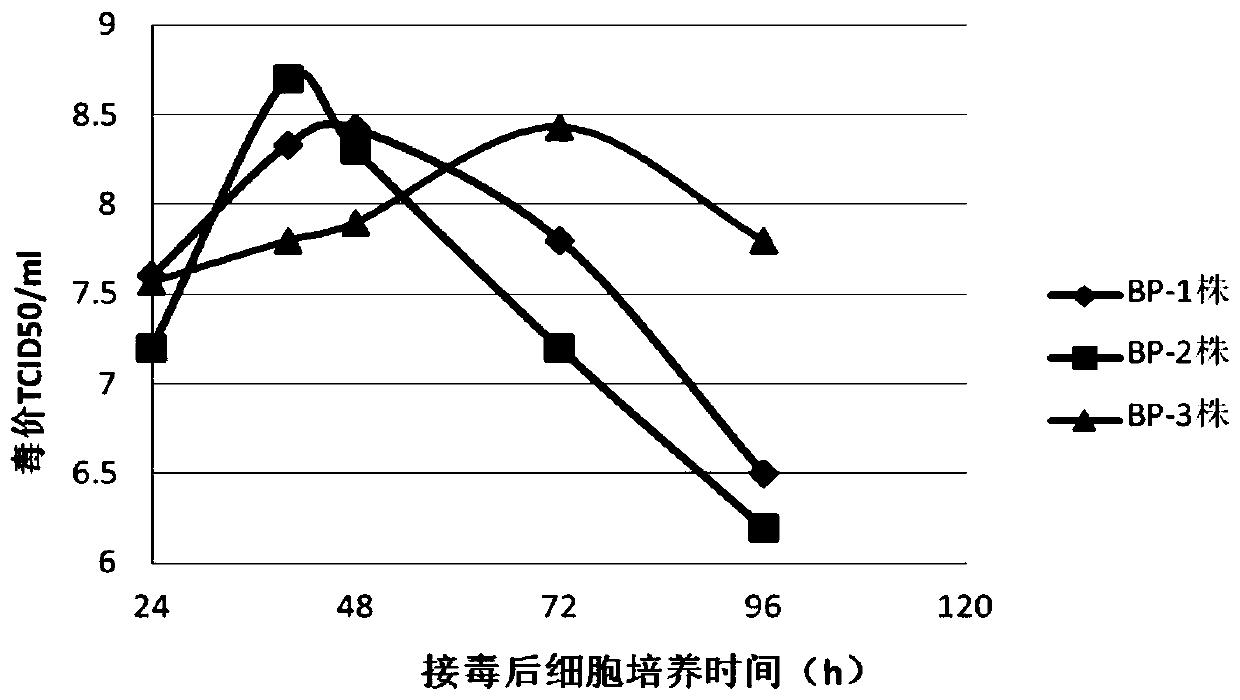
BHK-21 cell line BHK-21-BP-2 clones and application thereof in full suspension cell culture
ActiveCN110157659AImprove integrityLow total proteinMicroorganism based processesArtificial cell constructsTotal proteinImmunogenicity
The invention belongs to the technical field of veterinary biological products, and particularly relates to a BHK-21 cell line BHK-21-BP-2 clone and application thereof in full suspension cell culture. The provided BHK-21-BP-2 clone is preserved in the China general microbiological culture collection center, and the preservation number is CGMCC No. 17588. After the clone is inoculated with pseudorabies viruses during full suspension cell culture, the clone has rapid cytopathy, the virulence values of generated viruses are high, and the viruses can be collected after 24-48 hours; at the time ofvirus collection, only supernatant needs to be collected by centrifugation without repeated freezing and thawing, and the production process is simple. The total protein content of a produced virus antigen solution is low, the integrality and quality of obtained effective viral antigens are high, the viruses are excellent in immunogenicity and safety, and a prepared pseudorabies virus vaccine hasthe advantages of high stability, small difference between batches, a good immune effect and high safety.
Owner:CHINA ANIMAL HUSBANDRY IND
Porcine rotavirus G4-G5-G9 type trivalent inactivated vaccine as well as preparation method and application thereof
ActiveCN114854697AAdvantages of Guaranteed Seed PoisonImprove stabilityViral antigen ingredientsMicroorganism based processesAdjuvantPreventive vaccination
The invention provides a porcine rotavirus G4-G5-G9 type trivalent inactivated vaccine as well as a preparation method and application thereof, three genotype strains of current PoRV epidemic are adopted as seed viruses, are inactivated by BEI, and are emulsified with an adjuvant ISA201VG to prepare the PoRV trivalent inactivated vaccine. The trivalent inactivated vaccine prepared by the research has the characteristics of high immune protection rate, good stability, high yield, safety and reliability, and can be used for emergency vaccination when a PoRV epidemic situation outbreaks.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Antigen of epidemic encephalitis live vaccine and preparation method and application thereof
InactiveCN106754762AAvoid infectionSmall batch-to-batch varianceSsRNA viruses positive-senseViral antigen ingredientsAntigenAnimals vaccines
The invention relates to animal vaccines, and specifically discloses an antigen of an epidemic encephalitis live vaccine and a preparation method and application thereof. Vero cells are adopted to carry out roller bottle culture on epidemic encephalitis virus attenuated strains (SA14-14-2 strains); virus culture maintenance solution which comprises components such as 4-hydroxyethylpiperazine ethane sulfonic acid, arginine monohydrochloride and PEG2000 is used; the produced antigen has the advantages of being small in batch difference, good in immunogenicity and high in virus titer; and the epidemic encephalitis live vaccine prepared through the antigen is capable of effectively preventing the epidemic encephalitis virus infection of pigs.
Owner:CHINA ANIMAL HUSBANDRY IND
Mutated PCV2 (Porcine circovirus Type 2) virus free from being degraded by ubiquitination proteasome, and preparation method and application thereof
ActiveCN108148816AInfectious fastFast copyArtificial cell constructsGenetic engineeringWild typeUbiquitinated Proteins
The invention provides a mutated PCV2 (Porcine circovirus Type 2) virus free from being degraded by ubiquitination proteasome, and a preparation method and application thereof. Three loci (164K, 179Kand 191K) of porcine circovirus 2 type (PCV2, GenBank and No. EU366323) strains Cap are mutated, the mutated PCV2 can not be subjected to MKRN1-mediated ubiquitination degrading, in addition, high speed copying can be carried out through infectious clone, and virus price is high than virus price of wild-type PCV2. Through mutated viruses obtained by the infectious clone, the pathogenic mechanism of the PCV2 can be more favorably researched, and a potential control measure is provided for PCV2 outbreak.
Owner:NORTHWEST A & F UNIV
Chicken newcastle disease virus inactivated vaccine preparation method
InactiveCN106729689AImproving immunogenicityHigh protection rateViral antigen ingredientsAntiviralsSide effectEmbryo
The invention provides a chicken newcastle disease virus inactivated vaccine preparation method. According to the present invention, inoculation with chick embryo fibroblasts is used to replace inoculation with chick embryo allantocherion to breed chicken newcastle disease viruses, such that the vaccine preparation cost is saved, the quality difference between the batches is reduced, the yield is high, and the virus titer and the vaccine quality are improved; the vaccine is emulsified with the homogenizer, such that the prepared vaccine has advantages of low viscosity, small particle size and easy absorption compared to the traditional water-in-oil dosage form; and any surfactant components are not required to be added during the vaccine preparation process through the selected adjuvant, the prepared vaccine has good safety, and the side effect due to the difficult absorption of the traditional oil adjuvant is avoided.
Owner:鼎正生物药业(天津)有限公司
Novel method for preparing porcine circus-virus 2 type
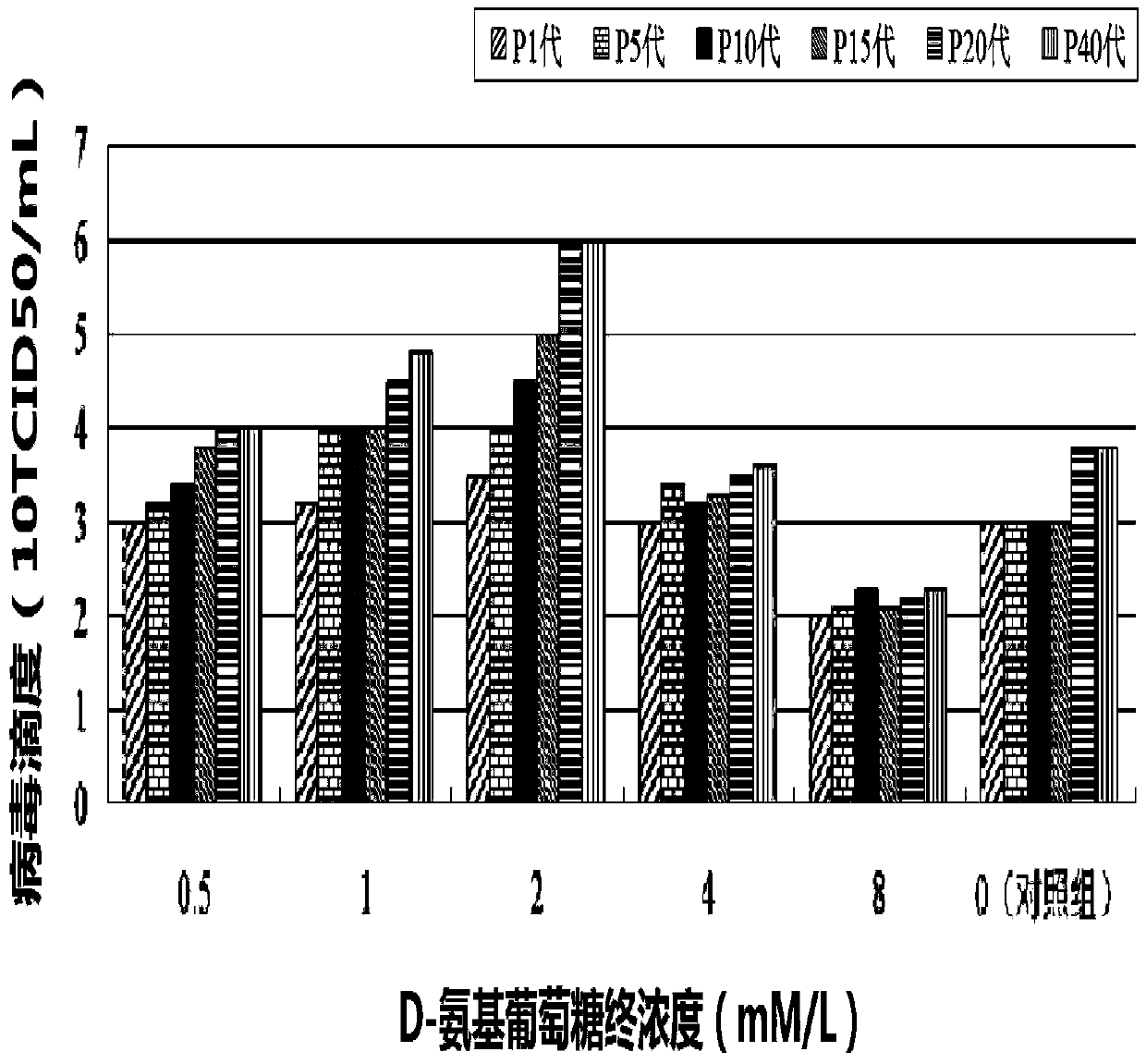
InactiveCN103740652AHigh titerShorten the timeMicroorganism based processesViruses/bacteriophagesConcentration gradientTiter
The invention provides a novel method for preparing porcine circus-virus 2 type, wherein inoculated PK-15 cells are treated by D-glucosamine with different concentration gradients, and is screened by IFA; the method not only makes the OK-15 cells normal grow, but also substantially raises PVC2 virus titer; the PVC2 virus titer acquired by the method is not less than 105TCID50, and a method for propagating and operating PVC 2 by screened proper D-glucosamine is used for researching a PVC2 vaccine and a relative diagnostic reagent, and preparing the products.
Owner:TECON BIOLOGY CO LTD
Method for producing swine fever vaccines
InactiveCN101905020AGenetic stabilityGuaranteed singularityInactivation/attenuationAntiviralsFreeze-dryingVirus strain
The invention relates to a method for producing swine fever vaccines, which comprises the following technical steps: (1) cloning and identifying hog cholera lapinized virus strain; (2) propagating seed viruses of the cloned strain; (3) propagating productive cells; (4) propagating productive virus solution; and (5) distributing vaccines, filling and freeze-drying. The method of the invention has the characteristics of simple and stable production process, high virus immunogenicity and genetic stability, small exogenous virus pollution risks, convenient examination, low production cost and the like. The swine fever vaccines produced by the method have high genetic stability, purity and immunogenicity.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Large-scale production method of porcine transmissible gastroenteritis virus
The invention provides a large-scale production method of a porcine transmissible gastroenteritis virus. The method comprises the steps that: in a tidal-type bioreactor, cells are inoculated to micro-carriers, such that cell absorption culturing and amplification culturing are carried out; porcine transmissible gastroenteritis virus is inoculated, and virus absorption culturing is carried out; virus proliferation culturing is carried out; and when cell CPE reaches 70% or higher, virus solution is collected. The porcine transmissible gastroenteritis virus large-scale production method provided by the invention shows a trend of animal cell and virus continuous culturing and large-scale production. Compared with a traditional shake flask culturing process, an antigen titer is improved by more than 30 times. Product quality is homogenous, production process is simplified, operation is simple, production efficiency is improved, and production cost is reduced.
Owner:PU LIKE BIO ENG
Application of siRNA sequences and target thereof in improving PEDV titer
PendingCN111808858AHigh poison priceEasy to operateSsRNA viruses positive-senseGenetically modified cellsTiterMolecular biology
The invention discloses two siRNA sequences and target gene sequences thereof. Two target spots of si-LNC_002015 gene in Vero-E6 culture cells are interfered through siRNA, so that the PEDV RNA copy number and the protein expression quantity in the Vero-E6 culture cells are obviously increased compared with those of a control group, and the virus titer is increased.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for constructing muscovy duck reovirus (MDRV) reverse genetic system
ActiveCN109321583AEfficiently obtainedHigh poison priceVirus peptidesFermentationEnzyme digestionComplete sequence
The invention discloses a method for constructing a muscovy duck reovirus (MDRV) reverse genetic system. Viral genome RNA is extracted from cell culture poison containing the muscovy duck reovirus; adesigned specific primer and the viral genome RNA serving as a template are utilized to perform RT-PCR amplification on gene segments of an MDRV complete sequence, enzyme digestion is performed on target gene segments to connect the target gene segments into 10 gene segments of the MDRV, the 10 gene segments are connected with a carrier, and positive bacterial colonies are picked to perform sequencing and verification; positive plasmids of the 10 segments of the MDRV are mixed according to the proportion to perform cotransfection on single-layer 293T cells. After passage is performed on the cells for 15th generation, cytopathy becomes stable, and MDRV recombinant virus can be obtained.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Cell growth regulator
InactiveCN103667178AProlonged growth retentionExtended durationVertebrate cellsArtificial cell constructsHydrolysateCulture fluid
The invention relates to a cell growth regulator. The cell growth regulator comprises the following ingredients by amount: 100 mg of ethyl ciprofloxacin, 50-100 ml of multi-cell extracting solution, 5 g of lactoalbumin hydrolysate, 3 g of N-(2-hydroxyethyl)piperazine-N'-2-sulfonic acid (HEPERS for short), and 1 L of culture medium with the lowest nutritional requirement. The preparation method comprises the steps of filtering the prepared liquid with a filter membrane of 0.2 microns to sterilize, split charging the filtered liquid for short term preservation at 4 DEG C, and long term preservation at -20 DEG C. According to the requirement, 40-60% of the cell growth maintaining regulator can be added in a normal cell culture fluid to culture cells; the culture fluid is not required to be changed in the halfway; the whole maintaining period of the cells is prolonged for 2-3 times; the cell growth regulator can be applied to a cell microneutralization test, a virus plaque titration test and the like. A maintenance medium containing 40-60% of the growth maintaining regulator is replaced for the grown cells before the biological test, and thus the cell maintaining period can be prolonged by about 2 times. The cell growth regulator can be applied to viral antigen production and the like.
Owner:YUNNAN ANIMAL SCI & VETERINARY INST
Serum-free full-suspension cell culture method for Newcastle disease VII type attenuated strain
ActiveCN112961837AStrong ability to cultivateReproductive stabilitySsRNA viruses negative-senseInactivation/attenuationVaccine ProductionGenotype
The invention relates to the field of cells and biological products, in particular to a serum-free full-suspension cell culture method for Newcastle disease VII type attenuated strain. In the method provided by the invention, the Newcastle disease virus strain is a Newcastle disease gene VII type virulent strain attenuated by F gene locus mutation. According to the method disclosed by the invention, a serum-free full-suspension cell culture is adopted, so that the method is not influenced by insufficient hatching eggs supply or uncontrollable factors; the method disclosed by the invention has the advantages of easy operation, large virus preparation amount and high virus content, and overcomes the defect that the supply of vaccines on the market is influenced by vaccine production stagnation caused by influence on virus reproduction due to insufficient supply of hatching eggs in virus chick embryo culture.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Production method of hog cholera live vaccine
InactiveCN102018957BGuaranteed singularityHigh genetic stabilityInactivation/attenuationAntiviralsFreeze-dryingVaccine Immunogenicity
The invention relates to a production method of hog cholera live vaccine, comprising the following technical steps: (1) cloning and identifying a hog cholera lapinized low virulent strain; (2) breeding cloned strain seed virus; (3) breeding cells for production; (4) breeding virus liquid for production; and (5) matching vaccine, carrying out split charging and freeze-drying. The method provided by the invention has the characteristics of simple and stable production technology, good virus immunogenicity and genetic stability, small risk of exogenous virus pollution, low production cost and the like, and is convenient for inspection. The hog cholera live vaccine produced by the production method has the advantages of good genetic stability, high purity and strong immunogenicity.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Porcine epidemic diarrhea and porcine parvovirus bivalent vaccine and application thereof
ActiveCN112877299AHigh titerHigh poison priceSsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaVariant strain
The invention relates to a porcine epidemic diarrhea and porcine parvovirus bivalent vaccine. The porcine parvovirus CH01 strain with the preservation number of CCTCC NO: V202085 and the porcine epidemic diarrhea virus variant 2a are inoculated into suspended ST cells, the bivalent vaccine is prepared through a full suspension method, and the obtained bivalent vaccine is high in immunogenicity, has high protection force on the sow and the farrowing capacity of the sow and can effectively guarantee normal reproduction of the sow. The bivalent vaccine provided by the invention has important significance in the field of common immunization of the porcine epidemic diarrhea virus and the porcine parvovirus.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Method for preparing porcine circovirus through basket reactor fermentation
ActiveCN102703391BHigh yieldHigh degree of automationAnimal cellsRecovery/purificationAntigenCircovirus
The invention discloses a method for preparing porcine circovirus through basket reactor fermentation. The method for preparing the porcine circovirus through the basket reactor fermentation is characterized by comprising the following steps: (1), preparing the host cell species; (2), preparing a basket reactor before inoculation; (3), performing host cell propagating culture; and (4), performing virus proliferating culture: emptying cell growing liquid in the reactor, supplementing cell maintaining liquid with the volume equal to that of the emptied cell growing liquid, inoculating the porcine circovirus, 36-48h after inoculation, harvesting a virus solution for the first time, after harvesting the virus solution, adding the cell maintaining liquid with the volume equal to that of the harvested virus solution into the reactor, propagating, reproducing, and harvesting the virus again, circularly repeating for multiple times until the sugar consumption of cells in the virus solution to be harvested is detected to be reduced down to 0.5g / L / day, and harvesting the last porcine circovirus solution in the batch. The method is simple and feasible; and by the method, the production cost can be greatly reduced, the unit titer of a porcine circovirus antigen is significantly improved, and the harvest of the virus solution antigen in each fermentation batch is increased.
Owner:WENS FOODSTUFF GRP CO LTD
Attenuated strain of canine parainfluenza virus, its application and vaccine
ActiveCN112899240BStrong targetingEnhanced antibodySsRNA viruses negative-senseSsRNA viruses positive-senseVeterinary DrugsTGE VACCINE
Owner:BEIJING HUAXIA XINGYANG BIOLOGICAL SCI & TECH +1
Method for producing swine fever vaccines
InactiveCN101905020BGuaranteed singularityHigh genetic stabilityInactivation/attenuationAntiviralsFreeze-dryingCvd risk
The invention relates to a method for producing swine fever vaccines, which comprises the following technical steps: (1) cloning and identifying hog cholera lapinized virus strain; (2) propagating seed viruses of the cloned strain; (3) propagating productive cells; (4) propagating productive virus solution; and (5) distributing vaccines, filling and freeze-drying. The method of the invention has the characteristics of simple and stable production process, high virus immunogenicity and genetic stability, small exogenous virus pollution risks, convenient examination, low production cost and thelike. The swine fever vaccines produced by the method have high genetic stability, purity and immunogenicity.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Production process for improving titer of swine fever live vaccine product
ActiveCN102973931BReduce lossExtended shelf lifeAntiviralsViruses/bacteriophagesVaccinationInfective Dose
The invention relates to a production process for improving titer of a swine fever live vaccine product, which comprises the following steps of selecting rabbit, breeding a production virus seed, preparing a swine fever live vaccine, producing the vaccine, subpackaging, lyophilizing and inspecting a finished product. The virus seed is diluted by adopting a swine fever virus specific protective agent in vaccination, and can quickly colonize and propagate in a target cell when entering a rabbit body, so that the virus titer can be increased; and loss of the swine fever virus can be reduced by using a specific spleen and lymph tissue protective agent, so that the preservation period of a semi-finished product can be prolonged, and the titer and quality of the product can be improved, wherein the preservation period of the semi-finished product is prolonged to 60 days from the previous 15 days, and the titer of the finished product is increased from the previous infective dose of 150 rabbits to the infective dose of 900 rabbits.
Owner:兆丰华生物科技(福州)有限公司
A kind of I group 11d type poultry adenovirus strain, inactivated vaccine and preparation method thereof
ActiveCN110079509BHigh poison priceImprove proliferative abilityViral antigen ingredientsMicroorganism based processesMicroorganismImmunogenicity
Owner:洛阳职业技术学院 +1
Porcine epidemic diarrhea vaccine strain and vaccine containing it
ActiveCN107955804AGood cell adaptabilityEasy to trainSsRNA viruses positive-senseViral antigen ingredientsEpidemic diarrheaAnimals vaccines
The invention relates to an animal vaccine, and particularly discloses a porcine epidemic diarrhea vaccine strain and a vaccine containing the porcine epidemic diarrhea vaccine strain. The porcine epidemic diarrhea vaccine strain adopts porcine epidemic diarrhea virus attenuated by separation and purification as virus seed, and is freeze-dried by suitable protecting agent; the virus is injected topigs by muscle injection, thus high neutralizing antibody level is generated and passive protection is provided for suckling pigs; the vaccine has the advantages of simple preparation technique, stable property, and good immunogenicity; besides, the vaccine can effectively prevent porcine epidemic diarrhea virus infection.
Owner:CHINA ANIMAL HUSBANDRY IND
Infectious clone containing rabies virus glycoprotein and application
ActiveCN113234692AEasy to operateIncrease success rateSsRNA viruses negative-senseSsRNA viruses positive-senseStructural proteinPromoter
The invention belongs to the technical field of biology, and particularly discloses infectious clone containing rabies virus glycoprotein and application of the infectious clone. The infectious clone is constructed by a method that on the basis of a Semliki forest virus vector, an SP6 promoter sequence in the Semliki forest virus vector is replaced by a CMV promoter sequence, a hepatitis D virus ribozyme sequence (HdvRz) and a bovine auxin polyadenylation signal sequence are inserted behind a polyadenylate tail sequence, 13 base mutations are introduced into a non-structural protein coding region, and finally, rabies virus glycoprotein coding sequences are inserted at multiple cloning sites. The rabies virus-like vesicles rescued by the reverse genetic manipulation system only have one kind of structural protein RABV-G, proliferation passage can be carried out on BHK-21 cells, the titer is as high as 108 FFU / ml, and the rabies virus-like vesicles can be used as ideal rabies candidate vaccine strains.
Owner:HUAZHONG AGRI UNIV
A bhk-21 cell line bhk-21-bp-2 clone and its application in full suspension cell culture
ActiveCN110157659BLesions fastHigh poison priceMicroorganism based processesArtificial cell constructsRabiesTotal protein
The invention belongs to the technical field of veterinary biological products, and in particular relates to a BHK-21 cell line BHK-21-BP-2 clone and its application in full suspension cell culture. The present invention provides BHK-21-BP-2 clone strain, which is preserved in the General Microorganism Center of China Microbiological Culture Collection Management Committee, and the preservation number is CGMCC No.17588. After the clone is inoculated with pseudorabies virus in full suspension cell culture, the cell changes rapidly, and the virus produced has a high virulence, and the virus can be harvested within 24h to 48h; The process is simple; the total protein content of the produced virus antigen solution is low, the obtained effective virus antigen has high integrity, the antigen quality is good, and has excellent immunogenicity and safety; the prepared pseudorabies virus vaccine has high stability and batch-to-batch The advantages of small difference, good immune effect and high safety.
Owner:CHINA ANIMAL HUSBANDRY IND
Duck virus hepatitis virus, duck virus hepatitis inactivated vaccine and preparation method of duck virus hepatitis inactivated vaccine
InactiveCN103834619AHigh poison priceGood immune protectionEgg immunoglobulinsDigestive systemMicroorganismDuck viral hepatitis
The invention provides a duck virus hepatitis virus, a duck virus hepatitis inactivated vaccine and a preparation method of the duck virus hepatitis inactivated vaccine. The duck virus hepatitis virus is preserved in China General Microbiological Culture Collection Center with the preservation number of CGMCC No.7804. The duck virus hepatitis virus has strong toxicity, good immunogenicity, and high toxicity evaluation, and the vaccine prepared by the duck virus hepatitis virus has good safety and long immune protection period.
Owner:北京中联康生物科技股份有限公司
Duck short beak dwarf syndrome gene engineering subunit inactivated vaccine and preparation method thereof
PendingCN112933222AImprove the level ofLong durationViral antigen ingredientsVirus peptidesGenetic engineeringMolecular biology
The invention relates to a duck short beak dwarf syndrome gene engineering subunit inactivated vaccine and a preparation method thereof. Aiming at a variant strain, the VP2 gene of the SBDS-GPV JS01 strain is subjected to codon optimization and is cloned to a baculovirus vector, so that a recombinant baculovirus rBac-JS01VP2 strain is successfully constructed; insect cell full suspension (or spinner bottle) culture is adopted, the technology is mature, and production transformation and step-by-step amplification culture are facilitated; compared with traditional duck embryo or duck embryo fibroblast culture, the virus titer is high, the quality is stable, the production period is short, and the cost is low; compared with an antibody product, the protection rate is high, the duration time of a maternal antibody is long, only female ducks are immunized, and labor and cost are saved; serum or egg source miscellaneous protein does not exist, and the side reaction is extremely low; the expressed VP2 protein can be assembled into virus-like particles (VLPs), and the immunogenicity is good; the expression quantity is high (when the virus titer is 8.0 Log10TCID50 / ml, the VP2 protein expression quantity reaches 50-100 mg / L), the antibody level is high, and the lasting time is long; the shelf life of the product is 18 months, and the immune period is 6 months; and the ELISA antibody detection method is used for screening susceptible ducks and evaluating efficacy.
Owner:北京维牧康动保生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com