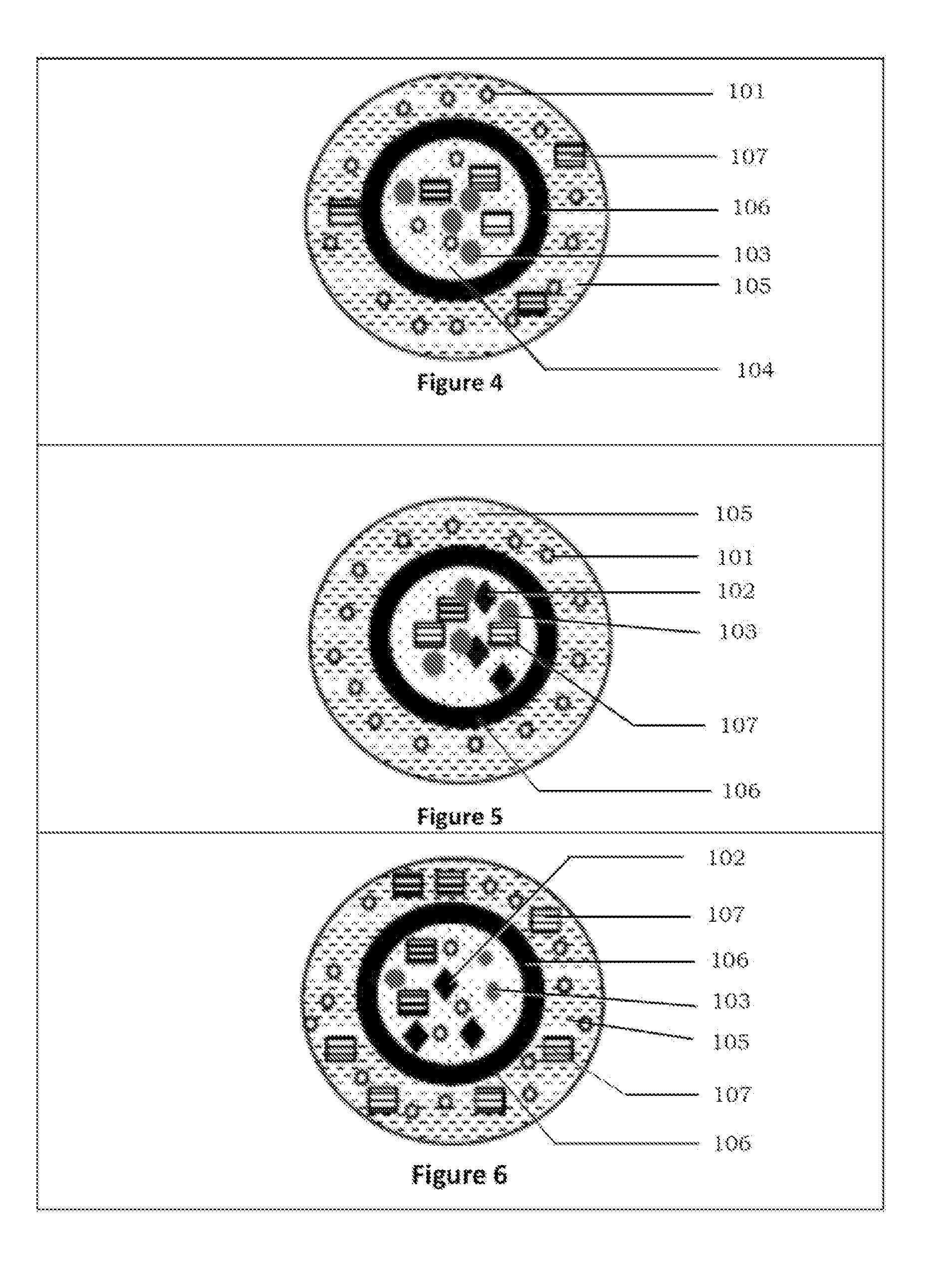
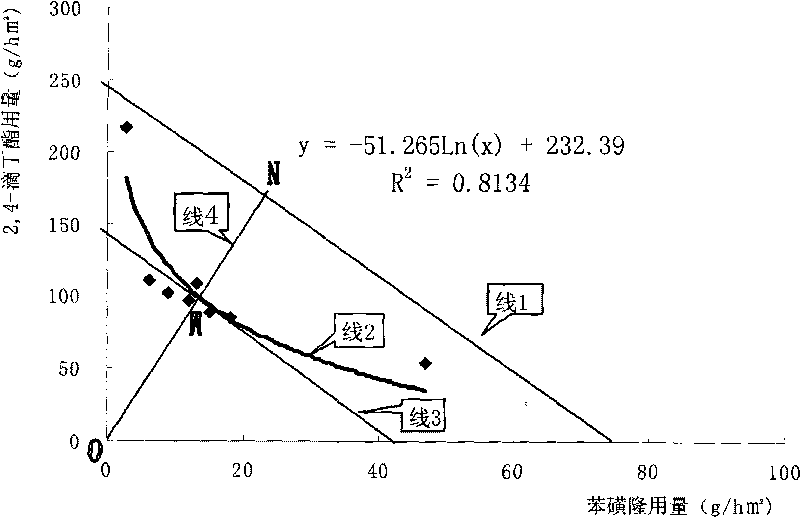
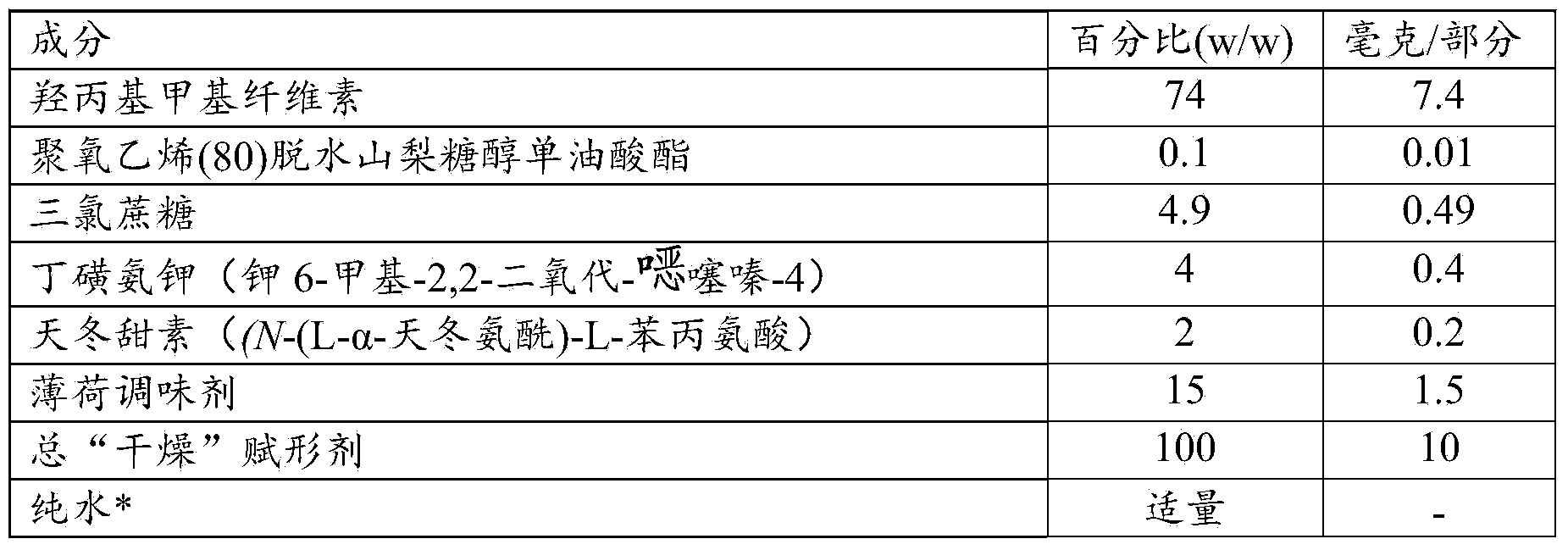
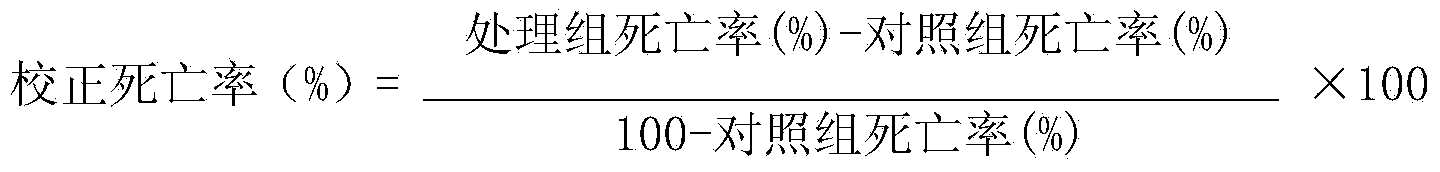
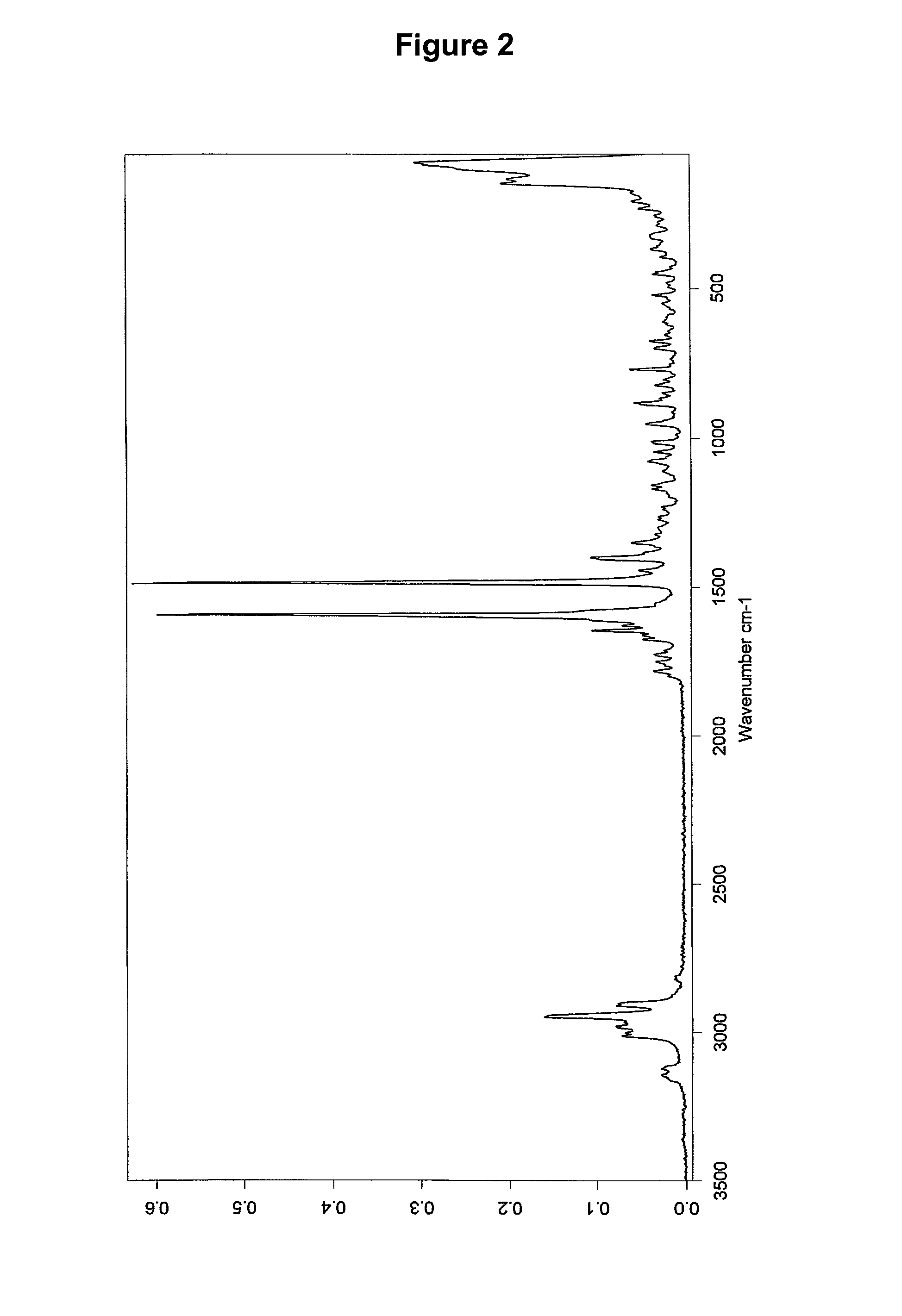
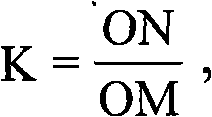Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Oil Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Controlled release oral dosage forms of poorly soluble drugs and uses thereof
InactiveUS20140018404A1Improve bioavailabilityExtend posting timeBiocidePharmaceutical non-active ingredientsControl releaseDosage form
Provided herein are controlled release oral dosage forms of poorly soluble drugs, methods of making the dosage forms, and methods of their use for the treatment of various diseases and / or disorders.
Owner:AMGEN INC
Dosage Form
InactiveUS20130330409A1Improve stress resistanceSmall diameterPowder deliveryBiocideParticulatesSedative/hypnotic
The present invention provides a dosage form, particularly a tamper resistant dosage form, comprising; non-stretched melt extruded particulates comprising a drug selected from an opioid agonist, a tranquilizer, a CNS depressant, a CNS stimulant or a sedative hypnotic; and a matrix; wherein said melt extruded particulates are present as a discontinuous phase in said matrix.
Owner:EURO-CELTIQUE SA
Fast-dispersing dosage forms containing fish gelatin
InactiveUS9192580B2Overcome problemsEasy to usePowder deliveryPeptide/protein ingredientsBULK ACTIVE INGREDIENTBuccal administration
The invention disclosed herein relates to a pharmaceutical composition comprising a carrier and an active ingredient, wherein the carrier is fish gelatin and the composition is a fast-dispersing dosage form designed to release the active ingredient rapidly on contact with a fluid. In one embodiment, the composition is designed for oral administration and releases the active ingredient rapidly in the oral cavity on contact with saliva. The fish gelatin can be obtained from cold water fish sources and is preferably the non-gelling, non-hydrolyzed form. A process for preparing such a composition and a method of using fish gelatin in a fast dispersing dosage form are also provided.
Owner:CATALENT USA WOODSTOCK INC +3
Controlled release dosage form
The present invention generally relates to a pharmaceutical dosage form and controlled release of biologically active agents, diagnostic agents, reagents, cosmetic agents, and agricultural / insecticide agents. In one embodiment, the dosage form has a substrate that forms a compartment, wherein the substrate includes at least a first piece and a second piece, wherein the first piece operably links to the second piece. The dosage form contains a drug content that is loaded into the compartment. The dosage form also has a releaser operably linked to the substrate which upon contact with water or body fluid is capable of separating the first and second piece to open the compartment and release the drug content.
Owner:TRIASTEK INC
Abuse deterrent immediate release biphasic matrix solid dosage form
InactiveUS20150272902A1Deter the abuse of drugsLow release ratePowder deliveryOrganic active ingredientsImmediate releasePsychiatry
An abuse deterrent immediate release biphasic matrix solid dosage form that releases the drug at a desired rate for quick onset of action when a single unit or prescribed units of the dosage form are orally administered but exhibits a reduced rate of release when more than the prescribed number of units, are administered.
Owner:SUN PHARMA INDS
Insecticidal composition containing pyriminostrobin
ActiveCN103548879AImprove securityMeet security requirementsBiocideAnimal repellantsAbamectinSuspending Agents
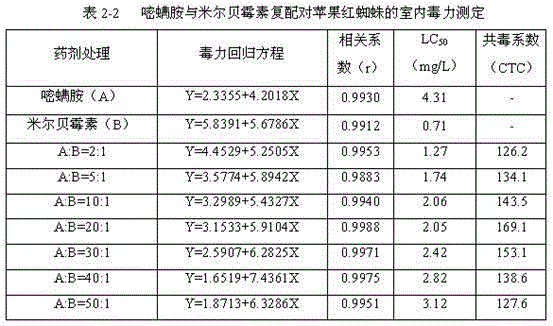
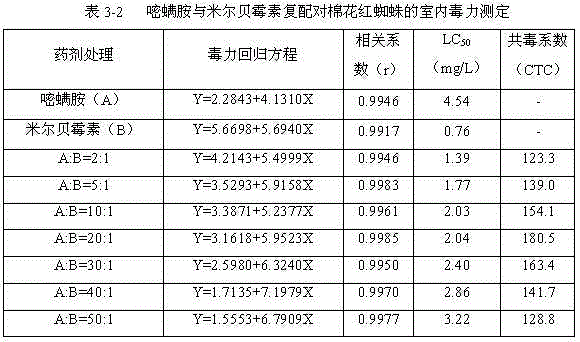
The invention discloses an insecticidal composition containing pyriminostrobin. The insecticidal composition is characterized in that the active ingredients of the insecticidal composition are pyriminostrobin and a biogenic insecticide abamectin or milbemycins; the weight ratio of pyriminostrobin to the biogenic insecticide is (2:1)-(50:1). The composition can be prepared into agriculturally allowable dosage forms such as suspension concentrates, wettable powder, water dispersible granules, dispersible oil suspension concentrates, microemulsion or emulsifiable concentrates. The insecticidal composition has the advantages that the insecticidal composition has reasonable components and good acaricidal effects; the activity and acaricidal effects of the insecticidal composition are not simple superposition of the activities of the components; compared with existing single preparations, the insecticidal composition has obvious synergistic effects besides obvious insecticidal effects, has good safety to crops and has obvious control effects on Panonychus citfi McGregor, Panonychus ulmi or Tetranychus cinnabarinnum Boisduval.
Owner:农心作物科技股份有限公司
Solid Nicotine-Comprising Dosage Form with Reduced Organoleptic Disturbance
Solid pharmaceutical dosage form for the release of nicotine in the oral cavity comprising a core encapsulated by at least one film coating, wherein the core comprises nicotine and wherein the film coating comprises at least one film-forming polymer and at least one component for reduction of one or more organoleptically disturbing sensations, and where the at least one film coating is devoid of nicotine and devoid of buffer.
Owner:MCNEIL AB
Avermectin and Carbosulfan Insecticide Composition
InactiveCN102265878AExpand insecticidal spectrumMany pest action sitesBiocideAnimal repellantsCecidomyiidaeAbamectin
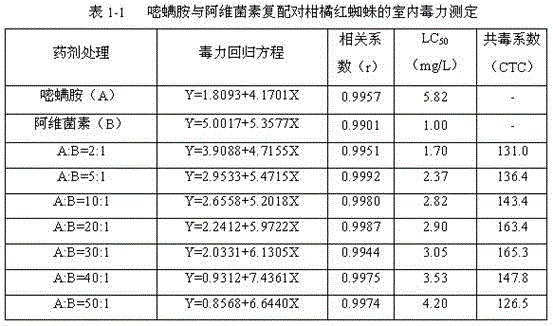
The invention discloses an abamectin-benfuracarb pesticide composition. The abamectin-benfuracarb pesticide composition is a water emulsion comprising abamectin, benfuracarb, one or more cosolvents, a solvent, one or more emulsifiers and one or more stabilizing agents. The abamectin-benfuracarb pesticide composition has not only a water emulsion dosage form, but also a missible oil dosage form, amicron emulsion dosage form, a powder form, a wettable powder dosage form and a microcapsule suspension dosage form. The abamectin-benfuracarb pesticide composition has obvious synergistic effects onthrip, aphid, wireworm, potato flea beetle, beet flea beetle, eriophyesoleivorus and the like living on vegetables, expands a control spectrum, and has excellent control effects on thrip, aphid, cabbage caterpillar, wireworm, whitefly, scale insect, carposinaniponensiswalsingham, oriental fruit moth, red spider, apple skin worm, apple cecidomyiidae and the like living on vegetables, fruit trees and cotton. In addition, the abamectin-benfuracarb pesticide composition improves pesticide effects, reduces use cost and delays drug resistance generated by pests.
Owner:GAUNGXI TIANYUAN BIOCHEM
Controlled release oral dosage forms of poorly soluble drugs and uses thereof
ActiveCN103442698AConvenient treatmentEasy to solveOrganic active ingredientsPill deliveryControl releaseDosage form
Provided herein are controlled release oral dosage forms of poorly soluble drugs, methods of making the dosage forms, and methods of their use for the treatment of various diseases and / or disorders.
Owner:AMGEN (EURO) GMBH
Oral dosage form
InactiveUS20170071988A1Achieve effectUnknown materialsUsing mechanical meansOrodispersible tabletDentistry
The present invention is in the field of delivering an active substance to the oral cavity and relates to mucoadhesive active composition and corresponding mucoadhesive dosage form, which can deliver an active substance within the oral cavity, especially an orodispersible tablet for delivering probiotic substance. The present invention also relates to a method of producing the said composition and a method of processing the composition into a mucoadhesive dosage form, especially an orodispersible tablet. The present invention moreover relates to a system and a method for testing the mucoadhesion of a dosage form.
Owner:SYMRISE GMBH & CO KG
Abuse deterrent immediate release coated reservoir solid dosage form
ActiveUS20150320689A1Deter the abuse of drugsLow release rateOrganic active ingredientsNervous disorderPack Dosage FormImmediate release
An abuse deterrent immediate release coated reservoir solid dosage form that releases the drug at a desired rate for quick onset of action when a single unit or prescribed units of the dosage form are orally administered but exhibits a reduced rate of release when more than the prescribed number of units, are administered.
Owner:SUN PHARMA INDS
Film dosage form with extended release mucoadhesive particles
ActiveUS9668970B2Avoid discomfortEasy to controlPharmaceutical delivery mechanismEther/acetal active ingredientsPack Dosage FormFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
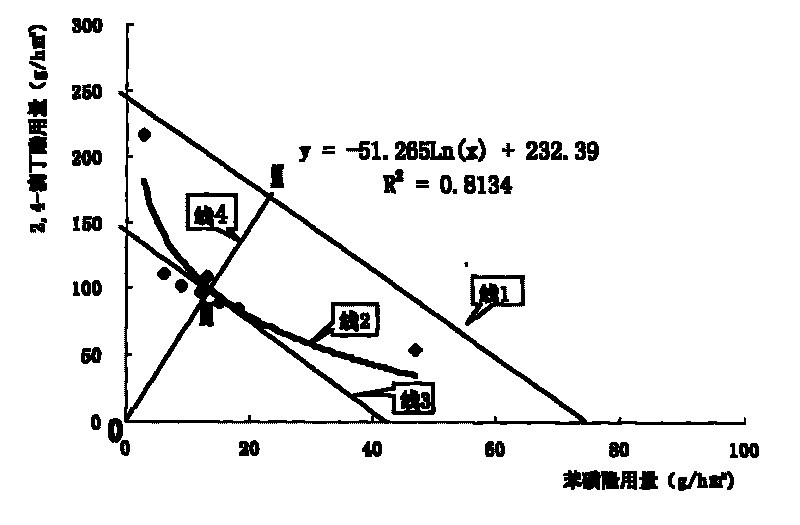
Tribenuron-methyl and 2,4-D butyl ester compound wheat field herbicide
InactiveCN101711524AEffective controlSynergistic effect is obviousBiocideAnimal repellantsDescurainiaSuspending Agents
The invention relates to a tribenuron-methyl and 2,4-D butyl ester compound wheat field herbicide, containing the following components in percent by weight: 50-75 percent of tribenuron-methyl and 2,4-D butyl ester and the balance of assistant, wherein the weight ratio of the tribenuron-methyl and the 2,4-D butyl ester is 1:5-12. The herbicide adopts assistants consistently used in the agricultural chemical field, such as wetting agents, dispersing agents, solid carriers and the like and can be made into various dosage forms of compound herbicides respectively, such as wettable powder dosage form, missible oil dosage form, oil suspending agent dosage form and the like. The invention has the advantages that the synergized action is obvious, broad leaved weeds in wheat fields can be effectively prevented and killed off, and especially, the prevention effect of weed Descurainia having drug resistance in the wheat fields is increased; meanwhile, the weed killing speed is increased, the safety to crops is increased and the drug hazard is lowered, thereby enabling the wheat to be increased by 2.9-8.1 percent in yield and the cost to be reduced by 13.4-40 percent.
Owner:INST OF CEREAL & OIL CROPS HEBEI ACAD OF AGRI & FORESTRY SCI
Solid nicotine-comprising dosage form with reduced organoleptic disturbance
InactiveCN104053433AIncrease weightOrganic active ingredientsNervous disorderMedicineFilm-coated tablet
Solid pharmaceutical dosage form for the release of nicotine in the oral cavity comprising a core encapsulated by at least one film coating, wherein the core comprises nicotine and wherein the film coating comprises at least one film-forming polymer and at least one component for reduction of one or more organoleptically disturbing sensations, and where the at least one film coating is devoid of nicotine and devoid of buffer.
Owner:MCNEIL AB
Botanical insecticide
InactiveCN104381314AImprove insecticidal effectNot easy to develop resistanceBiocideAnimal repellantsPesticide residueSolvent
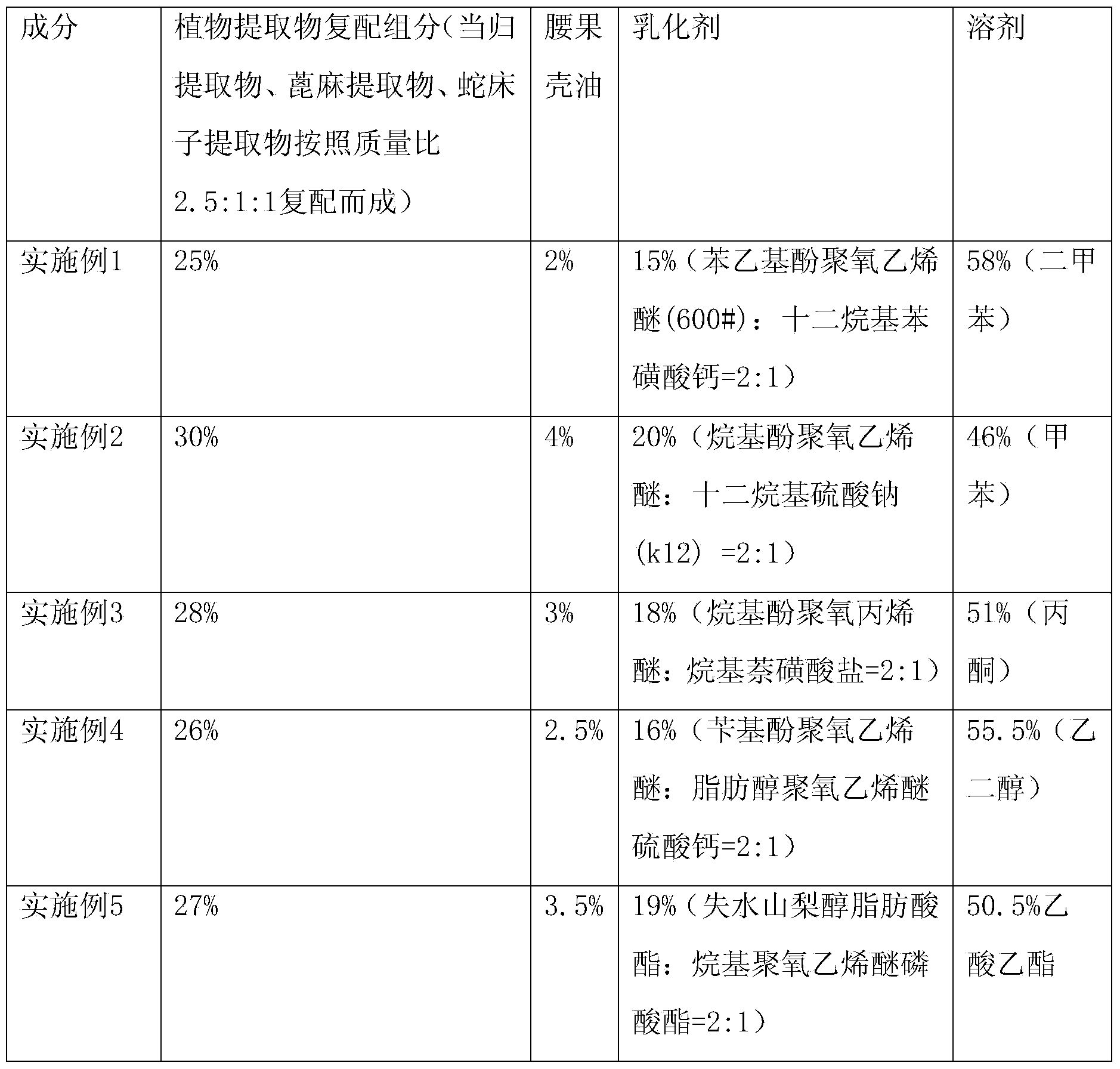
The invention provides a botanical agricultural insecticide, which is friendly to environment, good in insecticidal efficacy and not easy to generate resistance. A missible oil dosage form is prepared by compounding plant extract compound components, namely cashew nut shell oil, an emulsifier and a solvent. Through compound use of a botanical pesticide and a chemical pesticide, complementary advantages are facilitated; the usage amount of the chemical pesticide can be greatly reduced; the bad effects such as pesticide residue pollution are reduced; the drug resistance of pests is delayed; the use safety of the pesticide is improved; and the development direction of sustainable control on the pests at present is compounded.
Owner:夏征梅
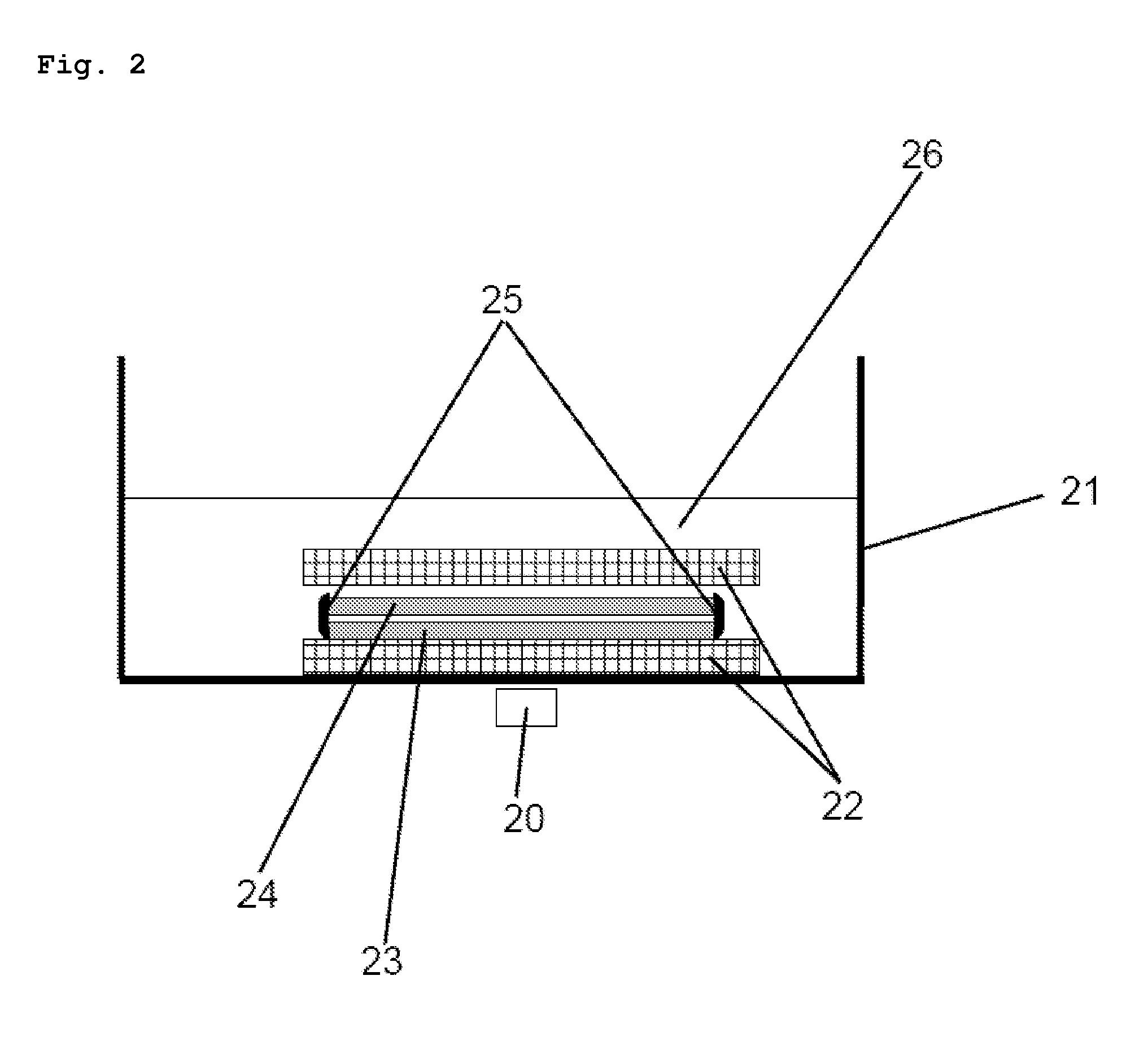
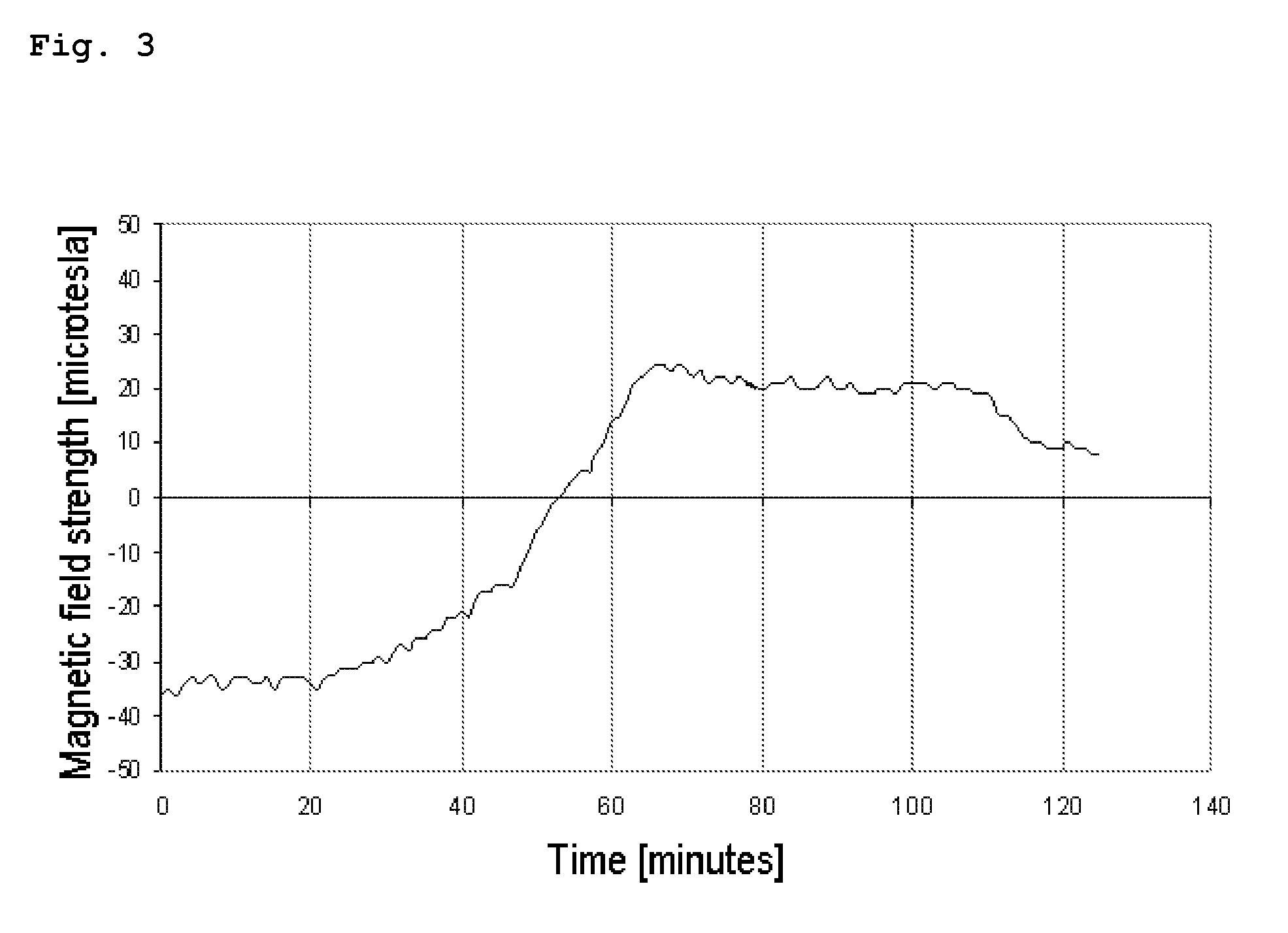
Oral dosage form, comprising at least one biologically active agent, formulation auxiliary substances and magnetizable particles
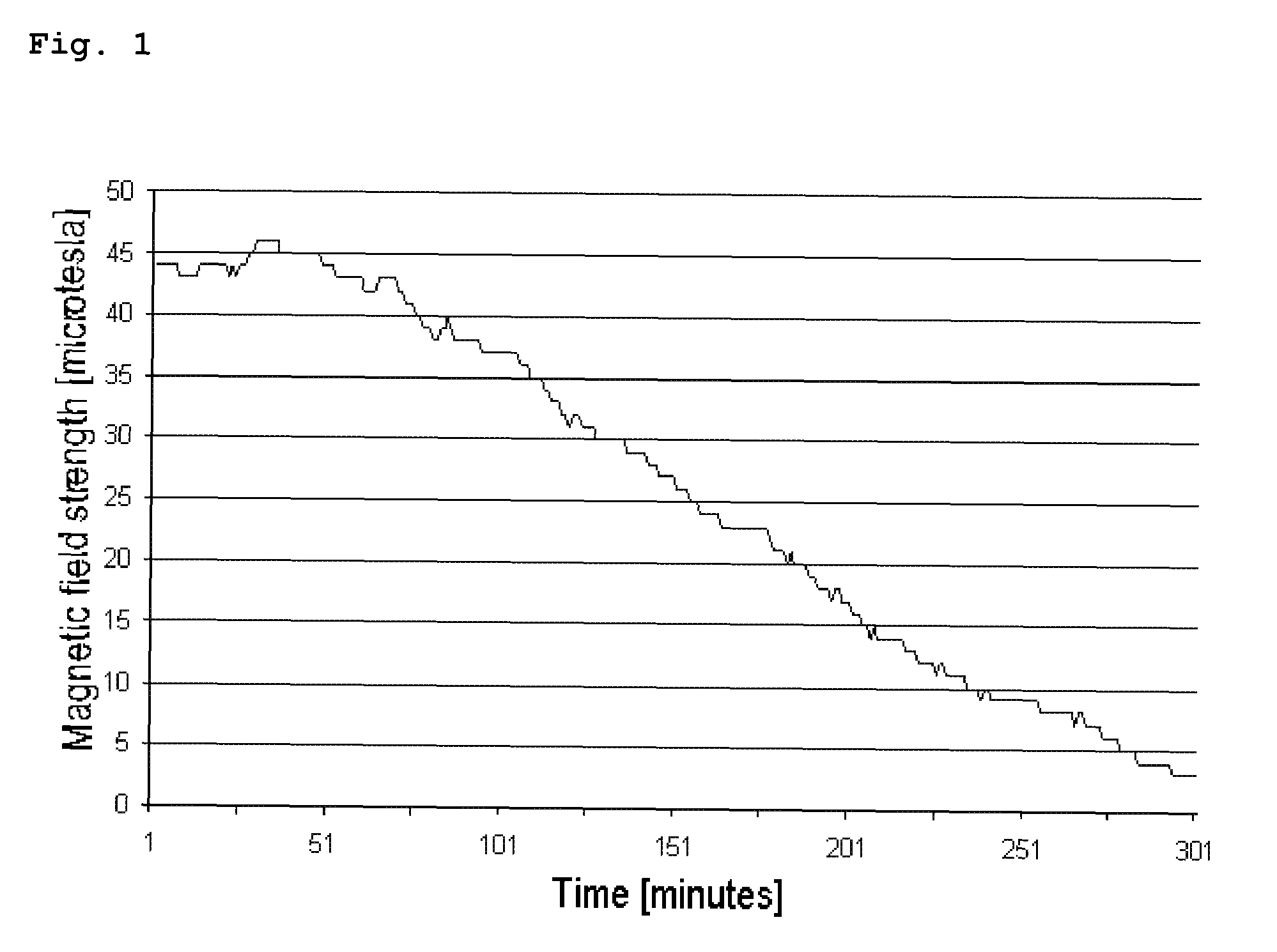
The invention relates to an oral dosage form, comprising at least one biologically active agent, formulation auxiliary substances and magnetizable particles, wherein the dosage form has an at least two phase composition, wherein the phases can dissolve in the body after oral administration due to their formulation and the magnetizable particles are bound in formulation auxiliary substances and are present in a magnetized state, wherein the magnetized particles are present in at least two phases of the dosage form and generate magnetic fields, wherein these phases dissolve at different times in the body after oral administration, and wherein the magnetic field strength with respect to time, position and movement in the body is acquired using a detection system and can be evaluated using a computer-based evaluation system.
Owner:EVONIK OPERATIONS GMBH
Film dosage form with extended release mucoadhesive particles
ActiveUS20170239172A1Improve permeabilityAvoid discomfortPharmaceutical non-active ingredientsSheet deliveryPack Dosage FormFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
Dosage form containing levorotation gossypol as active component
InactiveCN101269007APharmaceutical delivery mechanismAldehyde active ingredientsAdditive ingredientPotassium
The invention relates to a pharmaceutical preparation taking levorotatory gossypol as active ingredient, in particular to a compound preparation which contains the medicine such as levorotatory gossypol, potassium chloride, vitamin B1 and vitamin B6, etc. and is used for curing gynecological disease. The compound preparation comprises an oral solid preparation and an injection.
Owner:刘德强
Film dosage form with extended release mucoadhesive particles
ActiveUS20150150786A1Easy to controlAvoid discomfortBiocideEther/acetal active ingredientsPack Dosage FormFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
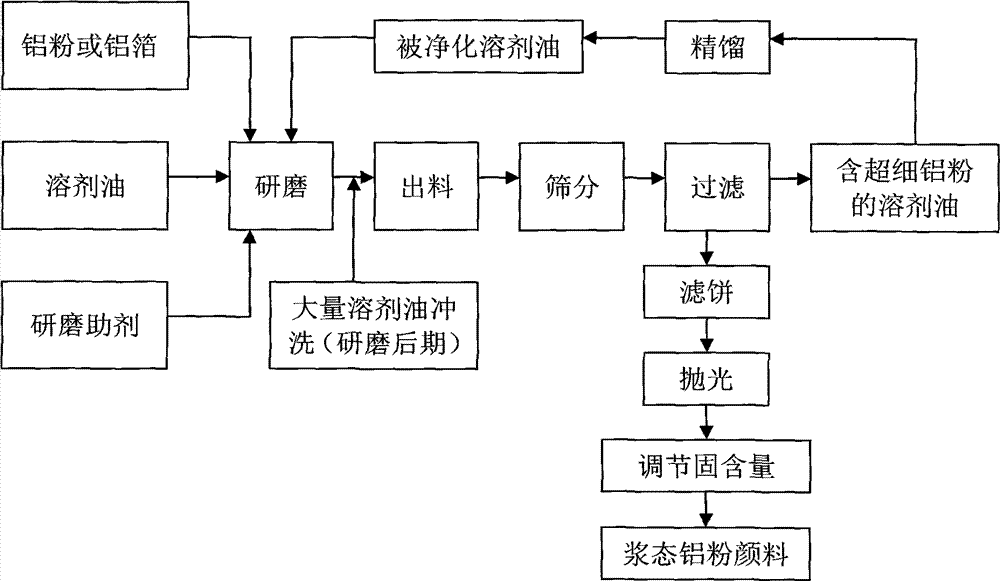
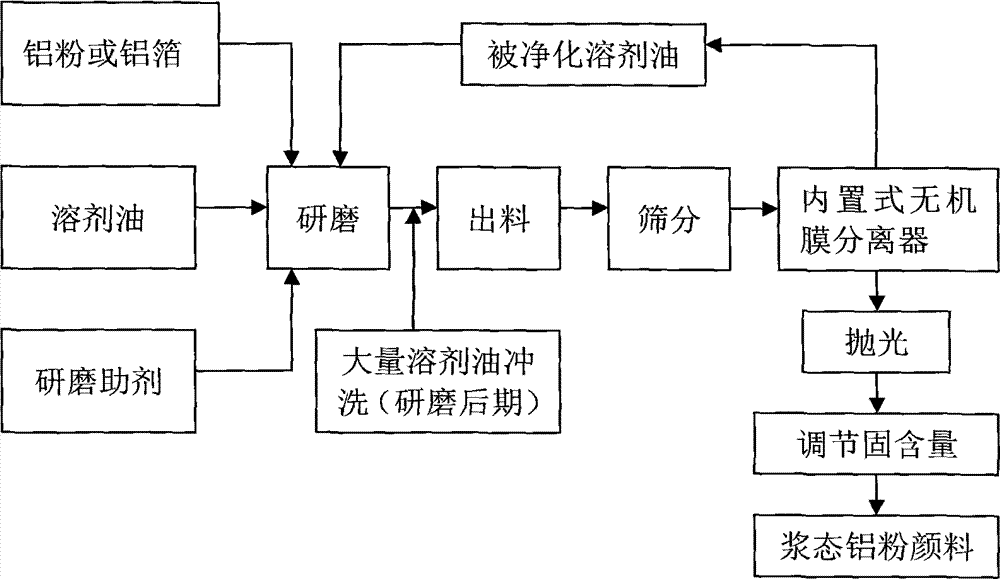
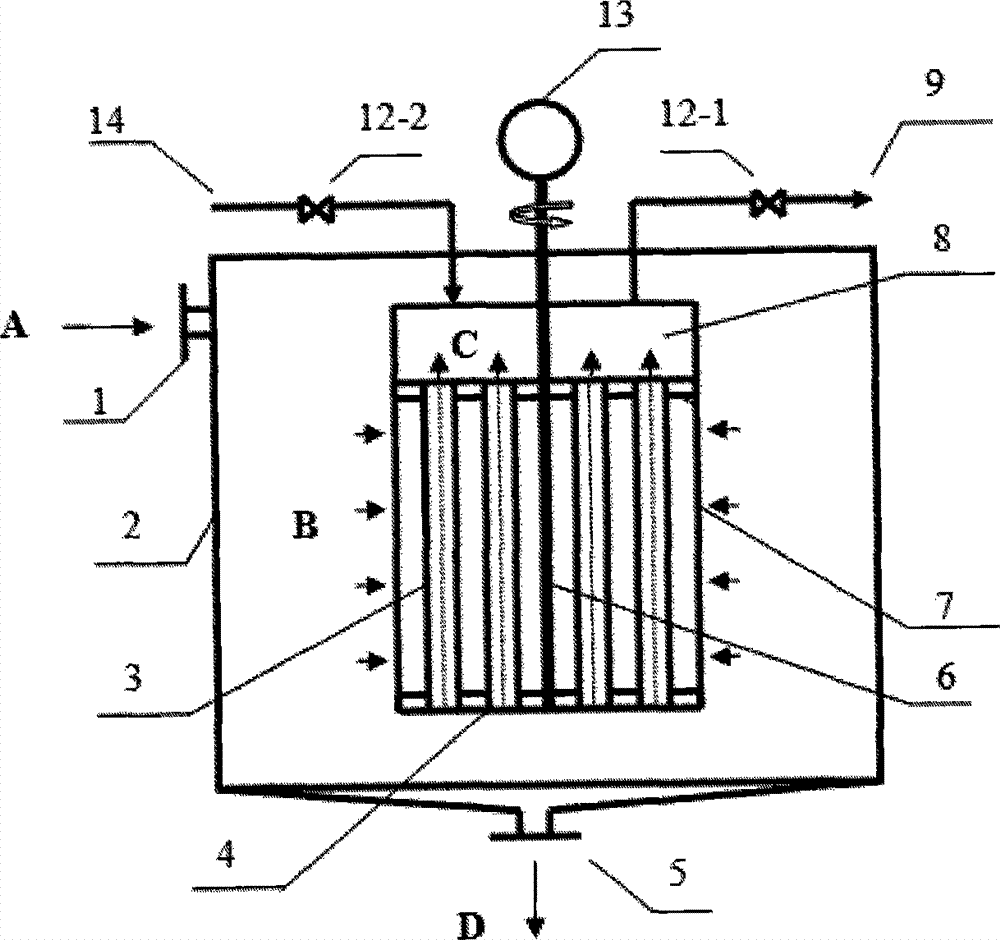
Method of producing oil-dosage-form aluminium powder pigment by utilization of inorganic membrane filtration and device for the method
InactiveCN104119706AReduce pollutionImprove throughputPigment physical treatmentPigment treatment with non-polymer organic compoundsFiltrationAluminium powder
The invention belongs to the technical field of pigment preparation, and particularly relates to a method of producing an oil-dosage-form aluminium powder pigment by utilization of inorganic membrane filtration and a device for the method. The production device comprises a grinding device, a sieving device and a separating device, wherein the sieving device and the separating device are connected after the grinding device in sequence. The separating device is a built-in type inorganic membrane separator. The inorganic membrane separator with a special structure is adopted to perform solid-liquid separation, thus achieving simultaneous performing of separation and purification of solvent oil, guaranteeing the product quality, simplifying processes at present, largely reducing the energy consumption in processes and saving the production cost.
Owner:HUNAN UNIV OF SCI & TECH
Novel dosage form
InactiveCN103987377AOrganic active ingredientsInorganic non-active ingredientsOral medicationBULK ACTIVE INGREDIENT
There is provided inter alia a pharmaceutical dosage form for oral administration comprising a sanglifehrin as active ingredient in which the sanglifehrin active ingredient is protected from acid degradation in the stomach environment following oral administration.
Owner:NEUROVIVE PHARMA AB
Insecticidal composition containing pyriminostrobin and hexythiazox
ActiveCN103548863AImprove securityMeet safety requirementsBiocideAnimal repellantsSuspending AgentsOrganic chemistry
The invention discloses an insecticidal composition containing pyriminostrobin and hexythiazox. The insecticidal composition is characterized in that the active ingredients of the insecticidal composition are pyriminostrobin and hexythiazox; the weight ratio of pyriminostrobin to hexythiazox is (1:5)-(10:1). The composition can be prepared into the dosage forms such as suspension concentrates, wettable powder, water dispersible granules, dispersible oil suspension concentrates, microemulsion or emulsifiable concentrates. The insecticidal composition has the advantages that the insecticidal composition has reasonable components and good acaricidal effects; the activity and acaricidal effects of the insecticidal composition are not simple superposition of the activities of the components; compared with existing single preparations, the insecticidal composition has obvious synergistic effects besides obvious insecticidal effects, has good safety to crops and has obvious control effects on Panonychus citfi McGregor, Panonychus ulmi or Tetranychus cinnabarinnum Boisduval.
Owner:农心作物科技股份有限公司
Novel Dosage Form
ActiveUS20140234414A1Improve bioavailabilityImprove the environmentBiocideOrganic active ingredientsOral medicationBULK ACTIVE INGREDIENT
There is provided inter alia a pharmaceutical dosage form for oral administration comprising a sanglifehrin as active ingredient in which the sanglifehrin active ingredient is protected from acid degradation in the stomach environment following oral administration.
Owner:ABLIVA AB
Controlled dosage form-dispensing system
ActiveUS10456332B2Drug and medicationsCoin-freed apparatus detailsPack Dosage FormMedication Dispenser
This invention provides a controlled dosage form-dispensing device, comprising a multi-chamber, bulk medicine storage and distribution unit, wherein said unit is provided with a plurality of individual dosage form-containing storage chambers with respective delivery ports; and a personal medication dispenser comprising a fixed dosage form extracting station comprising a dosage form receiving and extracting mechanism; wherein the medication dispenser is provided with a controller which moves the bulk medicine storage and distribution unit sequentially bringing a respective delivery port of a predetermined chamber into register with the fixed dosage form extracting station such that the dosage form receiving and extracting mechanism extracts and receives only one dosage form at a time. The dispensing device is further provided with at least one portable dosage form-dispensing cassette having a plurality of compartments and a first delivery controller for delivery of a predetermined dosage form from a predetermined compartment of the portable dosage form-dispensing cassette, the at least one portable cassette being releasably attachable to a personal medication dispenser; and a second delivery controller for the controlled delivery of predetermined dosage forms from the multi-chamber, bulk medicine storage and distribution unit to the portable dosage form-dispensing cassette via a conduit in the personal medication dispenser.
Owner:P C O A DEVICES LTD
Method of controlling the release of an active ingredient from a dosage form
ActiveUS20120029091A1Facilitated releaseIncrease loadAntibacterial agentsPowder deliveryMedicineBULK ACTIVE INGREDIENT
A method of controlling or adjusting release of an active ingredient from a dosage form comprising the active ingredient and a polysaccharide derivative has been found. The method comprises the steps ofa) providing a composition comprising a polysaccharide derivative and a controlled amount of a liquid diluent, based on the dry weight of the polysaccharide derivative,b) subjecting the composition to a dry-grinding operation to provide a dry-ground polysaccharide derivative, andc) incorporating the dry-ground polysaccharide derivative and an active ingredient into a dosage form.
Owner:DOW GLOBAL TECH LLC
Extraction method of pseudo-ginseng oil
InactiveCN104673482AHigh extraction rateIncrease profitAntibacterial agentsImmunological disordersAlcoholGinseng
The invention relates to an extraction method of pseudo-ginseng oil. The extraction method changes a traditional crushing and water-alcohol extraction method, and implements extraction by using superfinishing and supercritical carbon dioxide extraction method; and the extraction method comprises the following steps: (1) pretreatment; (2) primary extraction; (3) alkalization; 4) acidification; and (5) extraction. The extraction method both can improve the extraction rate of pseudo-ginseng oil, and can reduce the production cost, thereby providing raw material guarantee for the development of multiple pseudo-ginseng oil dosage forms.
Owner:TIANJIN ZHONGAO FEED
Controlled release oral dosage forms of poorly soluble drugs and uses thereof
ActiveUS9532977B2Improve bioavailabilityExtend posting timeBiocidePill deliveryControl releaseDosage form
Provided herein are controlled release oral dosage forms of poorly soluble drugs, methods of making the dosage forms, and methods of their use for the treatment of various diseases and / or disorders.
Owner:AMGEN INC
Gelling agent-based dosage form
ActiveUS9452135B2Easily administered and swallowedPrevent extractionPowder deliveryOrganic active ingredientsLarge doseBULK ACTIVE INGREDIENT
The present invention generally relates to dosage forms for oral administration including one or more gelling agents. In particular, the present invention is directed to gelling agent-based dosage forms that are easily administered and taken, or swallowed. The present invention is also directed to gelling agent-based dosage forms that exhibit relatively low syneresis, are thermally stable, exhibit substantially constant active ingredient concentration, and / or exhibit one or more advantageous rheological properties. In particular, the present invention is directed to such gels containing one or more omega-3 fatty acids. The gelling agent-based dosage forms of the present invention are suitable for administration of a relatively large dose of active ingredient. The gelling agent-based dosage forms of the present invention are also suitable for administration of multiple active ingredients. Dosage forms of the present invention also provide tamper resistance and, thus, prevent recovery or diversion of active ingredients contained therein. The gelling agent-based dosage forms are also suitable for use as gastro-retentive and sustained release dosage forms.
Owner:PARTICLE DYNAMICS INT
Dosage form with improved release of cefuroximaxetil
InactiveUS8747900B2Improve bioavailabilityEasy to processBiocideOrganic chemistryΛ carrageenanDosage form
The invention relates to a pharmaceutical composition comprising cefuroximaxetil and at least one carrageenan selected from the group consisting of κ-carrageenan, λ-carrageenan and -carrageenan. The invention furthermore relates to pellets, to a multiparticulate, pharmaceutical dosage form and to a novel crystalline modification of cefuroximaxetil.
Owner:GRUNENTHAL GMBH
Avermectin-containing pesticide in dosage form of missible oil
InactiveCN104304279AGood synergyReduce use costBiocideAnimal repellantsBULK ACTIVE INGREDIENTActive ingredient
The invention provides an avermectin-containing pesticide in the dosage form of missible oil. An active ingredient of the pesticide is avermectin. Formula of the pesticide contains the following components, by weight, 20% of flutenzine, 8% of avermectin, 1% of an emulsifier, 1% of a solvent, 1% of a cosolvent and deionized water added to 100%. Avermectin has good synergism. The pesticide provided by the invention has advantages of synergistic effect, low use cost, good control effect, wide acaricidal spectrum, high adaptability, long persistent period, low toxicity, low residue content, good safety and high compatibility, and can be used for killing both egg and larvae.
Owner:QINGDAO JINGYIXIN ELECTRONICS TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com