Solid nicotine-comprising dosage form with reduced organoleptic disturbance
A nicotine and dosage form technology, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of lack of nicotine, undisclosed, and lack of buffers in film coating.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0078] Manufacturing method
[0079]The composition of the batches for the tablet cores is given in Table A1 below. The material is sieved using a vibrating sieve with a mesh size of 1 mm, and thereafter blended for 10 to 30 minutes according to methods known in the art, eg, using a double cone mixer. The blended material is then compressed into tablets by means of direct compression. Powder compression can be performed, for example, using a rotary tablet press with 15mm round concave punches. The tablets are compressed to a sufficient hardness to allow an acceptable coating process and to achieve the desired in vivo dissolution time.
[0080] Table 1A: Components of Tablet Cores .
[0081] Element
percentage (w / w)
mg / part
Nicotine Resin Complex (20% Nicotine)
1.5
15*
89.0
890
5.75
57.5
0.25
2.5
0.5
5
mint flavoring
...
example 2
[0107] According to the manufacture method of the tablet of example 1
[0108] Table 2A: Components of Tablet Cores .
[0109] Element
percentage (w / w)
mg / part
Nicotine Resin Complex (20% Nicotine)*
1.67
10.0
93.56
561.36
0.5
3.0
mint flavoring
1.67
10.02
0.1
0.6
2
12.0
Silica (colloid)
0.5
3.0
total
100
600
[0110] *Equivalent to a 2.0mg dose of nicotine base. If a nicotine resin complex is used with other levels of nicotine loading such as 15%, the amount of polyol is adjusted accordingly.
[0111] Film coating of the tablets produced in 2A can be performed using, for example, a standard modern pan coater equipped with an air atomized spray nozzle dispensing the film coating fluid and a perforated drum of appropriate size. Membrane solutions were prepared by adding hydroxypr...
example 3
[0118] Following Example 2, a total tablet core weight of 650 mg was used, with oval 14.5 mm punches, but without sodium bicarbonate and / or sodium carbonate (which was compensated by the amount of mannitol). Additionally, the components of the film coating are provided in Table 3A.
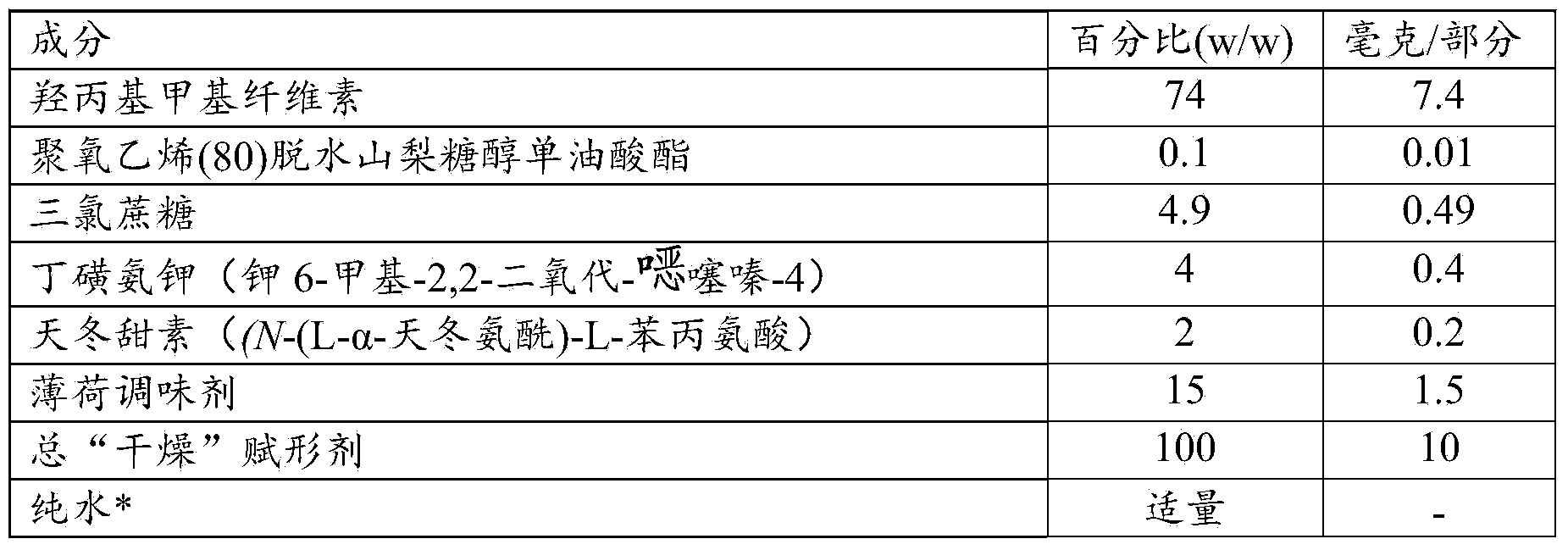
[0119] Table 2B: Components of the film coating .
[0120]
[0121]
[0122] *Total excipients except purified water
[0123] **Add the appropriate amount of pure water to achieve a dry content suitable for the coating process parameter settings to be applied, for example the dry content can be 16% w / w.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com