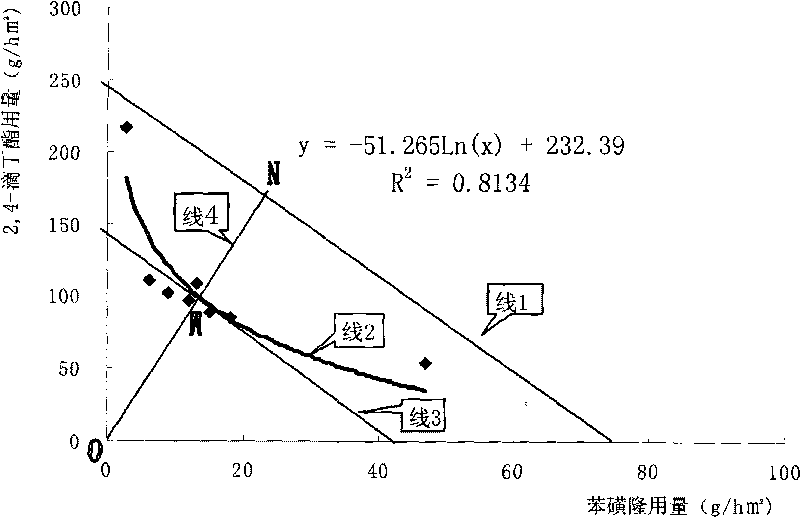
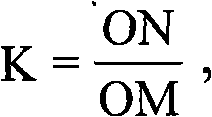Tribenuron-methyl and 2,4-D butyl ester compound wheat field herbicide
A technology of butyl drop and herbicide, applied in the directions of herbicides, algaecides, biocides, biocides, etc., can solve the problem that the control effect is not significantly improved, the price of chlorofluoropyridoxine is high, and the phytotoxicity of wheat leaves, etc. problems, to achieve the effect of improving the speed of action, reducing the cost of prevention and control, and reducing phytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: 50% Tribesulfuron-2,4-D Butyl Ester Oil Suspension Concentrate (1:5)
[0062] Raw material dosage (g)
[0063] Tribesulfuron 8.77
[0064] 2,4-D-butyl ester 43.40
[0065] Soitem 101 2
[0066] DBS-Ca2
[0067] Nongru No. 1601 3
[0068] Arnox BP series 0.2
[0070] canola oil 20
[0071] soybean oil 19.63
[0072] Preparation:
[0073] According to the above formula, the active ingredients Tribesulfuron-methyl, 2,4-D-butyl ester, Arnox BP series, soybean oil, and rapeseed oil were first weighed, and all of them were transferred into a sand mill, and then Soitem 101, DBS-Ca, and Nongru No. 1601, after being ground in a sand mill to a certain fineness (the fineness should reach 98% or more of the particles passing through the 0.043mm sieve), the material is transported to the storage tank, and transferred to homogeneous mixing after metering Then add the remaining additives and disperse evenly in a homomixer to obtain the p...
Embodiment 2
[0074] Embodiment 2: 60% Tribesulfuron-2,4-D Butyl Ester Oil Suspension Concentrate (1:6)
[0075] Raw material dosage (g)
[0076] Tribesulfuron 9.02
[0077] 2,4-D-butyl ester 53.57
[0078] Emulsogen M 5
[0079] OP-10 2
[0080] Silicone 0.5
[0081] Phosphate 1
[0082] Acetone 10
[0083] Xylene 18.91
[0084] According to the above formula, the active ingredients Tribesulfuron-methyl, 2,4-D-butyl ester, silicone, acetone, and xylene were weighed first, and all of them were transferred into a sand mill kettle, then Emulsogen M and OP-10 were added, and the After being ground in the kettle to a certain fineness (the fineness should be greater than or equal to 98% of the particles passing through the 0.043mm sieve), the material is transported to the storage tank, and then transferred to the homogeneous mixer after metering, and then the remaining additives are added , the product can be prepared after being uniformly dispersed by a homomixer.
Embodiment 3
[0085] Example 3: 70% Tribesulfuron-2,4-D Butyl Ester Oil Suspension Concentrate (1:5)
[0086] Raw material dosage (g)
[0087] Tribesulfuron 12.28
[0088] 2,4-D-butyl ester 60.76
[0089] ATLOX 3406F 2
[0090] Farm Milk 1601 3
[0091] Arnox BP series 0.3
[0093] Acetone 10
[0094] Ethanol 10.66
[0095] According to the above formula, the active ingredients Tribesulfuron-methyl, 2,4-D-butyl ester, Arnox BP series, acetone, and ethanol were first weighed, and all of them were transferred into a sand mill kettle, then ATLOX 3406F and Nongru 1601 were added, and the sand mill After being ground in the kettle to a certain fineness (the fineness should be greater than or equal to 98% of the particles passing through the 0.043mm sieve), the material is transported to the storage tank, and then transferred to the homogeneous mixer after metering, and then the remaining additives are added , the product can be prepared after being uniformly di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com