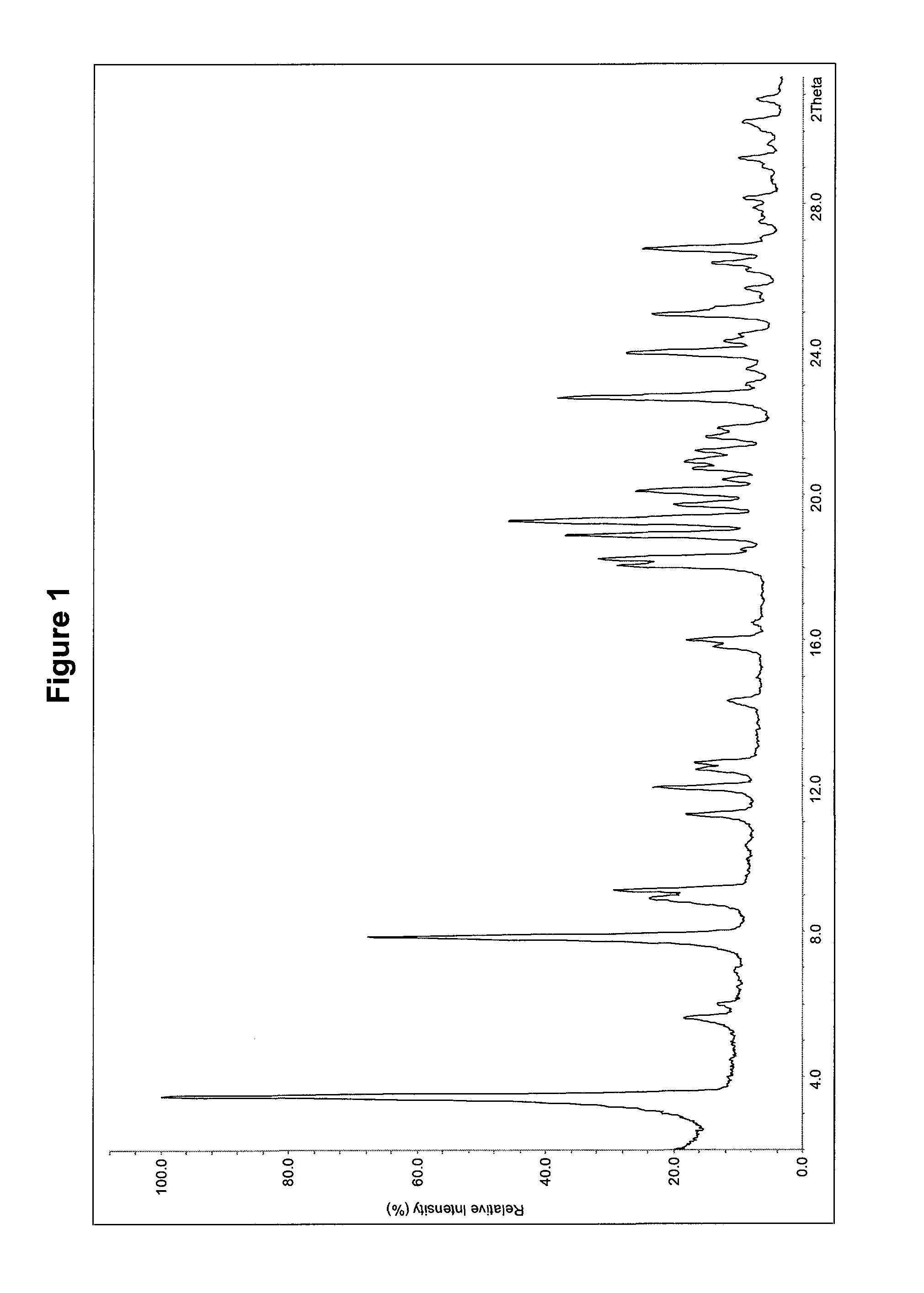
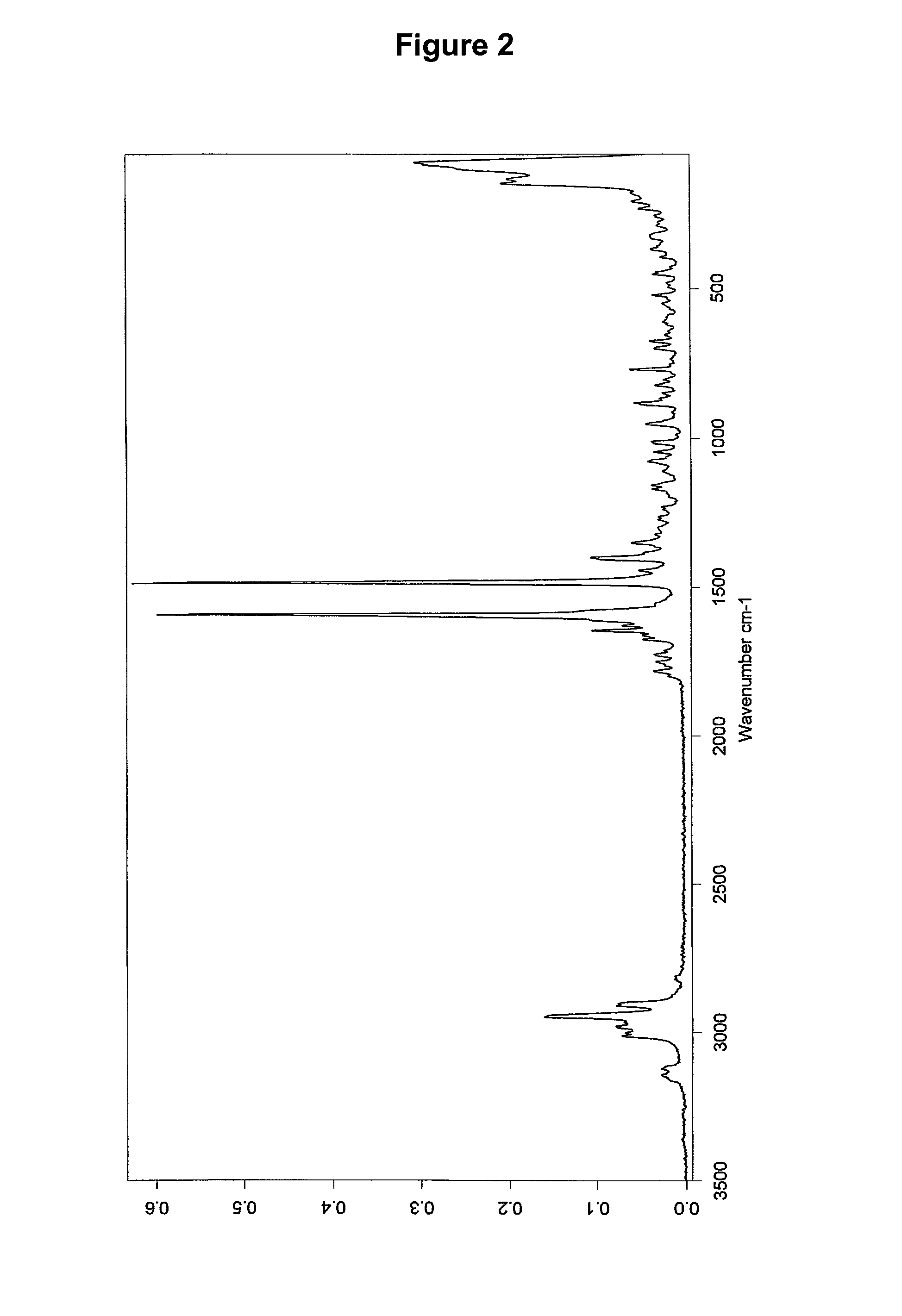
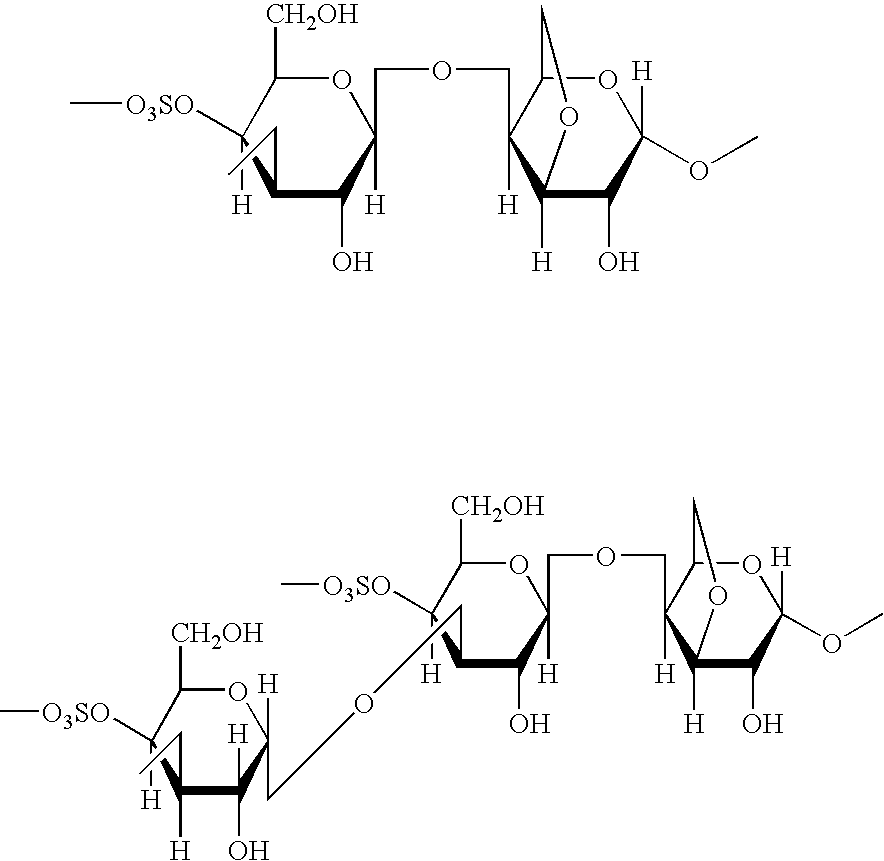
Dosage form with improved release of cefuroximaxetil
a technology of cefuroximaxetil and dosage form, which is applied in the field of dosage form with improved release of cefuroximaxetil, can solve the problems of affecting the use of amorphous form, affecting the effect of bioavailability, and affecting the effect of drug releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0107]Extruded Pellets of the Following Composition were Produced:
[0108]
Equivalent quantity ofcefuroxime per250 mgConstituentTotal150.5 mgCefuroximaxetil, microcrystalline, α-119.04 g modification150.5 mgCefuroximaxetil, amorphous119.04 g 60.2 mgTricalcium phosphate47.62 g120.4 mgGelcarin95.24 g 24.1 mgSugar ester S-157019.06 g505.7 mgTotal400.00 g
[0109]To this end, all the constituents were mixed in a Diosna mixer and wet granulated in this mixer. The moist granules were extruded in a Nica E142 extruder with a 0.5×0.5 mm die. The resultant extrudates were spheronised in a Nica S450 spheroniser. The resultant pellets were dried in a fluidised bed at 50° C. and then classified. Uncoated pellets with a particle diameter of 250-710 μm were used for determining cefuroximaxetil release.
[0110]Release was determined both in artificial gastric juice+Tween, and in artificial intestinal juice+SDS with in each case approx. 253 mg of uncoated pellets (total content of cefuroximaxetil approx. ...
example 2
[0119]Pellets of the Following Composition were Produced in a Similar Manner to Example 1:
[0120]
Equivalent quantity ofcefuroxime per250 mgConstituentTotal150.5 mgCefuroximaxetil, microcrystalline, α-129.18 gmodification150.5 mgCefuroximaxetil, amorphous129.18 g 34.0 mgTricalcium phosphate 29.19 g131.0 mgGelcarin112.45 g466.0 mgTotal400.00 g
[0121]Release of an Equivalent Dose of 125 or 62.5 mg of Cefuroxime in Artificial Gastric Juice+Tween 80:
[0122]
Average in %after125 mg cefuroxime62.5 mg cefuroxime30 min83.9103.060 min83.9103.290 min84.3103.1120 min 84.4102.7
[0123]The more rapid release at a dose of 62.5 mg is due to cefuroximaxetil's saturation solubility of 600 mg in the dissolution medium (1000 ml). Sink conditions may accordingly only be assumed up to a test dose of approx. 60 mg (max. 10% of the saturation solubility).
example 3
[0124]Pellets of the Following Composition were Produced in a Similar Manner to Example 1:
[0125]
Equivalent quantity ofcefuroxime per 250 mgConstituentTotal301.0 mgCefuroximaxetil,119.04 gamorphous 60.2 mgTricalcium phosphate 23.81 g120.4 mgGelcarin 47.62 g 24.1 mgSugar ester S-1570 9.53 g505.7 mgTotal200.00 g
[0126]Release in Artificial Gastric Juice+0.5% Tween 80 (Quantity used Corresponding to an Equivalent Dose of 125 mg of Cefuroxime):
[0127]
afterAverage in %30 min95.660 min100.490 min100.9120 min 101.0
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com