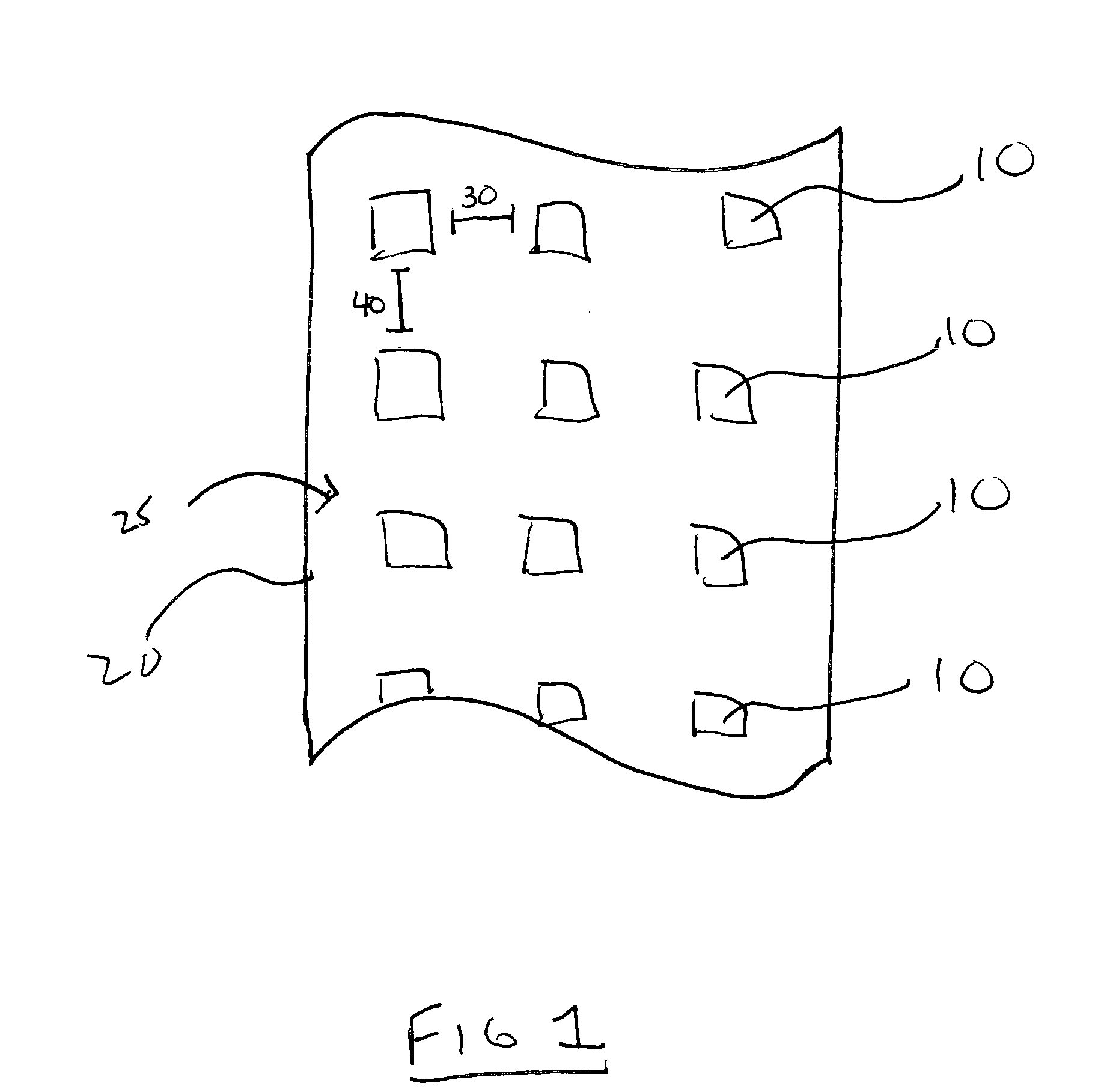
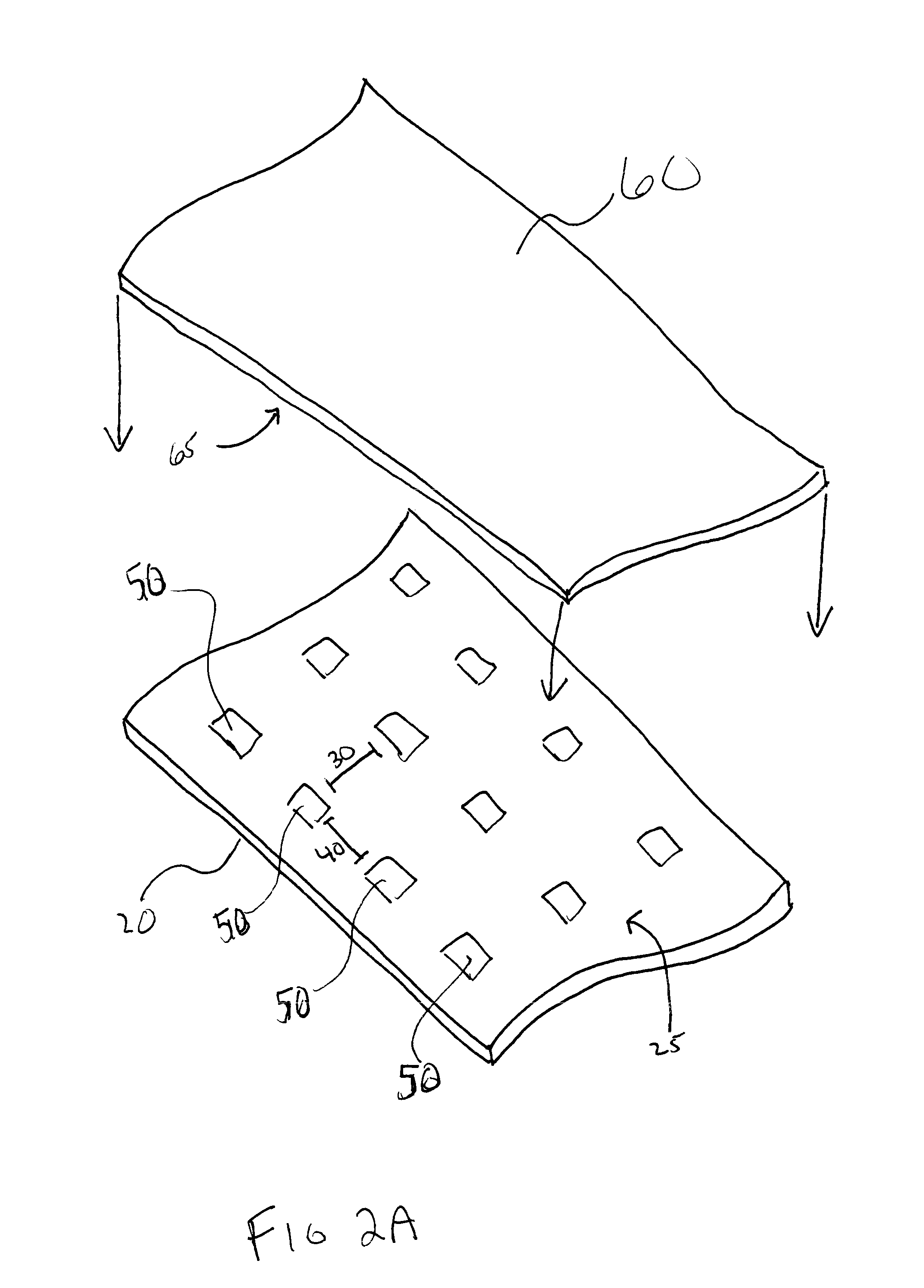
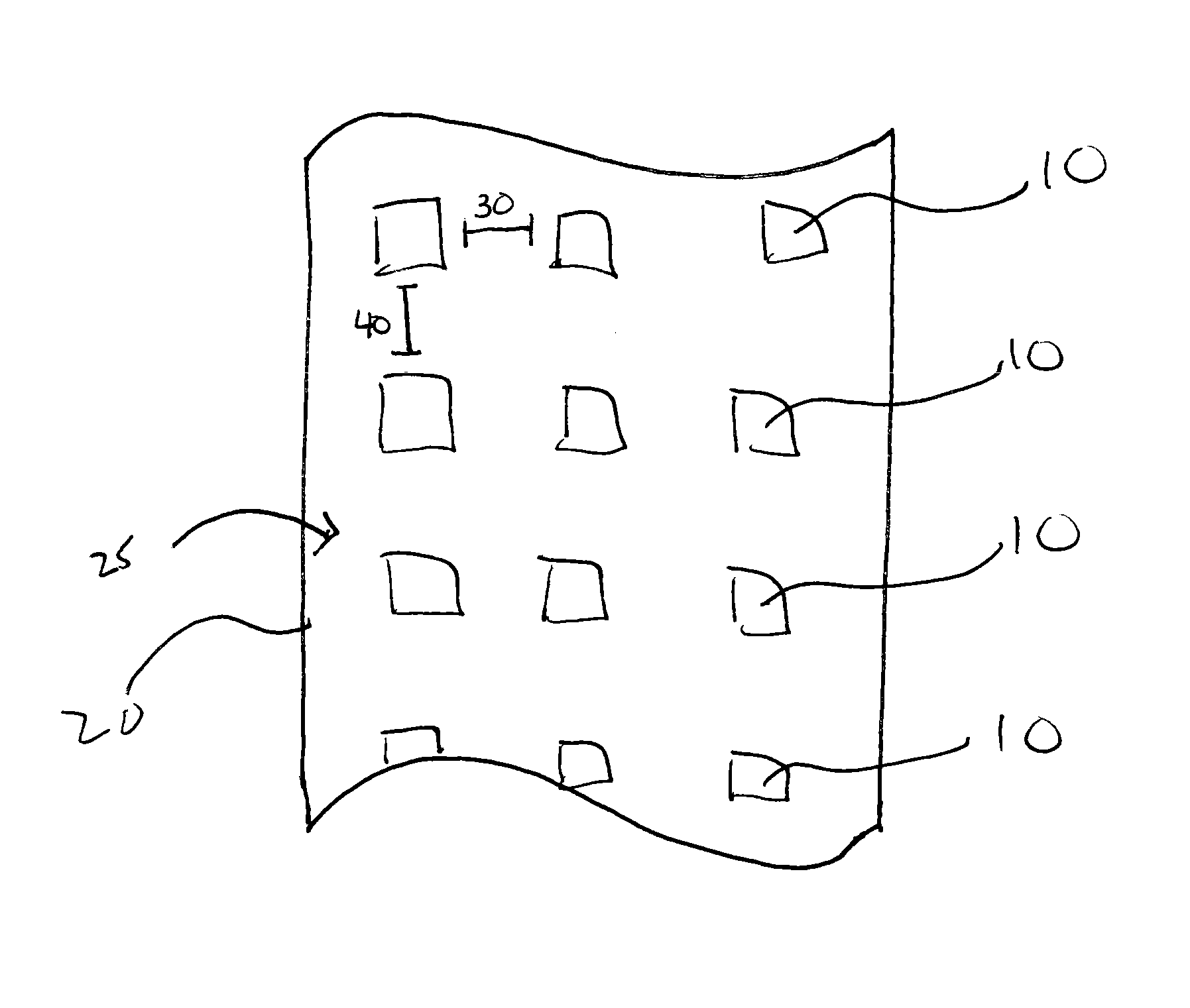
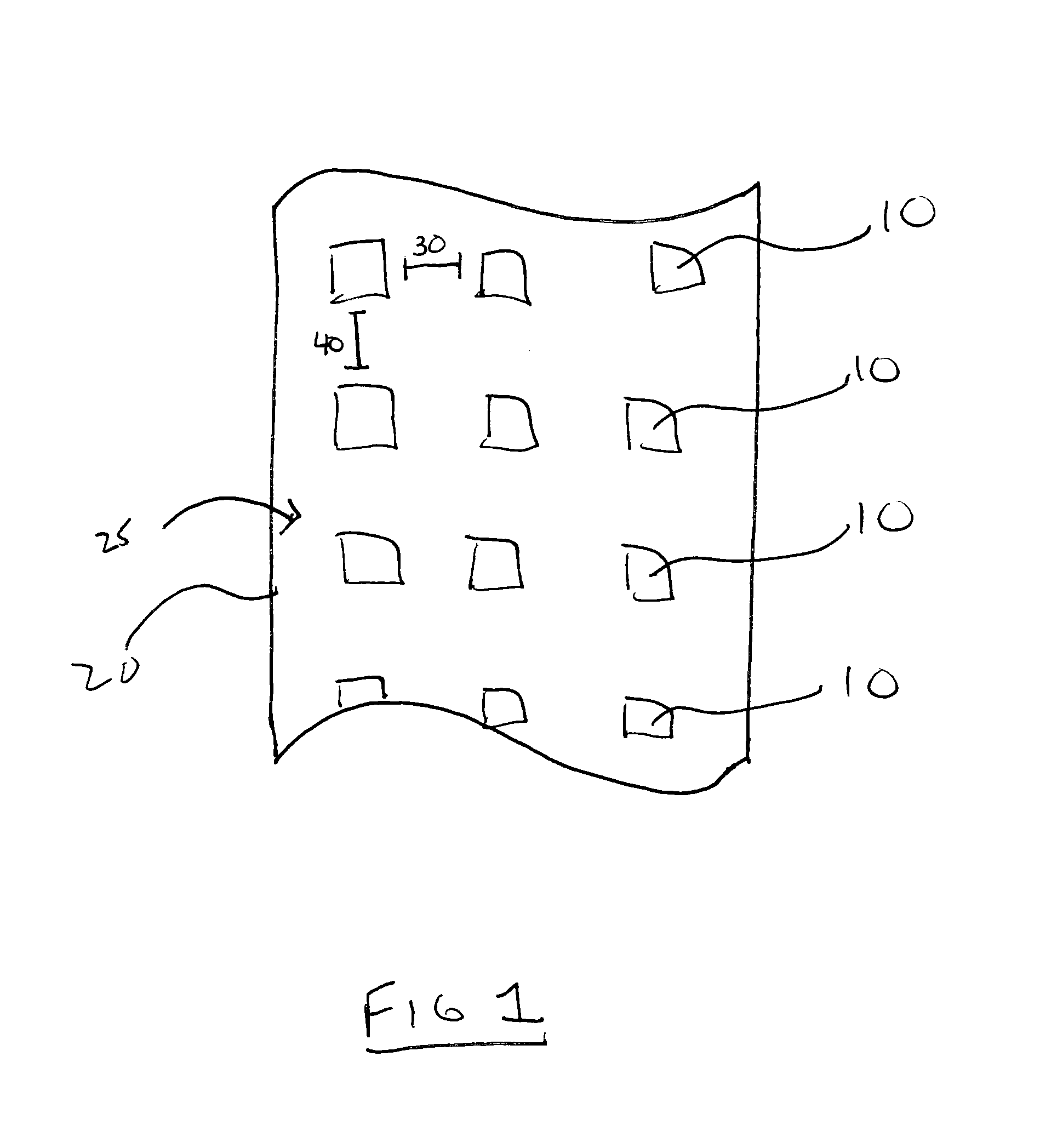
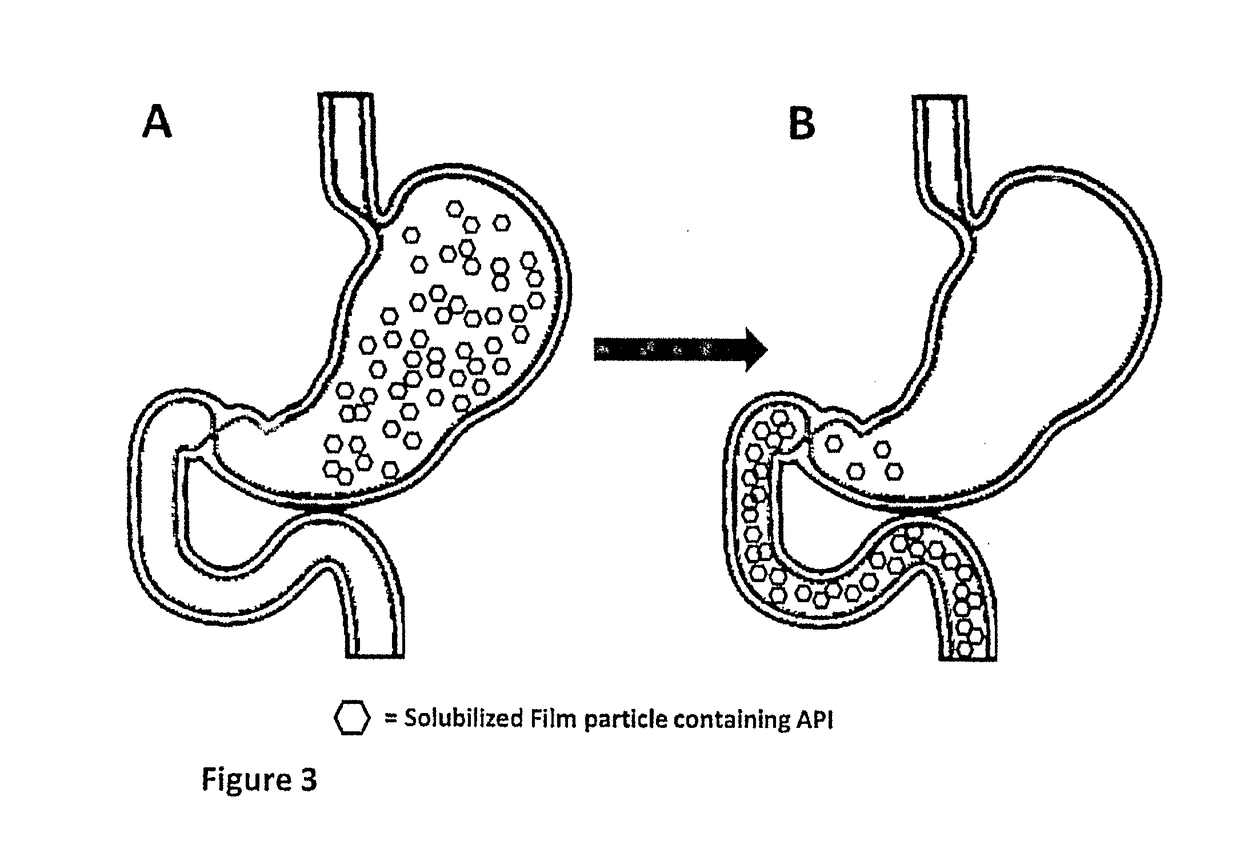
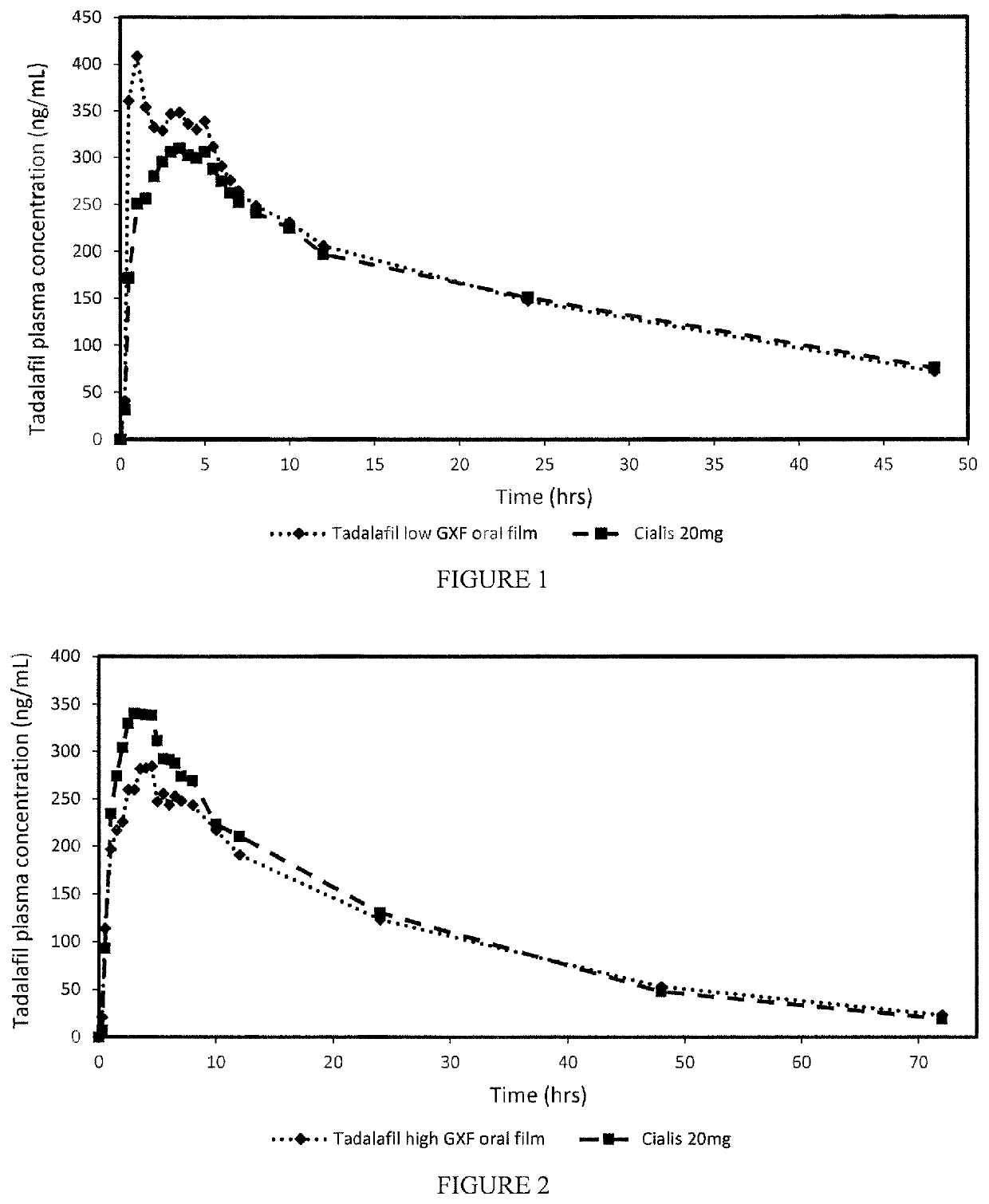
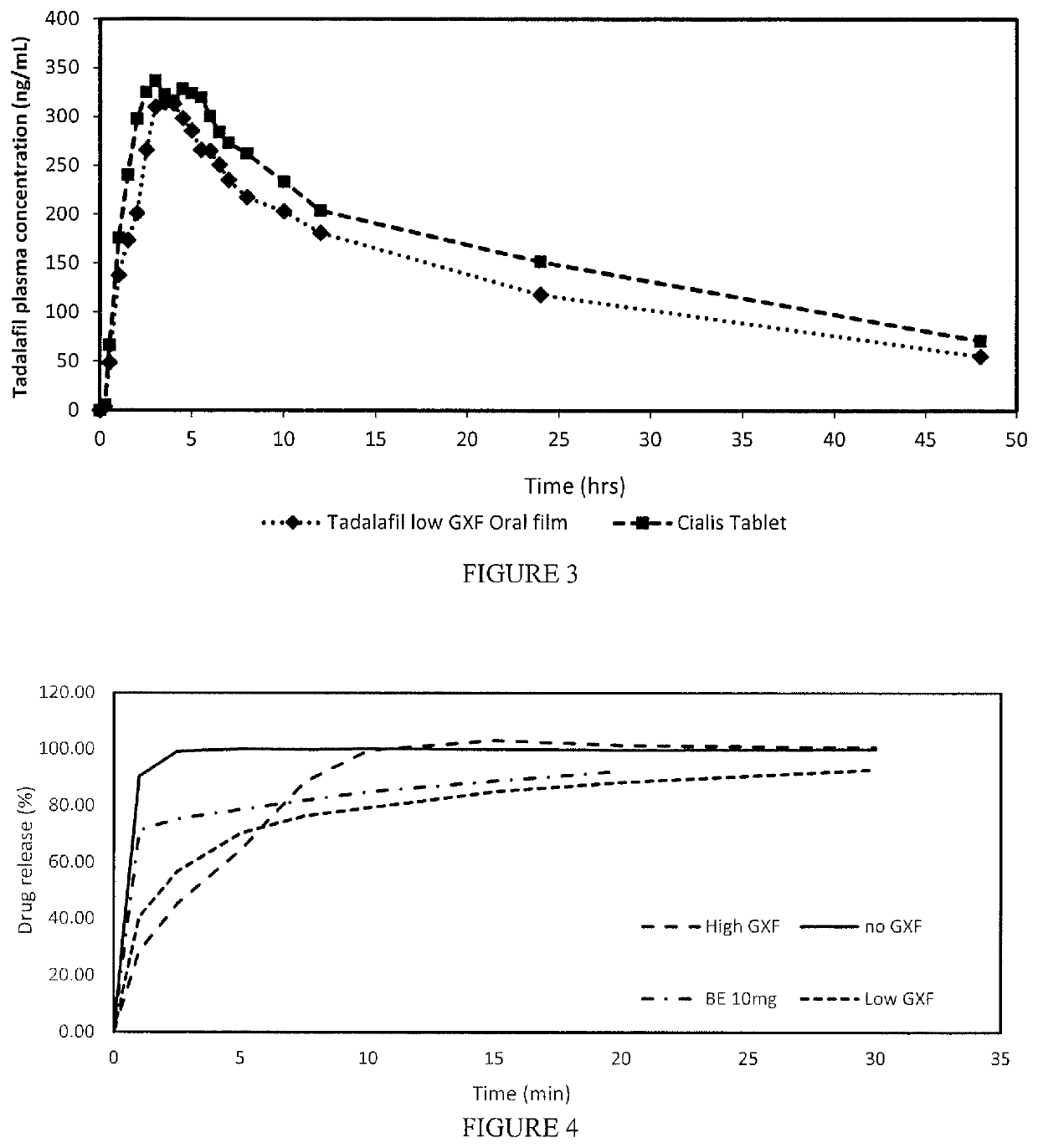
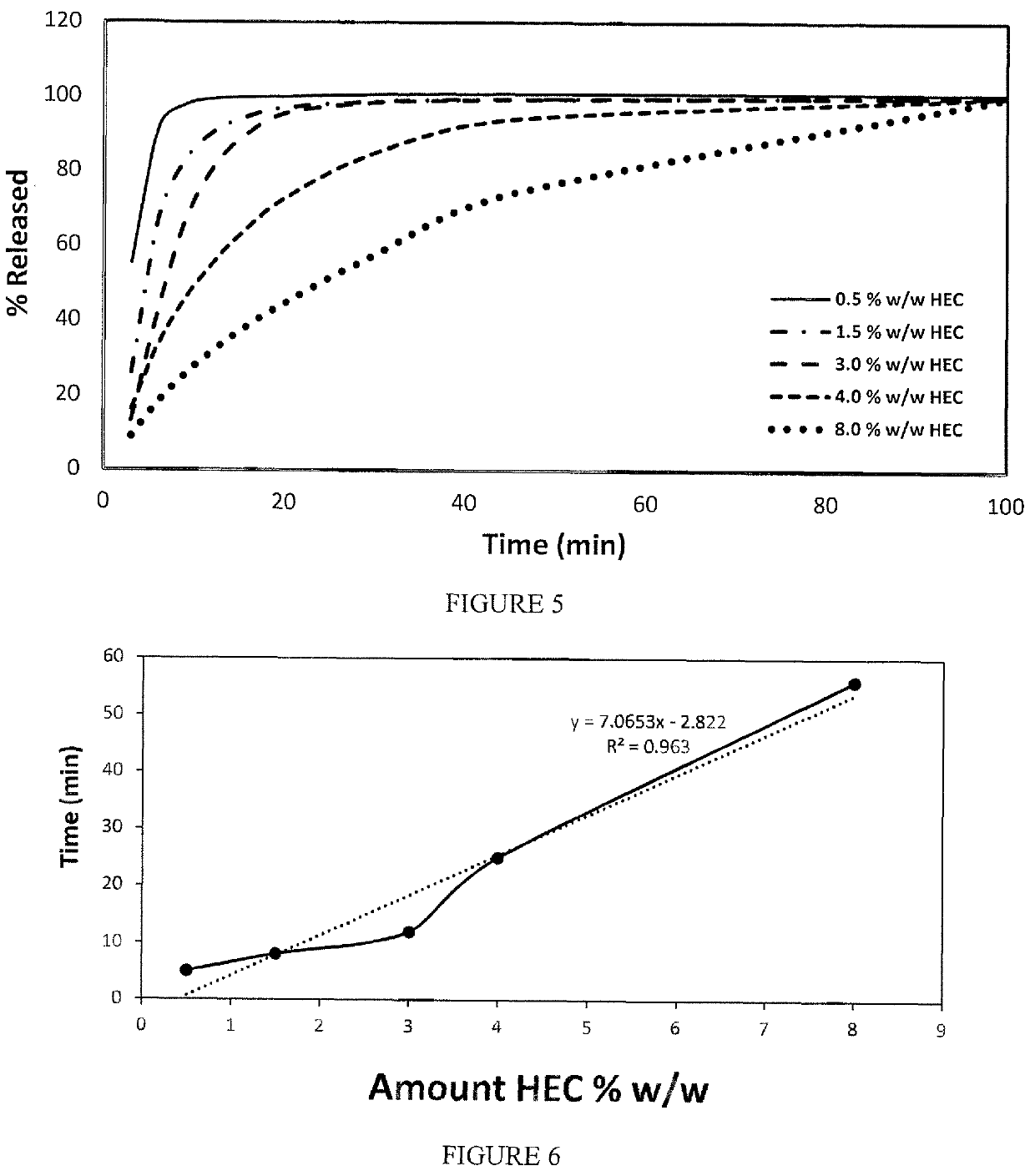
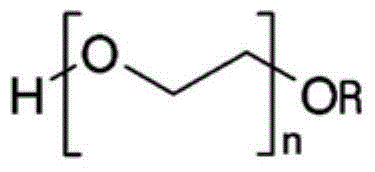Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Film Dosage Form" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
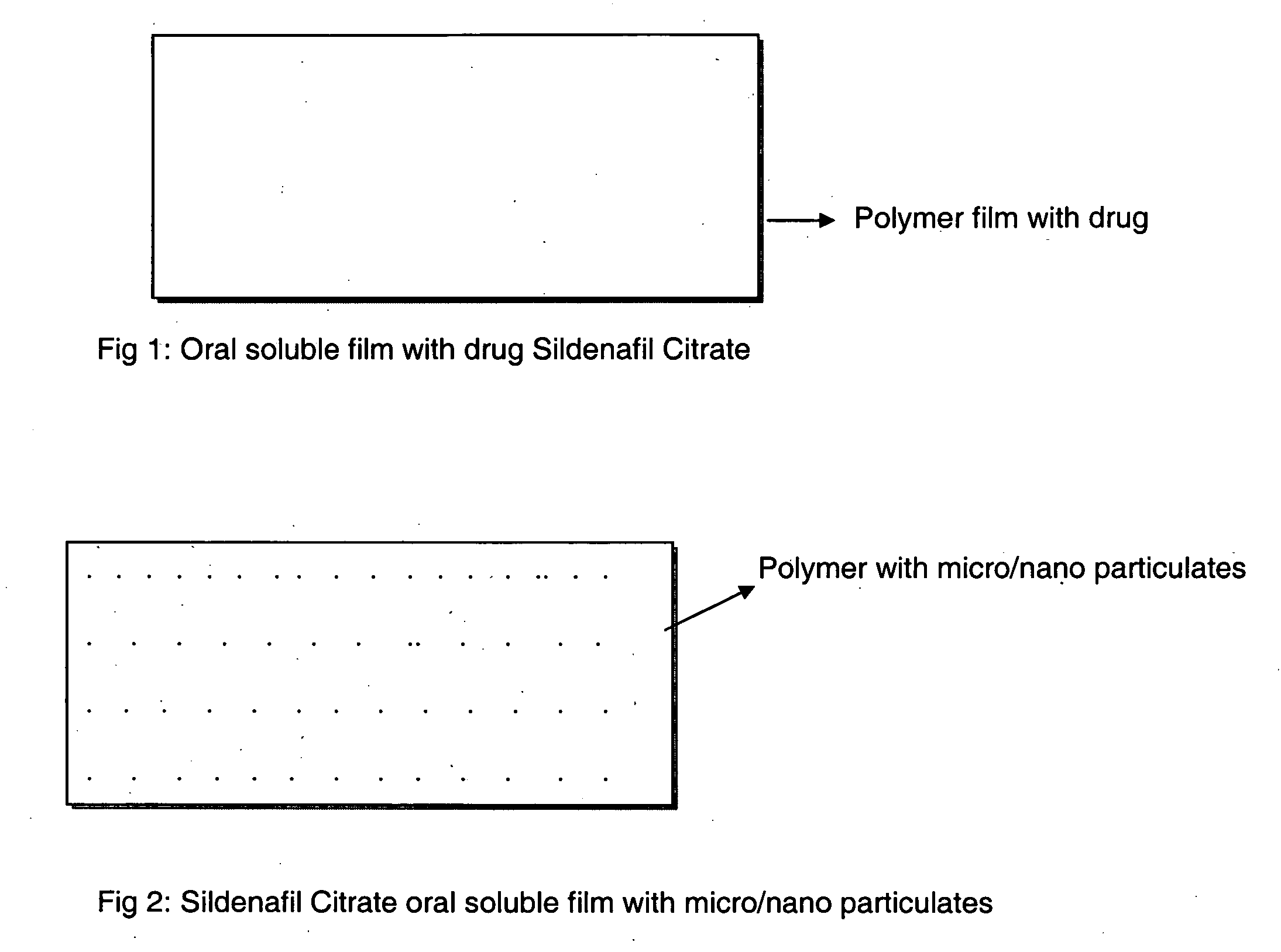
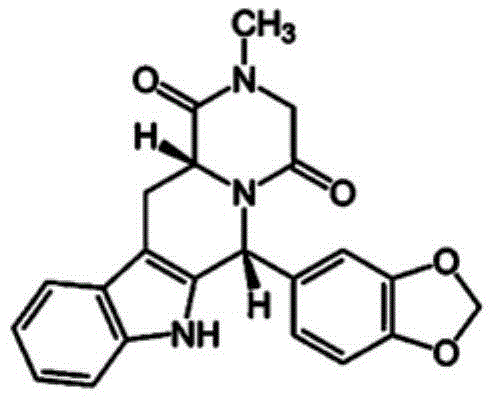
Oral fast dissolving films for erectile dysfunction bioactive agents
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
Oral film dosage form having indicia thereon
The present invention relates to rapidly dissolving edible film dosage form incorporating indicia. The indicia may correspond to an active ingredient that may be evenly distributed throughout the film. The indicia may be associated with at least one surface of the film composition and provide information to the consumer that is relevant to the edible film dosage form.
Owner:MONOSOL RX
Coating of complexed actives in film formulations
The present invention relates to products and methods of making products having a dual taste masked active component. In particular, the present invention relates to film dosage forms including at least one dual taste masked active, where the dual taste masked active includes a coated complexed active composition.
Owner:MONOSOL RX
Ondansetron film compositions
The present invention relates to products and methods of making products having a taste masked active component. In particular, the present invention relates to taste-masked film dosage forms including at least one active component and a slow dissolving basic composition.
Owner:MONOSOL RX
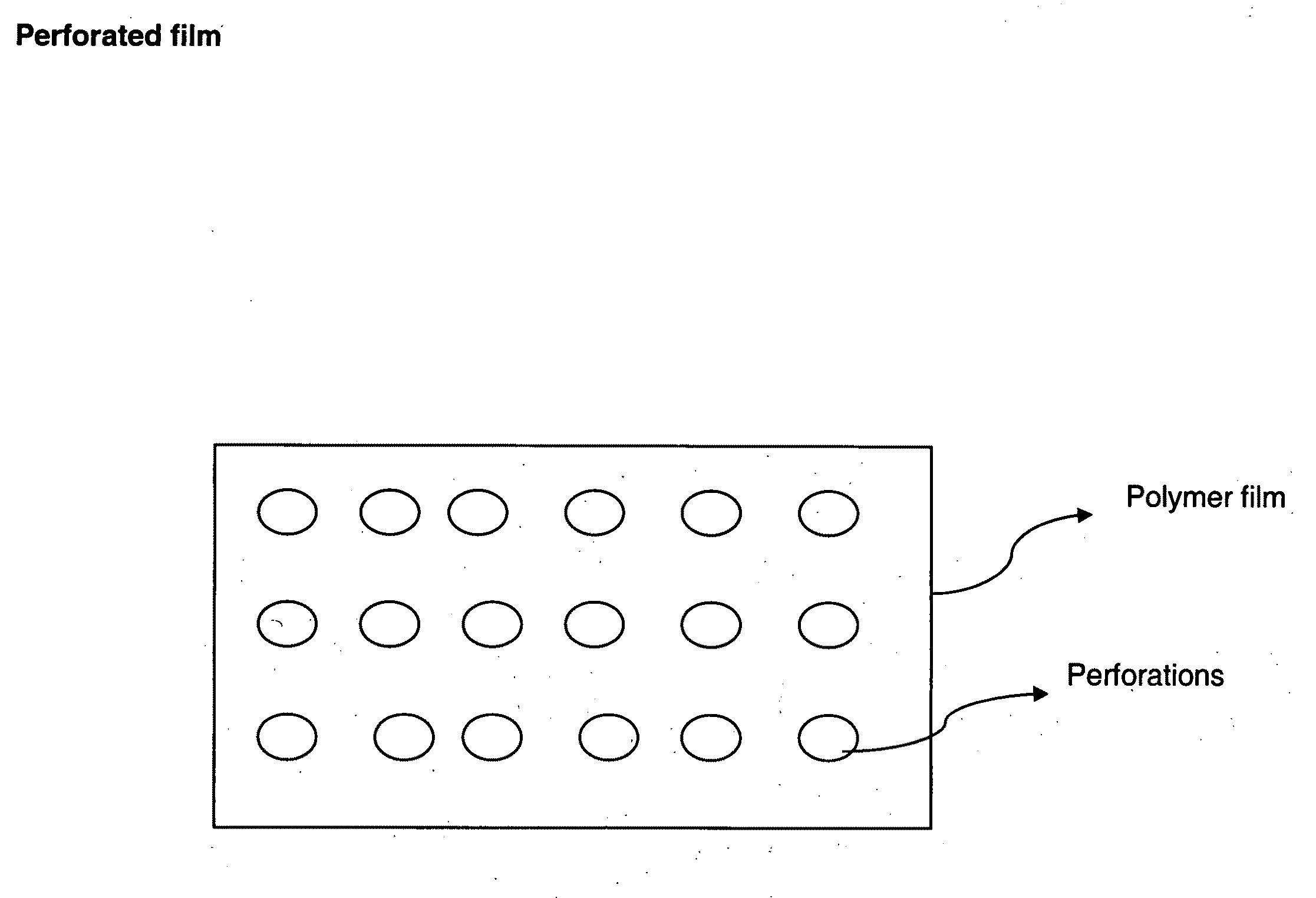
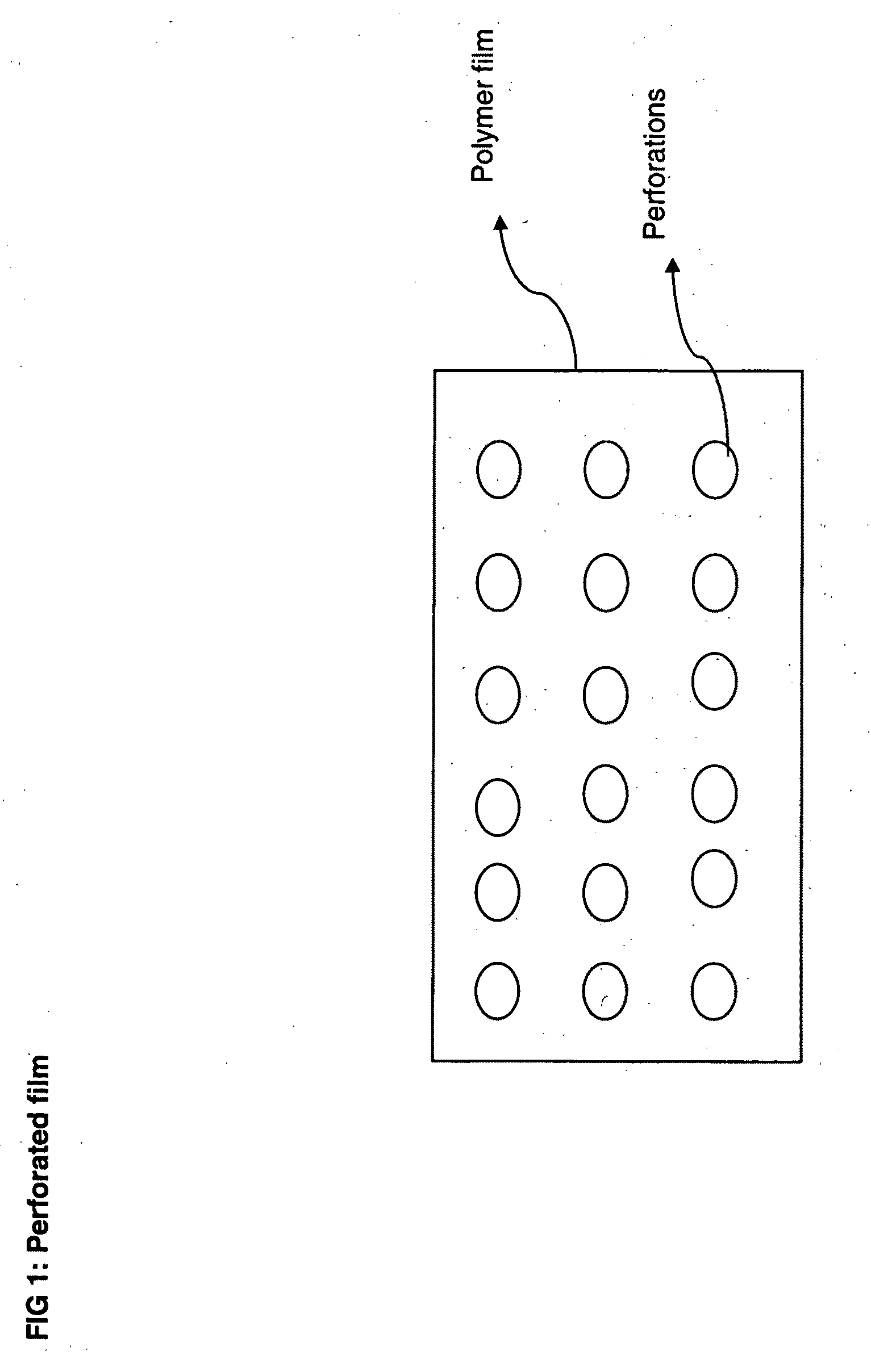
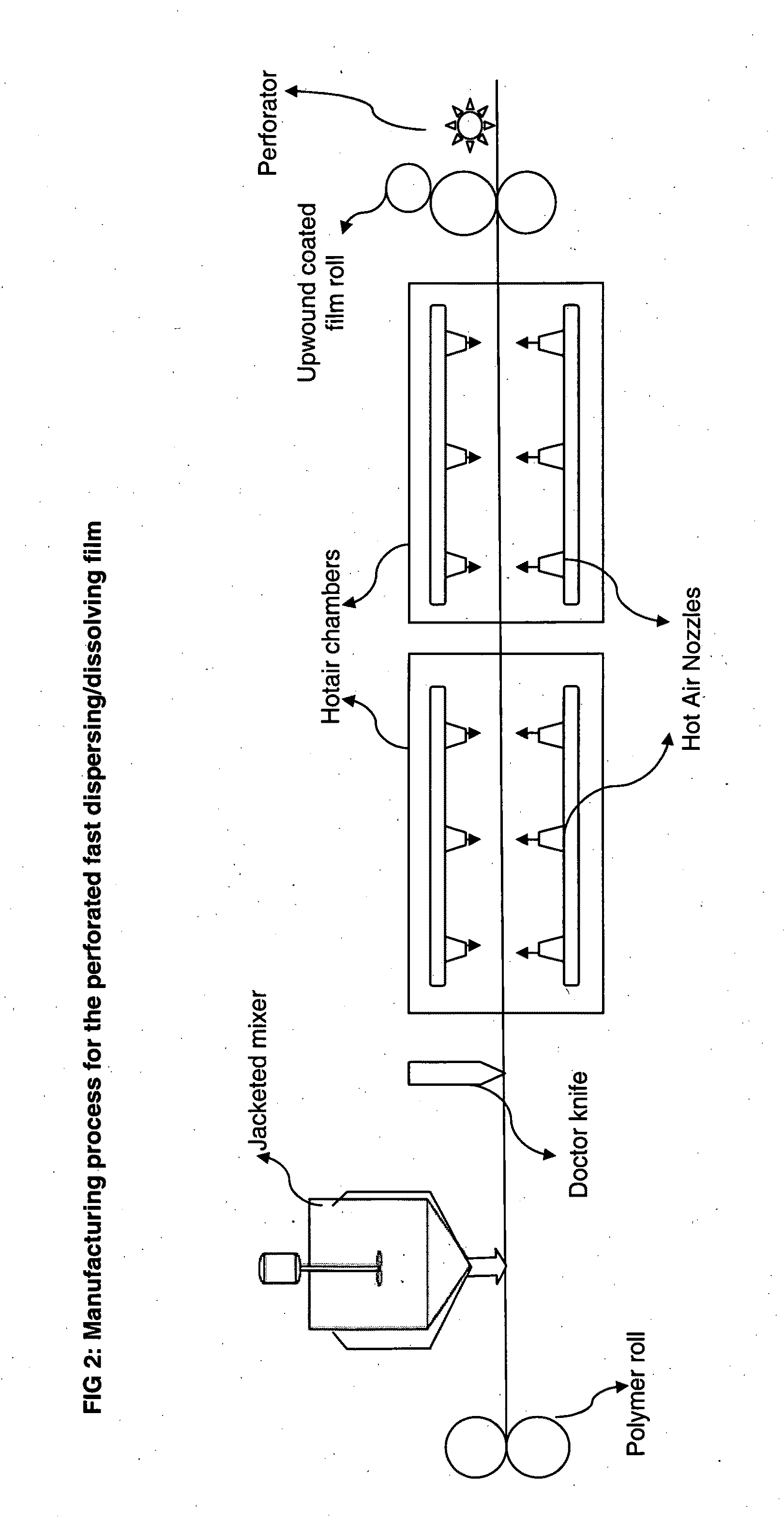
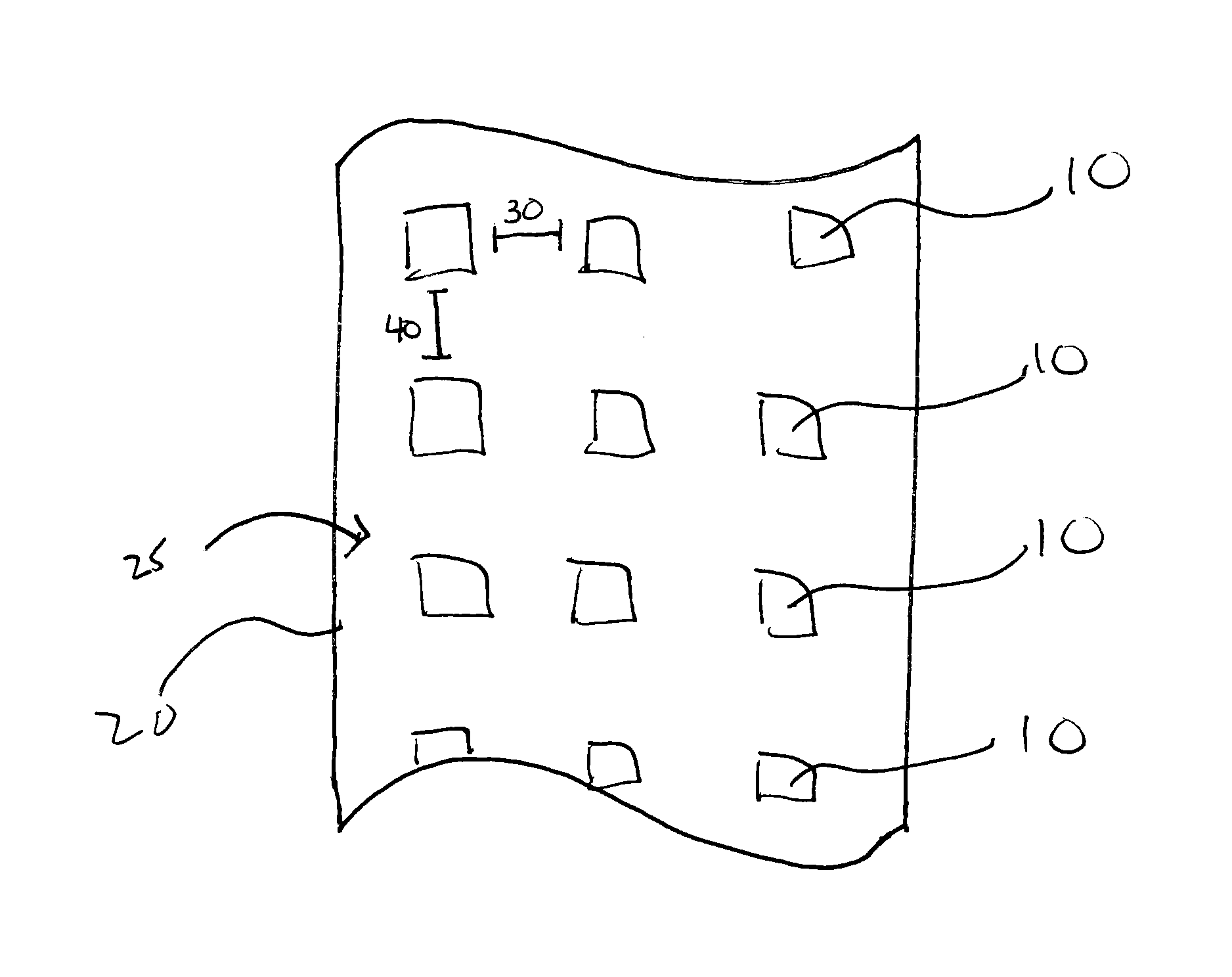
Perforated water soluble polymer based edible films
InactiveUS20090047350A1High drug loadingImprove abilitiesPowder deliveryOrganic active ingredientsSolubilityActive agent
Owner:BANGALORE RAMESH
Method and system for forming a pharmaceutical product directly onto a packaging surface
ActiveUS20120076921A1Improve sealingPretreated surfacesDermatological disorderFilm Dosage FormBiomedical engineering
The present invention relates to a method for forming a pharmaceutical product, such as a dissolvable film dosage form, onto a surface. Particularly, the present invention relates to a method of forming a pharmaceutical product directly onto the surface of a substrate.
Owner:AQUESTIVE THERAPEUTICS INC
Solid oral film dosage forms and methods for making same
InactiveUS20110136815A1Dissolve fastGood water solubilityBiocidePeptide/protein ingredientsCosmetic ingredientSublingual Absorption
Improved pharmaceutical solid oral film dosage forms for the buccal and / or sublingual delivery of pharmaceutical, nutraceutical or cosmetic ingredients are endowed with instant hydration potential and complete dissolution potentially enabling the active ingredient to become immediately available for enhanced buccal and / or sublingual absorption and / or reduced absorption through the gastrointestinal route. The improved delivery systems for solubilizing and stabilizing pharmaceutically active ingredients exhibit enhanced stability by the use of a combination of crystallization inhibitors, which together can maintain the active ingredient in a desired plurality of particles in an effective size range within a polymeric film matrix.
Owner:INTELGENX CORP
Method and system for forming a pharmaceutical product directly onto a packaging surface
ActiveUS8936825B2Improve sealingPowder deliveryPretreated surfacesFilm Dosage FormBiomedical engineering
The present invention relates to a method for forming a pharmaceutical product, such as a dissolvable film dosage form, onto a surface. Particularly, the present invention relates to a method of forming a pharmaceutical product directly onto the surface of a substrate.
Owner:AQUESTIVE THERAPEUTICS INC
Film dosage form with extended release mucoadhesive particles
ActiveUS20180360736A1Avoid discomfortEasy to controlHydroxy compound active ingredientsPharmaceutical delivery mechanismOral medicationFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
Film dosage forms containing amorphous active agents
InactiveUS20160243036A1Good dispersionAvoid rough feelingSalicyclic acid active ingredientsPeptide/protein ingredientsSolubilityActive agent
Oral thin film dosage form of a stable dispersion of non-solubilized amorphous or partially amorphous active agent(s), having a mean particle size diameter D50 equal or lower than 250 μm, that remains uniformly distributed within a film matrix and contains at least one film former polymer, and optional pharmaceutically-acceptable excipients, such as diluents, plasticizers, surfactants, sweeteners, and taste-masking agent(s). The oral thin film dosage exhibits increased solubility or rate of dissolution and enhanced bioavailability compared to a crystalline form of the active agent(s). The oral dosage form also exhibits long term stability confirmed by no changes in the dissolution profile over time.
Owner:INTELGENX CORP
Orally administrable film dosage forms containing ondansetron
The invention relates to orally administrable, disintegrating film dosage forms which include ondansetron and methods of orally administering the film dosage forms.
Owner:MONOSOL RX
Film dosage forms containing amorphous active agents
InactiveUS20160324773A1Preventing and retarding and growthPreventing and retarding nucleationSalicyclic acid active ingredientsPeptide/protein ingredientsActive agentMedicine
Oral thin film dosage form of a stable dispersion of non-solubilized amorphous or partially amorphous active agent(s), having a mean particle size diameter D50 equal or less than 250 μm, that remains uniformly distributed within a film matrix and contains at least one film forming polymer, and optional pharmaceutically-acceptable excipients, such as diluents, plasticizers, surfactants, sweeteners, and taste-masking agent(s), are prepared by a process including first providing the active agent in an amorphous particle form having a mean particle size diameter D50 equal or less than 250 μm. Next, the active agent is suspended in a liquid film-forming formulation without dissolving the active agent. Therefore, the solvent is removed to form a film.
Owner:INTELGENX CORP
Pharmaceutical microemulsion immobilized in a thin polymer matrix and methods of making them
ActiveUS20160317462A1Dissolve fastPeptide/protein ingredientsSulfonylurea active ingredientsMedicineAdditive ingredient
The invention comprises a ready to use film dosage form comprising microemulsion of an Active Pharmaceutical Ingredient embedded or immobilized in a thin polymeric matrix as a double microemulsion and a process of making the same. The microemulsion in the film dosage form of this invention is capable of being absorbed through mucosal route. The process of making the film dosage of this invention comprises steps of forming a film forming dispersion containing film forming polymers, excipients and microemulsion of active pharmaceutical ingredient, casting the same in the form of a film and drying the cast of the film being carried out by means of drying conditions that suit to retain stability of the active pharmaceutical ingredient being selected such that drying of the film is achieved retaining the moisture trapped in the microemulsion embedded in the polymeric film.
Owner:ZIM LAB LTD
Ondansetron film compositions
The present invention relates to products and methods of making products having a taste masked active component. In particular, the present invention relates to taste-masked film dosage forms including at least one active component and a slow dissolving basic composition.
Owner:MONOSOL RX
Oral film dosage form having physical-chemical identifier thereon
InactiveUS20130266520A1StampsPharmaceutical product form changeFilm Dosage FormBULK ACTIVE INGREDIENT
Owner:MONOSOL RX
Orally administrable film dosage forms containing ondansetron
The invention relates to orally administrable, disintegrating film dosage forms which include ondansetron and methods of orally administering the film dosage forms.
Owner:MONOSOL RX
Solid oral film dosage forms and methods for making same
ActiveUS20170157119A1Dissolve fastEnhanced local absorptionOrganic active ingredientsPharmaceutical delivery mechanismPlasticizerFatty acid glycerol esters
Oral film dosage forms that provide improved solubilization and stabilization of an active ingredient in particle form include at least one primary crystallization inhibitor in an amount that inhibits growth and / or agglomeration of the active ingredient, a polyoxyethylated fatty acid glycerides in an amount that further enhances inhibition of crystallization, growth and agglomeration of the particles of the pharmaceutically active ingredient; and at least one plasticizer present in an amount that is effective to increase flexibility and elasticity of the film dosage form.
Owner:INTELGENX CORP
Film dosage form with extended release mucoadhesive particles
ActiveUS9668970B2Avoid discomfortEasy to controlPharmaceutical delivery mechanismEther/acetal active ingredientsPack Dosage FormFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
Solid oral film dosage forms and methods for making same
ActiveUS20130137698A1Dissolve fastGood water solubilityBiocidePharmaceutical delivery mechanismCosmetic ingredientSublingual Absorption
Improved pharmaceutical solid oral film dosage forms for the buccal and / or sublingual delivery of pharmaceutical, nutraceutical or cosmetic ingredients are endowed with instant hydration potential and complete dissolution potentially enabling the active ingredient to become immediately available for enhanced buccal and / or sublingual absorption and / or reduced absorption through the gastrointestinal route. The improved delivery systems for solubilizing and stabilizing pharmaceutically active ingredients exhibit enhanced stability by the use of a combination of crystallization inhibitors, which together can maintain the active ingredient in a desired plurality of particles in an effective size range within a polymeric film matrix.
Owner:TEXAS INSTR INC
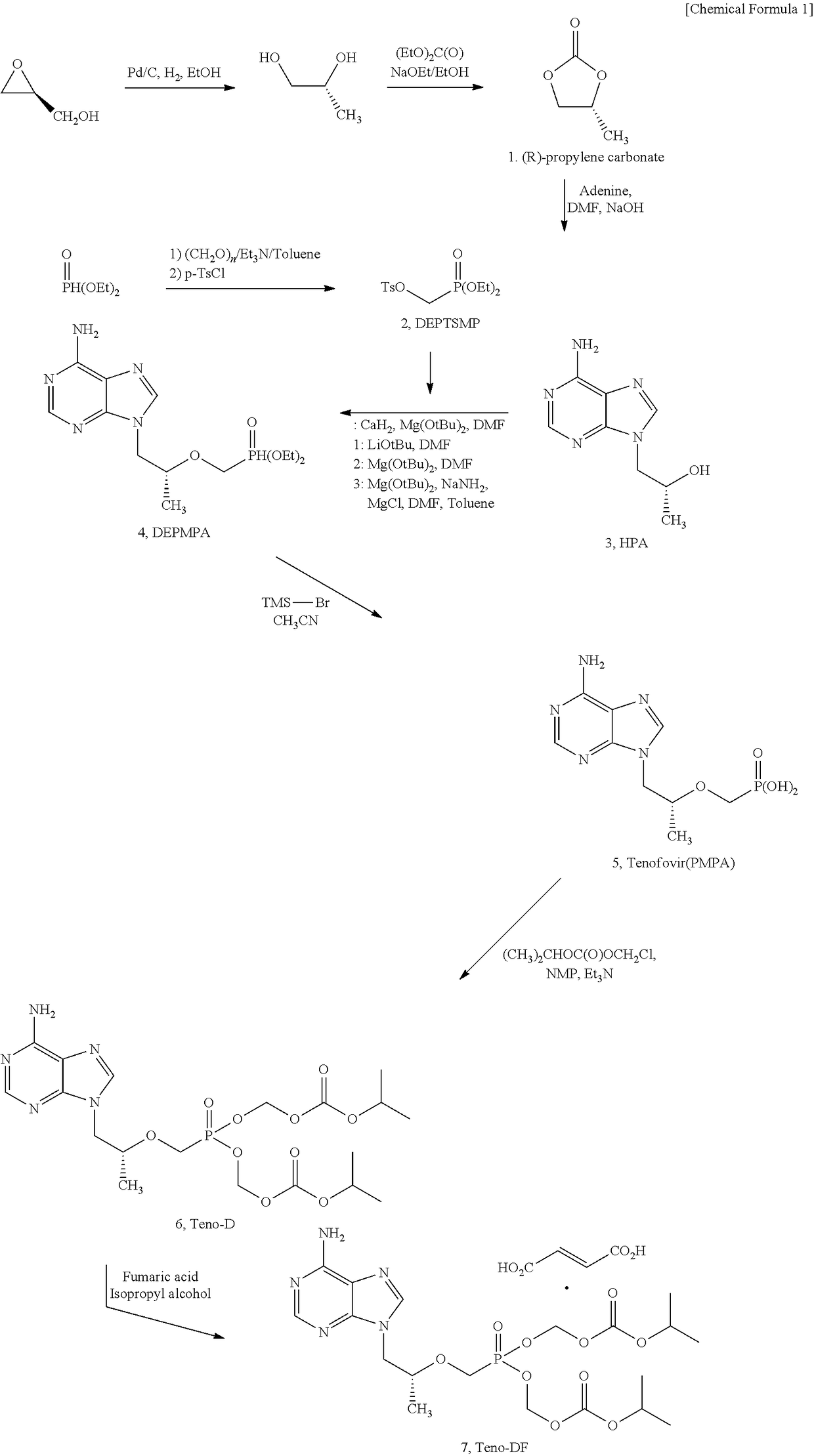
Synthesis Method For Improved Tenofovir Disoproxil Fumarate Using Ion-Exchange Resin And Method For Preparing Oral Dissolving Film Form Using The Same
InactiveUS20170354668A1Keep dryHigh purityAntiviralsPharmaceutical non-active ingredientsAdditive ingredientSynthesis methods
The present invention relates to a synthesis method of preventing the formation of impurities and byproducts in the synthesis of tenofovir disoproxil fumarate (Teno-DF) used as a medicine for hepatitis B and HIV treatment due to its function to promote bioactivities. In the synthesis method of the present invention, an ion-exchange resin (Dowex 50W hydrogen form, sulfonic acidic cation exchange resin) is used to enhance the yield and purity of the compound. The present invention also relates to a method of preparing an oral dissolving film dosage form in the manufacture of a medicine using the tenofovir compound with high purity obtained by the synthesis method of the present invention as an effective ingredient.
Owner:PERSON CO LTD
Method of treatment and device for the improved bioavailability of leukotriene receptor antagonists
InactiveUS20190133925A1Improve bioavailabilityInorganic non-active ingredientsPharmaceutical delivery mechanismDiseaseFilm Dosage Form
Disclosed is a method of administration and device for the improved bioavailability of leukotriene receptor antagonists. This method and device involve an alkaline surface pH oral film dosage form designed to deliver leukotriene receptor antagonists, such as Montelukast, to the stomach in an amorphous precipitate suspended in aqueous medium. Also disclosed is a device and method for treating a disease, such as a neurodegenerative disease or condition associated with neuroinflammation induced by a leukotriene. The device is a film unit dosage form having an alkaline surface pH film layer and a safe and effective amount of Montelukast. The device is configured and formulated to predominantly achieve enteral delivery of the Montelukast. The method includes enterally delivering to a human or an animal in need of treatment, a safe and effective amount of Montelukast capable of crossing the blood-brain barrier.
Owner:INTELGENX CORP
Film dosage form with extended release mucoadhesive particles
ActiveUS10272038B2Avoid discomfortEasy to controlPharmaceutical delivery mechanismPharmaceutical non-active ingredientsOral medicationFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
Traditional Chinese medicine film spraying agent for promoting blood circulation, stopping bleeding, removing necrotic tissues and promoting tissue regeneration, and preparation method of traditional Chinese medicine film spraying agent
PendingCN110680879APromote secretionInhibitory activityHydroxy compound active ingredientsAntipyreticCoptisNecrotic tissue
The present invention relates to a traditional Chinese medicine film spraying agent for promoting blood circulation, stopping bleeding, removing necrotic tissues and promoting tissue regeneration. Thetraditional Chinese medicine film spraying agent is prepared from the following components in 1,000 ml: Chinese rhubarb, coptis root, pseudo-ginseng, radix angelicae, donkey-hide gelatin, rhizoma bletillae, vinegar myrrh, radix rubiae, resina draconis, liquorice, pearl, borneol, a film forming material, a plasticizer, an aromatic, a preservative and 95% ethanol. Zhikang capsule is creatively improved into an external spraying film dosage form, during use, a spraying head of a spraying bottle is aimed at affected parts of traumatic injury for spraying, sprayed liquid medicine is quickly formedinto a film, so that effective components of the medicines are favorably soaked into the affected parts to generate a biochemical reaction, the medicines are not liable to lose, and a better treatment effect is generated; and the film spraying agent has obvious effects of promoting blood circulation, stopping bleeding, relieving swelling and pains, removing necrotic tissues, promoting tissue regeneration, inhibiting micro-organisms and diminishing inflammation, simultaneously is free of any hormone medicines, has advantages of high safety and low side effect, simultaneously sprays the liquidmedicine to form the film quickly, the effective components of the medicines are not liable to lose, and the better treatment effect is generated.
Owner:XIAN CHIHO PHARMA
Film dosage form with extended release mucoadhesive particles
ActiveUS20170239172A1Improve permeabilityAvoid discomfortPharmaceutical non-active ingredientsSheet deliveryPack Dosage FormFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
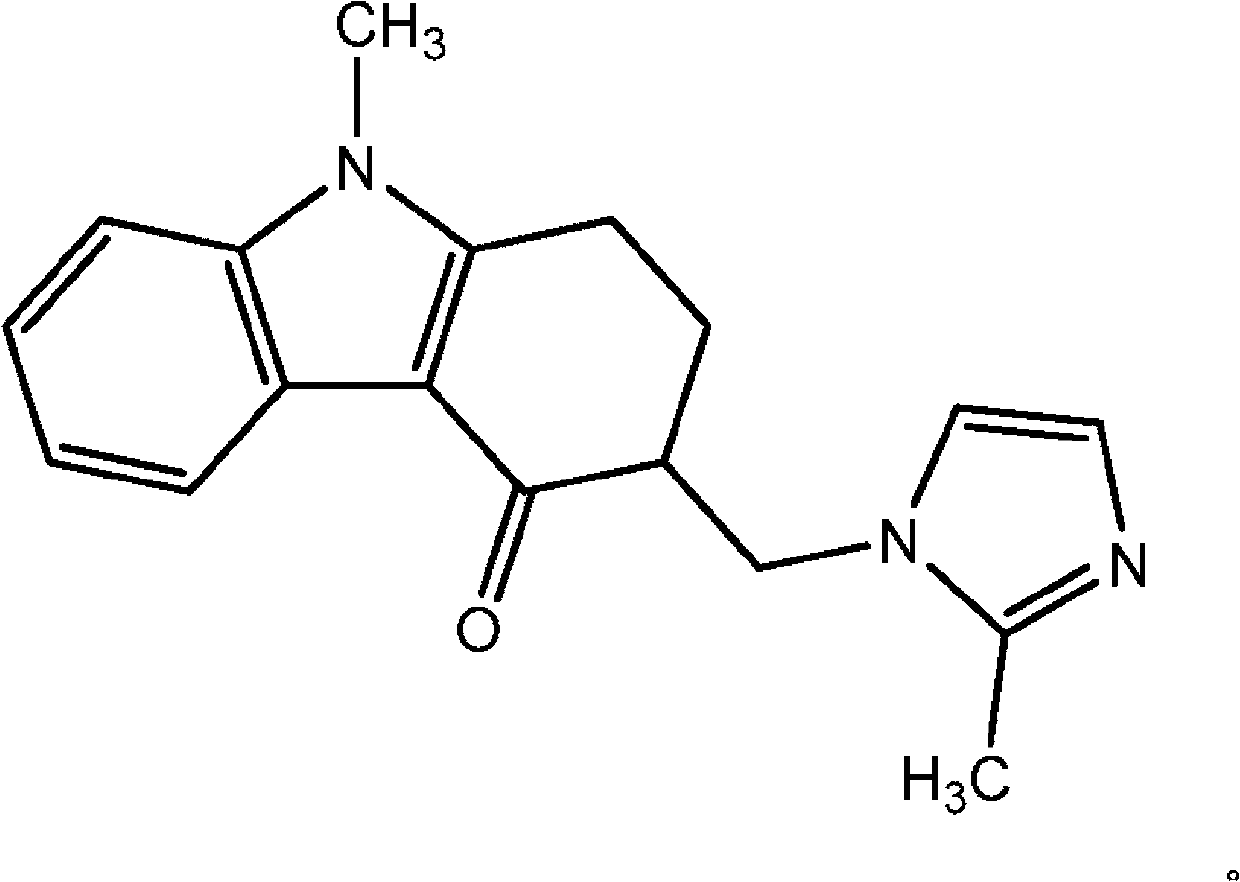
Tadalafil free base-containing film dosage form containing polyethylene glycol-based polymer and/or vinyl pyrrolidone-based polymer as dispersion stabilizer
InactiveUS20160074396A1Maximized dispersion stabilityReduce the amount requiredBiocidePharmaceutical non-active ingredientsDispersion stabilityOral medication
Owner:CTC BIO INC
Film dosage form with extended release mucoadhesive particles
ActiveUS20150150786A1Easy to controlAvoid discomfortBiocideEther/acetal active ingredientsPack Dosage FormFilm Dosage Form
An orally administered dosage form that facilitates delivery of an agent locally in the buccal cavity for a sustained period of time includes mucoadhesive particles that are made of at least a mucoadhesive material combined with the agent, and which are dispersed in a disintegrating film. The dosage form is capable of delivering an agent to a patient at the desired oral mucosa site over an extended period of time while reducing patient discomfort or annoyance associated with conventional sustained release mucoadhesive films that must reside on the oral mucosa during the period of sustained release.
Owner:INTELGENX CORP
Method of treatment and device for the improved bioavailability of leukotriene receptor antagonists
InactiveUS20180250240A1Improve bioavailabilityPharmaceutical non-active ingredientsSheet deliveryDiseaseBioavailability
Disclosed is a method of administration and device for the improved bioavailability of leukotriene receptor antagonists. This method and device involve an alkaline surface pH oral film dosage form designed to deliver leukotriene receptor antagonists, such as Montelukast, to the stomach in an amorphous precipitate suspended in aqueous medium. Also disclosed is a device and method for treating a disease, such as a neurodegenerative disease or condition associated with neuroinflammation induced by a leukotriene. The device is a film unit dosage form having an alkaline surface pH film layer and a safe and effective amount of Montelukast. The device is configured and formulated to predominantly achieve enteral delivery of the Montelukast. The method includes enterally delivering to a human or an animal in need of treatment, a safe and effective amount of Montelukast capable of crossing the blood-brain barrier.
Owner:INTELGENX CORP
Oral film formulation for modulating absorption profile
ActiveUS10828254B2Extending in vivo absorption and thus bioavailabilityMitigating the quick onset and high COrganic active ingredientsPharmaceutical delivery mechanismMedicineActive agent
An oral film dosage form includes a high viscosity polymer in an amount of from 1% to 5% by dry weight to reduce, modulate and / or control Cmax of an active agent. The high viscosity polymer has a viscosity of from 100 cps to 500 cps as determined at 2% concentration in water by weight at 25° C. using a Brookfield LVF viscometer with spindle no. 2 at 60 rpm.
Owner:INTELGENX CORP
Orally administrable, self-supporting dissolving film dosage forms
InactiveUS20140275148A1Relieve symptomsBiocidePharmaceutical non-active ingredientsPolyvinyl alcoholFilm Dosage Form
Orally administrable, self-supporting, dissolving film dosage forms comprising an active ingredient and a polyvinyl alcohol-polyethylene glycol graft copolymer, and methods of orally administering the film dosage forms are provided.
Owner:NOVUS PHARMA
Tadalafil free base-containing film dosage form containing polyethylene glycol-based polymer and/or vinyl pyrrolidone-based polymer as dispersion stabilizer
ActiveCN105209025AReduce clumpingImprove dispersion stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDispersion stabilityOral medication
The present invention relates to a film preparation for oral administration, containing tadalafil free base, and a preparation method therefor. The invention provides a film. It is possible to: maximize the dispersion stability of tadalafil free base in the film by using a small amount of a dispersion stabilizer without inducing a specific odor or taste which can occur when using other dispersion stabilizers known in the relevant art; provide a film having an extremely low rate of tadalafil free base particle reaggregation; and remarkably reduce the amount of bubbles during a preparation process.
Owner:CTC BIO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com