Preparation method for vaccine of porcine circovirus II
A technology for preparing porcine circovirus and vaccine, which is applied in the field of animal virus and porcine circovirus type II vaccine preparation, can solve the problems of affecting cell growth, high production cost, low single batch yield, etc., and achieves precise control of process parameters, The effect of product cost reduction and product quality stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 tidal type microcarrier suspension culture technology high-density cultivation of PK15 cells and porcine circovirus type II (PCV2)
[0033] The bioreactor used was TideCell-020 from CESCO, USA, and the carrier used was BioNocII polyester fiber from CESCO, USA.
[0034] (1) PK15 cells 4.0×10 9 The cell was inoculated in a 20L carrier tank and the carrier BioNocII polyester fiber 2.2×10 2 g, and DMEM growth medium containing 6% calf serum with a working volume of 500 L, cultivated by tidal microcarrier suspension culture technology until the total number of cells reaches 4.0×10 10 cell; when culturing cells, start the attachment program first, and switch to the culture program after 4 hours; the cell attachment program is: up: 2500mL / min hold 1min, Down: 2500mL / min hold 30s, set the maximum exchange volume of 18500mL; cell culture program : Up: 1900mL / min hold 1min, Down 1900mL / min hold 1min, set the maximum exchange volume of 18000mL;
[0035] (2) All the...
Embodiment 2
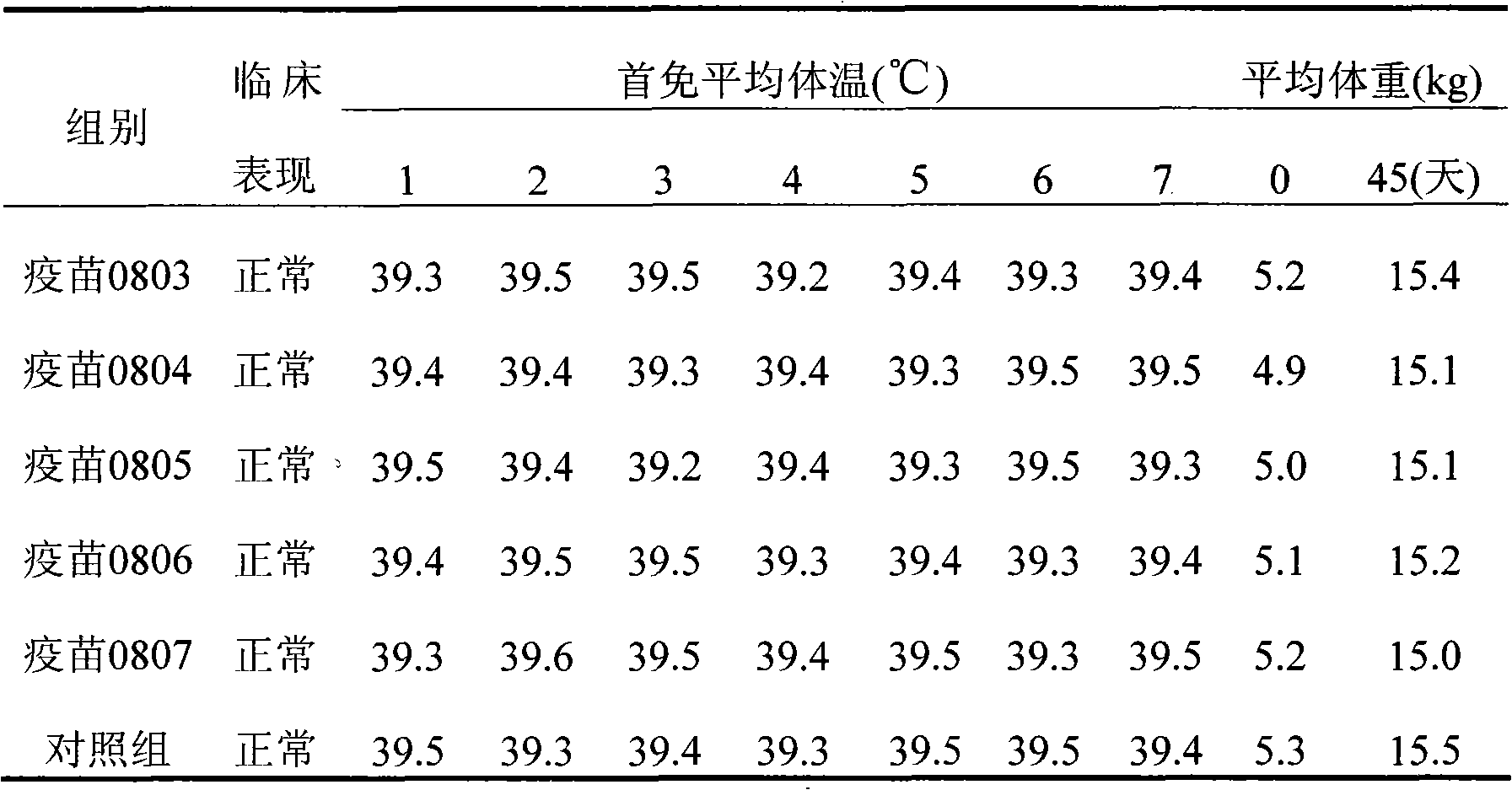
[0044] Preparation of inactivated porcine circovirus type 2 (PCV2) vaccine and evaluation of immune efficacy
[0045] 1. Materials and methods
[0046] 1.1 Vaccines
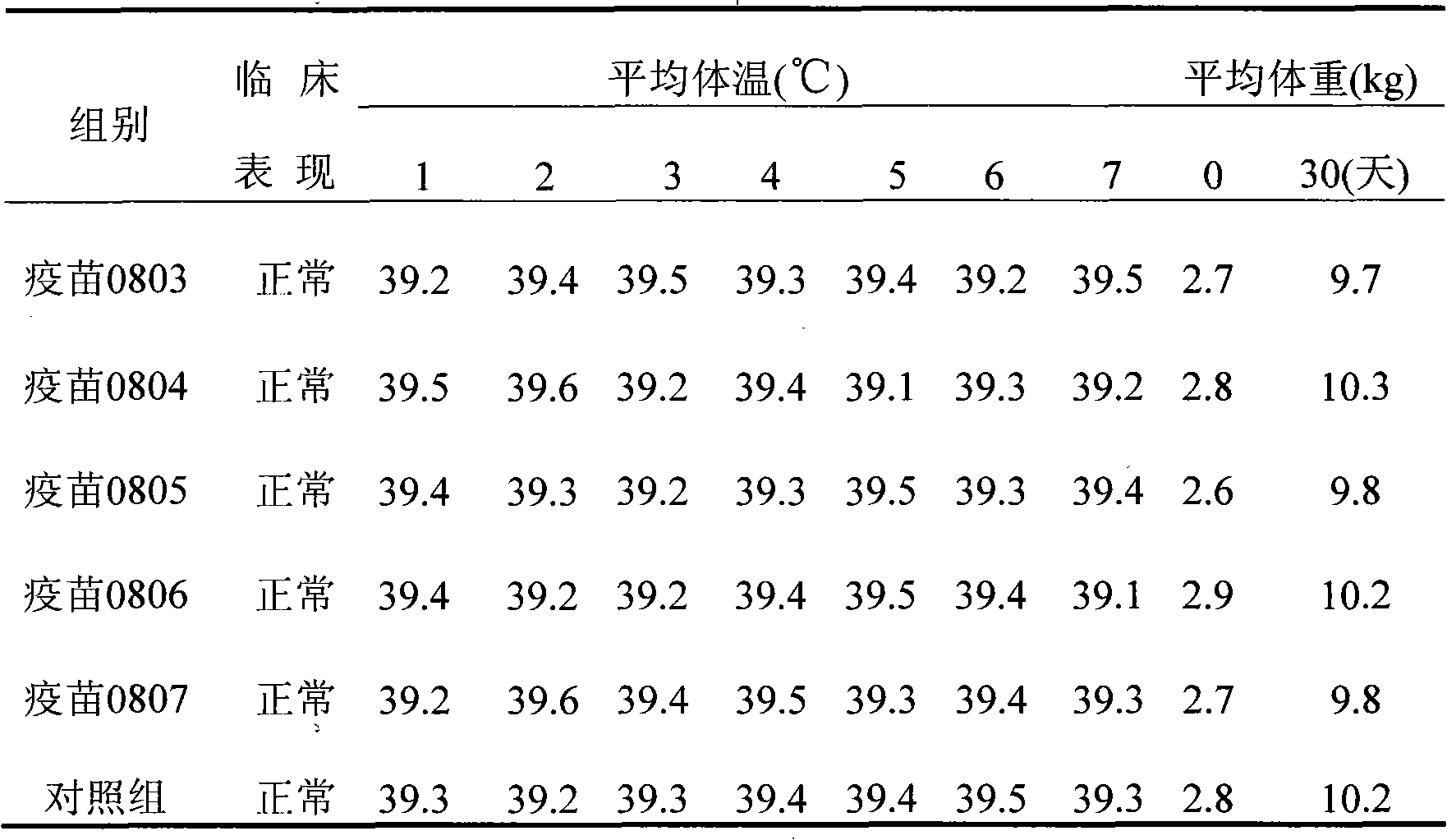
[0047] The porcine circovirus type II (PCV2) virus solution obtained in Example 1 was added with 206 adjuvant (French SEPPIC company product) at a ratio of 1:1, and fully mixed to get the final product (batch numbers are: 0803, 0804, 0805, 0806 and 0807 ). Finished product testing of the prepared vaccines to confirm compliance with various standards;
[0048] Character test: The seedling is a two-phase light red emulsion of water-in-oil-in-water, and the character test has reached the requirements of the "Quality Standard (Draft)". Take a clean straw, suck up a small amount of vaccine and drop it in cold water, all but the first drop will not spread. Stability test: no delamination and no demulsification when placed at 37°C for 21 days. Take 10ml of the vaccine and put it in a centrifuge tube, centrifuge at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com