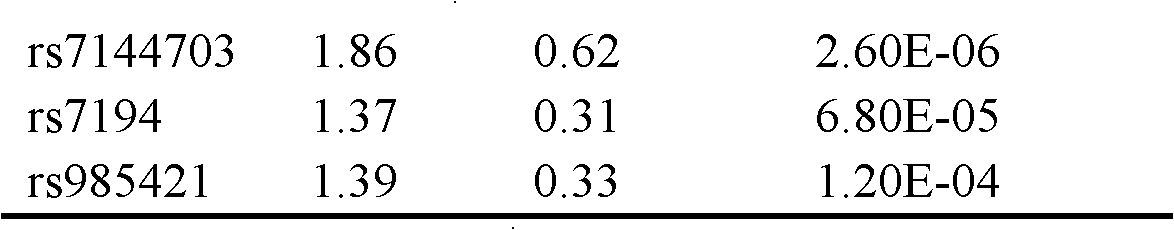Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Obstructive azoospermia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Obstructive azoospermia simply indicates a blockage somewhere in the reproductive tract. Sperm production is normal but the problem lies in the transport of sperm into the ejaculatory duct. Often, men with obstructive azoospermia will have reduced volumes of semen when the ejaculate.
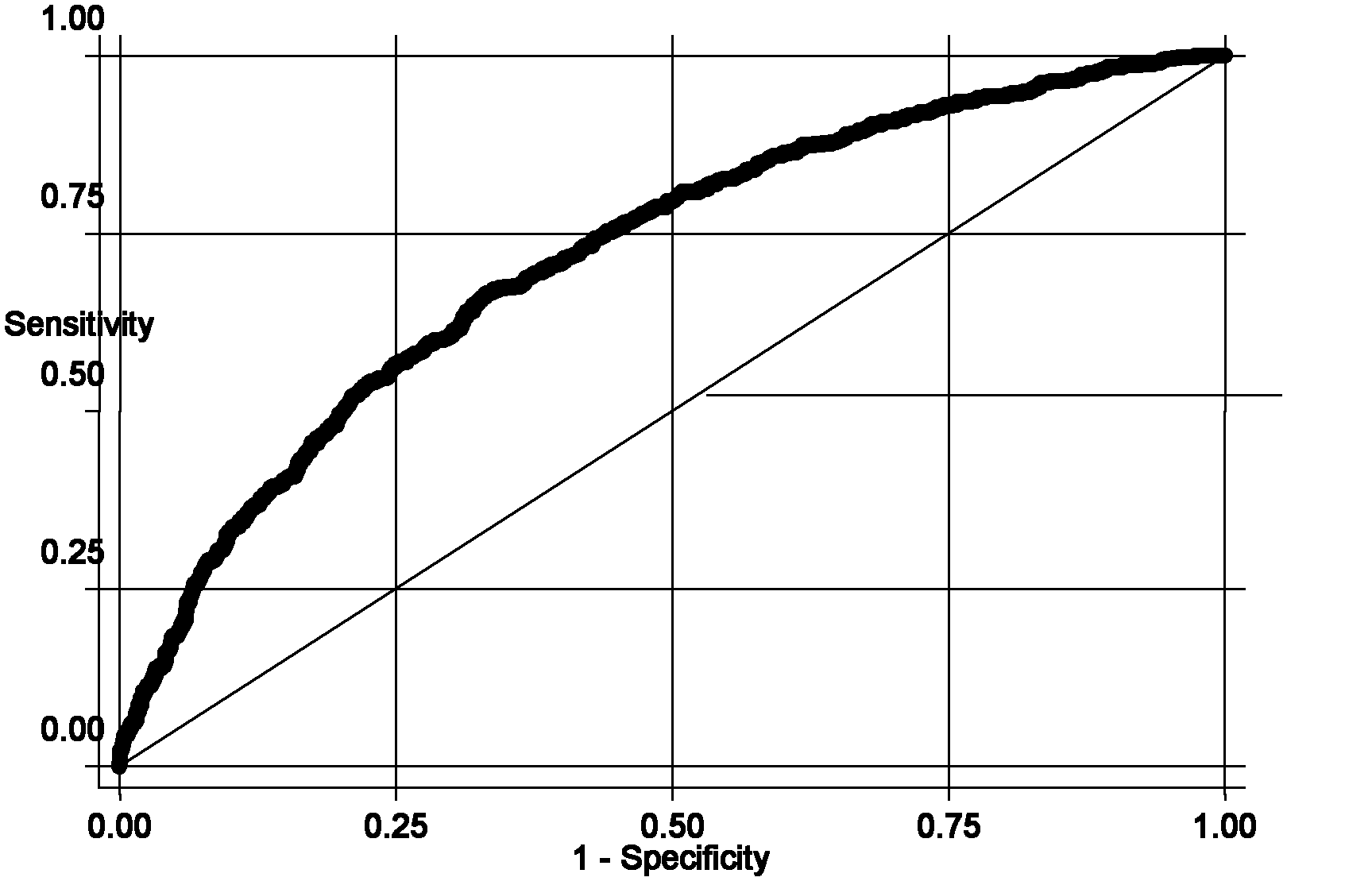
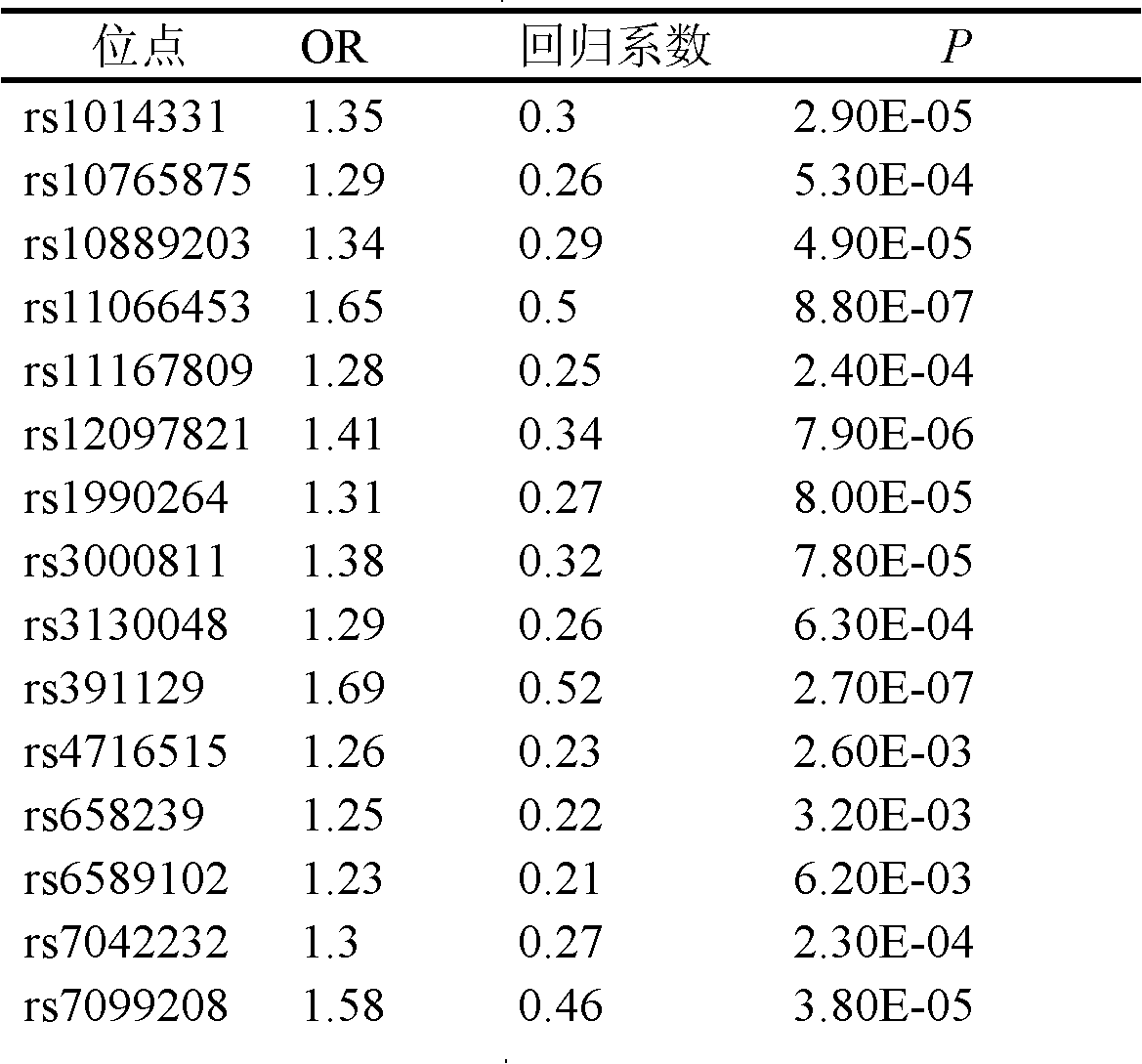
Single nucleotide polymorphism (SNP) marker related with clinically cryptogenic non-obstructive azoospermia aided diagnosis and application of SNP marker
ActiveCN102399898AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationGene engineeringReproductive medicine
The invention belongs to the fields of gene engineering and reproductive medicine, and discloses a single nucleotide polymorphism (SNP) marker related with clinically cryptogenic non-obstructive azoospermia aided diagnosis and application of the SNP marker. The marker is a combination of rs1014331, rs10765875, rs10889203, rs11066453, rs11167809, rs12097821, rs1990264, rs3000811, rs3130048, rs391129, rs4716515, rs658239, rs6589102, rs7042232, rs7099208, rs7144703, rs7194 and rs985421. The marker can be used for preparing a clinically cryptogenic non-obstructive azoospermia aided diagnosis kit.
Owner:NANJING MEDICAL UNIV
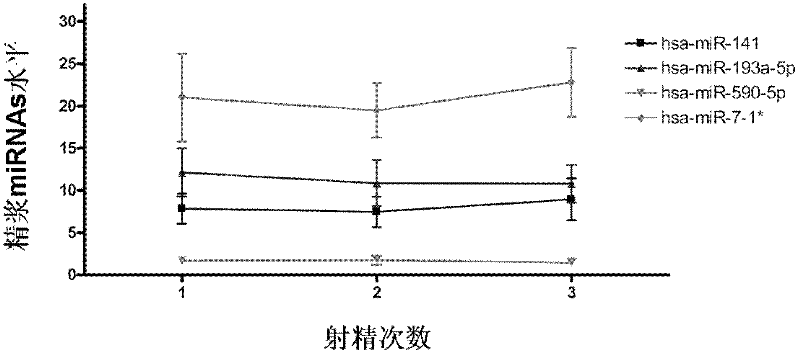
Seminal plasma microRNA markers associated with human non-obstructive azoospermia and their application
ActiveCN102296112AHigh sensitivityEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationDynamic monitoringGenetic engineering
The invention which belongs to the medical field of genetic engineering and reproduction discloses a seminal plasma miRNA marker associated with human non-obstructive azoospermia and an application thereof. The maker is selected from several of hsa-miR-141, hsa-miR-193a-5p, hsa-miR-590-5p and hsa-miR-7-1*. The maker which has specificities and sensitivities to the non-obstructive azoospermia can be used for the preparation of a reagent for non-obstructive azoospermia diagnosis or monitoring, so invasive diagnosis is avoided, repeated detection is realized, and the dynamic monitoring of the obstacle degree of sperm generation can be easily achieved.
Owner:NANJING MEDICAL UNIV
Separation and culture method and application of human testis mesenchymal stem cells
InactiveCN105331579AIncrease serum testosterone levelsMammal material medical ingredientsSkeletal/connective tissue cellsDiseaseTissue biopsy
The invention discloses a separation and culture method and application of human testis mesenchymal stem cells. The separation method comprises the steps that an obstructive azoospermia patient testis specimen which is processed through tissue biopsy separation is digested with type IV collagenase, digested cell suspension is filtered to remove tissue clumps, and human testis cells are obtained; the human testis cells are marked with a CD51 antibody, separation is performed with a flow cytometer, the cells expressing the CD51 are collected, and then the human testis mesenchymal stem cells with the positive CD51 are separated. The invention further provides the human testis mesenchymal stem cells which are obtained through the separation method and have the positive CD51, the culture method of the stem cells and application of the stem cells in preparation of medicine for treating related diseases caused by low testosterone level.
Owner:SUN YAT SEN UNIV
Reagent kit for detecting obstruction performance and non-obstruction performance non-spermatozoa symptom based on Eppin antibody
The invention relates to a reagent kit for detecting obstructive and non-obstructive azoospermia based on an Eppin antibody. The reagent kit comprises bovine serum albumin, a phosphate buffer solution, a TBST eluant, a rabbit anti-human Eppin antibody, a goat anti-rabbit IgG antibody, a chromogenic agent, a microfilter and a pyroxylin membrane arranged in the microfilter; in testing, seminal plasma which is to be tested is diluted through the phosphate buffer solution, is adsorbed and fixed on the pyroxylin membrane of the microfilter and is closed through the bovine serum albumin; and the pyroxylin membrane closed by the albumen is subjected to first washing through the TBST eluant, is incubated through the rabbit anti-human Eppin antibody, is subjected to secondary washing through TBST, is incubated through the goat anti-rabbit IgG antibody and is subjected to chromogenic discrimination through the chromogenic agent. The reagent kit has the advantages that the reagent kit has no wound, can alleviate the pain of a patient receiving seminal duct radiography and testicle biopsy, can save mass inspection expense needed by the prior seminal duct radiography and relevant inspection, has high sensitivity, can reach the molecular level, is simple, feasible and rapid and has important application prospect and practical value.
Owner:JIANGSU PROVINCE HOSPITAL
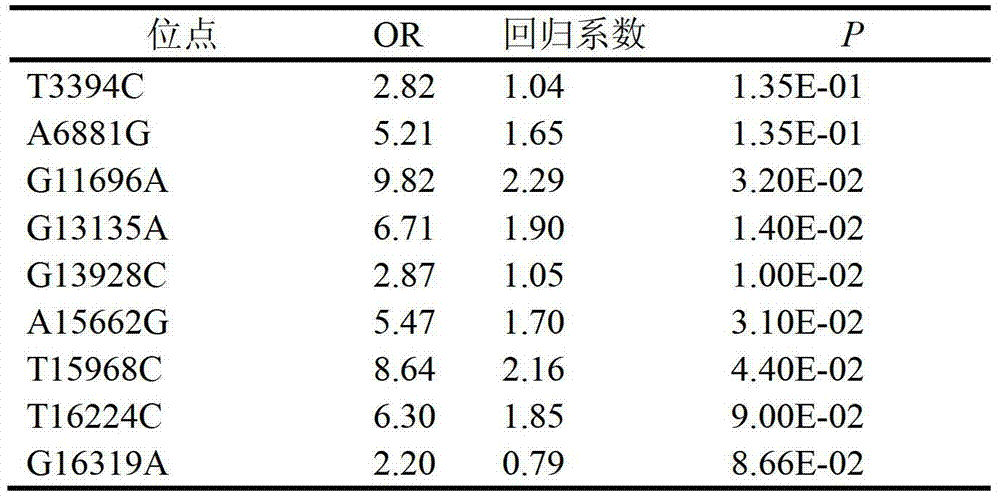
A clinically unexplained NOA-related mitochondrial DNA SNP marker and applications thereof
ActiveCN103290006AIncreased sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationDiseaseTrue positive rate
The invention relates to a clinically unexplained NOA (non-obstructive azoospermia)-related mitochondrial DNA SNP (single nucleotide polymorphism) marker and applications thereof, belonging to the fields of genetic engineering and reproductive medicine. The marker is a combination of the following mitochondrial DNA SNP sites: T3394C, A6881G, G11696A, G13135A, G13928C, A15662G, T15968C, T16224C and G16319A. The marker and specific primers and / or specific probes thereof can be used for auxiliary diagnosis of the clinically unexplained NOA, greatly improving the sensitivity and specificity of diagnosis.
Owner:夏彦恺
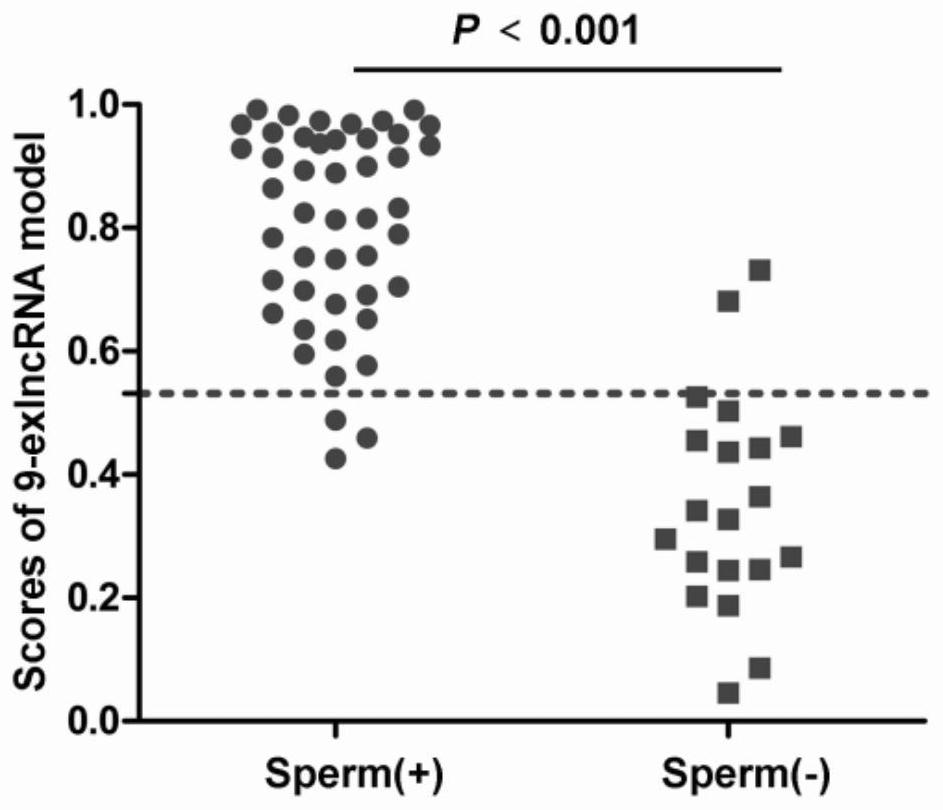
Seminal plasma exosome tsRNA markers related to non-obstructive azoospermia diagnosis and application of seminal plasma exosome tsRNA markers
ActiveCN112251508AEasy diagnosisImprove discriminationMicrobiological testing/measurementDNA/RNA fragmentationTesticular volumeBlood plasma
The invention discloses seminal plasma exosome tsRNA markers related to non-obstructive azoospermia diagnosis and application of the seminal plasma exosome tsRNA markers. The markers are tRF-Pro-AGG-003 and tRF-Val-AAC-010, the sequence of tRF-Pro-AGG-003 is SEQ ID NO.1, and the sequence of tRF-Val-AAC-010 is SEQ ID NO.2. The markers have specificity and sensitivity on non-obstructive azoospermiaand can be used for preparing a diagnostic kit for non-obstructive azoospermia. The two seminal plasma exosome markers tRF-Pro-AGG-003 and tRF-Val-AAC-010 are differentially expressed in seminal plasma of patients suffering from non-obstructive azoospermia and obstructive azoospermia, non-obstructive azoospermia can be well diagnosed, an azoospermia sperm taking result can be predicted, and the effect of the markers is superior to the indexes such as the common plasma follicle stimulating hormone level and the testicular volume.
Owner:江苏阔然生物医药科技有限公司
Rapid typing detection kit for ejaculated sperm cells and rapid typing detection method for ejaculated sperm cells
InactiveCN110286080AChange dependenciesLow application costIndividual particle analysisBiological testingGlycerolBovine serum albumin
The invention discloses a rapid typing detection kit for ejaculated sperm cells. The kit comprises a PBS solution, a calcium-free HBSS buffer solution, a fixing solution, a staining reagent and a quality control product. The PBS solution and the calcium-free HBSS buffer solution both comprise one or more of bovine serum albumin, fetal bovine serum, glycerol serum, protein and glycerol; the staining reagent comprises a cell permeabilizing agent and a fluorescently labeled antibody. The invention also provides a rapid typing detection method for ejaculated sperm cells by adopting the kit mentioned above. The rapid typing detection kit for ejaculated sperm cells and the rapid typing detection method for ejaculated sperm cells can be used for quickly determining the types and the quantity of spermatogenic cells in the ejaculated sperm cells, further determining whether the ejaculated sperm cells are obstructive or non-obstructive azoospermia, solve the problem that clinical evaluation aiming at the current semen cytology in China lacks a quick and accurate detection reagent in the current clinic, provide guidance for a clinician to select a treatment scheme, and practically solve the problem of a patient.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
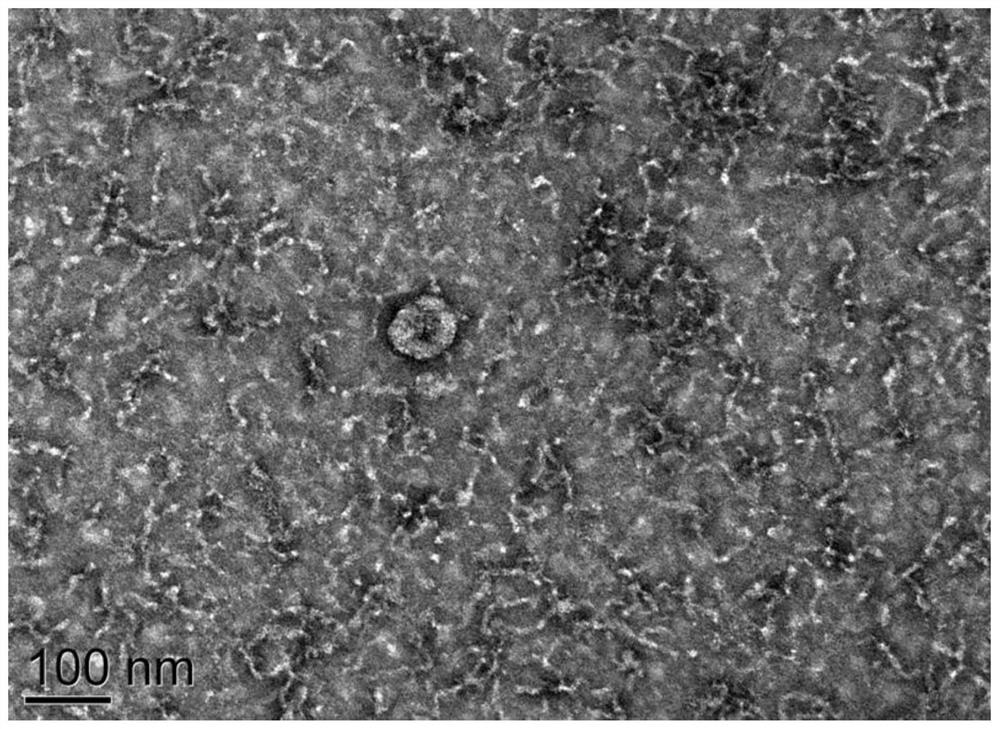
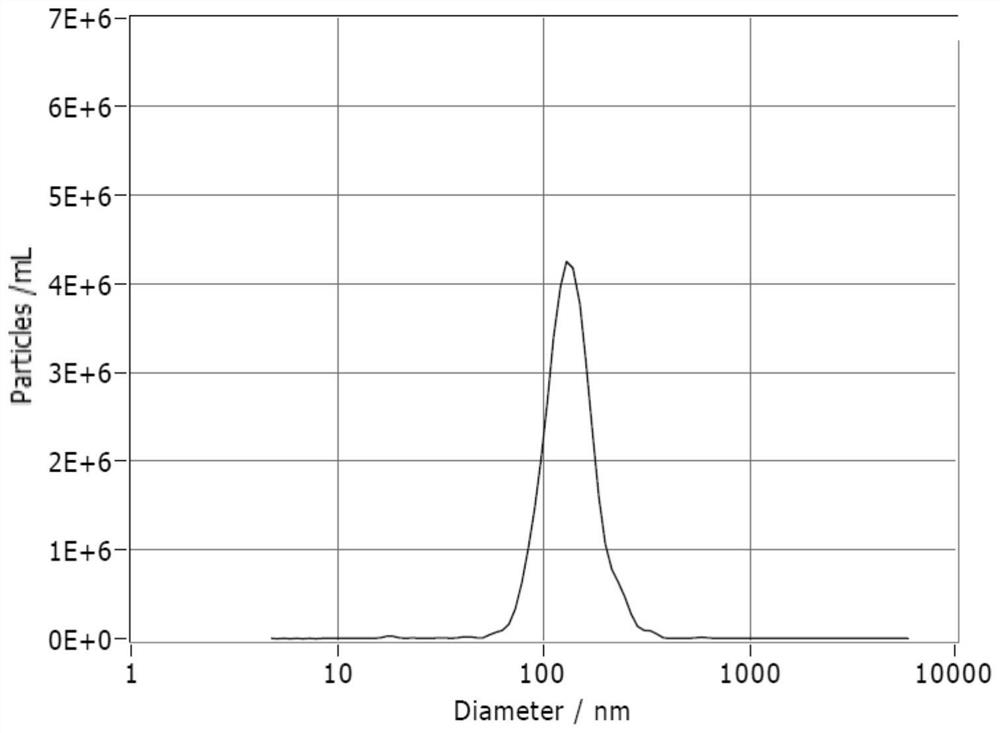
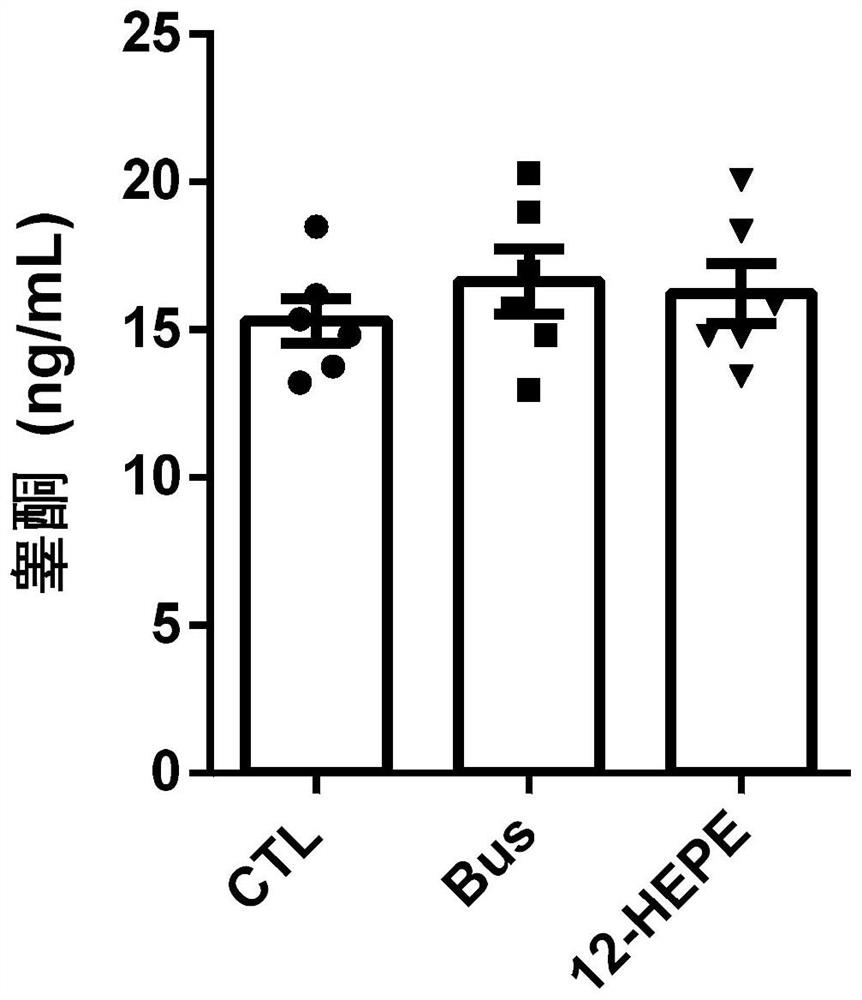
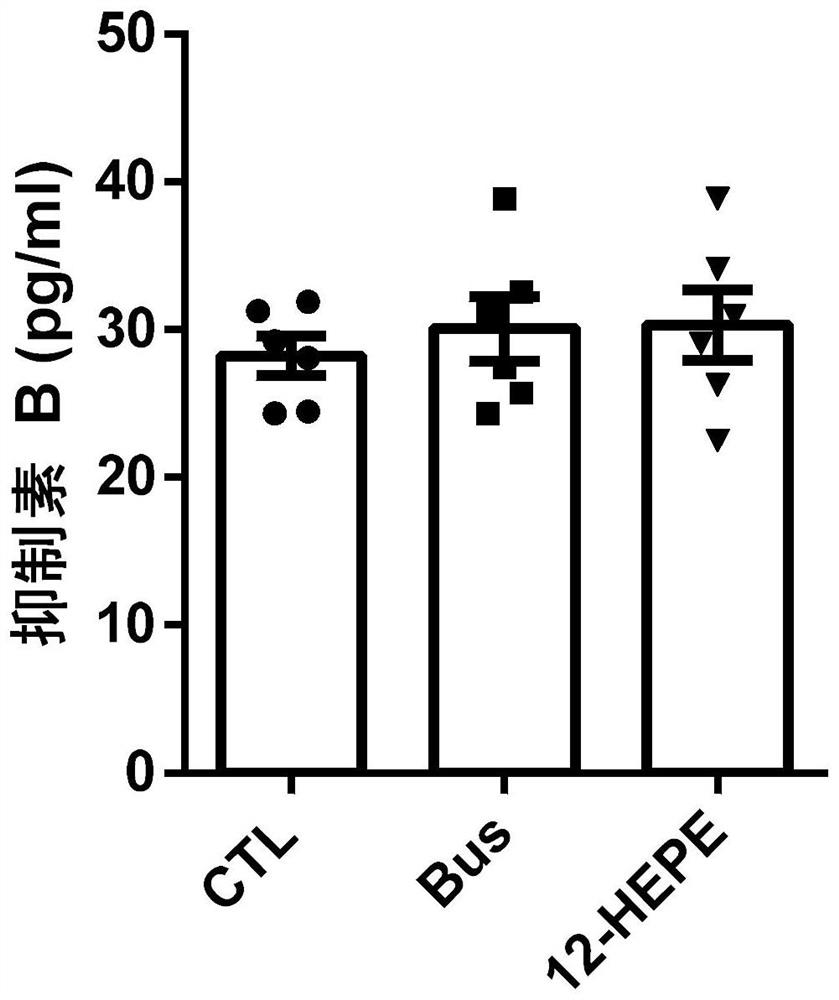
Application of 12-HEPE or pharmaceutically acceptable fatty acid thereof in alleviation of spermatogenesis disorder
ActiveCN113855659APromote proliferationGood treatment effectOrganic active ingredientsSexual disorderMouse TesticlePharmaceutical medicine
The invention discloses novel application of 12-HEPE or pharmaceutically acceptable fatty acid thereof, and comprises the application of the 12-HEPE or the pharmaceutically acceptable fatty acid thereof in preparation of a medicine for treating NOA. The 12-HEPE or the pharmaceutically acceptable fatty acid thereof can play a therapeutic role by promoting proliferation and differentiation of the endogenous spermatogonium of a mouse damaged by busulfan, and experiments prove that recovery of the thickness and the integrity of the spermatogenic epithelium of the testis and the recovery of the sperm concentration of the tail of epididymiscan be observed through intragastric administration of the 12-HEPE into the NOA mouse induced by the busulfan, meanwhile, proliferation and differentiation of spermatogonium are promoted, expression of the paracrine factors of cells is supported, so that the 12-HEPE can be used as a new target for alleviating the spermatogenesis dysfunction of the testis of the NOA mouse, and a new method is provided for treating the non-obstructive azoospermia.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A +3
Reagent kit for predicting semen collection outcome of patient suffering from non-obstructive azoospermia
ActiveCN111944895AAchieve three-stage amplificationMicrobiological testing/measurementHybridisationPhysiologySpermatid
The invention relates to the field of biological medicine, in particular to a reagent kit for predicting semen collection outcome of a patient suffering from non-obstructive azoospermia. The reagent kit comprises a reagent for detecting the expression level of lncRNA in a sample, and the lncRNA comprises at least one of SPATA42, CCDC37-DT, GABRG3-AS1, LOC440934, LOC100505685, LOC101929088 (XR_927561.2), LOC101929088 (XR_001745218.1), LINC00343 and LINC00301. According to the reagent kit, the sperm extraction outcome of the patient suffering from NOA can be noninvasively and accurately predicted before an operation, and the reagent kit has sufficient scientificity and practicability.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
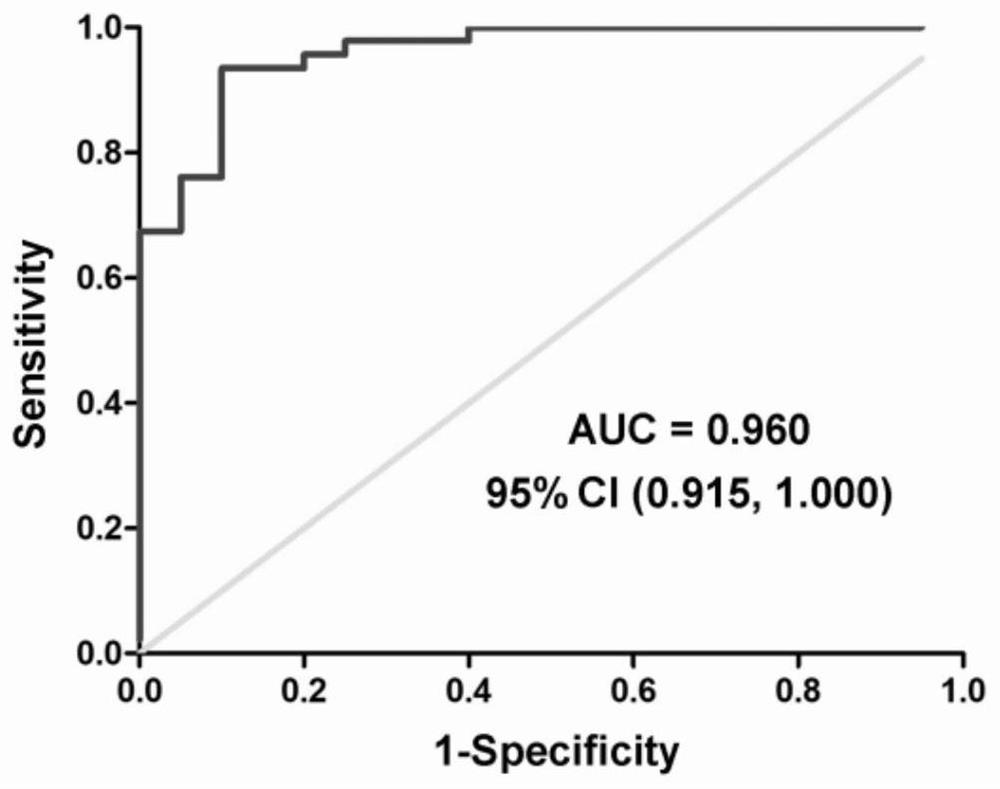
Plasma exosome tsRNA marker related to non-obstructive azoospermia diagnosis and application thereof
ActiveCN112176052APrediction of sperm retrieval resultsEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationTesticular volumeBlood plasma
The invention discloses a plasma exosome tsRNA marker related to non-obstructive azoospermia diagnosis and application thereof. The marker is tRF-Gly-GCC-002, wherein the sequence of the tRF-Gly-GCC-002 is shown as SEQ ID NO.1. The marker has specificity and sensitivity to non-obstructive azoospermia, and can be used for preparing a diagnostic kit for non-obstructive azoospermia; and the plasma exosome marker tRF-Gly-GCC-002 is differentially expressed in plasma of patients with non-obstructive azoospermia and obstructive azoospermia, the plasma exosome marker tRF-Gly-GCC-002 can well diagnosethe non-obstructive azoospermia, and can predict the azoospermia sperm taking result, and the effect of the plasma exosome marker tRF-Gly-GCC-002 is superior to common indexes such as the plasma follicle stimulating hormone level and the testicular volume.
Owner:XUZHOU MEDICAL UNIV
Application of mercury and cadmium mixed exposure detection in auxiliary diagnosis of non-obstructive azoospermia
PendingCN109254070AIncreased sensitivityImprove featuresMaterial analysis by electric/magnetic meansMixed exposureMedicine
The invention belongs to the field of analytical chemistry and clinical medicine, and discloses application of mercury and cadmium mixed exposure detection in auxiliary diagnosis of non-obstructive azoospermia. An exposed biomarker related to the non-obstructive azoospermia is mercury and cadmium mixed exposure. The concentration of mercury and cadmium in whole blood is detected by ICP-MS detection, the application can be used for auxiliary diagnosis of the non-obstructive azoospermia, a few amount of blood is used, and the application is simple, rapid and accurate to operate and has relatively good clinical promotion value.
Owner:NANJING MEDICAL UNIV
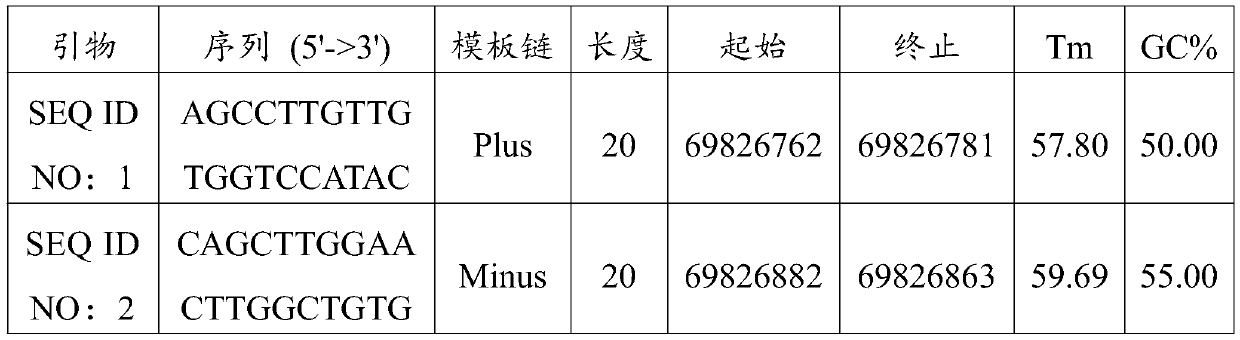
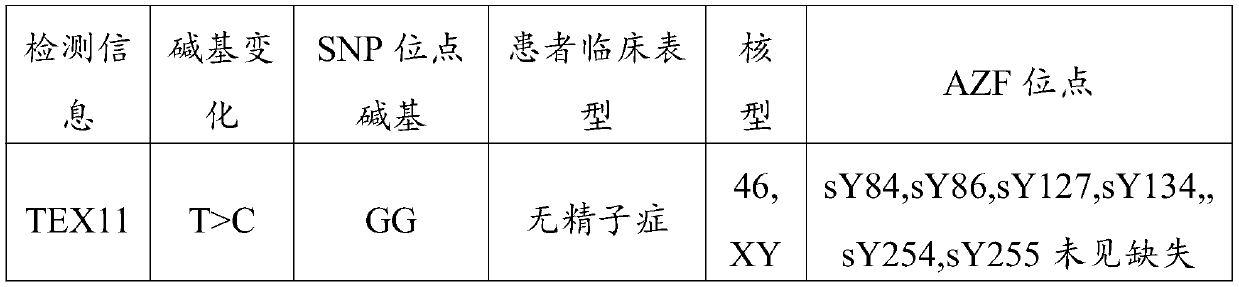
Non-obstructive azoospermia auxiliary diagnosis gene detection kit
The invention discloses a non-obstructive azoospermia auxiliary diagnosis gene detection kit, relates to the field of azoospermia diagnosis, and solves the problem of difficult clinical diagnosis of male infertility. The non-obstructive azoospermia auxiliary diagnosis gene detection kit comprises a specific PCR primer, a PCR buffer solution, dNTPs and DNA polymerase, wherein the specific PCR primer is a group of specific primers for detecting the 69826819 mutation site in the non-obstructive azoospermia TEX11 gene. A specific primer SEQ ID NO: 1 and a specific primer SEQ ID NO: 2 are designed,the specific primers have higher specificity, the non-obstructive azoospermia auxiliary diagnosis gene detection kit consisting of the specific primers is adopted to detect the 69826819 mutation sitein the TEX11 gene of a male patient, and the detection rate of the gene mutation is 100%.
Owner:YINFENG JILIN BIOLOGICAL ENG TECH CO LTD +2
Application of TEX11 gene pathogenic mutation in preparation of diagnostic kit for detecting non-obstructive azoospermia
ActiveCN113308531AReduce financial burdenQuick checkMicrobiological testing/measurementAgainst vector-borne diseasesGenes mutationGene screening
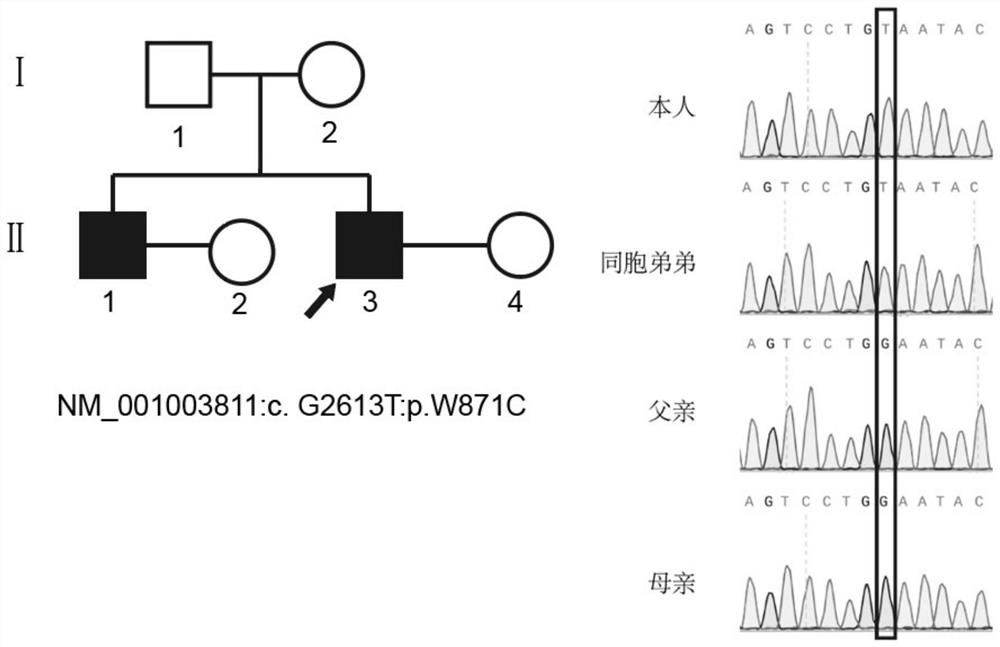
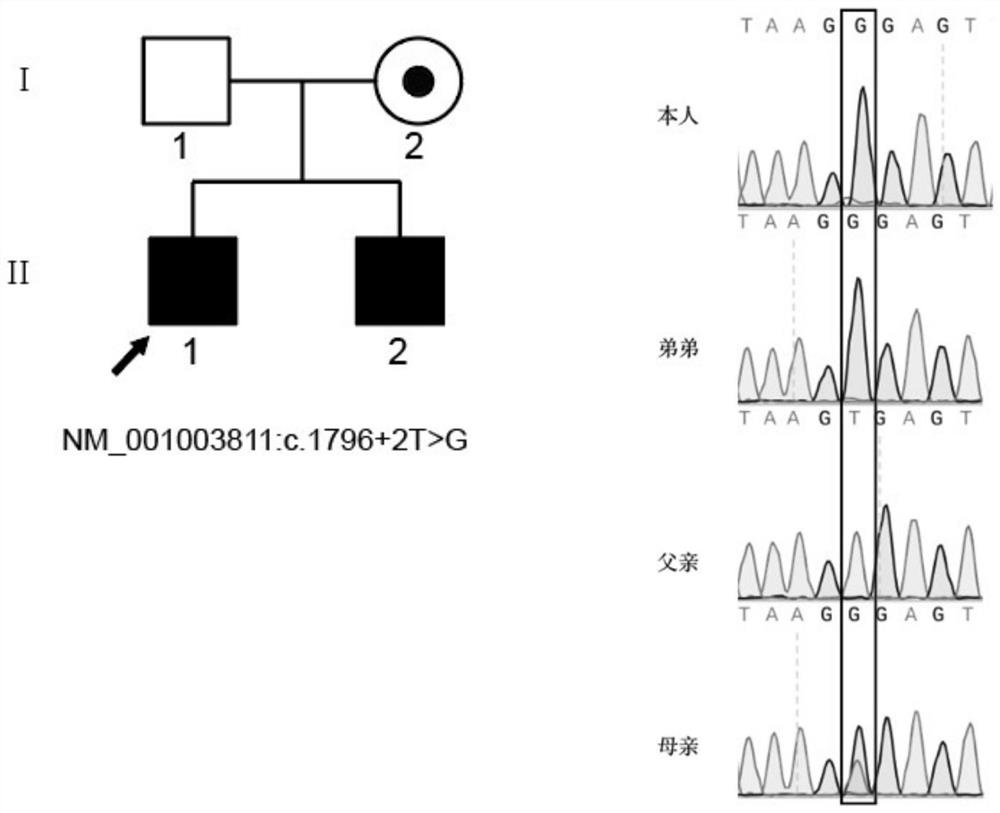
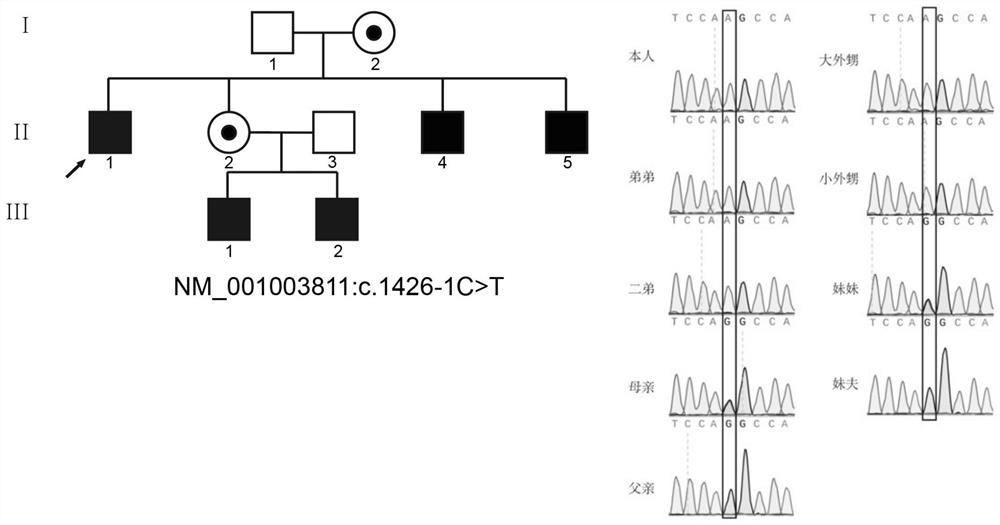
The invention relates to the technical field of biology, in particular to application of TEX11 gene pathogenic mutation in preparation of a diagnostic kit for detecting non-obstructive azoospermia. According to the method, peripheral blood DNA (deoxyribonucleic acid) is firstly extracted, then a target gene is subjected to PCR (polymerase chain reaction) amplification, and finally, the pathogenic mutation of the TEX11 gene is finally verified in four families: family 1: NM_001003811:c.G2613T:p.W871C, family 2: NM_001003811:c.1426-1C>T; family 3: NM_001003811:c.1796+2T>G; and family 4: NM_001003811:c.857delA:p.K286R fs*5. The method has the advantages that an effective way is provided for non-obstructive azoospermia gene diagnosis, prenatal gene screening and genetic counseling.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Serum miRNA combination as molecular marker for assessing non-obstructive azoospermia
InactiveCN110592204AEasy to operateImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationSerum igeSerum mirna
The invention discloses an application of a serum miRNA combination as molecular marker for assessing non-obstructive azoospermia. The serum miRNA combination includes hsa-miR-1263, hsa-miR-221-5p, hsa-miR-483-3p, hsa-miR-4275 and hsa-miR-194-3p. Through the miRNA combination, a reagent kit for assessing the non-obstructive azoospermia is prepared. The serum miRNA for assessing the non-obstructiveazoospermia is obtained, further a detection reagent kit containing primers used for quantitative RQ-PCR detection of the serum miRNA combination is obtained, detection is performed through the detection primers and the detection reagent kit, the operation is simple, the accuracy is high, and the serum miRNA combination is worth of extensive popularization.
Owner:广东省计划生育科学技术研究所
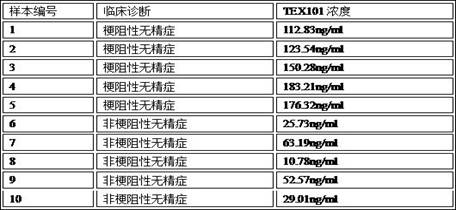
Preparation method and application of chemiluminescence detection kit for quantitatively detecting TEX101 concentration
PendingCN112630451AConcentration rapid quantificationIncreased sensitivityChemiluminescene/bioluminescenceDisease diagnosisBiochemistrySemen
The invention discloses a preparation method and application of a chemiluminiscence detection kit for quantitatively detecting TEX101 concentration, belongs to the field of in-vitro diagnostic reagents, and provides a TEX101 chemiluminiscence detection kit by researching TEX101 concentration difference results of obstructive azoospermia OA and non-obstructive azoospermia NOA. The kit can rapidly and quantitatively detect the concentration of TEX101 in seminal fluid, the sensitivity of identifying obstructive azoospermia OA and non-obstructive azoospermia NOA is 73%, the specificity is 100%, and the kit has a good prediction value for assisted reproduction results.
Owner:普迪特泰州生物科技有限公司
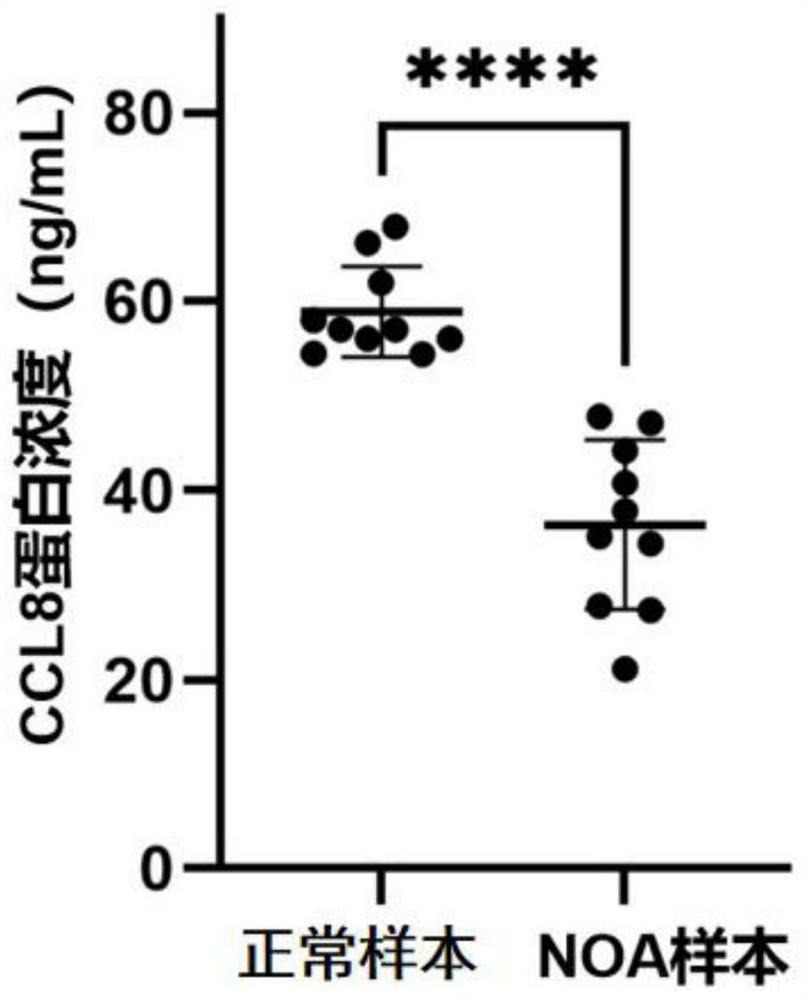
CCL8 protein as biomarker of non-obstructive azoospermia and application of CCL8 protein
The invention discloses a CCL8 protein serving as a biomarker of non-obstructive azoospermia and application of the CCL8 protein. The biomarker comprises a CCL8 protein. The invention finds that the concentration of the CCL8 protein in the seminal fluid of the NOA patient is obviously lower than that of a normal male for the first time, and proposes that the CCL8 protein can be used as a potential early warning molecule for evaluating the spermatogenic dysfunction of the NOA patient, so that expansion and diagnosis of NOA indexes are facilitated, the diagnosis range is expanded, and the CCL8 protein has important significance in the field of NOA diagnosis.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
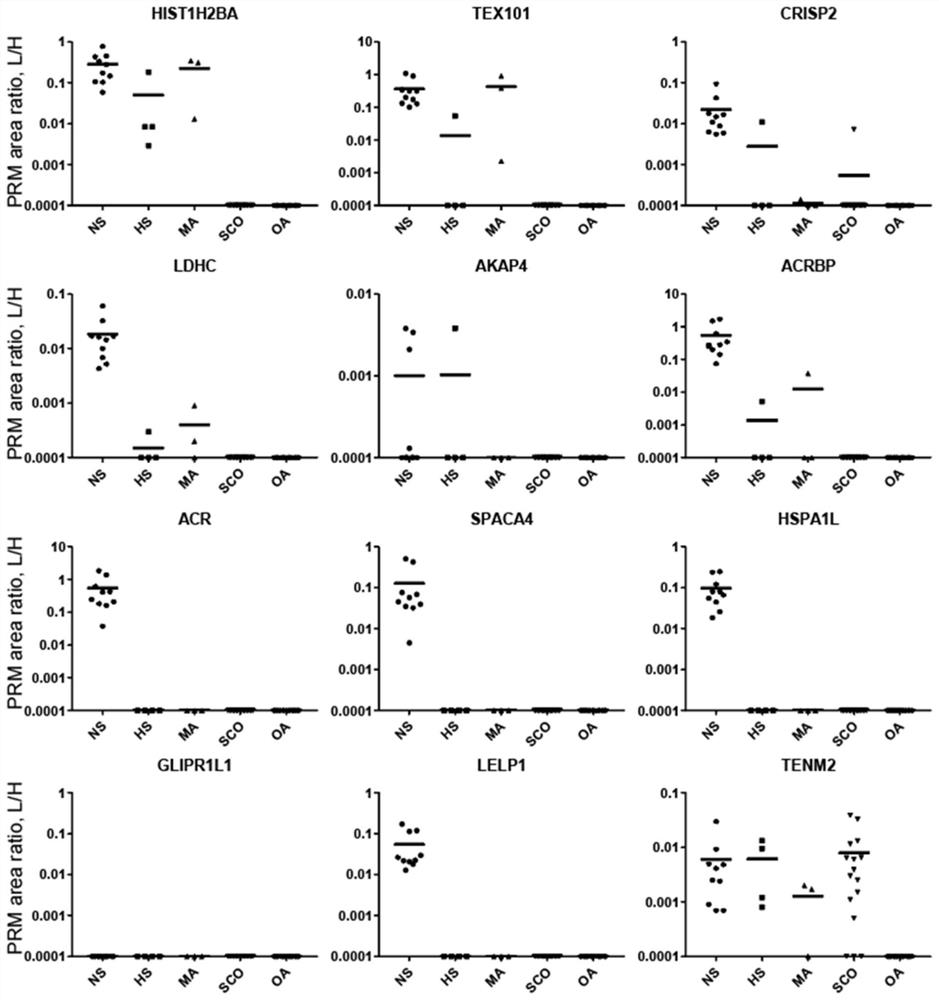
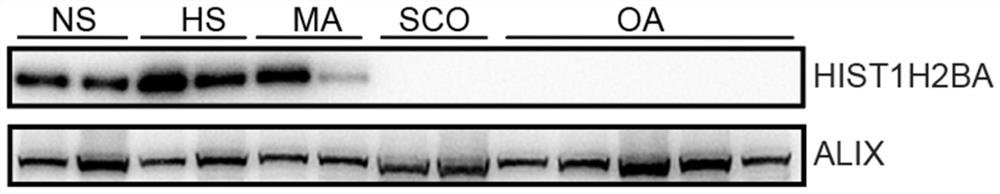
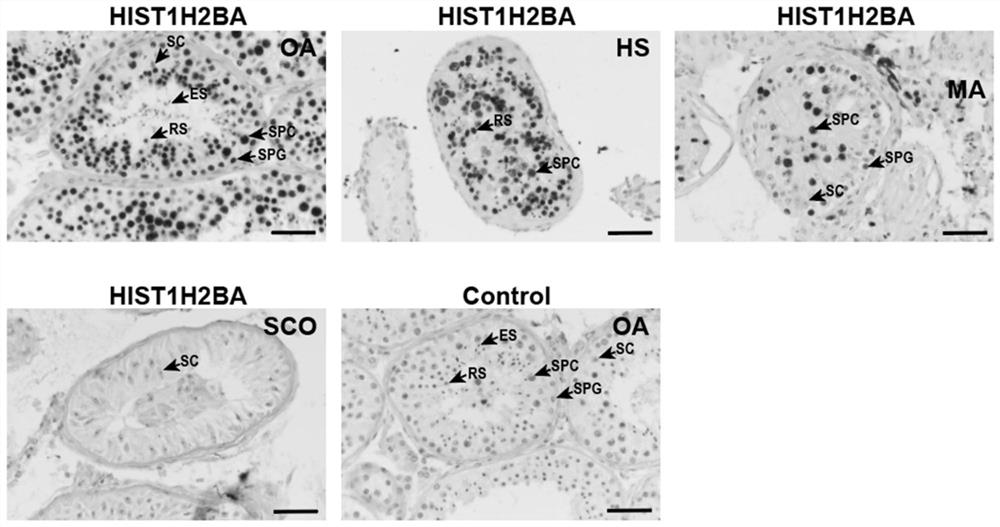
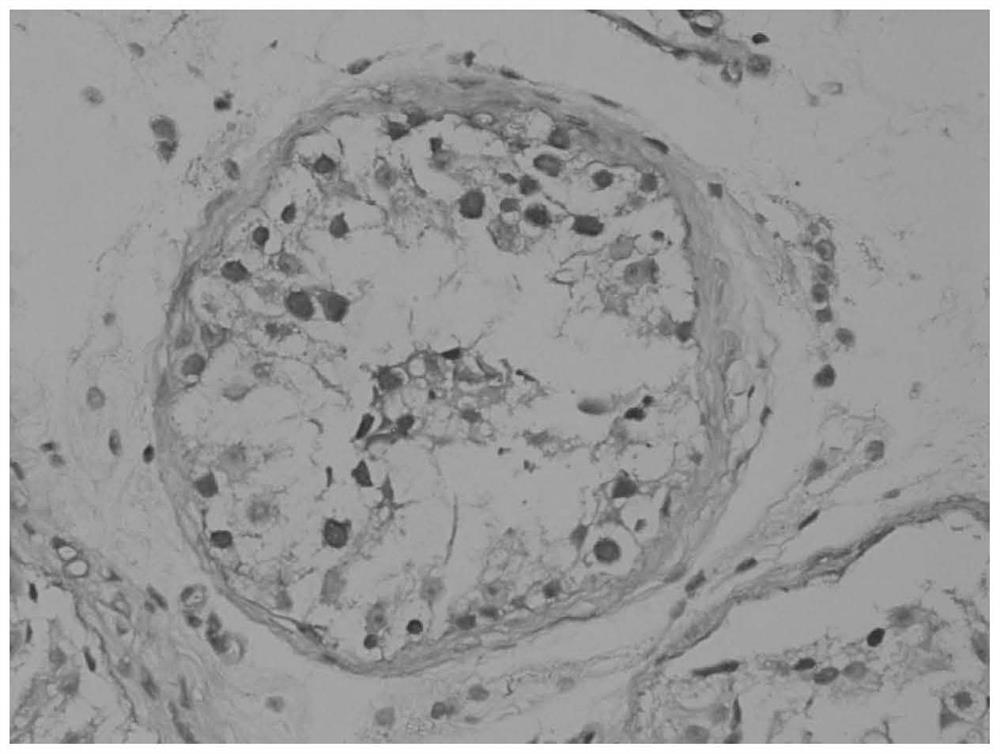
Application of seminal plasma extracellular vesicle HIST1H2BA protein
The invention discloses application of seminal plasma extracellular vesicle HIST1H2BA protein, belongs to the field of protein detection, and particularly relates to application of the seminal plasma extracellular vesicle HIST1H2BA protein in biomarkers of seminal plasma extracellular vesicles of different pathological types of non-obstructive azoospermia. The HIST1H2BA provided by the invention can be used for distinguishing NOA-SCO from other pathological types and predicting the spermatogenesis condition in testis.
Owner:NANJING MEDICAL UNIV
Application of HFM1 gene in preparation of diagnostic kit for detecting non-obstructive azoospermia
ActiveCN114438194AEfficient detectionReduce financial burdenMicrobiological testing/measurementDNA/RNA fragmentationNucleotideGene screening
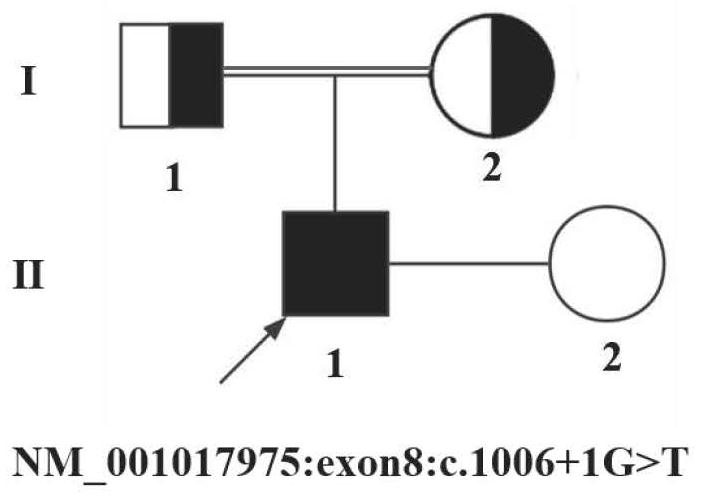
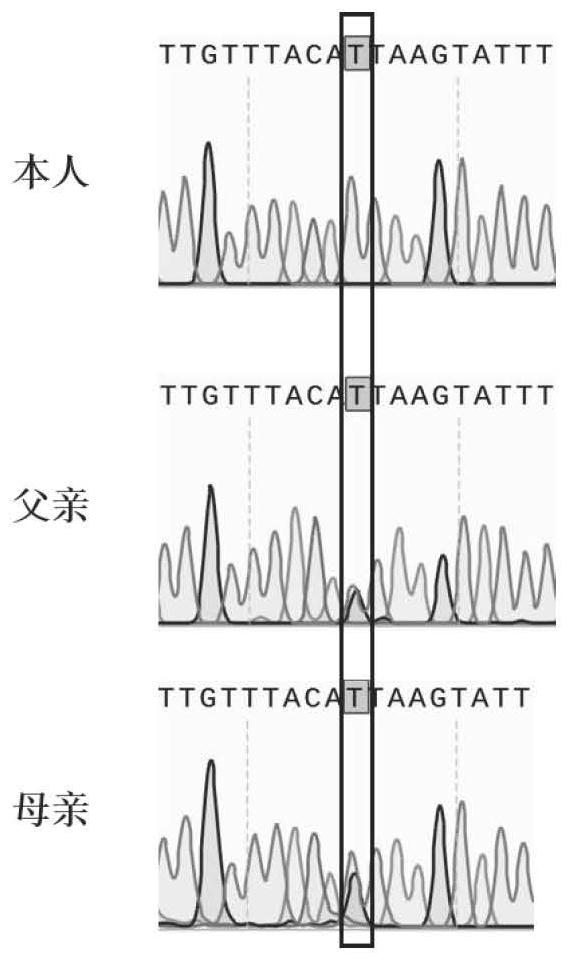
The invention relates to a non-obstructive azoospermia detection kit, the kit comprises a primer pair for detecting a genetic marker related to non-obstructive azoospermia, the nucleotide sequence of the genetic marker is the sequence of an HFM1 gene, and the mutation site of the sequence is c.1006 + 1Ggt; t. The invention also provides application of the detection kit in diagnosis of non-obstructive azoospermia and application of the HFM1 gene in preparation of the diagnostic kit for detecting non-obstructive azoospermia. The invention provides a new application of the HFM1 gene, so that an effective way for performing non-obstructive azoospermia gene diagnosis, prenatal gene screening and genetic counseling is provided; the application effect shows that the SNP site of the gene and the detection primer provided by the invention can be effectively used for rapid detection of the HFM1 gene mutation site in clinical patients and fetal villus or amniotic fluid.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Biomarker related to non-obstructive azoospermia and application thereof
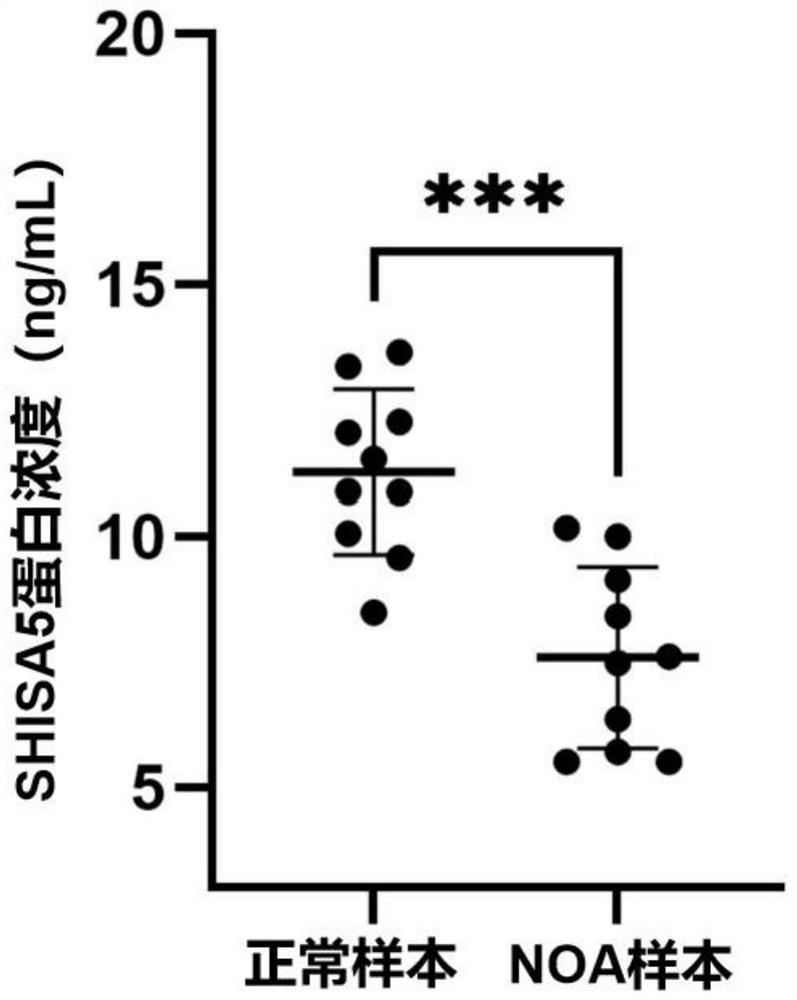
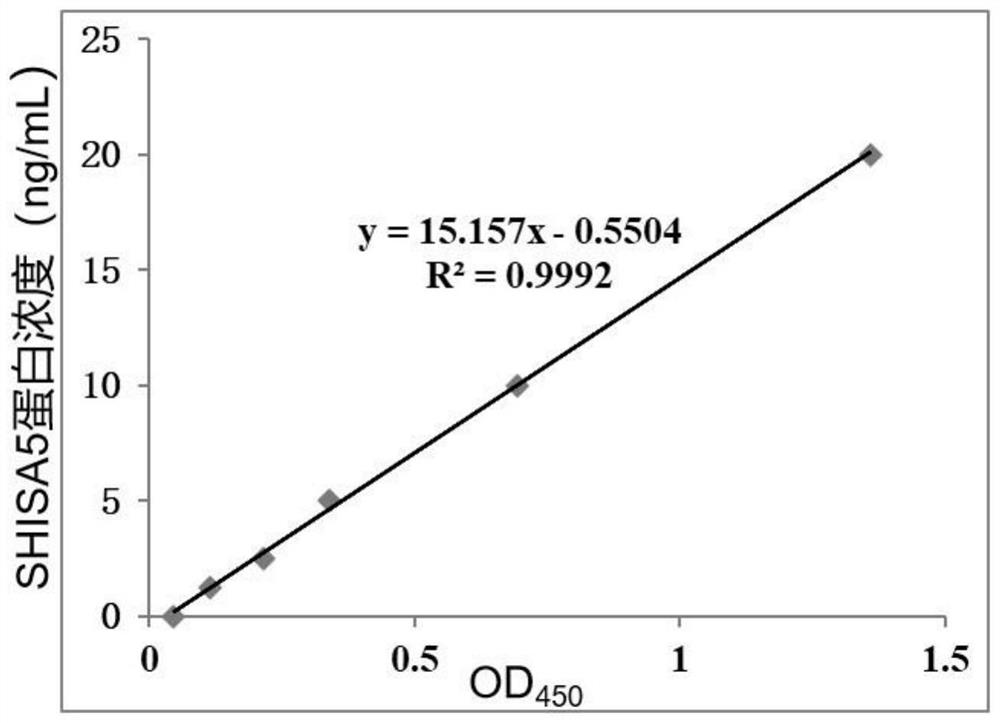
The invention discloses a biomarker related to non-obstructive azoospermia and an application of the biomarker. The biomarker comprises an SHISA5 protein. According to the present invention, the concentration of the Wnt receptor inhibition protein SHISA5 in the NOA patient semen is significantly lower than the concentration of the Wnt receptor inhibition protein SHISA5 in the normal male semen for the first time, and the SHISA5 protein can be adopted as the potential early warning molecule for evaluating the spermatogenic dysfunction of the NOA patient, such that the NOA diagnosis index can be easily expanded so as to expand the diagnosis range, and the important significance is provided for the NOA diagnosis field.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
Plasma exosome miRNA markers related to diagnosis of primary non-obstructive azoospermia and application thereof
ActiveCN112195232APrediction of sperm retrieval resultsEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationAzoospermiaTesticle
The invention discloses plasma exosome miRNA markers related to diagnosis of primary non-obstructive azoospermia and application thereof. The markers are hsa-miR-513c-5p and hsa-miR-202-5p, the sequence of the hsa-miR-513c-5p is shown as SEQ ID NO.1, and the sequence of the hsa-miR-202-5p is shown as SEQ ID NO.2. The markers have specificity and sensitivity to the non-obstructive azoospermia, andcan be used to prepare a diagnostic kit for the non-obstructive azoospermia. The two plasma exosome markers hsa-miR-513c-5p and hsa-miR-202-5p disclosed by the invention are differentially expressed in the plasma of patients with non-obstructive azoospermia and obstructive azoospermia, so that the non-obstructive azoospermia can be diagnosed better, the sperm taking result of azoospermia can be predicted, and the effect is superior to common indexes such as plasma follicle-stimulating hormone level and testis volume.
Owner:XUZHOU MEDICAL UNIV
A plasma exosomal miRNA marker associated with the diagnosis of primary non-obstructive azoospermia and its application
ActiveCN112195232BPrediction of sperm retrieval resultsEasy diagnosisMicrobiological testing/measurementDNA/RNA fragmentationTesticular volumeDisease
The invention discloses a plasma exosome miRNA marker related to the diagnosis of primary non-obstructive azoospermia and its application. The markers are hsa-miR-513c-5p and hsa-miR-202-5p, the sequence of hsa-miR-513c-5p is SEQ ID NO.1, and the sequence of hsa-miR-202-5p is SEQ ID NO. 2. The marker has specificity and sensitivity to non-obstructive azoospermia, and can be used to prepare a diagnostic kit for non-obstructive azoospermia. The two plasma exosome markers hsa-miR-202-5p and hsa-miR-513c-5p of the present invention are differentially expressed in the plasma of patients with non-obstructive azoospermia and obstructive azoospermia, which can better It can diagnose non-obstructive azoospermia, and can predict the result of sperm retrieval in azoospermia, and its effect is better than commonly used indicators such as plasma follicle stimulating hormone level and testicular volume.
Owner:XUZHOU MEDICAL UNIV
Stem cell exosome preparation for preventing and treating male sexual dysfunction
InactiveCN113171378AGood effectOrganic active ingredientsUnknown materialsSexual functioningMesenchymal stem cell
The invention provides a stem cell exosome preparation for preventing and treating male sexual dysfunction, the active ingredient of the stem cell exosome preparation is obtained by loading cryptotanshinone on exosome derived from mesenchymal stem cells, and the exosome is loaded with cryptotanshinone through an electrotransfection method. In the process of researching the mesenchymal stem cell exosome in the treatment of non-obstructive azoospermia, a researcher finds that the effect of using the mesenchymal stem cell-derived exosome alone was not obvious, but unintentionally finds that the effect is obviously enhanced after cryptotanshinone is loaded into the exosome through the electrotransfection method; in addition, the researcher also finds that different concentrations of exosomes loaded with cryptotanshinone have different effects.
Owner:奥启(深圳)生物科技有限公司
Application of Omega-3 or pharmaceutically acceptable fatty acid thereof to improvement of spermatogenesis disorder
PendingCN114146076APromote proliferationGood treatment effectOrganic active ingredientsSexual disorderMouse TesticlePharmaceutical medicine
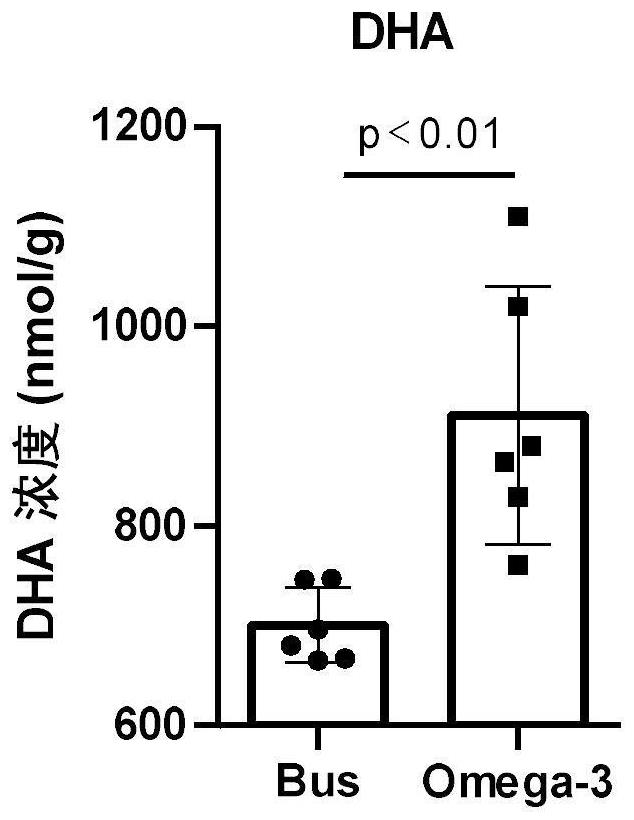
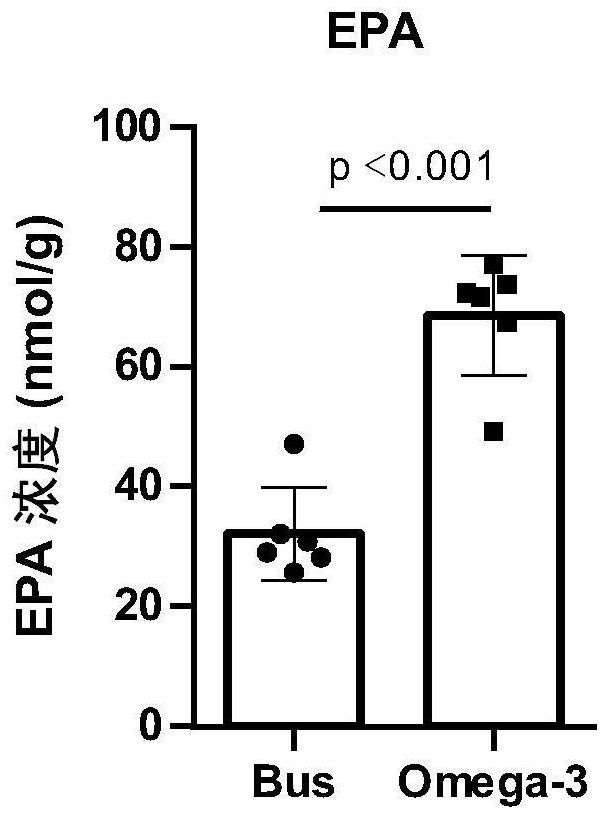
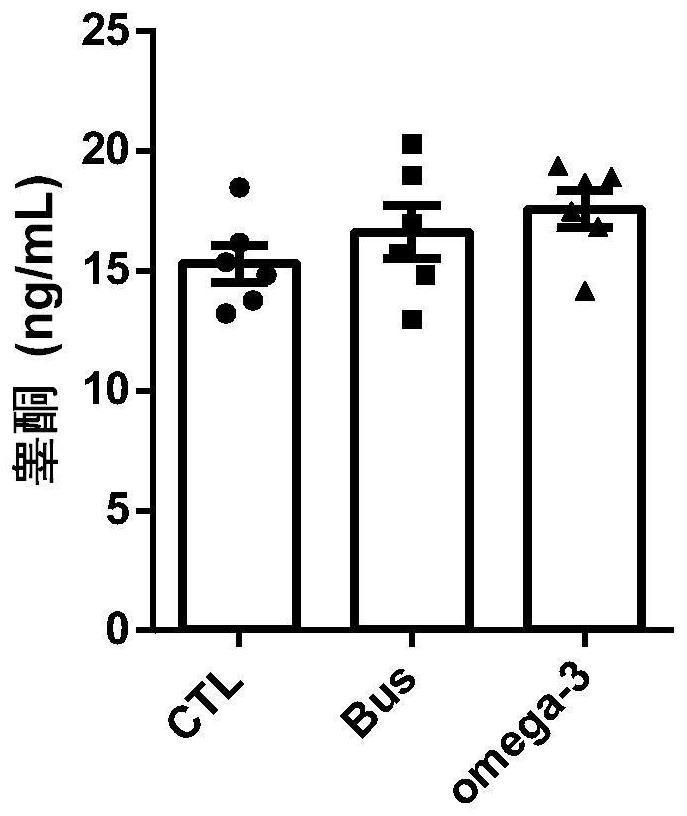
The invention discloses a novel application of Omega-3 or pharmaceutically acceptable fatty acid thereof, and the novel application comprises an application of Omega-3 or pharmaceutically acceptable fatty acid thereof in preparation of a medicine for treating NOA. Through eicosanoic acid targeted metabonomics, it is screened that DHA and EPA in testicular tissue of a polyunsaturated fatty acid Omega-3 gavage male mouse are obviously up-regulated. Experiments prove that Omega-3 is injected into the stomach of a busulfan-induced NOA mouse, the thickness and integrity of testis recovery spermatogenic epithelium and the concentration of epididymis tail sperms can be observed, and meanwhile, proliferation and differentiation of spermatogonium and expression of supporting cell paracrine factors are promoted, so that the Omega-3 can be used as a new target for improving the NOA mouse testis spermatogenic dysfunction; a novel method is provided for treating the non-obstructive azoospermia.
Owner:NANJING MEDICAL UNIV +1
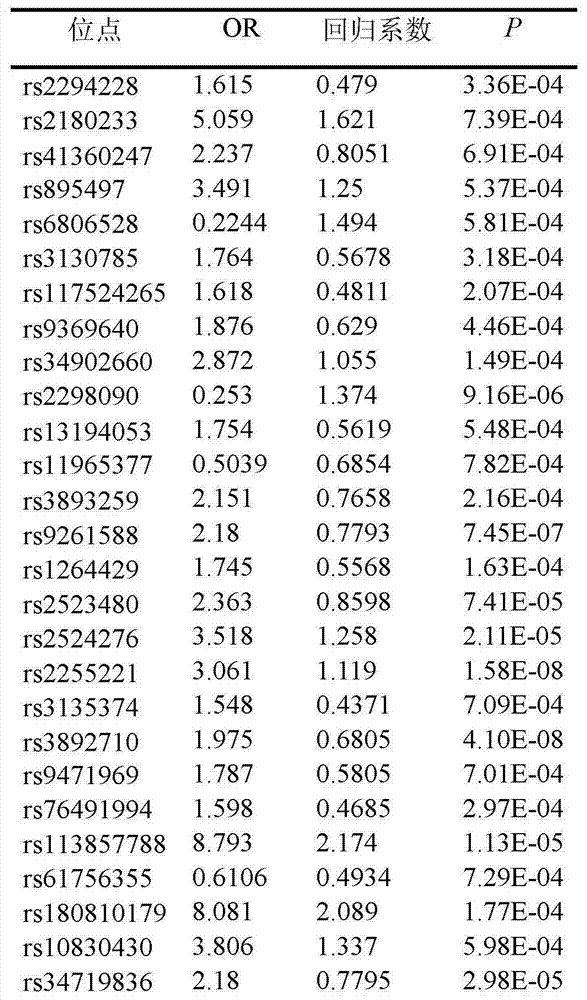
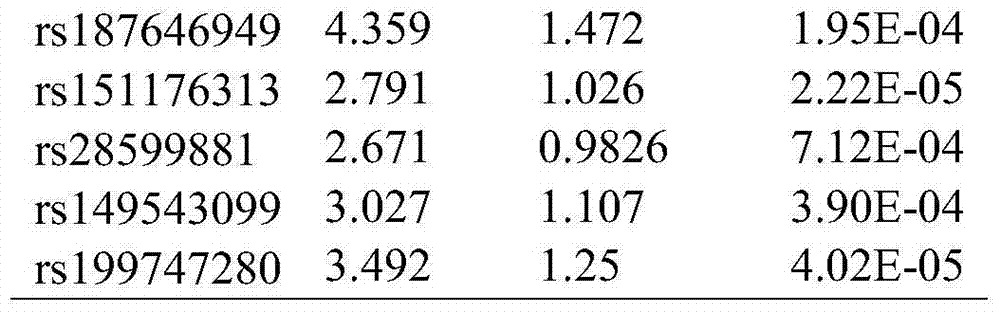
Low-frequency SNV (single nucleotide variant) marker related to assisted diagnosis of NOA (non-obstructive azoospermia) with unknown clinical reasons and application thereof
ActiveCN104263822AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationGene engineeringBiology
The invention belongs to the fields of gene engineering and reproductive medicine, and discloses a low-frequency SNV (single nucleotide variant) marker related to assisted diagnosis of NOA (non-obstructive azoospermia) with unknown clinical reasons and application thereof. The marker is a combination of 32 low-frequency SNVs, including rs2294228, rs2180233, rs41360247, rs895497, rs6806528, rs3130785, rs117524265, rs9369640, rs34902660, rs2298090, rs13194053, rs11965377, rs3893259, rs9261588, rs1264429, rs2523480, rs2524276, rs2255221 and the like. The marker can be used for preparing an assisted diagnosis kit for non-obstructive azoospermia with unknown clinical reasons.
Owner:NANJING MEDICAL UNIV
A kit for predicting sperm retrieval outcome in patients with non-obstructive azoospermia
ActiveCN111944895BAchieve three-stage amplificationMicrobiological testing/measurementHybridisationBiomedicineSpermatid
The invention relates to the field of biomedicine, in particular to a kit for predicting the outcome of sperm extraction for patients with non-obstructive azoospermia. The kit of the present invention includes a reagent for detecting the expression level of lncRNA in the sample, the lncRNA includes SPATA42, CCDC37-DT, GABRG3-AS1, LOC440934, LOC100505685, LOC101929088 (XR_927561.2), LOC101929088 (XR_001745218.1), LINC00343, LINC00343, LINC00343, LINC00343 at least one of them. The invention can realize the non-invasive and accurate prediction of the sperm retrieval outcome of NOA patients before operation, and has sufficient scientificity and practicability.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
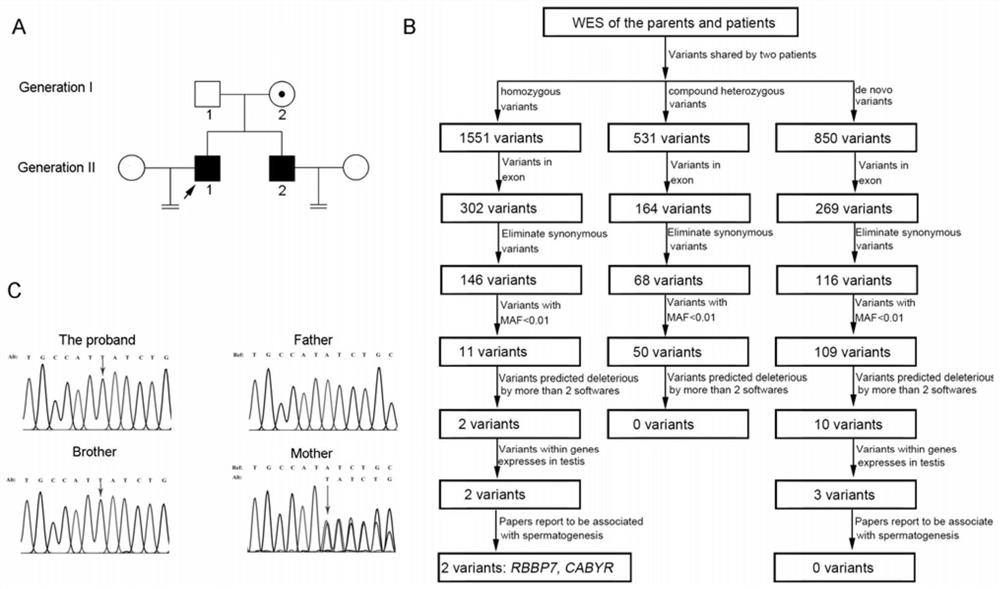
Application of RBBP7 gene/protein as drug target in preparation of products for diagnosing and treating male infertility diseases
PendingCN114854844AReduced expression levelHigh activityCompound screeningApoptosis detectionDiseasePhysiology
The invention provides application of an RBBP7 gene / protein as a drug target in preparation of a product for diagnosing and treating male infertility diseases. Experiments show that the RBBP7 mutant gene / protein causes male infertility, spermatogenesis disorder, spermatogenesis arrest and no sperm. The RBBP7 mutant gene / protein and one pathogenic mutation site of the RBBP7 mutant gene / protein can be used as a target gene for diagnosing male infertility. Meanwhile, the expression level of the RBBP7 of the RBBP7 gene / protein is remarkably reduced in non-obstructive azoospermia patients, and male infertility can be prevented and / or treated by improving the activity and / or expression quantity of the RBBP7 protein.
Owner:ZHEJIANG UNIV
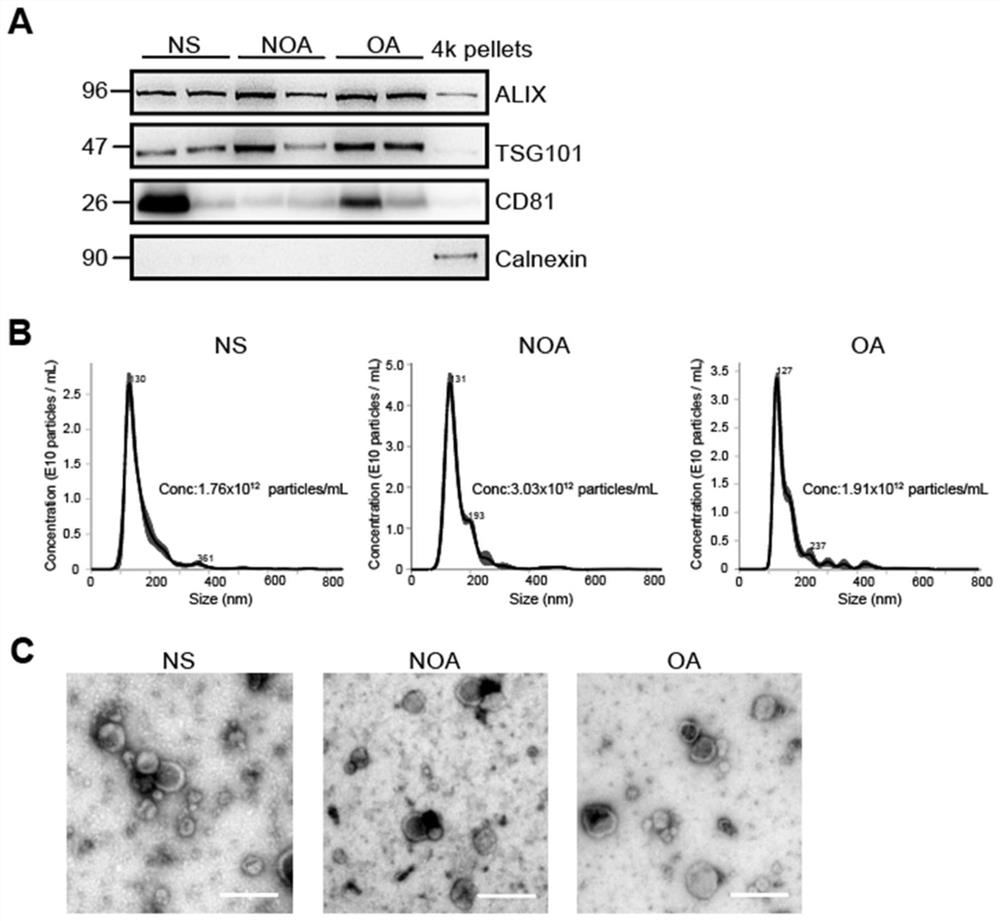
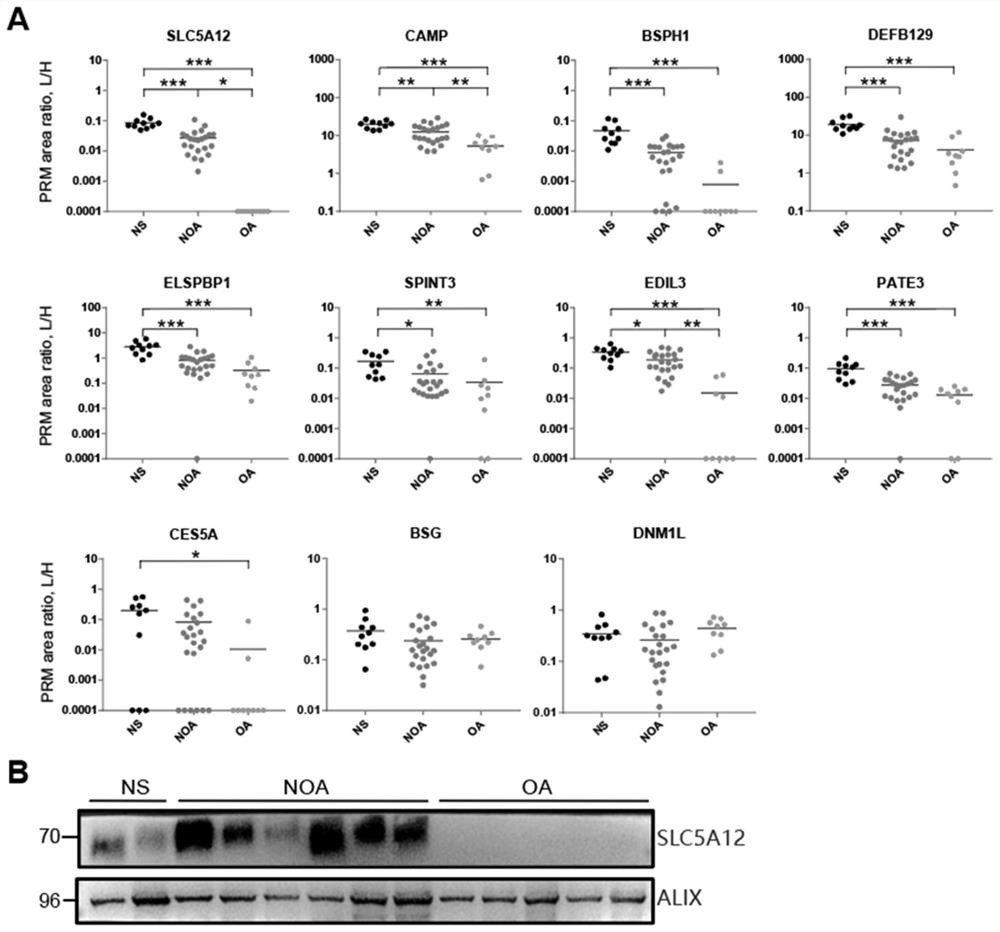
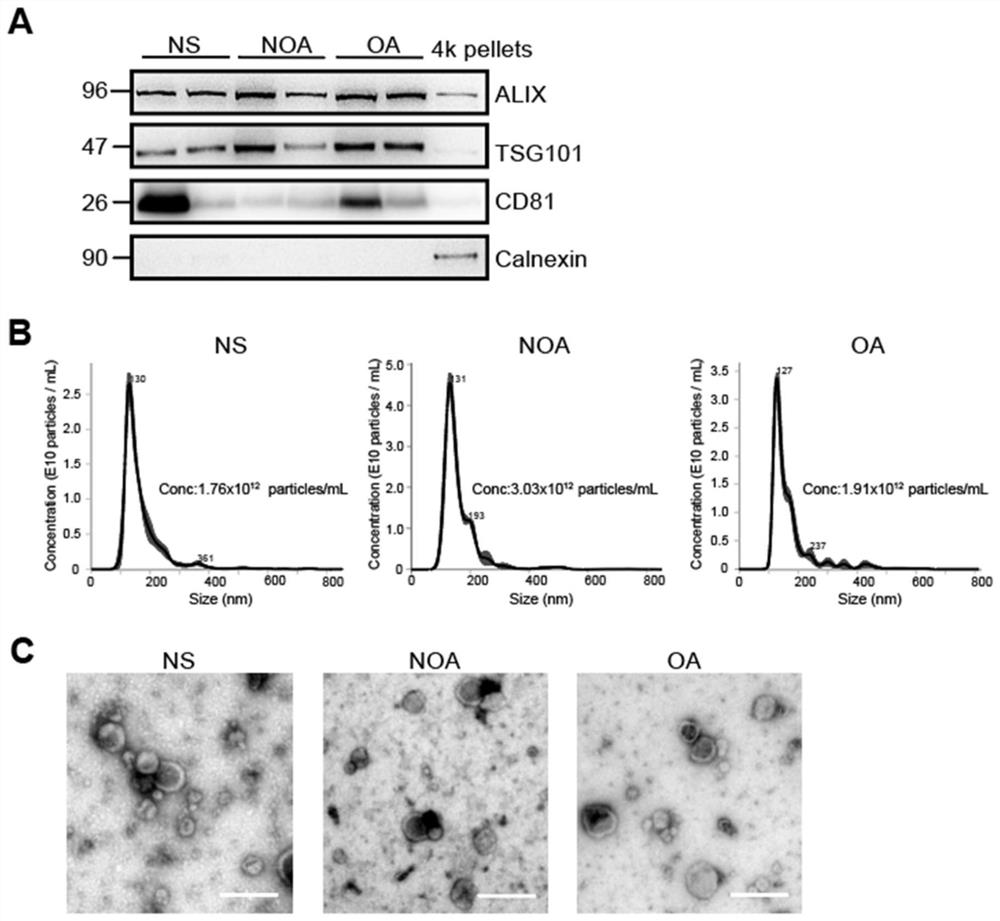
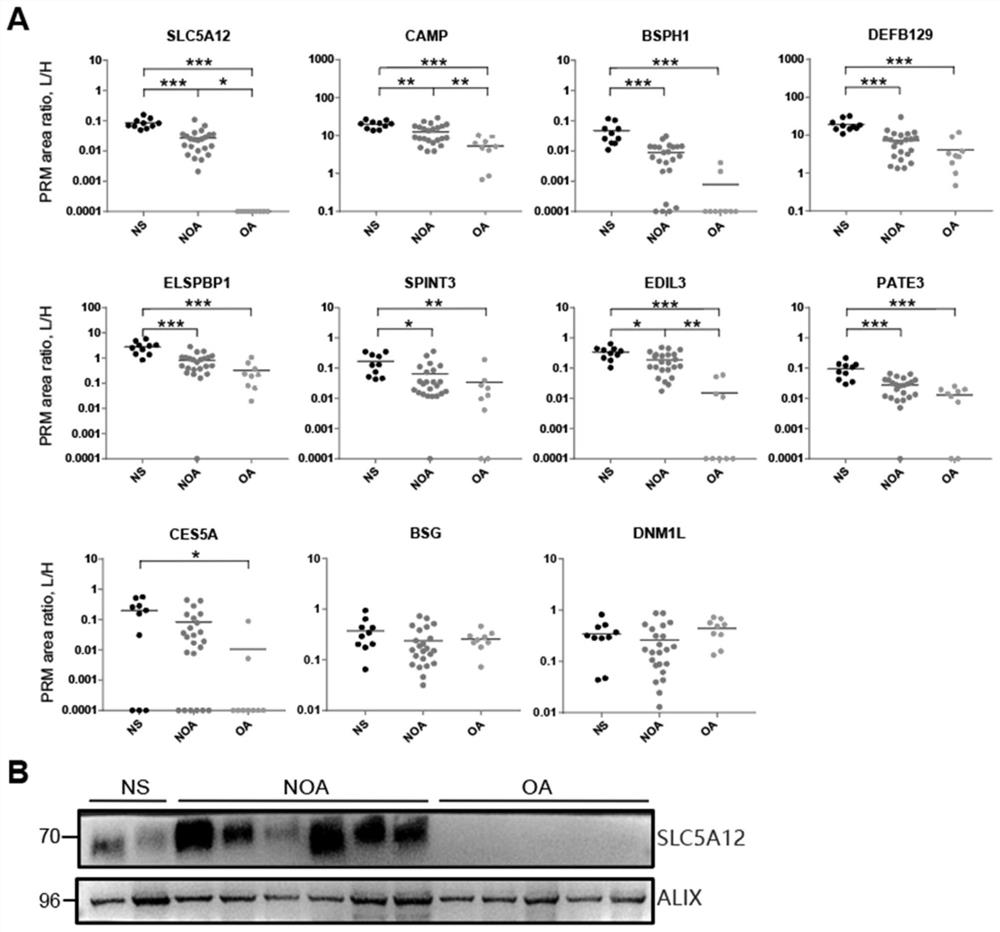
Application of seminal plasma extracellular vesicle SLC5A12 protein
ActiveCN112485322ACell dissociation methodsMaterial analysis by electric/magnetic meansExtracellular vesicleProtein detection
Application of seminal plasma extracellular vesicle SLC5A12 protein belongs to the field of protein detection. In the application, protein carried by sEV of NS, NOA and OA groups is quantitatively analyzed through adopting a TMT labeling technology, inter-group differential protein is identified, the biomarker SLC5A12 is successfully verified through a PRM technology, and the protein can be used for diagnosing and distinguishing non-obstructive azoospermia and obstructive azoospermia.
Owner:NANJING MEDICAL UNIV
Stasis-removing essence-replenishing soup
InactiveCN104069313AGood for infertilityGood for male infertilitySexual disorderPlant ingredientsMedicinal herbsSide effect
The invention provides stasis-removing essence-replenishing soup. The soup has the functions of eliminating dampness and heat, removing stasis and dissipating stagnation, tonifying kidney replenishing essence; the soup is mainly used for treating infertility caused by varicocele in chronic obstructive azoospermia scrotum, testis bearing-down pain or abdominal swelling pain, sperm abnormality and the like and has an excellent effect. The traditional Chinese medicine composition is prepared from the following various raw medicines by weight: angelica sinensis, dipsacus root, red peony root, white peony root, cowherb seed, rhizoma cyperi, red sage root, dried rehmannia root, prepared rehmannia root, honeysuckle flower, dodder, cortex moutan, Chinese yam, epimedium, dogwood, szechwan chinaberry fruit, tangerine seed, liquorice and the like. The soup provided by the invention has an excellent effect on treating the infertility caused by varicocele in chronic obstructive azoospermia scrotum, testis bearing-down pain or abdominal swelling pain, sperm abnormality and the like. The stasis-removing essence-replenishing soup adopts pure natural traditional Chinese medicine ingredients and has no side effect on a human body.
Owner:苟晓龙
Pathogenic mutation of genetic gametogenesis disorder and detection reagent thereof
ActiveCN112226439AScreening benefits are highGenetically heterogeneousMicrobiological testing/measurementDisease diagnosisPrimary ovarian insufficiencyBase J
The invention discloses pathogenic mutation of genetic gametogenesis disorder and a detection reagent thereof. The invention relates to a mutant Pof1b gene, the mutant Pof1b gene is located in a chromosome X and is deletion mutation c.312_316delp.S104fs, the gene number of the wild type Pof1b gene in an Ensemble database is ENSG00000124429, the physical position of the deletion mutation Pof1b geneis a third exon, five bases TACAT are deleted at chrX-84622737-84622742, and following sequences cause frameshift mutation. The mutant Pof1b gene or the mutant POF1B protein is used as a detection target to be applied to preparation of an auxiliary diagnosis reagent or detection equipment for male non-obstructive azoospermia or female hereditary primary ovarian insufficiency.
Owner:NANJING MATERNITY & CHILD HEALTH CARE HOSPITAL
Application of slc5a12 protein in seminal plasma extracellular vesicles
ActiveCN112485322BCell dissociation methodsMaterial analysis by electric/magnetic meansExtracellular vesicleProtein detection
The application of seminal plasma extracellular vesicle SLC5A12 protein belongs to the field of protein detection. The present invention uses TMT labeling technology to quantitatively analyze the proteins carried by sEVs in NS, NOA and OA groups, and identifies differential proteins between groups, and uses PRM technology to successfully verify The biomarker SLC5A12 was identified, which can be used to diagnose non-obstructive azoospermia and obstructive azoospermia.
Owner:NANJING MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com