MYBL2 epitope peptides and vaccines containing the same
A technology of vaccines and amino acids, which is applied in the field of drugs for the treatment and prevention of tumors, and can solve problems such as low objective response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0156] Materials and methods
[0157] cell line
[0158] A24 lymphoblastoid cell line (A24LCL) cells were established by transforming Epstein-Bar virus into HLA-A24 positive human B lymphocytes.
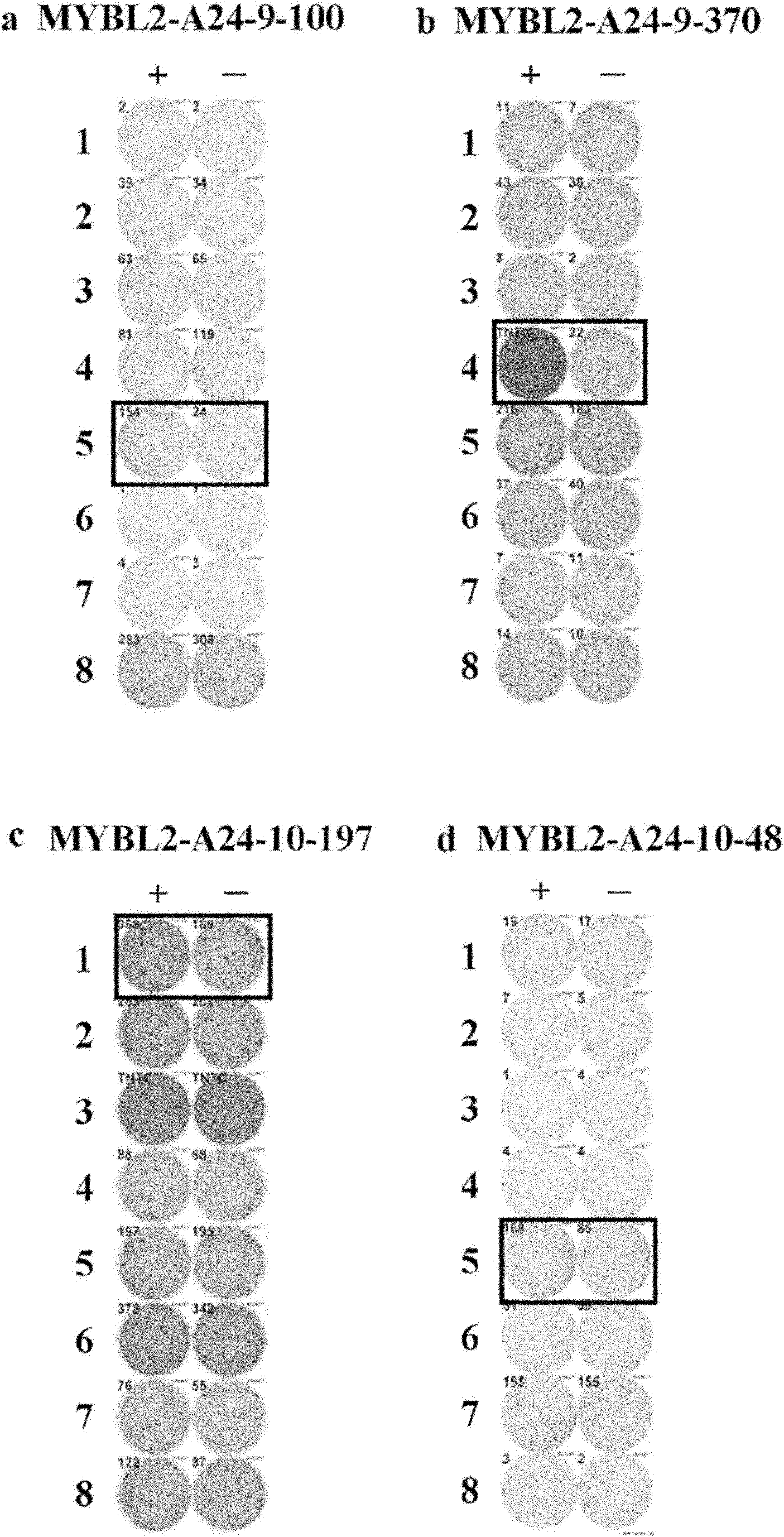
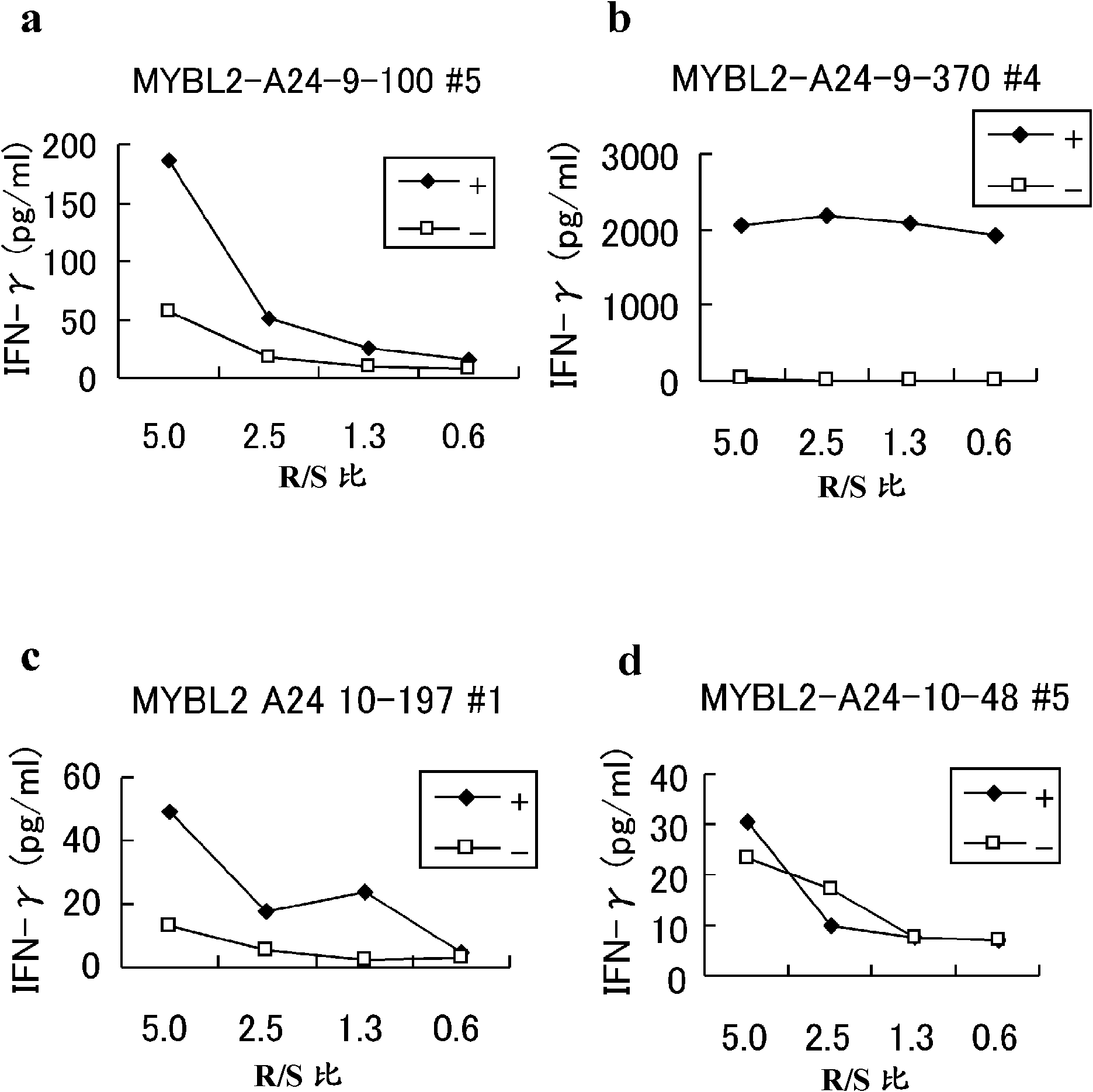
[0159] Selection of candidates for MYBL2-derived peptides
[0160] MYBL2-derived HLA-A*2402-binding 9- and 10-mer peptides were predicted using the binding prediction software "BIMAS" (http: / / www-bimas.cit.nih.gov / molbio / hla_bind), which It is described in Parker KC et al. J Immunol 1994, 152(1): 163-75 and Kuzushima K et al. Blood 2001, 98(6): 1872-81. The peptides were synthesized by Sigma (Sapporo, Japan) following standard solid phase synthesis and purified by reverse phase high performance liquid chromatography (HPLC). The purity (>90%) and identity of the peptides were determined by analytical HPLC and mass spectrometry analysis, respectively. Peptides were dissolved in dimethyl sulfoxide (DMSO) at 20 mg / ml and stored at -80C.
[0161] In vitro CTL induction
[0162...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com