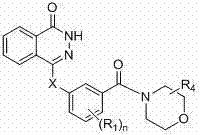
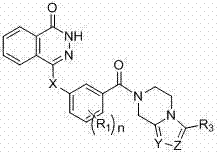
Novel phthalazinone derivatives and uses thereof
A compound and solvate technology, applied in the field of 2,3-naphthyridine derivatives and their use as medicine, can solve problems such as energy depletion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1: Preparation of 4-(3-(4-pyridineformylpiperazine-1-carbonyl)phenylamino)phthalazin-1(2H)-one (001)
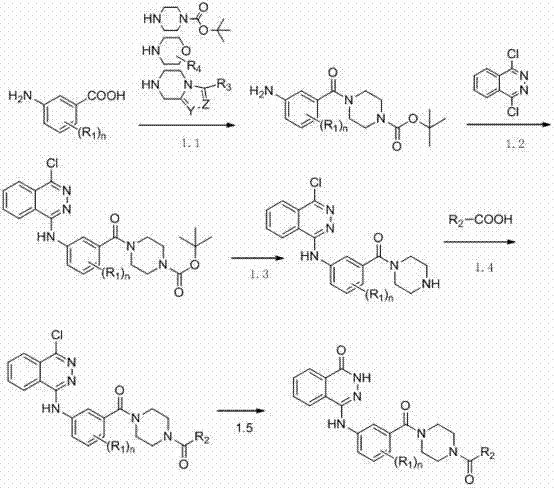
[0184] Dissolve tert-butoxycarbonylpiperazine (4.28g, 0.023mol), triethylamine (6.98g, 0.069mol), m-aminobenzoic acid (7.01g, 0.023mol) in N,N-dimethylformamide ( 100ml), add benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (8.72g, 0.023mol), stir at room temperature, add water to quench the reaction and precipitate The solid product was filtered and dried to give tert-butyl 4-(3-aminobenzoyl)piperazine-1-carboxylate.
[0185] Add trifluoroacetic acid (0.25ml) to a solution of triethylamine (0.3ml) in ethanol (50ml) in an ice bath (0°C), and add 4-(3-aminobenzoyl)piperene obtained in the previous step after 15 minutes tert-butyloxazine-1-carboxylate (1.65g, 0.0054mol) and 1,4-dichlorophthalazine (1.07g, 0.0054mol), heated to reflux, after the reaction (about 30min), add ethyl acetate (100ml ), washed with water (3*50ml), the organic phase was dr...
Embodiment 2
[0191]Example 2: Preparation of 4-(3-(4-(2-propylpentanoylpiperazine-1-carbonyl)phenylamino)phthalazin-1(2H)-one (003)
[0192] Preparation Method Reference Example 1: The target compound was obtained with a melting point of 204-205°C.
[0193] ESI-MS m / z: 475.7(m+H) + , Calculated: 475.3.
[0194]
Embodiment 3
[0195] Example 3: Preparation of 4-(3-(4-(cyclopropylformyl)piperazine-1-carbonyl)phenylamino)phthalazin-1(2H)-one (007)
[0196] The preparation method refers to Example 1, and the melting point is >230°C.
[0197] ESI-MS m / z: 415.9 (m-H) - , Calculated: 416.2.
[0198]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com