Peripheral blood memory T cell culture method
A cell culture and peripheral blood technology, applied in the field of peripheral blood memory T cell culture, can solve the problems of poor cell activity and low memory T cell magnification, and achieve the effect of increasing the ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] In some embodiments, the preparation method of the autologous tumor antigen comprises:
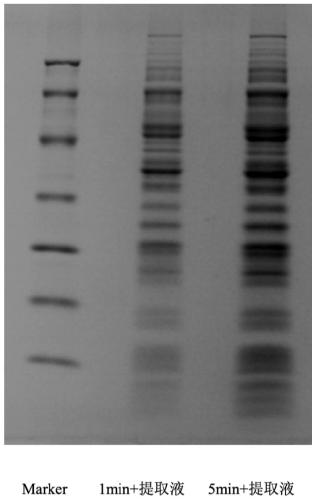
[0049] The tumor tissue was mechanically crushed and resuspended in the antigen extract solution, then ultrasonically crushed at 280W-320W for 50s-70s, shaken in an ice bath, centrifuged, and the supernatant was dialyzed at low temperature.
[0050] In some embodiments, the working concentration of the following components of the antigen extraction solution in the aqueous solution is within the following range:
[0051] -Metal ion chelating agent 4mM~6mM,
[0052] -Iodoacetamide 2nM~3nM,
[0053] -Zwittergent 0.18g / mL%~0.28g / mL%, and
[0054] - buffer components;
[0055] In addition, the pH of the antigen extraction solution during the antigen extraction process is 8.2-8.6.
[0056] In some embodiments, the working concentration of the following components of the antigen extraction solution in the aqueous solution is within the following range:
Embodiment 1
[0071] 1. Preparation of Tumor Antigens
[0072] 1) Under sterile conditions, the tumor tissue block was washed with an appropriate amount of normal saline to remove blood and other tissues.
[0073] 2) After being minced with scissors to the greatest extent, resuspended in antigen extraction solution (0.01M Tris-HCl, pH 8.4, containing 5mM EDTA, 2.5nM iodoacetamide and 0.2% Zwittergent 3-12).
[0074] 3) Ultrasonic wave at 300W for 1 min to further dissociate the small pieces of tissue, and shake in an ice bath at 4°C for 2 h.
[0075] 4) Centrifuge at 12000 rpm for 15 minutes, place the supernatant in a dialysis bag, and dialyze in distilled water overnight at 4°C.
[0076] 5) Tumor antigens are obtained after concentration.
[0077] 2. Peripheral Blood DC Cell Culture
[0078] 2.1. Peripheral blood DC cell culture medium
[0079] 1) DC culture: AIM-V medium (containing 1% autologous plasma), GM-CSF concentration 50 ng / mL, IL-4 concentration 50 ng / mL.
[0080] 2) DC mat...
Embodiment 2
[0103] 1. Preparation of Tumor Antigens
[0104] 1) Under sterile conditions, the tumor tissue block was washed with an appropriate amount of normal saline to remove blood and other tissues.
[0105] 2) After cutting to the maximum extent with scissors, resuspend in antigen extraction solution (0.012M Tris-HCl, pH8.2, containing 4mM EDTA, 3nM iodoacetamide and 0.18g / mL% Zwittergent 3-12).
[0106] 3) Ultrasonic wave 280W for 70 s to further dissociate small pieces of tissue, and shake in an ice bath at 4°C for 2 h.
[0107] 4) Centrifuge at 12000 rpm for 15 minutes, place the supernatant in a dialysis bag, and dialyze in distilled water overnight at 4°C.
[0108] 5) Tumor antigens are obtained after concentration.
[0109] 2. Peripheral Blood DC Cell Culture
[0110] With embodiment 1.
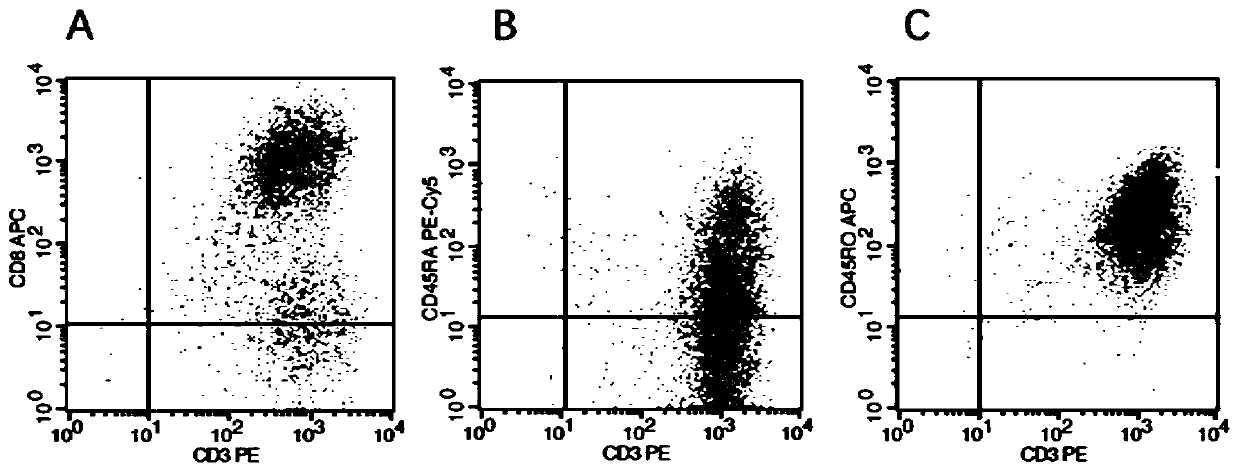
[0111] 3. Peripheral blood memory T cell culture
[0112] 3.1 Peripheral blood memory T cell culture medium
[0113] 1) Coating solution: D-PBS, CD3 monoclonal antibody concentration 450...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com