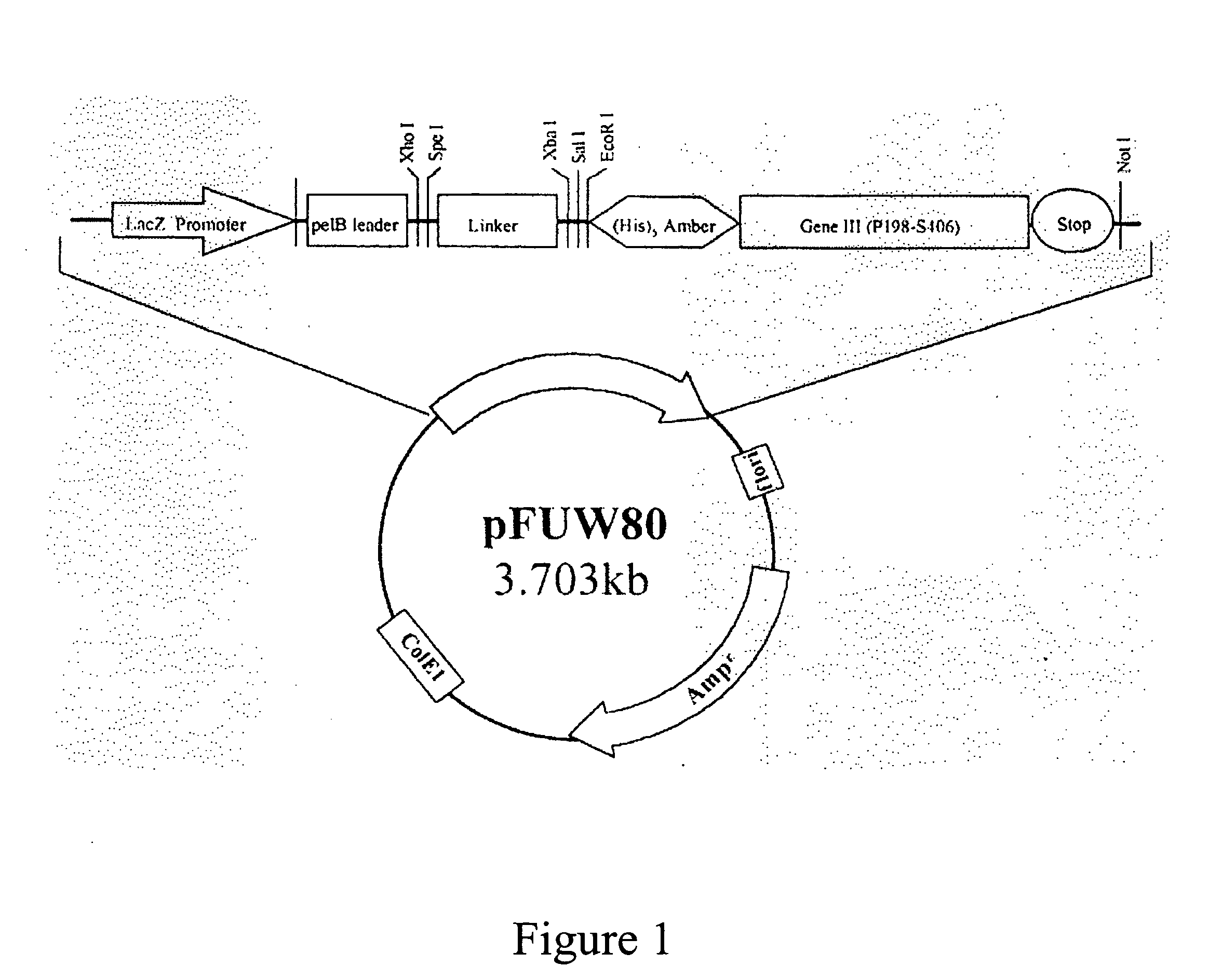
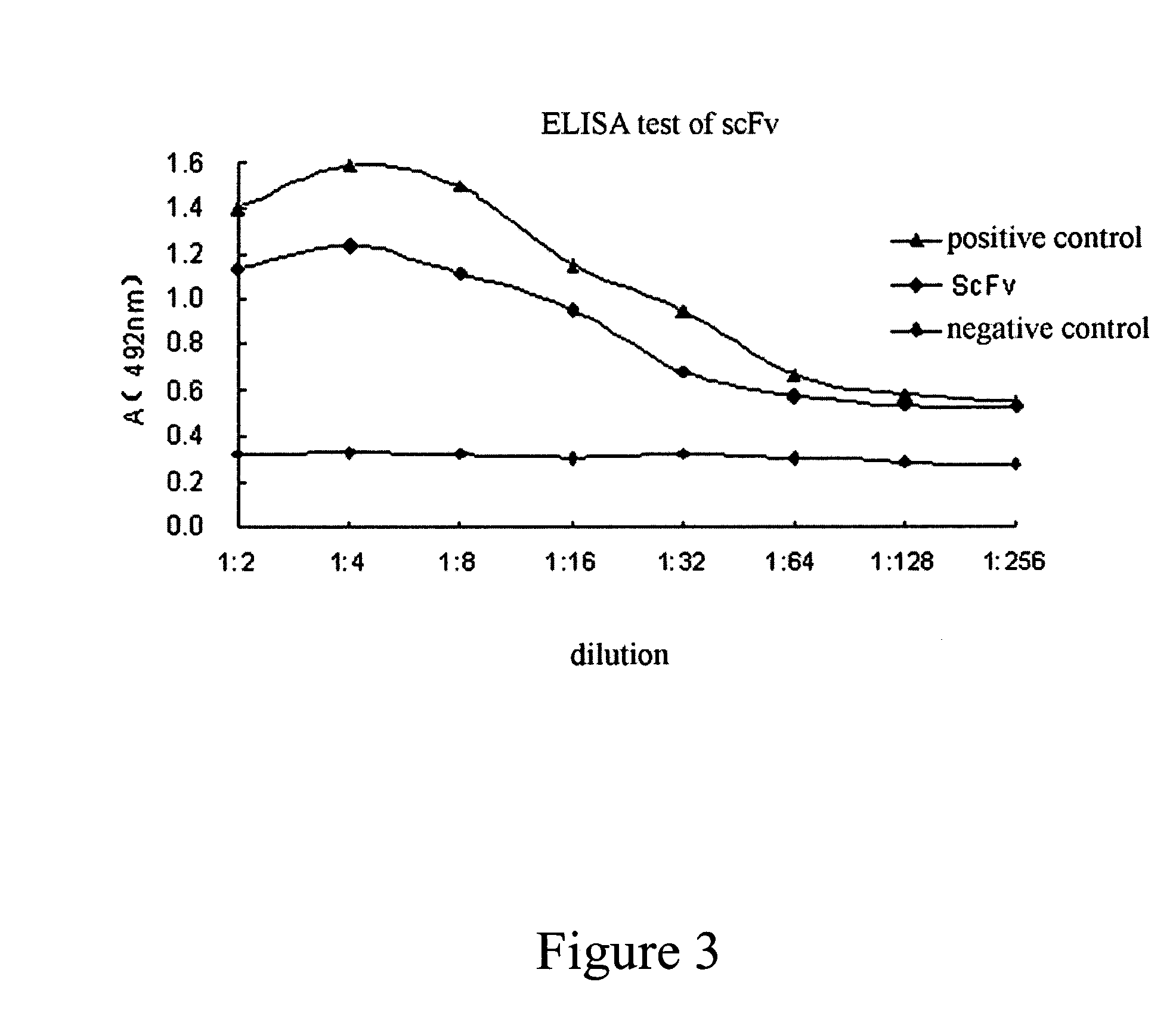
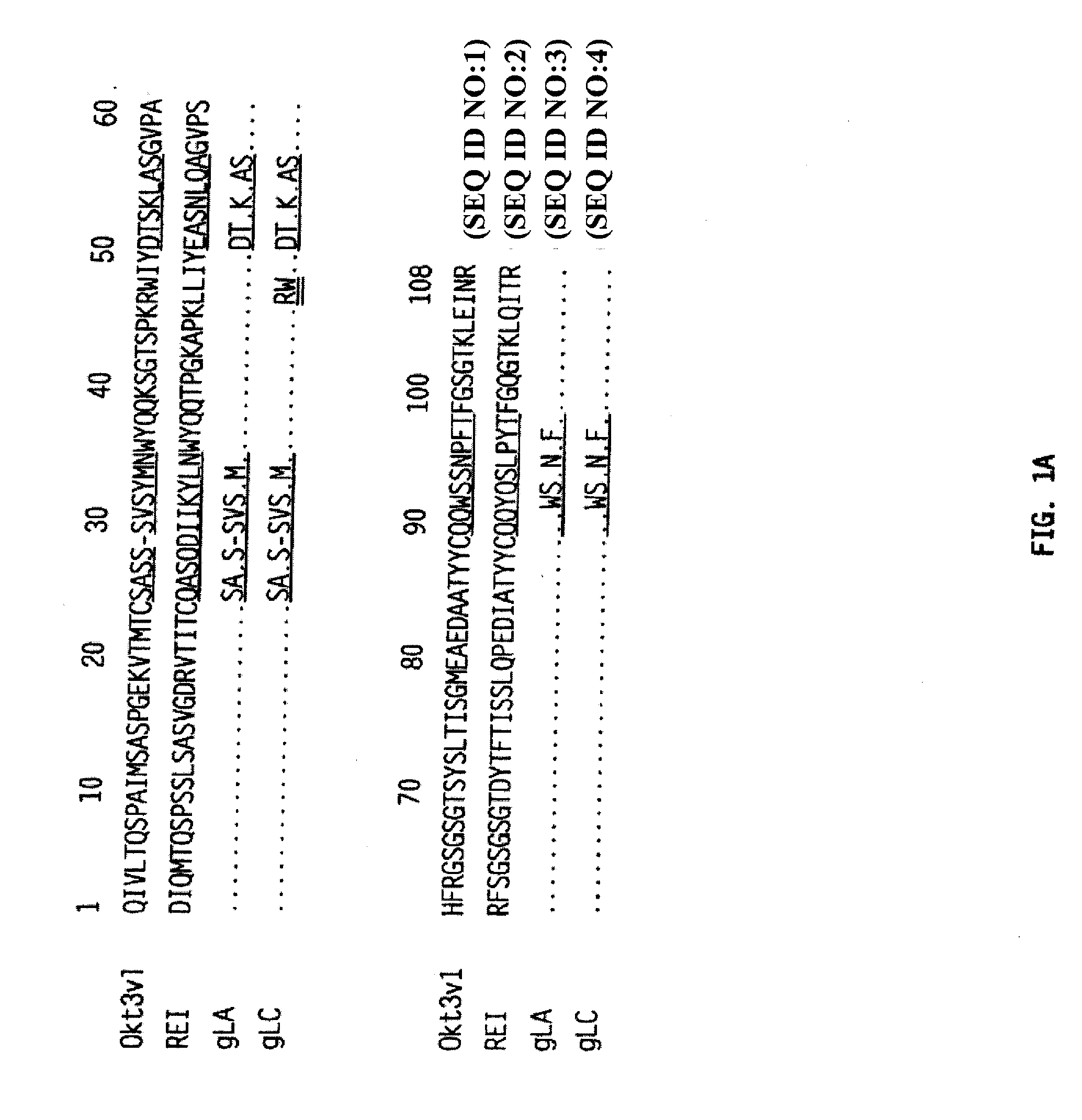
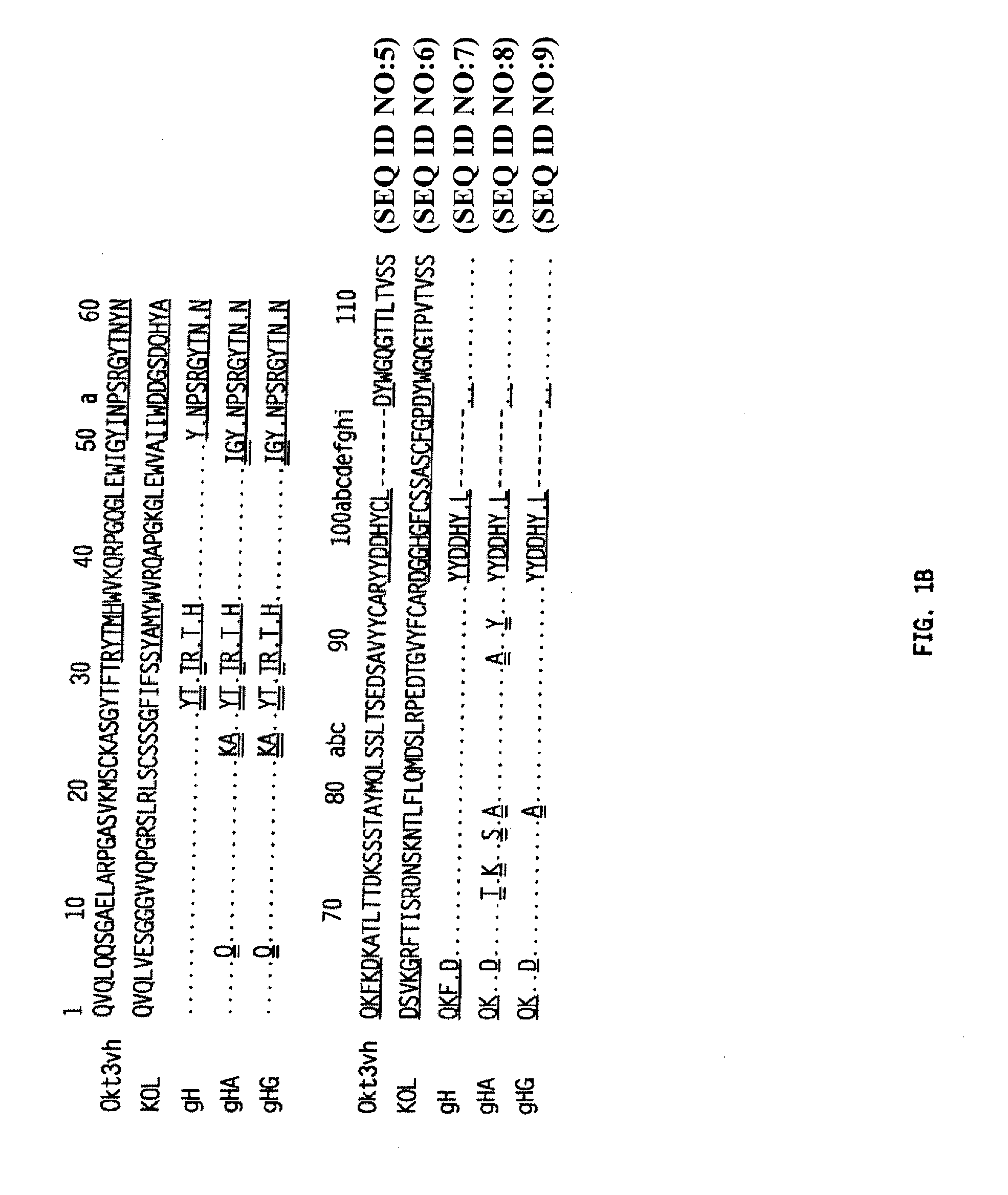
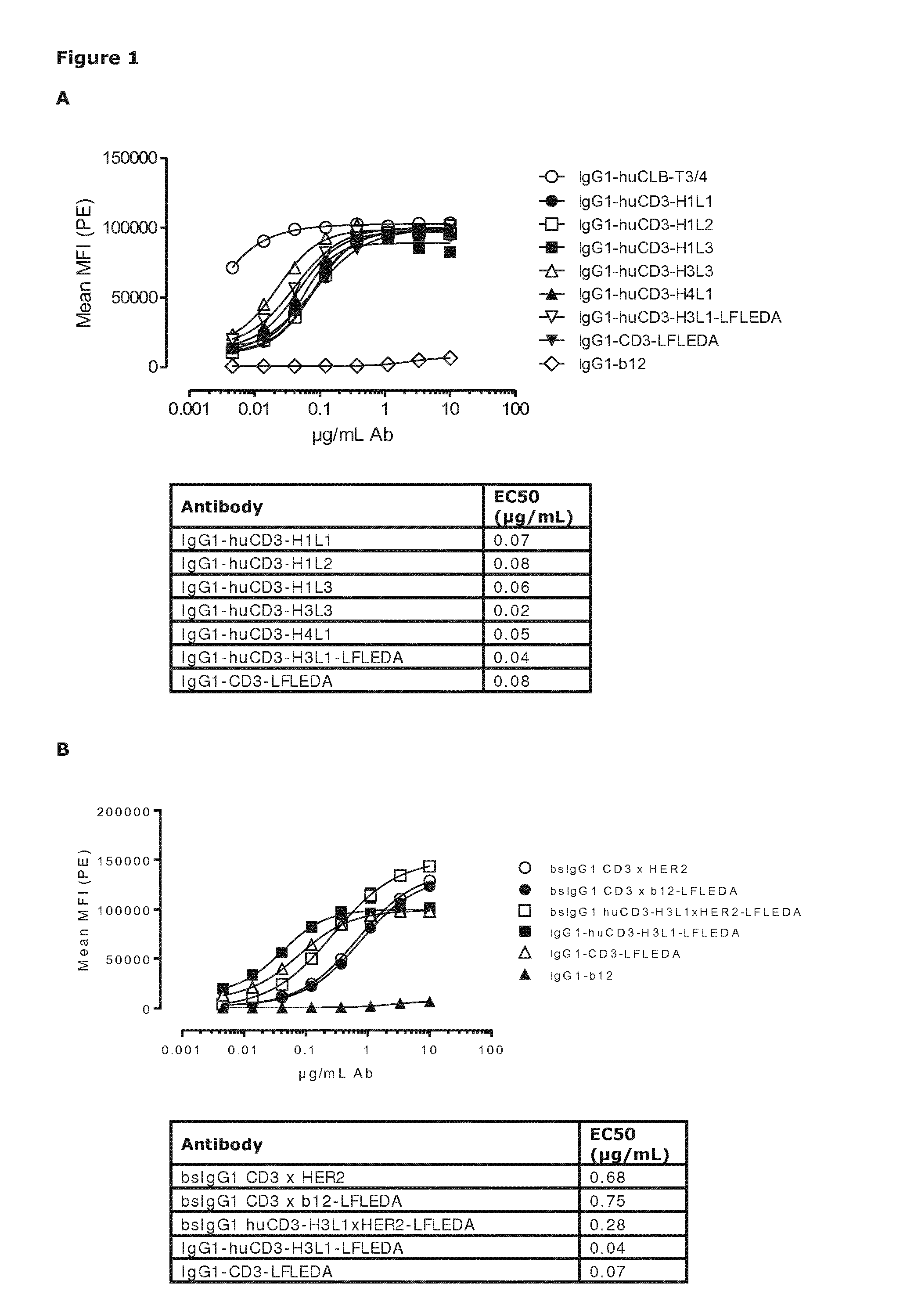
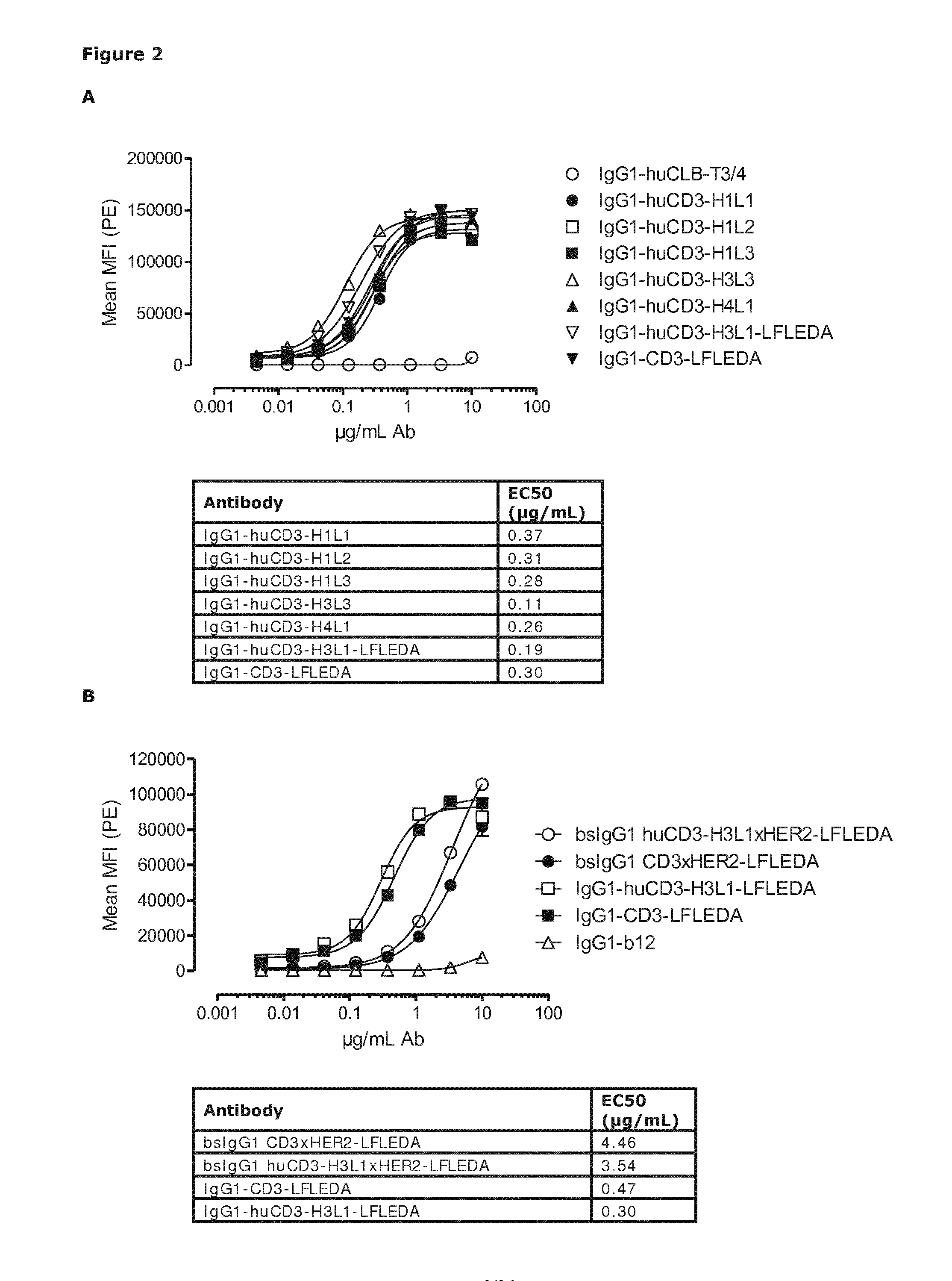
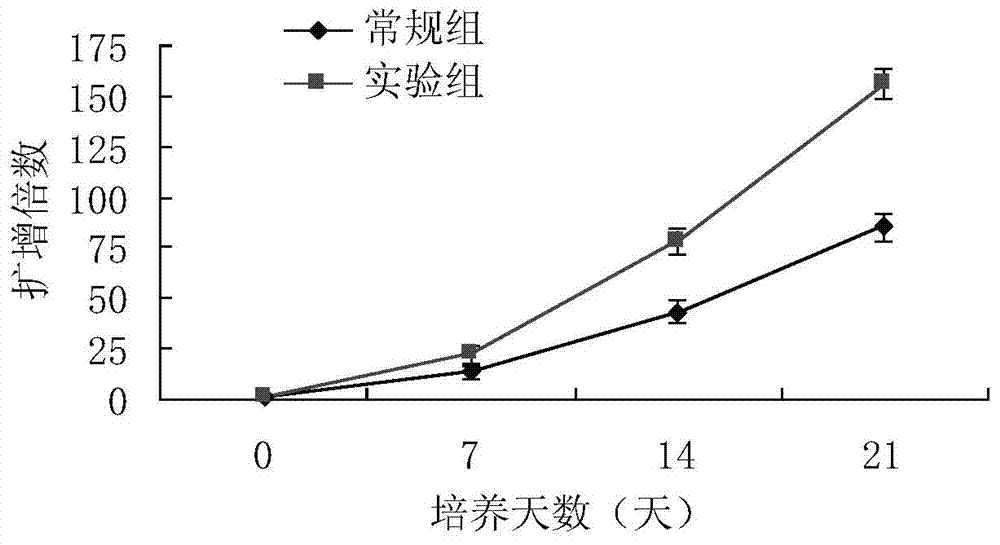
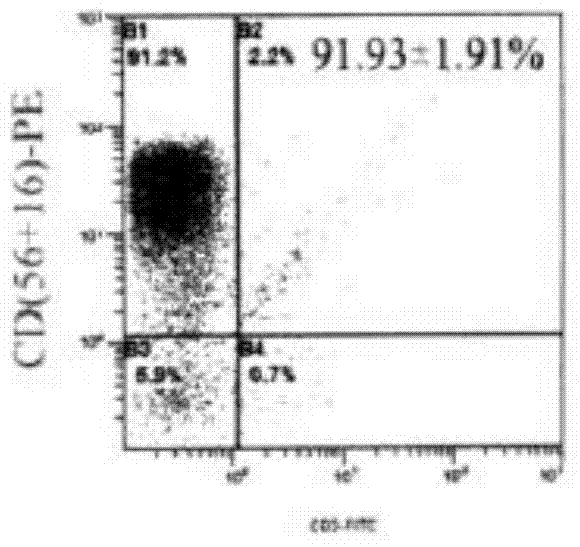
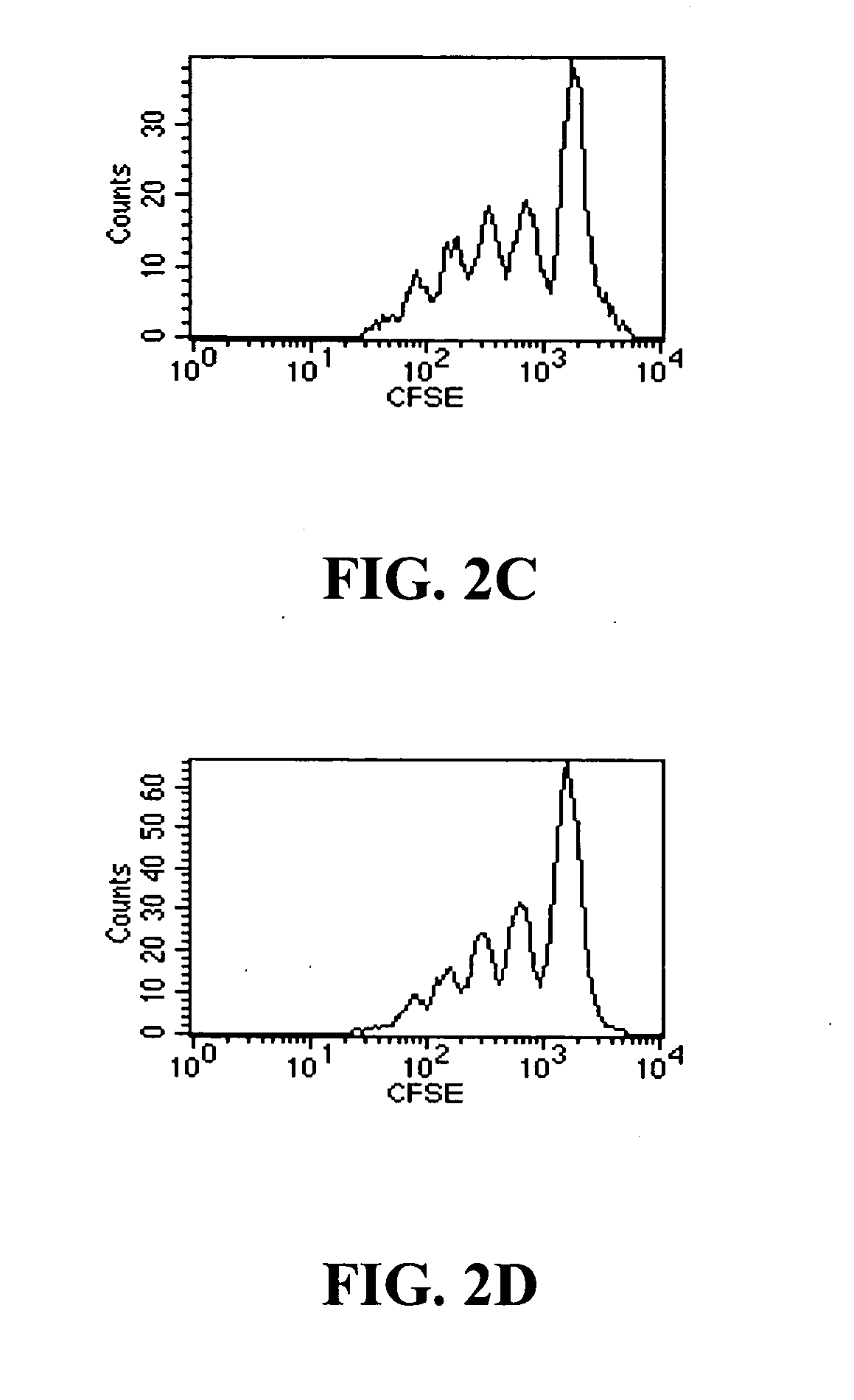
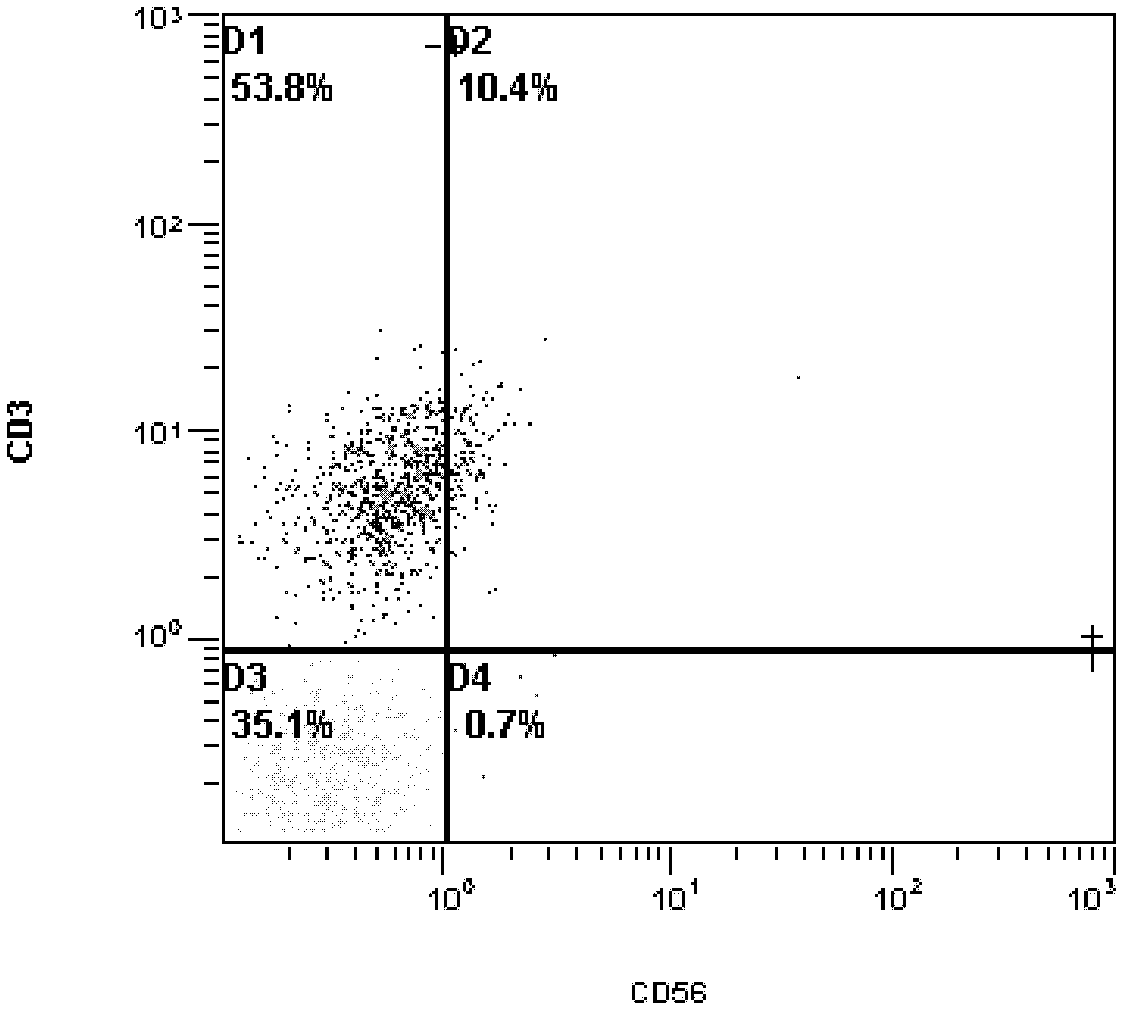
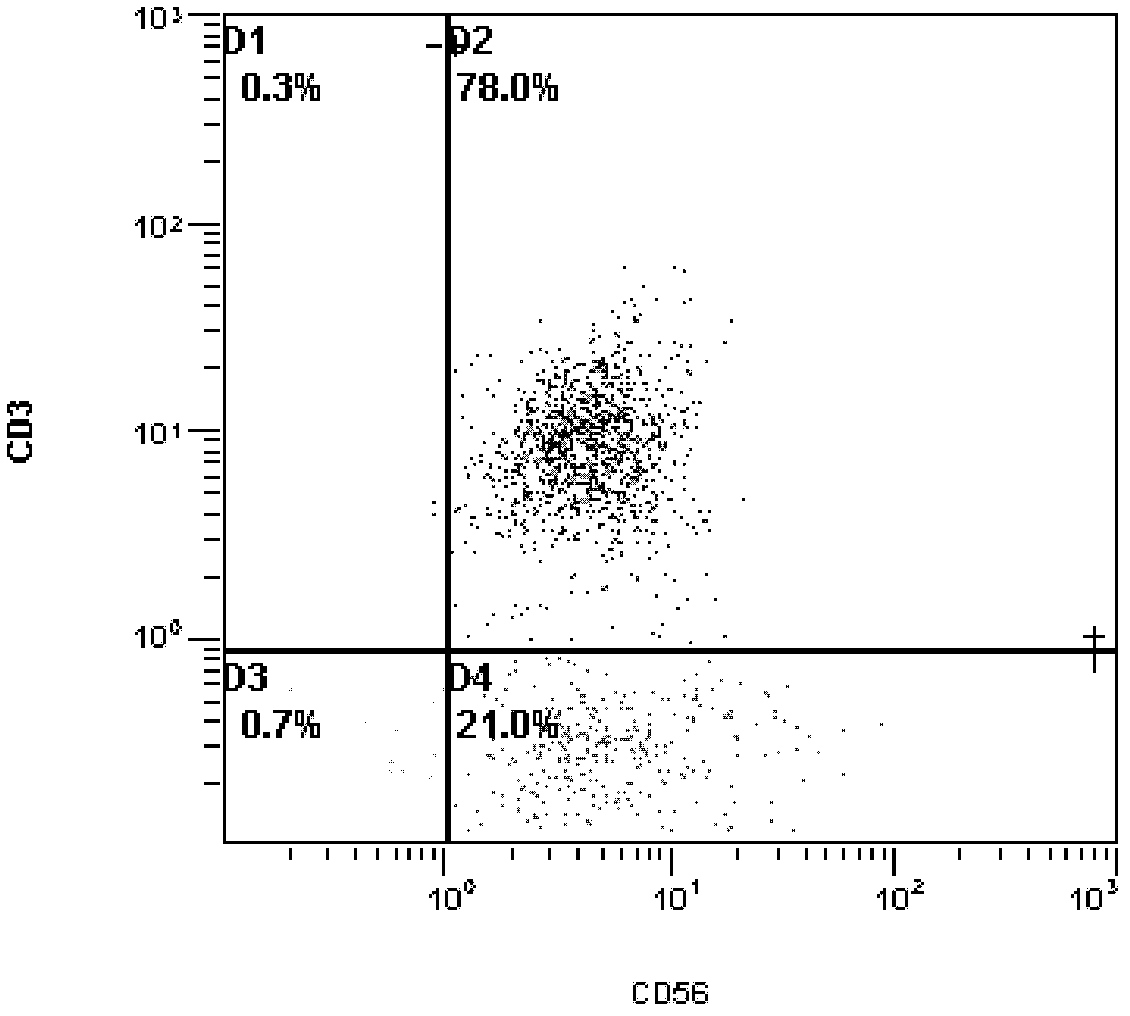
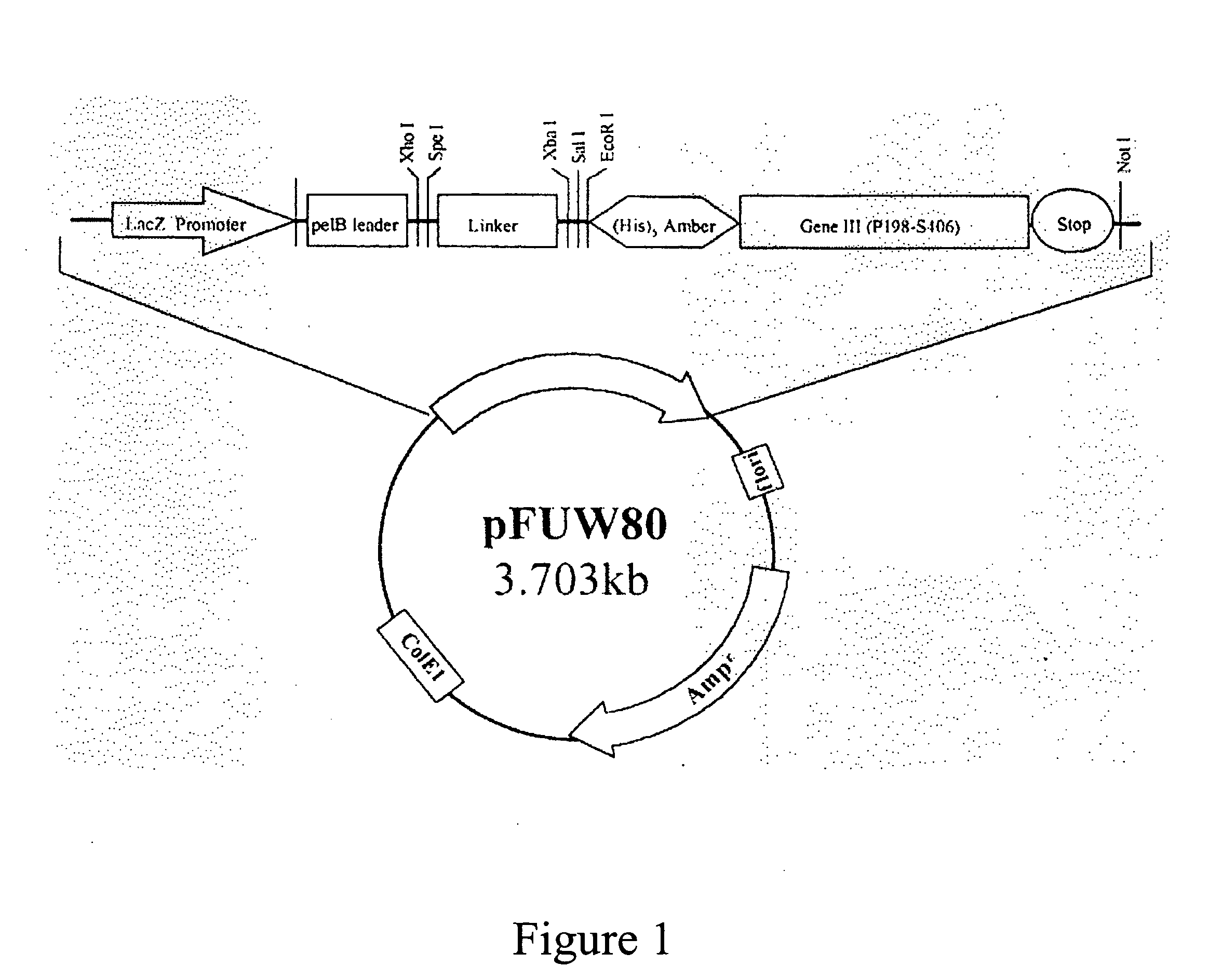
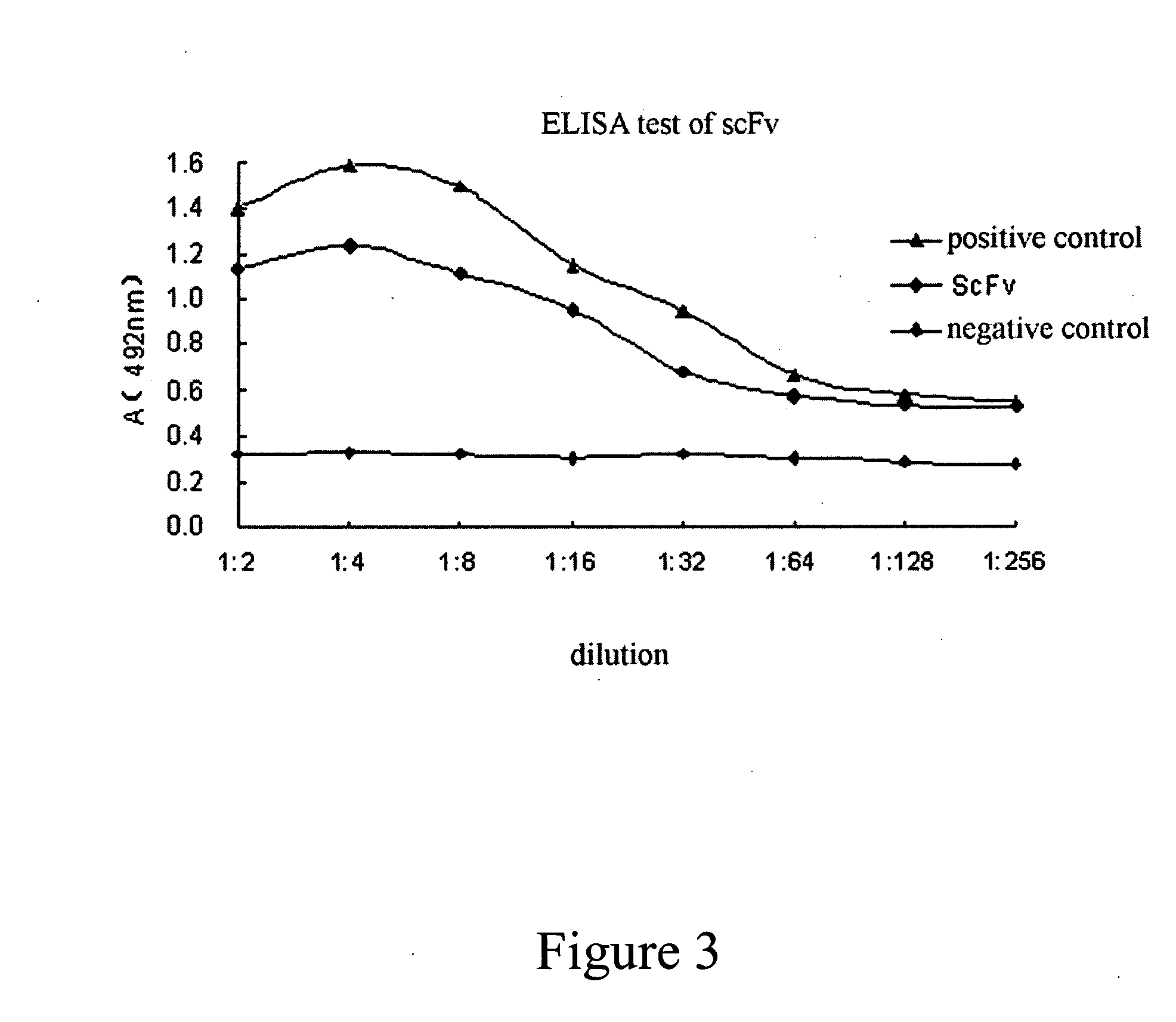
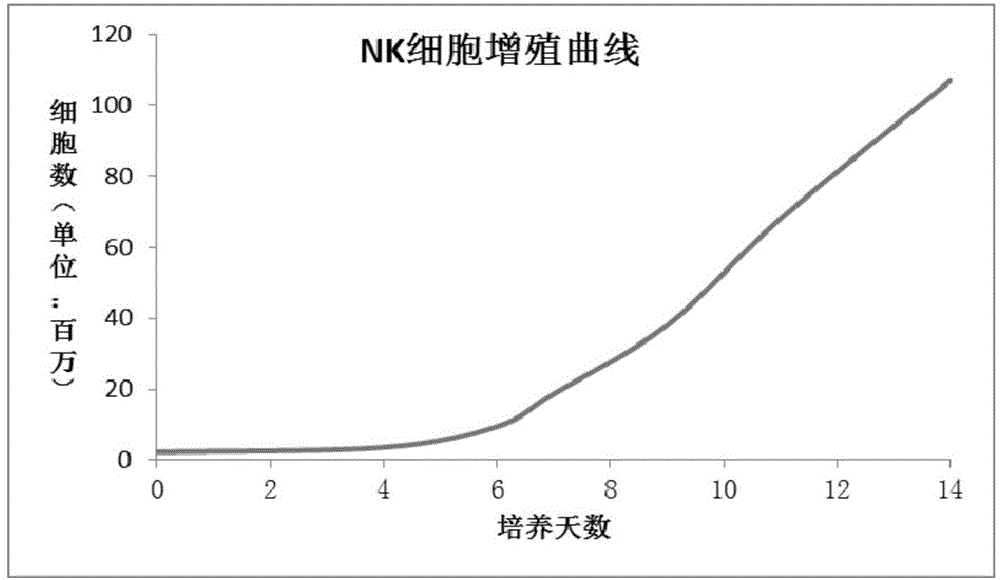
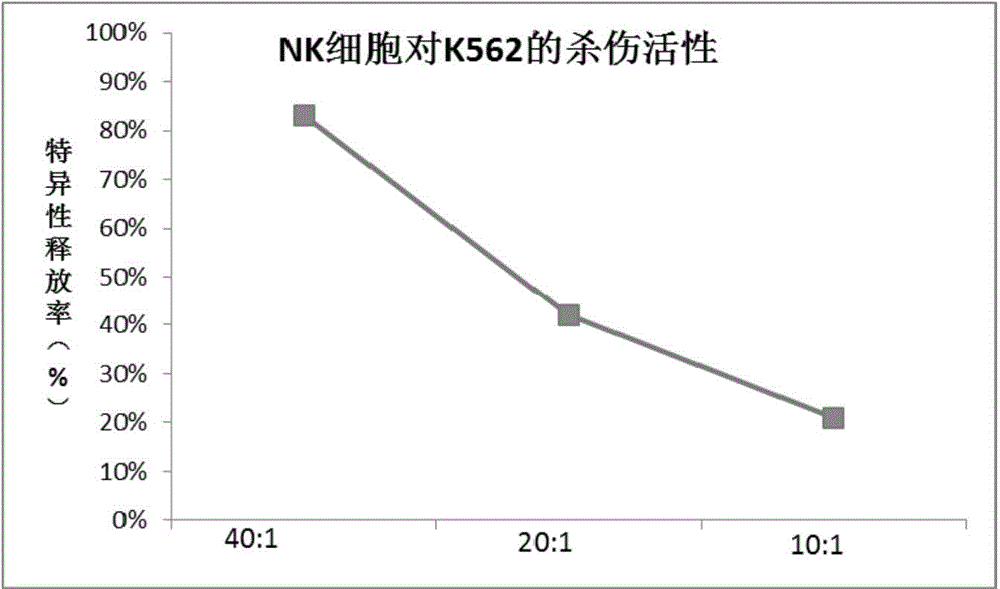
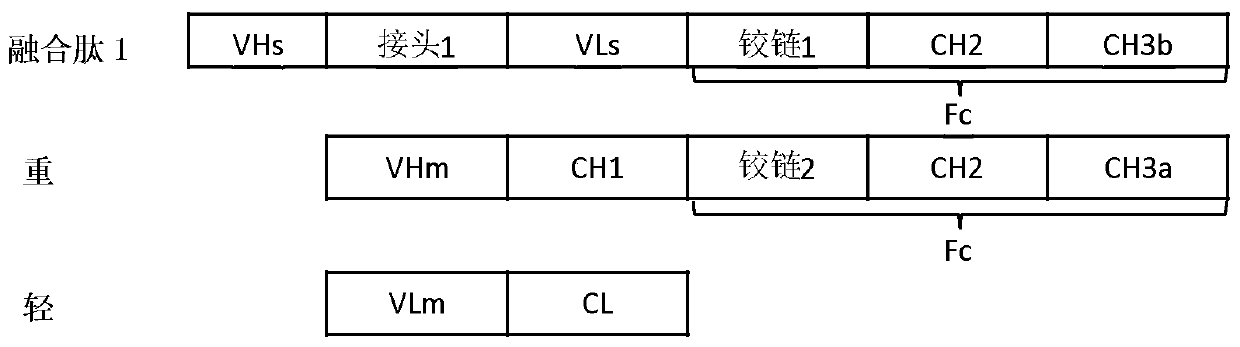
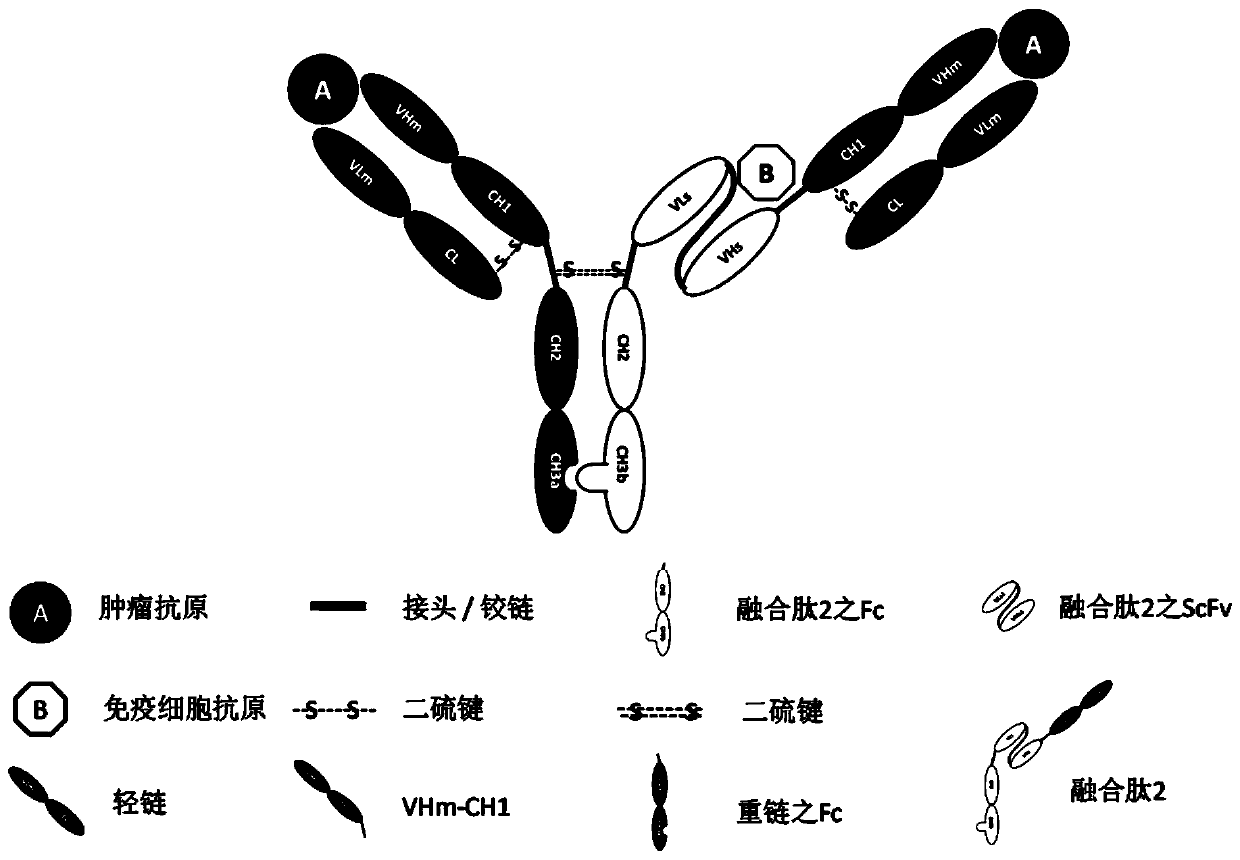
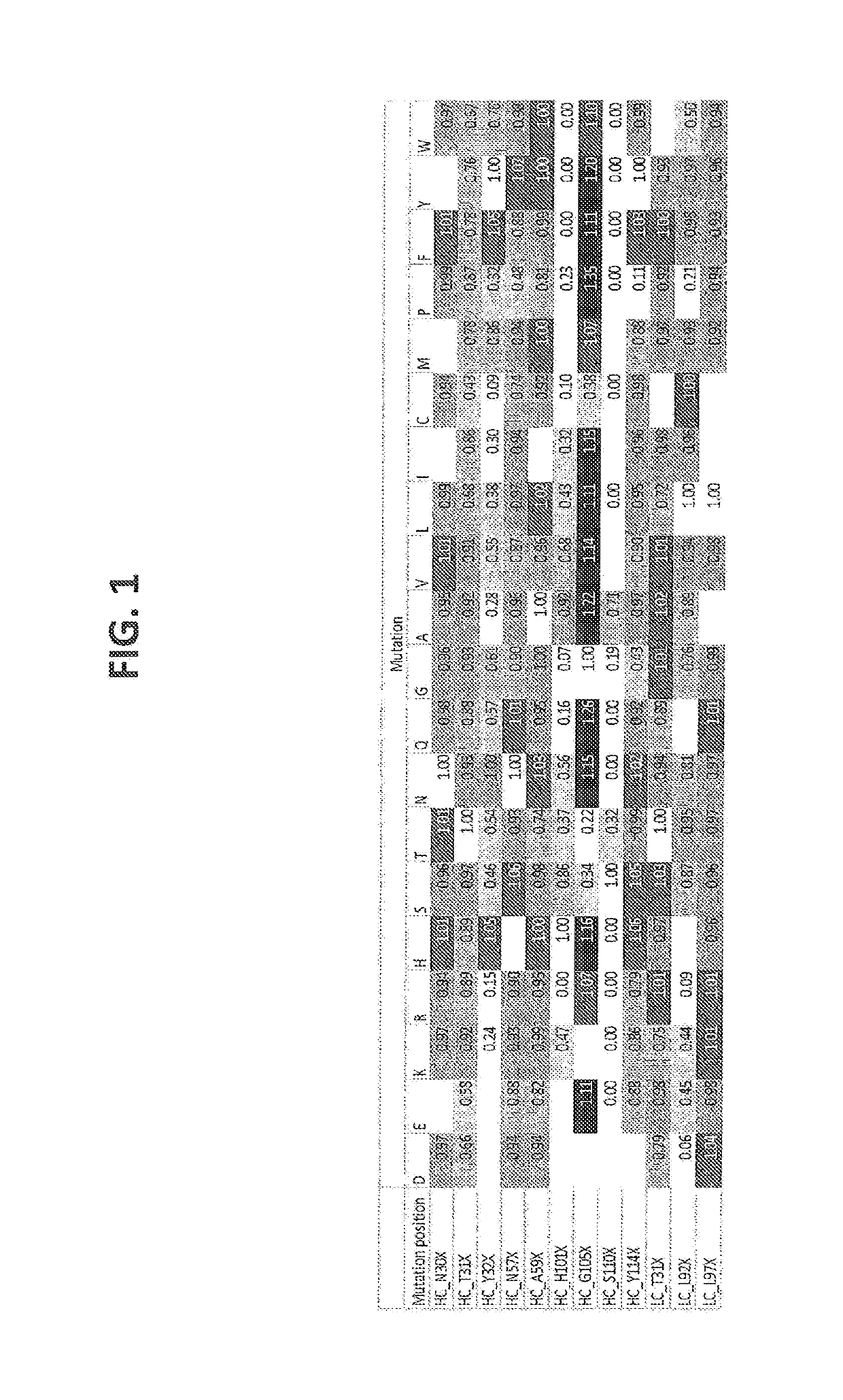
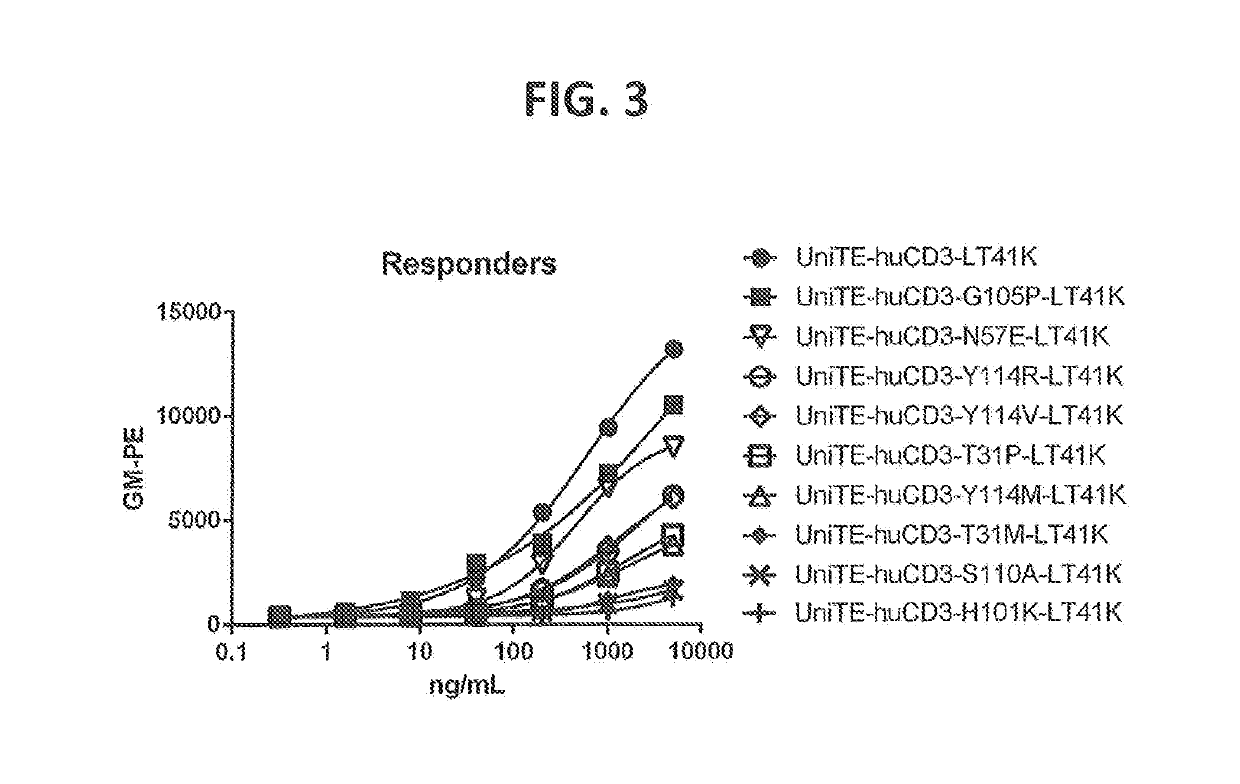
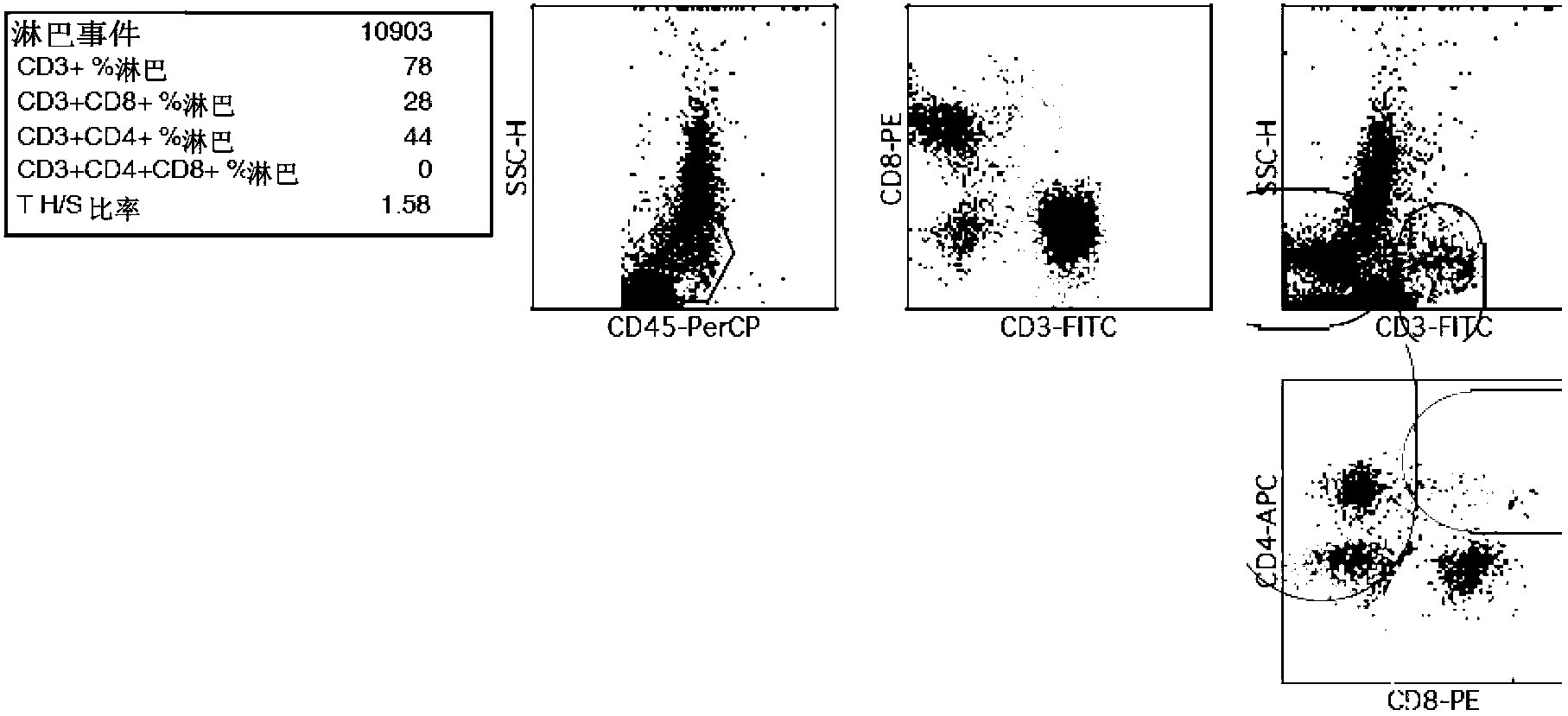
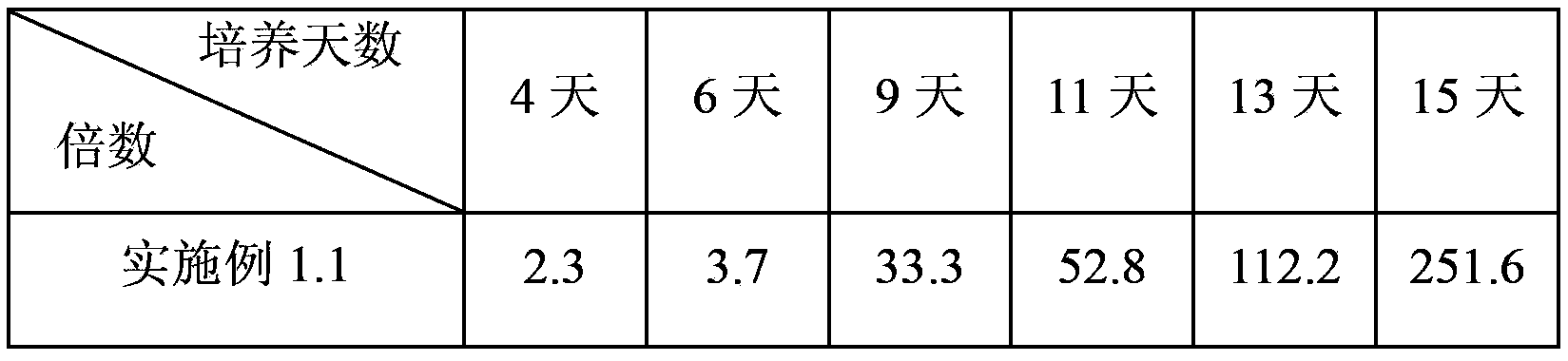
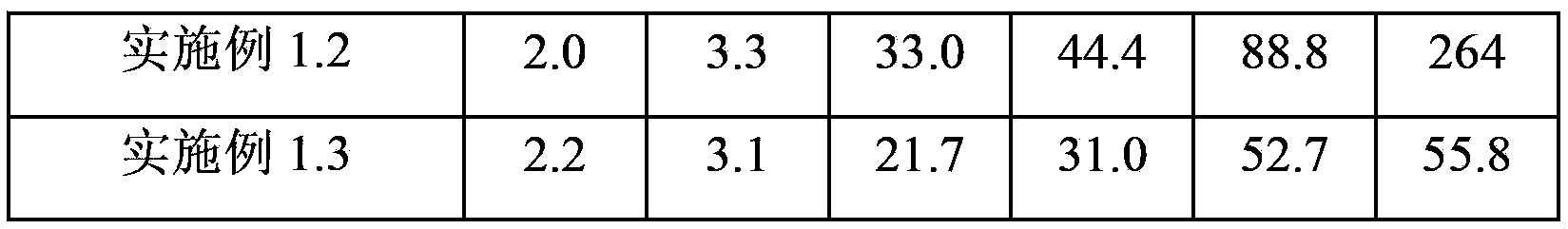
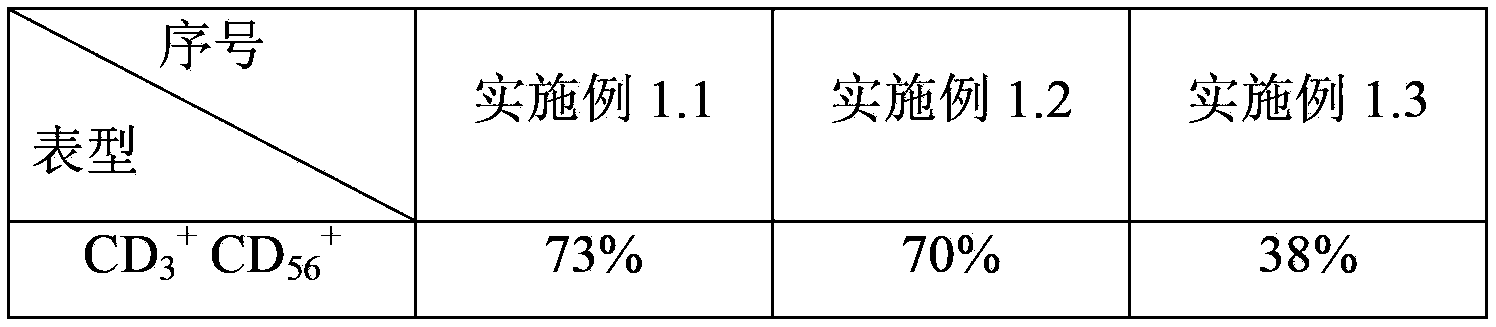
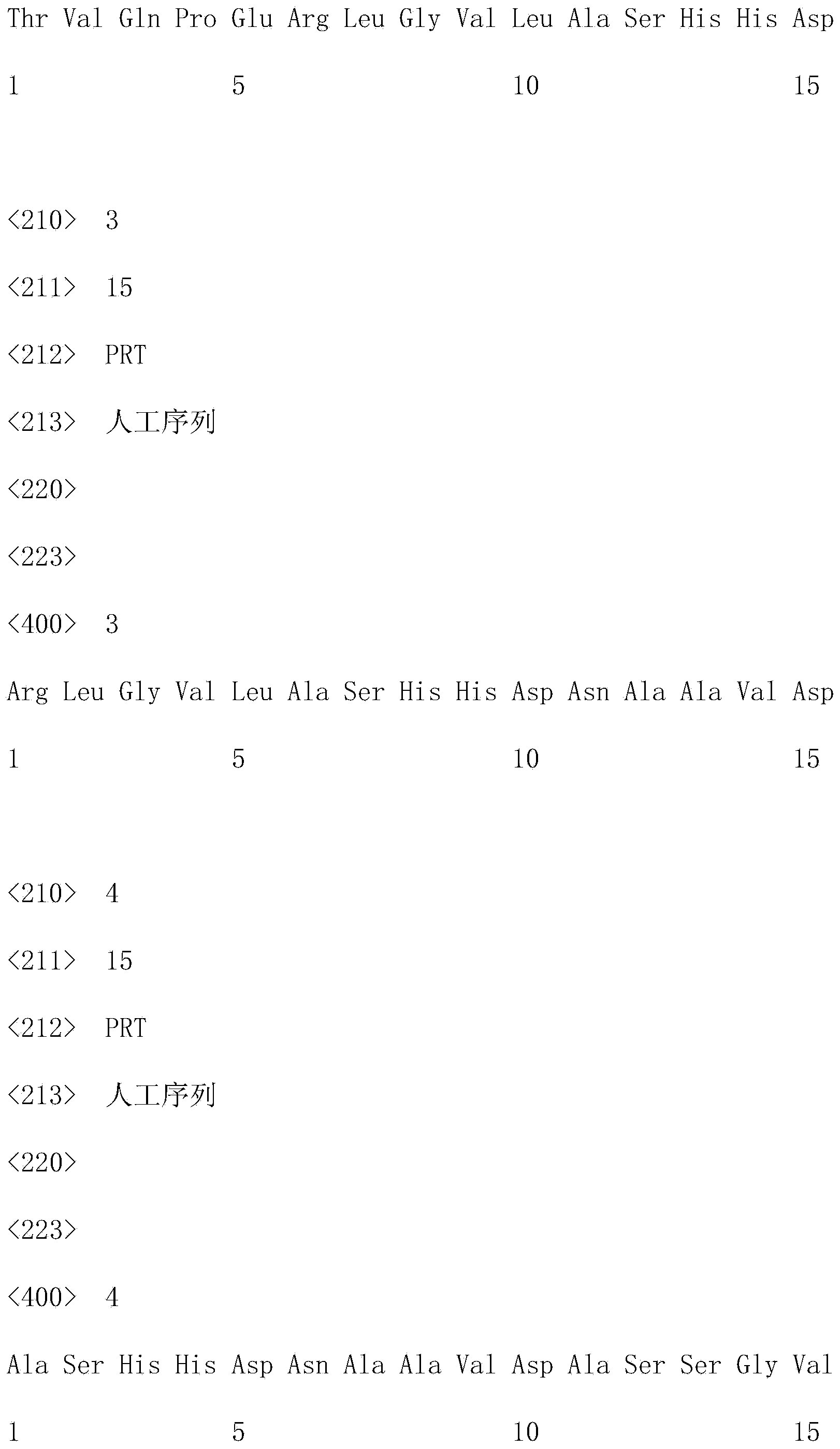Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
193 results about "CD3 Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An anti-CD3 monoclonal antibody is one that binds to CD3 on the surface of T cells. They are immunosuppresive drugs. The first to be approved was muromonab-CD3 in 1986, to treat transplant rejection.
Anti human ovarian cancer-anti CD3 bispecific antibody
InactiveUS7262276B2Low toxicityHigh efficiency and cost-effectivenessImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsComplementarity determining regionAntiendomysial antibodies
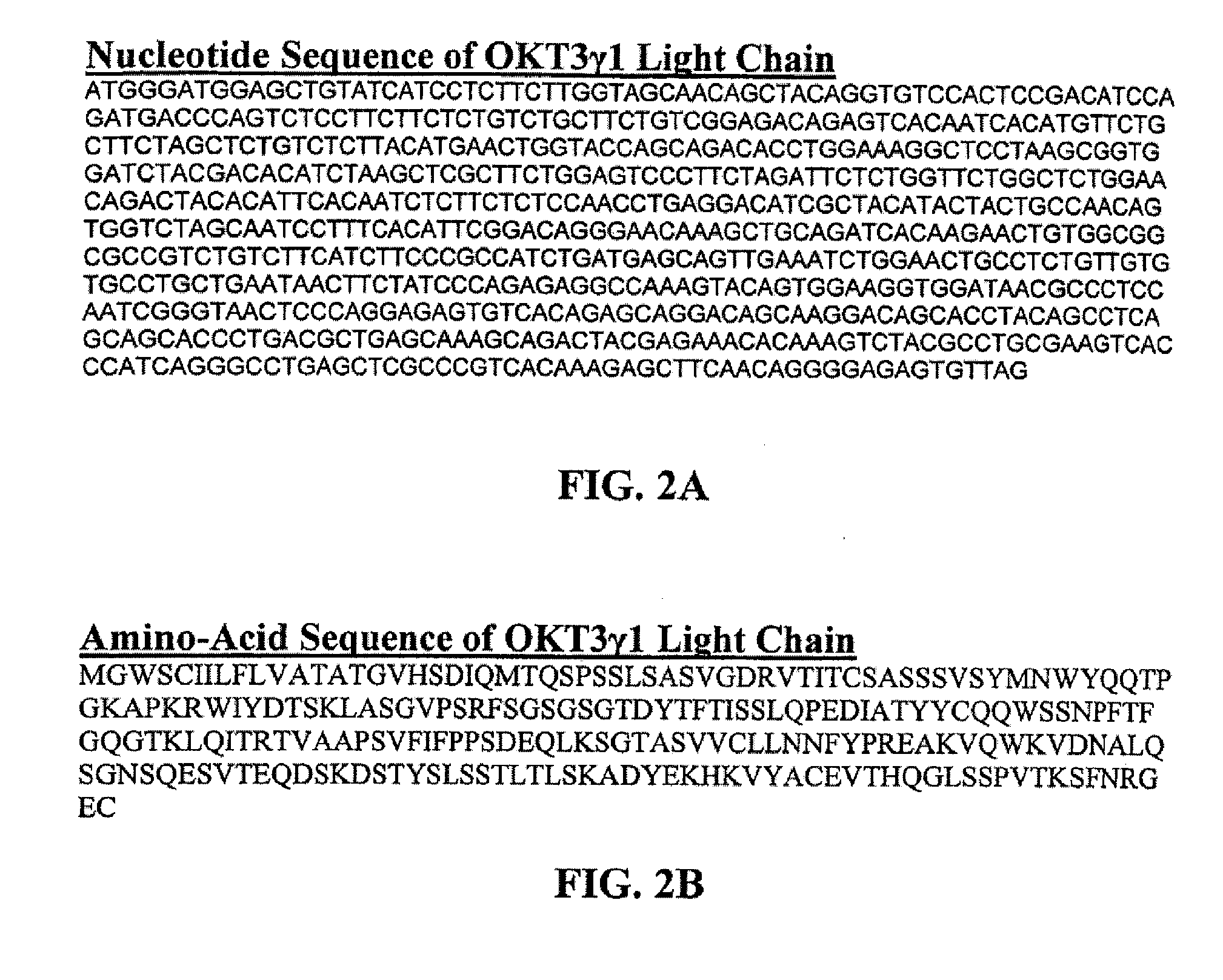
The present invention provides an anti-ovarian cancer bispecific antibody. Said antibody includes two polypeptide domains connected by a polypeptide linker, one is anti-ovarian cancer antibody, or its Fab fragment, single complementarity determining region (CDR) antibody or single chain Fv (scFv) and the other is anti-CD3 antibody, or its Fab fragment, single CDR antibody or scFv. The present invention also provides DNA sequences encoding said antibody, an expression vector containing said DNA sequence, and a host cell containing said expression vector.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
Methods for the Treatment of Autoimmune Disorders Using Immunosuppressive Monoclonal Antibodies with Reduced Toxicity
ActiveUS20080095766A1Reduce the possibilityIncreasing concentration of antibodySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
Gene Engineering Recombinant Anti-CEA, Anti-CD3, And Anti-CD28 Single-Chain Tri-Specific Antibody
InactiveUS20090117108A1Increase production costHigh expressionBacteriaSugar derivativesAntibody fragmentsSingle-domain antibody
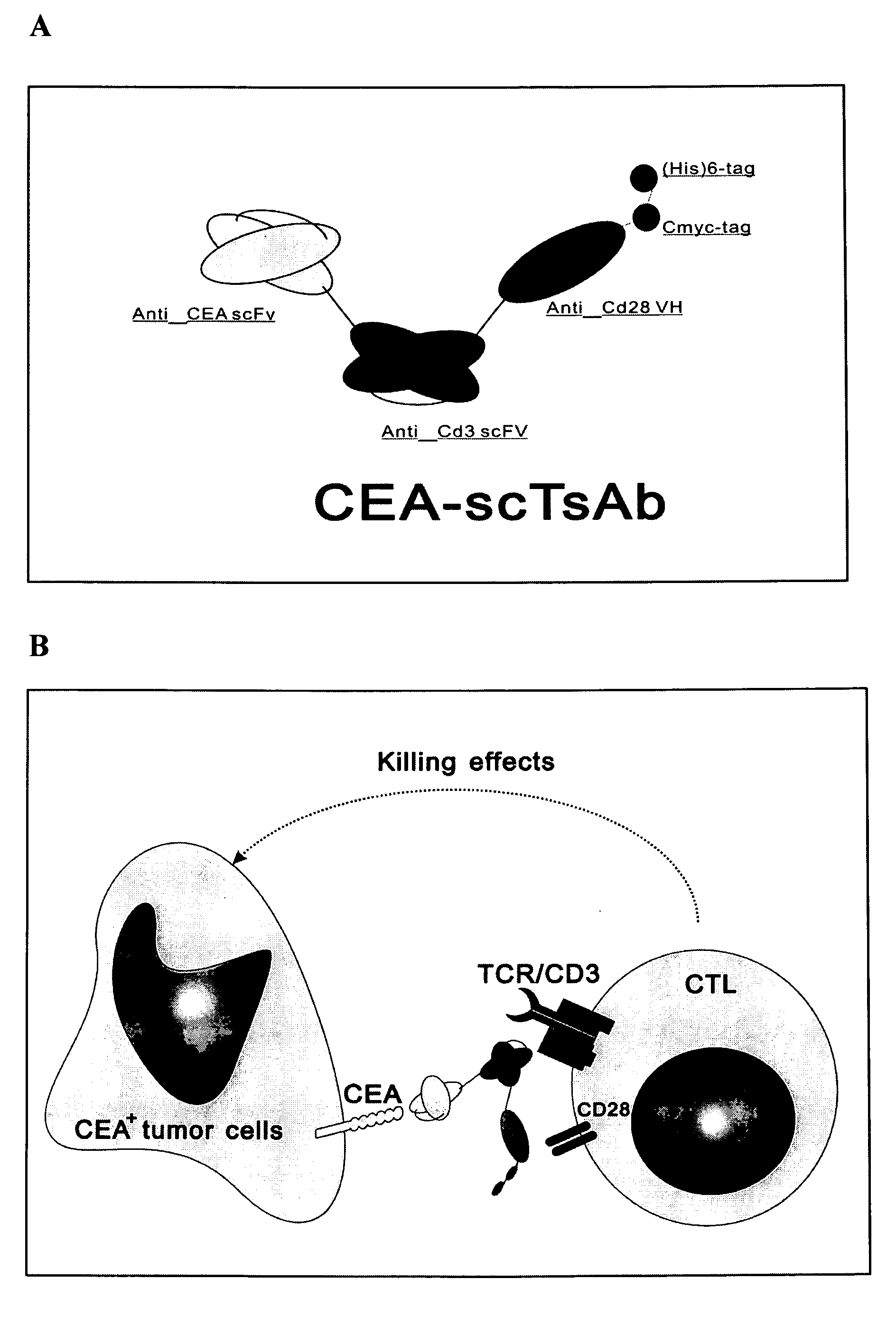
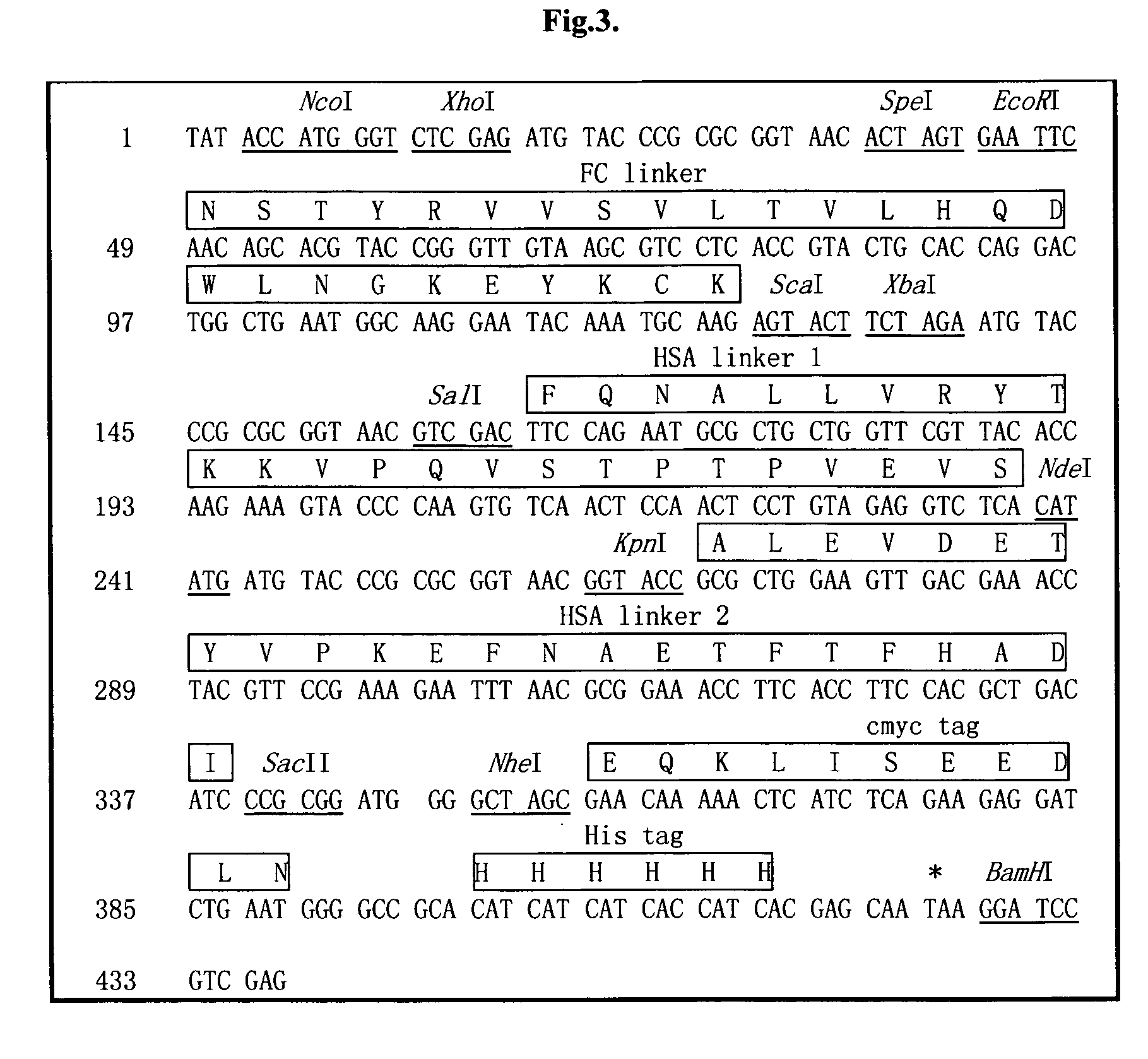
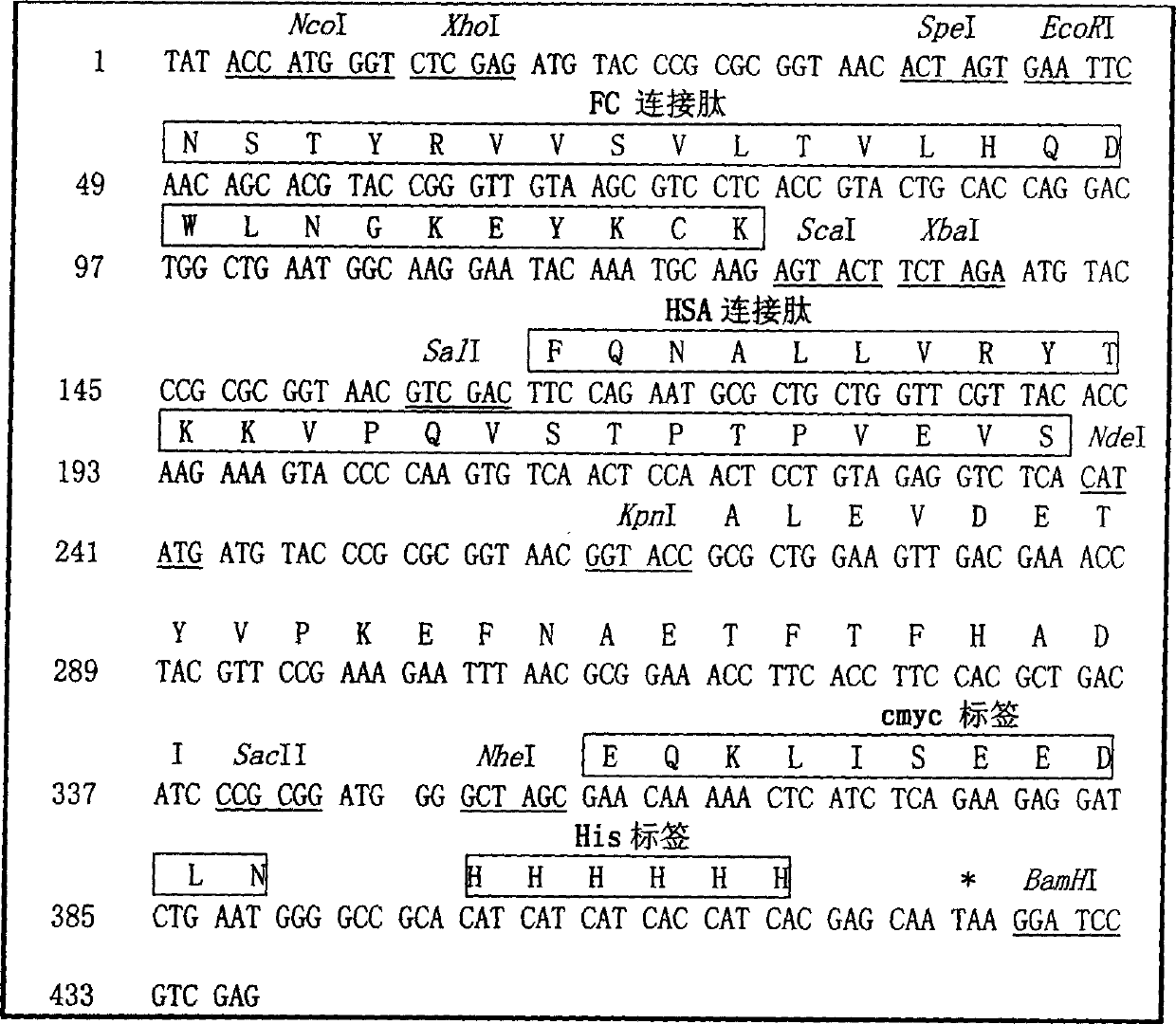
The invention is related to a recombinant single-chain tri-specific antibody made from anti-Tumor Associated Antigen (TAA) antibody, FC interlinker, anti-CD3 antibody, HSA interlinker and anti-CD28 antibody in turn. Particularly, the invention relates to an anti-CEA, anti-CD3, anti-CD28 recombinant single-chain tri-specific antibody, CEA-scTsAb, which was constructed with three tandem antibody fragments (anti-CEA scFv, anti-CD3 scFv and anti-CD28 single-domain antibody) linked by two interlinkers (FC interlinker, HSA interlinker), and could be appended by C myc tag or histidine tag ((His)6-tag) at the C terminal. It also concerns a method for construction, expression and purification of the antibody. It also offers the encoded DNA sequence of the antibody, expression vectors and host cells for the vectors.
Owner:WANG XIANGBIN +5
Humanized or chimeric cd3 antibodies
ActiveUS20160168247A1BacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsBispecific antibodyChimeric antibody
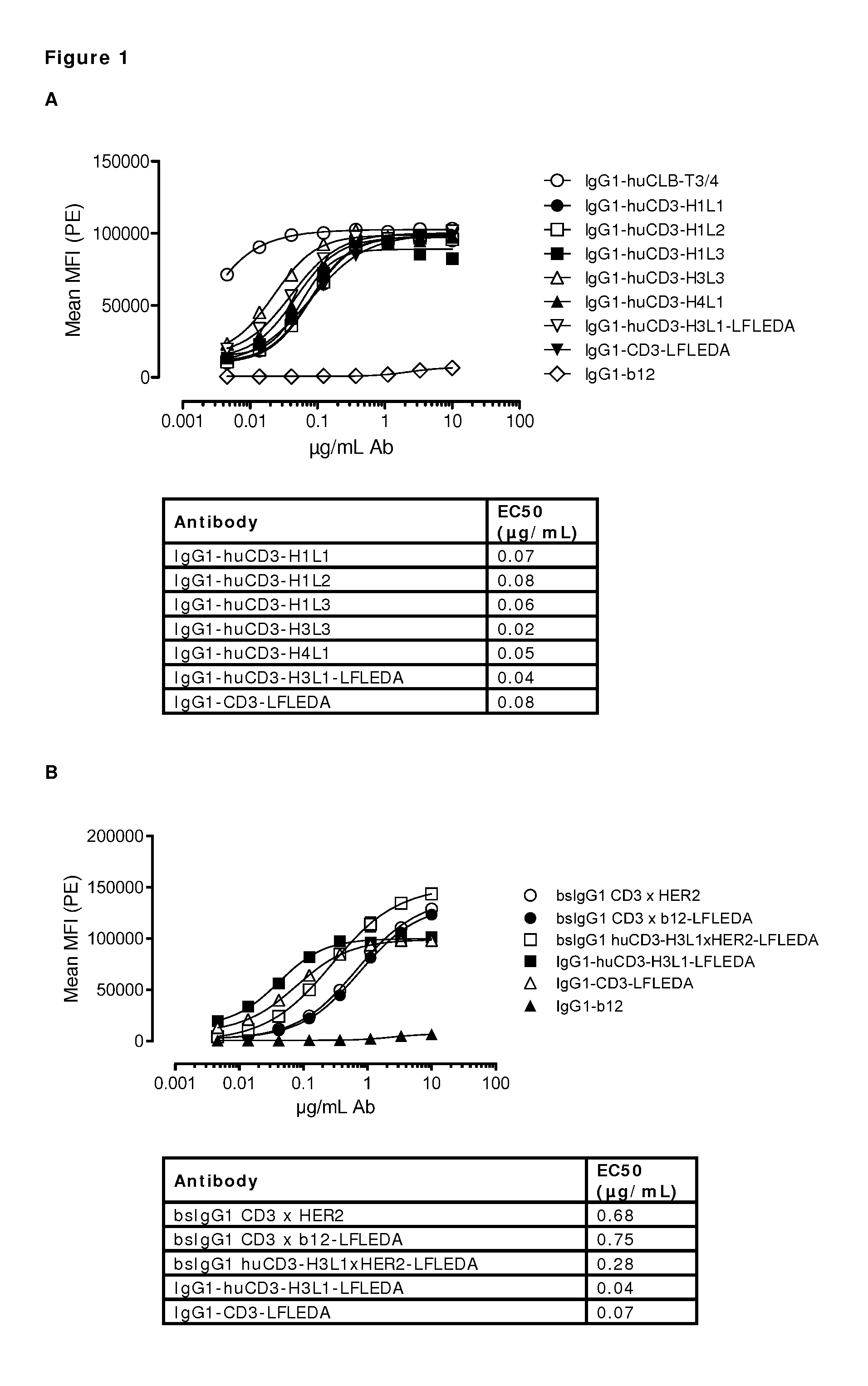
The present invention relates to humanized or chimeric antibodies binding CD3. It furthermore relates to bispecific antibodies, compositions, pharmaceutical compositions, use of said antibodies in the treatment of a disease, and method of treatment.
Owner:GENMAB AS
Method used for in vitro proliferation of NK cells
ActiveCN103756963AIncrease lethalityEasy to synthesizeBlood/immune system cellsSerum free mediaPeripheral blood mononuclear cell
Owner:SHANGHAI CLAISON BIOTECH
Recombining single chained three specific antibodies of anti CCA, anti CD 3, anti CD 28 through genetic engineering
InactiveCN1563092AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesSingle-Chain Antibodies
The invention relates to a recombinant single chain tri-specific antibody, and it is characterized by that it is made by successively connecting antibody of antineoplastic related antigen, FC onnecting peptide, antihuman CD3 antibody, HSA connecting peptide and antihuman CD28 antibody. More specifically, said invention relates to a recombinant single chain tri-specific antibody; CEA-TsAb of anti-CEA, anti-CD3 and anti-CD28. Said antibody is made up by using three antibody fragments (unti-CEA single chain antibody, anti-CD3 single chain antibody and anti-CD28 single chain antibody), utilizing two connecting peptides (FC connecting peptide and HSA connecting peptide) and series-connecting them together, and on its C end C-myc tag and (His)6-tag are added. Said invention also relates to a method for constructing expressing and purifying said antibody, including exonic DNA sequence of said antibody, expression vector and host cell containing said vector.
Owner:弘业新创抗体技术股份有限公司
Humanized or chimeric cd3 antibodies
ActiveUS20160333095A1Biological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseBispecific antibody
The present invention relates to humanized or chimeric antibodies binding CD3. It furthermore relates to bispecific antibodies, compositions, pharmaceutical compositions, use of said antibodies in the treatment of a disease, and method of treatment.
Owner:GENMAB AS
Regulatory CD8cells induced with anti-CD3 antibody
InactiveUS20070190052A1Prevent rejectionAntibody ingredientsUnknown materialsRegulatory T cellTolerability
The invention provides methods for treating autoimmunity, for reestablishing tolerance, and for generally dampening or suppressing the activation state of the immune system. The methods involve the induction or activation of a particular regulatory T cell population, characterized by its expression of CD8, CD25 and Foxp3.
Owner:RGT UNIV OF CALIFORNIA +1
Method for culturing natural killer (NK) and/or natural killer T (NKT) cells
InactiveCN102676453ATo proliferateHigh purityBlood/immune system cellsPeripheral blood mononuclear cellMagnetic bead
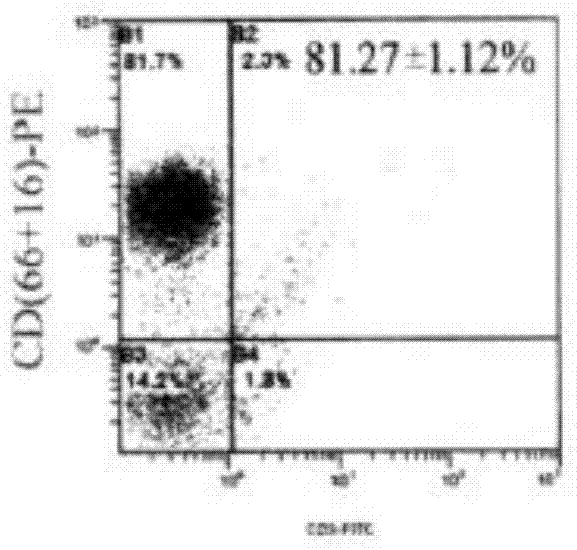
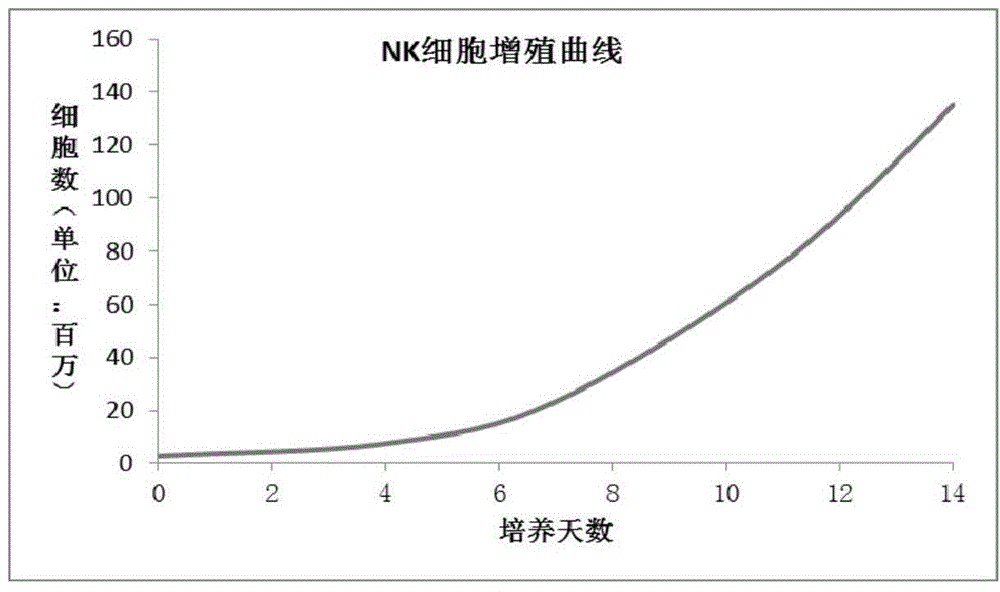
The invention discloses a method for culturing natural killer (NK) and / or natural killer T (NKT) cells. The method comprises the following step: inoculating isolated NK and / or NKT cells into a culture system A for culture to obtain propagated NK and / or NKT cells, wherein the culture system A consists of a buffer solution containing CD3 antibody and / or Retronectin and inducing factors. Experiments prove that peripheral blood mononuclear cells (PBMC) extracted from peripheral blood are separated and enriched through magnetic beads, high-purity CD56+ cells are obtained, two proteins, namely Retronectin and CD3mAb are added into an in-vitro culture system for joint stimulation, and IL-2 and IL-5 factors are used for assisting in induction, so that a culture method capable of obtaining massive NK and NKT cells with high killing activity is established.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Amplifying, freezing and storing and recovering method of activated lymphocyte with CD3+CD8+as major
InactiveCN102839153ASolve the problem of multiple blood collectionHigh purityDead animal preservationBlood/immune system cellsPatient needT lymphocyte
The invention discloses culturing, freezing and recovering methods of an activated lymphocyte with CD3+CD8+as major, which can solve problems that a patient needs carrying out blood sampling for many times caused by continuously utilizing the activated lymphocyte with CD3+CD8+as major. The method comprises the following steps of: (1) contacting the extracted lymphocyte of the peripheral blood with IL-2, IL-15, an anti-CD3 antibody and an anti-CD28 antibody, so as to amplify the activated lymphocyte with CD3+CD8+as major; (2) freezing and storing the activated lymphocyte; and (3) recovering the activated lymphocyte. The activated lymphocyte cultured via the method disclosed by the invention has clear components, and comprises few CD4+CD25+Treg cells and more CD8+T lymphocyte; feedback time and frequency of the activated lymphocyte can be adjusted according to other treatments for a patient, such as a radiotherapy or a chemotherapy, so that diseases, such as tumor, infectious diseases and immunodeficiency can be treated well.
Owner:JINAN TAISHENG BIOLOGICAL TECH CO LTD
Fully human anti-CD3 monoclonal antibodies
InactiveUS20060275292A1Prevent rejectionImprove survivalImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAutoimmune conditionAutoimmune disease
Non-toxic anti-CD3 antibody is useful for the treatment, prevention, and reversal of human autoimmune disease. Anti-CD3 antibody is generated by immunizing xenogenic mice capable of developing fully human antibodies. Because the inventive antibodies are not derived from other species, they do not the invoke the immune responses typically associated with humanized or grafted antibodies.
Owner:AMGEN FREMONT INC
Culture system and culture method for efficient amplification in vitro of autologous NK cells
InactiveCN105238752AAvoid uncertaintyHigh yieldBlood/immune system cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention provides a culture system and a culture method for efficient amplification in vitro of autologous NK (Natural Killer) cells. The culture system comprises a basic culture medium containing GT-T551, interleukin IL-2, interleukin 1L-12, interleukin IL-15, interleukin IL-18, interleukin 1L-17, interleukin IL-22 and natural traditional Chinese medicine substances. The culture method comprises the following steps of: collecting and separating autologous 40ml peripheral blood mononuclear cells; resuspending the cells by a culture medium containing 10 percent of self plasma; adding the cells into a cell culture bottle coated by CD3 antibodies or CD56 antibodiees; performing stimulation by a cell factor combination A; putting the cells in a 37 DEG C and 5-percent-CO<2> culture tank to perform incubation and shaking; after 6 to 7 days, transferring the cells into a cell culture bag to be cultured; complementally adding a culture medium containing a cell factor combination B; in the culture period, culturing the cells in a basic culture medium containing IL-2 every 3 to 4 days; and obtaining high-purity NK cells after the total culture time of 17 to 19 days. The culture system and the culture method have the advantages that the NK cells can be amplified by more than 1000 times in vitro; and the high-purity NK cells exceeding 5*10<9> can be obtained.
Owner:苏州科贝生物技术有限公司
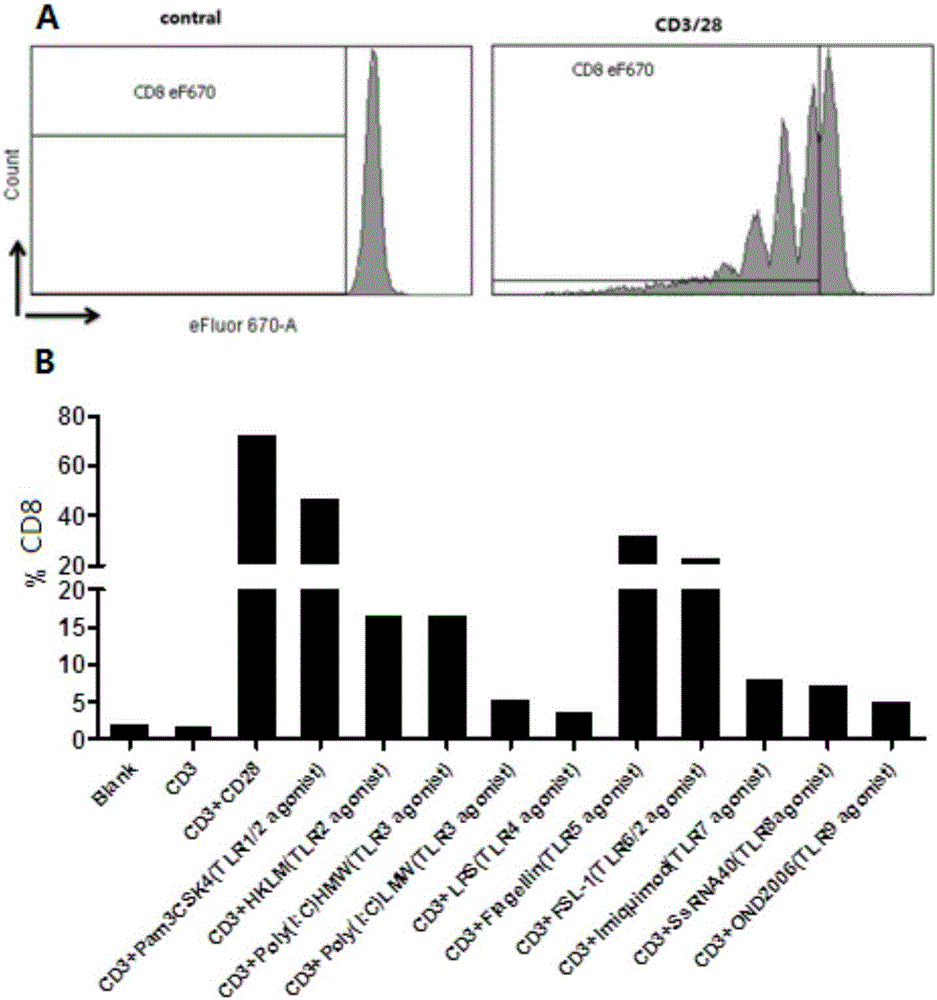
Method for in-vitro amplification of CD8+T cell and cell subset of CD8+T cell
ActiveCN106834228AImprove efficiencyImprove repair functionCell dissociation methodsBlood/immune system cellsTlr agonistsMagnetic bead
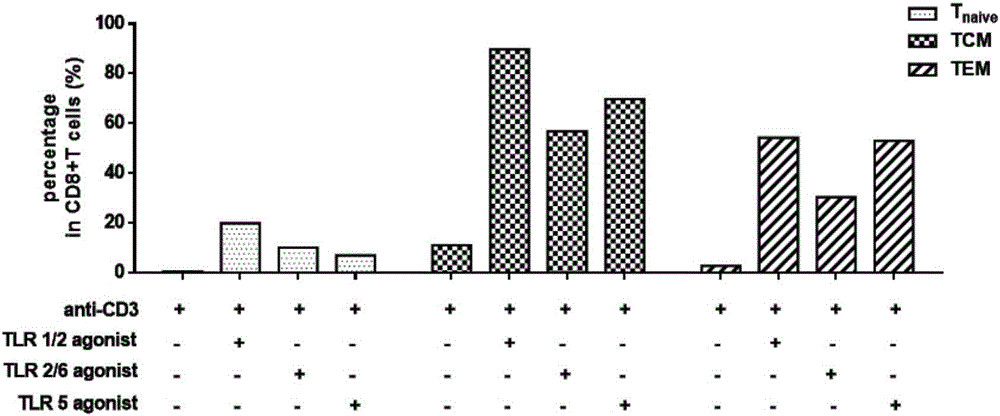
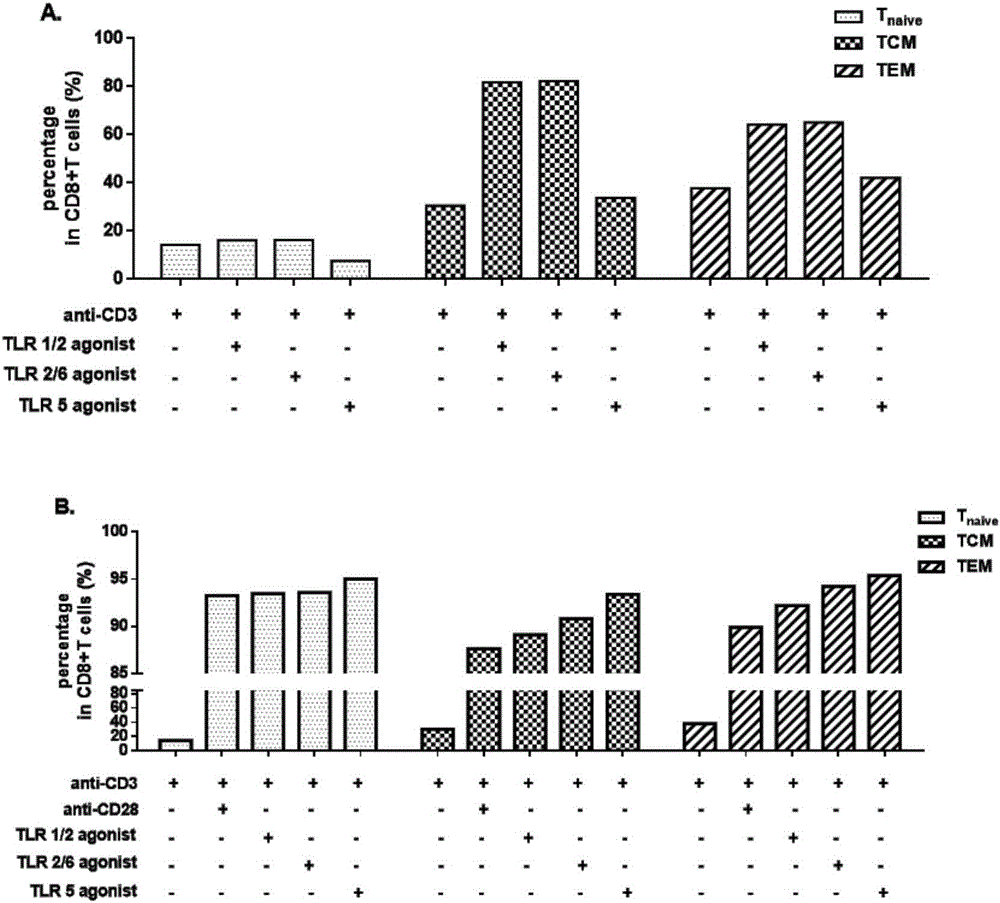
The invention establishes a method for in-vitro amplification of a CD8+T cell and a cell subset of the CD8+T cell. By adding a TLR1 / 2 agonist, a TLR2 / 6 agonist and a TLR5 agonist into an in-vitro culture system for conventionally culturing and amplifying the CD8+T cell or conjunctively utilizing the agonists, the amplification efficiency of the CD8+T cell can be remarkably improved; besides, by utilizing a TLC agonist, the functional CD8+T cell subsets which are difficult to amplify under a conventional culture condition are amplified, such as PD-1+CD8+T cells and TEM CD8+T cells; and the CD8+T cells and the functional cell subsets can be rapidly and largely propagated under the common and continuous stimulation of a TLRs agonist, recombinant cell factors IL-2, IL-7 and IL-15, an anti-human CD3 antibody and anti-human CD28 antibody coated magnetic beads.
Owner:SHANGHAI INNOVATIONAL CHANGAN BIOLOGICAL TECH CO LTD
Cell sorting magnetic bead, synthesis method thereof and application thereof in cell sorting
The invention provides a new cell sorting magnetic bead, which is a 50-70 nm gold plated Fe3O4 magnetic nanoparticle (Fe3O4 and Au nanoparticle) ostensibly coupled with proteinA by covalent bonds, interacted with the specificity of a Fc section of IgG through the proteinA, and directionally coupled with IgG type mouse CD3 antibodies. The invention relates to a method for synthesizing and modifying the magnetic bead, and a method for carrying out cell sorting by using the magnetic bead. The magnetic bead of the invention is used for removing the CD3 T cells in the mouse splenocytes so as to reduce the CD3 T cell content of the splenocytes from 36.5 percent to 0.7 percent, and has extremely high sorting efficiency. The magnetic bead also has the advantages of high protein-coupled quantity and superparamagnetism, and the preparation method has the advantages of simpleness, environmental protection, low cost and good application prospect.
Owner:PEKING UNIV
Bispecific antibody capable of resisting B cell lymphoma and application thereof
InactiveCN102250245AHighlighting the role of anti-B-cell lymphomaFungiHybrid immunoglobulinsAntiendomysial antibodiesT lymphocyte
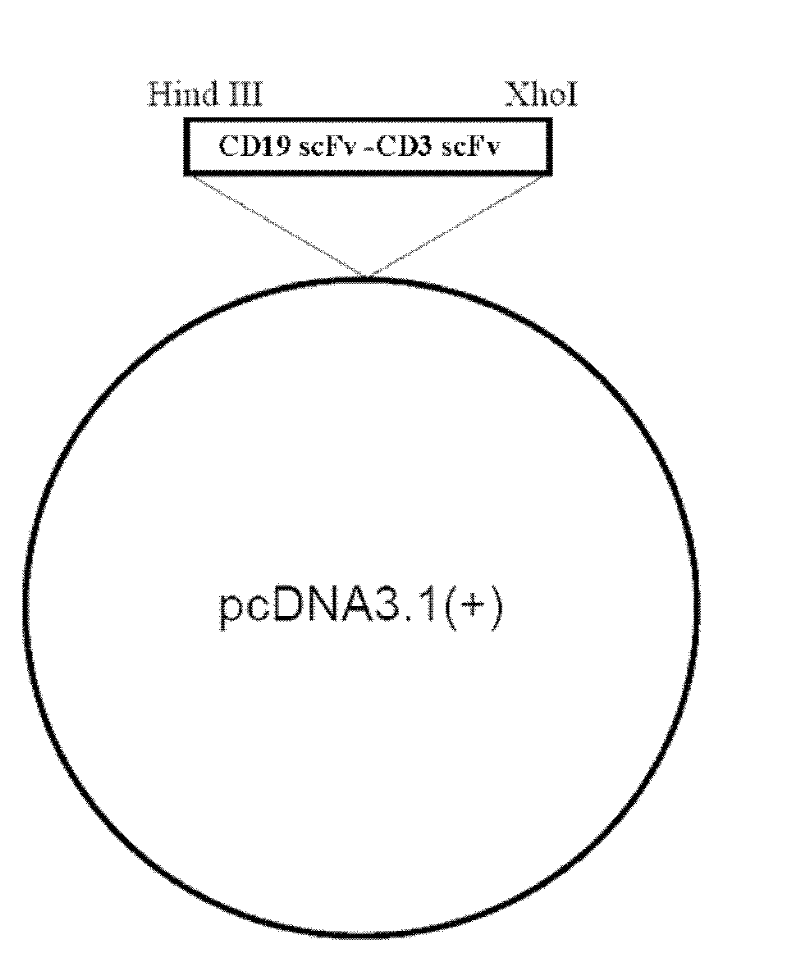
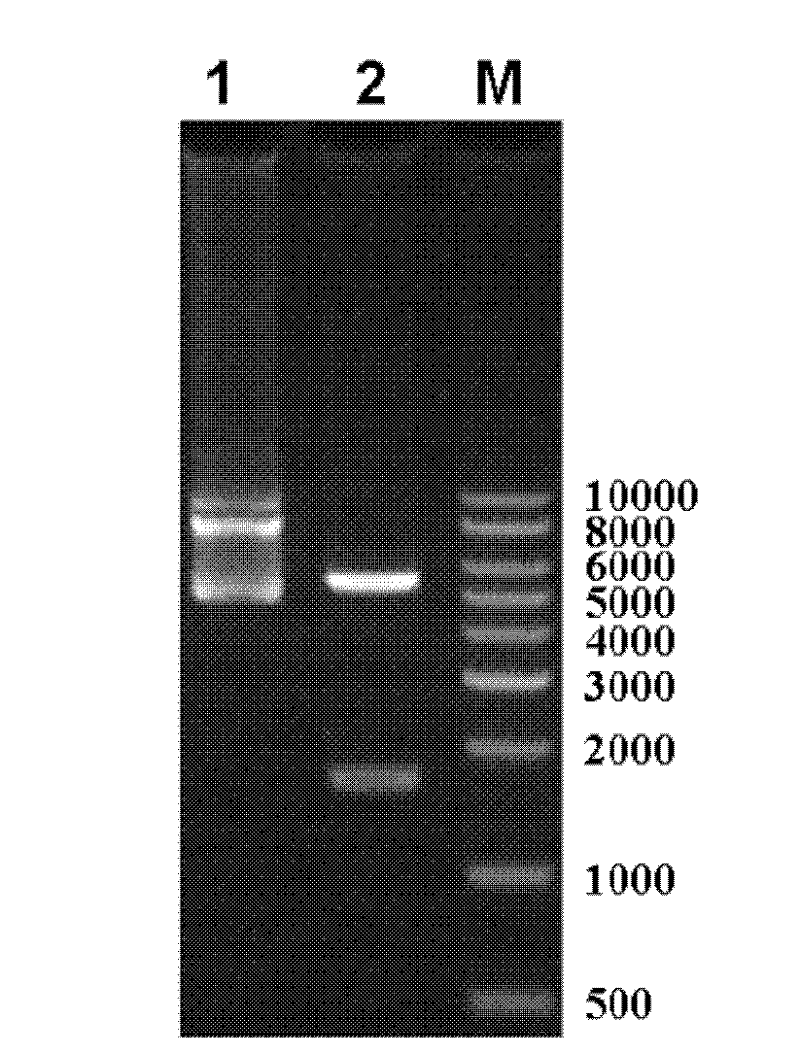
The invention relates to the technical fields of genetic engineering and protein engineering, in particular relates to DNA (deoxyribonucleic acid) for encoding recombinant fusion protein containing human CD19 antibody variable region and human CD3 antibody variable region fragments, fusion protein encoded by DNA, a production method of the fusion protein, pharmaceutical application of the fusion protein and a treatment method using the fusion protein. The invention provides bispecific antibody protein containing human CD19scFv and CD3scFv. The bispecific antibody protein can be combined with positive CD19 and CD3 positive cells, has good bioactivities in vivo and vitro, can activate human T lymphocyte, kill B lymphoma cells, and has good application prospects.
Owner:SICHUAN UNIV
Anti human ovarian cancer-anti cd3 bispecific antibody
InactiveUS20050255115A1Low toxicityImprove efficiencyAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionBispecific antibody
The present invention provides an anti-ovarian cancer bispecific antibody. Said antibody includes two polypeptide domains connected by a polypeptide linker, one is anti-ovarian cancer antibody, or its Fab fragment, single complementarity determining region (CDR) antibody or single chain Fv (scFv) and the other is anti-CD3 antibody, or its Fab fragment, single CDR antibody or scFv. The present invention also provides DNA sequences encoding said antibody, an expression vector containing said DNA sequence, and a host cell containing said expression vector.
Owner:DONGGUAN HAOFA BIOTECH DEVAL +2
NK (natural killer) cell culture medium and culture method of NK cell
ActiveCN104894065AStable proliferationImprove securityBlood/immune system cellsInsulin-like growth factorNatural Killer Cell Inhibitory Receptors
The invention provides a NK (natural killer) cell culture medium and a culture method of a NK cell, wherein the NK (natural killer) cell culture medium comprises the following components in parts by weight: 83 to 93 parts of serum-free basal culture medium; 4 to 6 parts of plasma; 0.5 to 1.5 parts of interleukin-2; 0.5 to 1.5 parts of anti-CD3 monoclonal antibody; 1 to 5 parts of insulin-like growth factor 1; 1 to 3 parts of lycium barbarum polysaccharide. According to the NK (natural killer) cell culture medium provided by the invention, the safety is better, and the NK cell cultured by the culture medium with the components can quickly and stably proliferate, and has better killing activity to tumor cell. Experimental results show that, after using the NK cell culture medium provided by the invention for culturing the NK cell for two weeks, the proliferation times soars almost fiftyfold; the killing activity to K562 reaches 91 percent at effector-target ratio being 40: 1.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Bispecific EGFRvIII x CD3 antibody engaging molecules
ActiveUS9249217B2Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsMutated proteinActivation cells
We have constructed bispecific antibody engaging molecules which have one arm that specifically engages a tumor cell which expresses the human EGFRvIII mutant protein on its surface, and a second arm that specifically engages T cell activation ligand CD3. The engaging molecules are highly cytotoxic and antigen-specific.
Owner:THE UNITED STATES GOVERNMENT AS REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES NIH +1
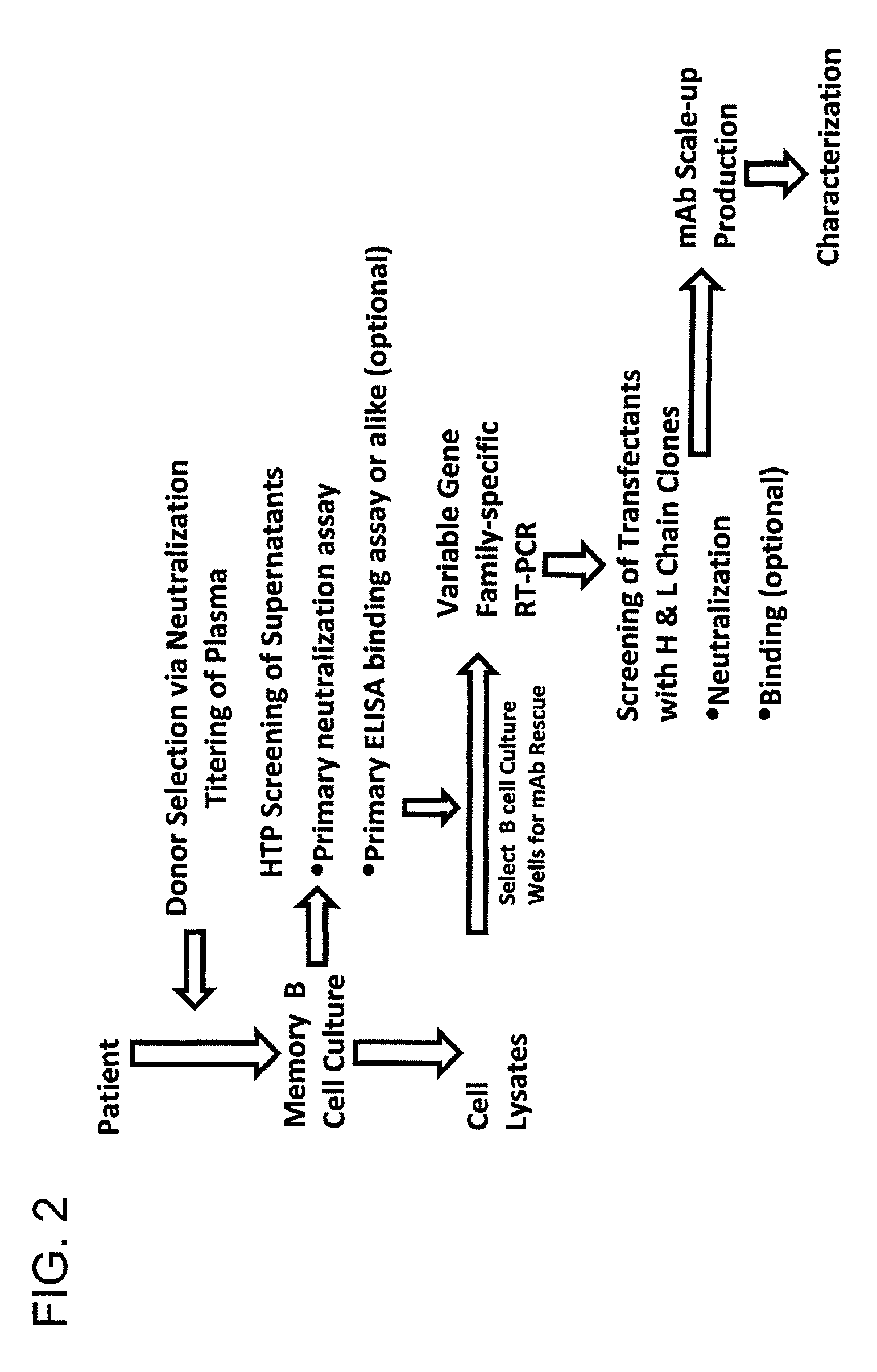
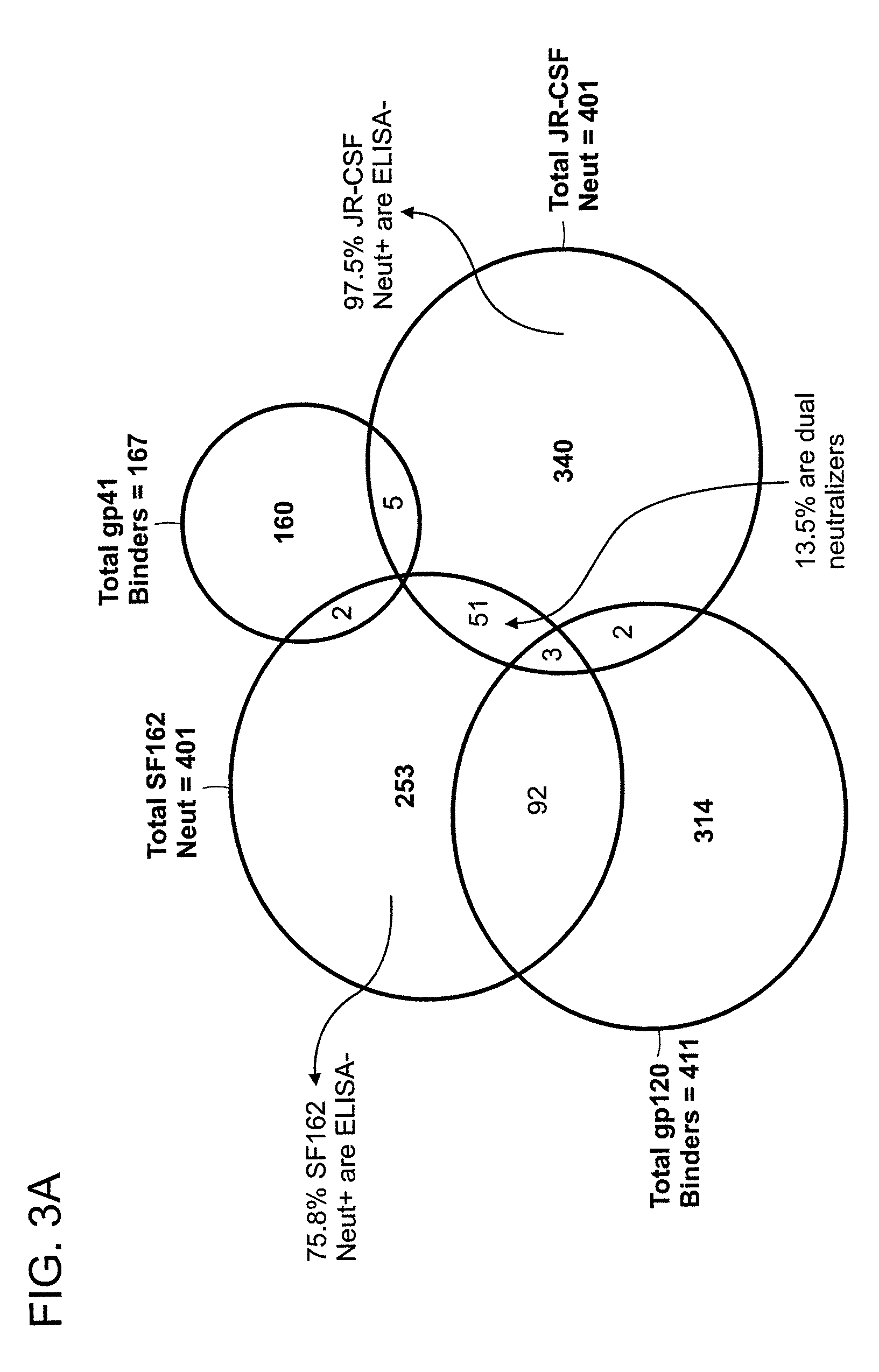
Monoclonal antibodies directed against trimeric forms of the HIV-1 envelope glycoprotein with broad and potent neutralizing activity
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
Preparation method and application of human TSCMs (T memory stem cells)
InactiveCN105543169AHave common characteristicsMammal material medical ingredientsBlood/immune system cellsFicollCulture fluid
The invention discloses a preparation method of human TSCMs (T memory stem cells), which comprises the following steps: (1) collecting peripheral venous blood with a blood cell separator or injector under aseptic conditions, and carrying out Ficoll-Hypaque density gradient centrifugation to obtain mononuclear cells; (2) putting the separated PBMCs (peripheral blood mononuclear cells) in a culture medium containing irritants and cell factors, regulating the cell density to (0.5-2)*10<6> / mL, transferring into a cell culture plate, culture bottle or culture bag, and culturing in an incubator; (3) after culturing the cells for 48-72 hours, replacing half of the culture solution, supplementing the equivalent culture solution to keep the cell density at (0.5-2)*10<6> / mL; and according to the cell growth state, harvesting the cells in the 10th-21st day. An anti-CD3 monoclonal antibody and an anti-CD28 monoclonal antibody are utilized as the irritants to activate the TSCMs, the cell factors rh IL-7, rh IL-15 and rh IL-2 are combined for irritation, and IM-12 is utilized to stop the stem cell differentiation, so that the cultured TSCMs have the common characteristics of both memory cells and stem cells, thereby providing a new optional scheme for clinical adoptive immunity cellular therapy.
Owner:SHENZHEN HORNETCORN BIOTECH
Compositions and methods for the treatment of type 1 diabetes
ActiveUS20190022154A1Avoid destructionBacteriaPeptide/protein ingredientsAntiendomysial antibodiesPancreatic hormone
Provided herein are compositions and methods for the treatment of type 1 diabetes (T1D) in mammalian subjects. The compositions include lactic acid fermenting bacteria (LAB) expressing an IL-2 gene and a T1D-specific self-antigen (e.g., proinsulin (PINS)) gene. Exemplary methods include: orally administering to a mammalian subject, a therapeutically effective amount of the composition. The composition can be administered to the subject mucosally, resulting in delivery of the LAB into the gastrointestinal tract, where the LAB is released. Bioactive polypeptides expressed by the LAB are thus administered via mucosal delivery. The LAB may be selected to deliver a low-dose of IL-2 to the subject. The methods may not require concomitant systemic anti-CD3 antibody treatment. The methods may be suited for subjects possessing residual beta-cell function, e.g., those with recent-onset T1D.
Owner:INTREXON ACTOBIOTICS NV
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS9056906B2Treating and preventing and slowing progression of and ameliorating symptomSlow and reduce autoimmunitySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
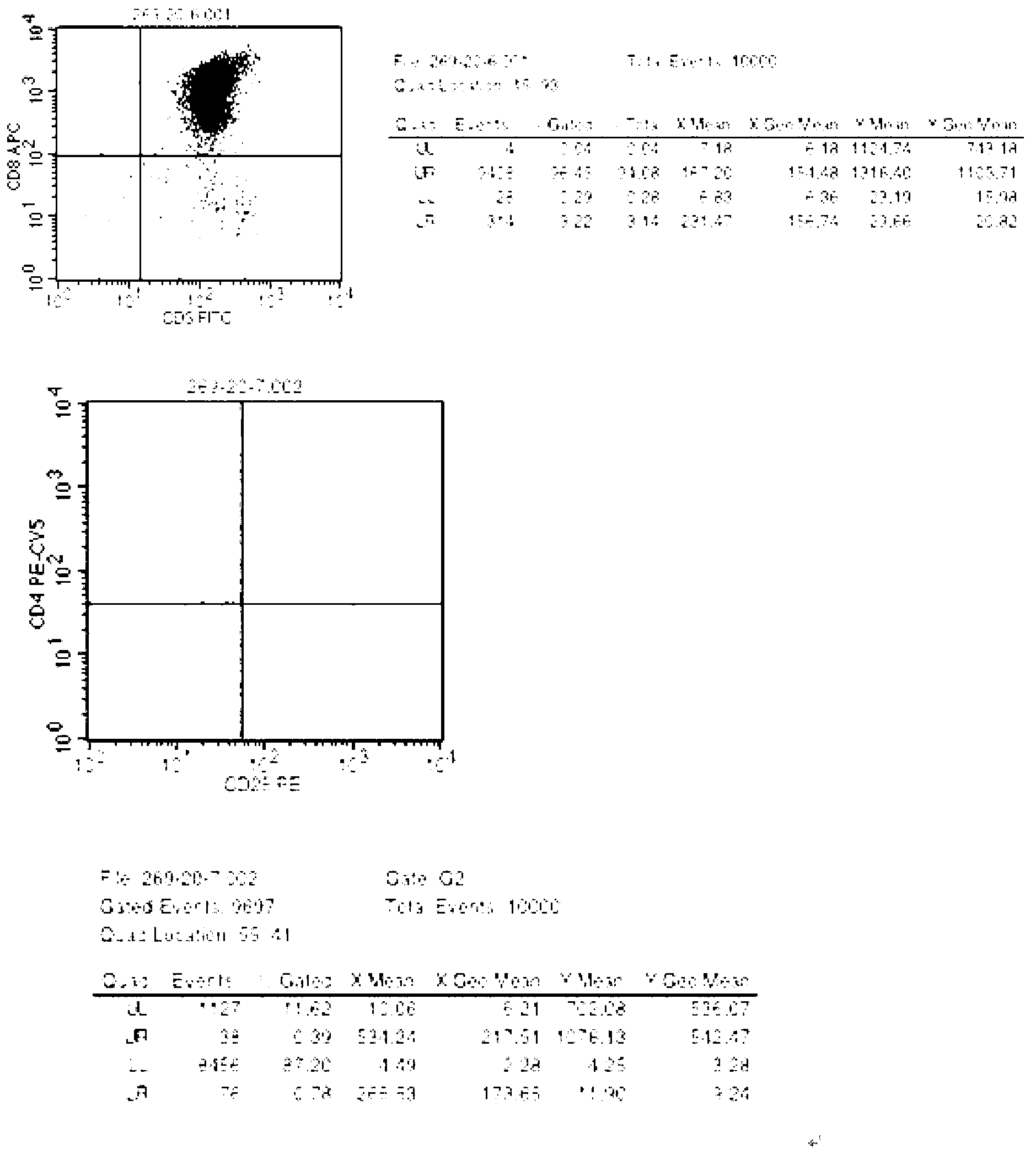
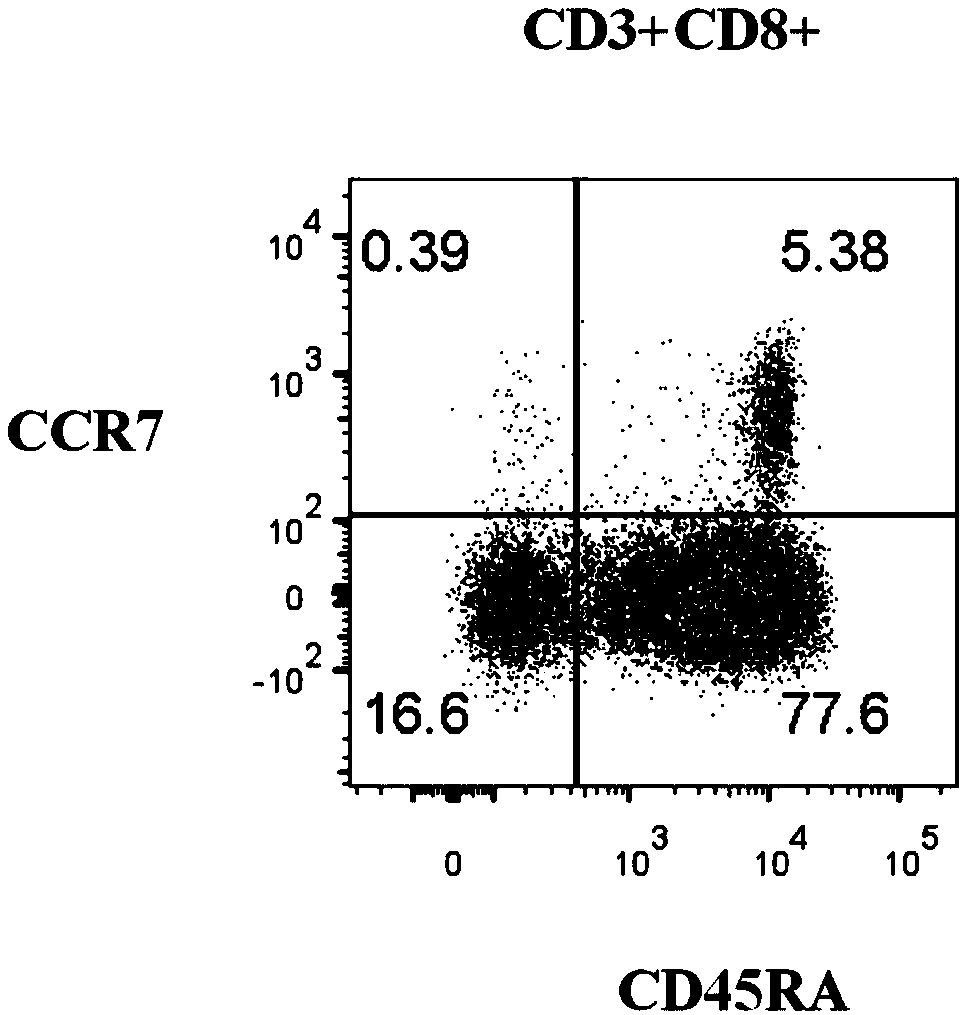
Kit for assessing function of cytotoxic immune cells in human peripheral blood and assessment method
The invention provides a kit for assessing a function of cytotoxic immune cells in human peripheral blood and an assessment method. The kit for assessing the function of cytotoxic immune cells in human peripheral blood provided by the invention comprises an anti-human CD3 antibody, an anti-human CD8 antibody, an anti-human CD45RA antibody, an anti-human CCR7 antibody, an anti-human CD28 antibody,an anti-human CD38 antibody, an anti-human CD57 antibody, an anti-human HLA-DR antibody, an anti-human PD-1 antibody, an anti-human CD56 antibody, an anti-human CD94 antibody, an anti-human NKP30 antibody, an anti-human NKP46 antibody, an anti-human NKG2D antibody, an anti-human KIR antibody, an anti-human Gamma Delta antibody and an anti-human V Delta 2 antibody, wherein all the antibodies are labeled by fluorescein. The kit for assessing the function of cytotoxic immune cells in human peripheral blood provided by the invention can be used for comprehensively assessing the immunity function of the cytotoxic immune cells in human peripheral blood, and is convenient and safe for use.
Owner:东莞暨南大学研究院
Modified Fc fragment, antibody comprising same, and use thereof
ActiveCN110914296AAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsFc-Gamma ReceptorAntiendomysial antibodies
The present invention relates to a modified Fc fragment, an antibody comprising the same, and uses thereof. The Fc fragment is a human IgG1 source, and the constant region CH2 domain of the Fc fragment comprises a plurality of substitutions. The substitution can significantly reduce the binding capacity of the Fc fragment and an Fc gamma receptor (Fc gamma R), and reduce the non-specific activation of the antibody (such as an anti-CD3 antibody) on T cells.
Owner:WUHAN YZY BIOPHARMA CO LTD
Humanized or chimeric cd3 antibodies
ActiveUS20190284278A1Reduced binding affinitySame cytolytic activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsBispecific antibodyCD3 Antibody
The present invention relates to humanized or chimeric antibodies binding CD3. It furthermore relates to bispecific antibodies, compositions, pharmaceutical compositions, use of said antibodies in the treatment of a disease, and method of treatment.
Owner:GENMAB AS
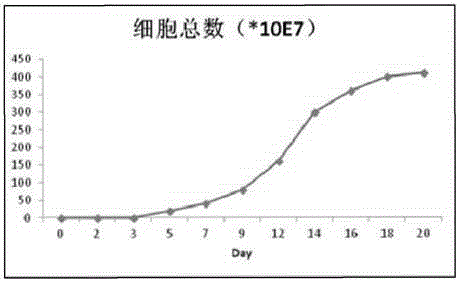
Method for in-vitro culture of killer T cells
ActiveCN103013914AHigh kill rateShort maturation timeBlood/immune system cellsLymphocyteBiological Immunotherapy
The invention relates to a method for in-vitro culture of killer T cells. Specifically, IL-2 and an anti-CD3 monoclonal antibody are combined with indigowoad leaf decoction for costimulation of lymphocytes taken from 50ml of in-vitro blood so as to get the T cells with cytotoxin activity by induction; and the T cells with the cytotoxin activity, which are obtained by an induction culture method, have the advantages of large proliferation number and good vitality, and the number of the CD8-positive T cells can be increased from 20%-30% to 80%-90%. Simultaneously, the method disclosed by the invention has the advantages of low taking quantity of the blood, simple operation steps, low cost of a reagent and short maturation time of the cells, above 1*10<9> cells can be achieved by 8 days and used for performing continuous intravenous infusion on a patient six times, and above 1*10<9> cells can be infused every time. The method is suitable for a clinical biological immunotherapy of the patients with tumors and can be widely popularized and applied.
Owner:JILIN TUO HUA BIOTECH
NK cell culture medium and NK cell culture method
InactiveCN106190976AHigh activityIncrease lethalityCulture processBlood/immune system cellsBlood plasmaBiological activation
An NK cell culture medium is prepared from, by weight, 94-98 parts of serum-free basic culture medium, 7-10 parts of plasma, 0.6-1.6 parts of anti-CD3 monoclonal antibody, 2-4 parts of first para-insulin growth factor, 20-30 parts of PBS buffer solution, 1-4 parts of squid ink, 2-6 parts of flos carthami polysaccharide, 2.5-4.5 parts of allicin, 1-3 parts of alfalfa polysaccharide and 2-4 parts of cinnamaldehyde. Serum-free basic culture medium, plasma, anti-CD3 monoclonal antibody and first para-insulin growth factor together form a human body marrow environment required by NK cell growth. Flos carthami polysaccharide, allicin, alfalfa polysaccharide and cinnamaldehyde can improve activity of the NK cells easily, and accordingly killing capacity of the NK cells is enhanced. Squid ink contains IL-2 which can induce activation and differentiation of the NK cells, and accordingly multiplication efficiency of the NK cells is easily improved. The activated NK cells can synthesize and secrete various cytokines, and accordingly tumor cell differentiation and multiplication are directly inhibited.
Owner:浙江译美生物科技有限公司
Induced culture composition capable of promoting proliferation of spleen-derived CD8 positive T cell and application of induced culture composition
The invention relates to an induced culture composition capable of promoting proliferation of a spleen-derived CD8 positive T cell and application of the induced culture composition. The induced culture composition comprises a culture medium and inducing factors added in the culture medium, the inducing factors comprises phytohemagglutinin, a CD3 antibody and a CD28 antibody, the final concentration of the phytohemagglutinin is 1 mu g / mL to 10 mu g / mL, the final concentration of the CD3 antibody is 1 mu g / mL to 10 mu g / mL, the final concentration of the CD28 antibody is 1 mu g / mL to 10 mu g / mL. The induced culture composition provided by the invention can promote proliferation of the spleen-derived CD8 positive T cell.
Owner:深圳灵赋拓普生物科技有限公司
Preparing method of human DCCIK immunocompetent cell
InactiveCN104073467AShorten the timeEasy to operateMammal material medical ingredientsBlood/immune system cellsLymphocyte cultureLymphocyte
A preparing method of a human DCCIK immunocompetent cell is disclosed. The method includes steps of: (1) separating a mononuclear cell from human peripheral blood; (2) inoculating the mononuclear cell to a culture medium suitable for lymphocyte culturing, adding IFN[gamma], IL-2, an anti-CD3 monoclonal antibody, GM-CSF and IL-4, and culturing for 3-5 days; (3) adding TNF[alpha], and continuously culturing for 1-2 days; and (4) adding IL-2, and continuously culturing for 7-10 days to obtain the DCCIK cell. A composition of various cytokines and stimulating factors is added directly into the mononuclear cell so that the DC cell and lymphocyte induce at the same time and mutually stimulate maturity, thus simplifying operation steps, reducing experiment consumable items and shortening cell culturing time. In addition, normalized experiment process technology can be easily mastered, thus facilitating clinic popularization and application.
Owner:SHANGHAI YUYAN BIOTECH CO LTD
Kit for detecting mycobacterium tuberculosis infection by using peripheral blood and application of kit
The invention discloses a kit for detecting mycobacterium tuberculosis infection by using peripheral blood. The kit provided by the invention comprises the following substances: (1) a mycobacterium tuberculosis Rv3615c mixed polypeptide library which consists of all or a part of 19 polypeptides consisting of amino acid sequences shown as sequences 1 to 19 in a sequence table, (2) an anti-CD3 antibody labeled with a fluorescent dye A, (3) an anti-gamma-interferon antibody labeled with a fluorescent dye B, and (4) a Golgi apparatus blocking agent, wherein the fluorescent dye A and the fluorescent dye B emit different fluorescent colors. A novel method which can be used for detecting mycobacterium tuberculosis infection is provided. As proved by experiments, antigenic stimulation can be performed by directly using the peripheral blood without separating single karyocytes of the peripheral blood. As indicated by experimental data, the kit has high sensitivity and high specificity when being used for detecting mycobacterium tuberculosis infection. Moreover, the kit is easy and convenient to operate and low in cost, and has high clinical application value.
Owner:北京同生时代生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com