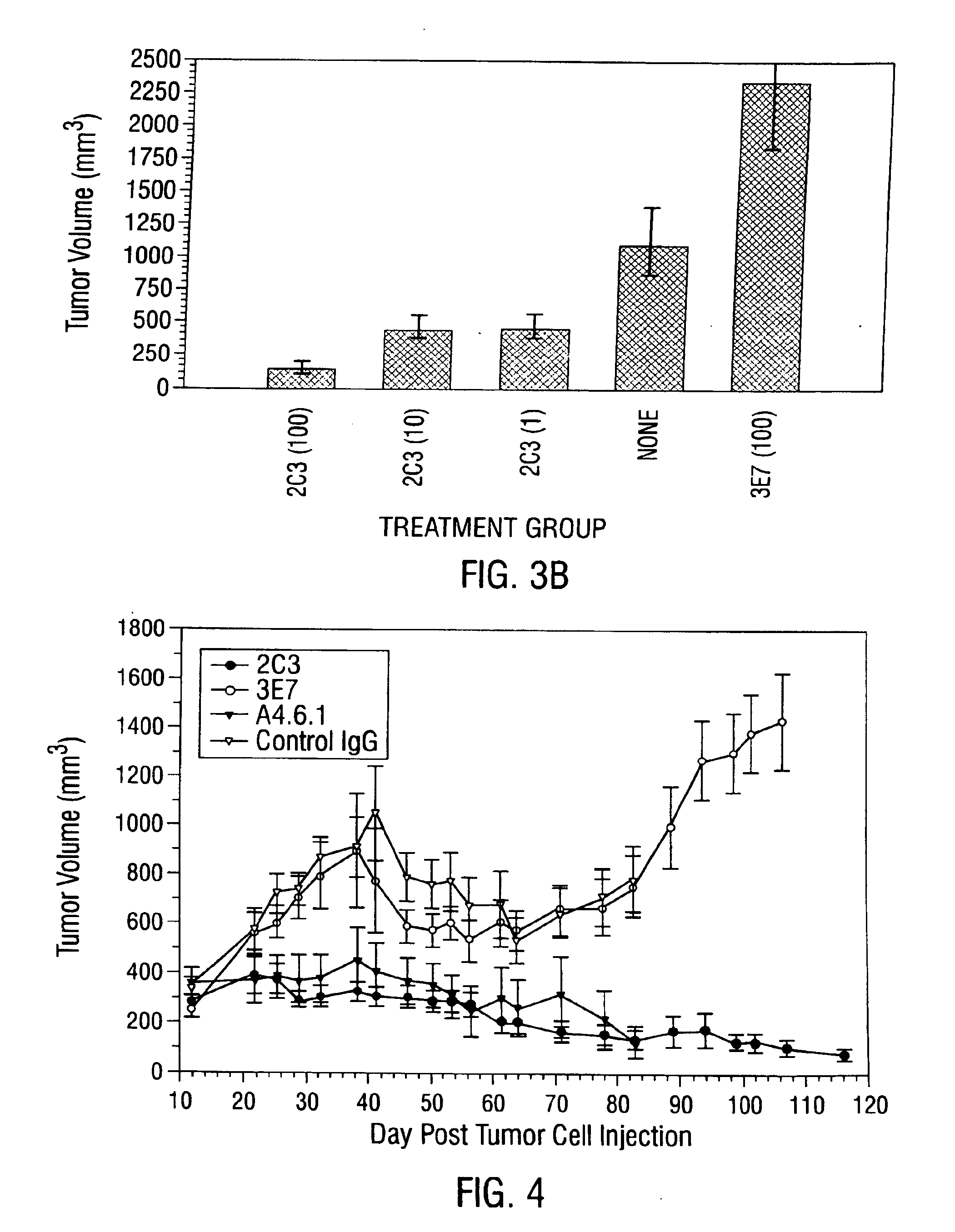
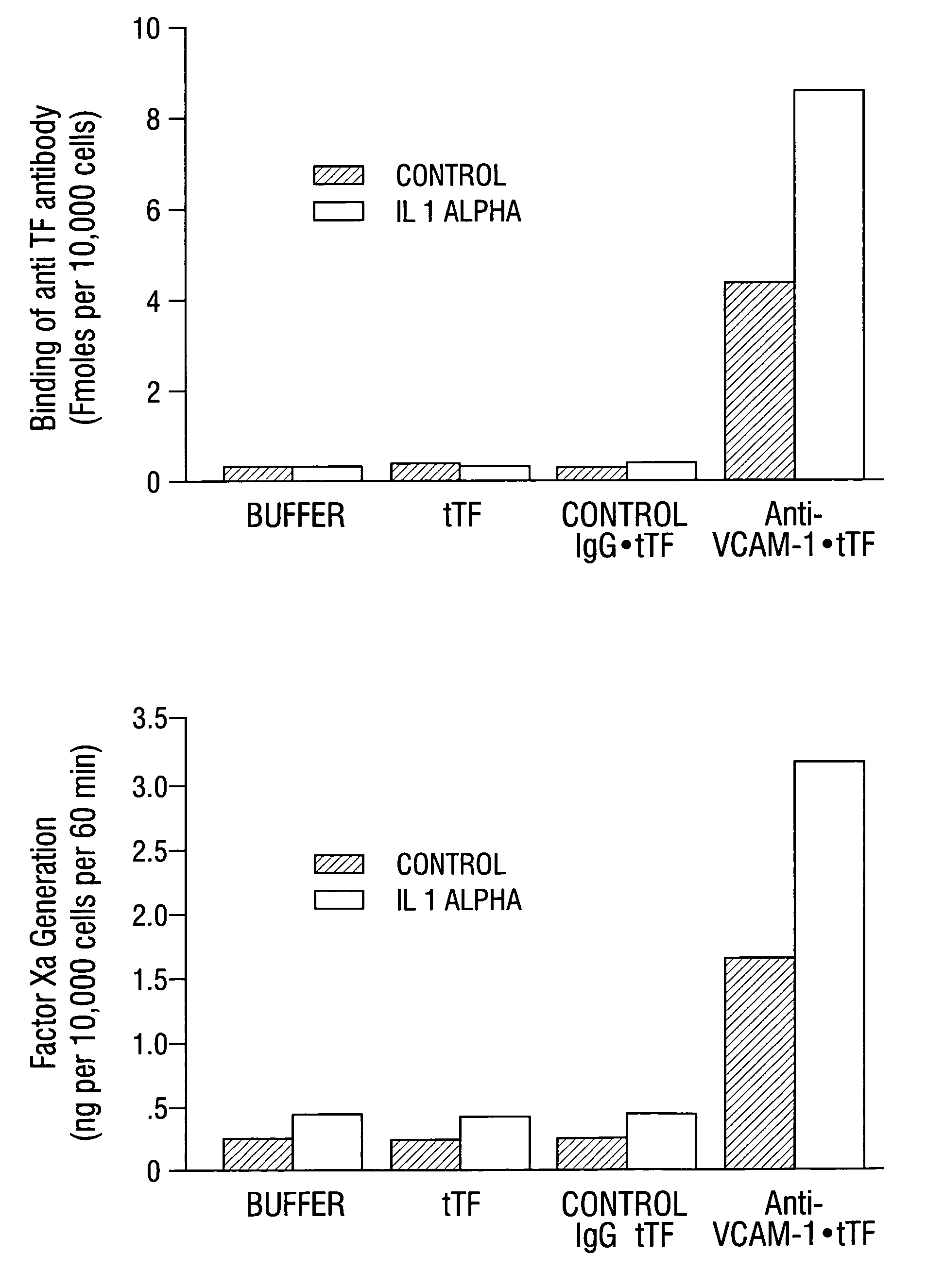
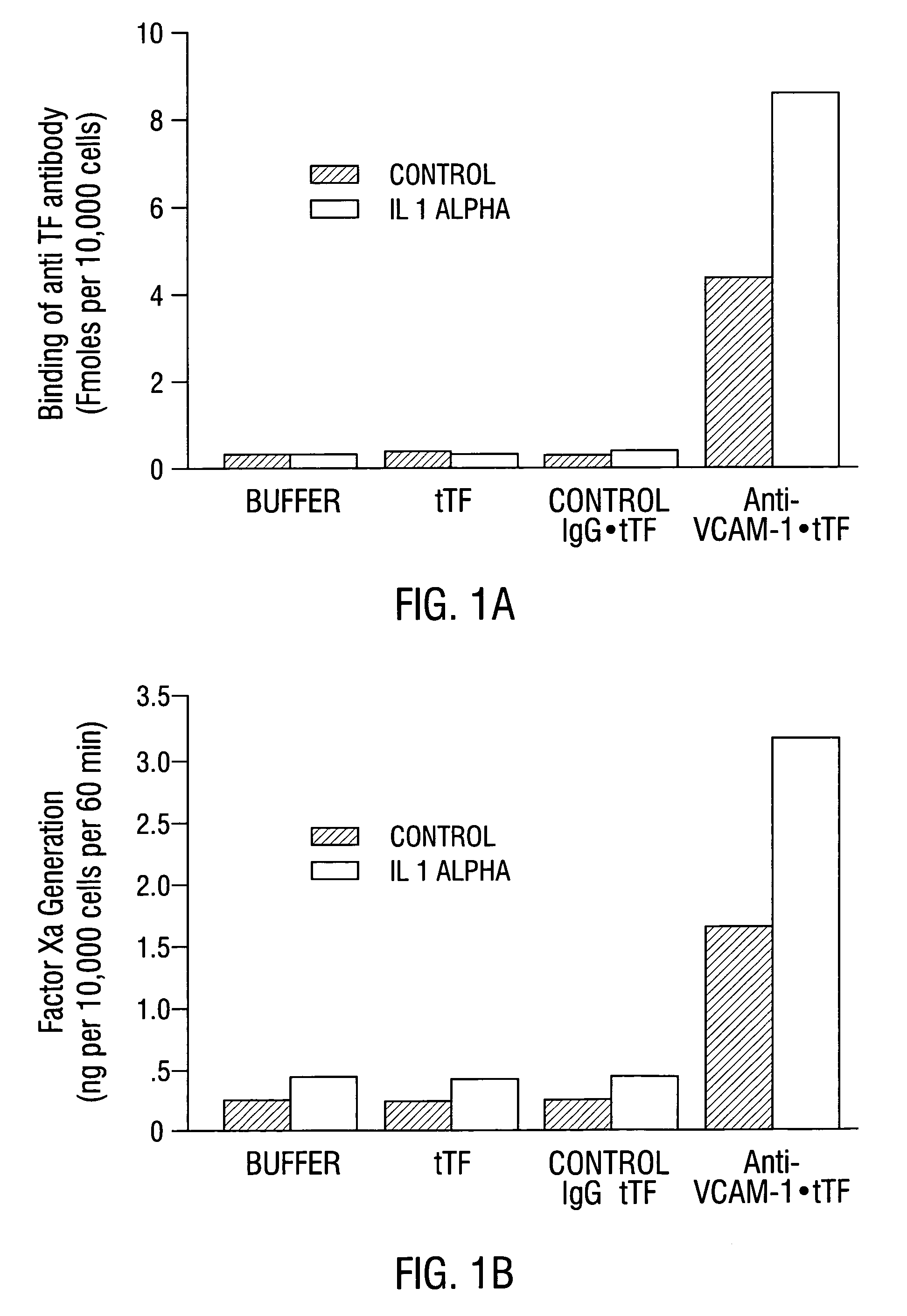
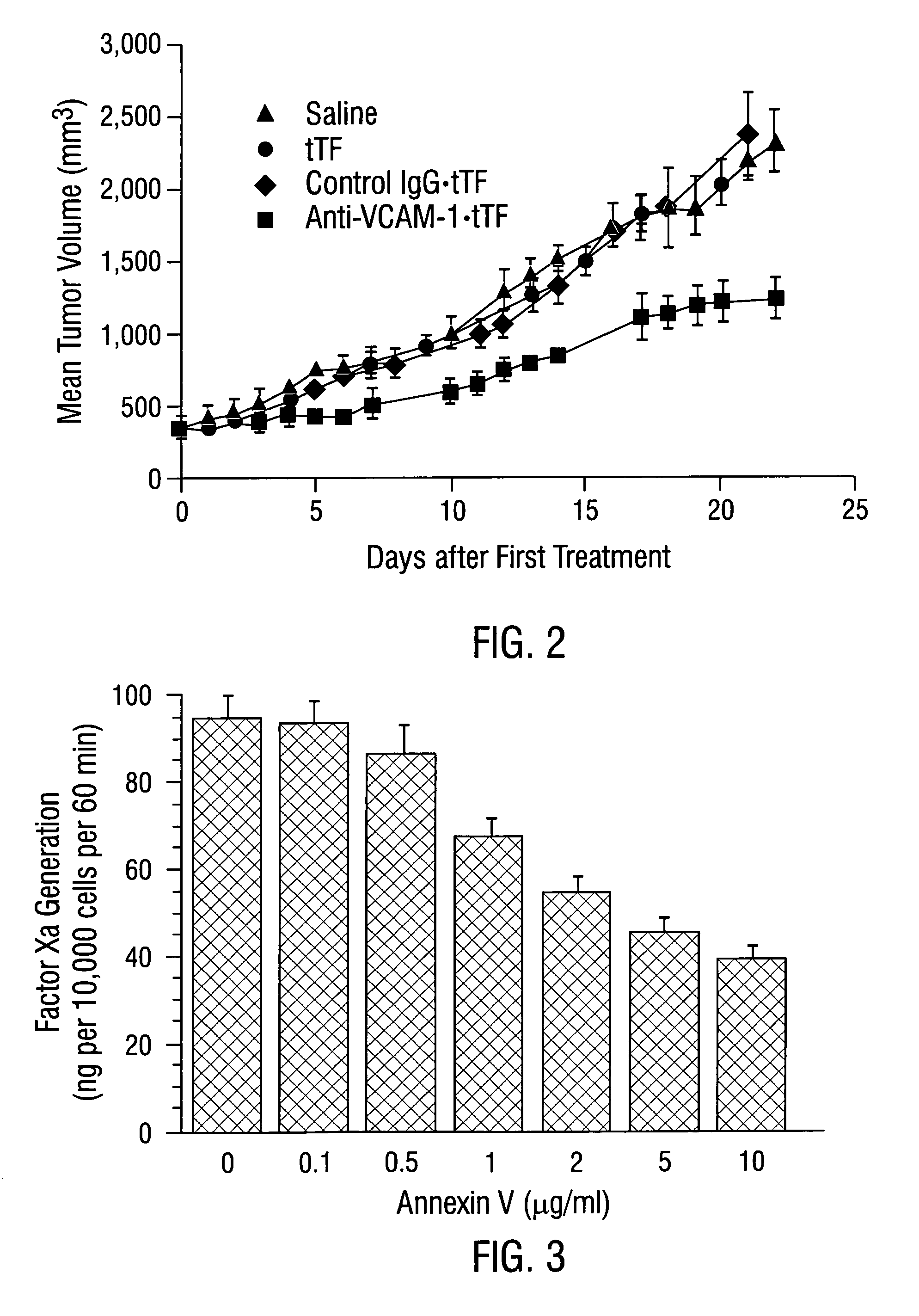
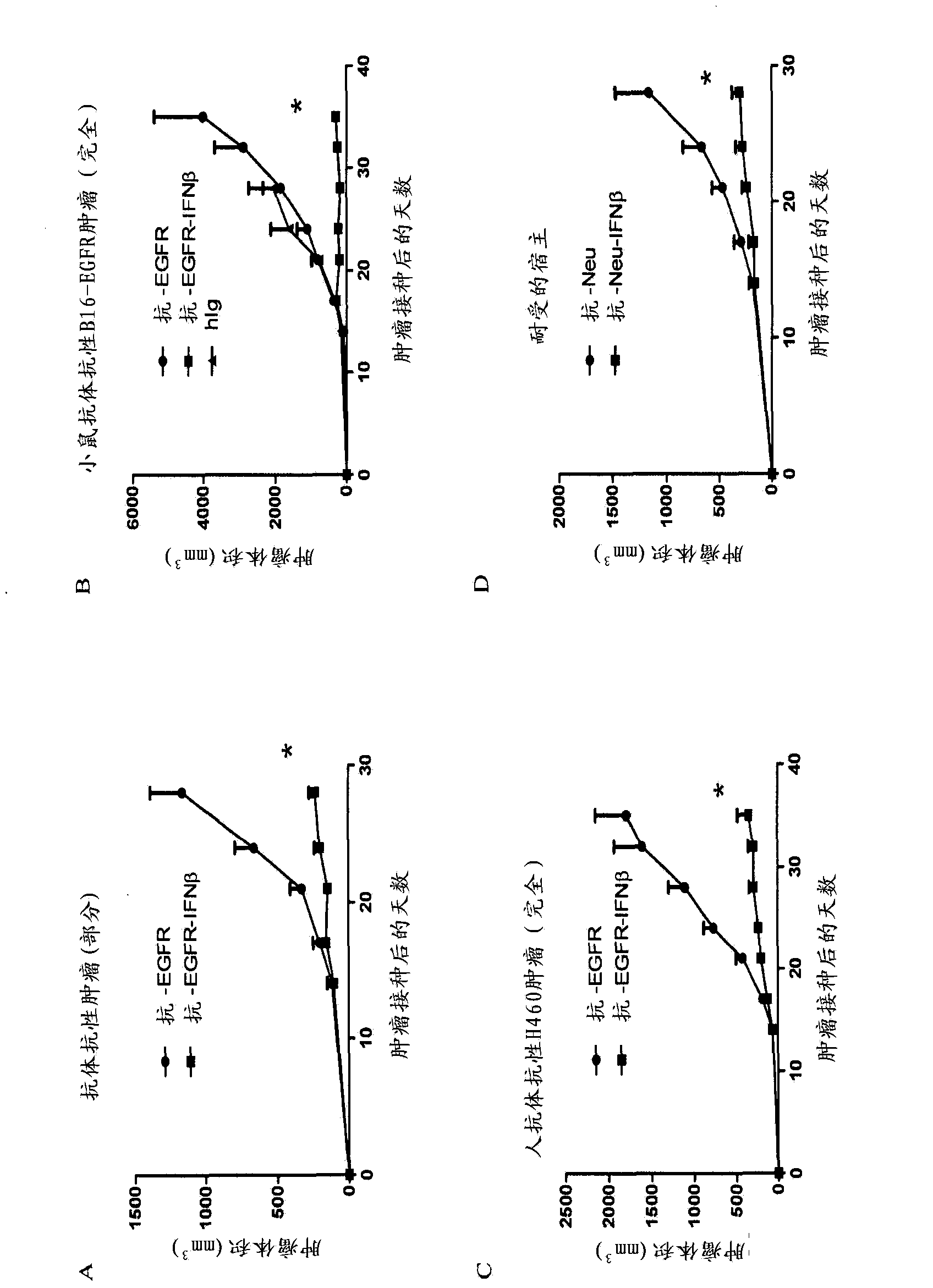
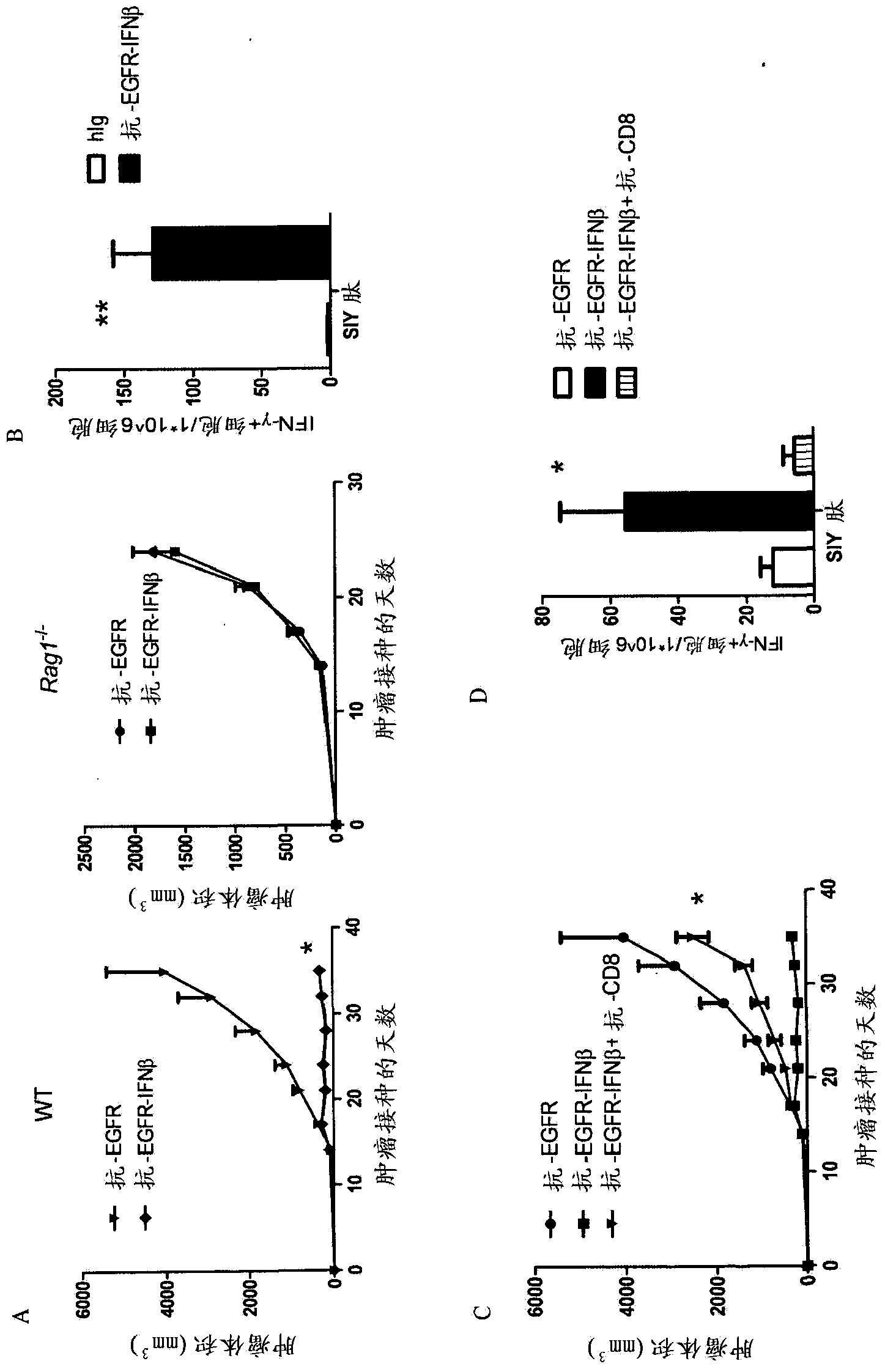
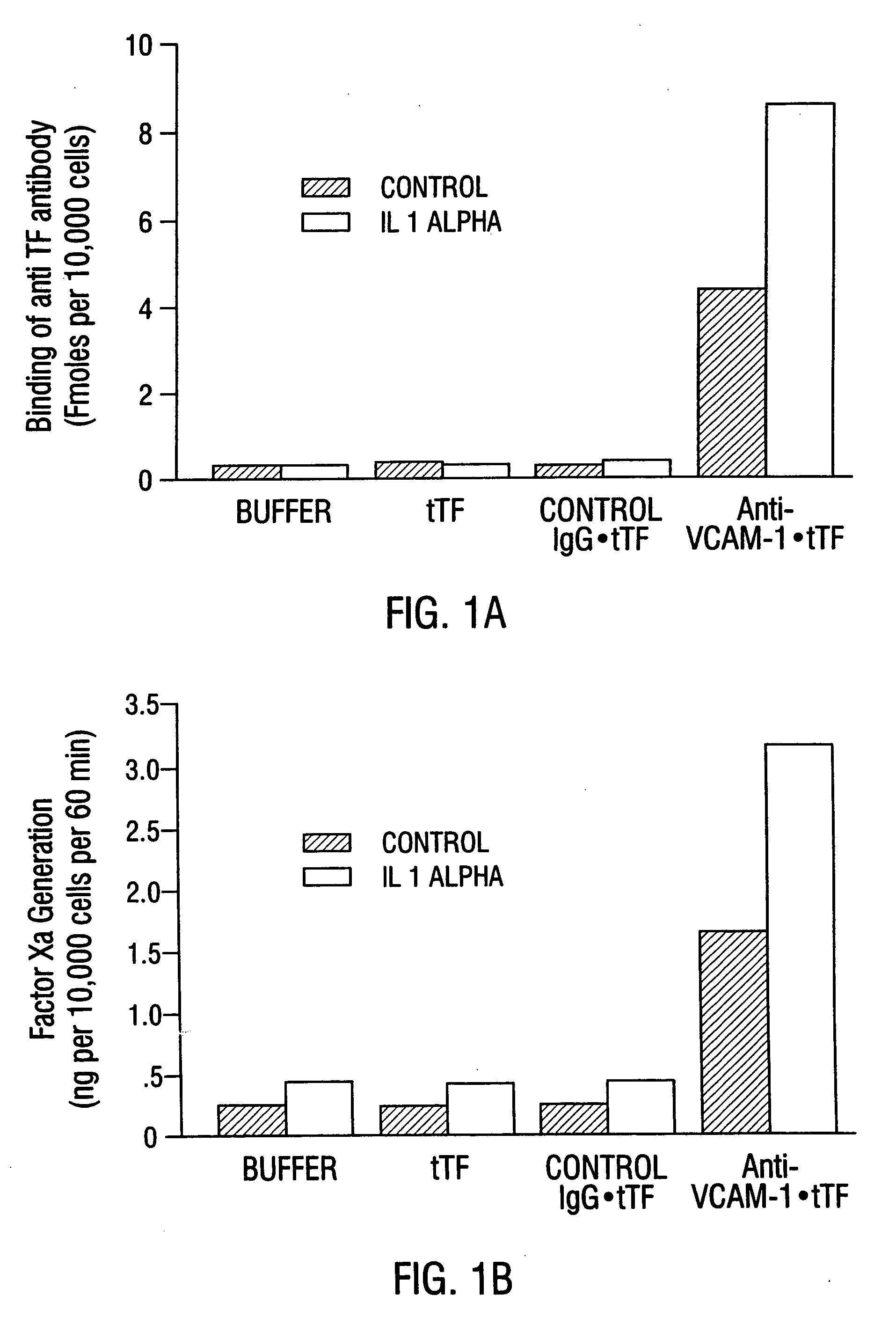
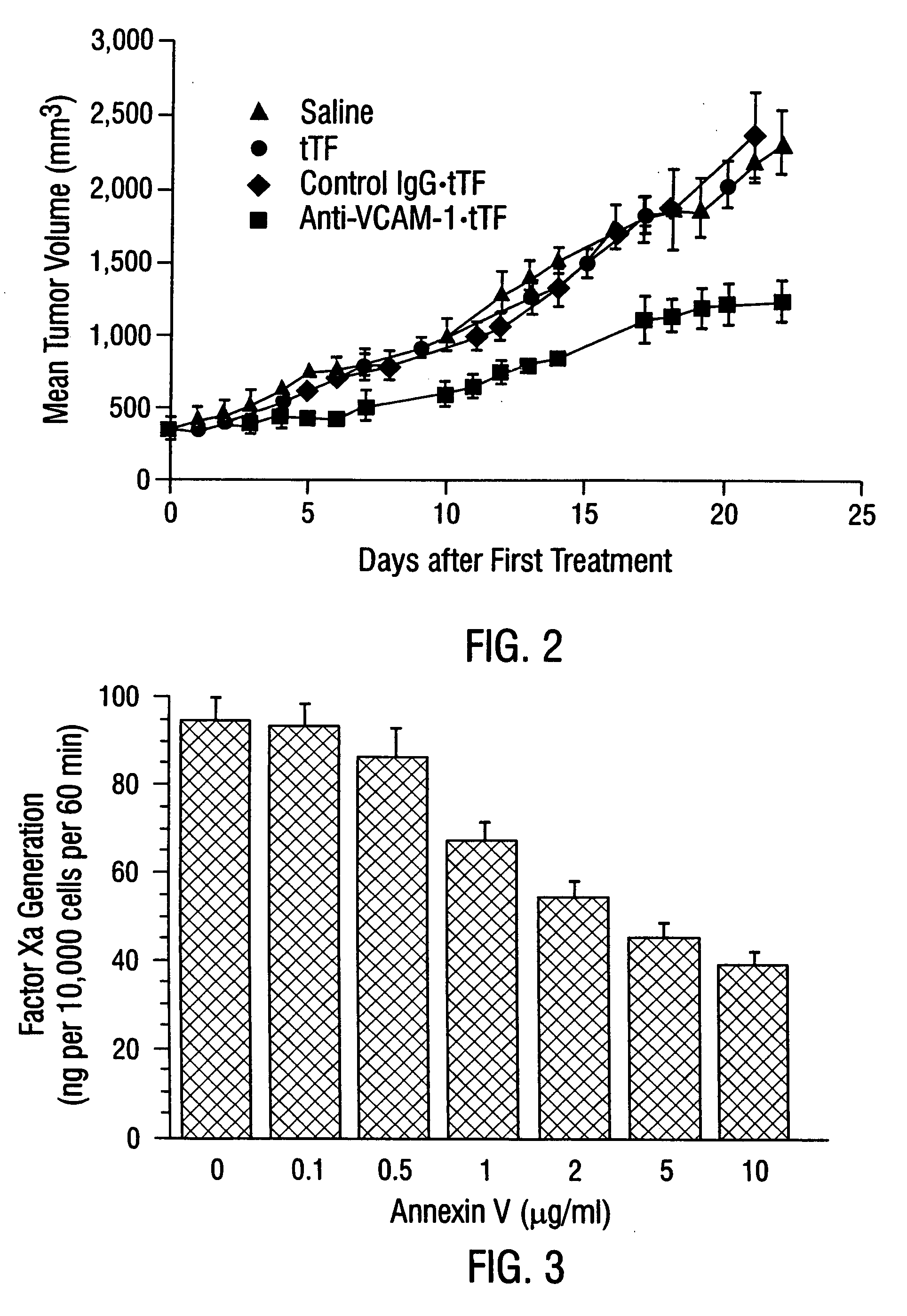
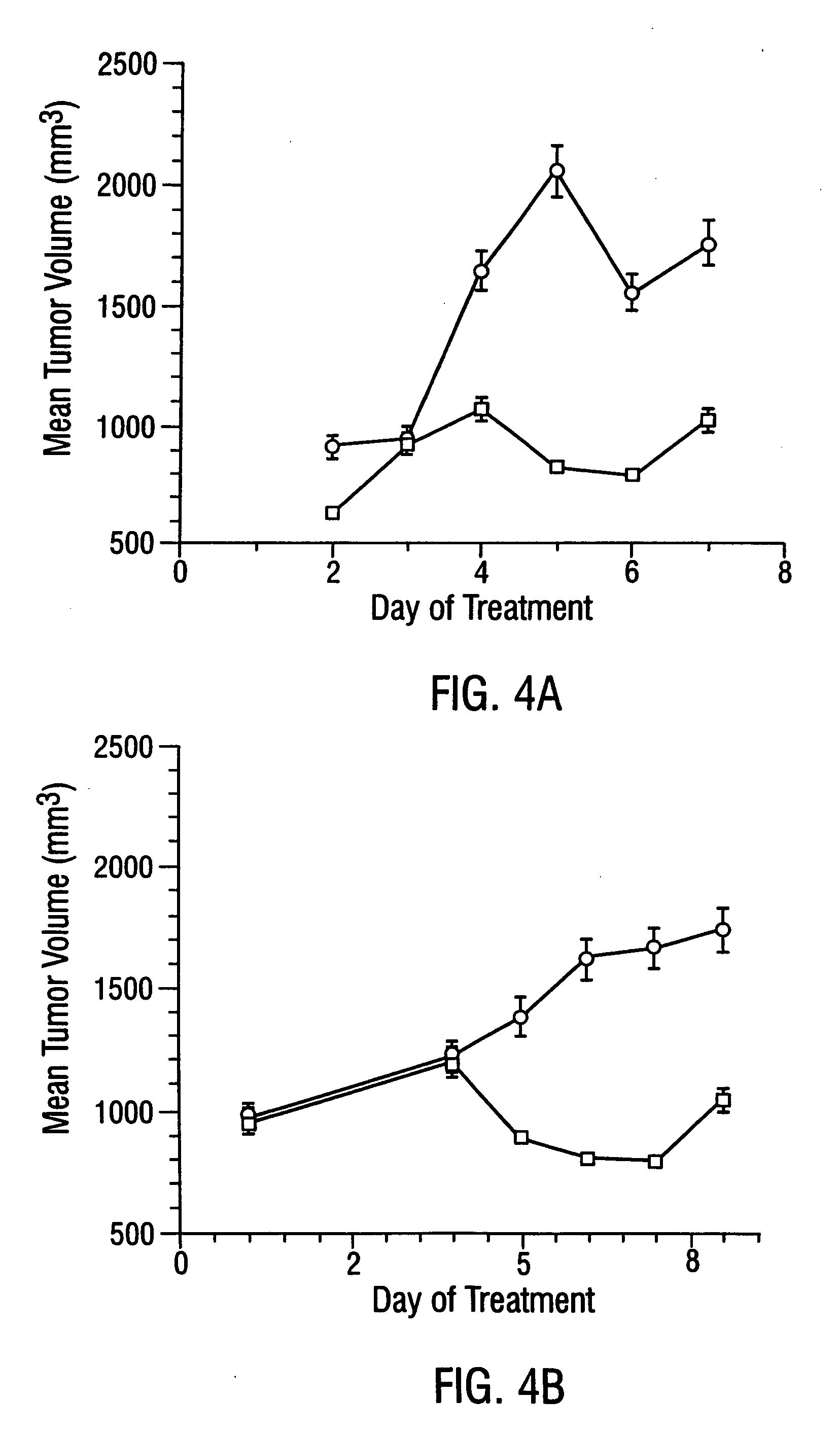
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Tumor regression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tumor regression is one of the most important factors determinating the tumor control probability after radiotherapy. Tumor regression grading is usually based on the presence of residual vital tumor cells in proportion to the total tumor size.
Tumour necrosis factor antibodies
InactiveUS6451983B2Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman tumorSingle-Chain Antibodies
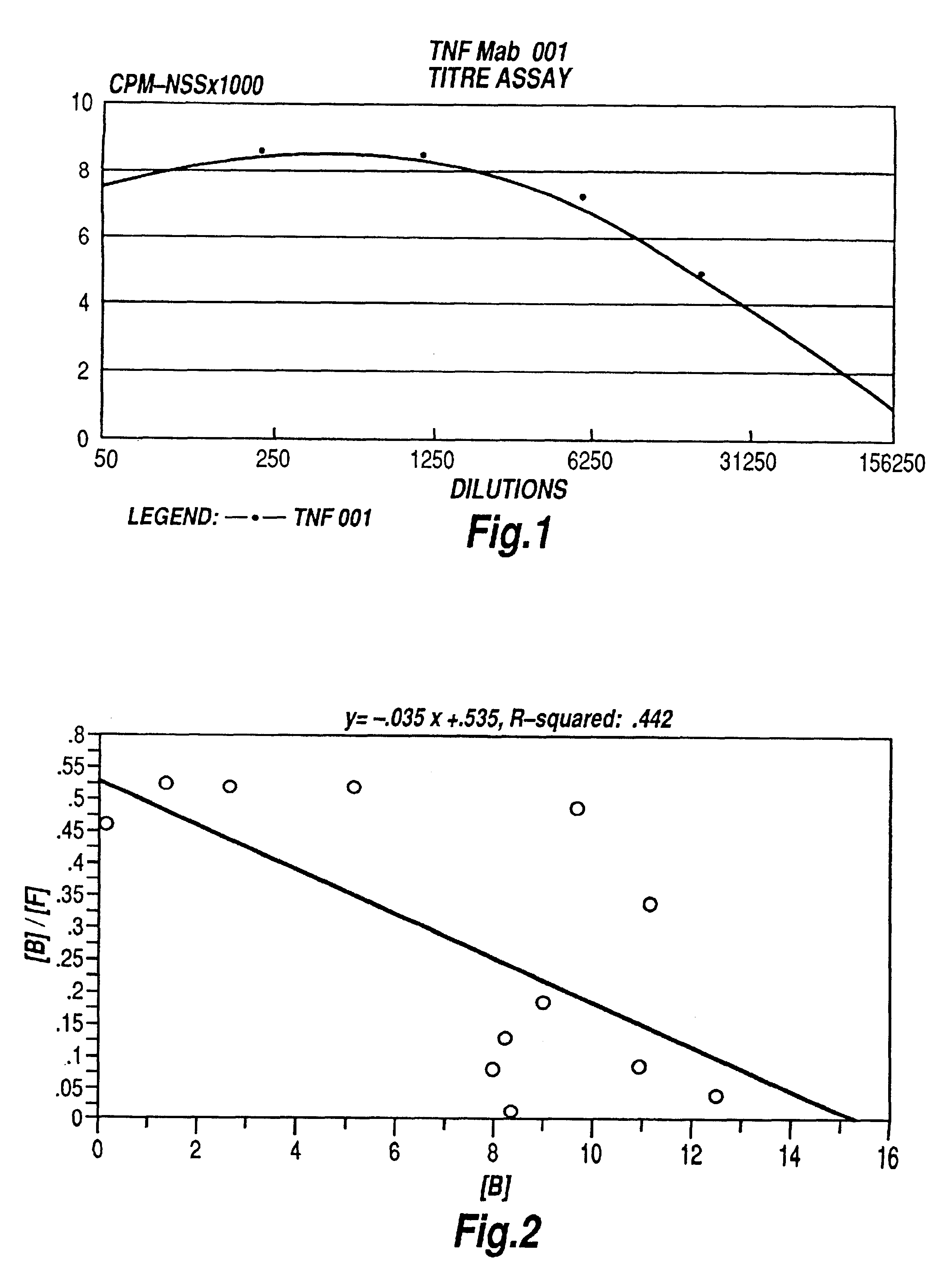
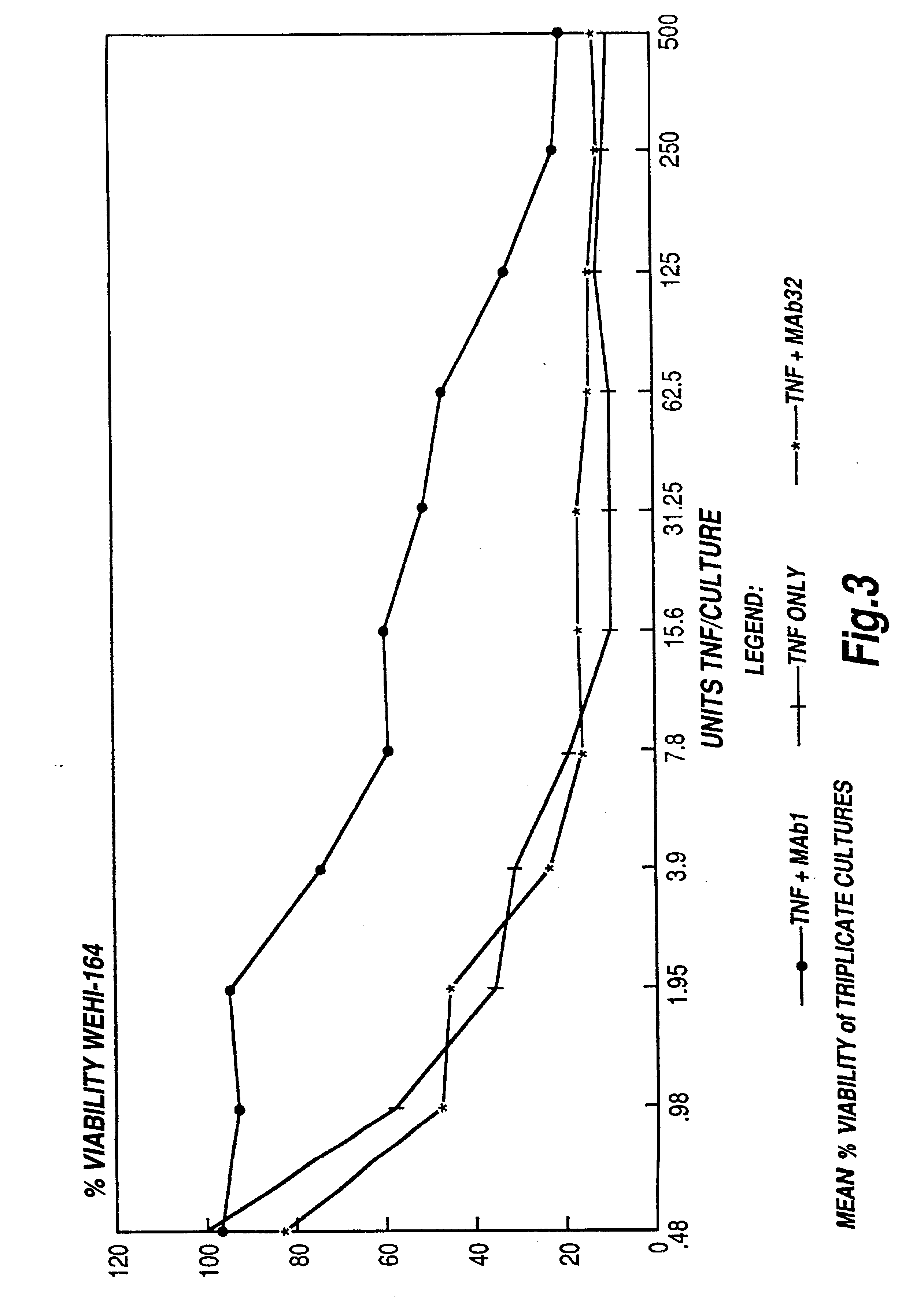
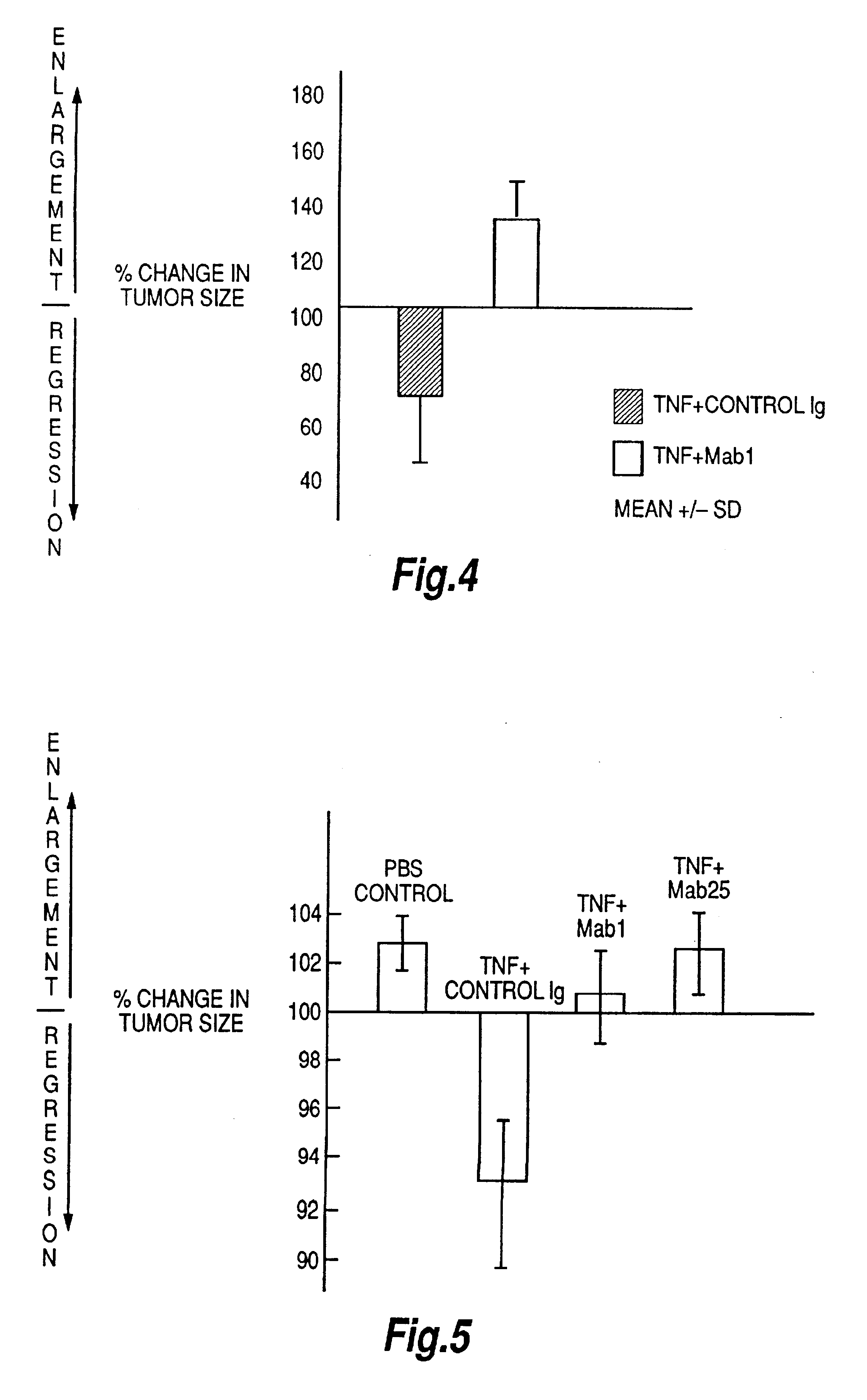
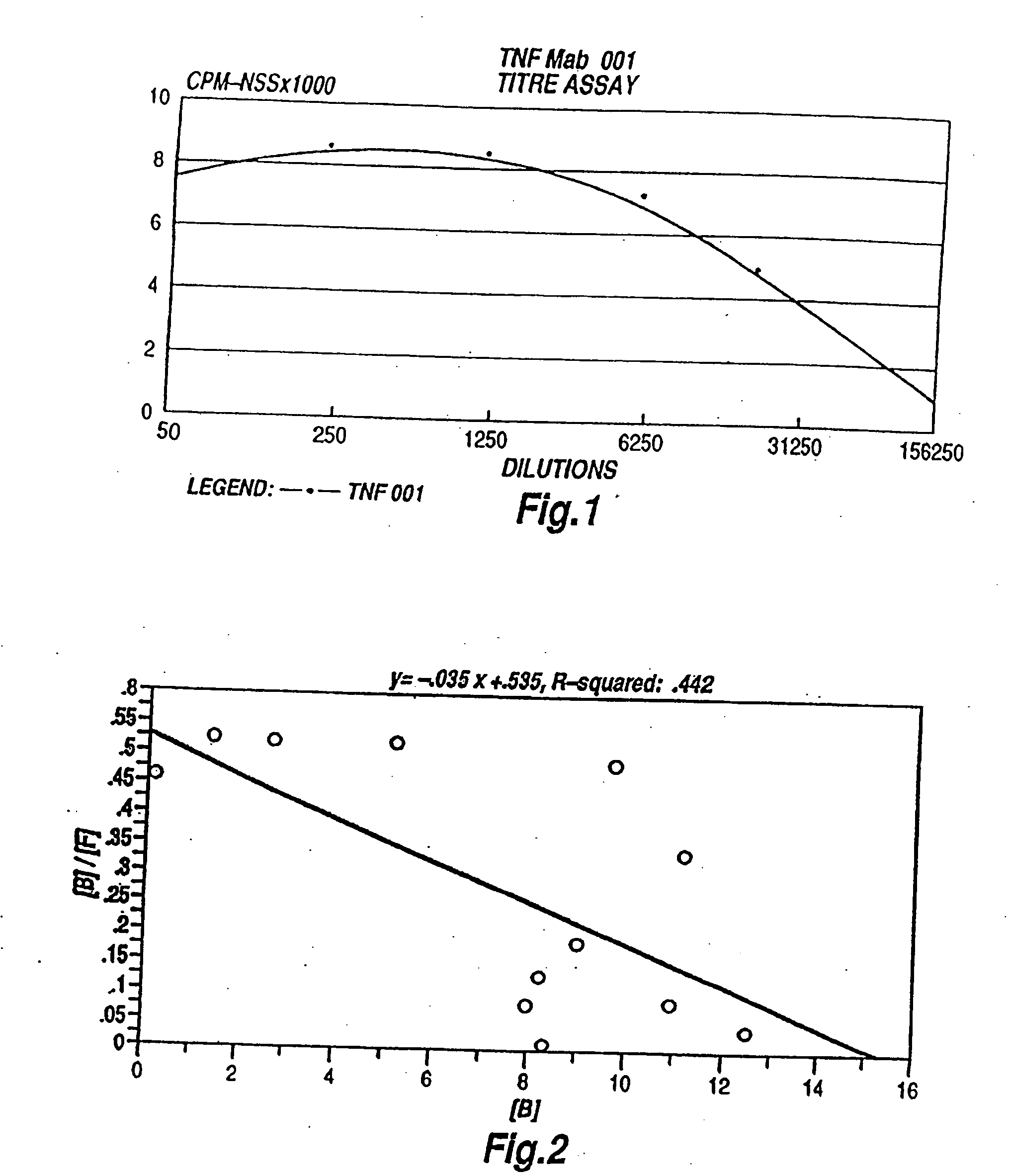
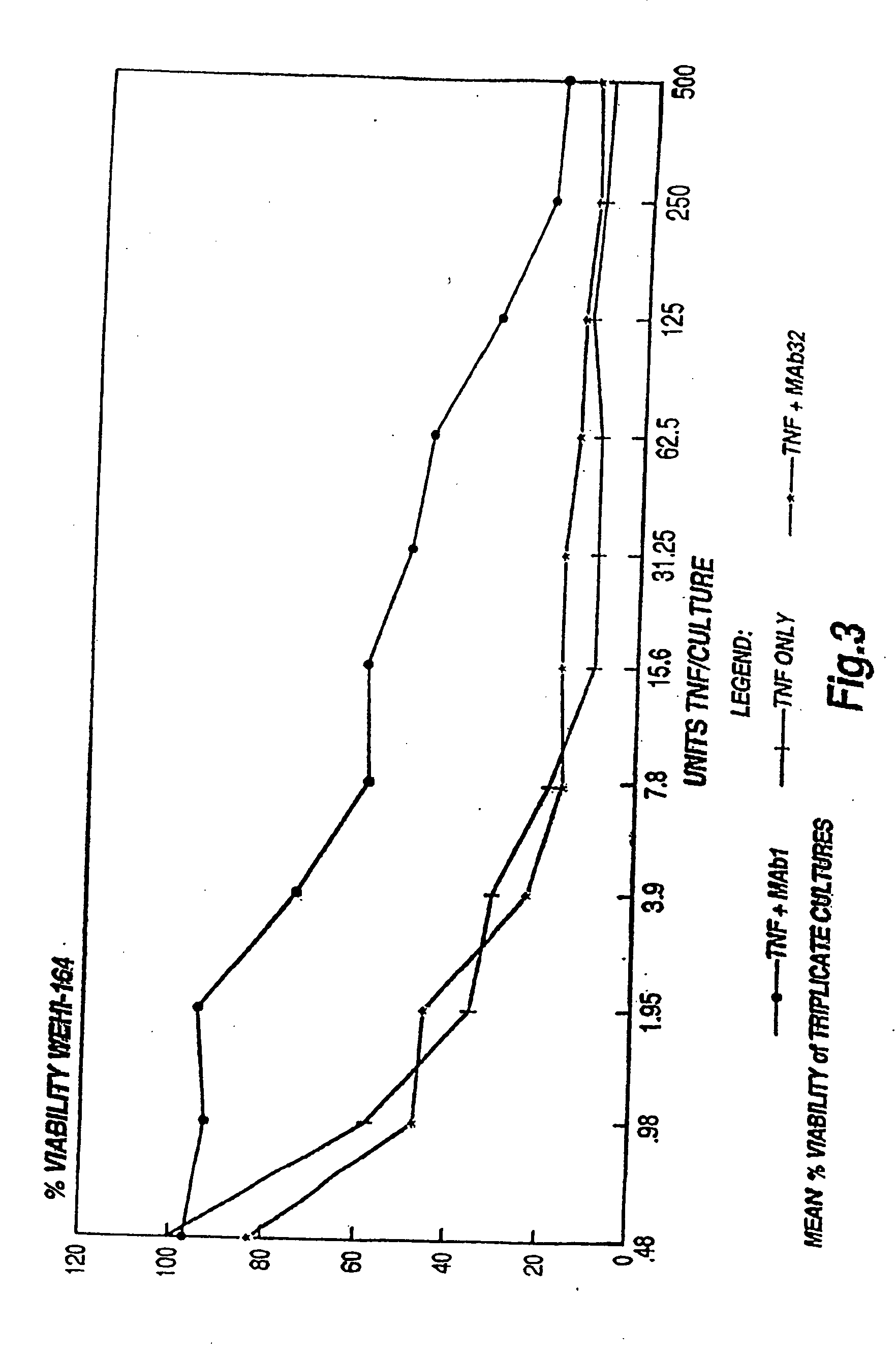
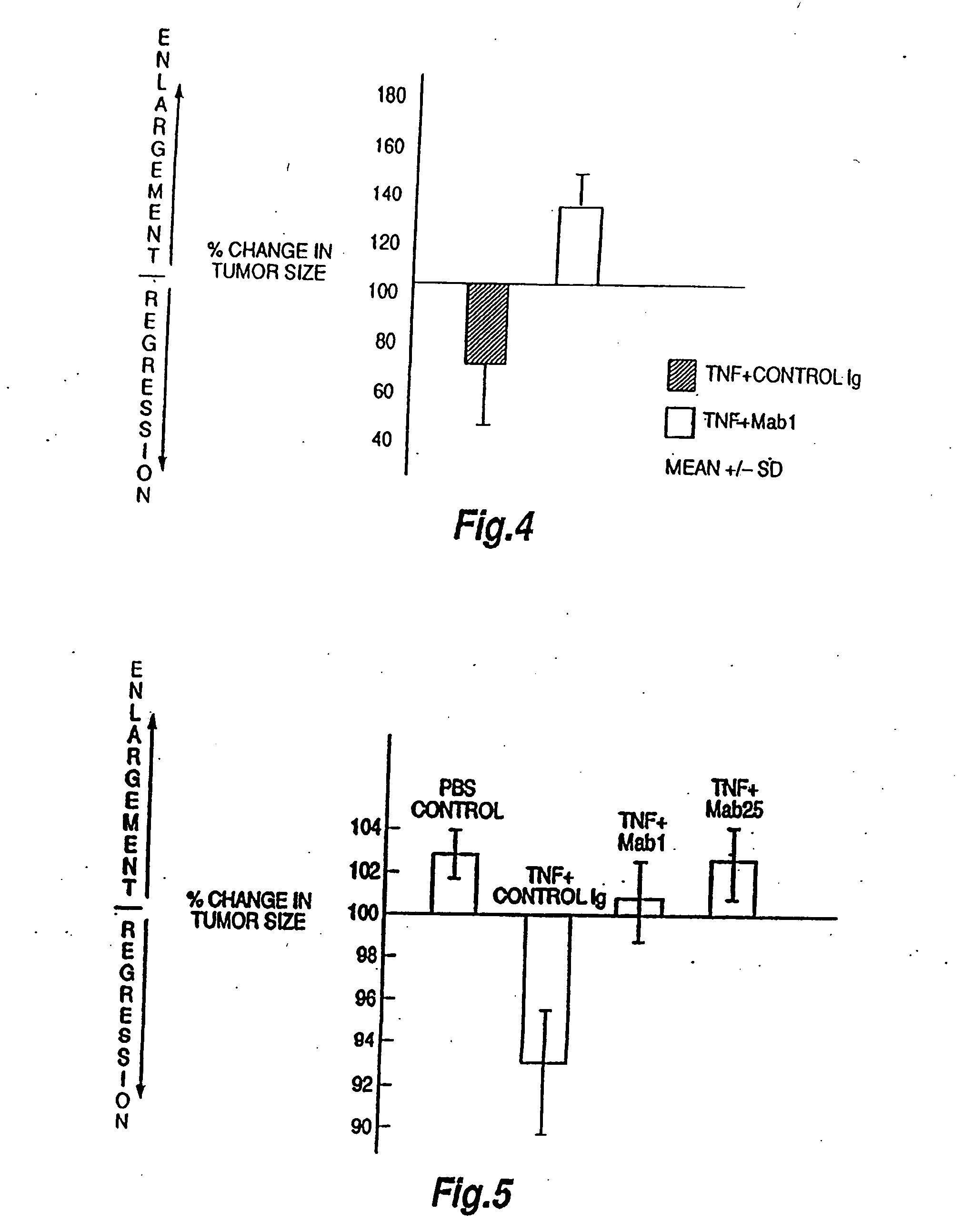
The present invention relates to ligands which bind to human tumor necrosis factor alpha (TNF) in a manner such that upon binding of these ligands to TNF the biological activity of TNF is modified. In preferred forms the ligand binds to TNF in a manner such that the induction of endothelial procoagulant activity of the TNF is inhibited; the binding of TNF to receptors on endothelial cells is inhibited; the induction of fibrin deposition in the tumor and tumor regression activities of the TNF are enhanced; and the cytotoxicity and receptor binding activities of the TNF are unaffected or enhanced on tumor cells. The ligand is preferably an antibody, F(ab) fragment, single domain antibody (dABs) single chain antibody or a serum binding protein. It is preferred, however, that the ligand is a monoclonal antibody or F(ab) fragment thereof.
Owner:CEPHALON AUSTRALIA
Antibody methods for selectively inhibiting VEGF
InactiveUS7056509B2Inhibit bindingAntibacterial agentsSenses disorderDiseaseAngiogenesis growth factor
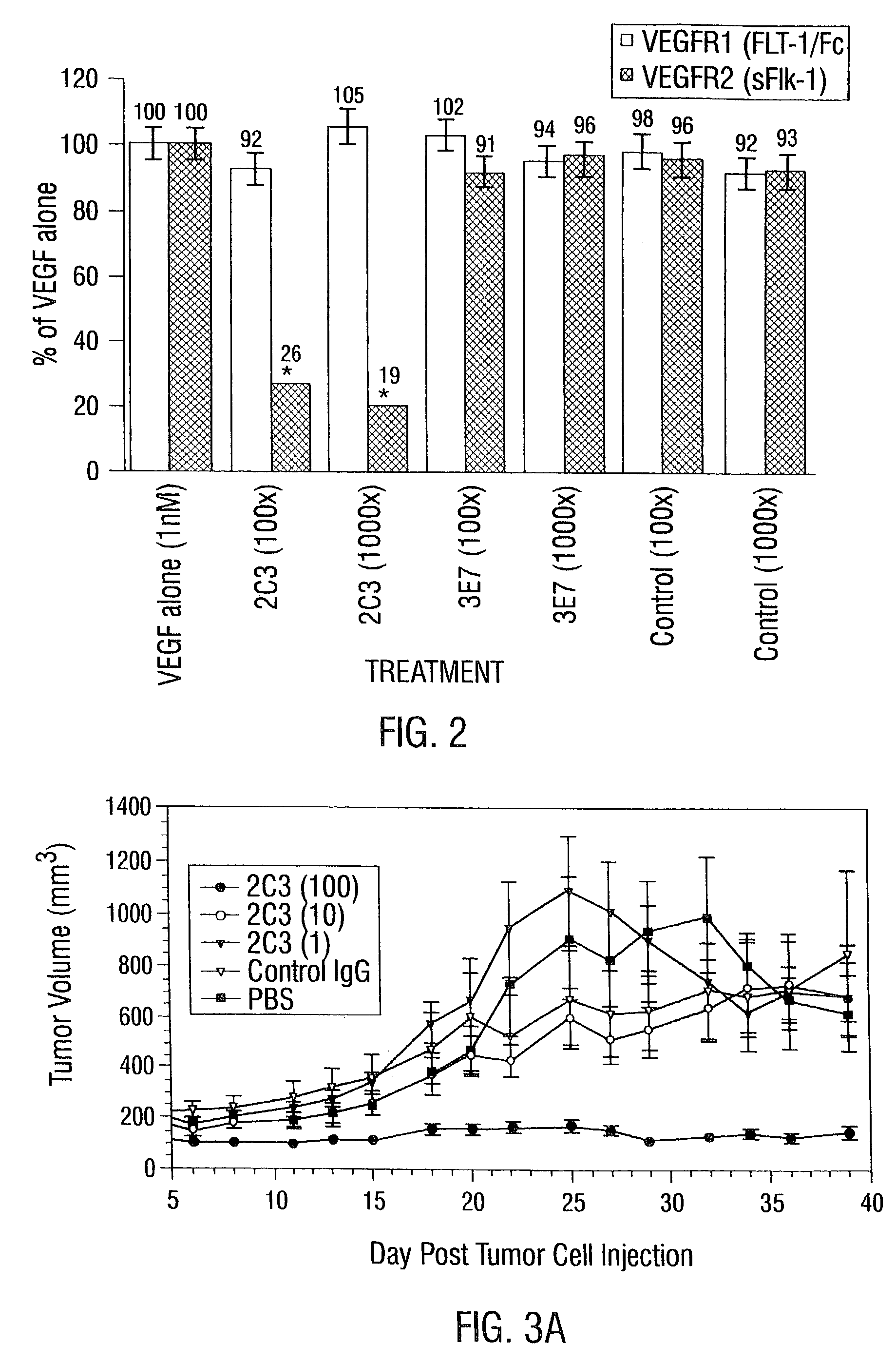
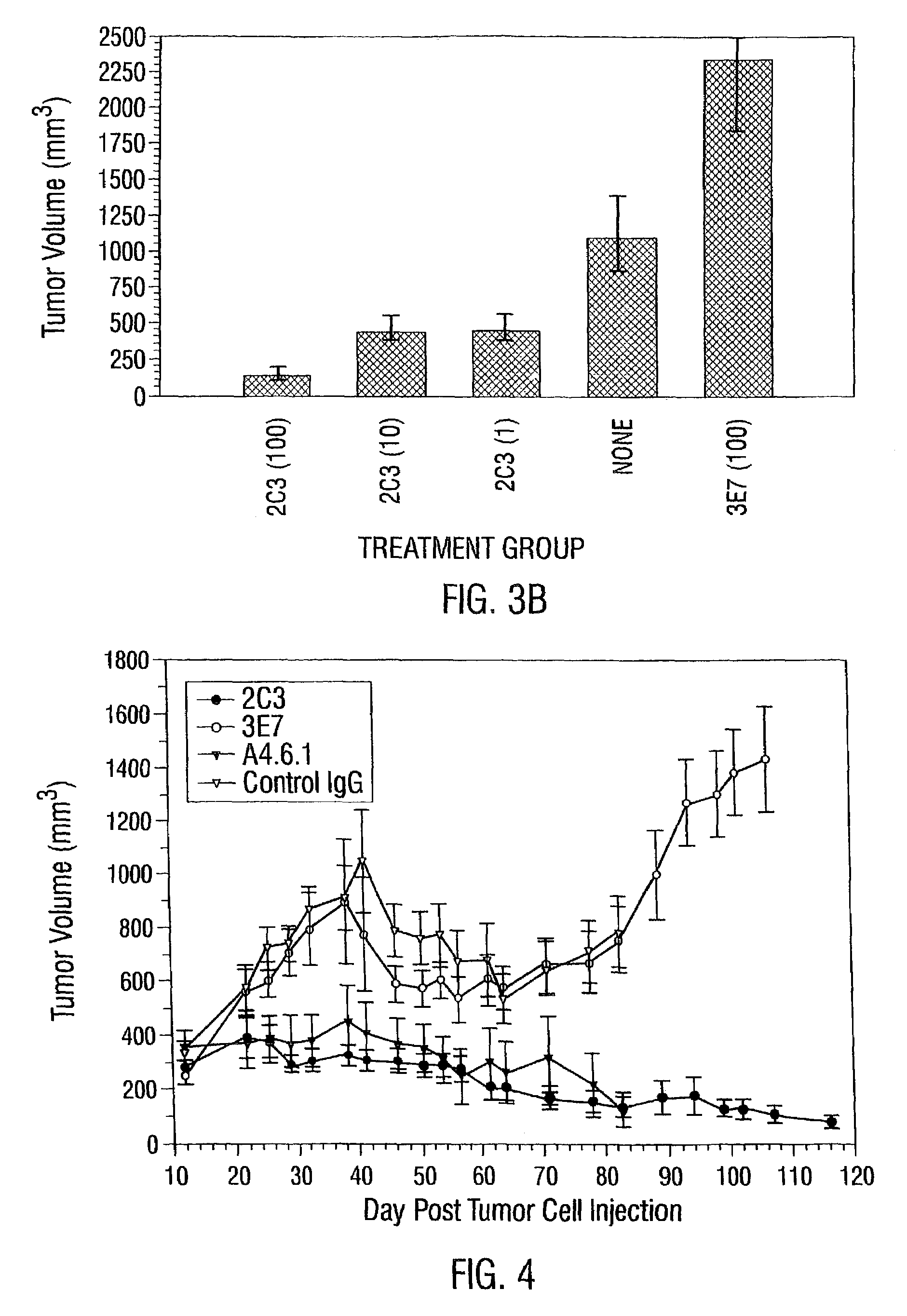
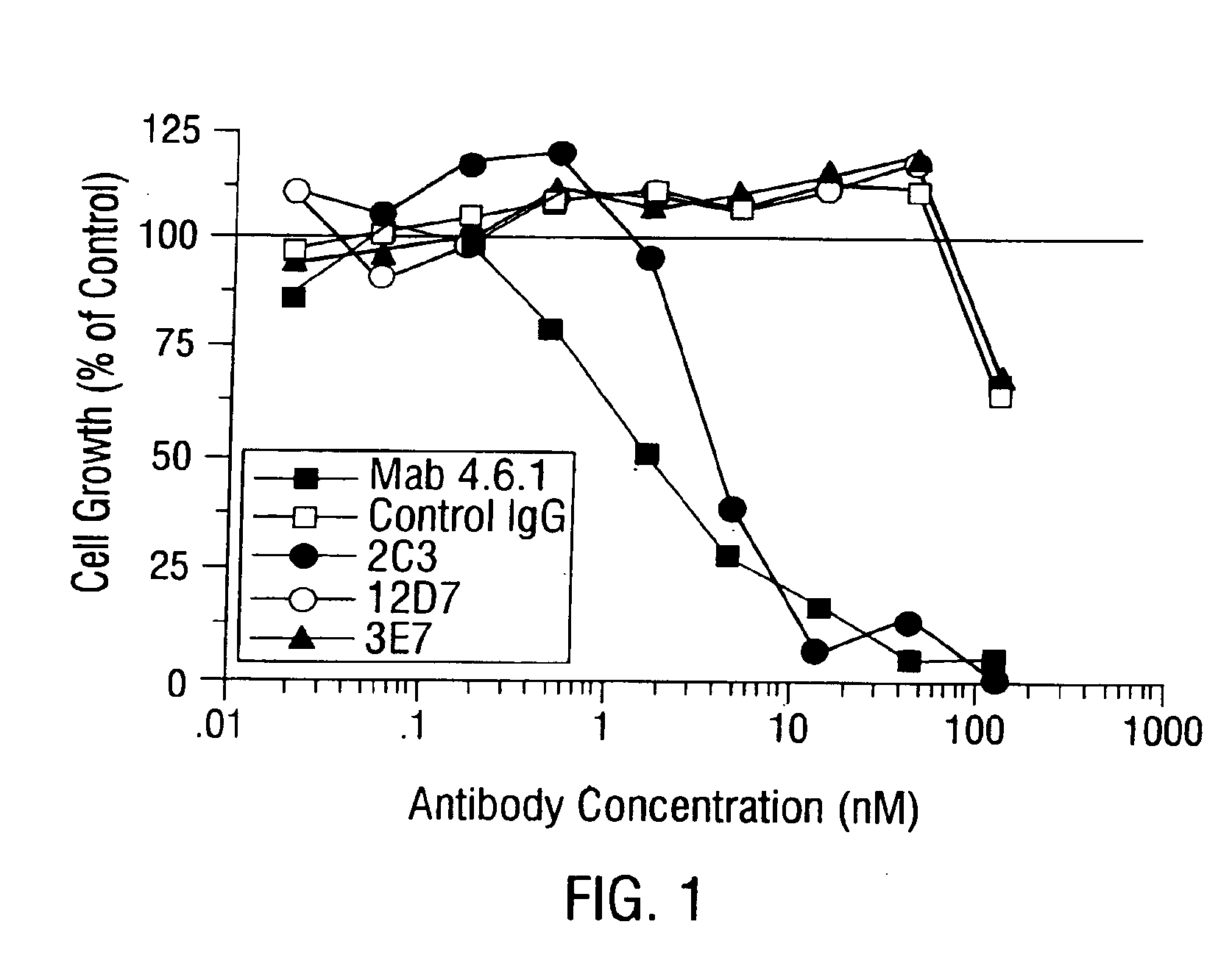
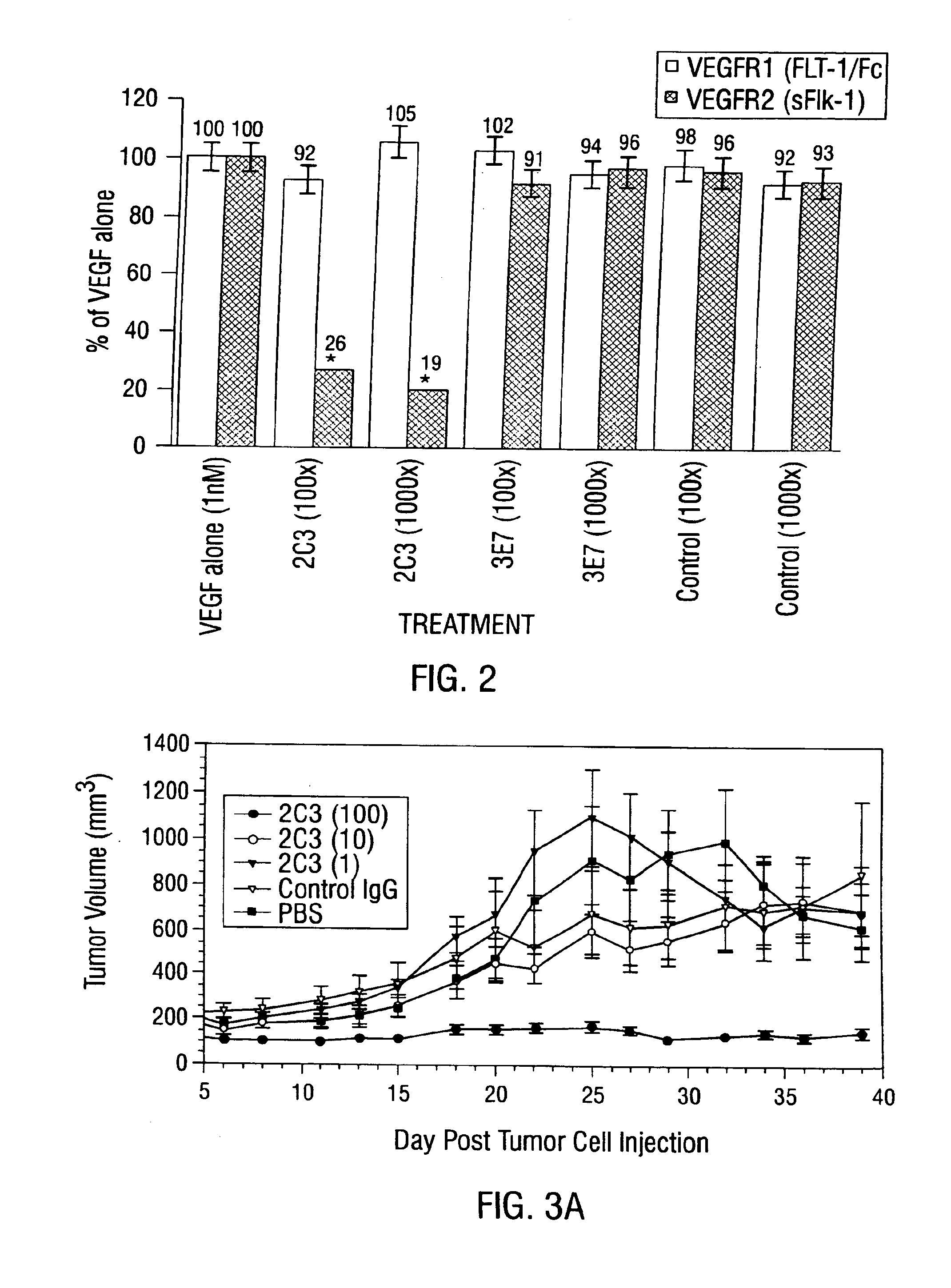
Disclosed are antibodies that specifically inhibit VEGF binding to only one (VEGFR2) of the two VEGF receptors. The antibodies effectively inhibit angiogenesis and induce tumor regression, and yet have improved safety due to their specificity. The present invention thus provides new antibody-based compositions, methods and combined protocols for treating cancer and other angiogenic diseases. Advantageous immunoconjugate and prodrug compositions and methods using the new VEGF-specific antibodies are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Antibody kits for selectively inhibiting VEGF
InactiveUS6887468B1Reduce control antibody bindingReduce the binding forceAntibacterial agentsSenses disorderDiseaseAngiogenesis growth factor
Disclosed are antibodies that specifically inhibit VEGF binding to only one (VEGFR2) of the two VEGF receptors. The antibodies effectively inhibit angiogenesis and induce tumor regression, and yet have improved safety due to their specificity. The present invention thus provides new antibody-based compositions, methods and combined protocols for treating cancer and other angiogenic diseases. Advantageous immunoconjugate and prodrug compositions and methods using the new VEGF-specific antibodies are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Cancer treatment kits comprising therapeutic conjugates that bind to aminophospholipids
InactiveUS7067109B1Stable expressionAmple targeting opportunitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsThrombusTumor regression
Disclosed is the surprising discovery that aminophospholipids, such as phosphatidylserine and phosphatidylethanolamine, are specific, accessible and stable markers of the luminal surface of tumor blood vessels. The present invention thus provides aminophospholipid-targeted diagnostic and therapeutic constructs for use in tumor intervention. Antibody-therapeutic agent conjugates and constructs that bind to aminophospholipids are particularly provided, as are methods of specifically delivering therapeutic agents, including toxins and coagulants, to the stably-expressed aminophospholipids of tumor blood vessels, thereby inducing thrombosis, necrosis and tumor regression.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
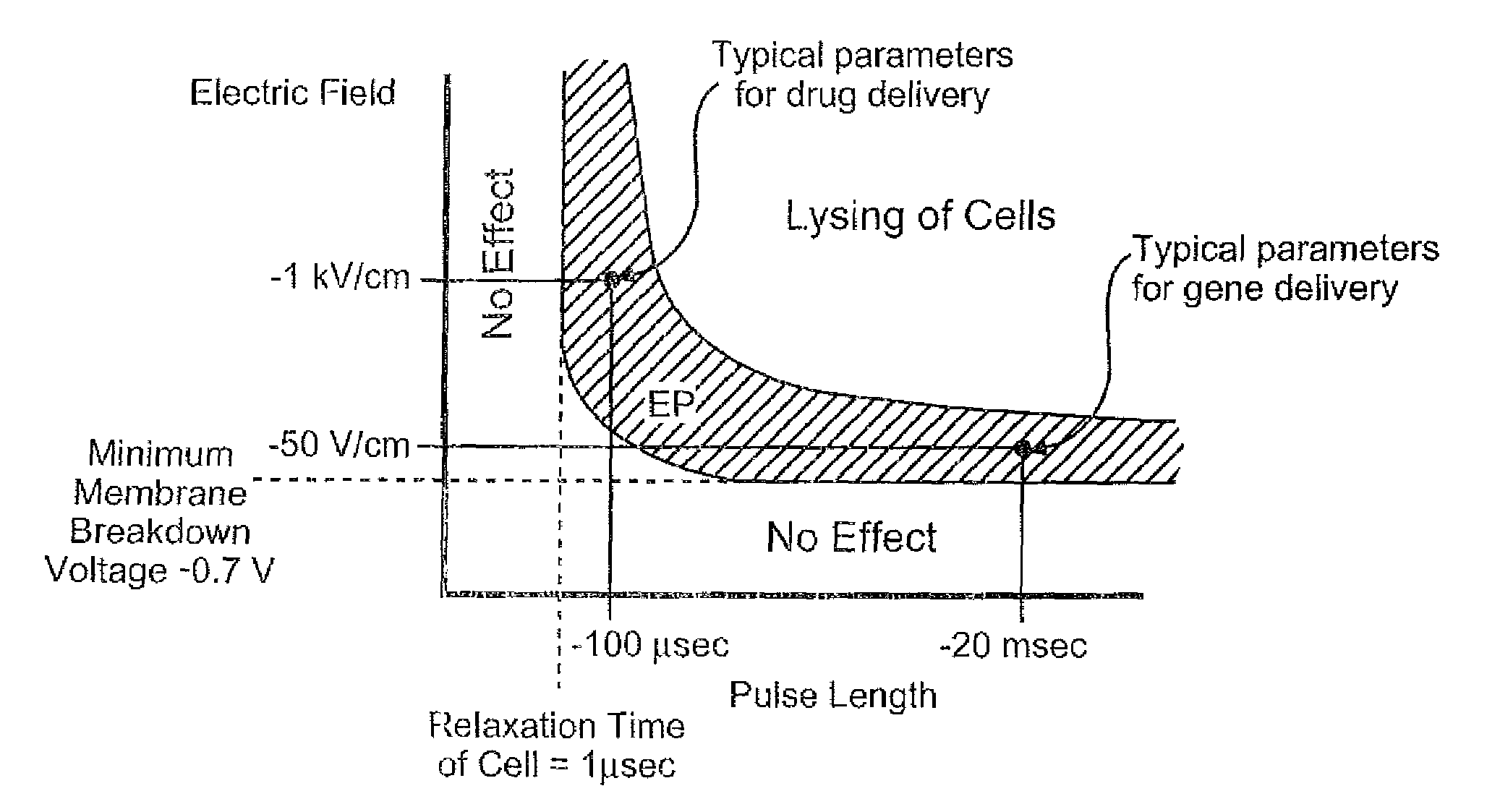
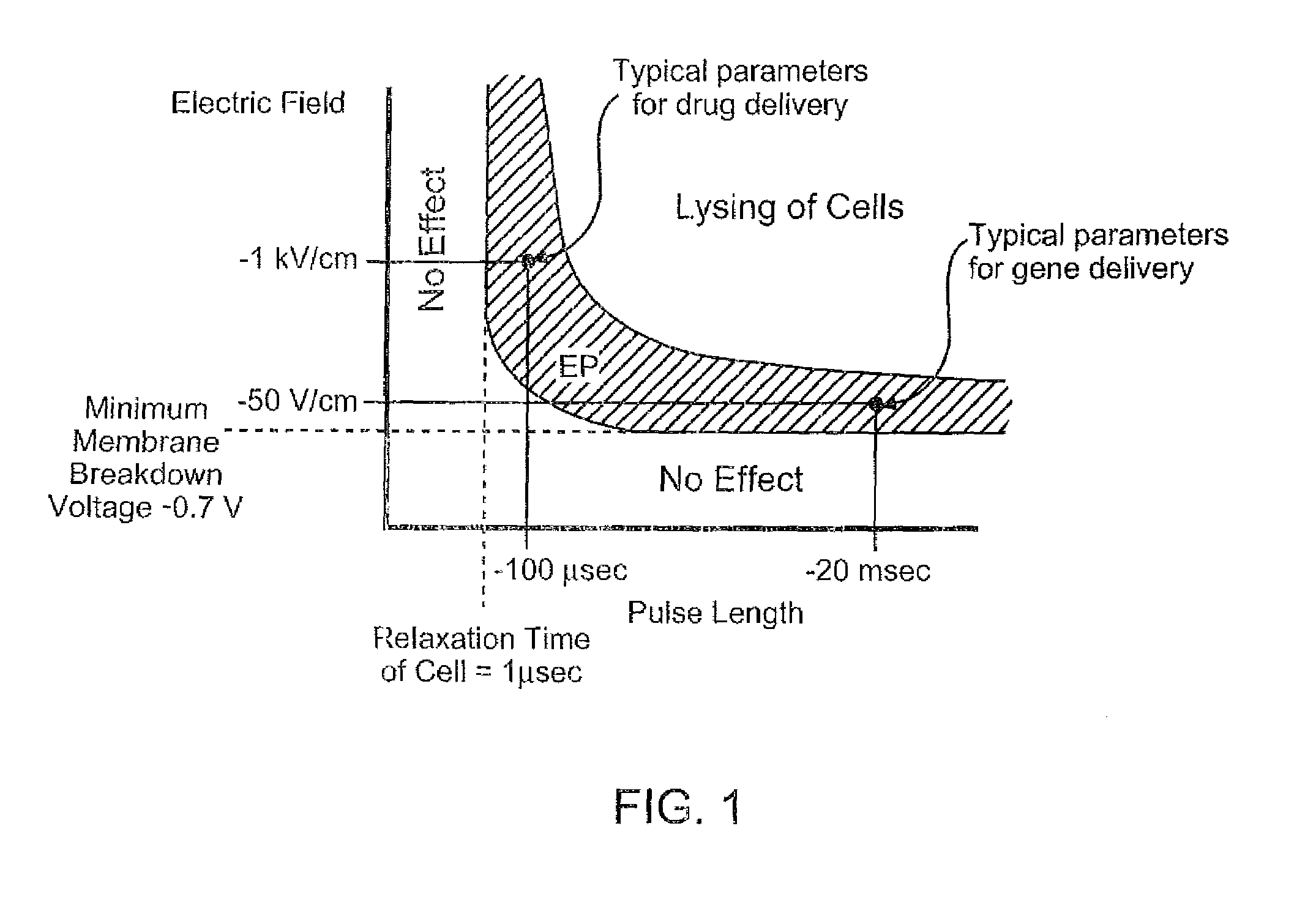
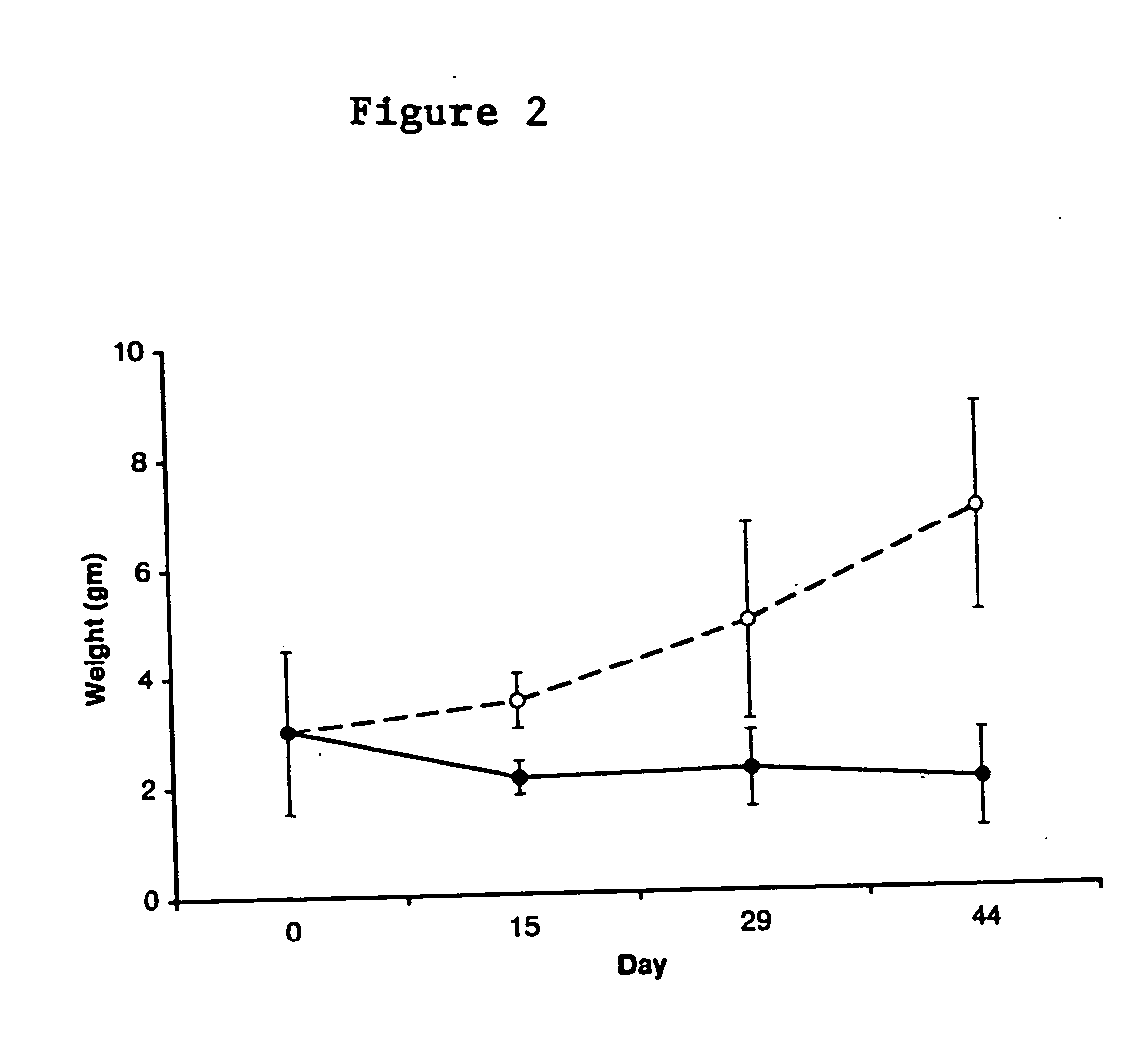
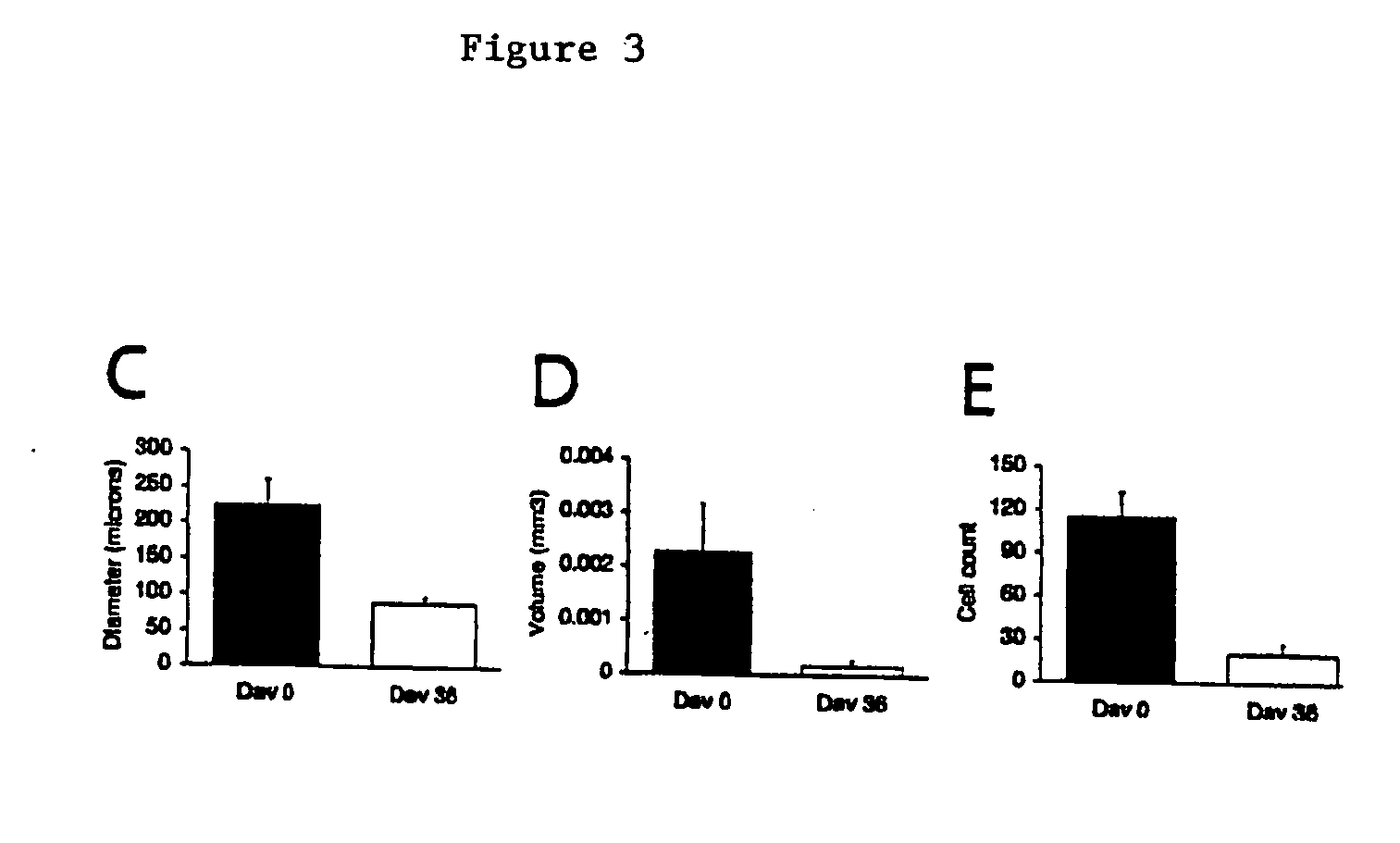
Electroporation to deliver chemotherapeutics and enhance tumor regression
ActiveUS20090326436A1Convenient introductionIncrease the number ofElectrotherapySurgical needlesAbnormal tissue growthCell membrane
A method is disclosed for disrupting capillary blood flow and trapping materials such as chemotherapeutic agents in undesirable tissue, including cells of a cancerous or non-cancerous tumor. The method involves the placement of electrodes into or near the vicinity of capillary vessels supplying blood to capillaries in the undesirable tissue, and application of electrical pulses causing capillary blood flow disruption. In some cases, the electric pulses irreversibly permeate the cell membranes, thereby invoking cell death. The irreversibly permeabilized cells are left in situ and are removed by the body's immune system. The process may further comprise monitoring blood flow and / or infusion of a material such as a chemotherapeutic agent or marker into the blood.
Owner:RGT UNIV OF CALIFORNIA
Therapeutic treatment of human cancers using simple salts of zinc
InactiveUS20110117210A1Functional impairmentOrganic active ingredientsBiocideDiseaseTumor regression
The present invention is a non-topical method of therapeutically treating human cancer patients which comprises an administration of an anti-cancer medicament comprising at least one simple organic or inorganic salt of zinc in at least a minimally effective concentration to suppress malignant tumor growth and induce tumor regression in-vivo. Administration can be performed by oral, parenteral, and / or body cavity routings; and the therapeutic treatment method is effective for the treatment of a diverse range of primary human cancers and metastatic diseases.
Owner:UGOLKOV ANDREY
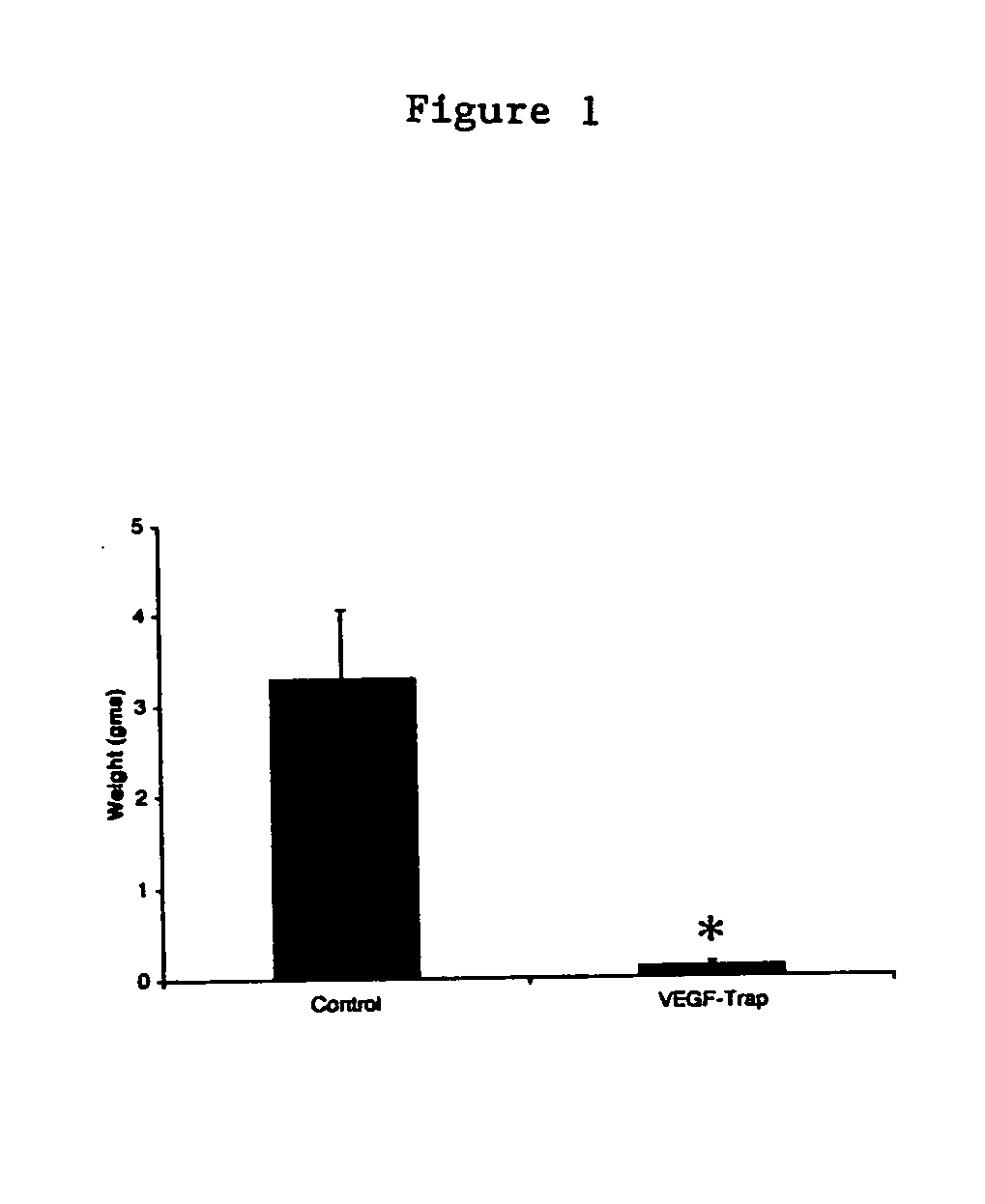
Method of tumor regression with VEGF inhibitors
ActiveUS20040265309A1Peptide/protein ingredientsAntibody mimetics/scaffoldsLymphatic SpreadFactor ii
Methods of regressing or inhibiting a tumor in a subject by administering an agent capable of blocking, inhibiting, or ameliorating vascular endothelial growth factor (VEGF)-mediated activity to a subject in need thereof such that the tumor is regressed or inhibited. The method of the invention results in a reduction of tumor size and inhibition of tumor metastases. This method is particularly useful for patients suffering from bulky, metastatic cancers.
Owner:REGENERON PHARM INC +1
Electroporation to deliver chemotherapeutics and enhance tumor regression
ActiveUS8298222B2Disruption in blood flowReducing minimal therapeutic doseElectrotherapySurgical needlesCell membraneTumor regression
A method is disclosed for disrupting capillary blood flow and trapping materials such as chemotherapeutic agents in undesirable tissue, including cells of a cancerous or non-cancerous tumor. The method involves the placement of electrodes into or near the vicinity of capillary vessels supplying blood to capillaries in the undesirable tissue, and application of electrical pulses causing capillary blood flow disruption. In some cases, the electric pulses irreversibly permeate the cell membranes, thereby invoking cell death. The irreversibly permeabilized cells are left in situ and are removed by the body's immune system. The process may further comprise monitoring blood flow and / or infusion of a material such as a chemotherapeutic agent or marker into the blood.
Owner:RGT UNIV OF CALIFORNIA
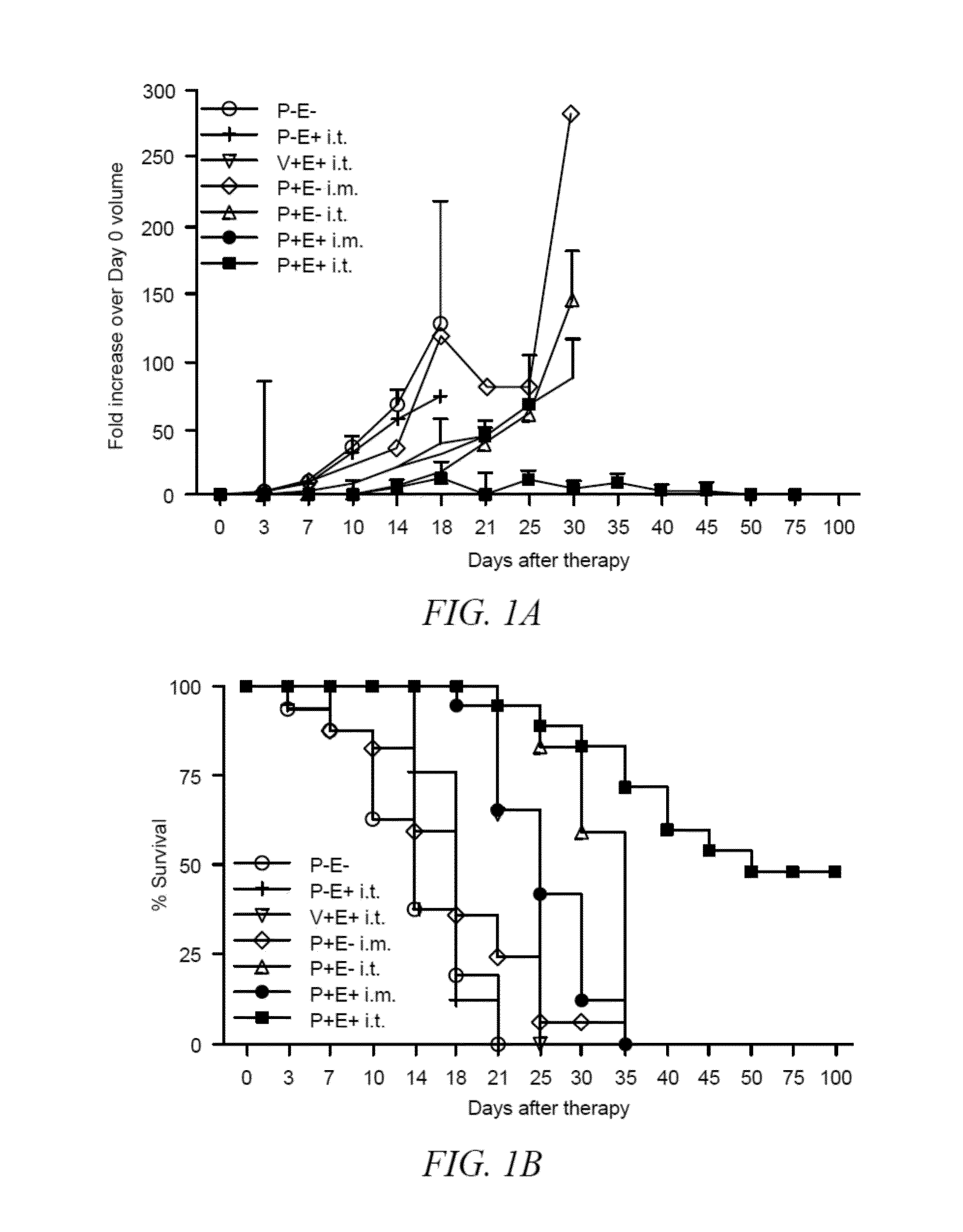
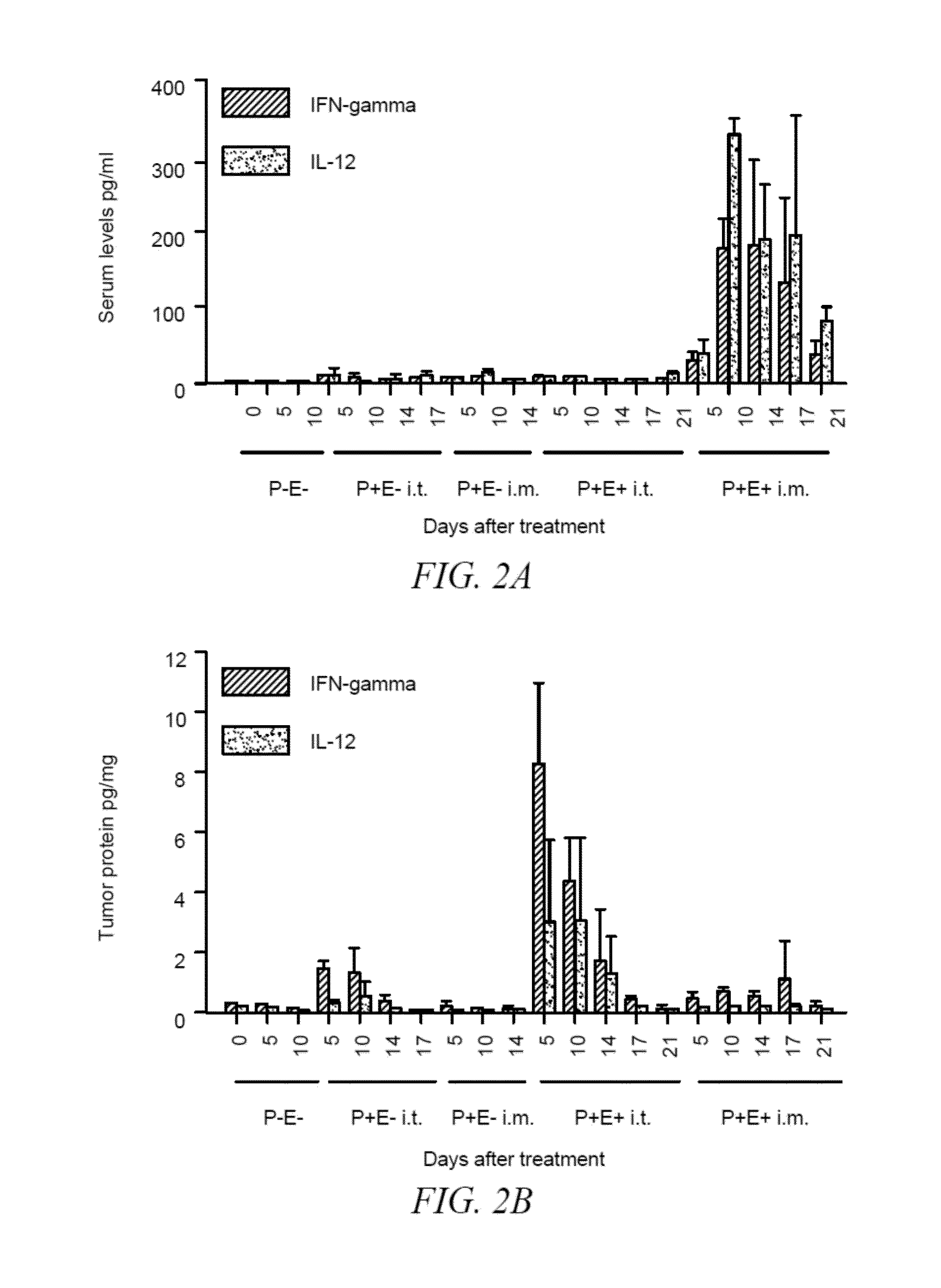
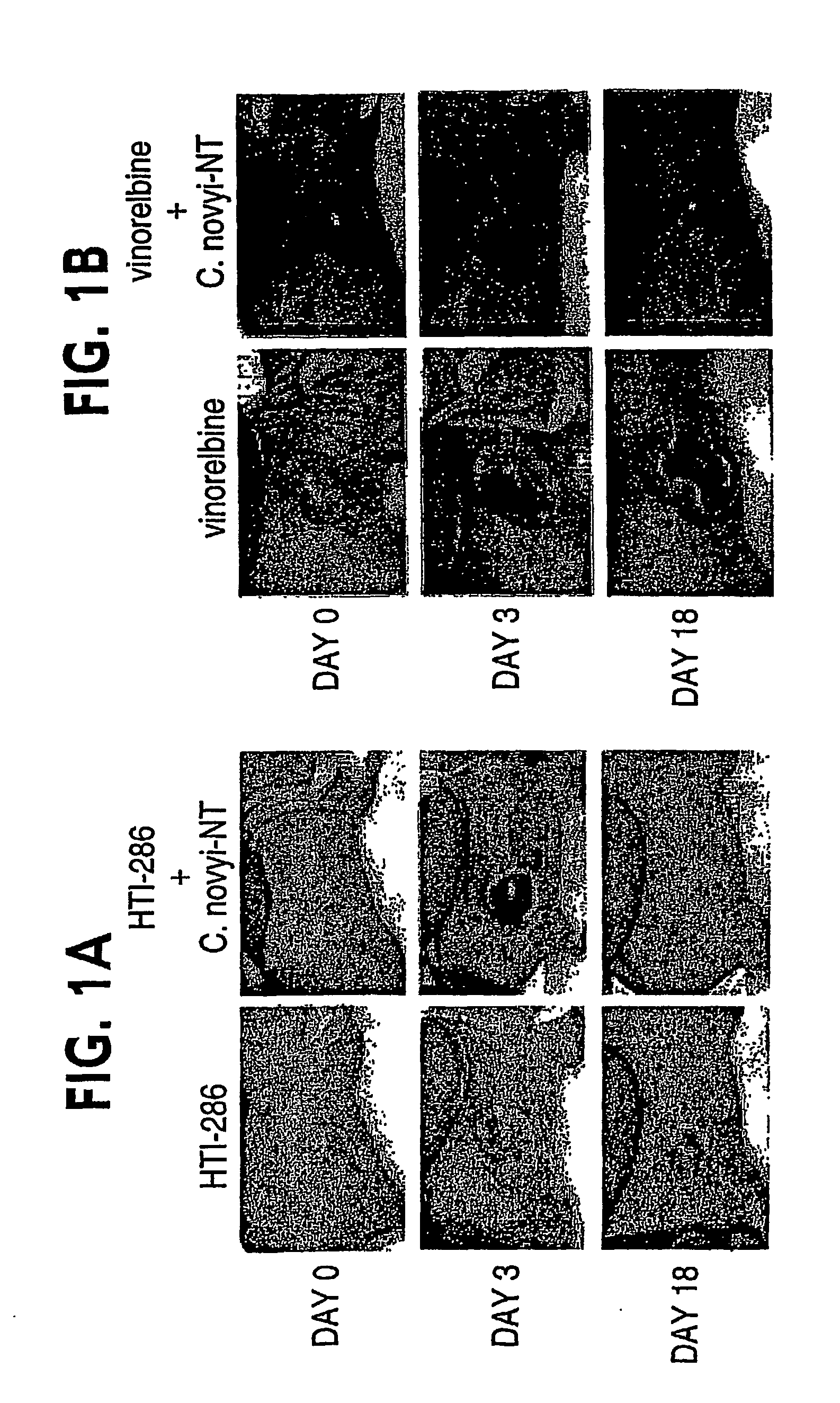
Combination bacteriolytic therapy for the treatment of tumors
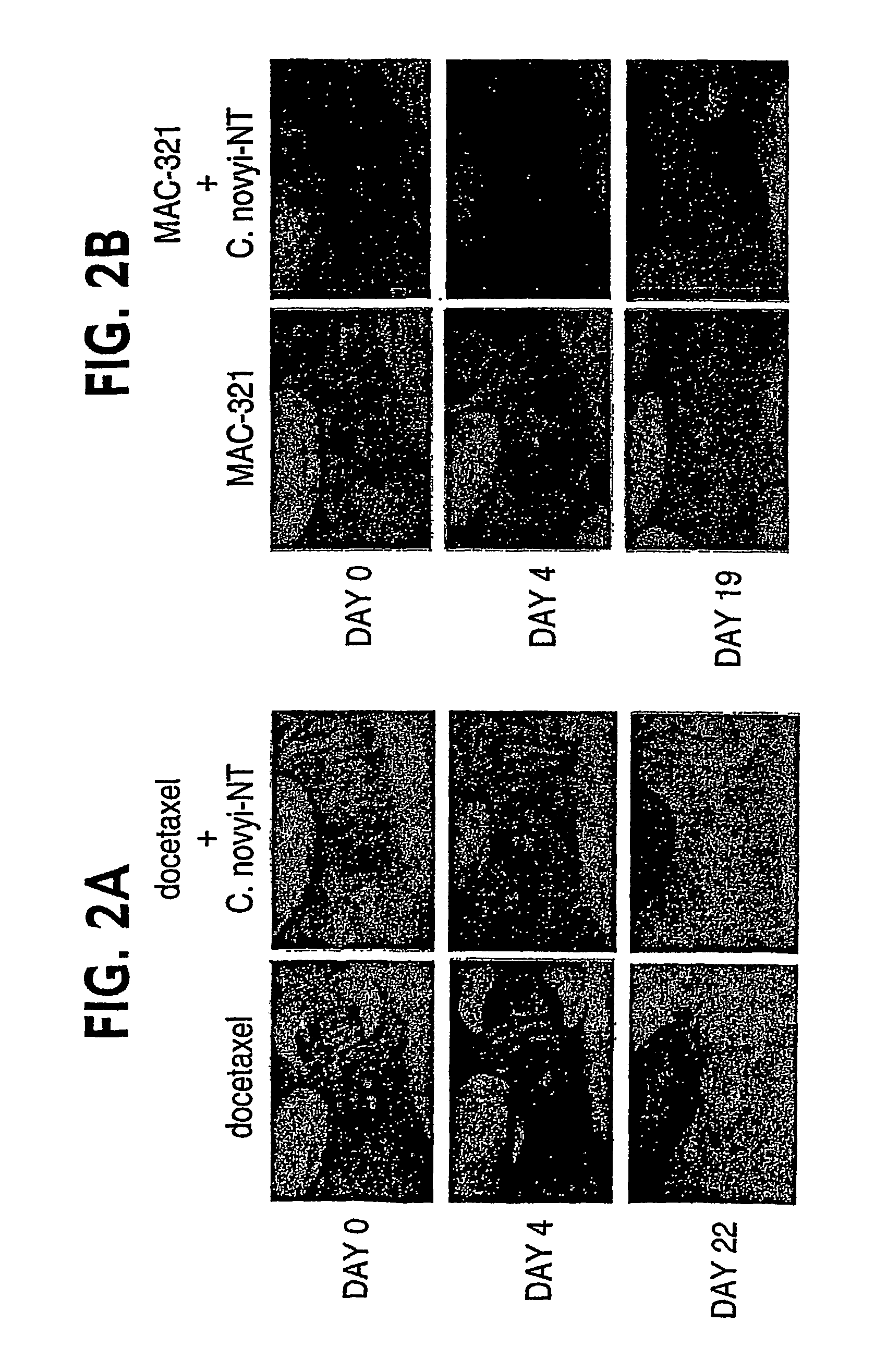
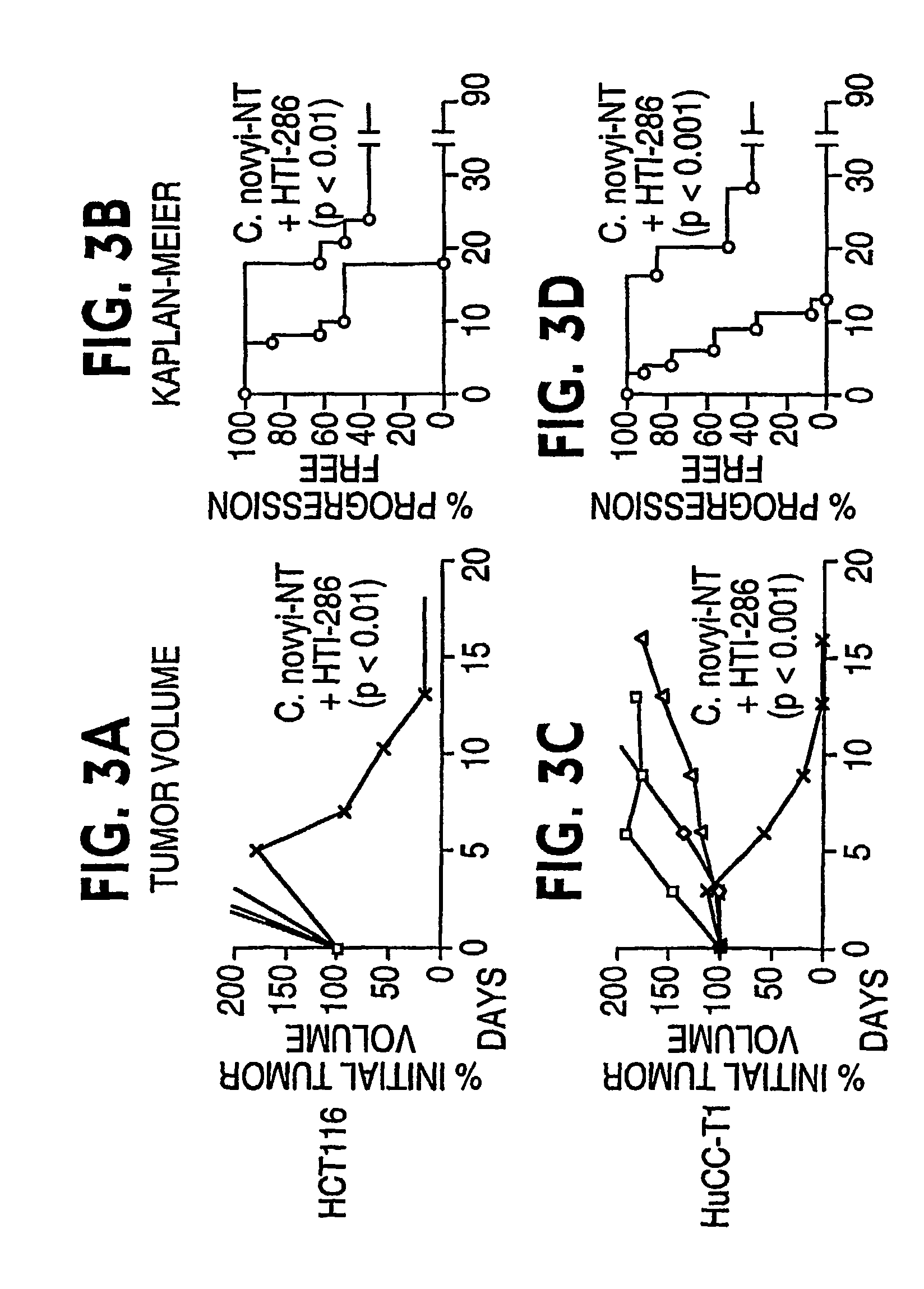
Current approaches for treating cancer are limited, in part, by the inability of drugs to affect the poorly vascularized regions of tumors. We have found that spores of anaerobic bacteria in combination with agents which interact with microtubules can cause the destruction of both the vascular and avascular compartments of tumors. Two classes of microtubule inhibitors were found to exert markedly different effects. Some agents that inhibited microtubule synthesis, such as vinorelbine, caused rapid, massive hemorrhagic necrosis when used in combination with spores. In contrast, agents that stabilized microtubules, such as the taxane docetaxel, resulted in slow tumor regressions that killed most neoplastic cells. Remaining cells in the poorly perfused regions of tumors could be eradicated by sponzlated bacteria. Mechanistic studies showed that the microtubule destabilizers, but not the microtubule stabilizers, radically reduced blood flow to tumors, thereby enlarging the hypoxic niche in which spores could germinate. A single intravenous injection of spores plus selected microtubule-interacting agents was able to cause regressions of several tumors in the absence of excessive toxicity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for the treatment of malignancies
ActiveUS8802643B1Improve survival rateElectrotherapyPeptide/protein ingredientsTumor regressionMalignancy
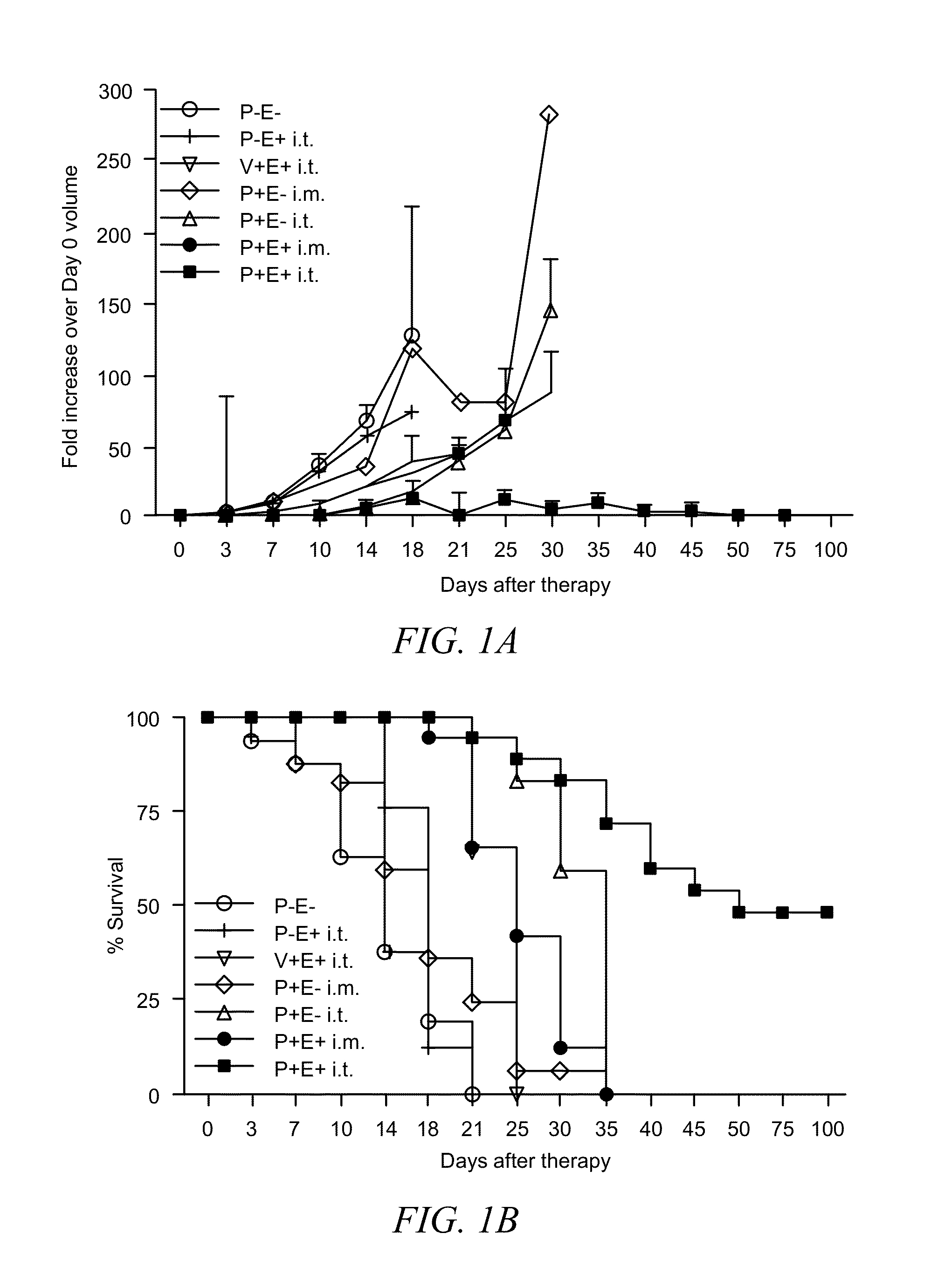
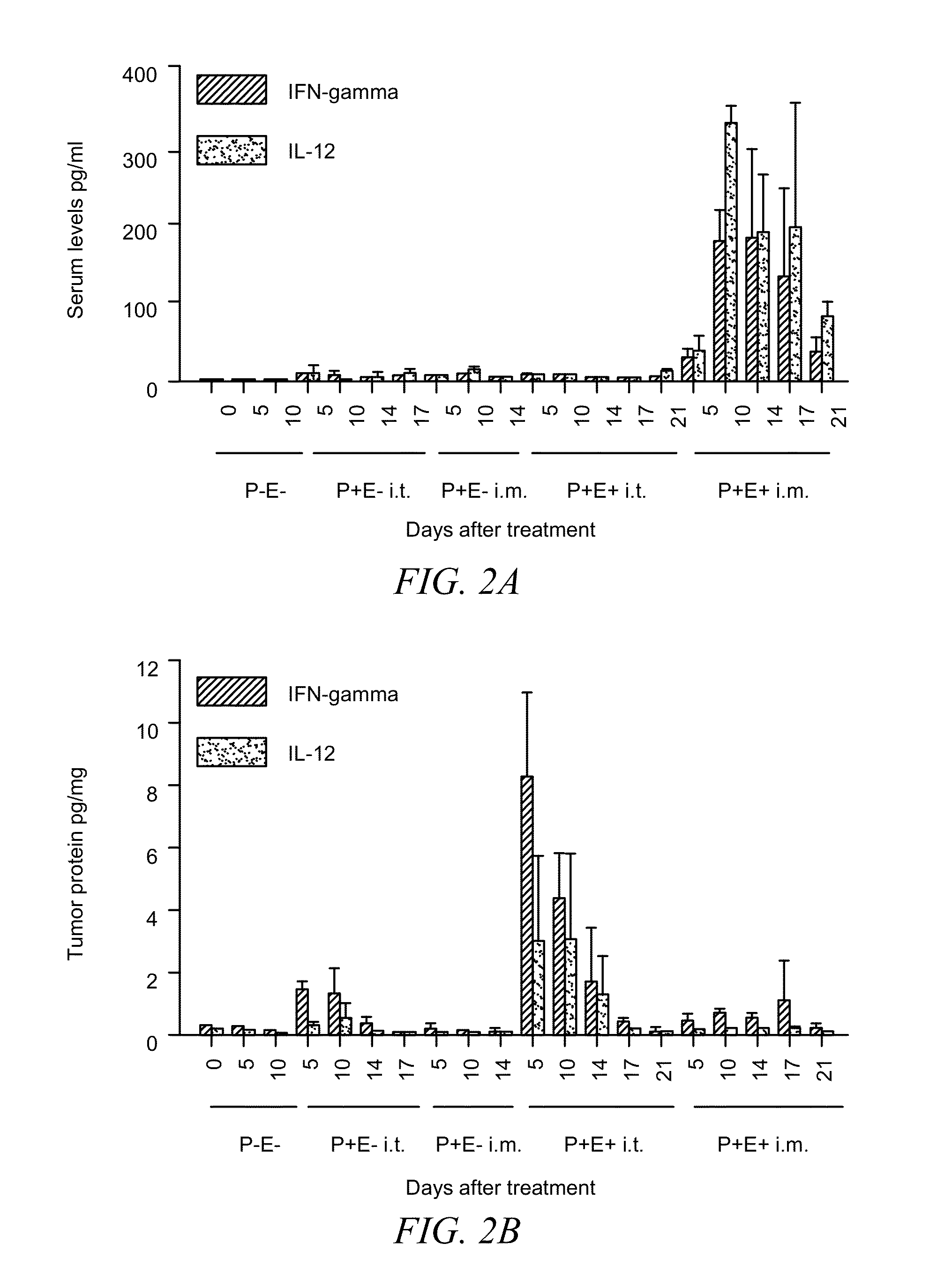
A method of treating cancerous tumors is presented herein. The method includes injecting an effective dose of a plasmid encoded for IL-12, B7-1 or IL-15 into a cancerous tumor and subsequently administering at least one high voltage, short duration pulse to the tumor. The electroporation pulses may be administered at least 700V / cm for a duration of less than 1 millisecond. The intratumor treatments with electroporation may be administered in at least a two-treatment protocol with the time between treatments being about 7 days. The intratumor treatments with electroporation may be administered in a three-treatment protocol with a time of four days between the first and second treatments and a time of three days between the second and third treatments. It was found that the intratumor treatments using electroporation not only resulted in tumor regression but also induced an immune memory response which prevented the formation of new tumors.
Owner:UNIV OF SOUTH FLORIDA
Combined methods for tumor coagulation and tumor treatment
InactiveUS7691380B2Significant utilityPeptide/protein ingredientsImmunoglobulins against growth factorsBispecific antibodyTumor therapy
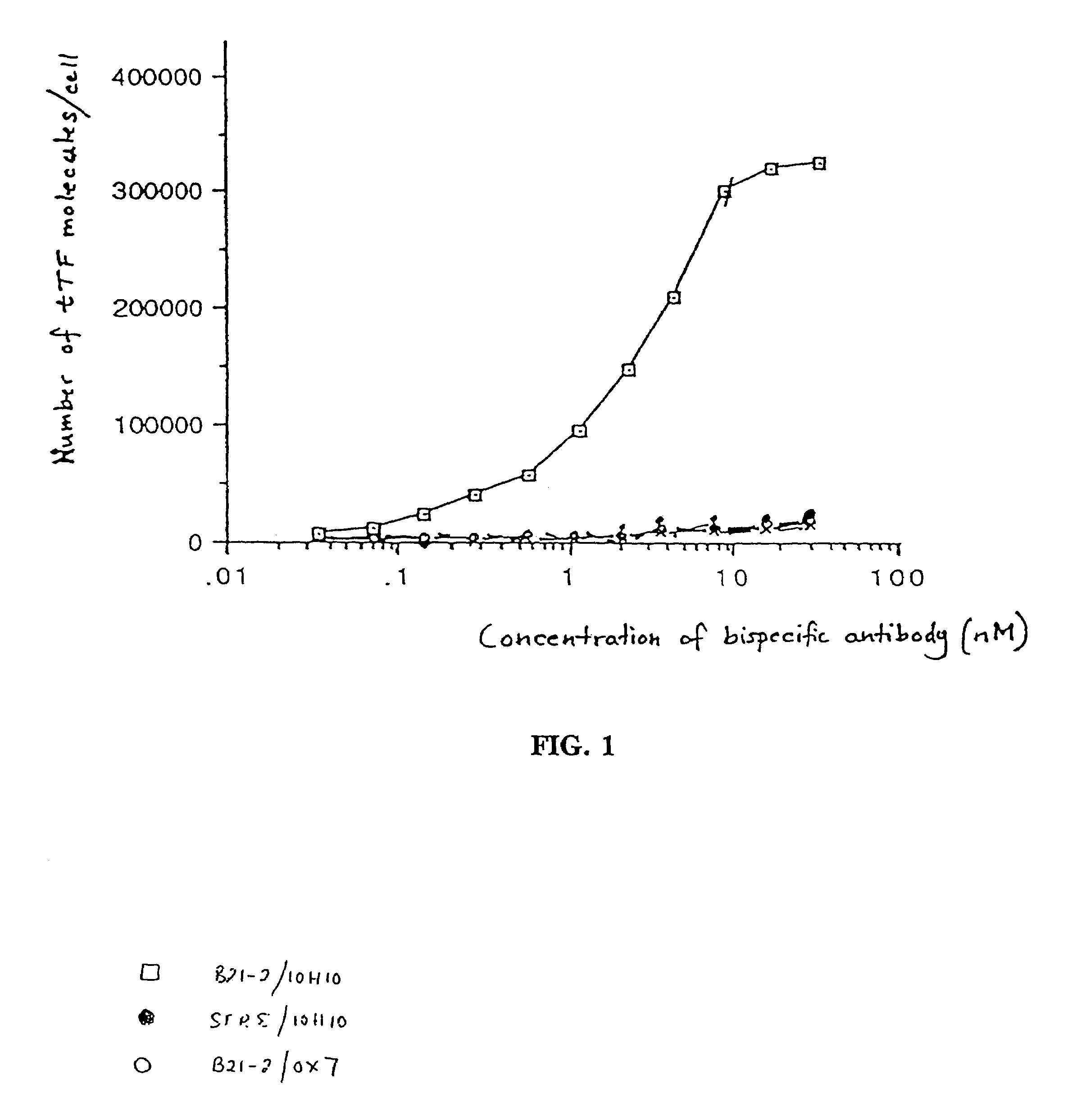
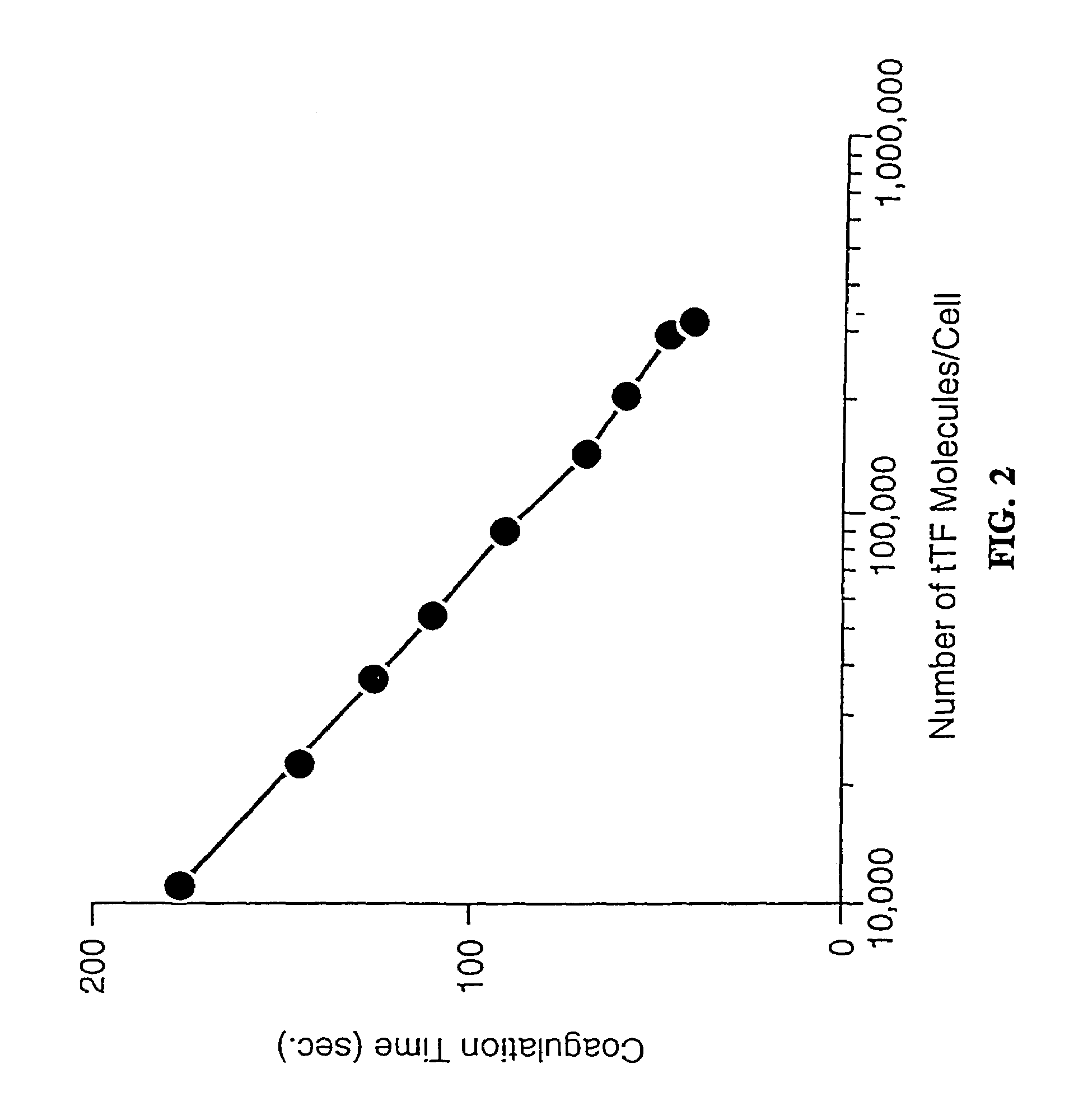
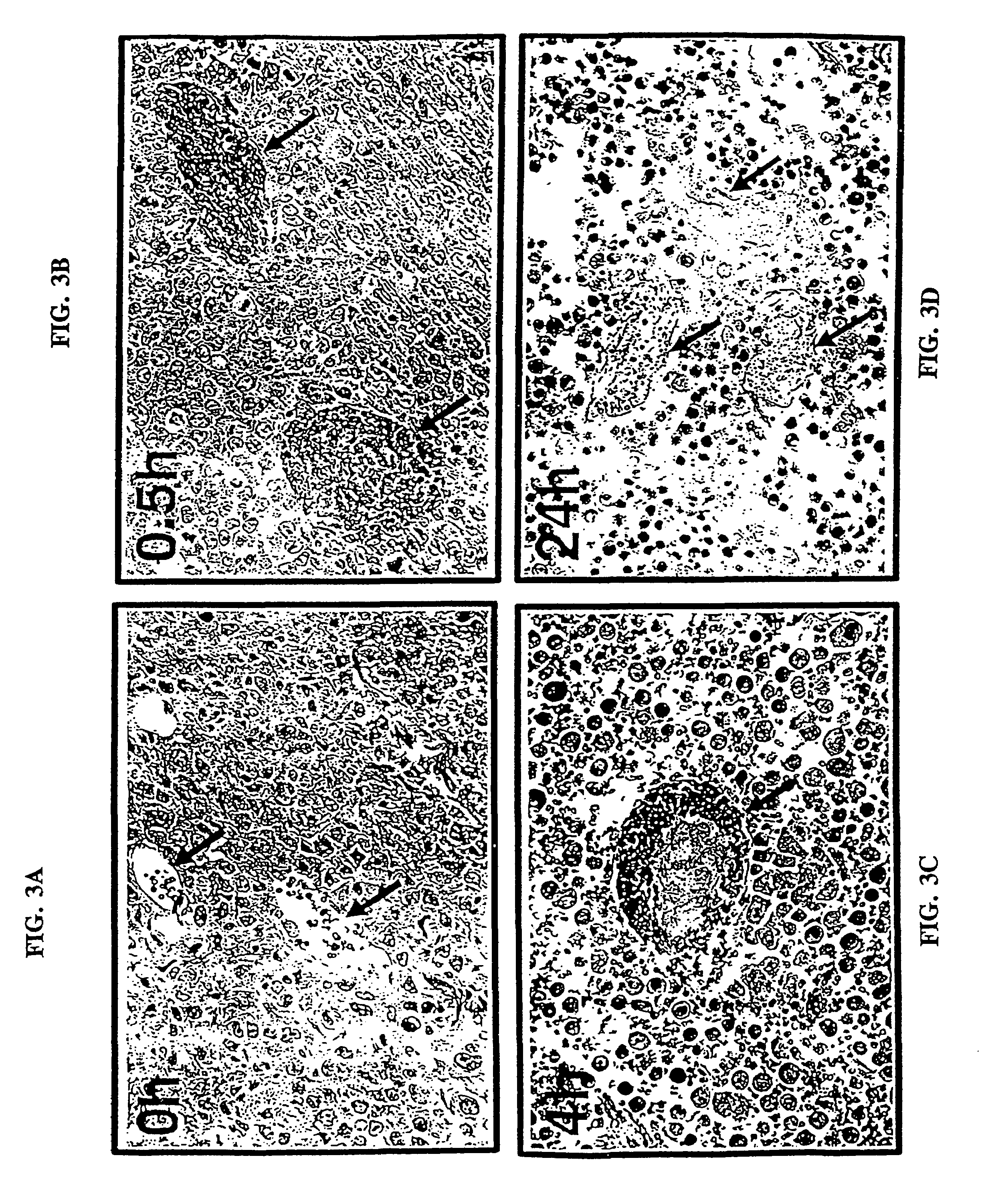
Disclosed are various compositions and methods for use in achieving specific blood coagulation. This is exemplified by the specific in vivo coagulation of tumor vasculature, causing tumor regression, through the site-specific delivery of a coagulant using a bispecific antibody.
Owner:THE SCRIPPS RES INST +1
Method for tumor treatment using infusion of xenogeneic cells to induce hyperacute rejection and innocent bystander effect
A method for treating tumors. Through infusion or xenotransplantation of xenogeneic cells, such as infusion of murine cells into the peritoneal cavity of humans, a hyperacute rejection response to the cells is induced. This in turn creates a bystander effect to the tumor. This effect creates tumor regression. This treatment can be used alone or in conjunction with gene therapy or chemotherapy treatments.
Owner:HUMAN GENE THERAPY RES INST
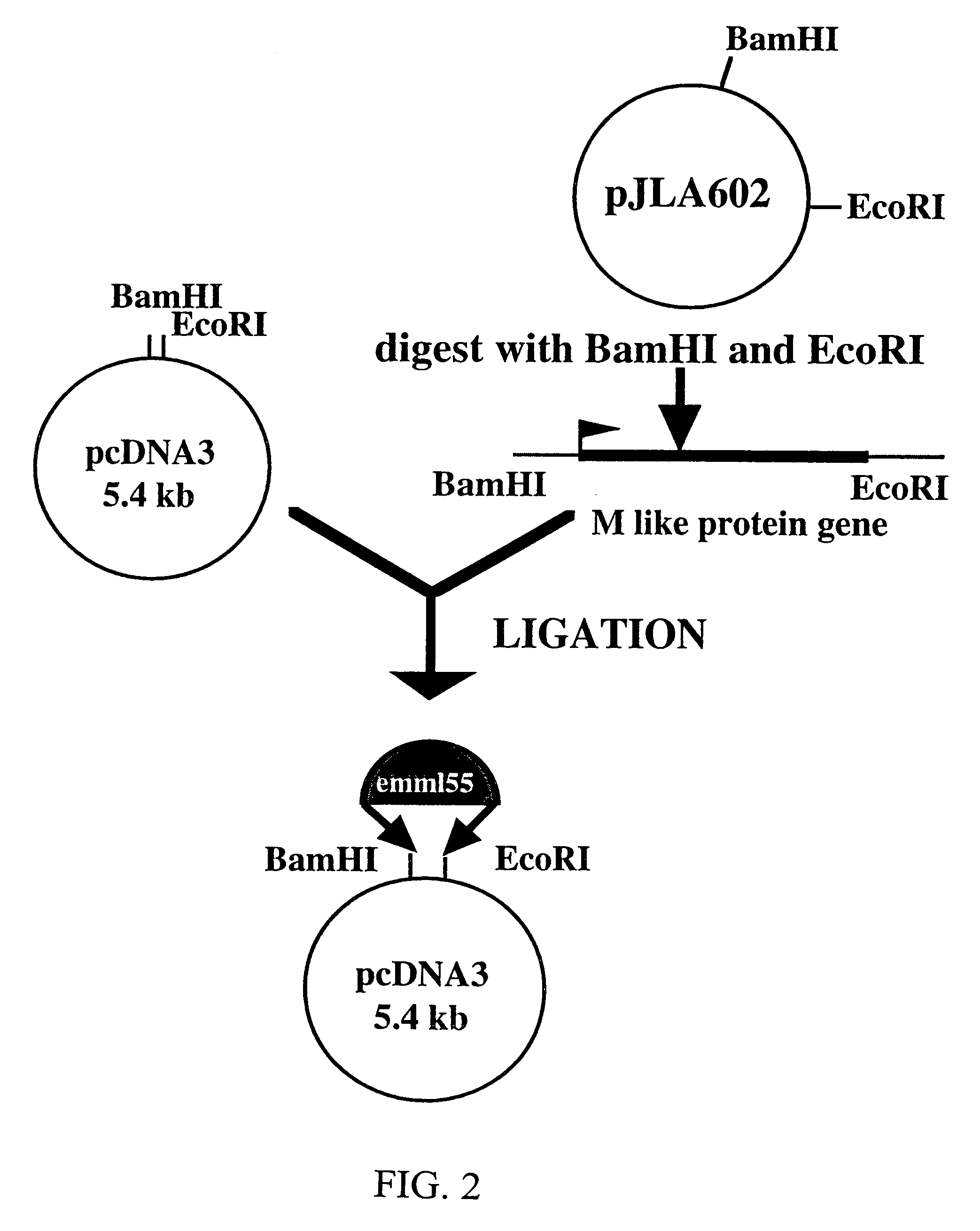
Materials and methods for treating oncological disease
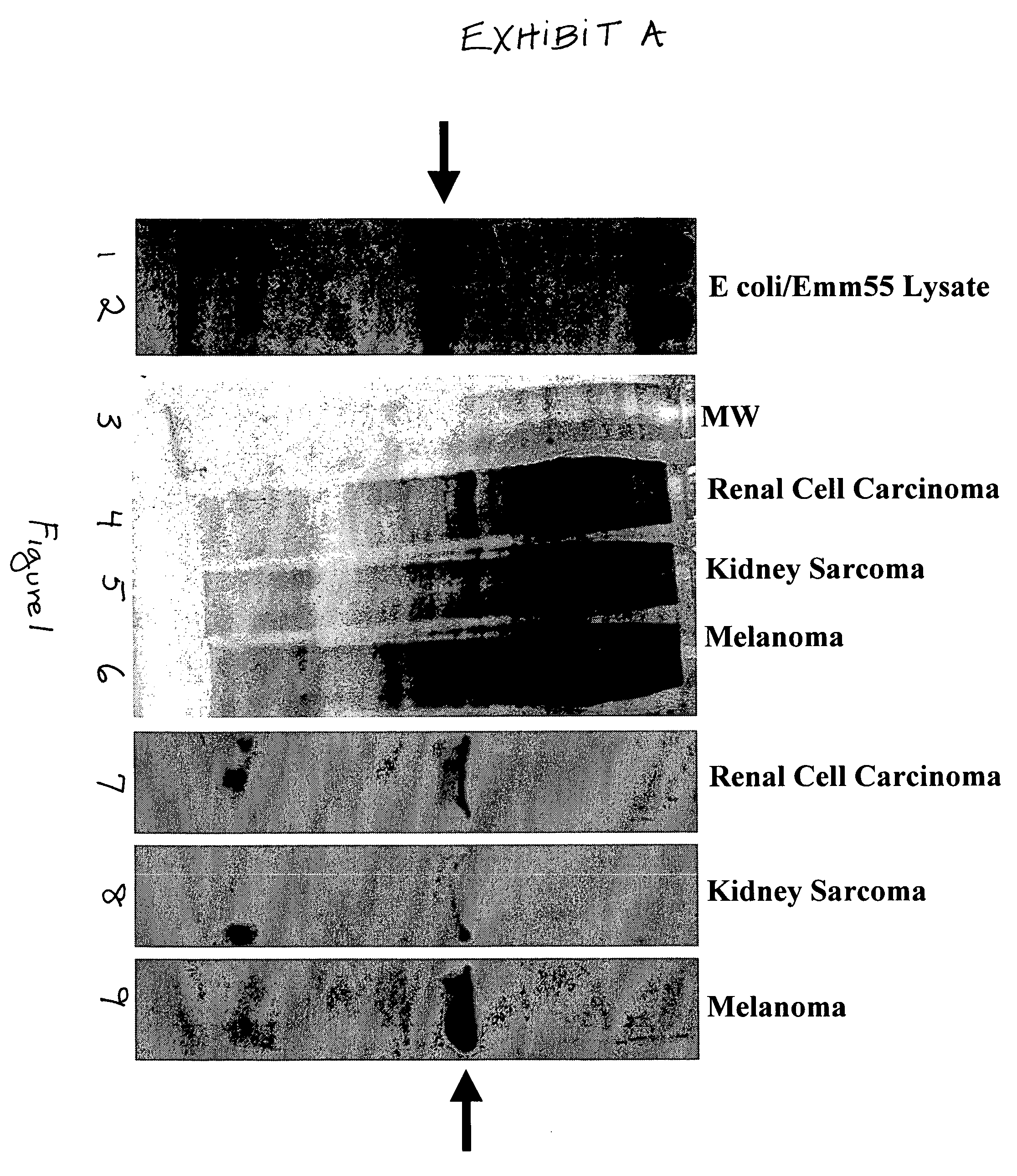
Novel methods are disclosed for treating oncological disorders in an individual or animal using a superantigen expressed in tumor cells. A gene encoding a superantigen, such as an M-like protein of group A streptococci, can be introduced into a tumor cell in order to make the tumor cell more immunogenic in the host. Also contemplated are methods wherein a cell expresses a superantigen or superantigens, and immunogenic or immunostimulatory proteins, such as foreign MHC, cytokines, porcine-derived hyperacute rejection antigen, Mycobacterium-derived antigens, and the like. The subject invention also pertains to cells transformed with polynucleotides encoding a superantigen and foreign MHC antigen, cytokines, and other immunogenic or immunostimulatory proteins. Transformed cells according to the subject invention are then provided to an individual or animal in need of treatment for an oncological disorder. The immune response to tumor cells transformed according to the present invention inhibits in vivo tumor growth and results in subsequent tumor regression. The subject invention also pertains to cell lines transformed with genes encoding a superantigen and, optionally, a foreign Class II MHC antigen and / or a cytokine.
Owner:MORPHOGENESIS
Tumor lesion regression and conversion in situ into autologous tumor vaccines by compositions that result in anti-Gal Antibody Binding
InactiveUS20060251661A1Reduce the overall heightBiocideSugar derivativesAntigenAbnormal tissue growth
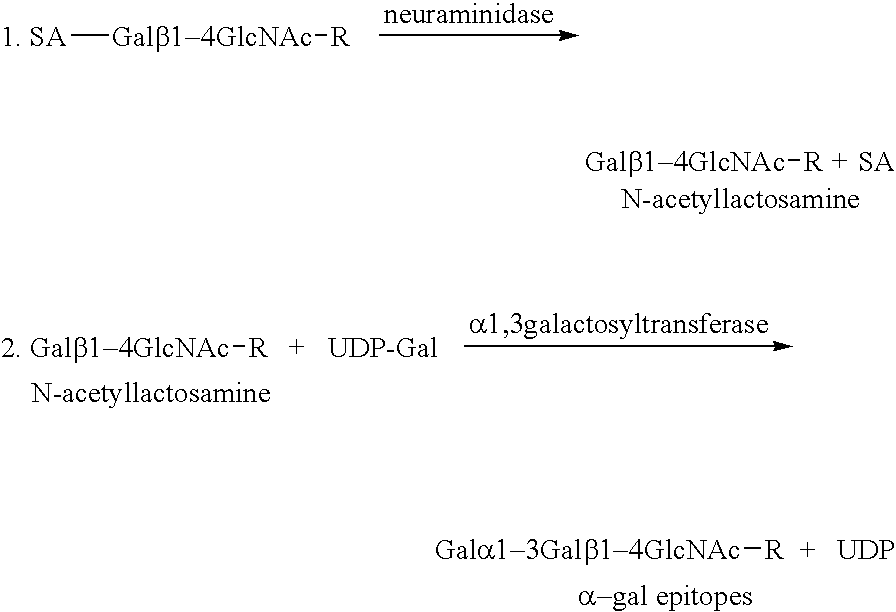
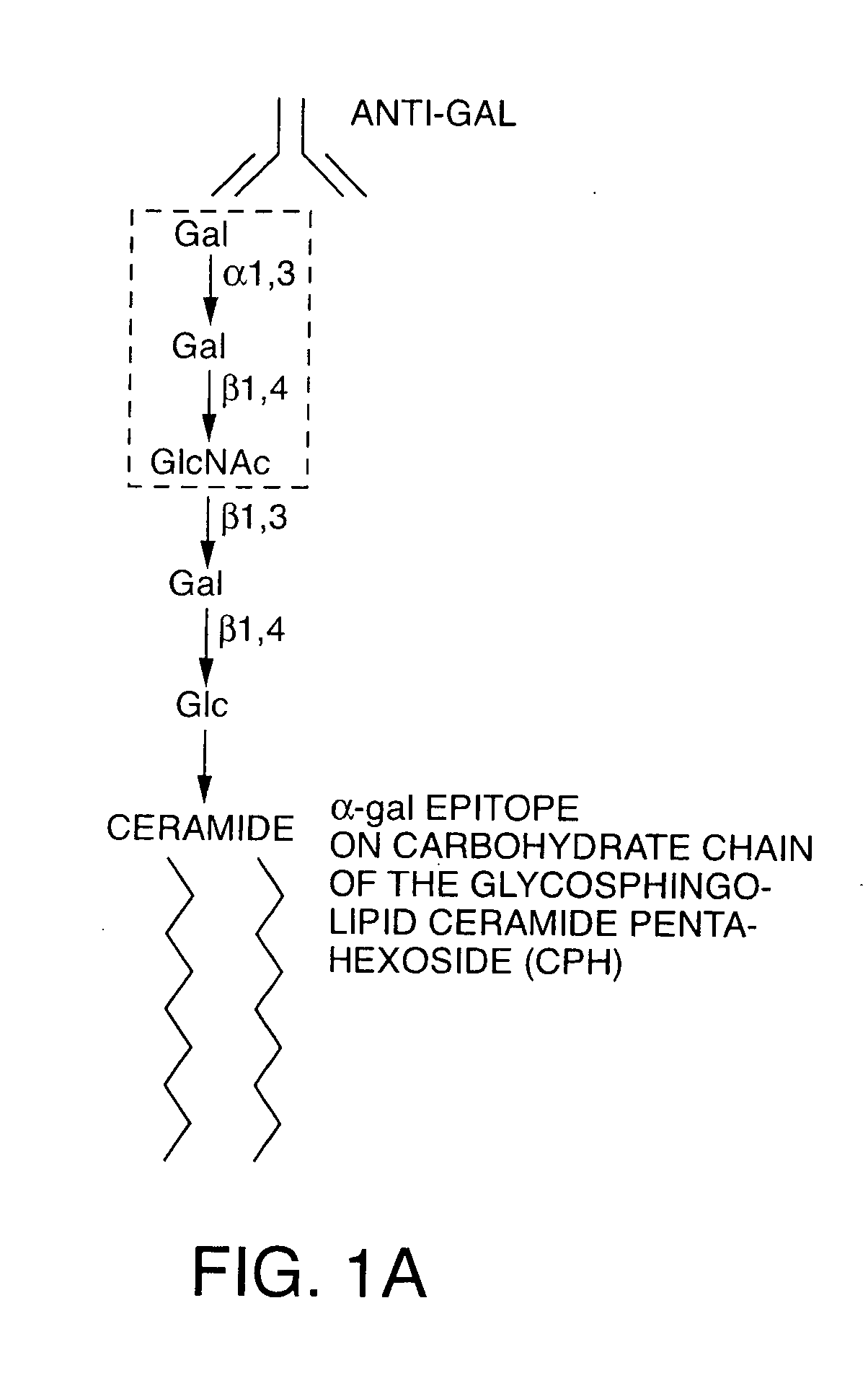
The present invention discloses that an intratumoral injection of: i) glycolipids with α-gal epitope; ii) gene vectors comprising an α1,3galactosyltransferase gene; or iii) a mixture of α1,3galactosyltransferase, neuraminidase, and uridine diphosphate galactose results in tumor regression and / or destruction. Binding of the natural anti-Gal antibody to de novo expressed tumoral α-gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Anti-icam3 antibody and use thereof
InactiveUS20120321557A1Improve anti-tumor effectEffective treatmentSugar derivativesMicrobiological testing/measurementCytotoxicityTumor regression
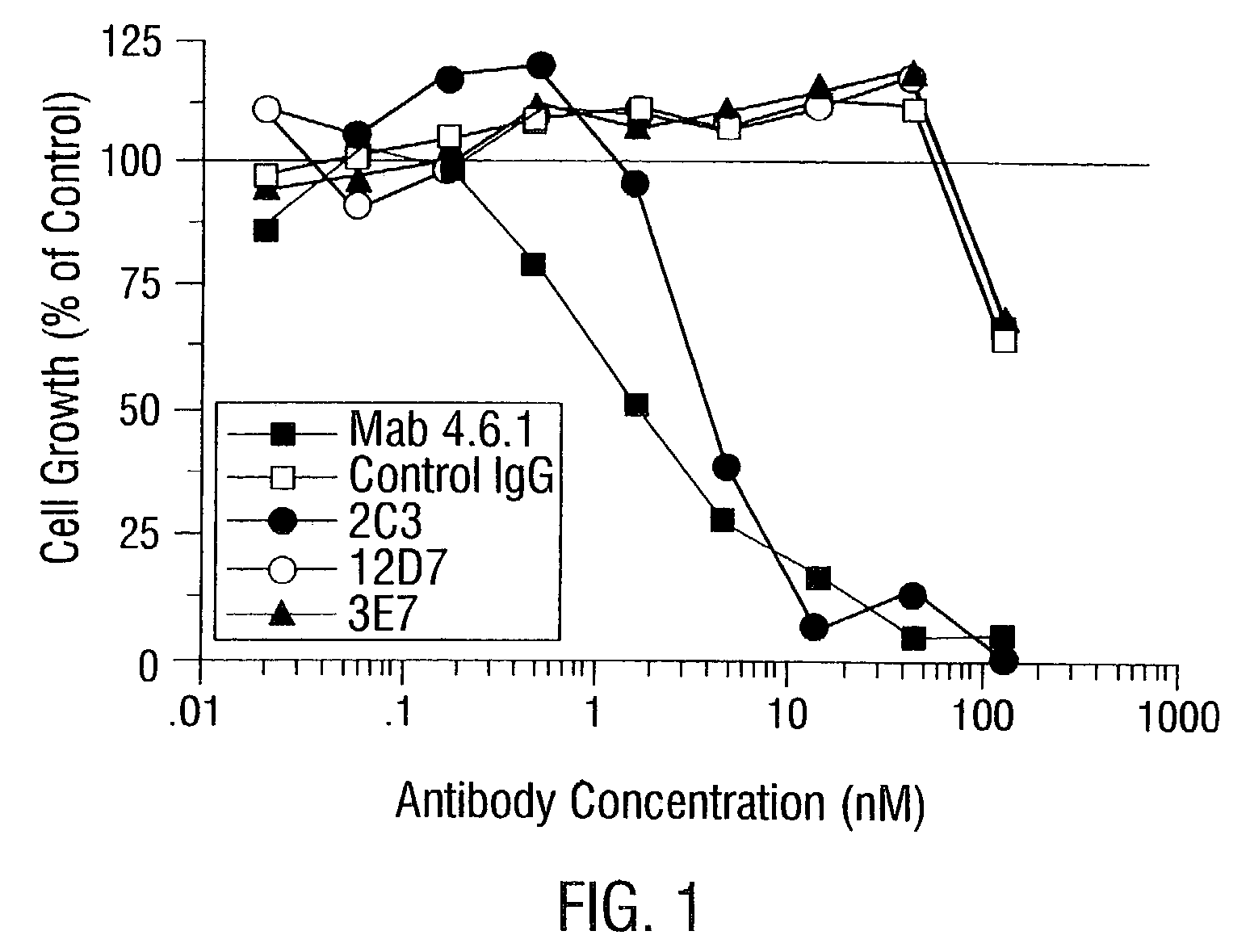
Based on an anti-ICAM3 antibody obtained by immunizing a mouse with BaF3 cells having artificially over-expressed ICAM3, a chimeric antibody has been successfully prepared which exerts cytotoxic actions, including both a proliferation-suppressing activity and an ADCC activity, on a blood cancer cell line, and which exerts a tumor regression activity in vivo.
Owner:CHUGAI PHARMA CO LTD
Tumor lesion regression and conversion in situ into autologous tumor vaccines by compositions that result in anti-Gal antibody binding
The present invention discloses that an intratumoral injection of: i) glycolipids with α-gal epitope; ii) gene vectors comprising an α1,3galactosyltransferase gene; or iii) a mixture of α1,3galactosyltransferase, neuraminidase, and uridine diphosphate galactose results in tumor regression and / or destruction. Binding of the natural anti-Gal antibody to de novo expressed tumoral α-gal epitopes induces inflammation resulting in an anti-Gal antibody mediated opsonization of tumor cells and their uptake by antigen presenting cells. These antigen presenting cells migrate to draining lymph nodes and activate tumor specific T cells thereby converting the treated tumor lesions into in situ autologous tumor vaccines. This therapy can be applied to patients with multiple lesions and in neo-adjuvant therapy to patients before tumor resection. In addition to the regression and / or destruction of the treated tumor, such a vaccine will help in the immune mediated destruction of micrometastases that are not detectable during the removal of the treated tumor.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
Cancer treatment kits using antibodies to aminophospholipids
InactiveUS8486391B2Method securityEasy to getPeptide/protein ingredientsAntibody mimetics/scaffoldsAntibody conjugateThrombus
Disclosed are the surprising discoveries that aminophospholipids, such as phosphatidylserine and phosphatidylethanolamine, are stable and specific markers accessible on the luminal surface of tumor blood vessels, and that the administration of an anti-aminophospholipid antibody alone is sufficient to induce thrombosis, tumor necrosis and tumor regression in vivo. This invention therefore provides anti-aminophospholipid antibody-based methods and compositions for use in the specific destruction of tumor blood vessels and in the treatment of solid tumors. Although various antibody conjugates and combinations are thus provided, the use of naked, or unconjugated, anti-phosphatidylserine antibodies is a particularly important aspect of the invention, due to simplicity and effectiveness of the approach.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
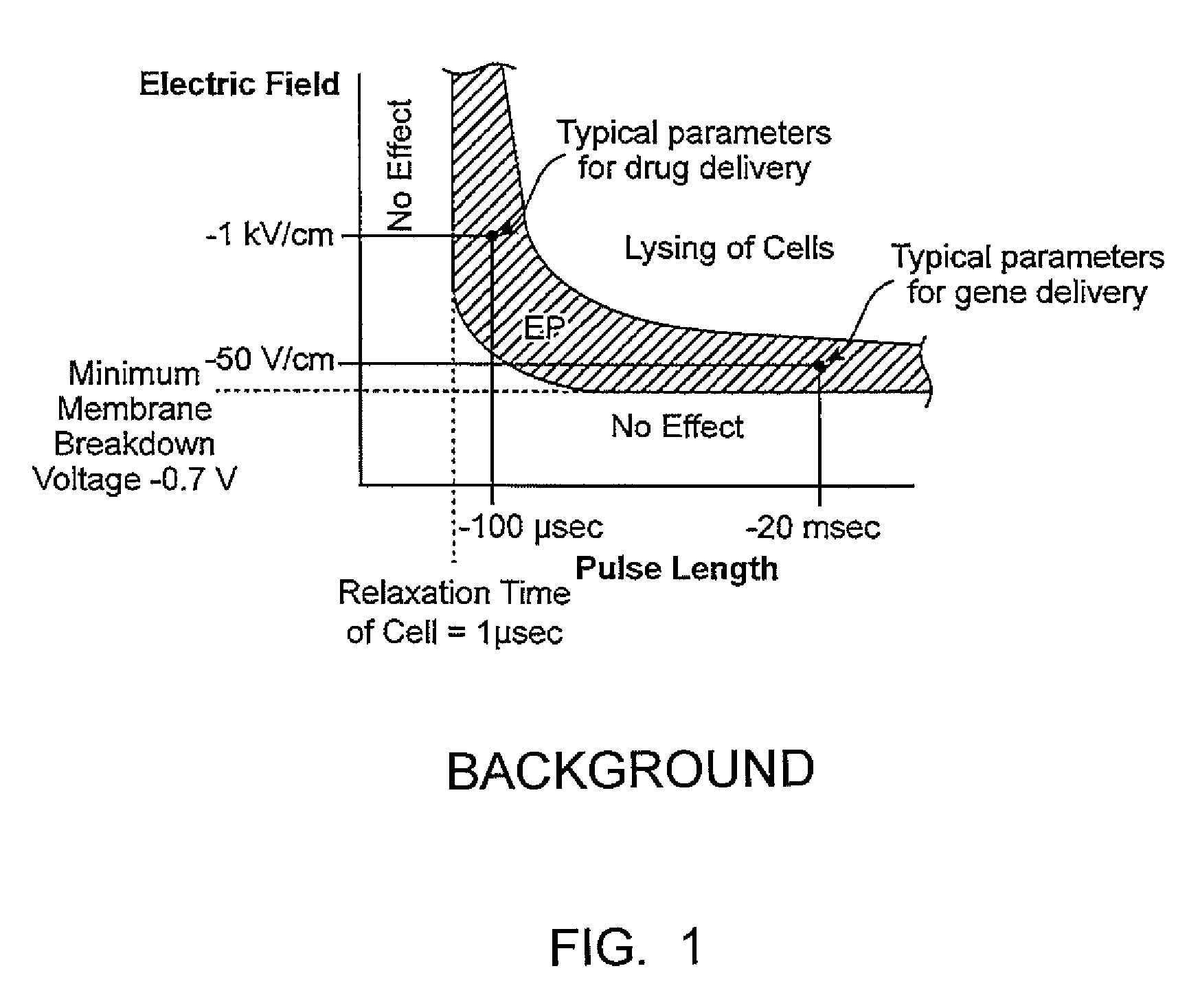
Apparatus for electroporation mediated delivery for drugs and genes
InactiveCN1768873AEasy to placeEffective placementOrganic active ingredientsPeptide/protein ingredientsElectroporation therapyLow voltage
A method and apparatus for in vivo electroporation therapy. Using electroporation therapy (EPT) as described in the invention, tumors treated by a combination of electroporation using the apparatus of the invention and a chemotherapeutic agent caused regression of tumors in vivo. In one embodiment, the invention provides a method of EPT utilizing low voltage and long pulse length for inducing cell death. One embodiment of the invention includes a system for clinical electroporation that includes a needle array electrode having a "keying" element that determines the set point of the therapy voltage pulse and / or selectable array switching patterns. A number of electrode applicator designs permit access to and treatment of a variety of tissue sites. Another embodiment provides a laparoscopic needle applicator that is preferably combined with an endoscope for minimally invasive EPT.
Owner:GENETRONICS INC
Use of interferon in treatment of tumor, and related product and method
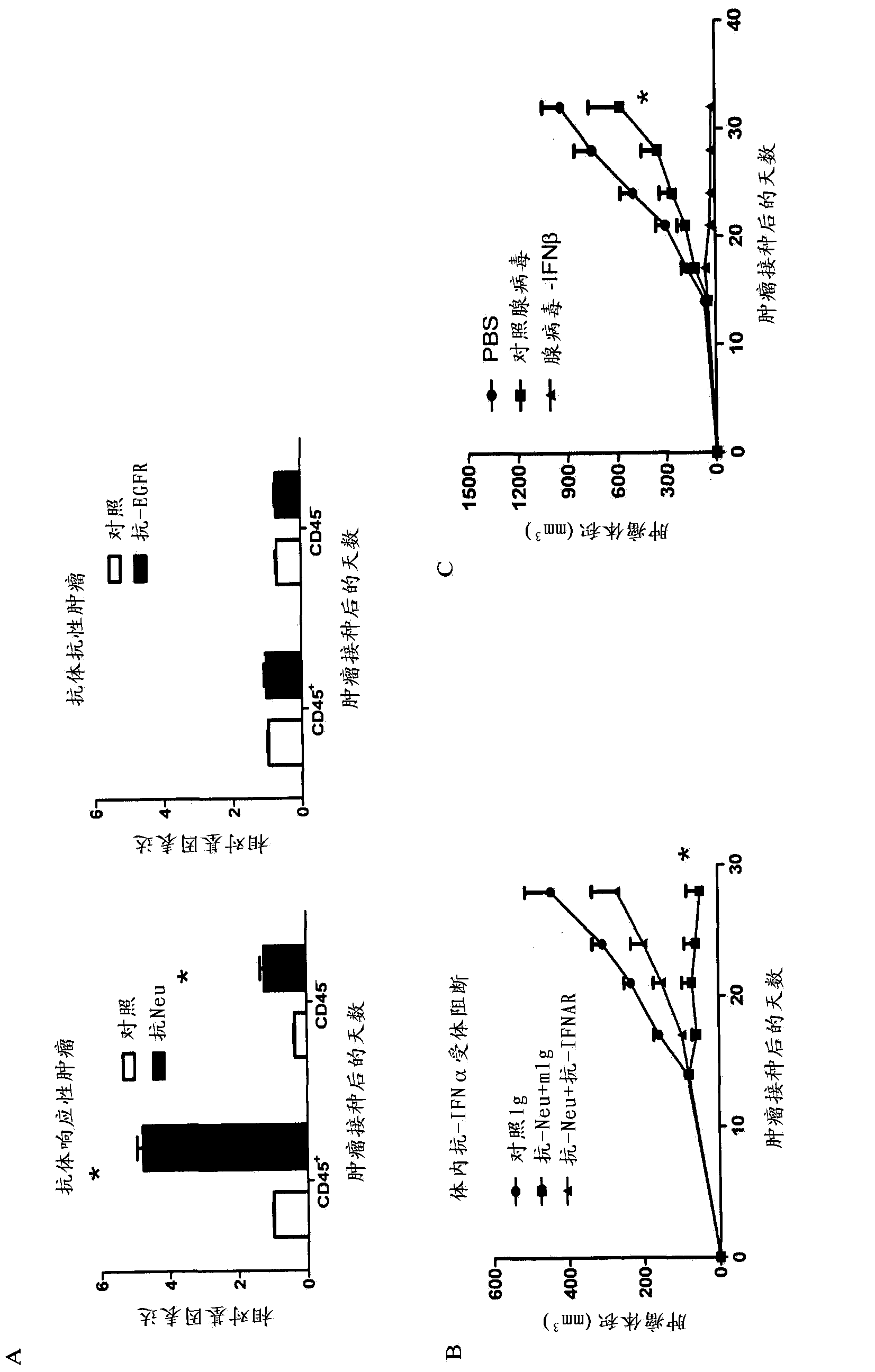
ActiveCN103536917AImprove efficiencyGood treatment effectPeptide/protein ingredientsAntibody ingredientsMedicineTreatment field
The invention relates to use of interferon in treatment of a tumor, and related products and a method, particularly relates to the field of tumor treatment, and especially aims at overcoming the resistance of the tumor on an antibody therapy. Particularly, the invention relates to application, and related product and method for inhibiting growth and recurrence of the tumor and causing tumor regression by combined utilization of interferon, especially I-type interferon, and a compound for blocking up a programmed death 1 or programmed death ligand (PD-1 / PDL) passage.
Owner:DINGFU BIOTARGET
Cancer treatment kits comprising therapeutic conjugates that bind to aminophospholipids
InactiveUS20060083745A1Improve in vivo stabilityGood “ release ” characteristicAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthThrombus
Disclosed is the surprising discovery that aminophospholipids, such as phosphatidylserine and phosphatidylethanolamine, are specific, accessible and stable markers of the luminal surface of tumor blood vessels. The present invention thus provides aminophospholipid-targeted diagnostic and therapeutic constructs for use in tumor intervention. Antibody-therapeutic agent conjugates and constructs that bind to aminophospholipids are particularly provided, as are methods of specifically delivering therapeutic agents, including toxins and coagulants, to the stably-expressed aminophospholipids of tumor blood vessels, thereby inducing thrombosis, necrosis and tumor regression.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Enclosures housing cell-coated supports for treating tumors
InactiveUS7056503B2Bioreactor/fermenter combinationsPowder deliveryAbnormal tissue growthTherapeutic protein
The present invention relates to devices, systems and methods for treating tumors. In particular, the present invention relates to enclosures housing cell-coated supports for promoting regression of tumors, such as cancerous tumors, papillomas, and warts. In preferred embodiments, the present invention provides methods of promoting tumor regression employing enclosures secreting therapeutic proteins.
Owner:RGT UNIV OF MICHIGAN
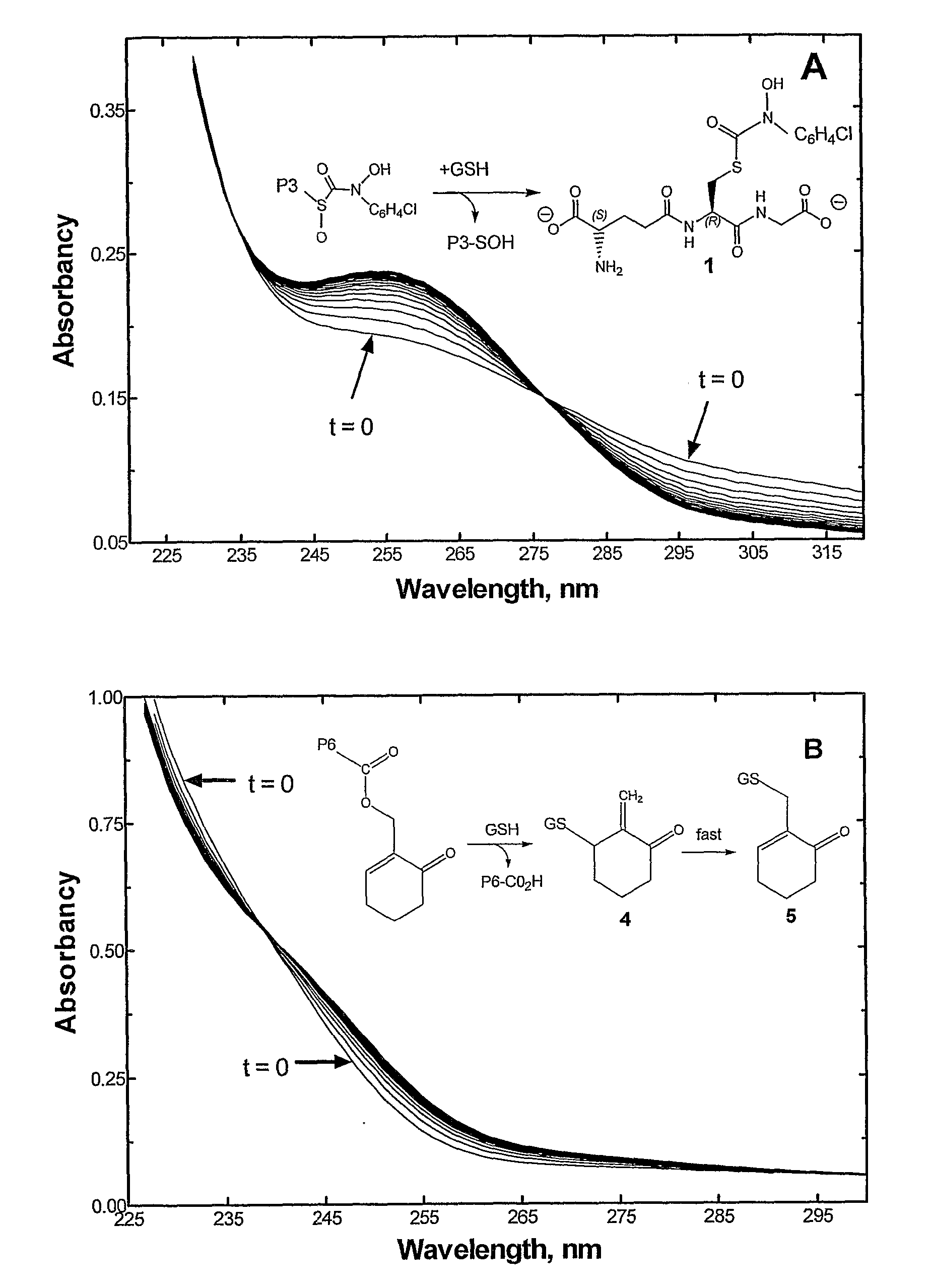
Macromolecular Gsh-Activiated Glyoxylase I Inhibitors
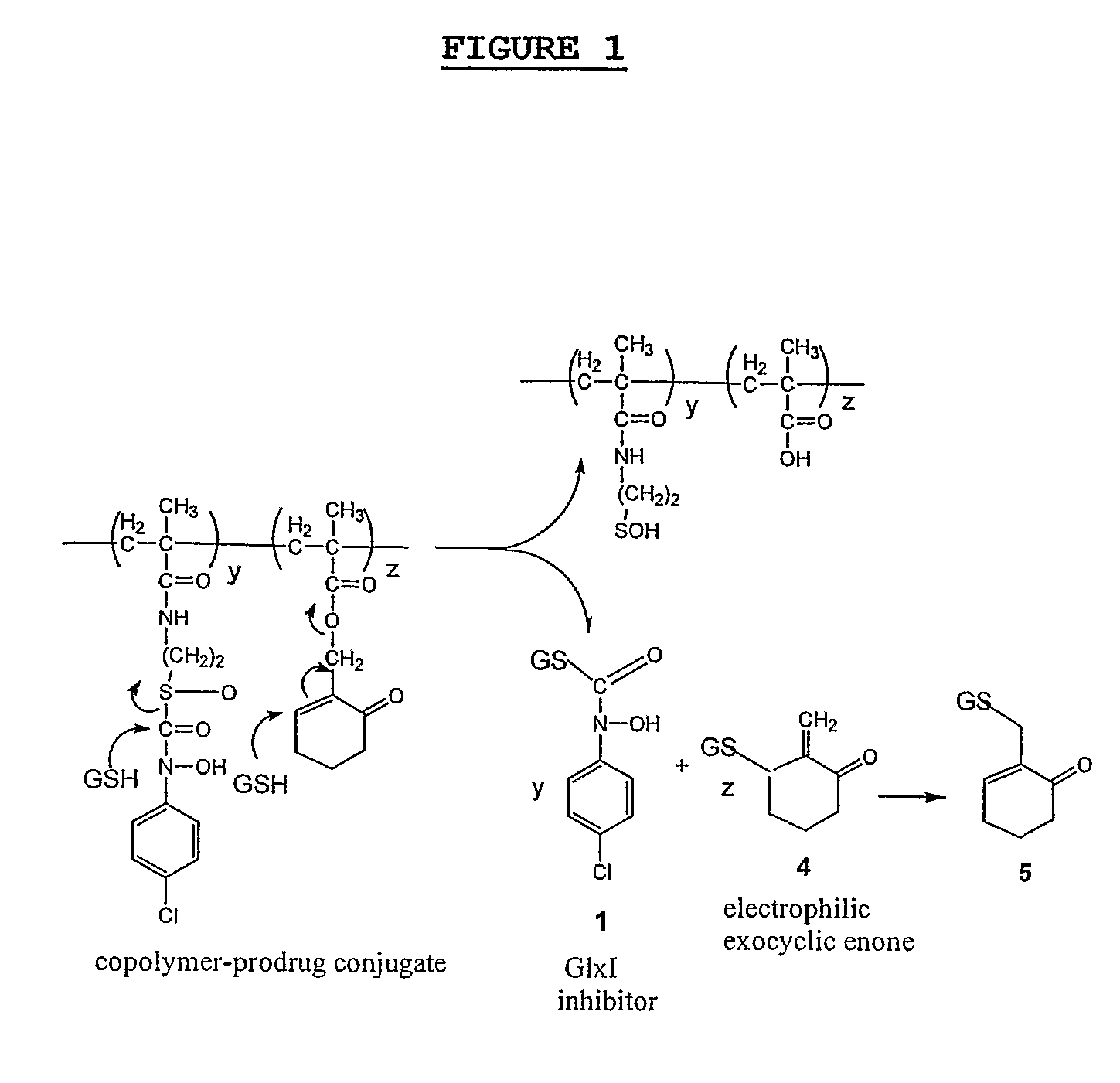
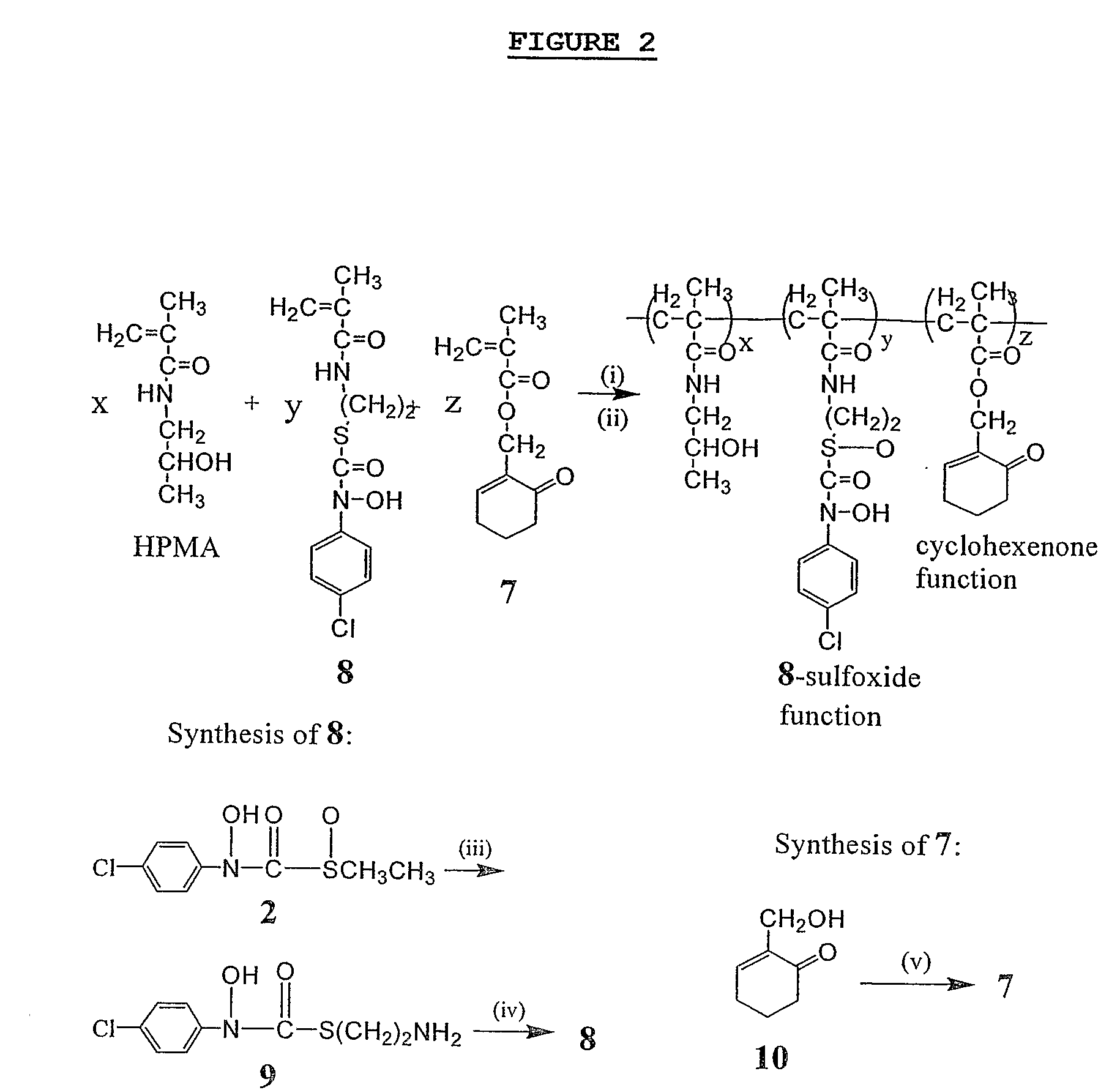
InactiveUS20070287672A1Prevent proliferationImprove permeabilityBiocideCosmetic preparationsReactive siteTumor regression
This invention relates to macromolecular prodrugs having antitumor activity when activated by GSH, and methods for synthesizing and administering these to patients. More particularly, this invention relates to, inter alia, the synthesis and use of polyacrylamide carriers to target anticancer prodrugs to tumors, and to release active antitumor agents selectively in tumor cells. These active antitumor agents target the active site of the methylglyoxal-detoxifying enzyme glyoxalase I to thereby cause tumor regression.
Owner:MARYLAND UNIV OF BALTIMORE COUNTY
Non-mammalian GnRH analogs and uses thereof in tumor cell growth regulation and cancer therapy
InactiveUS7834141B1Avoid actionHigh affinityHormone peptidesPeptide/protein ingredientsAbnormal tissue growthD-Arginine
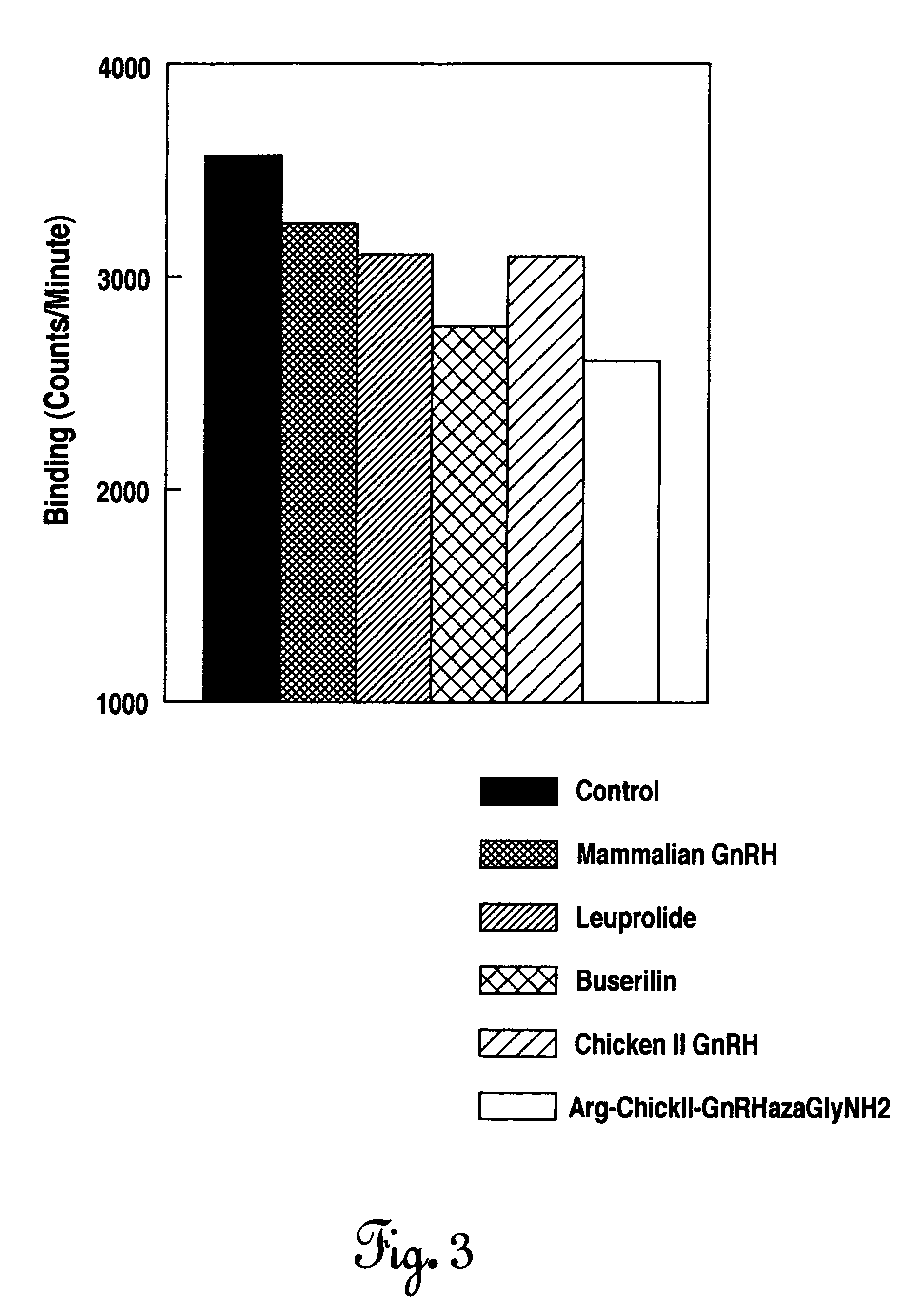
Specially designed non-mammalian GnRH analogs resistant to degradation by the tumor tissue enzymes, post-proline peptidases as well as endopeptidases, are disclosed. The GnRH analogs are further defined as analogs of chicken II GnRH, salmon GnRH, or herring GnRH, but can include any non-mammalian GnRH analog with similar amino acid structure. These non-mammalian analogs incorporate D-arginine, D-leucine, D-tBu-Serine or D-Trp or other similar amino acids at position 6 and ethylamide or aza-Gly-amide or similar amides at position 10. These analogs demonstrate preferential binding to tumor cell GnRH receptors that is greater relative to the binding of the mammalian analogs to the tumor cell GnRH receptor. These non-mammalian GnRH analogs may be used in pharmaceutical preparations, and it specifically in various treatments as an anti-tumor, anti-proliferation, anti-metastatic and / or an apoptotic agent. The non-mammalian GnRH analogs are also provided in pharmaceutical preparations that may be used clinically for tumor regression when used in very low doses and administered in pulsatile fashion.
Owner:SILER KHODR THERESA +1
Tumour necrosis factor binding ligands
InactiveUS20060182746A1Enhance or inhibit TNF alpha activityPeptide/protein ingredientsAntibody mimetics/scaffoldsSingle-Chain AntibodiesHuman tumor
The present invention relates to ligands which bind to human tumor necrosis factor alpha (TNF) in a manner such that upon binding of these ligands to TNF the biological activity of TNF is modified. In preferred forms the ligand binds to TNF in a manner such that the induction of endothelial procoagulant activity of the TNF is inhibited; the binding of TNF to receptors on endothelial cells is inhibited; the induction of fibrin deposition in the tumor and tumor regression activities of the TNF are enhanced; and the cytotoxicity and receptor binding activities of the TNF are unaffected or enhanced on tumor cells. The ligand is preferably an antibody, F(ab) fragment, single domain antibody (dABs), single chain antibody or a serum binding protein. It is preferred, however, that the ligand is a monoclonal antibody or F(ab) fragment thereof.
Owner:CEPHALON AUSTRALIA
Method for the treatment of malignancies
ActiveUS8927518B1Improve survival rateElectrotherapyPeptide/protein ingredientsTumor regressionElectroporation
A method of treating cancerous tumors is presented herein. The method includes injecting an effective dose of a plasmid encoded for IL-12, B7-1 or IL-15 into a cancerous tumor and subsequently administering at least one high voltage, short duration pulse to the tumor. The electroporation pulses may be administered at at least 700V / cm for a duration of less than 1 millisecond. The intratumor treatments with electroporation may be administered in at least a two-treatment protocol with the time between treatments being about 7 days. The intratumor treatments with electroporation may be administered in a three-treatment protocol with a time of four days between the first and second treatments and a time of three days between the second and third treatments. It was found that the intratumor treatments using electroporation not only resulted in tumor regression but also induced an immune memory response which prevented the formation of new tumors.
Owner:UNIV OF SOUTH FLORIDA
Combination bacteriolytic therapy for the treatment of tumors
Current approaches for treating cancer are limited, in part, by the inability of drugs to affect the poorly vascularized regions of tumors. We have found that spores of anaerobic bacteria in combination with agents which interact with microtubules can cause the destruction of both the vascular and avascular compartments of tumors. Two classes of microtubule inhibitors were found to exert markedly different effects. Some agents that inhibited microtubule synthesis, such as vinorelbine, caused rapid, massive hemorrhagic necrosis when used in combination with spores. In contrast, agents that stabilized microtubules, such as the taxane docetaxel, resulted in slow tumor regressions that killed most neoplastic cells. Remaining cells in the poorly perfused regions of tumors could be eradicated by sponzlated bacteria. Mechanistic studies showed that the microtubule destabilizers, but not the microtubule stabilizers, radically reduced blood flow to tumors, thereby enlarging the hypoxic niche in which spores could germinate. A single intravenous injection of spores plus selected microtubule-interacting agents was able to cause regressions of several tumors in the absence of excessive toxicity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Recyclable conformal radioactive particle cabin
PendingCN105727432ATake out the realizationInhibit sheddingX-ray/gamma-ray/particle-irradiation therapyTumour volumeHigh doses
The invention discloses a recyclable conformal radioactive particle cabin which comprises a fixing plate, a guide column and a plurality of moveable partitioning plates, wherein the guide column is longitudinally arranged at the lower end of the fixing plate; the guide column is made of a flexible material; the moveable partitioning plates are overlapped with one another up and down and are arranged on the guide column in a sliding sleeve manner. According to a preoperative plan, under the image guide, a puncture needle is penetrated into a tumor; the tip position is a tail end particle position of the preoperative plan; a needle core is pulled out, the recyclable conformal radioactive particle cabin is placed into a puncture needle tube and pushed to a particle distribution source position specified in the preoperative plan. As the guide column is made of the flexible material, the guide column can have conformal deformation along with change of the tumor, and the recyclable conformal radioactive particle cabin can be taken out after the tumor is eliminated, radioactive particles can be taken out, the consequence that the distribution sources of the radioactive particles in the tumor can be accumulated with one another to generate high-dosage areas along with reduction of the tumor size and radioactive damage of normal tissue around is caused be effectively prevented, and meanwhile particle migration and complication such as lumen obstruction and thromboembolism can be prevented.
Owner:牛洪欣
Method of inducing tumor cell apoptosis using trail/Apo-2 ligand gene transfer
The present invention is directed to methods for inhibiting tumor cell growth, causing tumor regression or eliminating tumor cells in a mammal afflicted with a tumor by administering to a TRAIL-sensitive cell a vector having a DNA expression cassette containing a promoter and a DNA sequence encoding TRAIL, wherein the expression of TRAIL results in tumor inhibition, regression or elimination.
Owner:UNIV OF IOWA RES FOUND
Kit for detecting cancer radiosensitivity and application of kit
The invention relates to a kit for detecting cancer radiosensitivity and application of the kit to curative effect prediction. According to the kit of the invention, the screening of molecular markersis completely based on whole-genome RNAi library screening which is a more practical and effective screening mode in international research level. Identification is carried out step by step in vitroand vivo based on a radiotherapy tolerance induction process; it is finally found that RFC4 is a gene highly correlated with radiotherapy tolerance of colorectal cancer; clinical retrospective analysis and in-vitro and in-vivo functional analysis are performed on the identified RFC4 gene, it is revealed that the high expression levels of the RFC4 gene can be positively correlated with tumor regression bad performance, and the RFC4 gene can be used to predict radiotherapy tolerance / sensitivity. Based on the above technical schemes of the invention, the kit used for predicting the efficacy of local advanced neoadjuvant radiotherapy is obtained, and is of great realistic significance for solving the problem of differences between individuals in clinical efficacies and the prediction blanks ofregression / prognosis effects, and better achieving accurate treatment.
Owner:SUN YAT SEN UNIV CANCER CENT
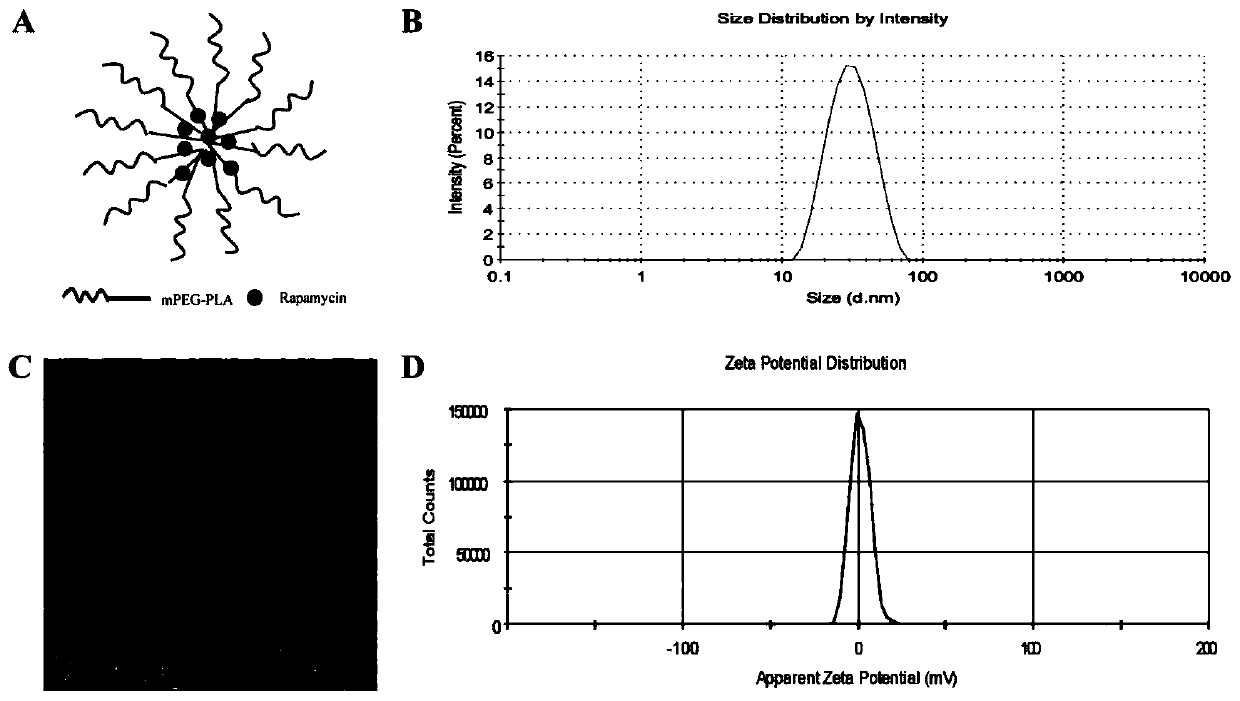
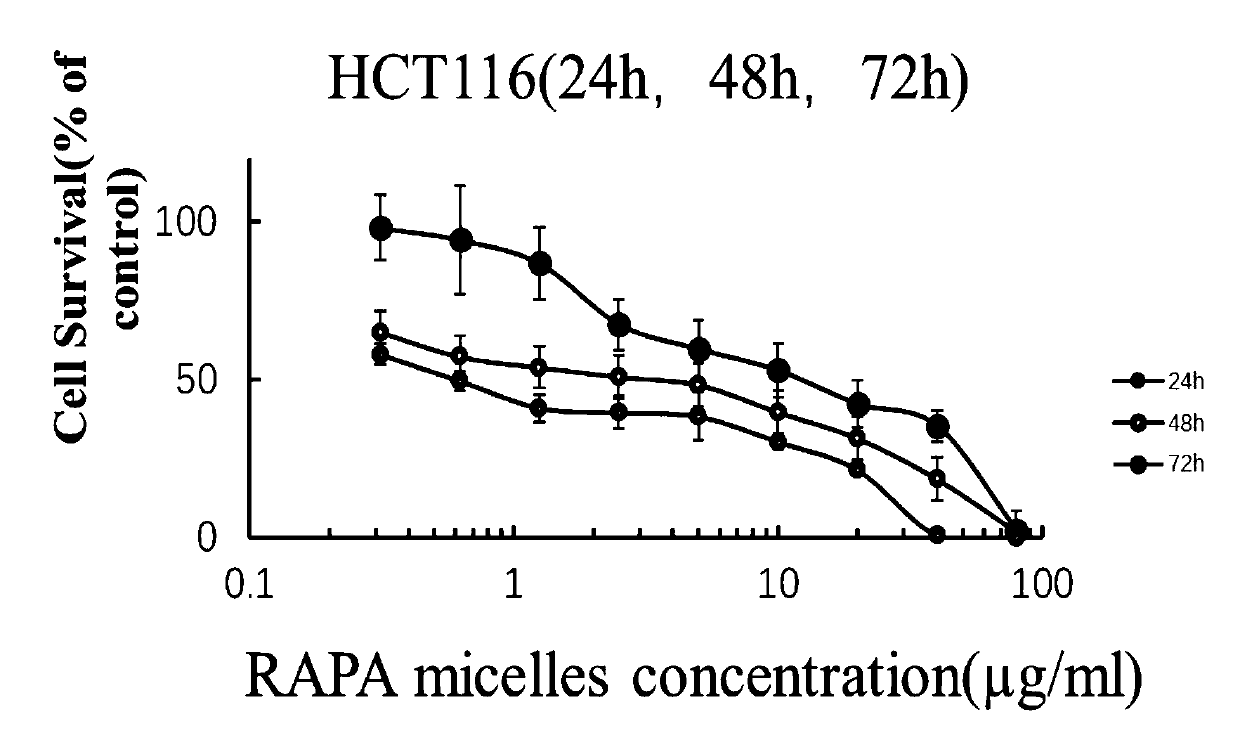
Rapamycin nano slow-release agent and preparation method thereof
ActiveCN110623925AControllable half-lifeHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryOrganic solventHalf-life
The present invention discloses a rapamycin nano slow-release agent. The rapamycin nano slow-release agent is made of the following raw materials in parts by weight: 1 part of rapamycin, 0.5-20 partsof a soluble high molecular polymer carrier, 40-200 parts of an organic solvent and 400-20,000 parts of an aqueous phase solution. The present invention also provides a preparation method of the rapamycin nano slow-release agent. The rapamycin nano slow-release agent has a nano micellar structure and particle size between 10-200 nm, and is low in risks to blood vessels. The half-life of the rapamycin nano slow-release agent in blood can be as high as 50 hours or more, the rapamycin nano slow-release agent can directly reach affected areas of tumors, is subjected to continuous administration and has a tumor regression rate of 50%.
Owner:严鹏科
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com