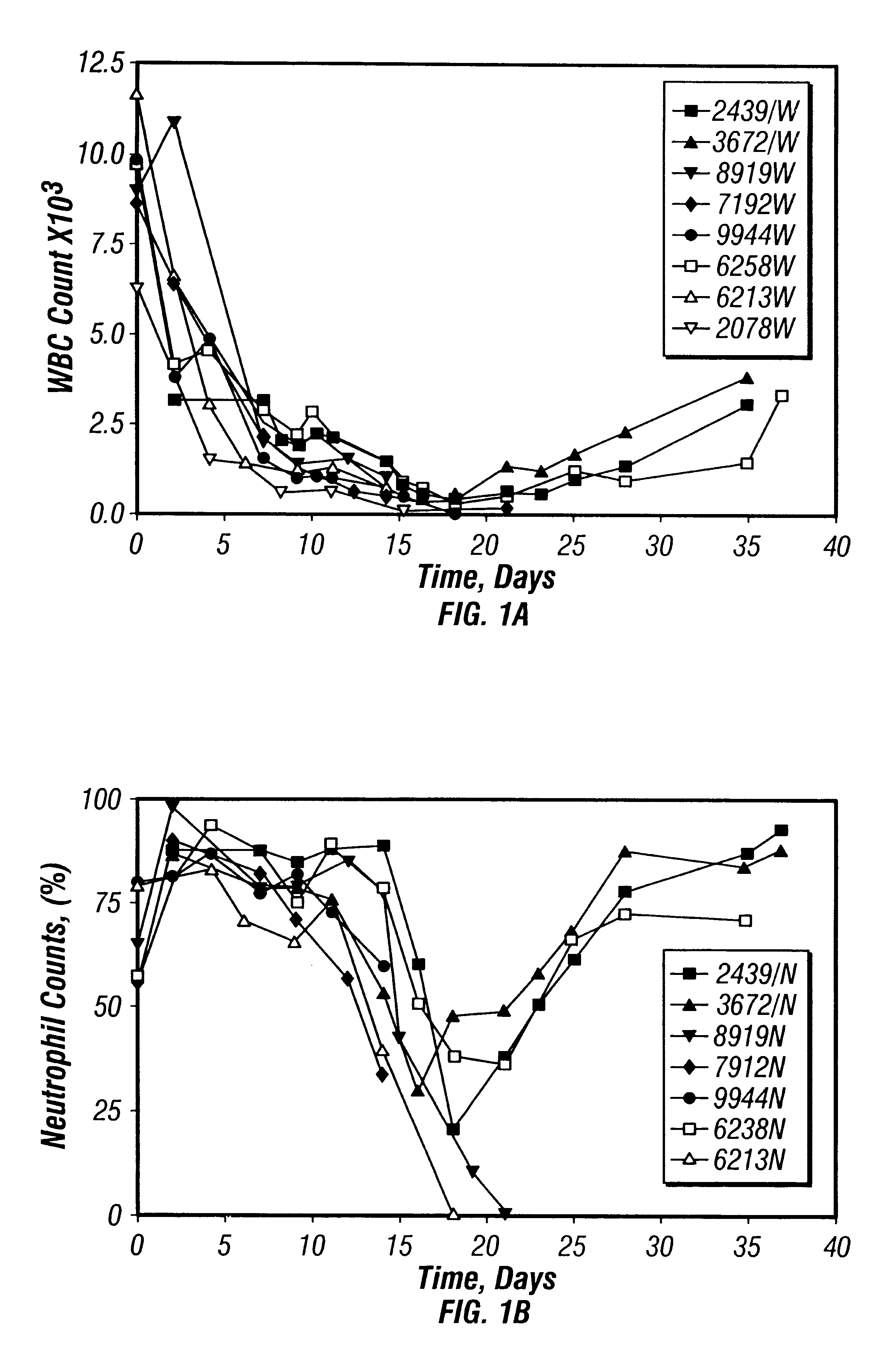
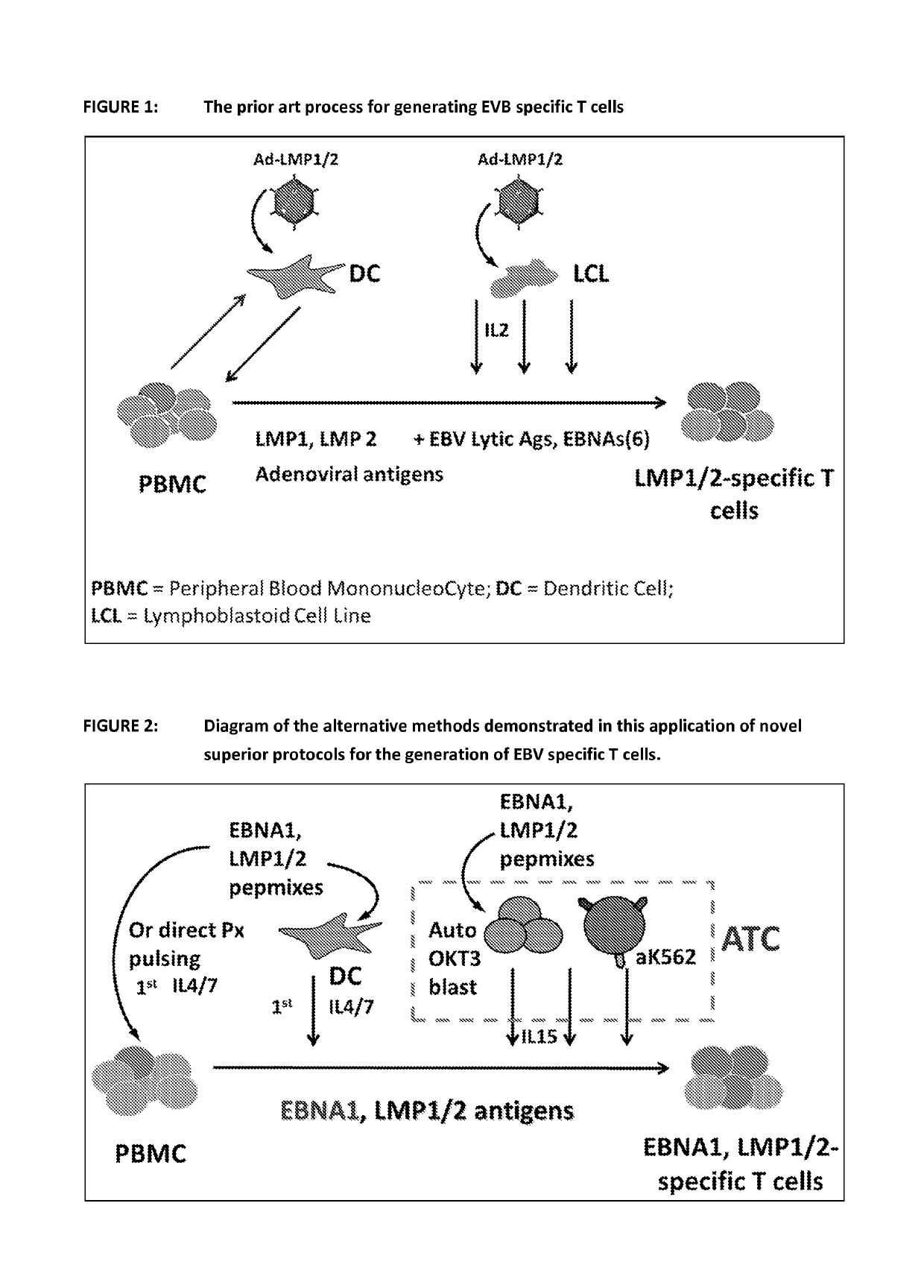
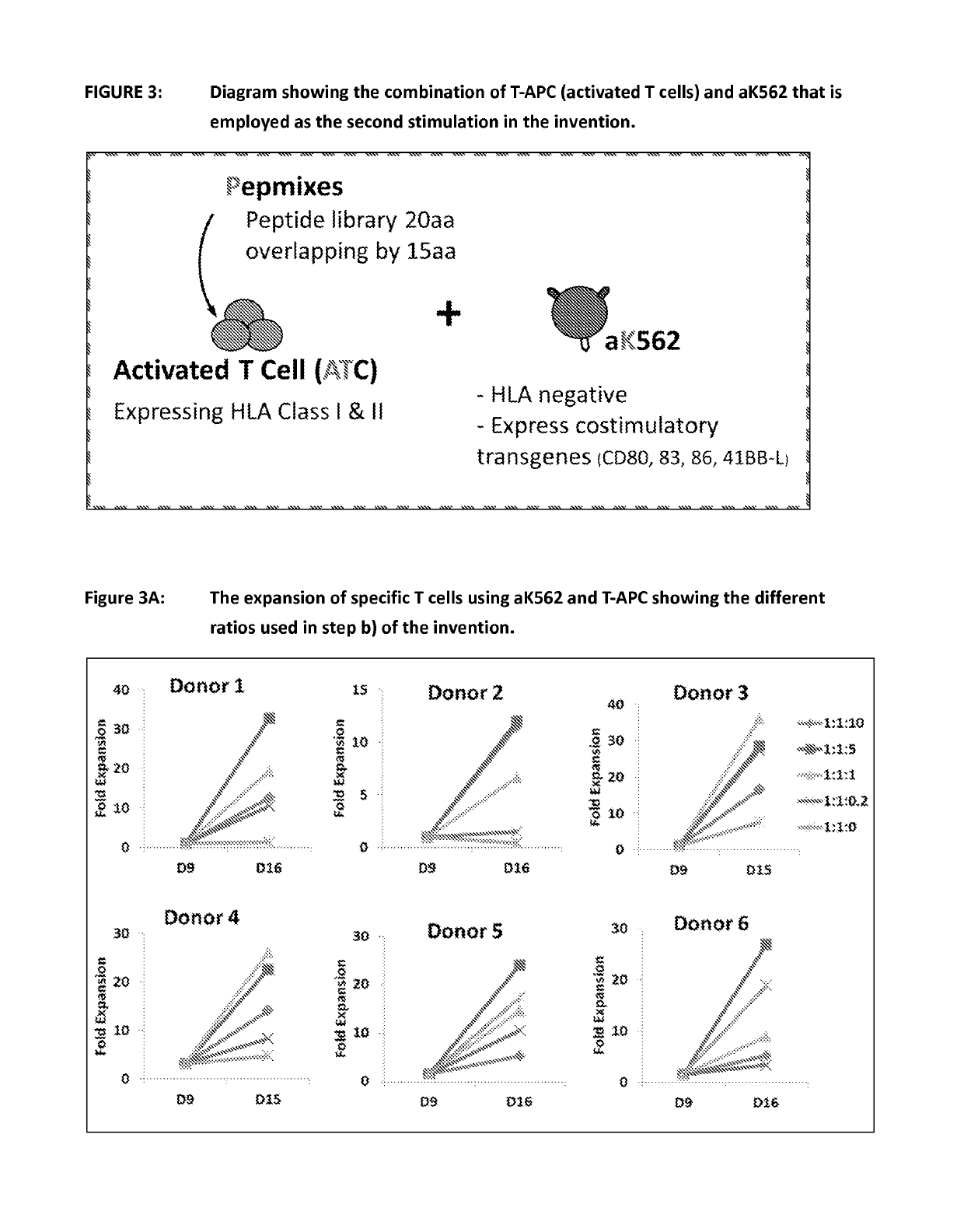
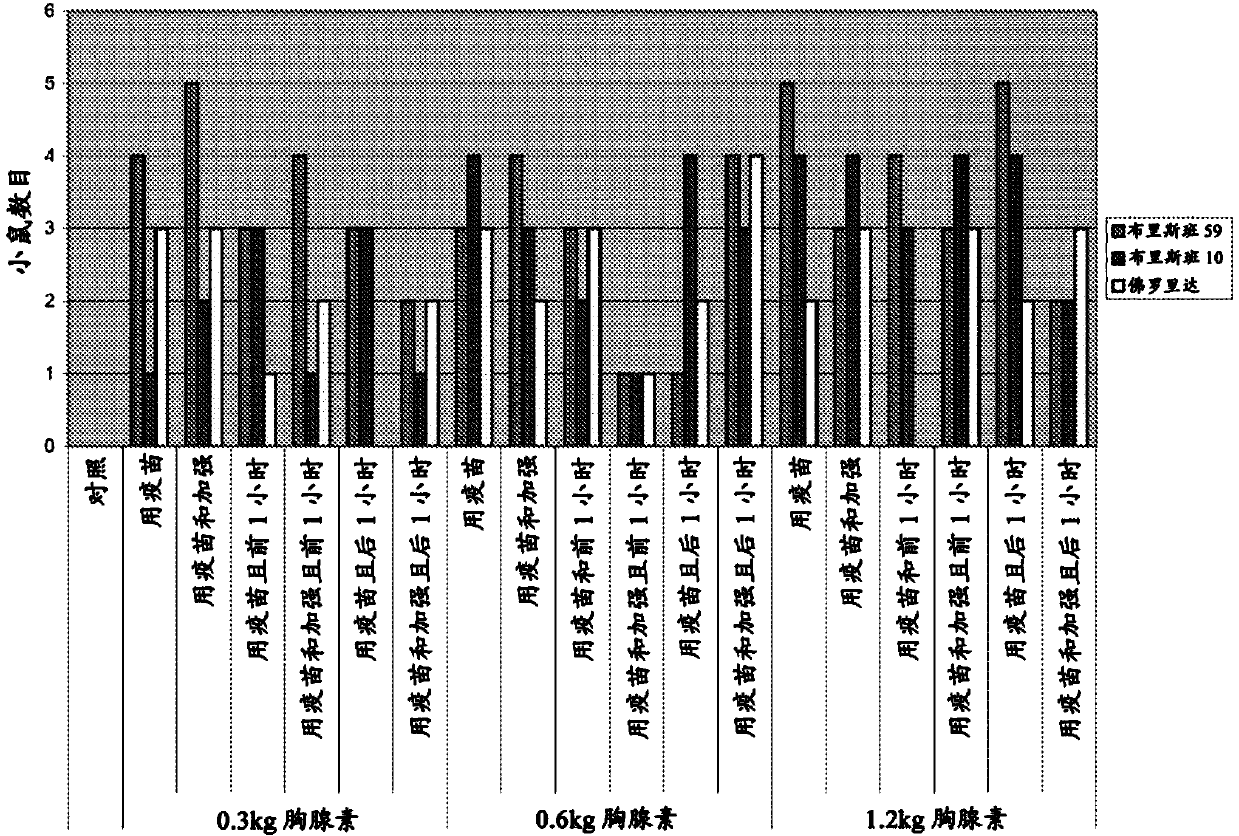
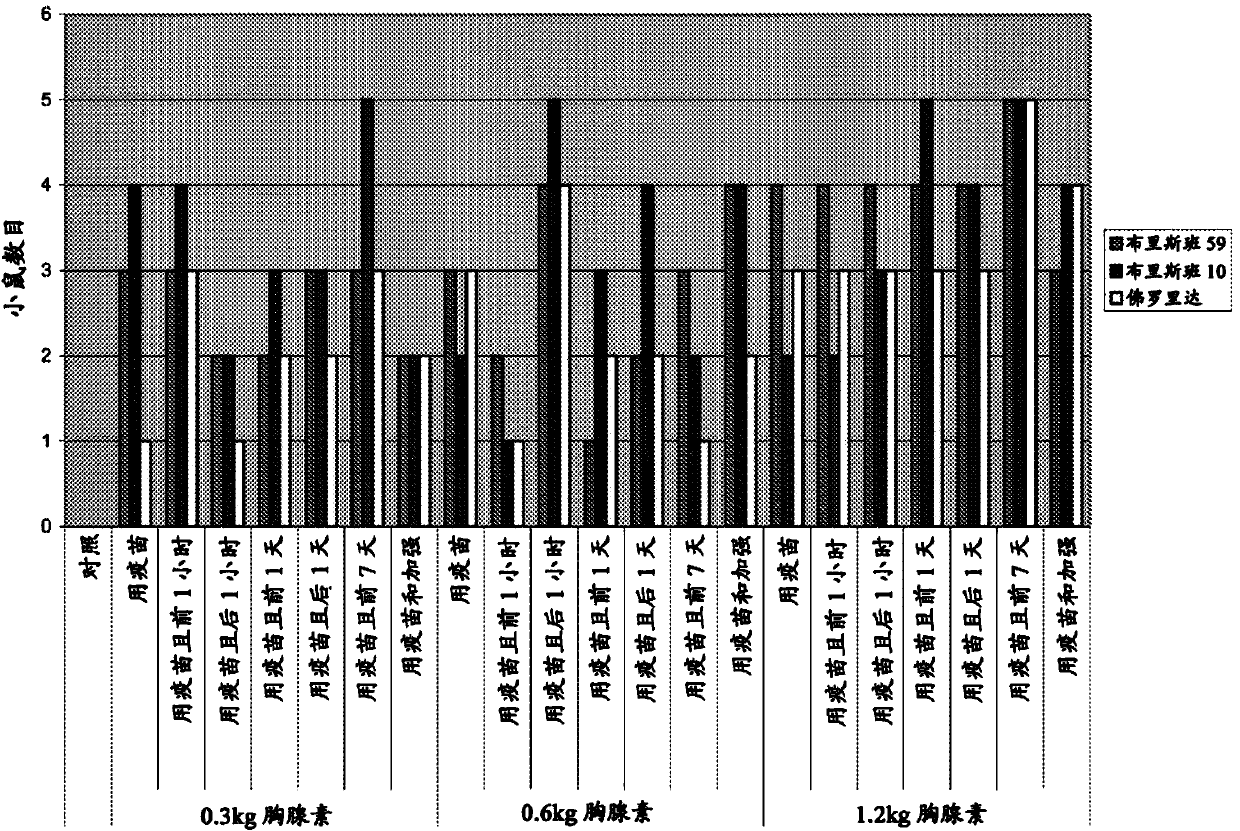
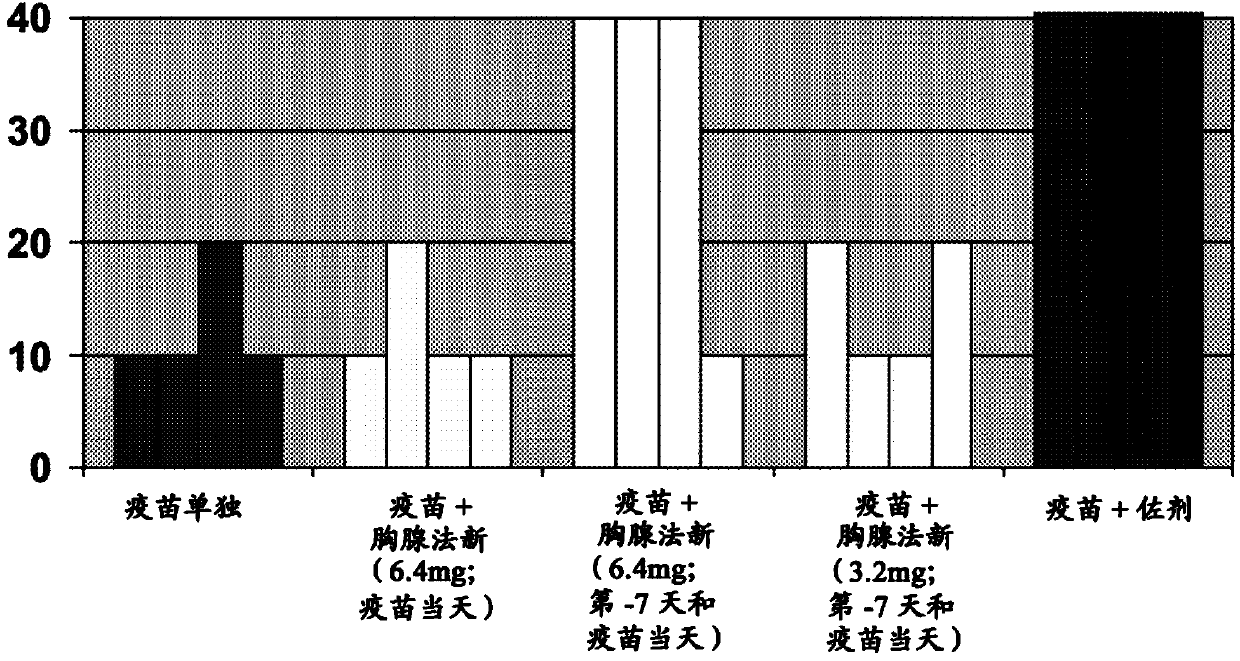
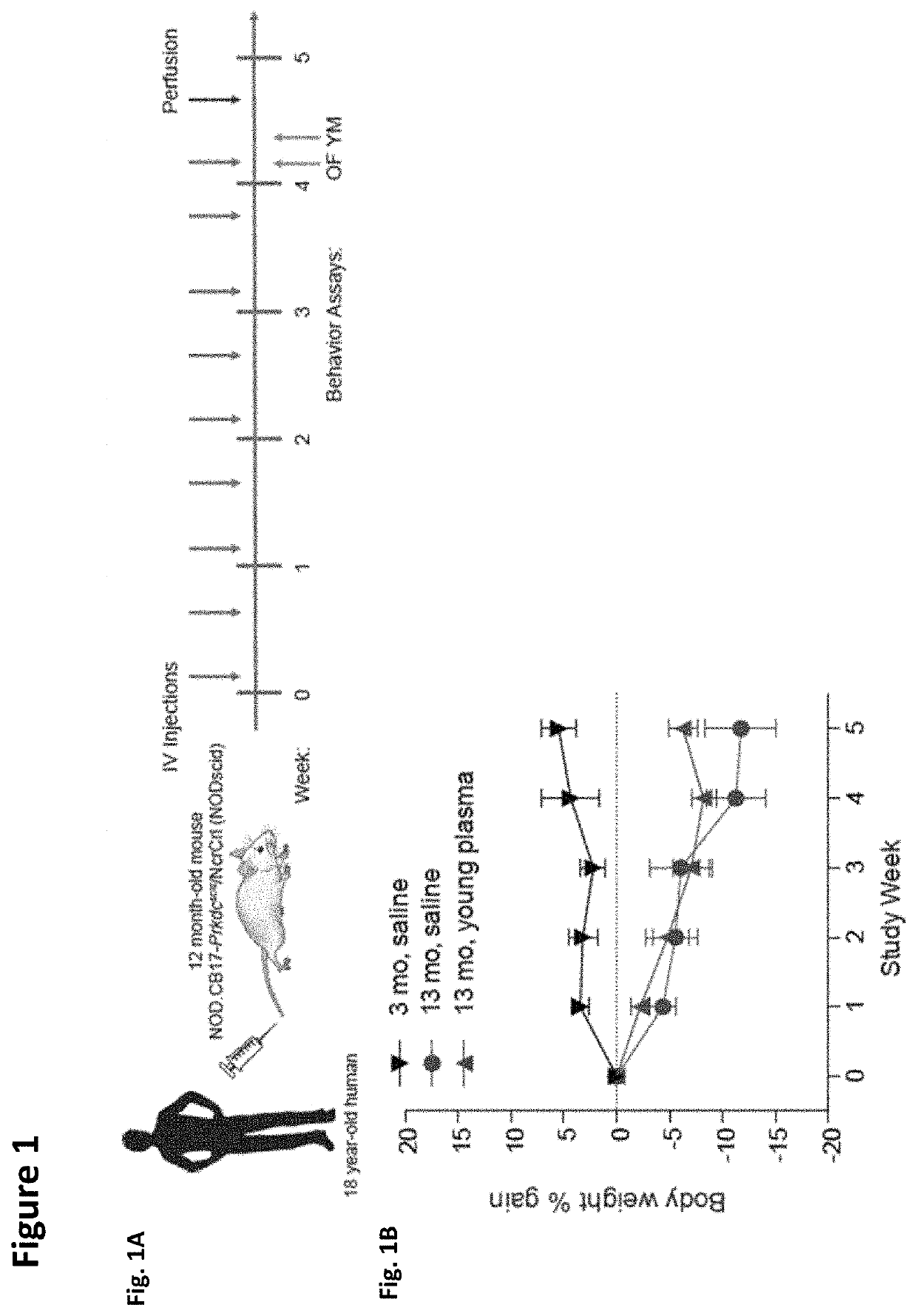
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Immune compromised" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune compromise, also referred to as immunocompromise or immunosuppression, is a condition in which the immune system does not work as well as it does in normal healthy workers. Immune compromised personnel are at higher risk of illness and/or more serious side effects of illness caused by an infectious disease.
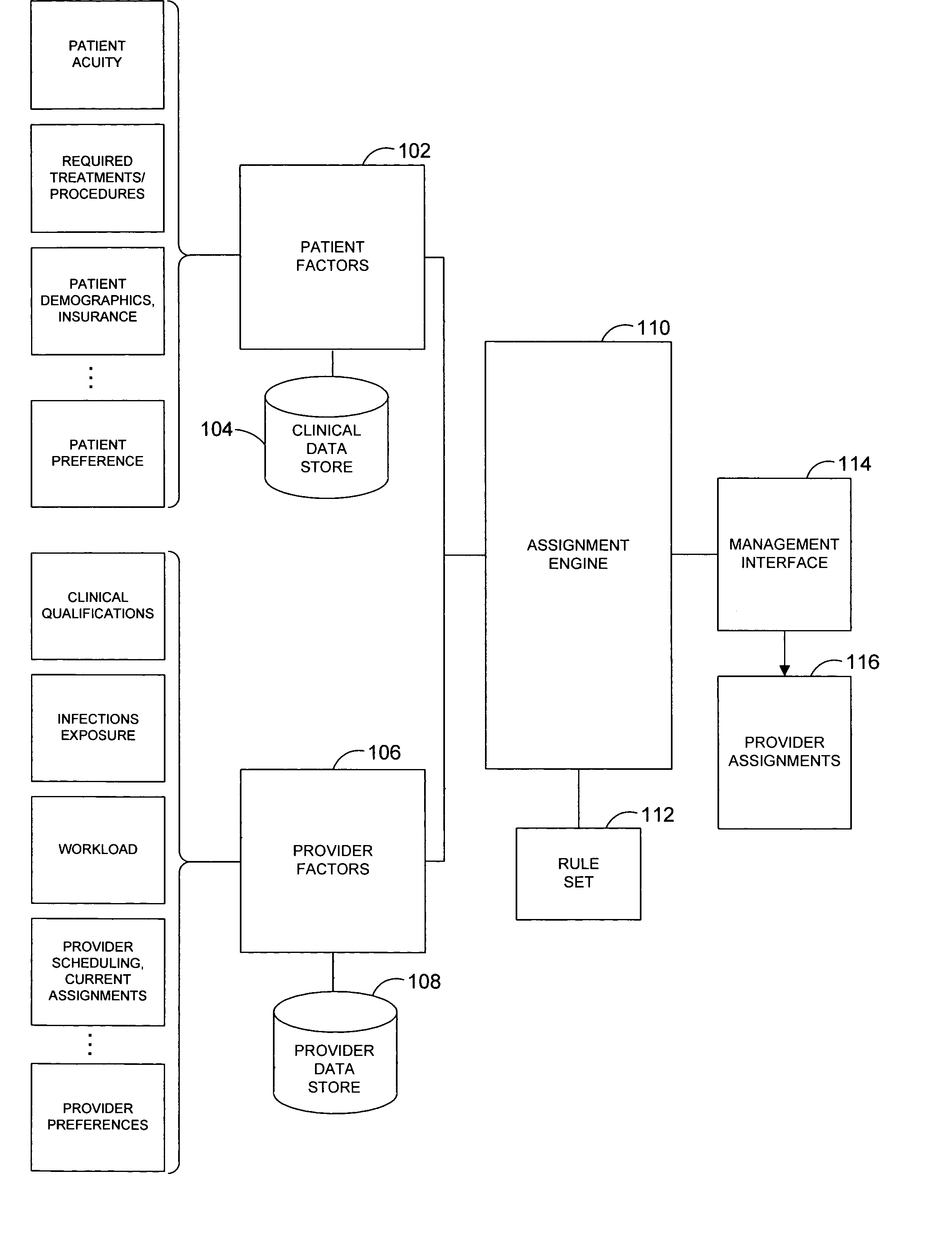
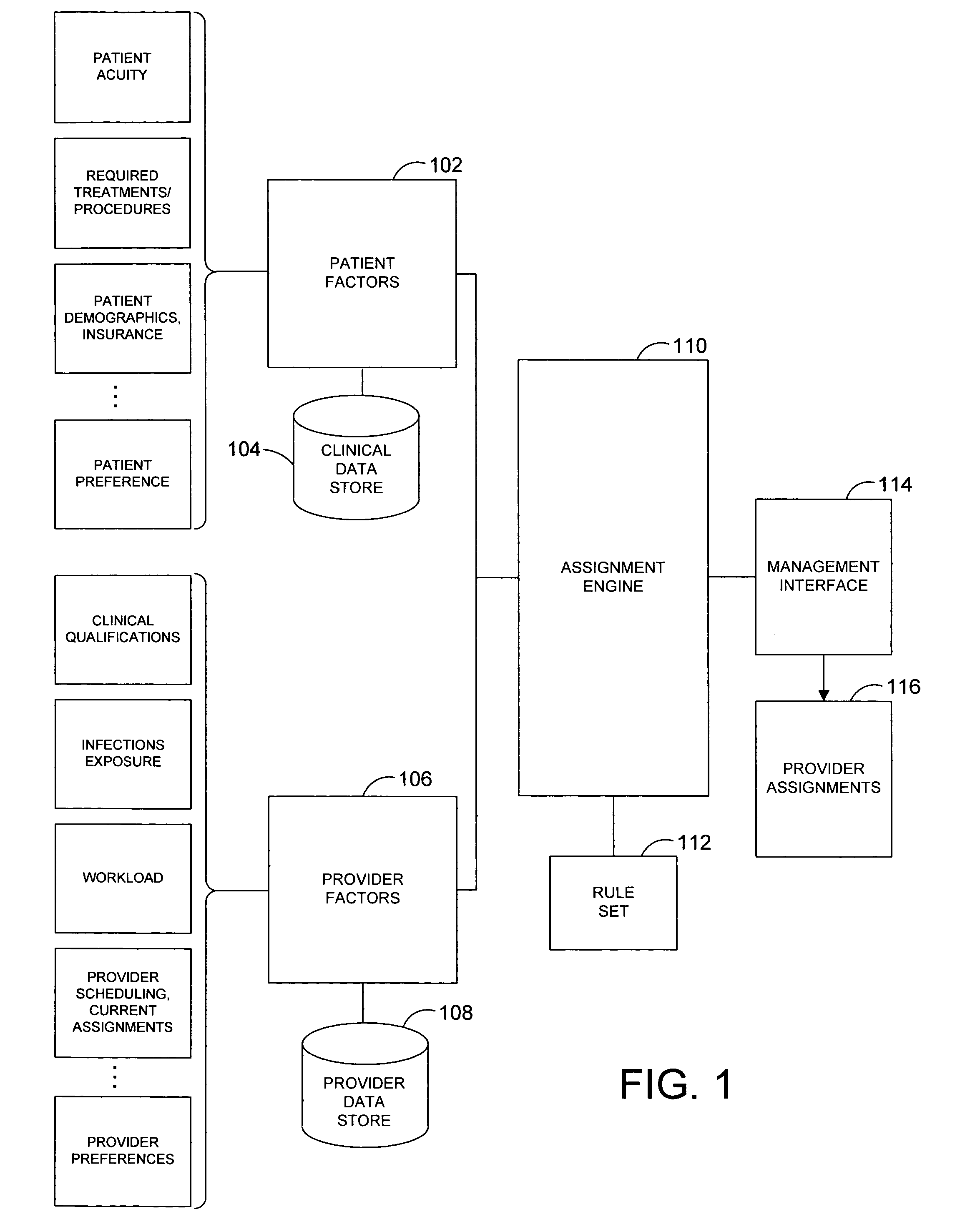
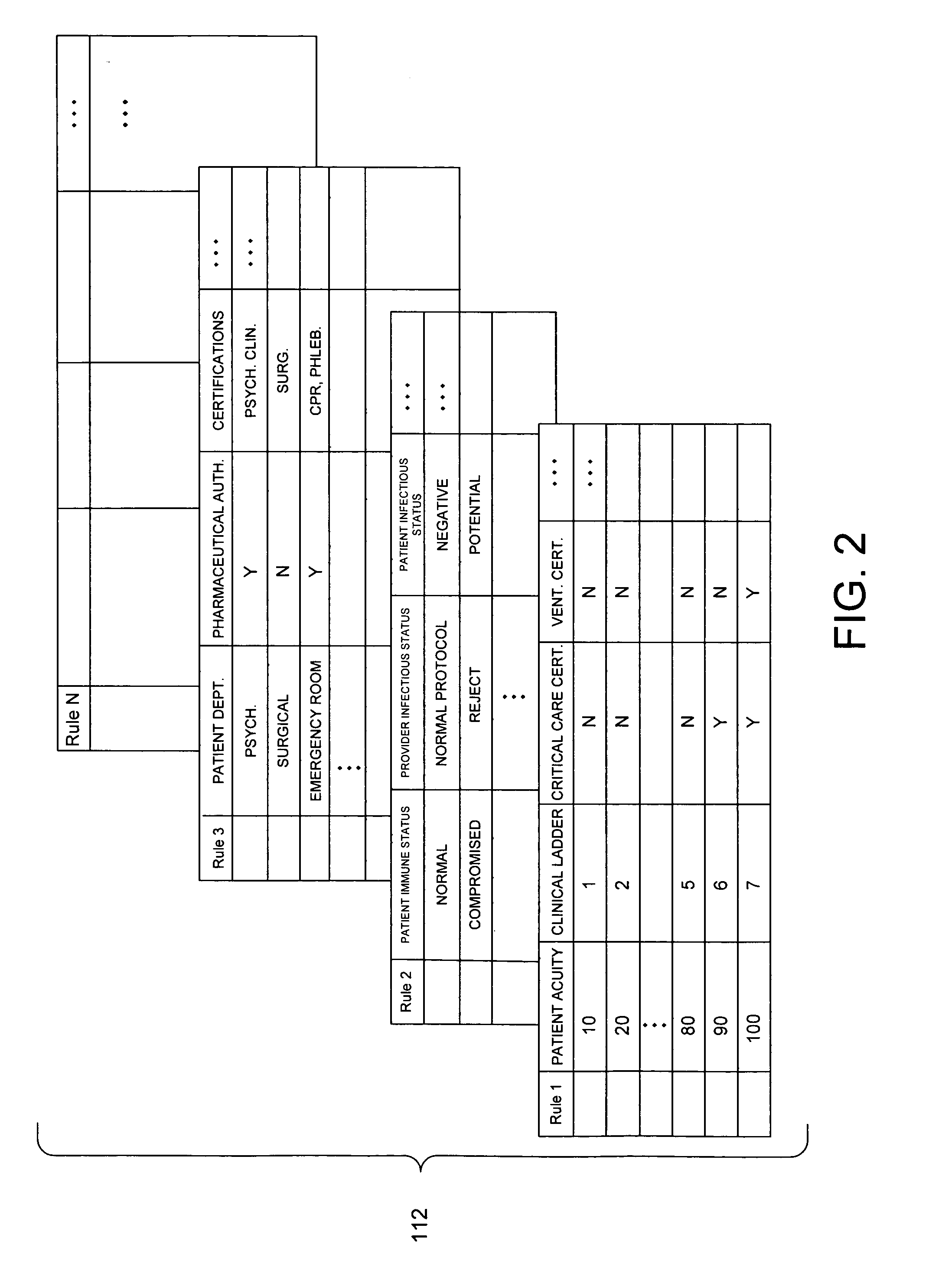
System and method for automatically generating evidence-based assignment of care providers to patients
A system and related techniques automatically generate optimized, best-match or sufficient assignments of care providers, such as physicians, nurses or technicians, to a patient based on clinical evidence, documentation, workload, infectious status and other factors. In embodiments, the patient's chart or other clinical record may be accessed by a rules-based engine configured with rules which relate a patient's clinical status and needs to the qualifications, certifications, capabilities and skills of care providers and select the care provider best qualified to service that patient's clinical requirements. The pool of available care providers may for example be ascertained from personnel systems recording staff schedules and estimated workload, while the qualifications of each provider indicating the categories of patient care and support that provider is qualified to provide may be accessed from a provider data store. For instance, nurses or technicians trained or certified in acute, emergency or surgical care may be identified for assignment to high acuity patients, or those presenting special or advanced care demands. According to the invention in a further regard, the provider's potential infectious exposure to other patients or from other sources may be screened to prevent that provider from being assigned to immune-compromised or other patients. Embodiments of the invention may present floor managers with a graphical display of available providers and generated assignments, which in embodiments the manager may override at their clinical discretion. Because patient needs are automatically aligned with provider capabilities, availability and other factors, the errors, oversights and inefficiencies of manual or informal assignment systems are avoided and better health care delivery can be realized.
Owner:CERNER INNOVATION
Compositions and methods for altering immune function
InactiveUS7601355B2Overcome problemsGood curative effectSnake antigen ingredientsAntibody ingredientsImmune compromisedInfectious Disorder
The present invention relates to molecules that stabilize and / or enhance CD154 activity, compositions comprising these molecules, methods for using these molecules and compositions (e.g., pharmaceutical compositions) comprising them. Specifically, the molecules can be used for enhancing immune function (e.g., enhancing Th1 cytokine expression), stimulating immune responses, and / or treating certain disorders (e.g., cancer, infectious disease, and immune compromised states). The invention also relates to kits and compositions comprising the molecules.
Owner:NORTHWESTERN UNIV
Prevention of opportunistic infections in immune-compromised subjects
ActiveUS20100260720A1Reduce the risk of infectionAvoid stickingAntibacterial agentsBiocideFucosylationImmune compromised
This invention relates to a composition suitable for use in the prevention of opportunistic infections in immune-compromised individuals comprising a probiotic Bifidobacterium lactis, Bifidobacterium infantis, Bifidobacterium breve or Bifidobacterium longum and a fucosylated oligosaccharide selected from the group comprising 2′-fucosyllactose, 3′fucosyllactose, difucosyllactose, lacto-N-fucopentaose, lacto-N-fucohexaose, fucosyllacto-N-hexaose and fucosyllacto-N-neohexaose. The invention further extends to the use of such a composition in the prevention of opportunistic infections in immune-compromised individuals.
Owner:SOC DES PROD NESTLE SA
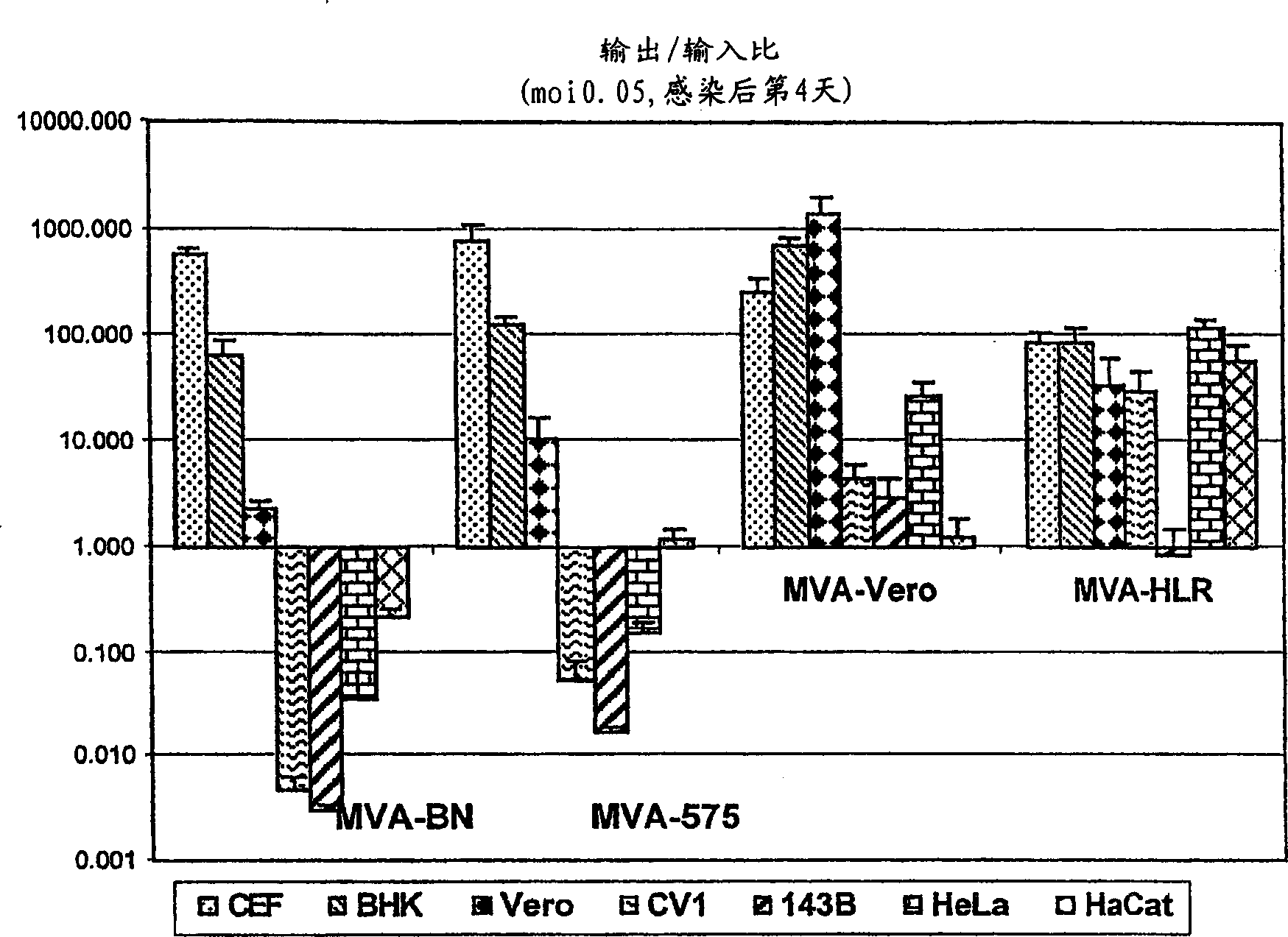
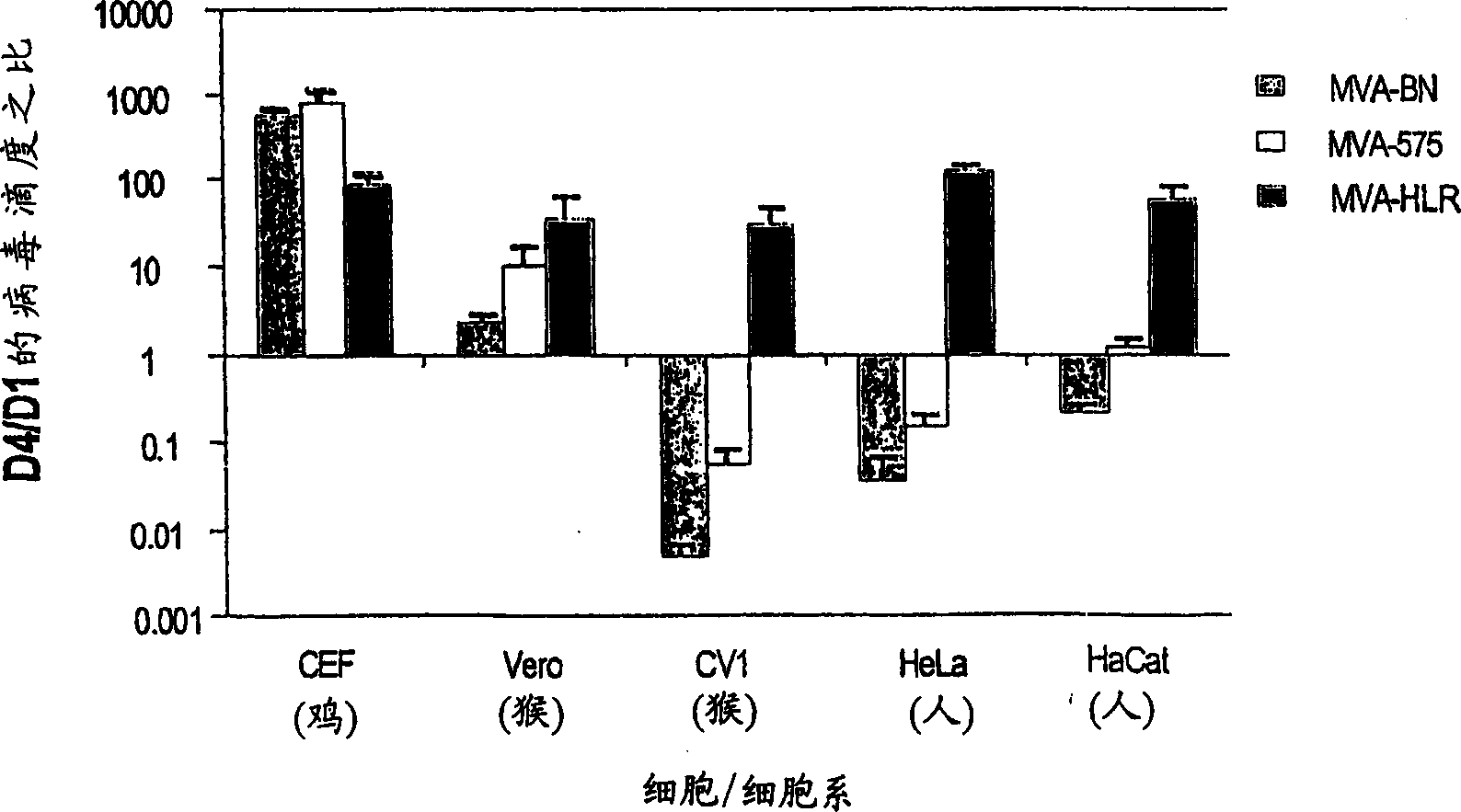
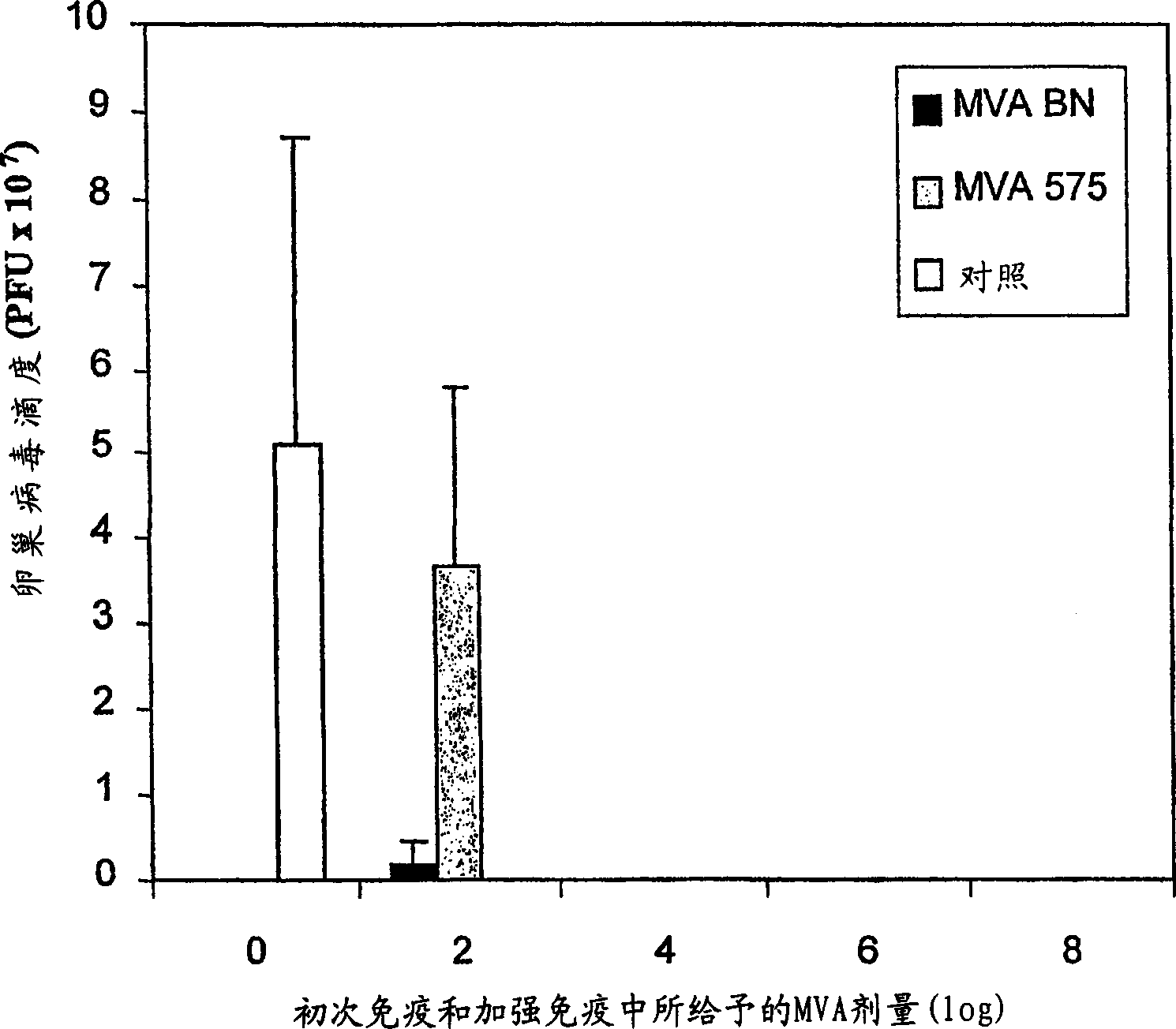
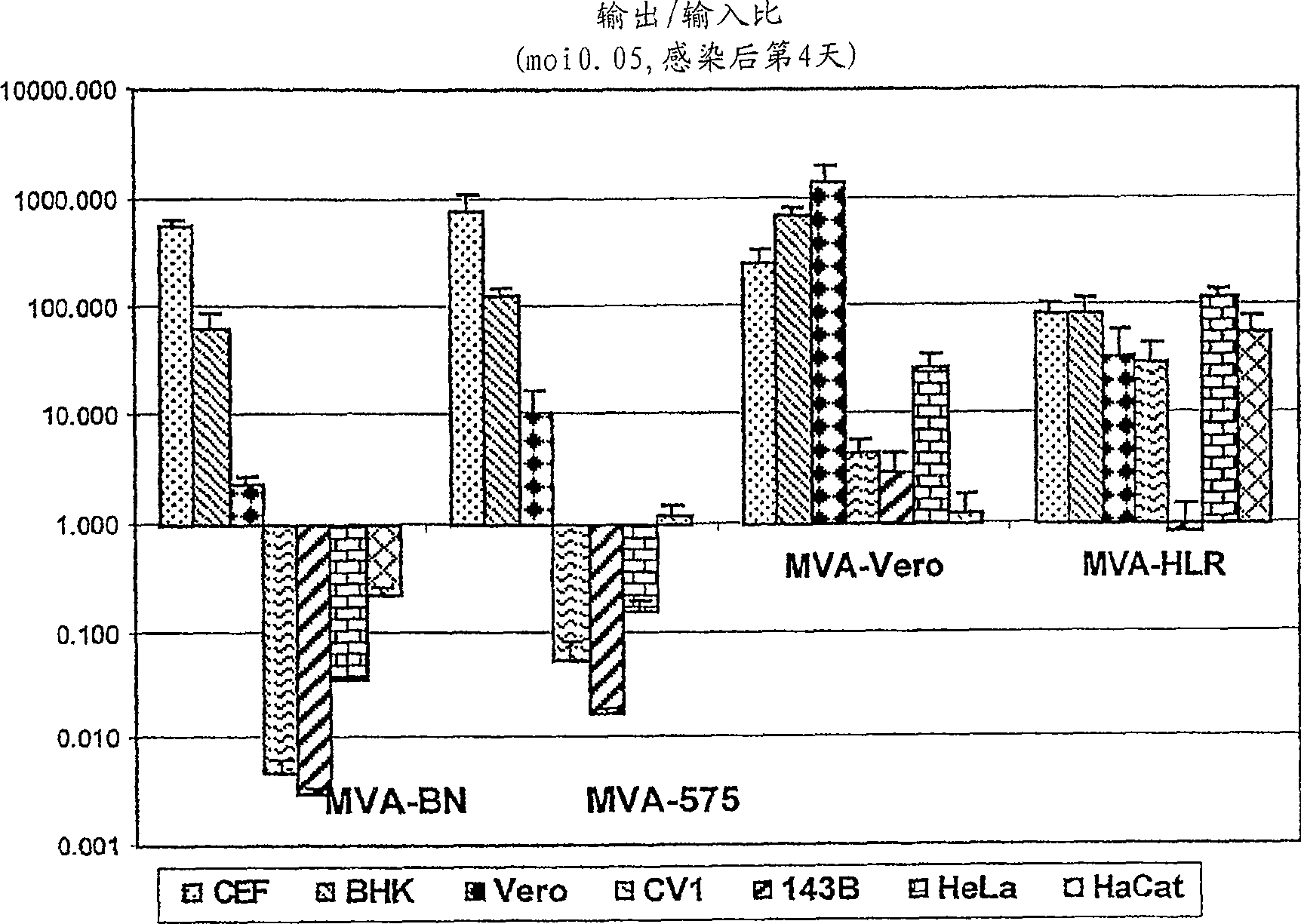
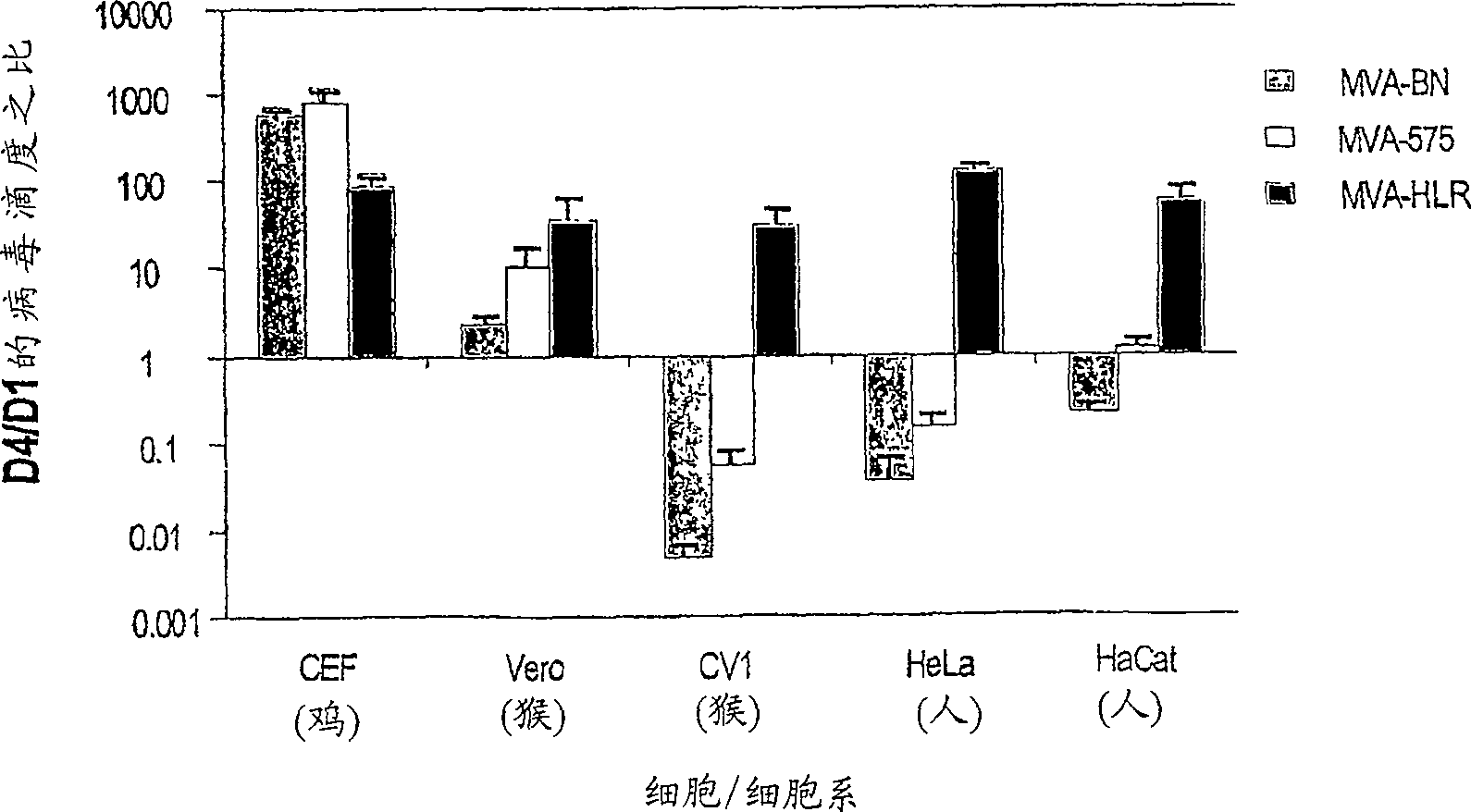
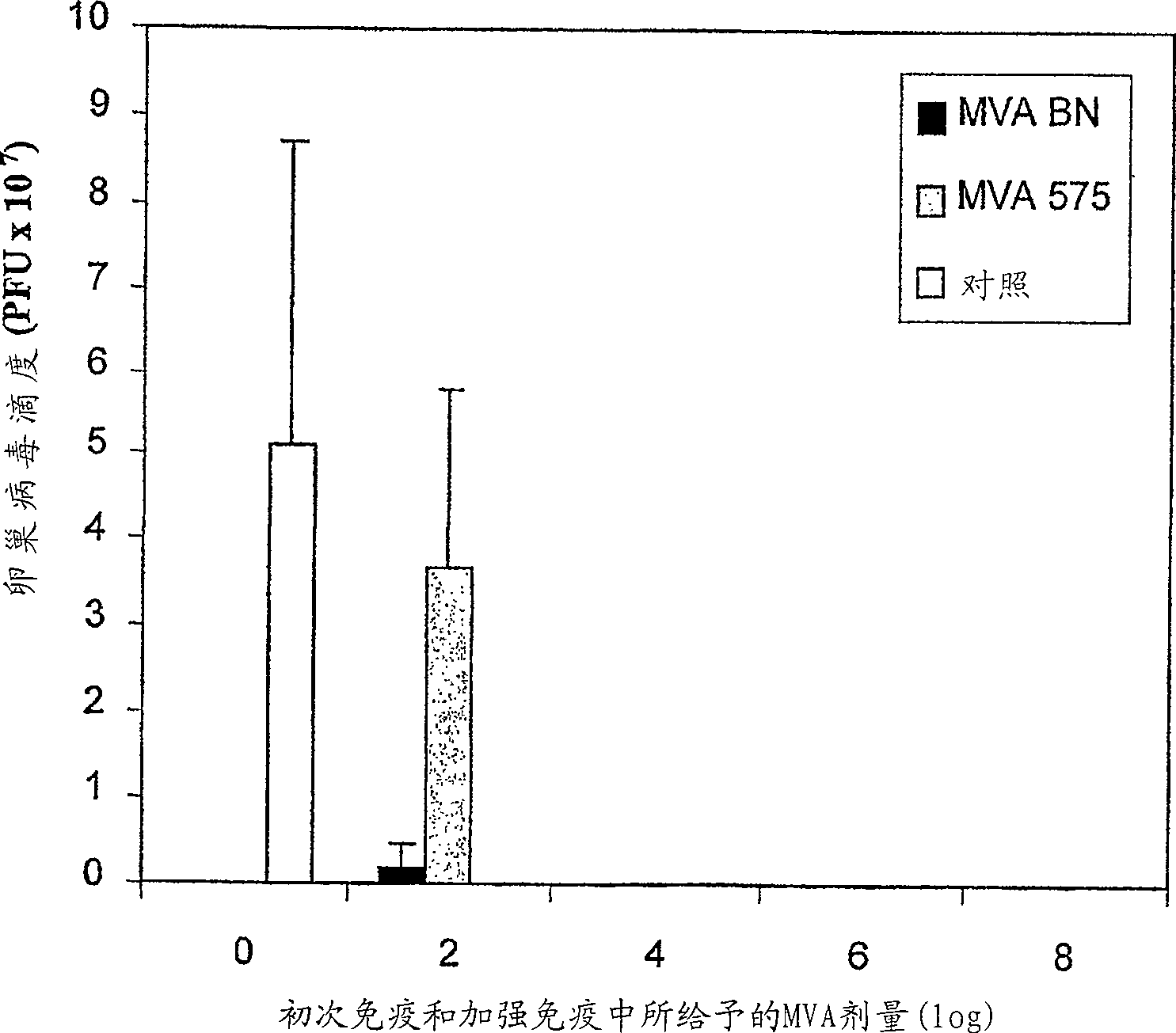
Modified Vaccinia Ankara virus variant and cultivation method
InactiveUS7445924B2Reduce riskGenetic material ingredientsVirus peptidesSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Modified vaccinia ankara virus variant and cultivation method
InactiveUS20050214323A1Reduce riskViral antigen ingredientsGenetic material ingredientsSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Immune modulating compounds from fungi
The present invention relates to compositions comprising polypeptides and polysaccharides. The compositions are in general immune modulating. The invention also discloses methods of producing these compositions using filamentous fungi cultivated in liquid medium. The compositions are useful for example in the treatment of immune compromised conditions.
Owner:GLYCANOVA
Lactobacillus reuteri to inhibit cryptosporidiosis in mammals
InactiveUS6103227AAvoid infectionImprove the immunityBiocideBacteriaChronic diarrheaImmune compromised
Cryptosporidium parvum (the cause of cryptosporidiosis) has become one of the most common enteropathogens causing diarrhea worldwide. Symptoms associated with cryptosporidiosis are very debilitating especially in the immunocompromised subject (e.g., AIDS patient). Clinical features include severe, chronic diarrhea, abdominal cramps, fatigue, weight loss, etc. which lead to increased health care costs and increased mortality. There is disclosed herein a method of inhibiting the severity of Cryptosporidium parvum infection by enterally administering a therapeutically effective amount of Lactobacillus reuteri.
Owner:WOLF BRYAN WARREN +1
Reduction in bacterial colonization by administering bacteriophage compositions
InactiveUS7459272B2Reduce and eliminate colonizationReduce riskAntibacterial agentsBiocideBacteroidesAcquired immunodeficiency
The present invention provides a method for reducing the risk of bacterial infection or sepsis in a susceptible patient by treating the susceptible patient with a pharmaceutical composition containing bacteriophage of one or more strains which produce lytic infections in pathogenic bacteria. Preferably, treatment of the patient reduces the level of colonization with pathogenic bacteria susceptible to the bacteriophage by at least one log. In a typical embodiment, the susceptible patient is an immunocompromised patient selected from the group consisting of leukemia patients, lymphoma patients, carcinoma patients, sarcoma patients, allogeneic transplant patients, congenital or acquired immunodeficiency patients, cystic fibrosis patients, and AIDS patients. In a preferred mode, the patients treated by this method are colonized with the pathogenic bacteria subject to infection by said bacteriophage.
Owner:INTRALYTIX
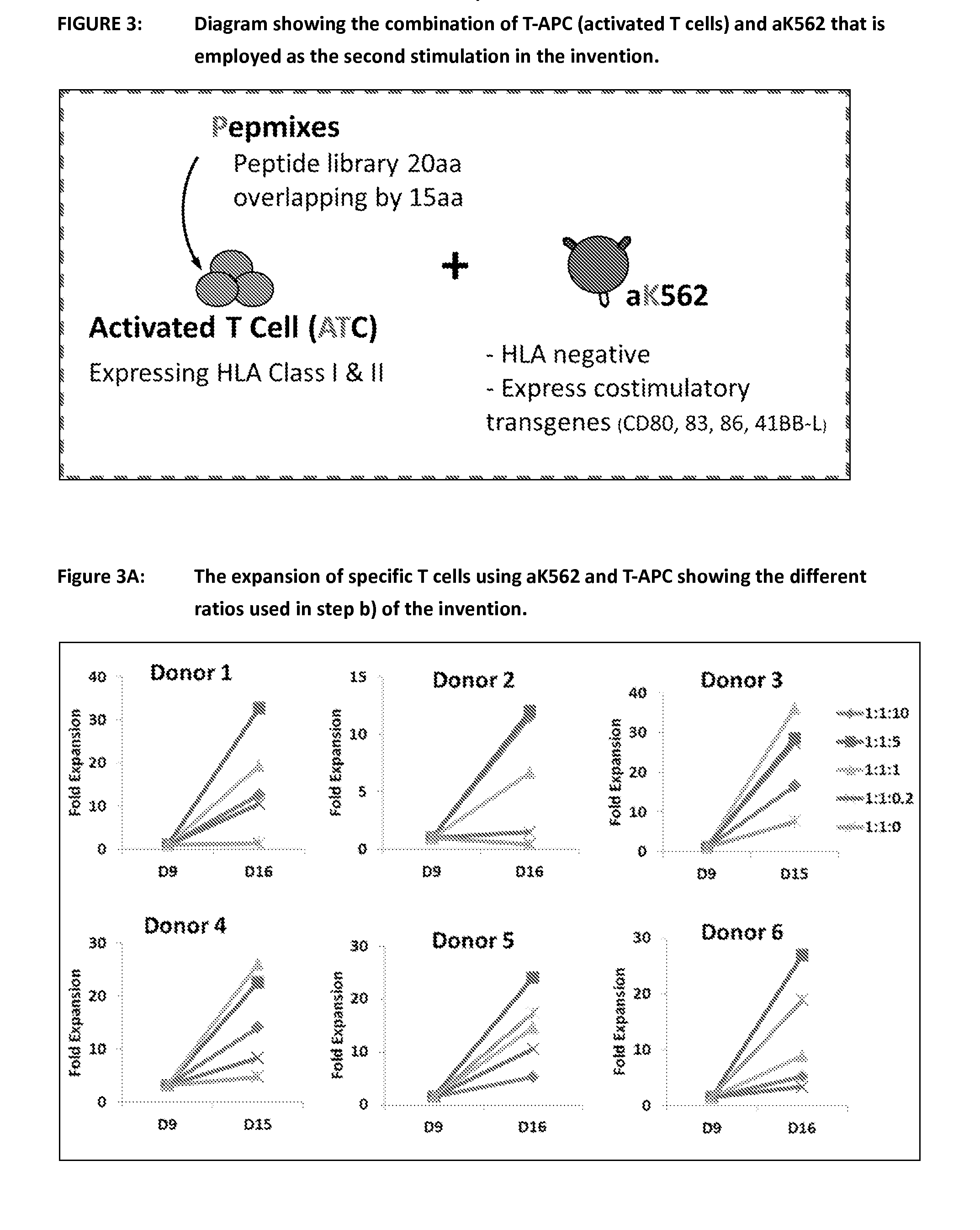
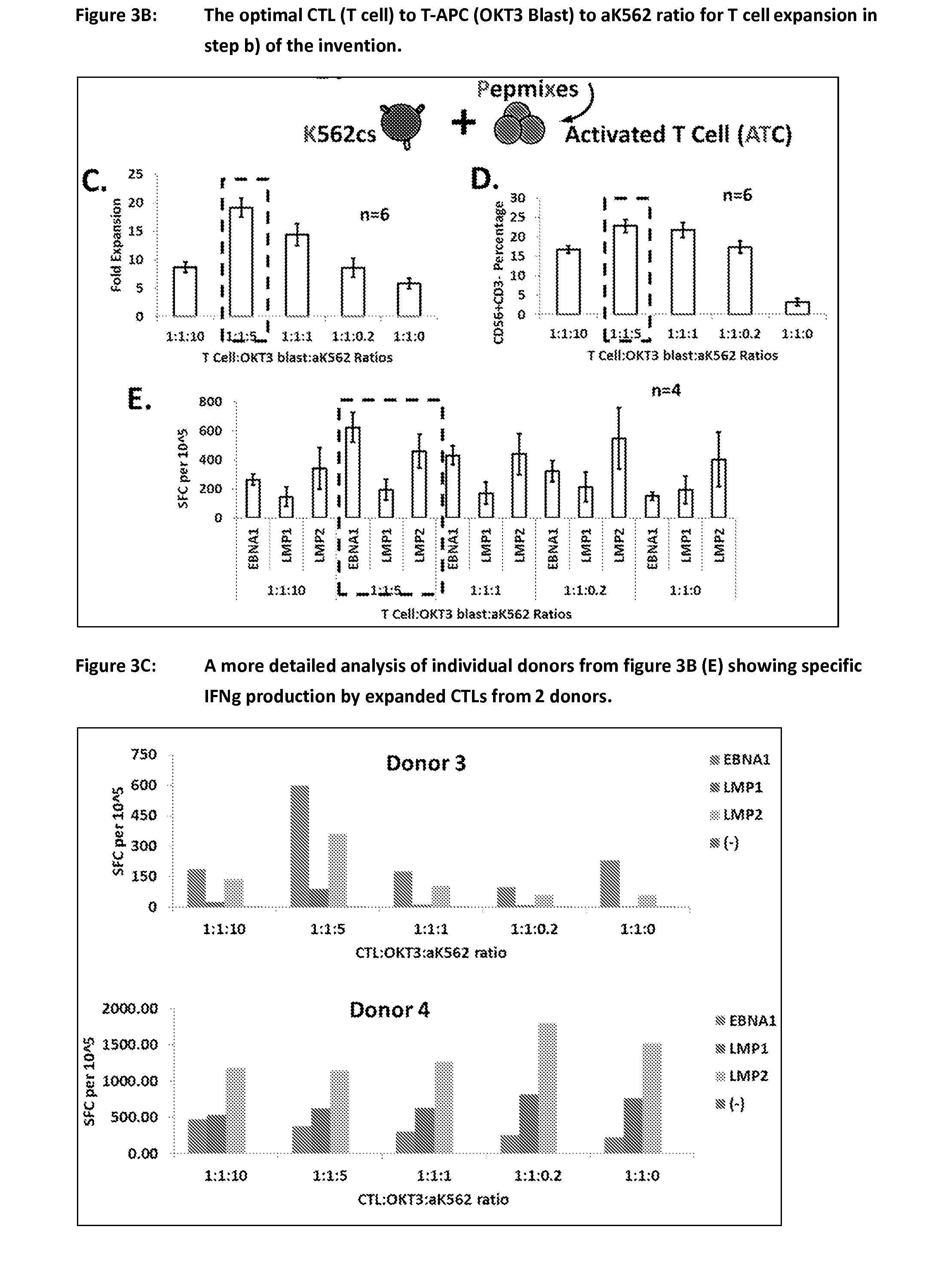
Process of expanding t cells
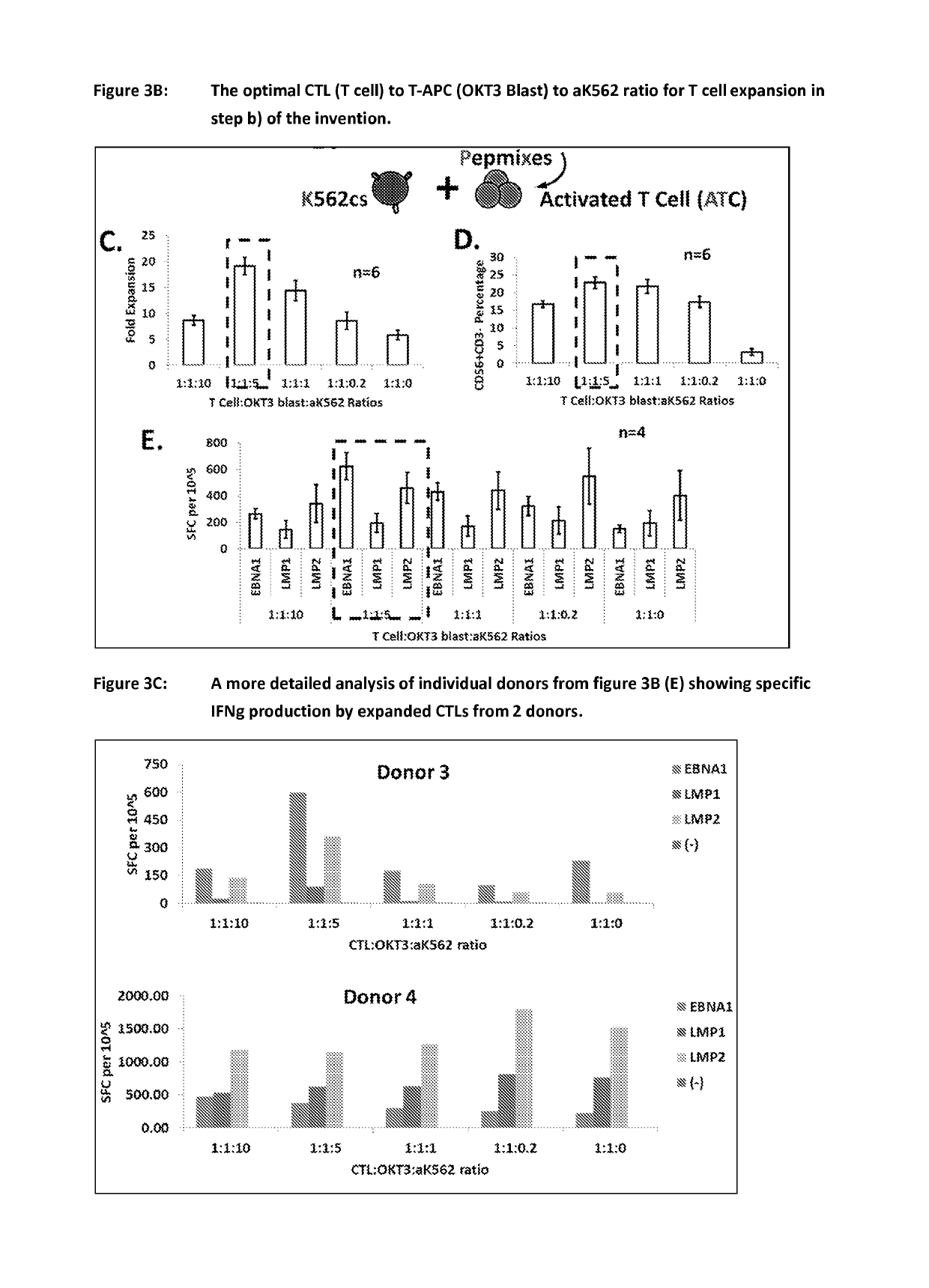
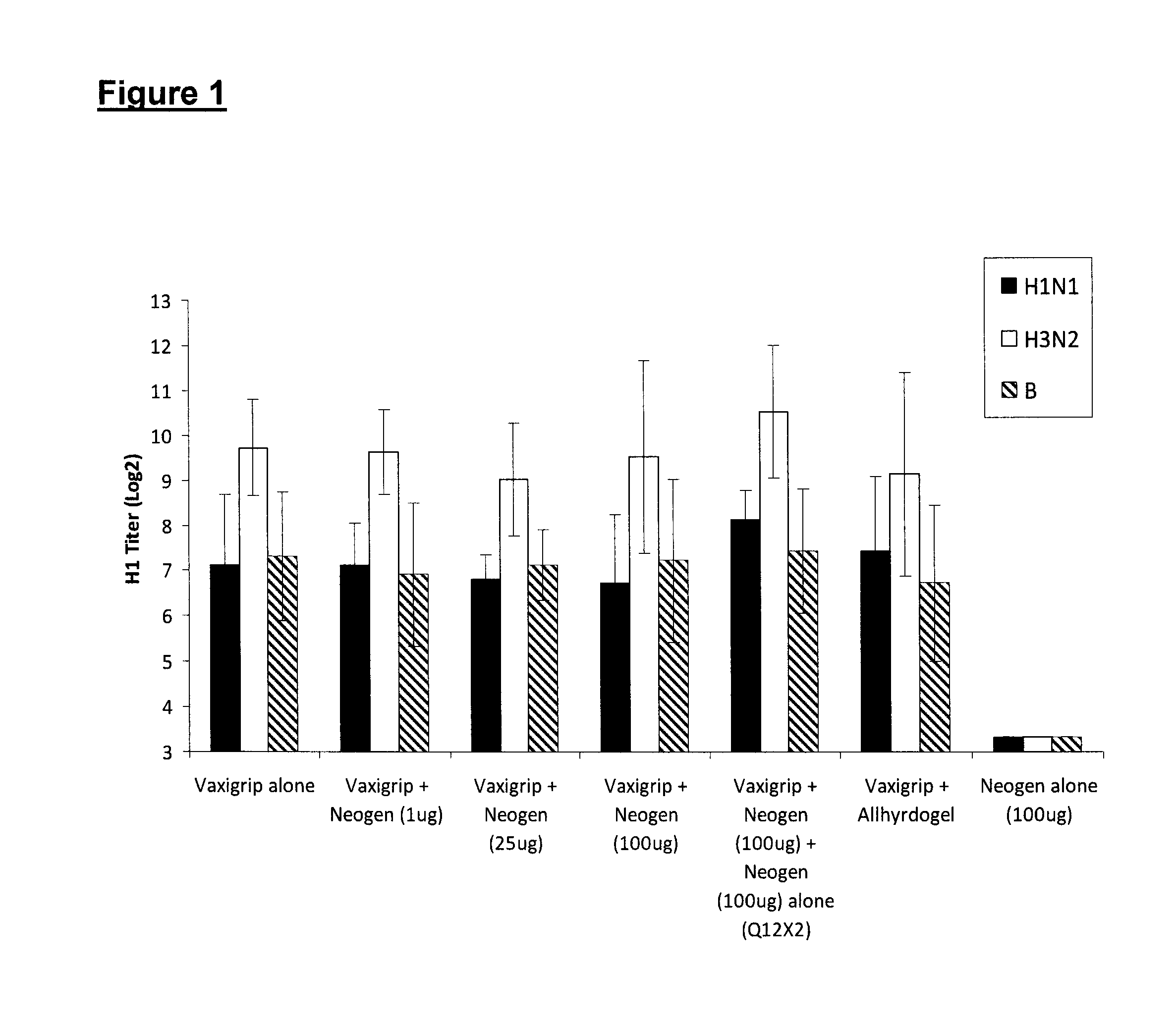
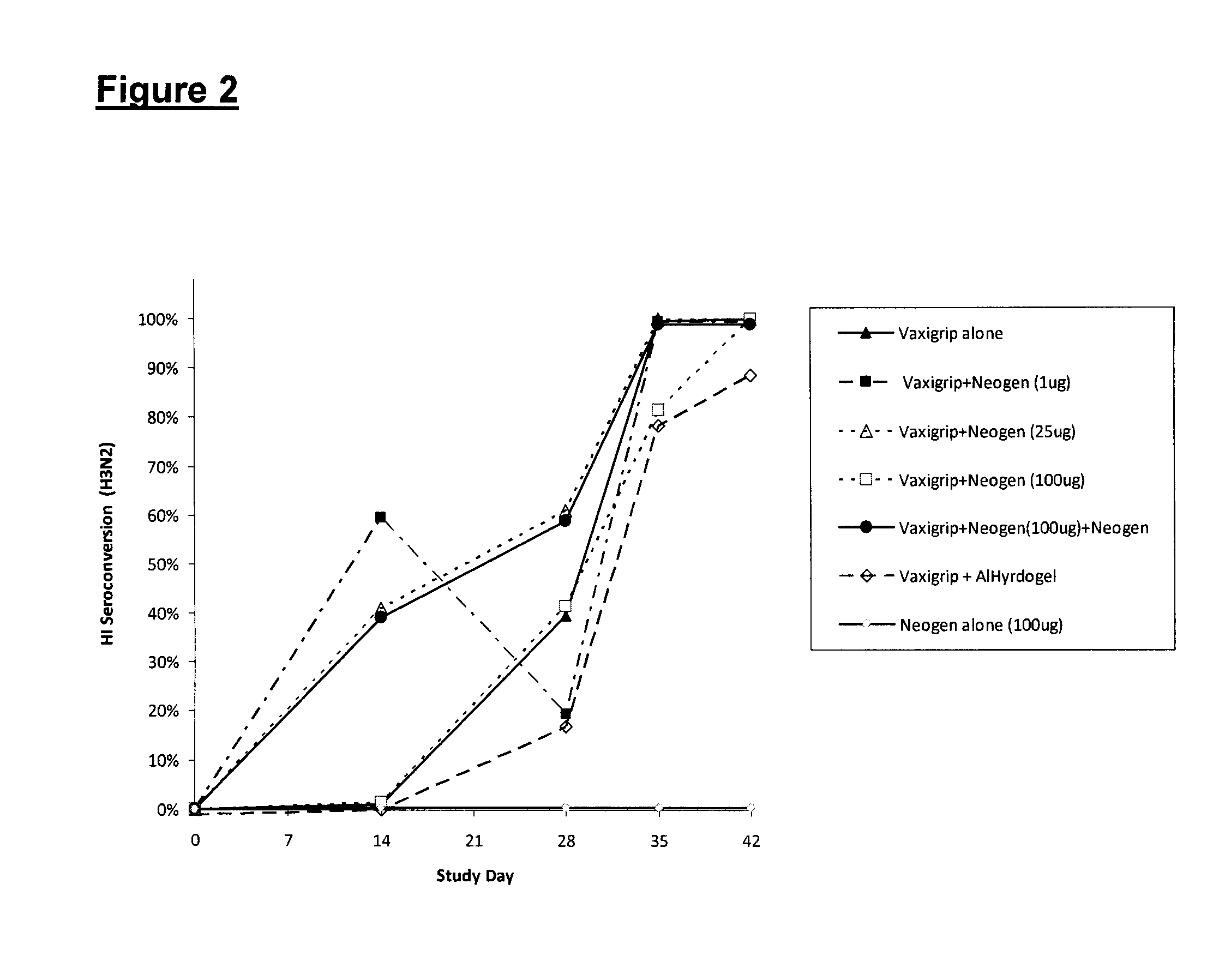
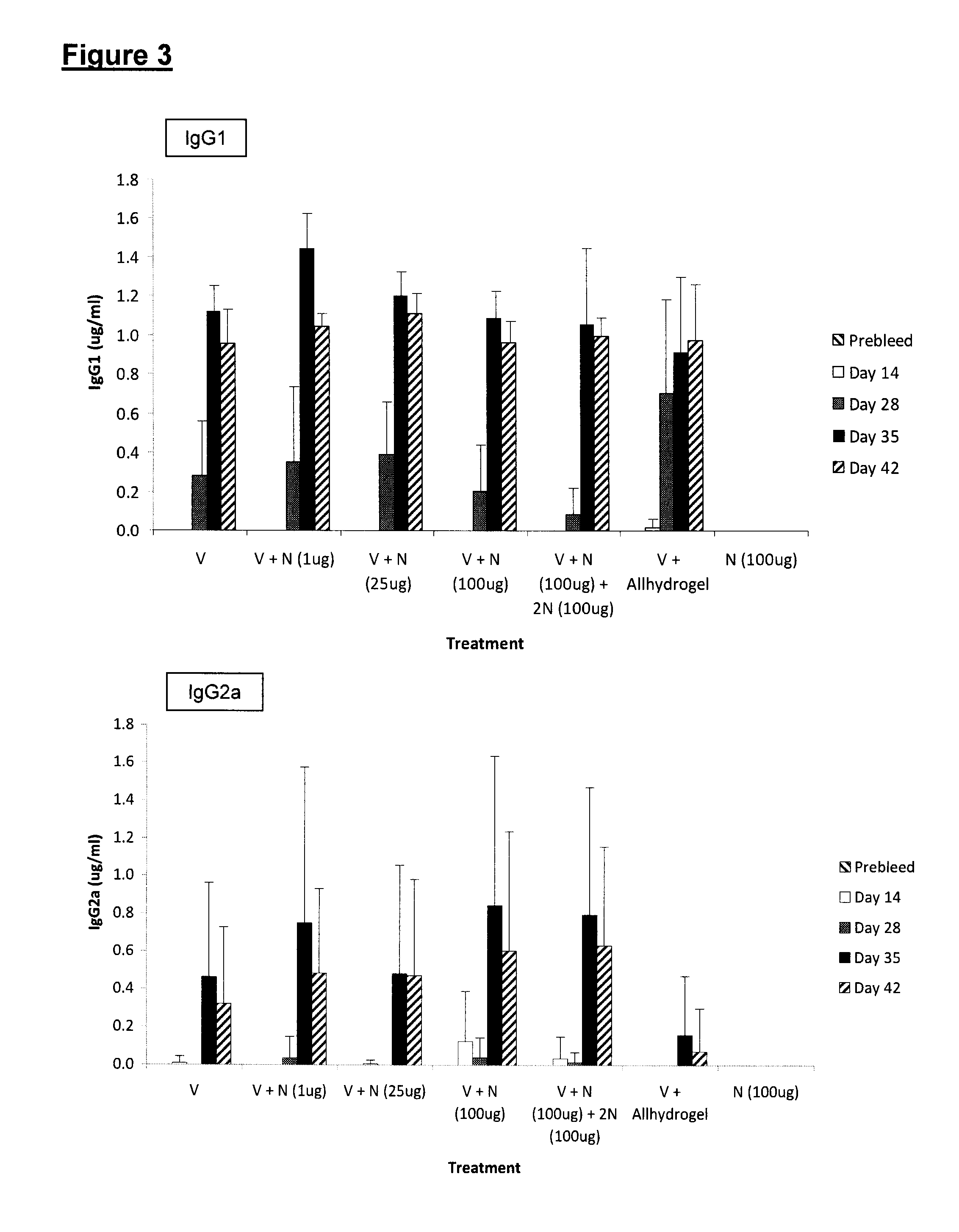
ActiveUS20150017723A1Minimises opportunity for contaminationMinimised of interventionGenetically modified cellsMammal material medical ingredientsImmune compromisedAutologous T-cells
The present disclosure relates to a novel process for expanding T cells, such as autologous T cells, cell populations therefrom, pharmaceutical compositions comprising the said cell populations and use of the cells and compositions for treatment, particular the treatment or prophylaxis of virus infection and / or cancer, for example in immune compromised or immune competent human patients.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Immune modulating compounds from fungi
The present invention relates to compositions comprising polypeptides and polysaccharides. The compositions are in general immune modulating. The invention also discloses methods of producing these compositions using filamentous fungi cultivated in liquid medium. The compositions are useful for example in the treatment of immune compromised conditions.
Owner:GLYCANOVA
Functional oligodendrocytes derived from pluripotent stem cells and methods of making and using the same
ActiveUS20170183627A1Low costComparable myelination potentialNervous disorderNervous system cellsProgenitorInduced pluripotent stem cell
Described is the efficient and robust generation of oligodendrocyte progenitor cells (OPCs) and oligodendrocytes from pluripotent stem cells (PSCs). The protocols provided recapitulate the major steps of oligodendrocyte differentiation, from neural stem cells to OLIG2+ progenitors, and then to 04+ OPCs, in a significantly shorter time than the 120-150 days required by previous protocols. Furthermore, 04+ OPCs are able to differentiate into MBP+ mature oligodendrocytes in vitro, and to myelinate axons in vivo when injected into immuno-compromised Shiverer mice, providing proof of concept that transplantation of PSC-derived cells for remyelination is technically feasible.
Owner:NEW YORK STEM CELL FOUND
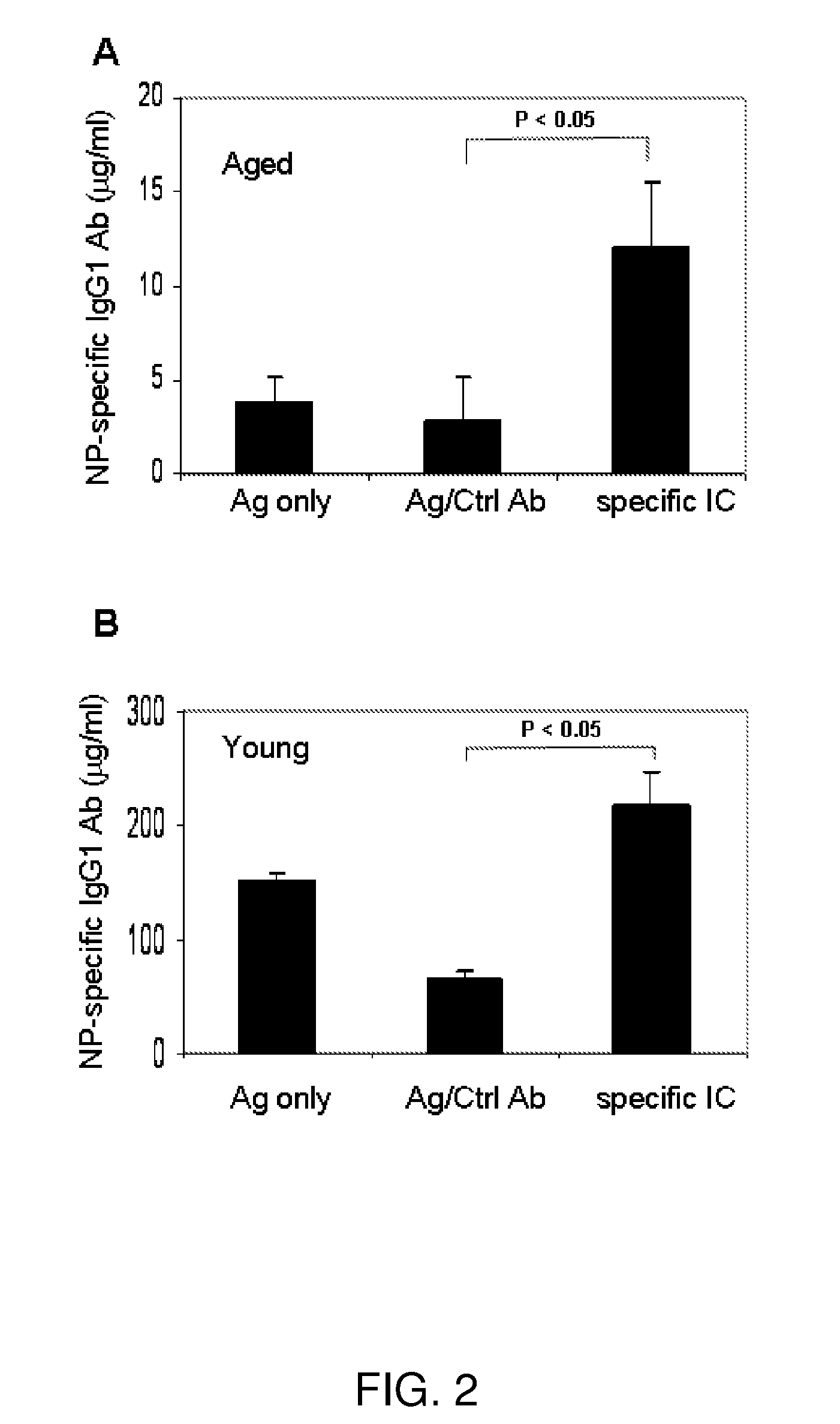
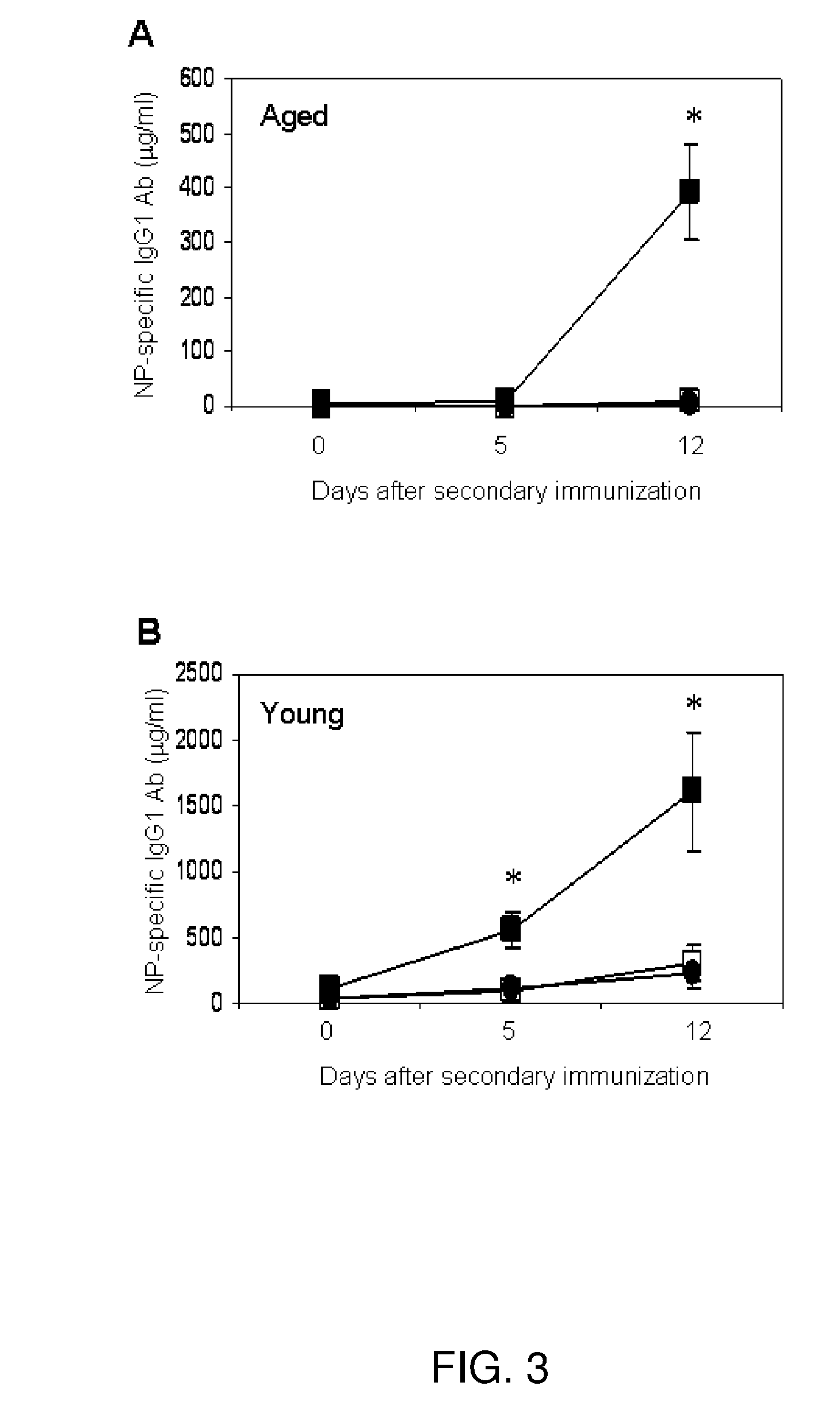
Immune complex vaccination as a strategy to enhance immunity in the elderly and other immune compromised populations
InactiveUS20080311135A1Reduced responseEnhance antibody responseAntibody ingredientsImmunoglobulinsImmune compromisedVaccination
The present invention generally concerns methods and compositions for improving the immune system of an individual that is an immune-compromised individual. In particular aspects, the immune-compromised individual is elderly or is immunosuppressed, such as from chemotherapy or immunosuppressants following organ or tissue transplantation. In specific embodiments, the invention relates to delivery to the immune-compromised individual of immune complexes harboring an antigen and an antibody that immunologically recognizes the antigen. The antigen may be viral, bacterial, or fungal, for example.
Owner:BAYLOR COLLEGE OF MEDICINE
Modified vaccinia ankara virus variant and cultivation method
InactiveUS20080317778A1Reduce riskBiocideGenetic material ingredientsSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost inoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
Prevention of opportunistic infections in immune-compromised subjects
ActiveUS9217133B2Avoid problemsEffective preventionAntibacterial agentsOrganic active ingredientsFucosylationImmune compromised
This invention relates to a composition suitable for use in the prevention of opportunistic infections in immune-compromised individuals comprising a probiotic Bifidobacterium lactis, Bifidobacterium infantis, Bifidobacterium breve or Bifidobacterium longum and a fucosylated oligosaccharide selected from the group comprising 2′-fucosyllactose, 3′fucosyllactose, difucosyllactose, lacto-N-fucopentaose, lacto-N-fucohexaose, fucosyllacto-N-hexaose and fucosyllacto-N-neohexaose. The invention further extends to the use of such a composition in the prevention of opportunistic infections in immune-compromised individuals.
Owner:SOC DES PROD NESTLE SA
Topical antifungal treatment
The present invention is a topical skin preparation for treatment of fungal infections of the skin and nails. The preparation comprises triacetin in combination with an antifungal agent. In a preferred form, the preparation further comprises, a fatty acid source such as a fish oil. In a preferred embodiment, cod liver oil and tolnaftate are used in combination with triacetin. The concentrations of these constituents are 96.0-99.0% concentration triacetin, 0.5-3.0% concentration tolnaftate and 0.5-1.0% concentration cod liver oil, in one preferred embodiment. Other compounds, such as ethyl alcohol, amino acids such as n-acetylcysteine, and herbal additives may also be added to the preparation. Further, other antifungal agents such as nystatin, clortimazole, econazole, ketoconazole, miconazole, solconazole, oxiconizole, naftifine, terbinafine, and butenafine, for example, may be substituted for the antifungal agent tolnaftate. The preparation of the present invention is effective in treating immune compromised patients and those with diabetes, as well as relatively healthy persons.
Owner:BOMMARITO ALEXANDER A
Topical antifungal treatment
The present invention is a topical skin preparation for treatment of fungal infections of the skin and nails. The preparation comprises triacetin in combination with one or more antifungal agents. In a preferred form, the preparation comprises a combination of the antifungal agents tolnaftate and grisiofulvin are used in combination with triacetin. The concentrations of these constituents are 40 to 50% concentration triacetin, 30 to 50% acetone, 0.5-3.0% concentration tolnaftate and 0.5-4.0% concentration grisiofulvin, in one preferred embodiment. Other compounds, such as ethyl alcohol, acetone, amino acids such as n-acetylcysteine, and herbal additives may also be added to the preparation. Further, other antifungal agents such as nystatin, clortimazole, econazole, ketoconazole, miconazole, solconazole, oxiconizole, naftifine, terbinafine, and butenafine, for example, may be substituted for the antifungal agents tolnaftate and grisiofulvin. The preparation of the present invention is effective in treating immune compromised patients and those with diabetes, as well as relatively healthy persons.
Owner:BOMMARITO ALEXANDER A
Modified vaccinia ankara virus variant
InactiveCN1476483AAntibacterial agentsGenetic material ingredientsModified vaccinia AnkaraHuman cell line
The present invention provides an attenuated virus derived from a modified vaccinia Ankara virus characterized by loss of the ability to replicate repeatedly in human cell lines. The present invention further describes recombinant viruses derived from the virus and the use of the virus or recombinants thereof as drugs or vaccines. Furthermore, the present invention provides a method of inducing an immune response even in immunocompromised patients, patients who are already immune to vaccine viruses, or patients who are receiving antiviral therapy.
Owner:BAVARIAN NORDIC AS
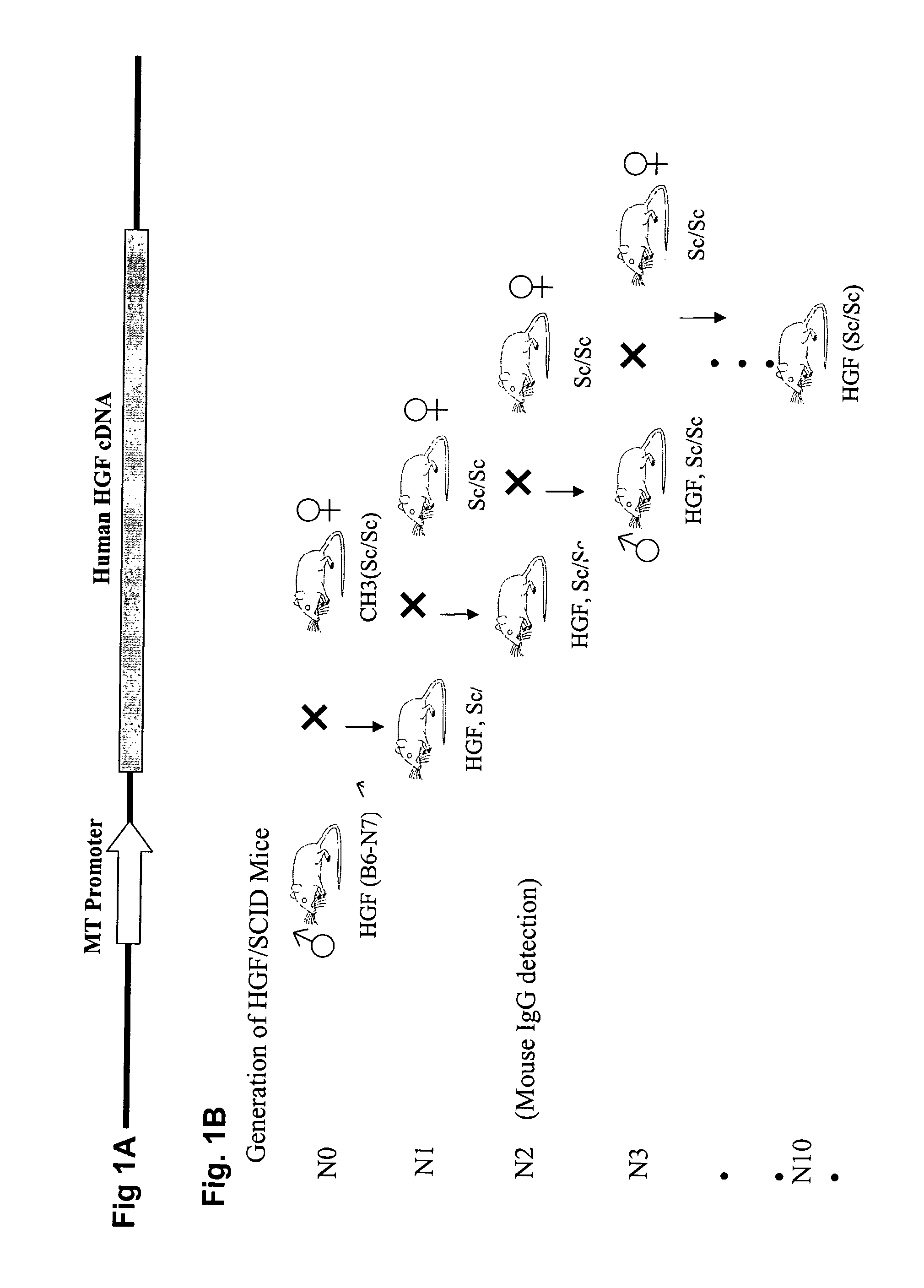
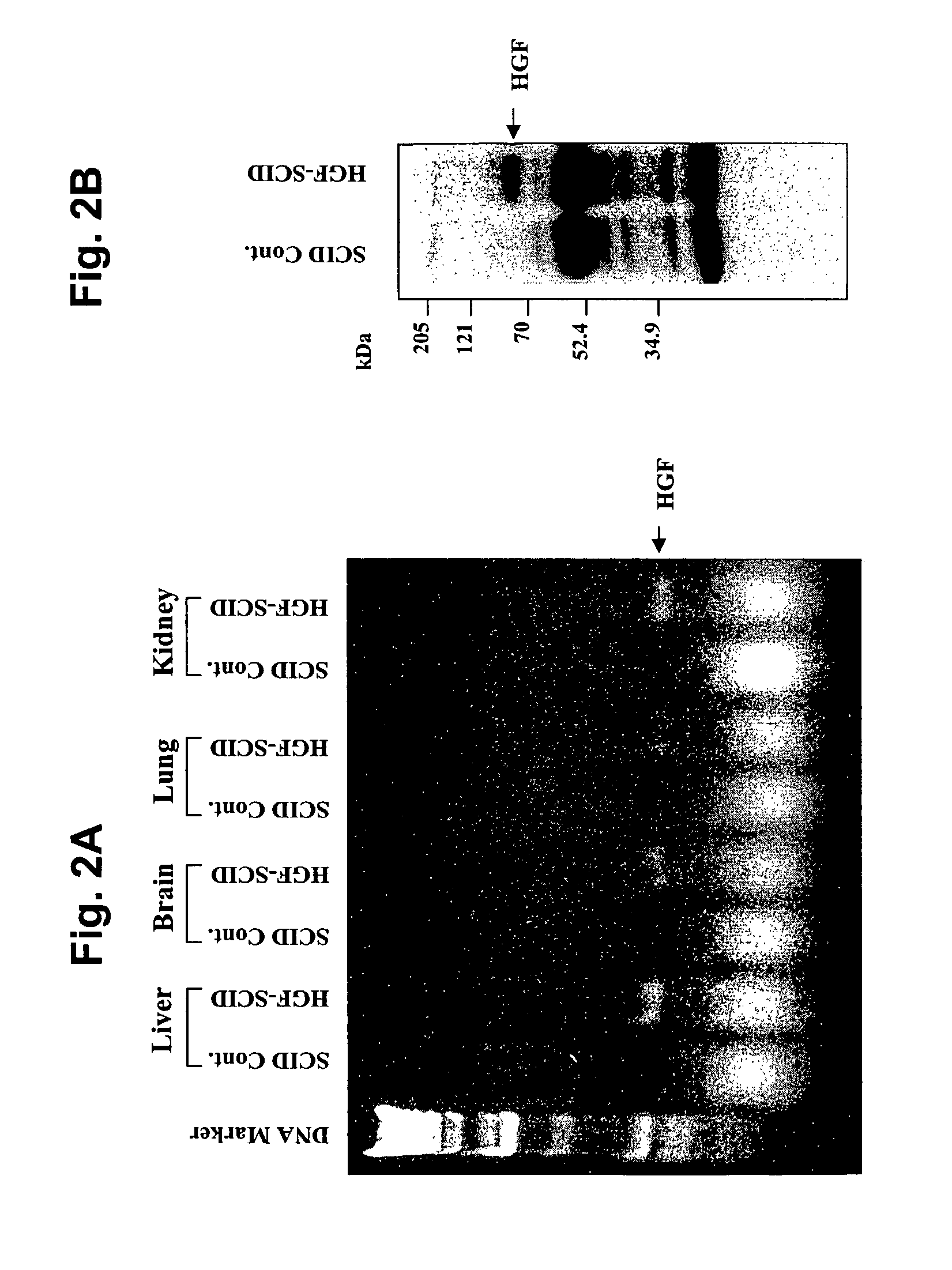
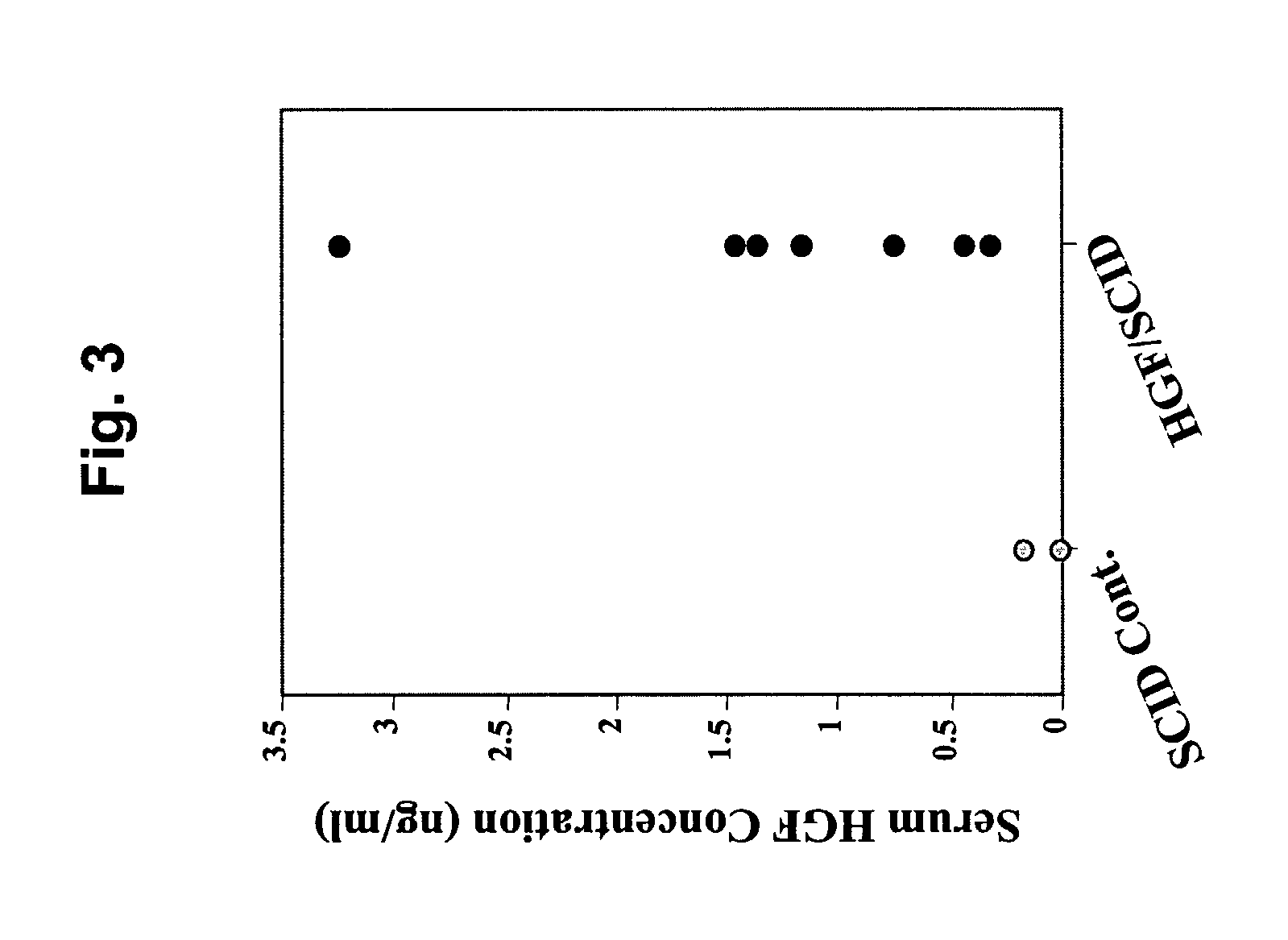
Immune-compromised transgenic mice expressing human hepatocyte growth factor (hHGF)
InactiveUS7968762B2Animal cellsHepatocyte-growth/scatter/tumor-cytotoxic factorImmune compromisedLymphatic Spread
A transgenic animal model for evaluating growth, survival and / or metastasis of xenotransplanted normal or tumor cells or tissue is disclosed, in which a human growth factor, hHGF stimulates growth in vivo of human cells or tissue. A strain of Tg mice on the C3H background that is immunocompromised as a result of a homozygous scid gene has been bred which express a nucleic acid encoding hHGF / SE The ectopically expressed hHGF / SF ligand significantly enhances growth of human tumor cell lines and explanted tumor cells or tissue that express the Met receptor for hHGF. Such animals also have an enlarged normal livers and greater than normal liver regenerative capacity. Any Met-expressing hHGF-dependent human cells, including hepatocytes and various stem cells can survive and grow in such animals.
Owner:VAN ANDEL RES INST
Large animal model of invasive pulmonary aspergillosis in an immunocompromised host
InactiveUS6444872B1High propensityEfficient designCompounds screening/testingMicrobiological testing/measurementClinical settingsImmune compromised
A model of systemic mold / Aspergillus infection in a profoundly immunocompromised host has been established in the beagle dog. The beagle was rendered immunosuppressed using a combination of total body irradiation and daily steroids, which provided a window of time where the mold could be successfully inoculated through a bronchoscope. This created a localized infection in one lung lobe, which subsequently spread diffusely throughout the lung parenchyma, and uniformly resulted in the animal's death. The invention contemplates the further study of the pathophysiology of opportunistic mold infections in vivo and also provides examples for the development of new antifungal agents and more effective combinations of agents. Finally, the invention contemplates the development of technology for the early detection of systemic mold infections. The inventors envision, that the use of the model should help save patients from clinical trials of antifungal drugs that may be effective in vitro without living up to the expectations in a clinical setting.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Composition and method for treating acute respiratory tract infections
ActiveUS20180036327A1Avoid symptomsEffective preventionOrganic active ingredientsAntiinfectivesImmune compromisedLacto-N-tetraose
A composition and method for using it to prevent and / or relieve symptoms of acute respiratory tract infections, particularly bronchitis, in immune-compromised persons, particularly adults. The composition contains 2′-fucosyllactose and lacto-N-neotetraose and / or lacto-N-tetraose.
Owner:GLYCOM AS
Process of expanding T cells
ActiveUS10351824B2Efficient and robust 2-step culture processImprove propertiesPeptide/protein ingredientsGenetically modified cellsImmune compromisedImmunocompetence
The present disclosure relates to a novel process for expanding T cells, such as autologous T cells, cell populations therefrom, pharmaceutical compositions comprising the said cell populations and use of the cells and compositions for treatment, particular the treatment or prophylaxis of virus infection and / or cancer, for example in immune compromised or immune competent human patients.
Owner:BAYLOR COLLEGE OF MEDICINE +1
Novel Cancer Indications of Mannan-Binding Lectin (Mbl) in the Treatment of Immunocompromised Individuals
InactiveUS20080214435A1Sufficient immune responseAntibacterial agentsAntimycoticsImmune compromisedOncology
The present invention pertains to the use of subunits and oligomers of mannan-binding lectin (MBL) in prophylactic and / or curative treatment of an immunocompromised individual such as subjects suffering from solid tumors or haematological cancers. Solid tumors include such as female cancers, male cancers, cancers of the respiratory system, cancers of the gastro intestinal system, the renal system and further subjects suffering from thyroid cancer and melanomas. Haematological cancers include leukaemia, lymphoma and myeloma. The immunocompromised condition of the individual may be due to a cancer disease as mentioned herein or the treatment of said cancer disease.
Owner:NAT IMMUNE
Vaccine Having a Peptide Adjuvant for Eliciting a Specific Immune Response to Treat Viral Infection and Other Conditions
InactiveUS20120231025A1Suitable for useSsRNA viruses negative-senseViral antigen ingredientsSide effectImmune compromised
This invention provides a family of immunogenic compositions and vaccines, each containing a target antigen or antigen mixture, and an oligopeptide adjuvant, exemplified by the tripeptide Ile-Glu-Trp. The adjuvant has a low side effect profile, and may be especially effective in generating a rapid and specific Th1 or cellular immune response where the antigen is poorly immunogenic, or the patient is elderly or immunocompromised. In some circumstances, effectiveness of the vaccine can be substantially enhanced by administering follow-on injections of the tripeptide alone. The vaccine has been used to generate an enhanced response to multiple strains of influenza simultaneously, and is suitable for preventing or treating other infectious and disease conditions.
Owner:PHARMA BIO
Glycoconjugate vaccines for use in immune-compromised populations
InactiveUS7449189B2Improve tolerancePrevent bacteremiaAntibacterial agentsBacterial antigen ingredientsAntigenImmune compromised
Staphylococcal and Enterrococcal glycoconjugate vaccines are disclosed for use in preventing or treating bacterial infection in an immune-compromised individual. Such vaccines contain an immunocarrier and a conjugate of a polysaccharide or glycopeptide surface antigen from a clinically-significant bacterial strain. The vaccines can be used for active protection in immune-compromised individuals who are to be subjected to conditions that place them at immediate risk of developing a bacterial infection, as would be case in the context of a catheterization or a surgical procedure.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Thymosin alpha peptide for preventing, reducing the severity of, and treating infection
The present invention provides methods for preventing, treating, or reducing the severity of infection, including bacterial, viral, and fungal infections, and including infections of more complex etiology. The invention involves the administration of an alpha thymosin peptide regimen, so as to prime or enhance a patient's immune response for pathogen exposure. In certain embodiments, the alpha thymosin regimen is scheduled or timed with respect to potential or expected pathogen exposures. The regimen of alpha thymosin peptide as described herein provides the patient with a more robust immune response to pathogen exposure, including higher antibody titers and / or a more rapid antibody response. In certain embodiments, the patient is immunodeficient or immunecompromised, and / or the patient is hospitalized or scheduled for hospitalization, such that the regimen of alpha thymosin peptide helps to protect the patient from, or reduce the severity of, nosocomial infection or illness.
Owner:SCICLONE PHARMACEUTICAL INC
Methods for screening human blood products comprising plasma using immunocompromised rodent models
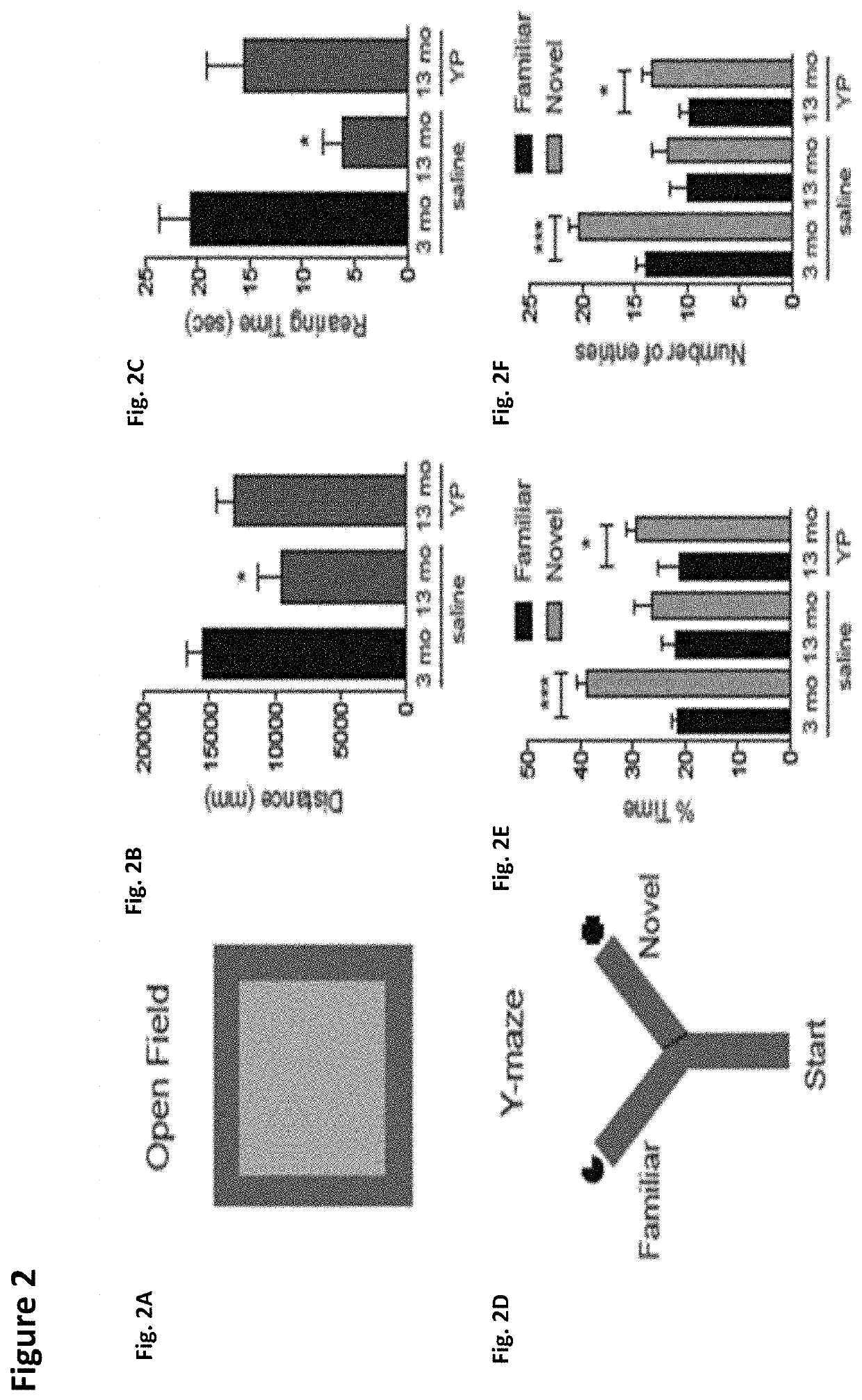
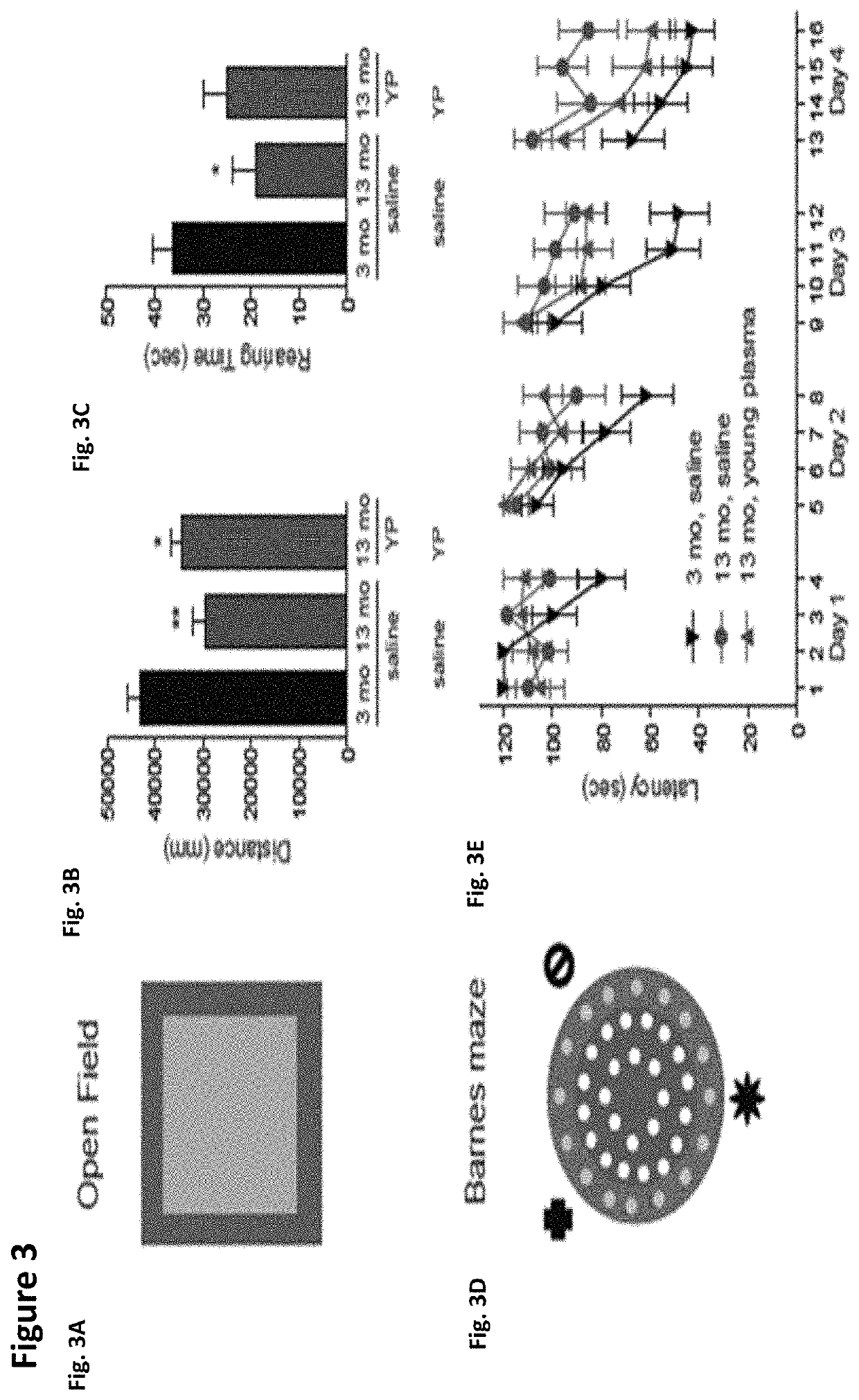
ActiveUS10905779B2Compounds screening/testingMammal material medical ingredientsImmune compromisedNeurogenesis
Owner:ALKAHEST INC +2
Intestinal flora related to immune recovery and application thereof
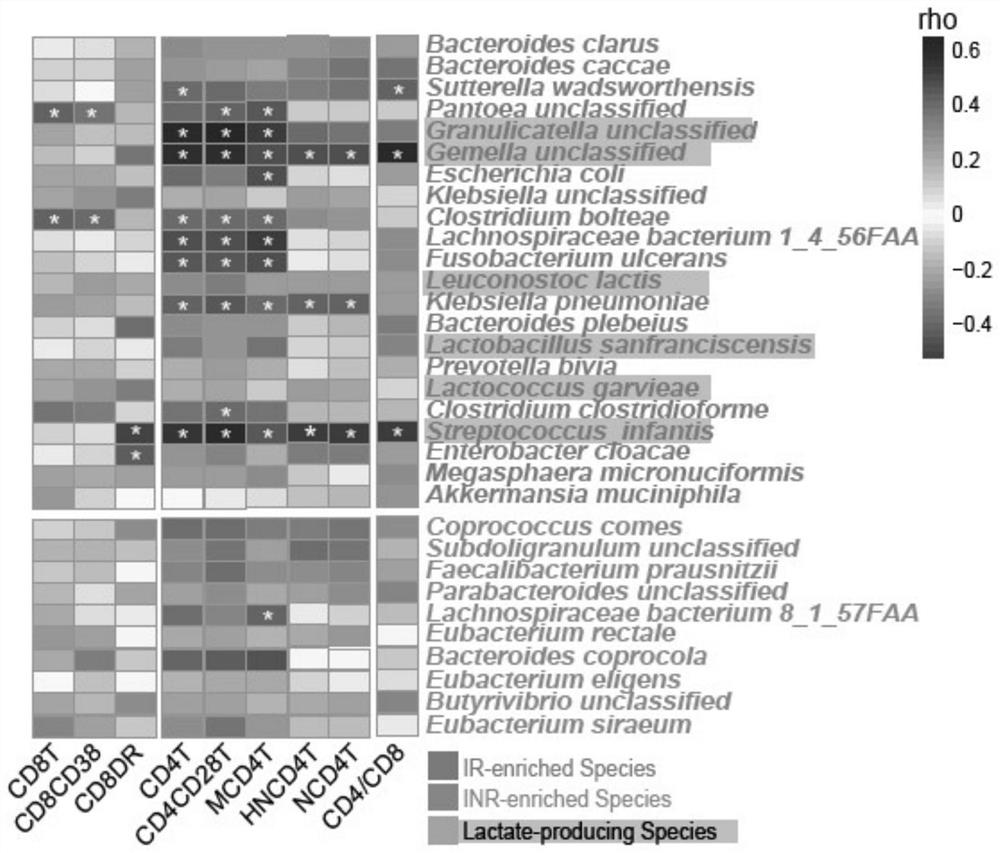
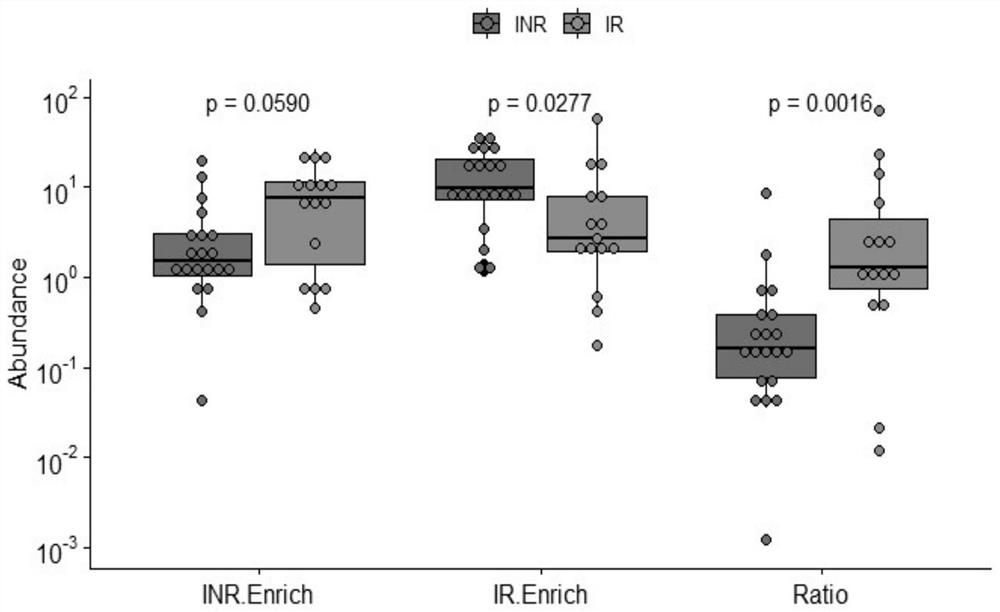
InactiveCN112011602APromote immune recoveryImprove accuracyMicrobiological testing/measurementDigestive systemLactic acid bacteriumImmune compromised
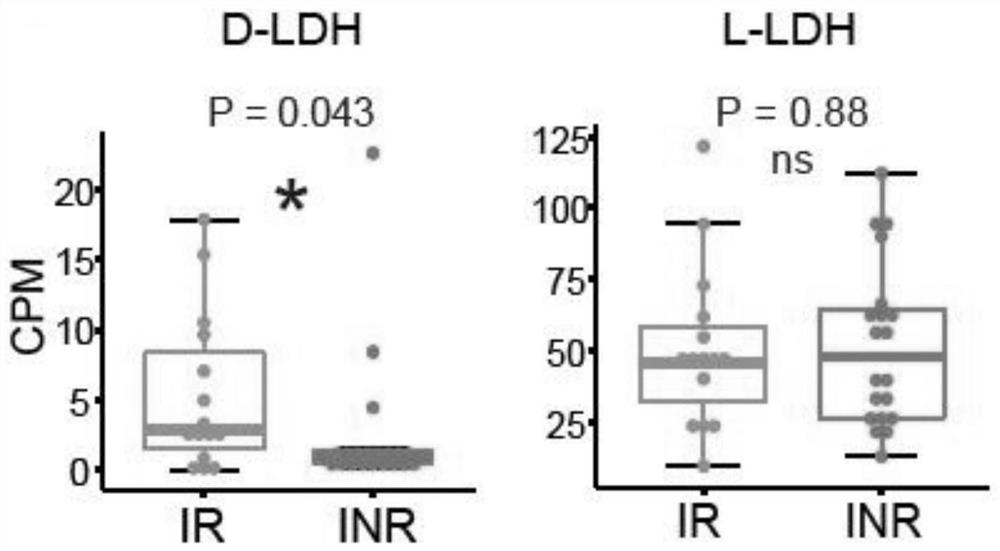
The invention provides an intestinal flora related to immune recovery and application thereof. As found in the invention for the first time, specific intestinal bacteria species have the effect of promoting body immune recovery. A biomarker related to immune recovery provided by the invention comprises 32 intestinal bacteria species, dextral lactate dehydrogenase and 18 lactic acid bacteria species. By utilizing the biomarker, risk assessment can be carried out on immune recovery prognosis after immune impairment of a patient, accuracy, sensitivity and specificity are high, sample collection is convenient, no body injury is generated, detection is convenient and fast, regular detection can be carried out, and the intestinal species can be used to develop preventive and therapeutic productsand assist the treatment of patients with impaired immune functions in order to reduce the incidence rate of poor immune recovery.
Owner:翊康生物科技发展(辽宁)有限公司
Autologous tumor vaccines and methods
ActiveUS20180008686A1Preserving heterogeneityEfficient responseEnergy modified materialsVaccinesImmune compromisedVaccine Production
Autologous anti-cancer vaccines and methods of manufacture and treatment are provided, including expansion of individual patient-derived tumor cells in an immune-compromised animal(s) to attain, quantitatively and qualitatively, sufficient material for efficacious vaccine production and utilization, to elicit an immune response against micrometastases and / or recurrence in the individual patient following tumor excision.
Owner:VACCINOGEN
Autologous tumor vaccines and methods
InactiveUS20150093416A1Minimize cytotoxicityEliminate tumorigenicityEnergy modified materialsVaccinesImmune compromisedVaccine Production
Autologous anti-cancer vaccines and methods of manufacture and treatment are provided, including expansion of individual patient-derived tumor cells in an immune-compromised animal(s) to attain, quantitatively and qualitatively, sufficient material for efficacious vaccine production and utilization, to elicit an immune response against micrometastases and / or recurrence in the individual patient following tumor excision.
Owner:VACCINOGEN
Modified vaccinia ankara virus variant
InactiveCN100537773CAntibacterial agentsGenetic material ingredientsModified vaccinia AnkaraImmunopotency
The present invention provides an attenuated virus derived from a modified vaccinia Ankara virus characterized by loss of the ability to replicate repeatedly in human cell lines. The present invention further describes recombinant viruses derived from the virus and the use of the virus or recombinants thereof as drugs or vaccines. Furthermore, the present invention provides a method of inducing an immune response even in immunocompromised patients, patients who are already immune to vaccine viruses, or patients who are receiving antiviral therapy.
Owner:BAVARIAN NORDIC AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com