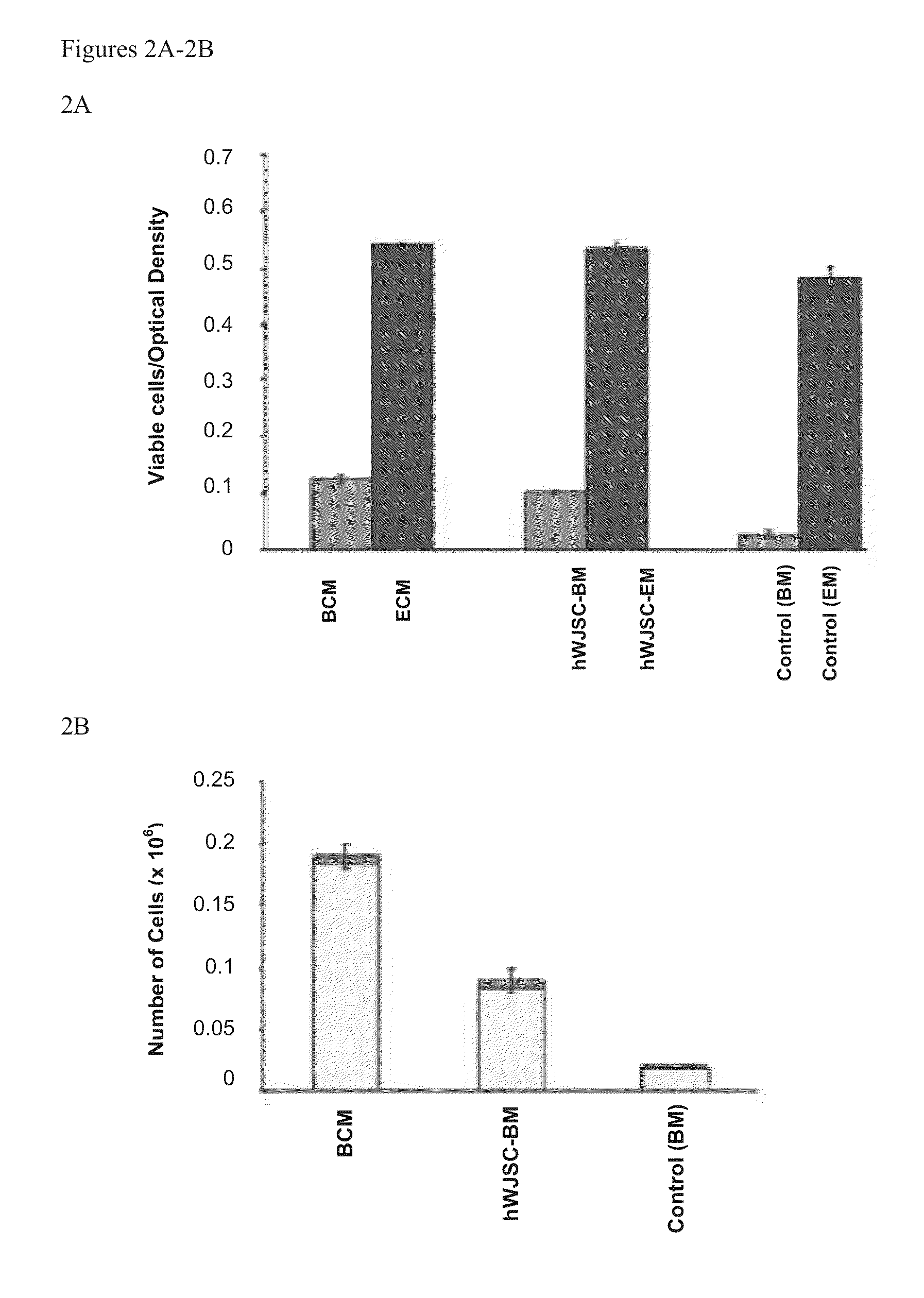
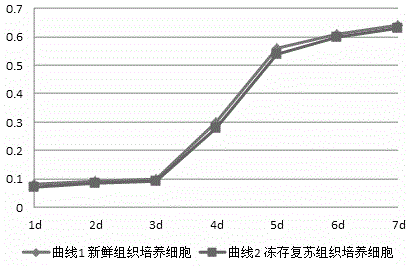
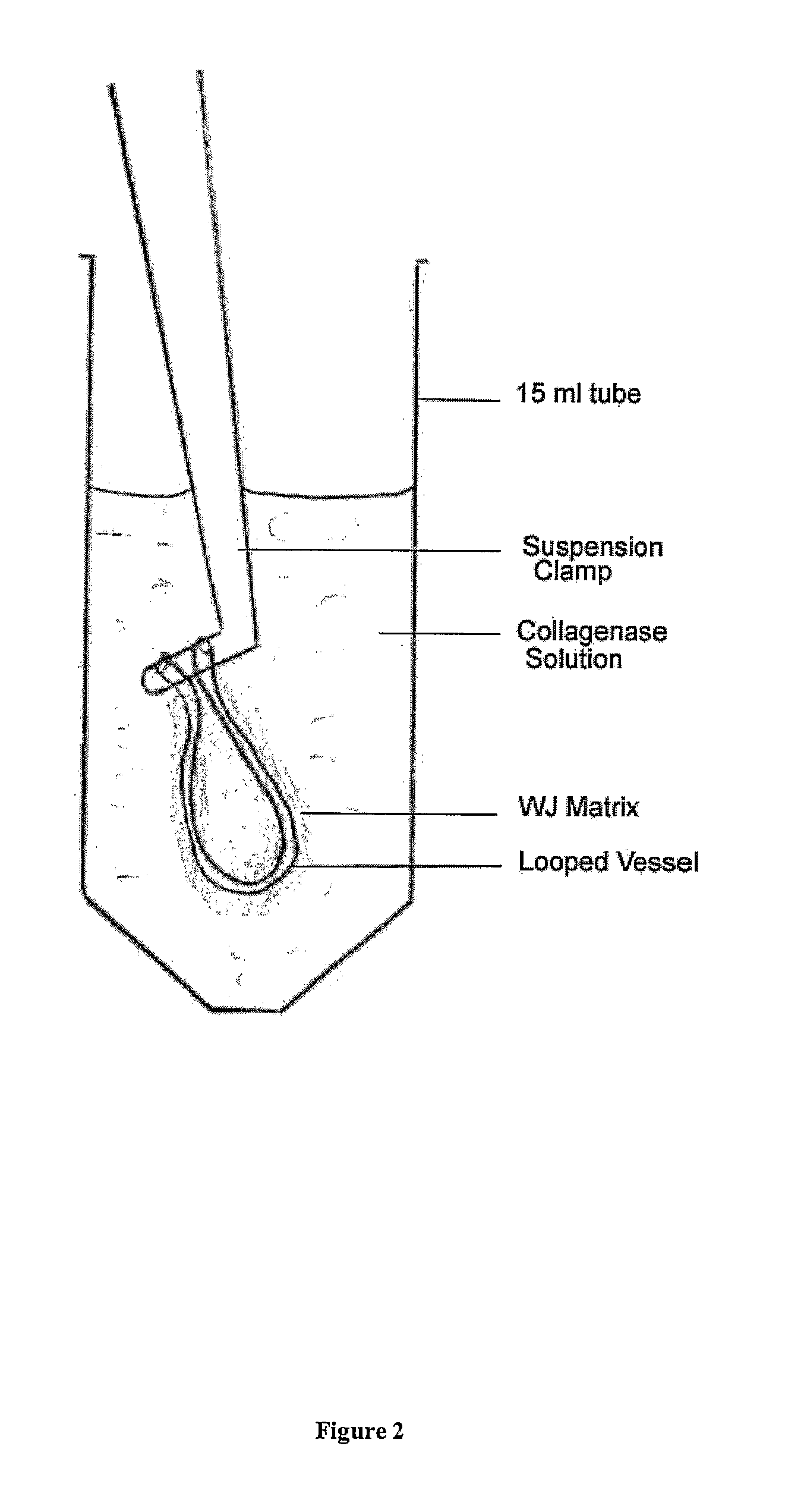
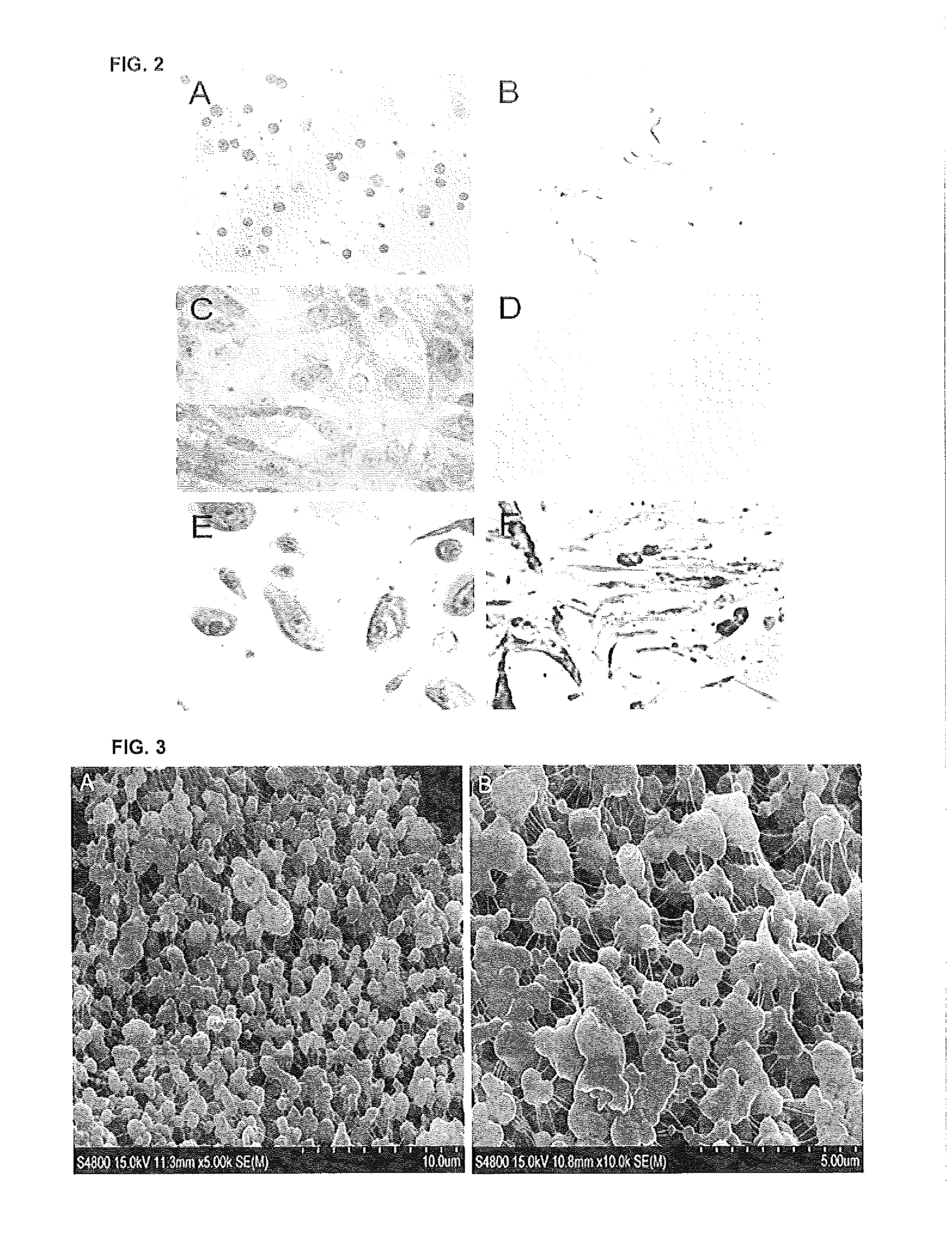
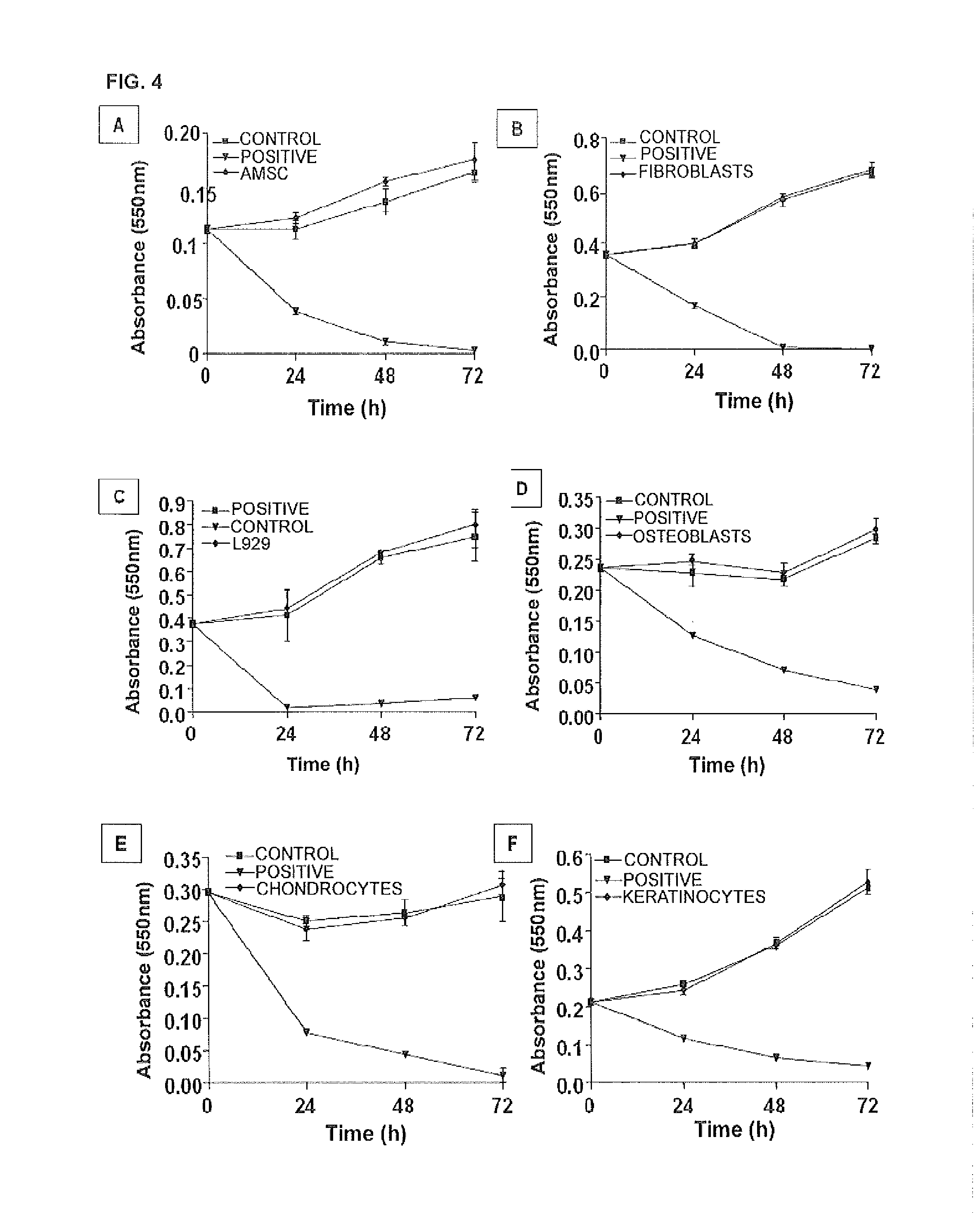
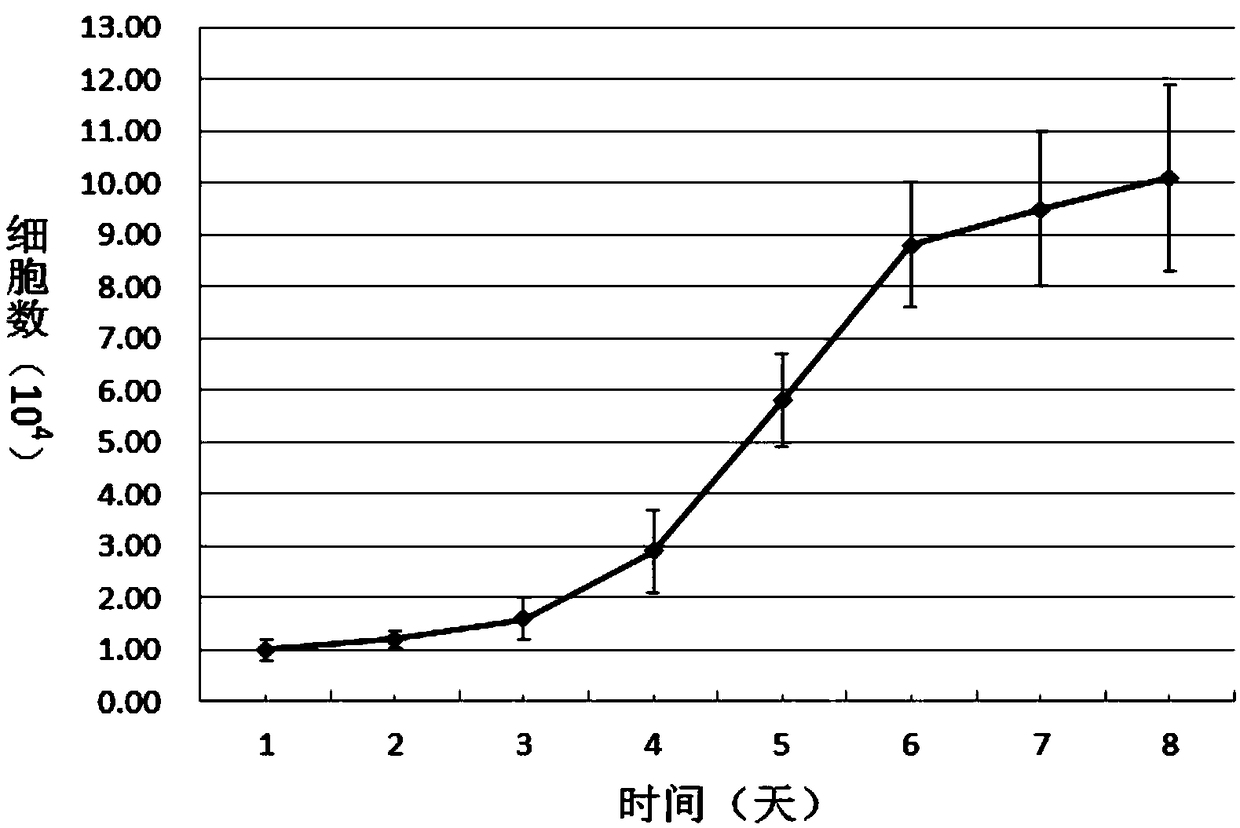
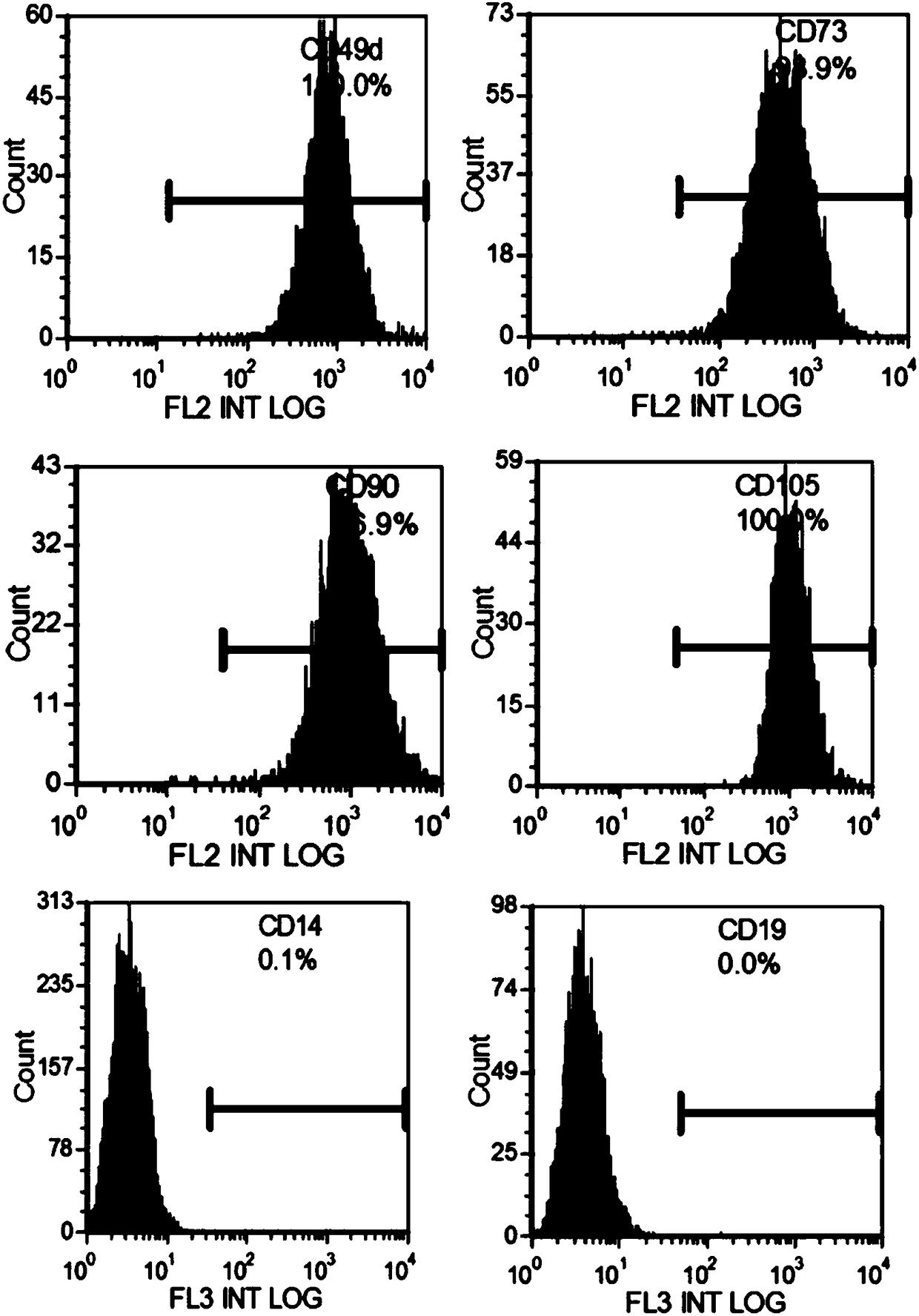
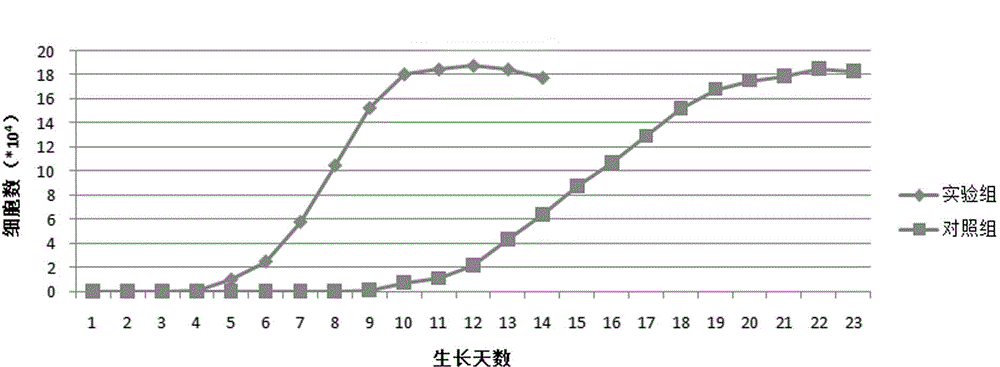
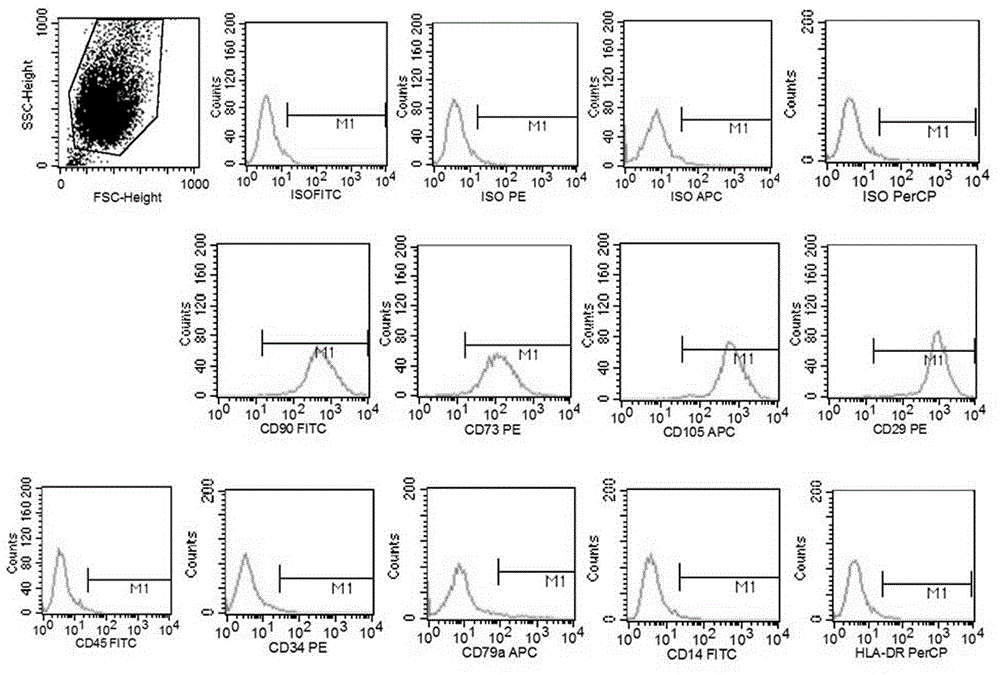
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
120 results about "Wharton's jelly" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
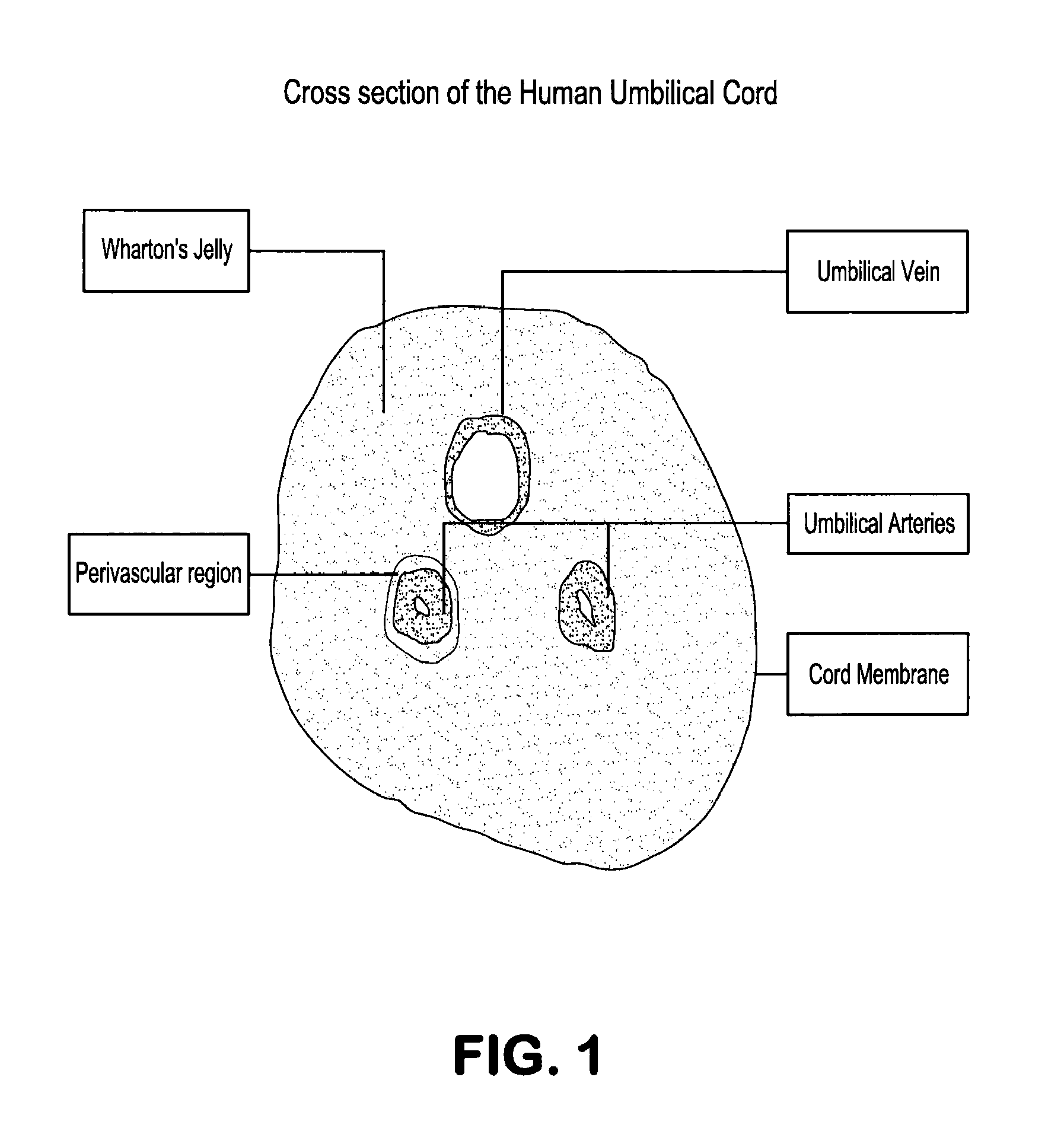
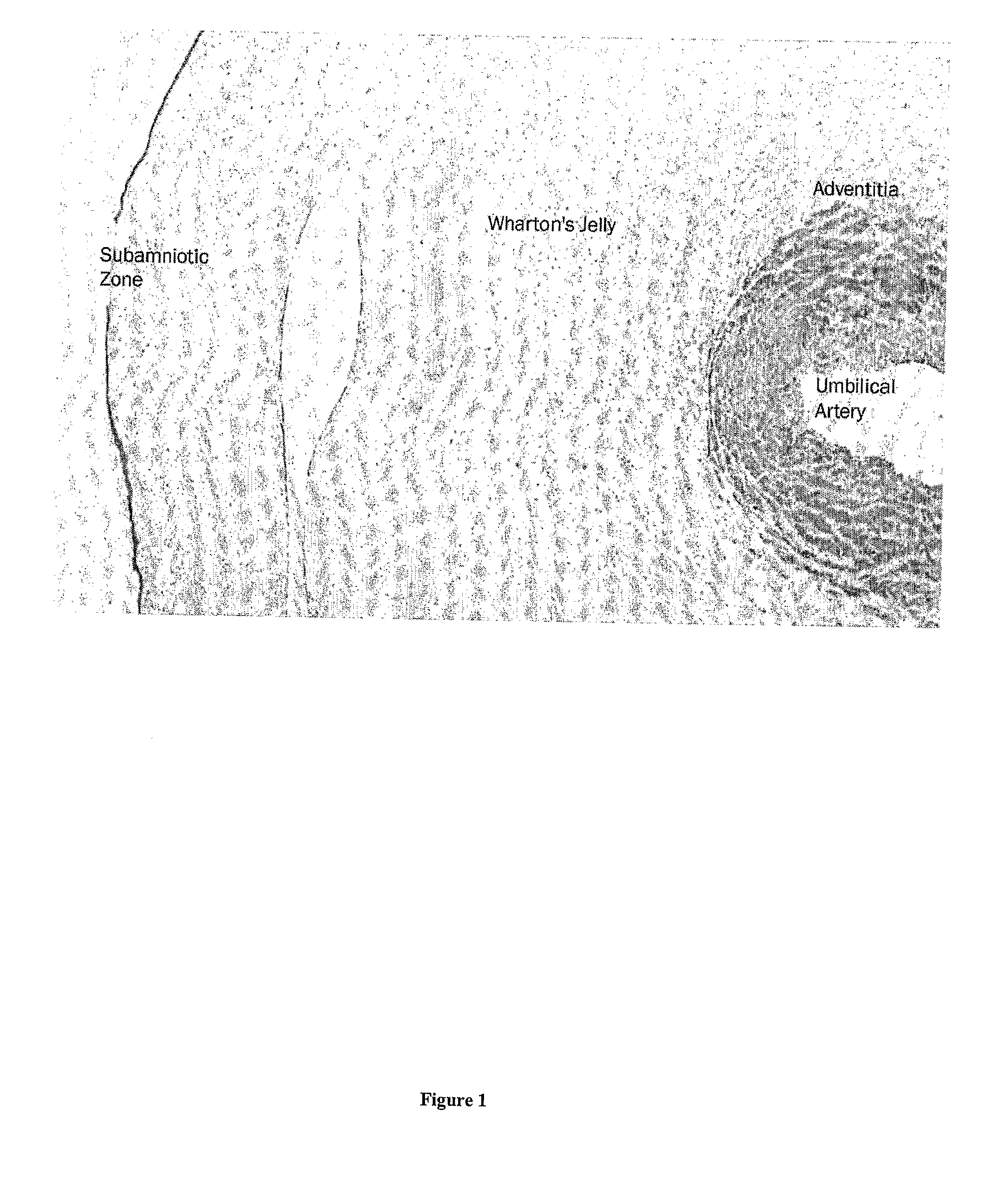
Wharton's jelly (substantia gelatinea funiculi umbilicalis) is a gelatinous substance within the umbilical cord, largely made up of mucopolysaccharides (hyaluronic acid and chondroitin sulfate). It also contains some fibroblasts and macrophages. It is derived from extra-embryonic mesoderm.
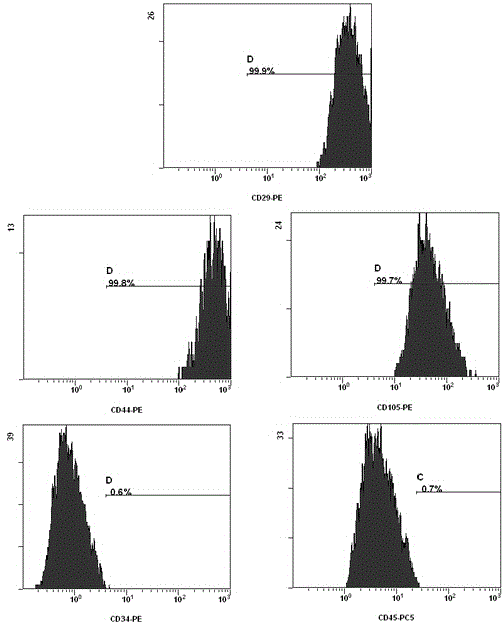
Wharton's Jelly Mesenchymal Stem Cells and Uses Thereof
ActiveUS20130302285A1Stable supportBiocideSkeletal/connective tissue cellsWharton's jellyCell culture media
In one aspect, the invention is directed to methods of expanding hematopoietic stem cells (HSCs) comprising culturing the HSCs with Wharton's Jelly mesenchymal stem cells (WJSCs), a cell culture medium that has been conditioned with WJSCs, or a combination thereof, thereby producing a HSC culture; and maintaining the HSC culture under conditions in which the HSCs expand in the culture, thereby expanding the HSCs. In another aspect, the invention is directed to a method of transplanting the expanded HSCs in an individual in need thereof. In yet another aspect, the invention is directed to compositions comprising HSCs and Wharton's Jelly mesenchymal stem cells (WJSCs). The composition can further comprise a cell culture medium that has been conditioned with WJSCs.
Owner:NAT UNIV OF SINGAPORE
Separation method and culture method for umbilical cord mesenchymal stem cells
ActiveCN103589683AClear chemical compositionAvoid heterogeneous contaminationSkeletal/connective tissue cellsPenicillinPhosphate
The invention relates to a separation method and a culture method for umbilical cord mesenchymal stem cells. The separation method comprises the following steps: thoroughly cleaning umbilical cord tissue of a healthy newborn by using a PBS (phosphate buffer solution) containing streptomycin and penicillin, and removing blood; shearing the umbilical cord into small sections uniform in length, and mechanically separating, bluntly stripping Wharton' s jelly, and removing umbilical arteries and umbilical veins; uniformly shearing the Wharton' s jelly; re-suspending the sheared Wharton' s jelly through an MSCs (mesenchymal stem cells) culture medium, inoculating to a culture dish with laid gelatin, and putting in a CO2 culture box for cultivation; conducting centrifugal separation to obtain tissue blocks and a cell resuspension solution. The culture method comprises the following steps: enwrapping the culture dish, discarding the gelatin, and washing with the PBS; inoculating the separated out tissue blocks and the cell resuspension into the culture dish; performing digestive subculture after cell fusion growth rate reaches 80-90%.
Owner:BEIJING DONGFANG HUAHUI BIOMEDICAL TECH
Method for treating brain ischemic injury through transplantation of human umbilical mesenchymal stem cells
A method for treating or preventing an ischemic brain injury or neurological damage due to ischemia in a subject includes transplanting a therapeutically effective amount of human umbilical mesenchymal stem cells (HUMSCs) obtained from Wharton's Jelly to the ischemic areas of the brain injury or the neurological damage of the subject. Recovery from neurological behavior deficits also is improved according by the method.
Owner:FU YU SHOW +1
Compositions and populations of cells obtained from the umbilical cord and methods of producing the same
InactiveUS20090280093A1Extensive mechanical processingFacilitate typeBiocideMammal material medical ingredientsProgenitorCord blood stem cell
The present invention relates to populations and compositions of stem and progenitor cells derived from the umbilical cord, and methods of obtaining the same. In some embodiments, one or more entire umbilical cords or sections thereof are subjected to a process where a cell population is derived without prior removal of any blood vessel. The population may be derived using mechanical and chemical means. The presently disclosed process may be applied to a single umbilical cord or to a plurality of umbilical cords, for example, as a batch process. Optionally, this process includes removing some or all cord blood before deriving the population. In some embodiments, presently disclosed cell populations include mesenchymal stem cells derived from Wharton's jelly and endothelial progenitor cells derived from a wall of a blood vessel of an umbilical cord. Optionally, the cell population includes stem cells derived from cord blood. The presently disclosed cell populations and compositions may be banked and / or used in a number of clinical or other applications. Exemplary applications include but are not limited to applications related to regenerative medicine, for screening compounds, for research, and for gene therapy.
Owner:THE REGENERATIVE MEDICINE INST
Cryopreservation protection liquid for Wharton jelly tissues of human umbilical cord and preparation and application of cryopreservation protection liquid
The invention relates to cryopreservation protection liquid for Wharton jelly tissues of a human umbilical cord. The cryopreservation protection liquid comprises 5-10 percent of a permeable cryoprotectant, 1-5 percent of a non-permeable cryoprotectant, 10-20 percent of a knockout serum replacement and 70-80 percent of a serum-free basal culture medium. The invention also discloses a preparation method of the cryopreservation protection liquid. The preparation method specifically comprises the steps of (1) precooling the serum-free basal culture medium, the non-permeable cryoprotectant and the knockout serum replacement, mixing and uniformly shaking; (2) adding the permeable cryoprotectant; and (3) performing refrigeration under a temperature condition of 2-6 DEG C for more than 30min. The invention also discloses a cryopreservation method for the Wharton jelly tissues of the human umbilical cord by the cryopreservation protection liquid. The cryopreservation method comprises the following steps of (1) pretreating the umbilical cord; (2) peeling off the Wharton jelly tissues; and (3) cryopreserving the Wharton jelly tissues of the human umbilical cord. The cryopreservation protection liquid and the cryopreservation method which are used for treating the Wharton jelly tissues of the human umbilical cord have the advantages that injury to umbilical cord mesenchymal stem cells in a cryopreservation process can be alleviated, so that the activity and the clinical use safety of the umbilical cord mesenchymal stem cells are guaranteed.
Owner:重庆市红汇脐血干细胞中心有限公司
Viable cells from frozen umbilical cord tissue
Viable progenitor cells are extracted from frozen umbilical cord tissue. In embodiments, the umbilical cord tissue is a blood vessel bearing perivascular Wharton's jelly, and the extracted progenitor cells are HUCPVCs.
Owner:TISSUE REGENERATION THERAPEUTICS
Methods for isolating mononuclear cells that include a subpopulation of mesenchymal progenitor cells and vascular cells that include a subpopulation of endothelial progenitor cells from umbilical cord tissue
ActiveUS20110151556A1Bioreactor/fermenter combinationsBiological substance pretreatmentsProgenitorWharton's jelly
Provided herein are methods and kits for the isolation, processing and cryopreservation of mesenchymal cells from the Wharton's Jelly and vascular progenitor cells from umbilical cord tissue. Also provided are isolated mesenchymal cells or vascular progenitor cells obtained by the invention methods, and compositions thereof.
Owner:C B B LIFELINE BIOTECH
Micronized wharton's jelly
The present invention provides compositions and formulations of micronized Wharton's jelly having a controlled viscosity such that when delivered to the injured region of a subject, it remains substantially localized with little or no migration out of the injured region for the repair and / or regeneration thereof. Micronized Wharton's Jelly can be suspended in a pharmaceutically acceptable aqueous carrier, such as saline, sterile water, or any suitable buffer, to form a suspension or a gelatinous gel composition, or it can be in the form of a paste, suitable for delivery into the space adjacent the articular surface cartilage injured region of a subject. The micronized Wharton's jelly when employed at sufficient concentrations can be hydrated into a gel or paste and administered topically, or it can be injected into the body through the use of a needle and syringe. Accordingly, micronized Wharton's Jelly, compositions, or formulations thereof, can be delivered in a manner that is more convenient than Wharton's jelly that has not been micronized in accordance with the present invention.
Owner:MIMEDX GROUP
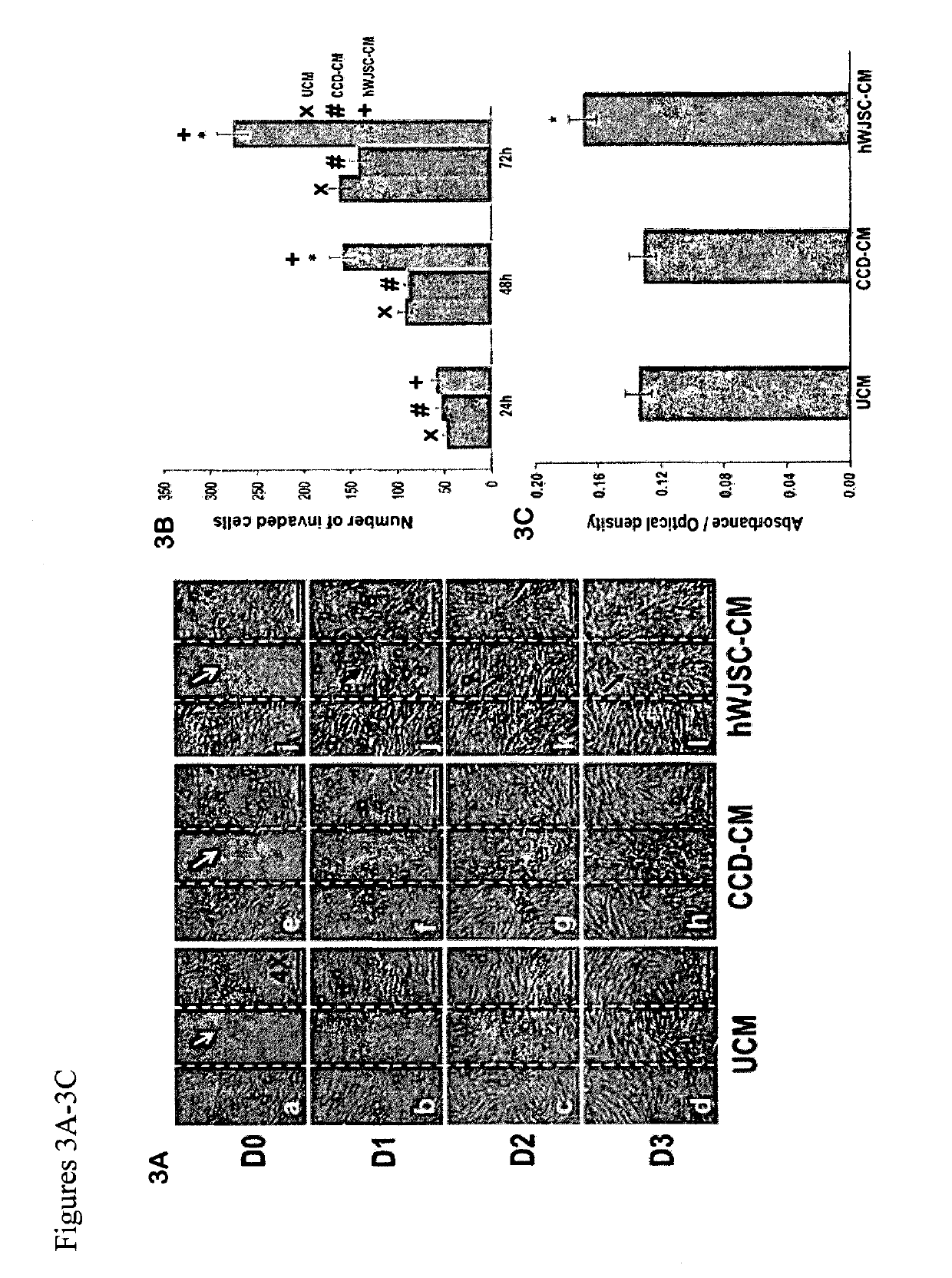
Wound dressing nanomesh impregnated with human umbilical cord Wharton's jelly stem cells
ActiveUS10413574B2Peptide/protein ingredientsPharmaceutical delivery mechanismWharton's jellyCell culture media
Owner:NAT UNIV OF SINGAPORE
Method for isolation and serum gradient switching culture of human umbilical cord mesenchymal stem cells
InactiveCN102533643AWide variety of sourcesReduce the cost of trainingSkeletal/connective tissue cellsSerum igeWharton's jelly
The invention discloses a method for isolation and serum gradient switching culture of human umbilical cord mesenchymal stem cells. The method comprises the following steps: digesting human umbilical cord with collagenase I until Wharton's jelly is fully digested, collecting digested single cells, inoculating the single cells into alpha-MEM medium, carrying out suspension culture until 80-90% of cells are fused, and digesting with trypsin; inoculating the suspension of trypsin-digested cells into alpha-MEM medium, carrying out suspension culture until 80-90% of cells are fused, digesting with trypsin, subcuturing and inoculating the subcultured cells into alpha-MEM medium, and carrying out subculture repeatedly. The concentration of fetal bovine serum in alpha-MEM medium in the steps of subculture decreases with the increase of the passage number until fetal bovine serum disappears. According to the method, the material human umbilical cords are easily accessible; the preparation of human umbilical cord mesenchymal stem cells is rapid and safe, and is not subject to ethical restrictions; and the subsequent culture is independent on the characteristics of fetal calf serum, resulting in greatly reduced culture cost and risk in clinical use. The method of the invention has a good application prospect.
Owner:肖扬
Viable cells from frozen umbilical cord tissue
Viable progenitor cells are extracted from frozen umbilical cord tissue. In embodiments, the umbilical cord tissue is a blood vessel bearing perivascular Wharton's jelly, and the extracted progenitor cells are HUCPVCs.
Owner:TISSUE REGENERATION THERAPEUTICS
Freezing storage protective solution for human umbilical Wharton's jelly tissue block
InactiveCN101971799AGuaranteed osmotic balanceLow toxicityDead animal preservationSucroseCell membrane
The invention discloses freezing storage protective solution for a human umbilical Wharton's jelly tissue block. 1 liter of the freezing storage protective solution comprises 0.8 to 1.2 mol of glycol, 0.4 to 0.6 mol of dimethyl sulfoxide, 0.1 to 0.3 mol of cane sugar, 100 to 200 milliliters of cord blood autoserum and the balance of culture solution DMEM / F12. In the freezing storage protective solution, the cord blood autoserum and the culture solution DMEM / F12 serve as base solution to provide nutrient for the tissue block, so osmotic equilibrium inside and outside cells is guaranteed, the cell environment in the tissue block is guaranteed to be similar to the in vivo environment, and the freezing storage effect is good; the low-concentration glycol and dimethyl sulfoxide serve as a permeability freezing protective agent, so the toxicity of the freezing storage protective solution to the cells is reduced; and the cane sugar serves as a non-permeability freezing protective agent, so the viscosity of the solution is increased and the osmotic pressure inside and outside cell membranes is relieved when the cells are frozen and stored. The human umbilical Wharton's jelly tissue block can be directly frozen and stored by the freezing storage protective solution, so the freezing storage effect is good, the cell damage is little, and high-quality human umbilical mesenchymal stem cells can be provided for clinic.
Owner:江苏省北科生物科技有限公司
Therapeutic preparations containing wharton's jelly
InactiveUS20110002883A1Improve skin appearanceIncrease elasticityOrganic active ingredientsCosmetic preparationsWharton's jellyBULK ACTIVE INGREDIENT
Therapeutic preparations for rejuvenation and anti-aging of human skin incorporates Wharton's Jelly or substantial fractions thereof as an active ingredient.
Owner:PETRIKOVSKY BORIS M +1
Method for separating and extracting hUC-MSC (human Umbilical Cord mesenchymal stem cells) from wharton jelly tissue of umbilical cord
InactiveCN105462919AReduce incubation timeImprove securityCell dissociation methodsCulture processSerum free mediaUmbilical cord tissue
The invention provides a method for rapidly separating and extracting hUC-MSC (human Umbilical Cord mesenchymal stem cells). The method comprises the following steps: taking the freshly collected umbilical cord tissue of a healthy newborn baby, carrying out on-ice transportation on the freshly collected umbilical cord tissue in umbilical cord storage transportation liquid containing double antibodies, carrying out cleaning and disinfection by adopting 75% alcohol and normal saline, removing blood vessels, carrying out blunt dissection on wharton jelly, carrying out mechanical pulverization, treating the obtained product I by adopting red blood cell lysis buffer for 3 min, digesting the obtained product II by adopting IV collagenase, screening the obtained product III by adopting a 100-200-mesh sieve, carrying out suspension culture on the obtained product IV by adopting a serum-free medium, wherein the liquid is changed every 3-5 days, taking supernatant, detecting cell pollution, after the adherent rate in a plate reaches 30-70%, carrying out trypsinization, carrying out centrifugation, collecting cells, carrying out passage amplification, carrying out merging when the cell merging rate reaches 90% or above, collecting the cells, carrying out cryopreservation on the cells, and detecting the biological characteristics of hUC-MSC.
Owner:郭镭 +1
Biomaterial based on Wharton's jelly from the human umbilical cord
The present invention relates to a biomaterial, specifically a hydrogel, formed from the extracellular matrix of the umbilical cord for its application in regenerative medicine. The invention particularly relates to a biomaterial made up of glycosaminoglycans isolated exclusively from the Wharton's jelly of the umbilical cord which can optionally contain cells, and also to the methods for the production and use thereof.
Owner:HISTOCELL SL
Micronized wharton's jelly
ActiveUS20160346332A1Reduce deliveryCell dissociation methodsPowder deliveryArticular surfacesWharton's jelly
The present invention provides compositions and formulations of micronized Wharton's jelly having a controlled viscosity such that when delivered to the injured region of a subject, it remains substantially localized with little or no migration out of the injured region for the repair and / or regeneration thereof. Micronized Wharton's Jelly can be suspended in a pharmaceutically acceptable aqueous carrier, such as saline, sterile water, or any suitable buffer, to form a suspension or a gelatinous gel composition, or it can be in the form of a paste, suitable for delivery into the space adjacent the articular surface cartilage injured region of a subject. The micronized Wharton's jelly when employed at sufficient concentrations can be hydrated into a gel or paste and administered topically, or it can be injected into the body through the use of a needle and syringe. Accordingly, micronized Wharton's Jelly, compositions, or formulations thereof, can be delivered in a manner that is more convenient than Wharton's jelly that has not been micronized in accordance with the present invention.
Owner:MIMEDX GROUP
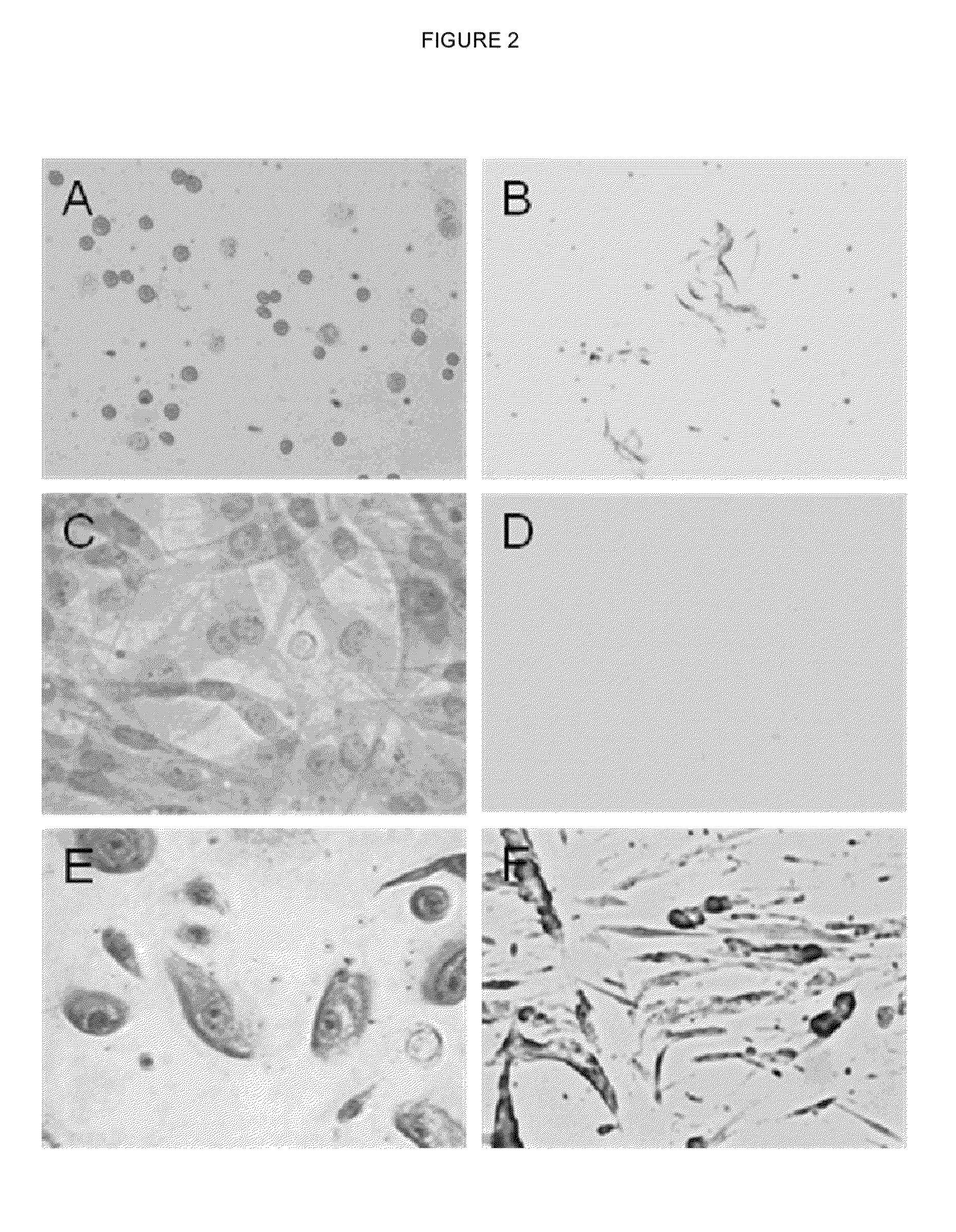
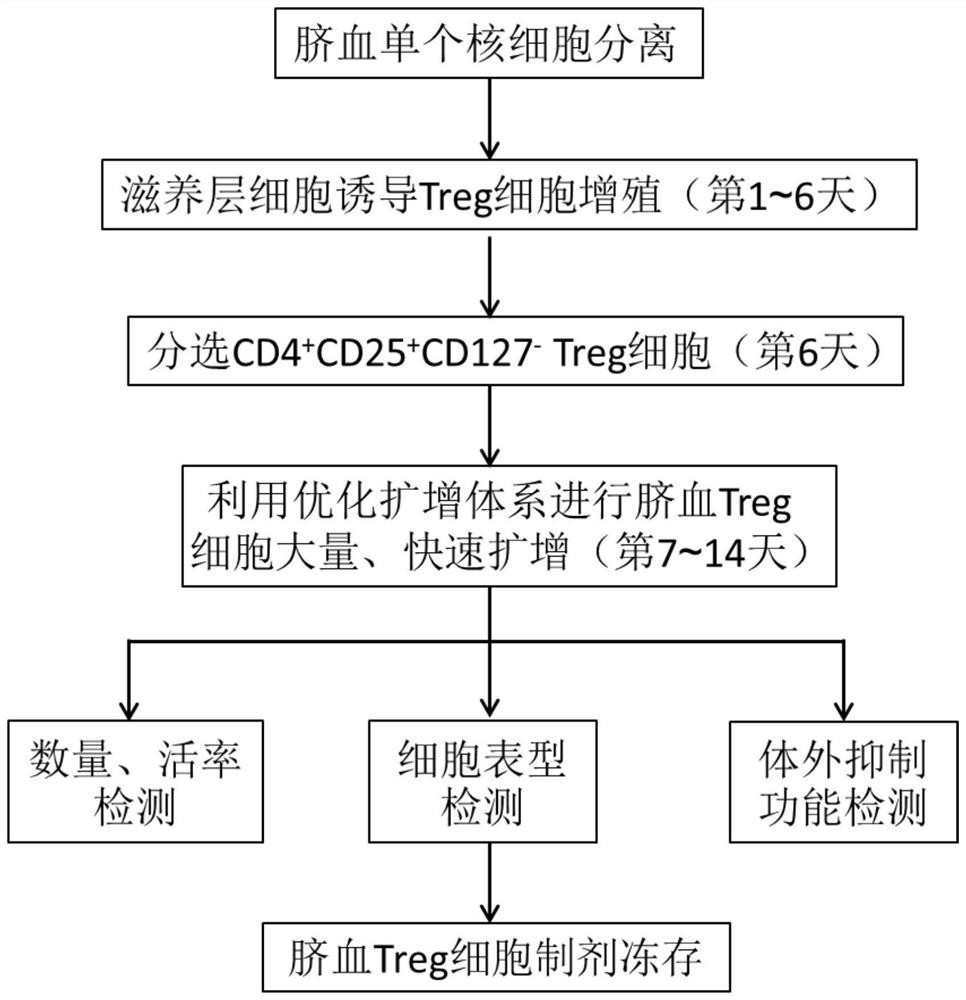
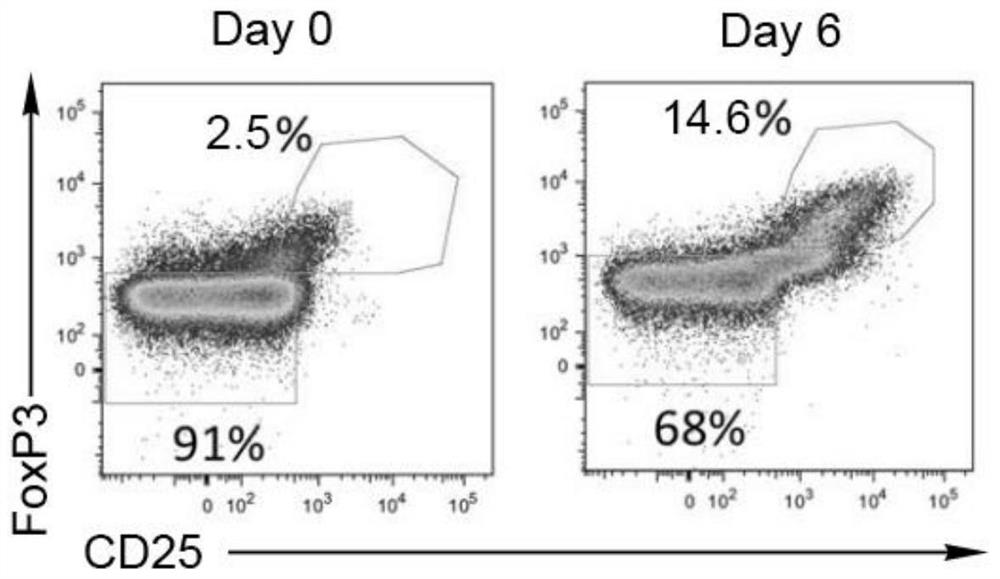
Umbilical cord blood Treg cell in-vitro amplification method based on trophoblastic cells and application
ActiveCN112458053APromote amplificationRaise the ratioAntipyreticDigestive systemAutoimmune diseaseTrophoblast
The invention discloses an umbilical cord blood Treg cell in-vitro amplification method based on trophoblastic cells and application. The specific technical method comprises the steps that firstly, umbilical cord Wharton's jelly mesenchymal stem cells are adopted as the trophoblastic cells to induce preliminary proliferation of Treg cells in umbilical cord blood mononuclear cells; then, pure Tregcells are obtained through magnetic bead sorting; and finally, the Treg cells are stimulated to be rapidly amplified by using optimized amplification factors. According to the amplification method, human AB plasma, IL-2, rapamycin, an RARA agonist and a DNA methyltransferase inhibitor are used as the optimized amplification factors, and a large number of umbilical cord blood Treg cells with high purity and high activity can be prepared within two weeks. Umbilical cord blood is used as a raw material for Treg cell amplification, batch preparation can be achieved, and Treg cell quality fluctuation caused by individual differences of samples can be reduced. The umbilical cord blood Treg cells have low immunogenicity and can be used as universal cells for clinical research, such as autoimmunediseases, graft-versus-host diseases and the like.
Owner:成都云测医学生物技术有限公司
Human umbilical cord mesenchymal stem cell culture method
InactiveCN107189982AReduce the risk of contaminationEasy to useCell dissociation methodsSkeletal/connective tissue cellsWharton's jellyUmbilical cord tissue
The invention discloses a human umbilical cord mesenchymal stem cell culture method, which comprises: (1) obtaining an umbilical cord tissue; (2) separating Wharton's Jelly; (3) cutting the Wharton's Jelly into tissue blocks, inoculating the tissue blocks into a T75 culture bottle, adding an appropriate amount of a primary culture medium, placing into a CO2 incubator, carrying out standing culture for 3 days, supplementing 5 ml of the primary culture medium at the 4th day, changing the culture medium every 2-3 days from the 4th day, removing the tissue block when cells start to migrate from the majority of the tissue blocks, changing the culture medium, and carrying out passage amplification when the cell density achieves 80% and above; and (4) after the treatment, re-suspending with a passage culture medium. According to the present invention, the animal-derived additive is not used during the culture process, such that the safety is provided; and the operation is simple and convenient, and the culture time is short.
Owner:北京焕生汇生物技术研究院有限公司
Method for preparing and preserving umbilical arterial and vein vascular peripheral stem cells
ActiveCN105695401AHigh purityReduce harmDead animal preservationSkeletal/connective tissue cellsZymogenLow glucose
The invention discloses a method for preparing and preserving umbilical arterial and vein vascular peripheral stem cells. The umbilical arterial and vein vascular peripheral stem cells are cultured by a cell culture fluid (DMEM low glucose, 10% of fetal calf serum and 1% of penicillin-streptomycin double anti-body). The operation is carried out under strict aseptic conditions; and after umbilical cord collection, the inconvenience caused by blood coagulation is effectively avoided by use of an anticoagulant. Compared with a conventional coating method, the method disclosed by the invention has the advantages that umbilical perivascular sourced mesenchymal stem cells with higher purity are obtained; allergic reaction and cross infection caused by animal-based protein can be avoided since digestive enzymes are not adopted; and the umbilical perivascular stem cells separated by a non-zymogen digestion method have higher positive rate than that of the Wharton's jelly sourced mesenchymal stem cells.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
Native wharton's jelly stem cells and their purification
Owner:AUXOCELL LAB
Biomaterial from wharton's jelly umbilical cord
ActiveUS20130095143A1Wide versatilityElasticity resistanceCosmetic preparationsBiocideWharton's jellyCell-Extracellular Matrix
The present invention relates to a biomaterial, specifically a hydrogel, based on the extracellular matrix of the umbilical cord for its application in regenerative medicine. The invention particularly relates to a biomaterial made up of glycosaminoglycans present exclusively in the Wharton's jelly of the umbilical cord (which can optionally be combined with cells as a combination therapy), and also to the methods for the production and use thereof.
Owner:HISTOCELL
Intrathecal administration of mesenchymal stem cells and derivatives thereof for treatment of pain
InactiveUS20180036348A1Pharmaceutical delivery mechanismUnknown materialsWharton's jellyIntrathecal use
The invention discloses means of treating pain through administration of mesenchymal stem cells or derivatives thereof via an intrathecal manner at a concentration and frequency sufficient to reduce pain. In one embodiment of the invention, treatment of discogenic pain is provided by administration of mesenchymal stem cells intrathecally that are derived from the Wharton's Jelly. In another embodiment the invention teaches administration of supernatants from mesenchymal stem cells, in one embodiment said supernatants comprising of concentrated microvesicles.
Owner:AIDAN RES & CONSULTING
Preparation method for improving yield of umbilical cord derived mesenchymal stem cell primary cells
ActiveCN108220229AImprove utilizationIncrease the number ofCell dissociation methodsSkeletal/connective tissue cellsWharton's jellyPrimary cell
The invention relates to the technical field of cell culture and cell separation and in particular discloses a preparation method for improving the yield of umbilical cord derived mesenchymal stem cell primary cells. On the basis of culturing and separating mesenchymal stem cells on a traditional Wharton's jelly tissue block in a disposable adherence manner, Wharton's jelly tissue blocks are finely collected at different time of a later period of culture and are repeatedly adhered; P0 generation mesenchymal stem cells can be continuously collected at different time. According to the method provided by the invention, the utilization rate of Wharton's jelly is maximized through a property that the mesenchymal stem cells continuously move out from Wharton's jelly tissues to grow; compared with Wharton's jelly tissue block disposable adherence culture, the obtained quantity of the P0 generation mesenchymal stem cells can be increased by two times at least; the culture density of the mesenchymal stem cells, the concentration of serum replacements in a culture medium and the culture time are subsequently controlled; when the cells are sub-cultured to a P3 generation, the obtained cell quantity can completely meet the requirements of cell storage and clinical transplantation.
Owner:北京中科易微生物科技有限公司
Method for acquiring bioactive proteins by utilizing umbilical cord mesenchymal stem cells
InactiveCN104651305AFast growthImprove stabilitySkeletal/connective tissue cellsFermentationCell-Extracellular MatrixCulture fluid
The invention discloses a method for acquiring bioactive proteins by utilizing umbilical cord mesenchymal stem cells. The method comprises the following steps: digesting Wharton's jelly of umbilical cord in short time by using type I collagenase, thereby obtaining a paste mixture containing extracellular matrix proteins and tissue blocks; performing primary culture; adding a stem cell growth culture medium into a packed bed type bioreactor for amplifying the umbilical cord mesenchymal stem cells; adding a protein production culture medium for performing continuous culture when the cell fusion rate in the reactor reaches the standard; collecting the culture solution, and treating the solution, thereby obtaining the bioactive proteins. According to the method for acquiring the bioactive proteins by utilizing umbilical cord mesenchymal stem cells disclosed by the invention, the purity of the obtained umbilical cord mesenchymal stem cells is high, the multiplication capacity is high, and the stem cell culture time is shortened; and moreover, the yield of the obtained bioactive proteins is high, the quality control process is convenient, and large-scale production is easily realized. The stem cells and bioactive proteins do not contain animal source components, and the safety of clinical application is improved.
Owner:SHENYANG BESTSTEM BIOTECH
Method for constructing cell transplantation slice by using human umbilical cord mesenchymal stem cells (hUC-MSCs) and amniotic membrane
InactiveCN105647859AInhibition formationWide variety of sourcesArtificial cell constructsSkeletal/connective tissue cellsHuc mscsWharton's jelly
The invention relates to a method for constructing a cell transplantation slice by using human umbilical cord mesenchymal stem cells (hUC-MSCs) and anthropogenic amniotic membrane. The method comprises the following steps: taking the freshly collected anthropogenic amniotic membrane and umbilical cord, separating chorion from the amniotic membrane, treating the separated amniotic membrane by normal saline, and then cryopreserving for later use; cutting the umbilical cord into small pieces, and tearing Wharton's jelly off; cutting the Wharton's jelly into pieces, putting the cut Wharton's jelly onto a sterile culture vessel, adding a culture solution into the sterile culture vessel, and putting the sterile culture vessel into an incubator for culturing to obtain primary generation hUC-MSCs; adding pancreatin into a culture medium of the primary generation hUC-MSCs, digesting, and continuously culturing in the incubator to obtain the hUC-MSCs; carrying out sterile hydration on the cryopreserved standby amniotic membrane, then flatly laying the amniotic membrane on an amniotic membrane carrier thimble, and then moving into the sterile culture vessel; inoculating the hUC-MSCs in the culture vessel, and then culturing in the incubator to obtain the cell transplantation slice with cell confluence reaching up to 80-90%. The cell transplantation slice prepared by the method is capable of carrying out damage repair at specific parts, so that the defects of using the hUC-MSCs or amniotic membrane alone for treatment are overcome; therefore, a new treatment strategy is provided for refractory diseases, and a new therapy approach is opened up for the clinical application of stem cells.
Owner:秦方园 +1
Method for efficiently separating umbilical cord mesenchymal stem cells
InactiveCN104862274AAvoid pollutionReduce pollutionSkeletal/connective tissue cellsParenchymaMesenchymal stem cell
The invention provides a method for efficiently separating umbilical cord mesenchymal stem cells. Wharton jelly is directly separated from umbilical cord, so that parenchyma cell contamination is avoided, multi-time passage is not required, and the cell purity and activity are ensured; contamination in an umbilical cord separation process can be effectively alleviated, and the separation cost can be reduced. The umbilical cord does not need to be mechanically crushed in the separation process, so that the damage of mechanical shear force to the cells is reduced and the viability of the obtained cells is higher; heterologous digestive enzymes and heterologous serum are not adopted in the separation process, so that the obtained cells do not contain heterologous proteins and are also safer, the needs of clinical use can be met, and a technical guarantee is provided for establishing an umbilical cord mesenchymal stem cell library; a tissue block method is adopted, is more convenient and quick and small in cell damage, and can protect the mesenchymal stem cells from being not damaged and enable the mesenchymal stem cells to have higher viability.
Owner:GUIZHOU BEIKE FACTORR BIOTECH CO LTD
Method using umbilical cord tissue cryopreservation to preserve various stem cells, thawing method and application
InactiveCN109122665AIncrease profitFrozen storage achievedDead animal preservationSkeletal/connective tissue cellsFresh TissueUmbilical cord tissue
The invention relates to a method using umbilical cord tissue cryopreservation to preserve various stem cells, a thawing method and application. The method specifically includes: cleaning umbilical cord tissue, shearing into tissue sections, and stripping mesenchyme Wharton's jelly tissue; using an alpha-MEM protecting agent containing DMSO for preprocessing, performing concentration balance under4-10 DEG C for 10-20 minutes, performing low-temperature centrifuging, and removing supernate; adding dextran or fetal calf serum to allow the dextran or fetal calf serum and the alpha-MEM protectingagent containing DMSO to jointly form cryopreservation liquid, and using a program-controlled temperature lower or graded temperature lowering manner to perform cryopreservation on the tissue. The cryopreservation method has the advantages that the cryopreserved umbilical cord tissue can be effectively protected, subsequent thawing operation is facilitated, and the activity of cells cultured by the thawed tissue is the same as that of cells in fresh tissue.
Owner:YINFENG BIOLOGICAL GRP
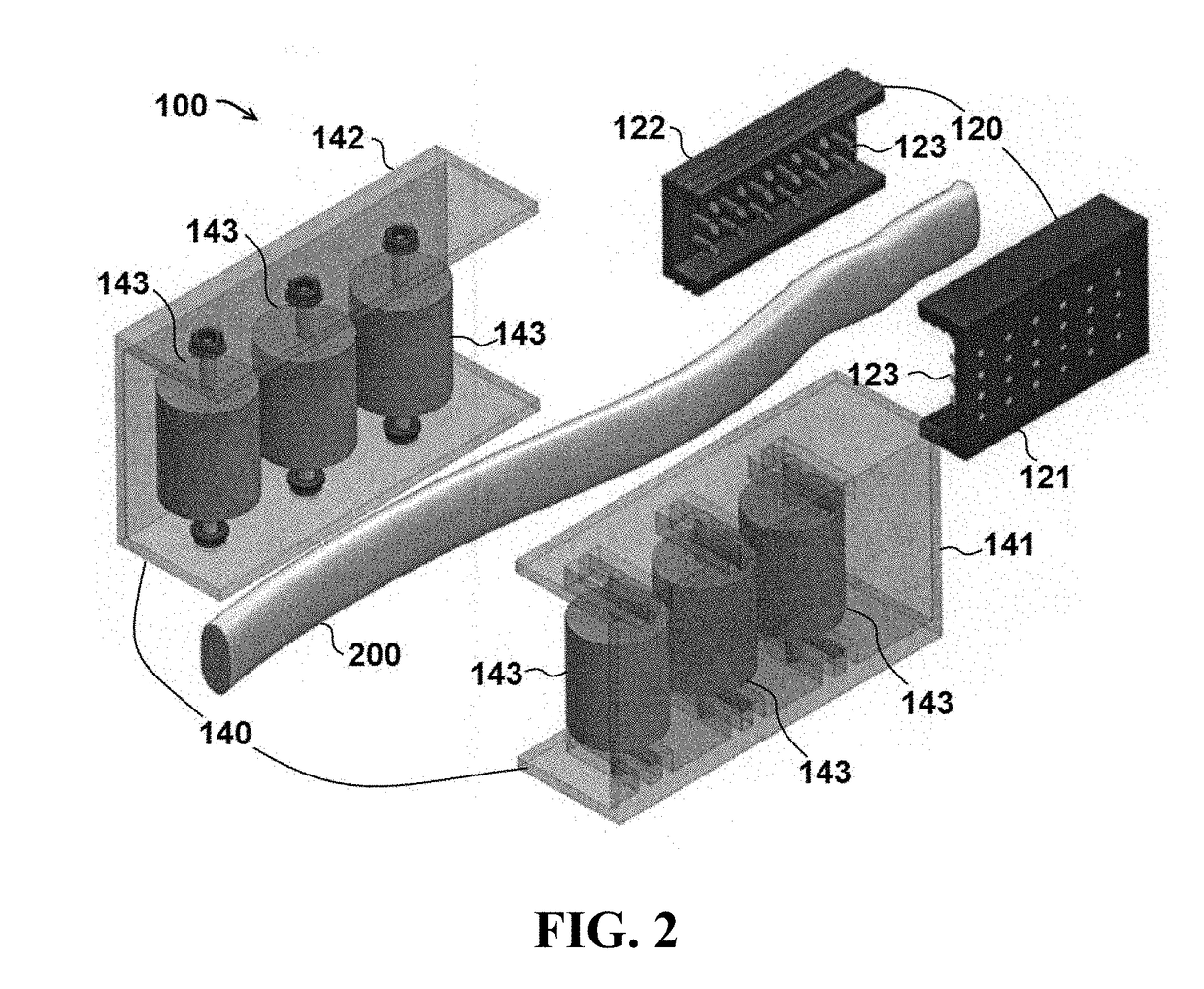
Devices and methods for umbilical cord processing
Devices and methods for extraction and processing of substantia gelatinea funiculi umbilicalis (Wharton's Jelly) from an umbilical cord. Isolated pluripotent cell compositions and methods of using the same are also provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Cryopreserved protection liquid of human umbilical cord watton's gum tissues, and preparation and application thereof
InactiveCN108184819AHigh activityImprove stress resistanceDead animal preservationAdditive ingredientPermeation
The invention provides cryopreserved protection liquid of human umbilical cord watton's gum tissues, and preparation and application thereof. The electrolyte concentration inside and outside cells arerespectively balanced through a permeating protection agent and a non-permeating protection agent; meanwhile, the free water content is reduced; the formation of ice crystals is reduced; on one hand,the cell damage caused by cell excessive dehydration due to external high-permeation environment can be avoided; on the other hand, the physical damage of the ice crystals on the cells can be reduced; a protein composition is added, so that the required nutrients can be provided for the cells; the cell survival rate is improved; lyophilic colloids can form thixotrope in the water, so that the distribution of all ingredients in the permeating protection agent is uniform; the cells can be uniformly dispersed; precipitates cannot easily occur; in addition, the taking, the use and the storage areeasy. Through the combined use of the ingredients, the activity and the stress resistance of the watton's gum tissues can be effectively improved; the survival rate of the cells during cryopreservation and recovery can be improved; the cell activity loss due to cryopreservation is reduced.
Owner:北京臻溪谷医学研究中心(有限合伙)
Native wharton's jelly stem cells and their purification
Owner:TAGHIZADEH ROUZBEH R
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com