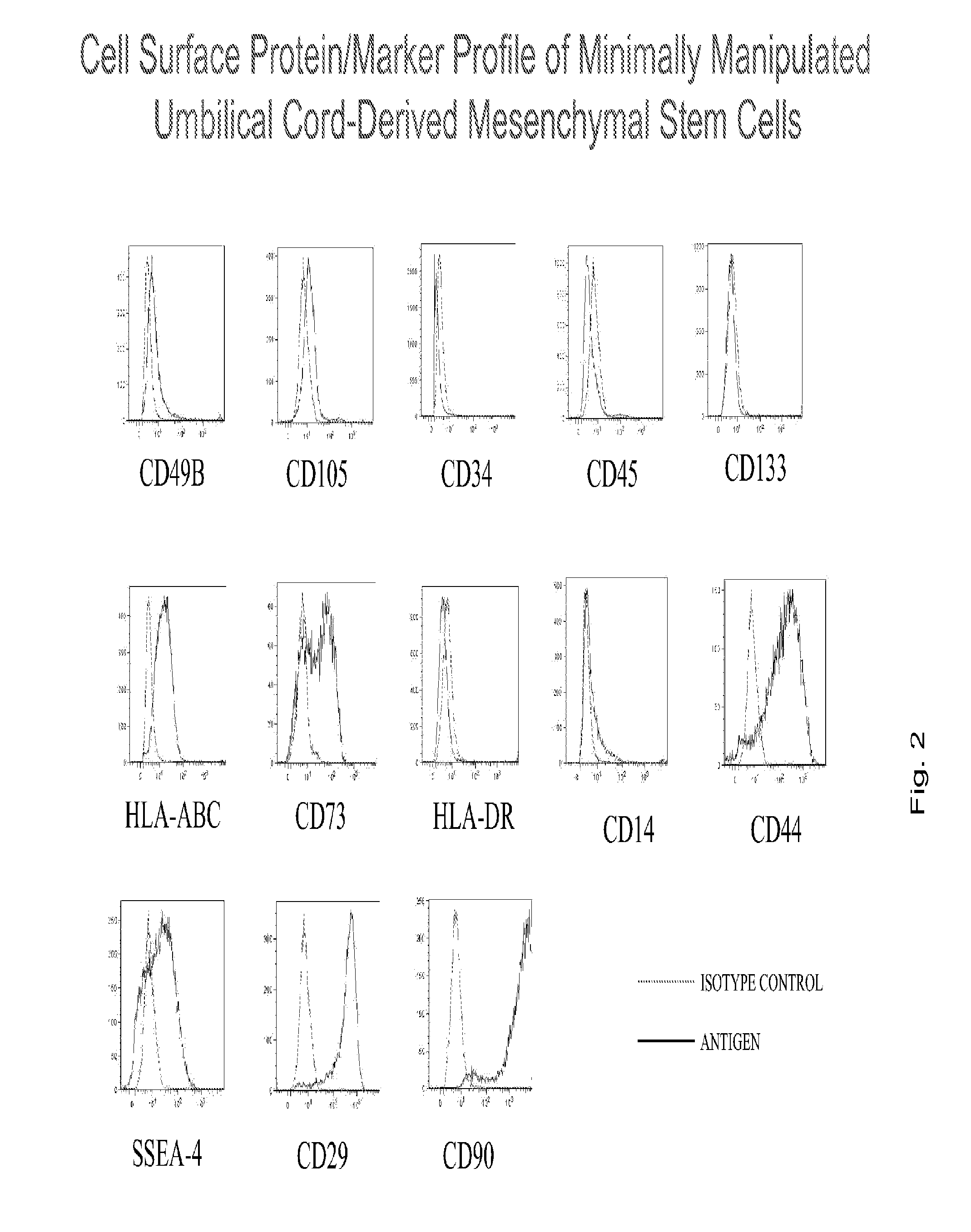
Native wharton's jelly stem cells and their purification
a technology of stem cells and whartons, applied in the field of native whartons jelly stem cells and their purification, can solve the problems of undigested tissue fragments left behind by incomplete digestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Purification of Native Wharton's Jelly Stem Cells and Homogeneous Wharton's Jelly Solution
[0026]Umbilical cords were collected in sterile specimen containers within 48 hours of the time of delivery. In a bio safety cabinet, 10 mL of Buffer B (50 μg / mL gentamicin, 100 units / mL penicillin and 100 μg / mL streptomycin in sterile Dulbecco's phosphate buffered saline) were added to the umbilical cord in an umbilical cord collection chamber. Other antibiotics, such as 0.25 μg / mL amphotericin B, 100 μg / mL streptomycin and / or 10 μg / mL ciprofloxacin, can also be added to Buffer B or substituted for any of the antibiotics in Buffer B. The contents of the collection chamber were then mixed by swirling and maintained at room temperature for fewer than 72 hours. The contents were swirled again for approximately 10-15 seconds to clean the umbilical cord tissue. Coagulated blood, if evident on the surface of the umbilical cord, was carefully removed using dissection tools.
[0027]The umbilical cord wa...
example 2
Storage of Noncultured Wharton's Jelly Stem Cells
[0039]The purified cell suspension of Example 1 was cryopreserved in a 25 mL freezing bag. Using a 60 mL syringe with an 18 G needle, 16 mL of autologous plasma, 5% human serum albumin, or a combination thereof were added to the 4 mL purified Wharton's Jelly stem cell suspension. An alcohol pad was used to wipe the top of a vial of 55% DMSO / 5% Dextran. Next, 5 mL of the DMSO / Dextran mixture were removed using a 60 mL syringe with an 18 G needle and slowly added to the cell suspension. The cell suspension tube was capped tightly and gently inverted to mix, taking care not to make foam or bubbles. Using the same 60 mL syringe, 25 mL of the cell suspension were transferred to the freezing bag. The freezing bag was stored in a metal canister in a Styrofoam holder at −80° C. for 16 to 24 hours, optionally followed by an intervening period in a liquid nitrogen freezer in which the cells were exposed only to the vapor phase of the liquid nit...
example 3
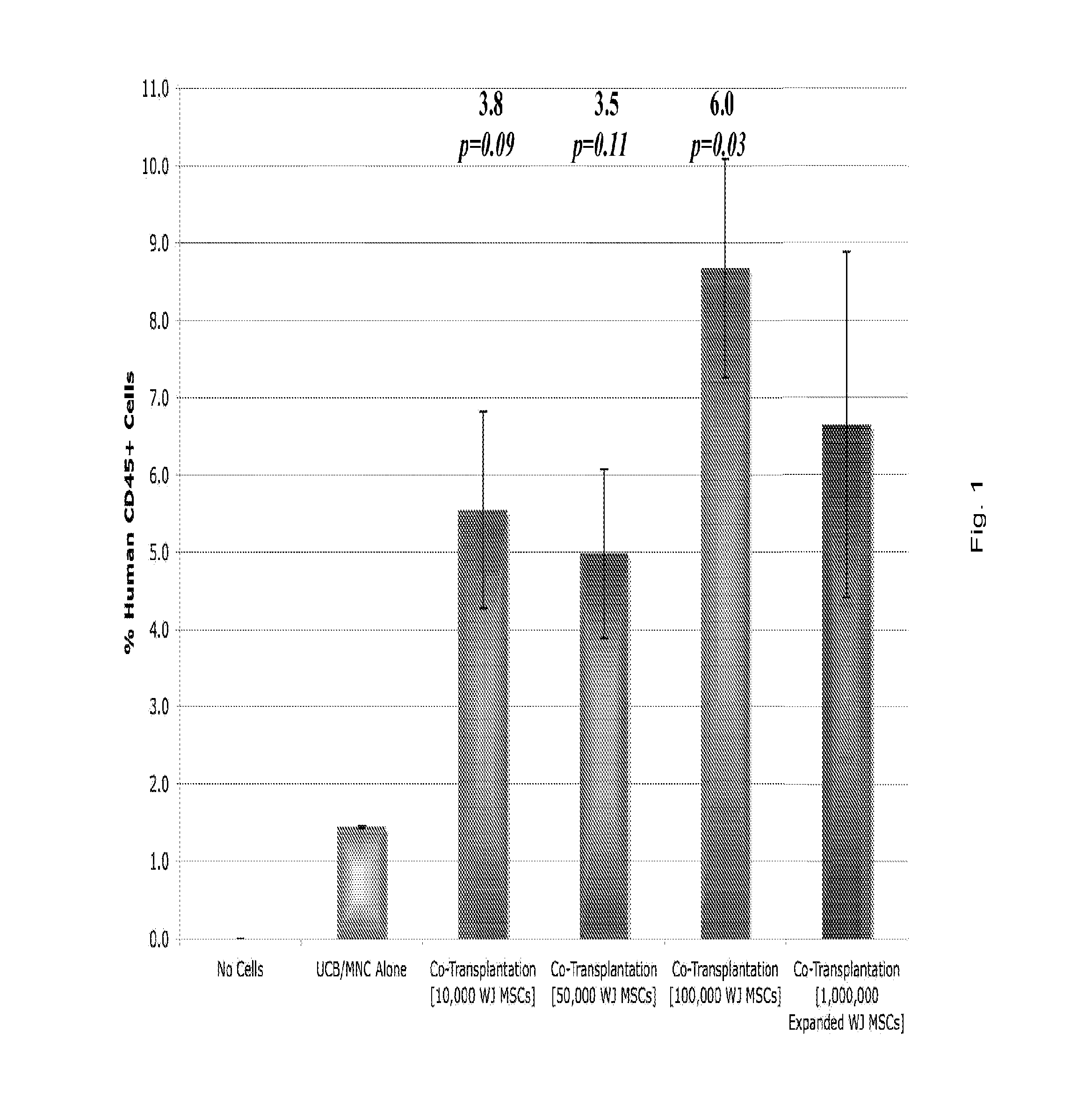
[0040]The therapeutic efficacy of noncultured Wharton's Jelly stem cells was demonstrated in a co-transplantation assay with hematopoietic stem cells from umbilical cord blood to renew a mammalian hematopoietic system.
[0041]Hematopoietic stem cells from umbilical cord blood can be administered to a mammal to reconstitute a hematopoietic system damaged, for example, by radiation. Co-transplantation of Wharton's Jelly stem cells improves the reconstitution process, enhancing the engraftment of the administered hematopoietic stem cells and, therefore, their ability to proliferate and recreate a hematopoietic system in their new host.
[0042]To test the efficacy of noncultured Wharton's Jelly stem cells, they were co-administered with hematopoietic stem cells from umbilical cord blood to (NOD / SCID IL2Rγ-null) mice that had been sublethally irradiated the day before with 300 cGy of gamma-radiation which ablated the bone marrow. 1,000,000 mononuclear umbilical cord blood cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com