Fluorine benzene ruthenium compound and preparation method and application thereof
A fluorobenzene ruthenium compound and a ruthenium compound technology are applied in the field of ruthenium compound preparation, and achieve the effects of easy availability of raw materials, simple preparation method and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
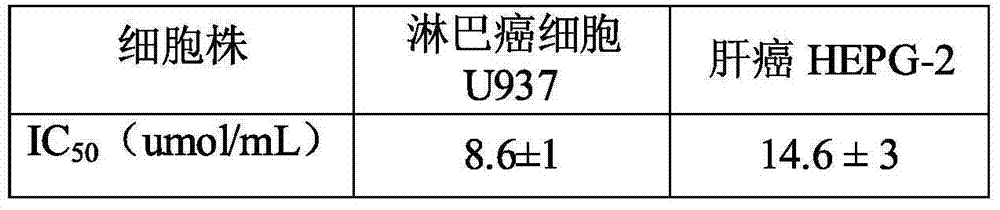
Image
Examples
Embodiment 1
[0026] A fluorobenzeneruthenium compound, the fluorobenzeneruthenium compound has the structure of (I):
[0027]
[0028] A preparation method of a fluorobenzene ruthenium compound, comprising the following steps:
[0029] Step 1. Dissolve 1 g of hydrated ruthenium trichloride and 30 mL of γ-terpinene in 70 mL of dehydrated ethanol, heat under reflux for 6 hours, and stand to separate out to obtain dichloro-di-methylisopropyl dichloride. diruthenium benzene;
[0030] Step 2: Weigh 8g of difluorobenzenesalicylic acid and dissolve it in 40mL of solvent, add 4g of N,N-dimethylformamide as a catalyst, then add substituted phenylacetyl chloride, react at 65°C for 8 hours, remove After the solvent, phenylacetyl fluorobenzene salicylic acid is obtained;
[0031] Step 3. Dissolve 1g of dichloro-di-methylisopropylbenzenediruthenium dichloride and 3g of phenylacetylfluorobenzenesalicylic acid in 80mL of tetrahydrofuran, react at 65°C for 6 hours, and purify, That's it.
[0032] W...
Embodiment 2
[0037] A preparation method of a fluorobenzene ruthenium compound, comprising the following steps:.
[0038] Step 1. Dissolve 6g of hydrated ruthenium trichloride and 80mL of γ-terpinene in 200mL of dehydrated ethanol, heat under reflux for 6 hours, and stand to separate out to obtain dichloro-di-methylisopropyl dichloride. diruthenium benzene.
[0039] Step 2: Weigh 12g of difluorobenzenesalicylic acid and dissolve it in 80mL of solvent, add 8g of N,N-dimethylformamide as a catalyst, then add substituted phenylacetyl chloride, react at 75°C for 12 hours, remove After solvent, phenylacetylfluorobenzenesalicylic acid is obtained.
[0040] Step 3. Dissolve 3g of dichloro-di-methylisopropylbenzene diruthenium dichloride and 9g of phenylacetylfluorobenzenesalicylic acid in 100mL of tetrahydrofuran, react at 75°C for 10 hours, and purify, That's it.
[0041] Wherein, the hydrated ruthenium trichloride in the first step is hydrated ruthenium trichloride with a ruthenium weight co...
Embodiment 3
[0046] A preparation method of a fluorobenzene ruthenium compound, comprising the following steps:.
[0047] Step 1. Dissolve 3g of hydrated ruthenium trichloride and 60mL of γ-terpinene in 120mL of absolute ethanol, heat under reflux for 6 hours, and stand to separate out to obtain dichloro-di-methylisopropyl dichloride. diruthenium benzene;
[0048] Step 2: Weigh 10g of difluorobenzenesalicylic acid and dissolve it in 60mL of solvent, add 6g of N,N-dimethylformamide as a catalyst, then add substituted phenylacetyl chloride, react at 70°C for 10 hours, remove After the solvent, phenylacetyl fluorobenzene salicylic acid is obtained;
[0049] Step 3. Dissolve 2g of dichloro-di-methylisopropylbenzenediruthenium dichloride and 6g of phenylacetylfluorobenzenesalicylic acid in 90mL of tetrahydrofuran, react at 70°C for 8 hours, and purify, That's it.
[0050] Wherein, the hydrated ruthenium trichloride in the first step is hydrated ruthenium trichloride with a ruthenium weight con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com