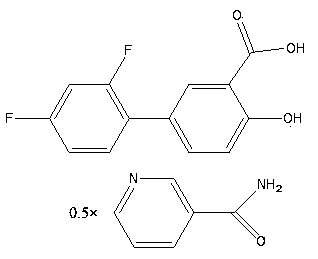
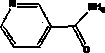
Eutectic of diflunisal and pyridine formamide compounds and preparation method thereof
A technology of pyridinecarboxamide and diflunisal, which is applied in the field of chemical pharmaceuticals, can solve problems in the initial stage and achieve good solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0052] Preparation of co-crystals with diflunisal and nicotinamide: the reactant diflunisal and nicotinamide are fed according to the material ratio of 1:1. Accurately weigh 131.0 mg of diflunisal and 63.9 mg of nicotinamide and dissolve them in 2.6 ml and 1.1 ml of ethanol, respectively, and ultrasonicate for 10 min to fully dissolve the two, and then slowly add the ethanol solution of nicotinamide to the two In the ethanol solution of flunisal, mix thoroughly, let it stand for one day, filter, rinse with a small amount of ethanol, and then place the filtered product in a vacuum drying oven at 40°C for one day to dry.
[0053] Figures 1 to 5 They are respectively the X-ray diffraction spectrum and solid-state nuclear magnetic resonance CP-MAS of diflunisal / nicotinamide co-crystal and its bulk drug in Example 1. 13 C spectrum, infrared spectrum and thermogram.
[0054] Depend on Figure 1 to Figure 5 It can be seen that the melting point of diflunisal and nicotinamide co-c...
example 2
[0056] Preparation of co-crystal with diflunisal and nicotinamide: the reactant diflunisal and nicotinamide are fed according to the material ratio of 2:1. Accurately weigh 131.0 mg of diflunisal and 32 mg of nicotinamide and dissolve them in 2.6 ml and 1.1 ml of ethanol, respectively, and ultrasonicate for 10 min. In the ethanol solution of flunisal, mix thoroughly, let it stand for one day, filter, rinse with a small amount of ethanol, and then place the filtered product in a vacuum drying oven at 40°C for one day to dry.
example 3
[0058] Preparation of co-crystal with diflunisal and isonicotine: the reactant diflunisal and isonicotine are fed according to the material ratio of 1:1. Accurately weigh 196.5 mg of diflunisal and 95.8 mg of isonicotine and dissolve them in 2.6 ml and 1.1 ml of ethanol respectively, and ultrasonicate for 10 min to fully dissolve the two, then slowly add the ethanol solution of isonicotine dropwise Put it into the ethanol solution of diflunisal, mix well, let stand for one day, filter, wash with a small amount of ethanol, then place the filtered product in a vacuum drying oven at 40°C, and dry for one day.
[0059] Figures 6 to 10 They are the X-ray diffraction spectrum and the solid-state nuclear magnetic resonance CP-MAS of diflunisal / isonicotine co-crystal and its bulk drug in Example 3 respectively. 13 C spectrum, infrared spectrum, thermogram and dissolution curve in water.
[0060] Depend on Figure 6 to Figure 10 It can be seen that the melting point of diflunisal / i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com