Diflunisal solid dispersion and preparation method thereof
A technology of solid dispersion and diflunisal, which is applied in the field of medicine, can solve the problems of thermal decomposition of drugs and carriers and limit their wide application, and achieve the effects of increasing solubility, reducing dosage, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In this embodiment, a solid dispersion of diflunisal is prepared from diflunisal and Soluplus as active ingredients, and Soluplus accounts for 80% of the mass percentage of the solid dispersion of diflunisal.
[0044] Take 2g of diflunisal and 8g of Soluplus and mix well. Set the extrusion temperature of the twin-screw hot-melt extruder to 160°C, start the screw after reaching the preset temperature, and the screw speed is 150 revolutions / min. The resulting physical mixture is added to the extruder, and the mixture passes through the screw into a strip Extrusion; the hot-melt extruded strips are crushed and passed through an 80-mesh sieve to obtain diflunisal solid dispersion powder.
[0045] The powder X-ray diffraction of the solid dispersion prepared in this example ( figure 1 ) Shows that the drug is dispersed in the carrier material in an amorphous or molecular state. The results of high performance liquid chromatography showed that the drug content and related substan...
Embodiment 2
[0047] In this example, a solid dispersion of diflunisal was prepared from diflunisal and copovidone VA64 as the active ingredients. The content of copovidone VA64 in the mass percentage of the solid dispersion of diflunisal was 70%. %.
[0048] Take 3g of diflunisal and 7g of copovidone VA64 (PVPVA64) and mix well. Set the extrusion temperature of the twin-screw hot melt extruder to 100°C, start the screw after reaching the preset temperature, and the screw speed is 50 revolutions / min. The physical mixture made is added to the extruder, and the mixture passes through the screw and becomes a strip. Extrusion; the hot-melt extruded strips are crushed and passed through an 80-mesh sieve to obtain diflunisal solid dispersion powder.
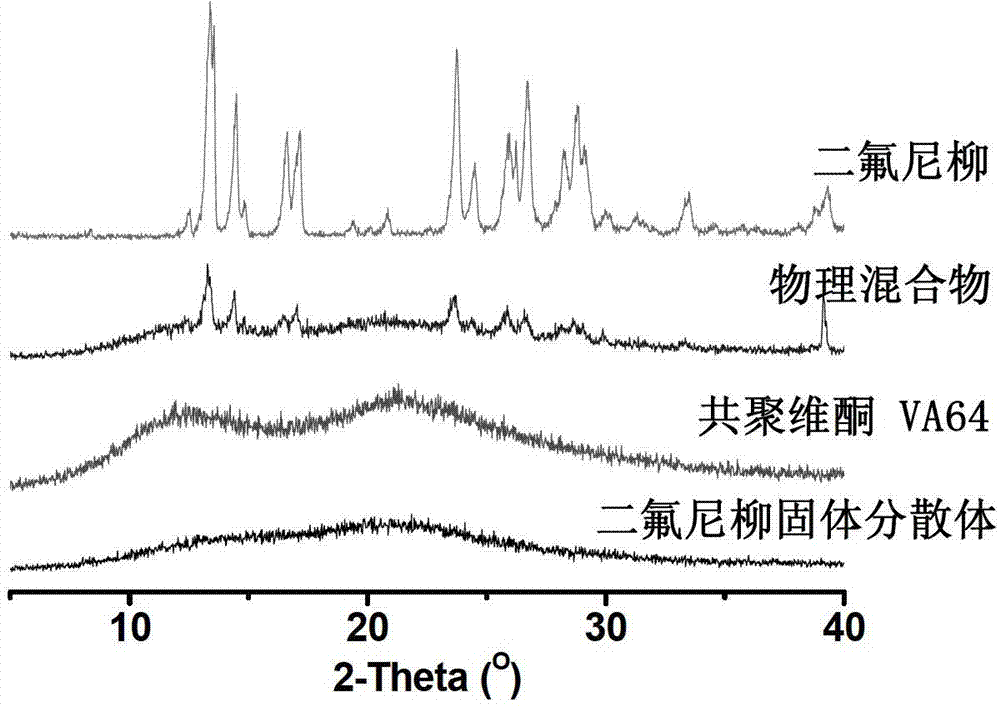
[0049] This example obtained powder X-ray diffraction ( image 3 ) Shows that the drug is dispersed in the carrier material in an amorphous or molecular state. The results of high performance liquid chromatography showed that the drug content and relat...
Embodiment 3
[0051] In this example, a solid dispersion of diflunisal was prepared from diflunisal and copovidone VA64 as the active ingredients. The content of copovidone VA64 in the mass percentage of the solid dispersion of diflunisal was 70%. %.
[0052] Take 3g of diflunisal and 7g of copovidone VA64 (PVPVA64) and mix well. Set the extrusion temperature of the twin-screw hot-melt extruder to 140°C. After reaching the preset temperature, start the screw, and the screw speed is 250 rpm. The resulting physical mixture is added to the extruder. Shape extrusion; the hot-melt extruded strips are crushed and passed through an 80-mesh sieve to obtain a solid dispersion powder of medicine.
[0053] The solid dispersion powder X-ray diffraction ( Figure 5 ) Shows that the drug is dispersed in the carrier material in an amorphous or molecular state. The results of high performance liquid chromatography showed that the drug content and related substances did not change significantly, in line with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com