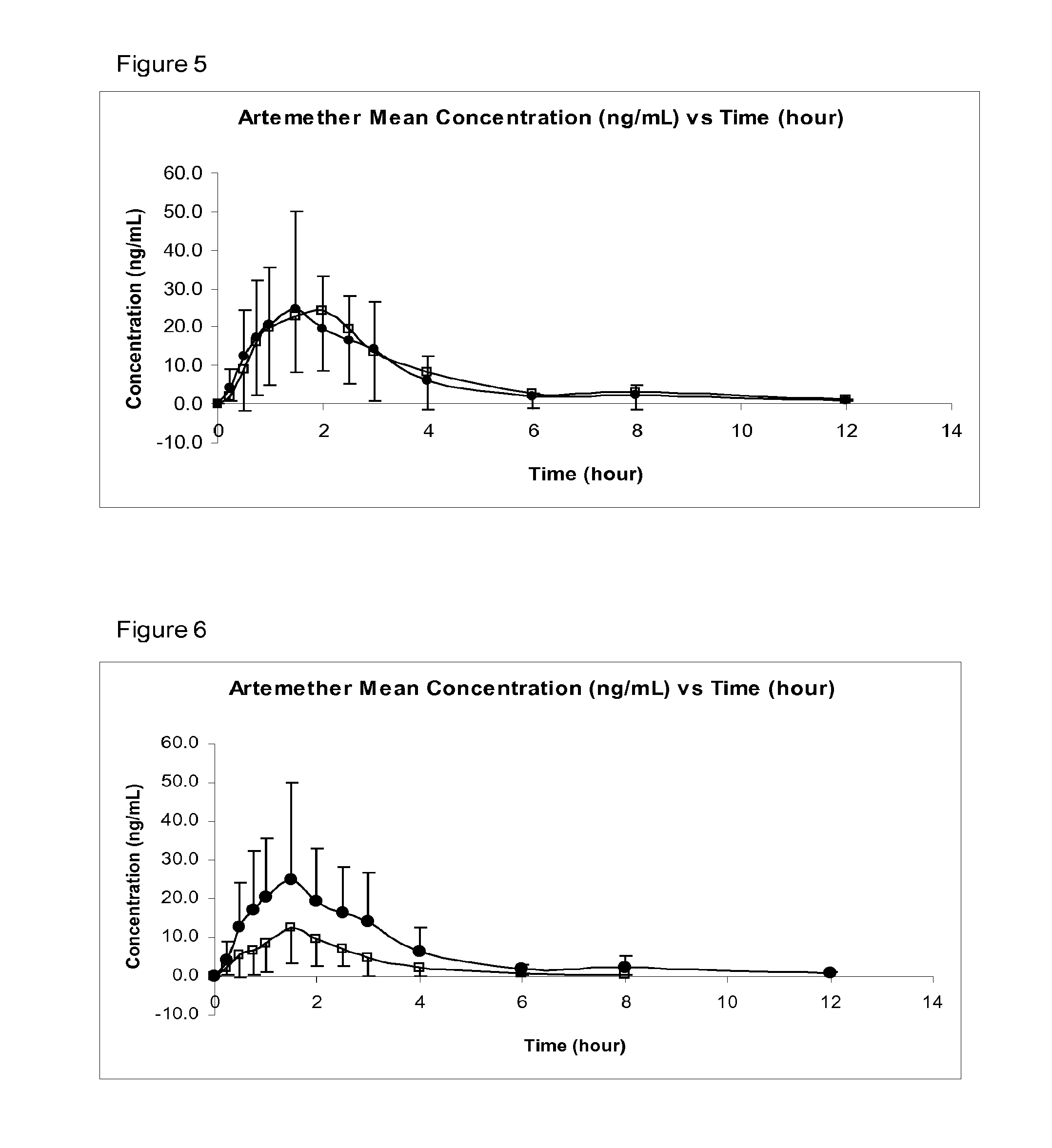
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Sublingual spray" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Administration of dihydroergotamine as a sublingual spray or aerosol for the treatment of migraine
The present invention is an improvement in the treatment of migraine headaches. By administering dihydroergotamine as a sublingual spray or aerosol, major limitations of past treatments are circumvented thereby allowing for higher efficacy and fewer side effects of treatment at lower doses.
Owner:R T ALAMO VENTURE I
Administration of dihydroergotamine as a sublingual spray or aerosol for the treatment of migraine
InactiveUS20030017994A1Easy and inexpensive to manufactureQuick releaseBiocidePowder deliveryDihydroergotamineSide effect
The present invention is an improvement in the treatment of migraine headaches. By administering dihydroergotamine as a sublingual spray or aerosol, major limitations of past treatments are circumvented thereby allowing for higher efficacy and fewer side effects of treatment at lower doses.
Owner:R T ALAMO VENTURE I
Naloxone hydrochloride sublingual spraying drug delivery system or composition and its preparation method
InactiveCN101057830APromote absorptionImprove bioavailabilityOrganic active ingredientsNervous disorderMouth mucosaPreservative
The invention discloses a chlorhydric acid naloxone hypoglossal spraying drug delivery system or compound and the preparing method, belonging to pharmacology field. It is characterized in that: it is hypoglossal spraying agent, and the comprised components and their weight proportion are as follows: (1) chlorhydric acid naloxone or naloxone free alkali or naloxone medical salt: 0. 1-30%, (2) absorption promoter: 0. 5-10%; (3) osmotic pressure regulator: 0. 1 -5%; (4) bodying agent: 0. 5-30%; (5) corrigent: 0. 01-20%; (6) conservative 0. 01-0. 5%, (7) water: 45-95%. The invention is characterized by good adjustment, especially suitable for patient in comatose state; high biological utilization rate, convenient utilization. The product is characterized by stable performance, controllable quality and no stimulation to mouth mucosa.
Owner:BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
Sublingual spray for the treatment of pain
This invention relates to a spray for sublingual administration, used in the treatment and management of pain, especially acute pain. Also provided are methods of treatment and management of pain, a metered dosage system for administration of the spray, and combination therapies.
Owner:UNIV OF KENTUCKY RES FOUND
Ketorolac Sublingual Spray for the Treatment of Pain
InactiveUS20090246273A1Improve bioavailabilityAmenable to ameliorationBiocidePowder deliveryKetorolacHeadache severe
The present invention provides for compositions and methods for accelerating the rate of delivery of ketorolac to the systemic circulation by sublingual spray administration under the tongue to provide a rapid response in the treatment of pain, especially acute pain associated with postoperative pain and migraine headache. Compositions of ketorolac formulated for sublingual delivery as liquid spray are provided. Also provided are methods of treatment and management of pain.
Owner:US WORLDMEDS
Formulation and method to induce a deep state of relaxation
ActiveUS20130210758A1Enhance beneficial synergistic effectMaximize transportBiocidePowder deliveryMedicineGamma-Aminobutyric acid
A relaxation formulation structured to induce a deep state of relaxation in a person comprises amounts of tryptophan, melatonin, vitamin B3, and vitamin B6. Another relaxation formulation also includes an amount of tyrosine, and yet another formulation includes an amount of vitamin B12. At least one embodiment of a relaxation formula comprises a physiologically effective amount of gamma-aminobutyric acid (“GABA”). A delivery system is provided to facilitate administration of the relaxation formulation to a person. The delivery system may include an edible high carbohydrate matrix, such as a chocolate brownie. Alternatively, the delivery system may comprise an inert vaporizable compound to allow the components of the relaxation formulation to be inhaled directly into the lungs of a person. Other delivery systems include an aqueous sublingual spray and a beverage.
Owner:KELLER RAYMOND M
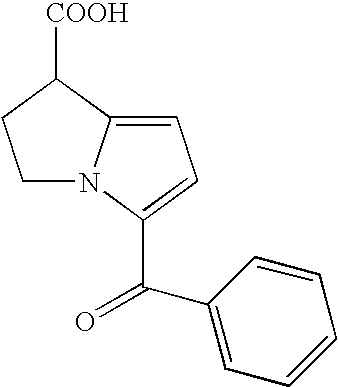
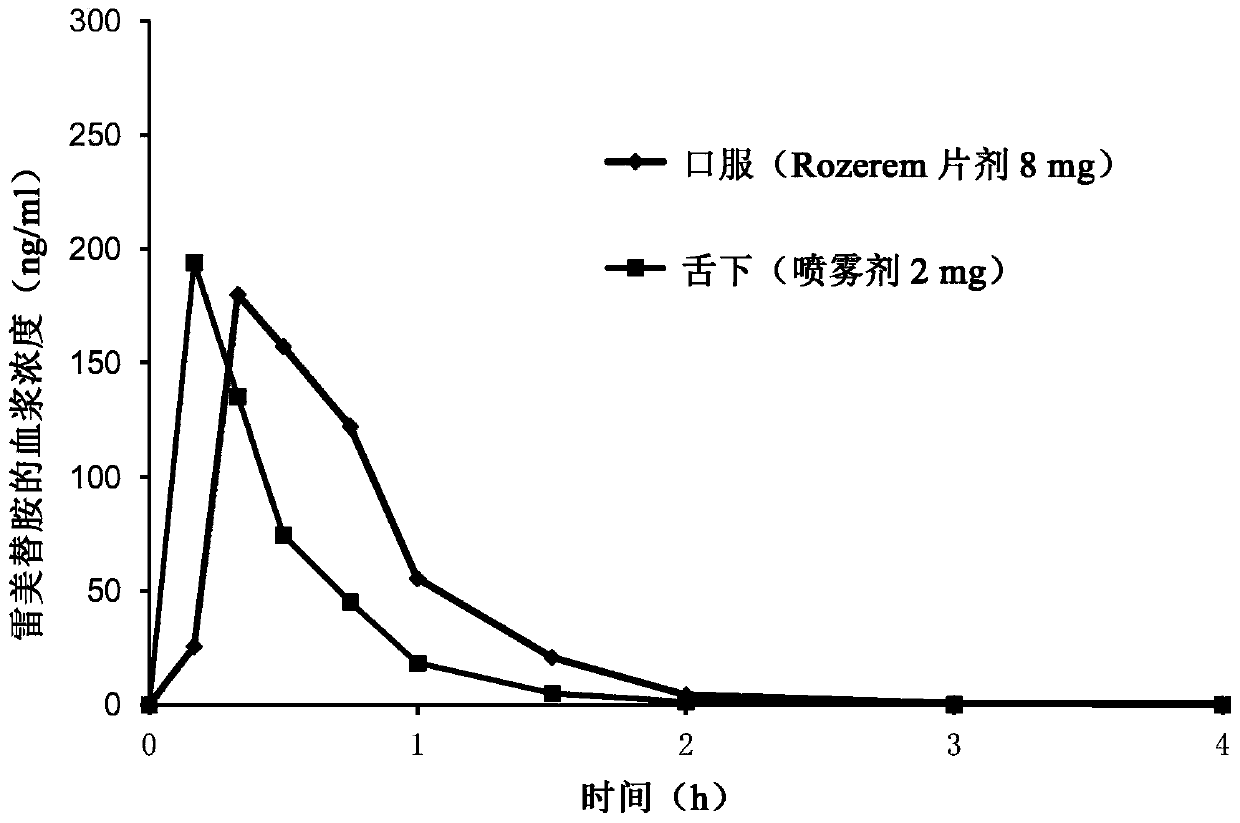
Pharmaceutical compositions of ramelteon and methods of use thereof
Pharmaceutical compositions of ramelteon for use in the treatment of insomnia or jet lag by transmucosal administration. More specifically, the pharmaceutical composition is formulated into nasal spray or nasal drop for intranasal administration, or formulated into sublingual spray for sublingual administration, or formulated into a solid dosage for sublingual administration.
Owner:MAXINASE LIFE SCI LTD
Sildenafil sublingual spray formulation
ActiveUS20150133459A1Organic active ingredientsPharmaceutical delivery mechanismSildenafilMale sexual dysfunction
The invention is directed to sublingual spray formulations containing sildenafil. The invention is further directed to methods of treating male sexual dysfunction or pulmonary arterial hypertension by administering sublingual spray formulations containing sildenafil to patients in need of such treatments.
Owner:BENUVIA OPERATIONS LLC
Sildenafil sublingual spray formulation
ActiveUS9370518B2Organic active ingredientsPharmaceutical delivery mechanismSildenafilPulmonary hypertension
The invention is directed to sublingual spray formulations containing sildenafil. The invention is further directed to methods of treating male sexual dysfunction or pulmonary arterial hypertension by administering sublingual spray formulations containing sildenafil to patients in need of such treatments.
Owner:BENUVIA OPERATIONS LLC
Ondansetron sublingual spray formulation
The invention is directed to sublingual spray formulations containing ondansetron or a pharmaceutically acceptable salt thereof and water. The invention is further directed to methods for treating or preventing nausea and emesis associated with cancer treatments by administering sublingual spray formulations containing ondansetron or a pharmaceutically acceptable salt thereof to a patient in need thereof.
Owner:BENUVIA OPERATIONS LLC
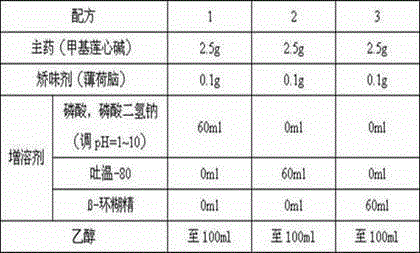
Cyclobenzaprine hydrochloride sublingual spray and preparation method thereof
ActiveCN107496355AImprove applicabilityQuality improvementOrganic active ingredientsAntipyreticPeppermintsDistilled water
The invention relates to a cyclobenzaprine hydrochloride sublingual spray and a preparation method thereof, and belongs to the field of pharmaceutical preparations. Preferably, the cyclobenzaprine hydrochloride sublingual spray consists of 15 parts of a cyclobenzaprine hydrochloride-DM-[beta]-cyclodextrin inclusion compound, 7 parts of vitamins, 3 parts of aspartame, 4 parts of peppermint essence, 0.3 part of sorbic acid, a proper amount of a pH regulator and 105 parts of distilled water. The sublingual spray provided by the invention is prepared by including the cyclobenzaprine hydrochloride with DM-[beta]-cyclodextrin as an inclusion material; and the sublingual spray is convenient for administration and rapid in actions, and the sublingual spray is low in administration dose, strong in applicability to patients, stable in quality and obvious in effect.
Owner:CP PHARMA QINGDAO CO LTD
Tosufloxacin tosylate sublingual spray for children and preparation method thereof
ActiveCN107375211AImprove use adaptabilityReduce dosageAntibacterial agentsOrganic active ingredientsDistilled waterChloroform
The invention relates to tosufloxacin tosylate sublingual spray for children and a preparation method thereof and belongs to the field of pharmaceutical preparation. The tosufloxacin tosylate sublingual spray preferably comprises 100 parts of glucosyl-beta-cyclodextrin inclusion of tosufloxacin tosylate, 0.3 parts of cherry essence, 0.03 parts of acetone chloroform, an appropriate amount of a pH adjusting agent and 100 parts of distilled water. The glucosyl-beta-cyclodextrin is used as an inclusion material so that an unexpected effect of solubilizing tosufloxacin tosylate is obtained and the tosufloxacin tosylate can be used in a form of sublingual spray. The sublingual spray is seasoned through cherry essence so that the sublingual spray has a slight cherry fragrance. The tosufloxacin tosylate sublingual spray has a small dosage, strong compliance to use of children, stable quality and significant effects.
Owner:CP PHARMA QINGDAO CO LTD
Formulation and method to induce a deep state of relaxation
ActiveUS9040582B2Enhance beneficial synergistic effectMaximize transportPowder deliveryBiocideMedicineGamma-Aminobutyric acid
A relaxation formulation structured to induce a deep state of relaxation in a person comprises amounts of tryptophan, melatonin, vitamin B3, and vitamin B6. Another relaxation formulation also includes an amount of tyrosine, and yet another formulation includes an amount of vitamin B12. At least one embodiment of a relaxation formula comprises a physiologically effective amount of gamma-aminobutyric acid (“GABA”). A delivery system is provided to facilitate administration of the relaxation formulation to a person. The delivery system may include an edible high carbohydrate matrix, such as a chocolate brownie. Alternatively, the delivery system may comprise an inert vaporizable compound to allow the components of the relaxation formulation to be inhaled directly into the lungs of a person. Other delivery systems include an aqueous sublingual spray and a beverage.
Owner:KELLER RAYMOND M
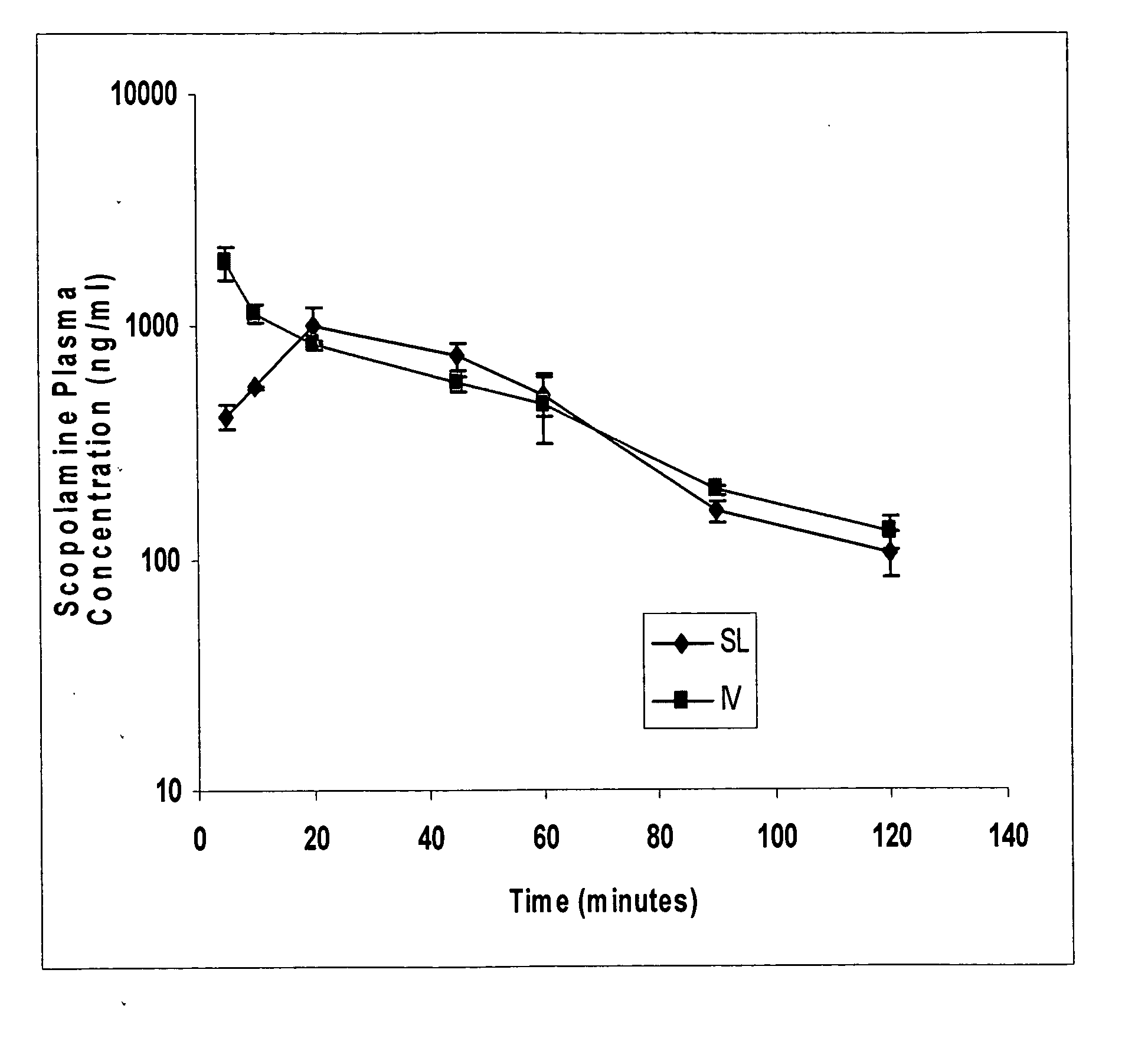
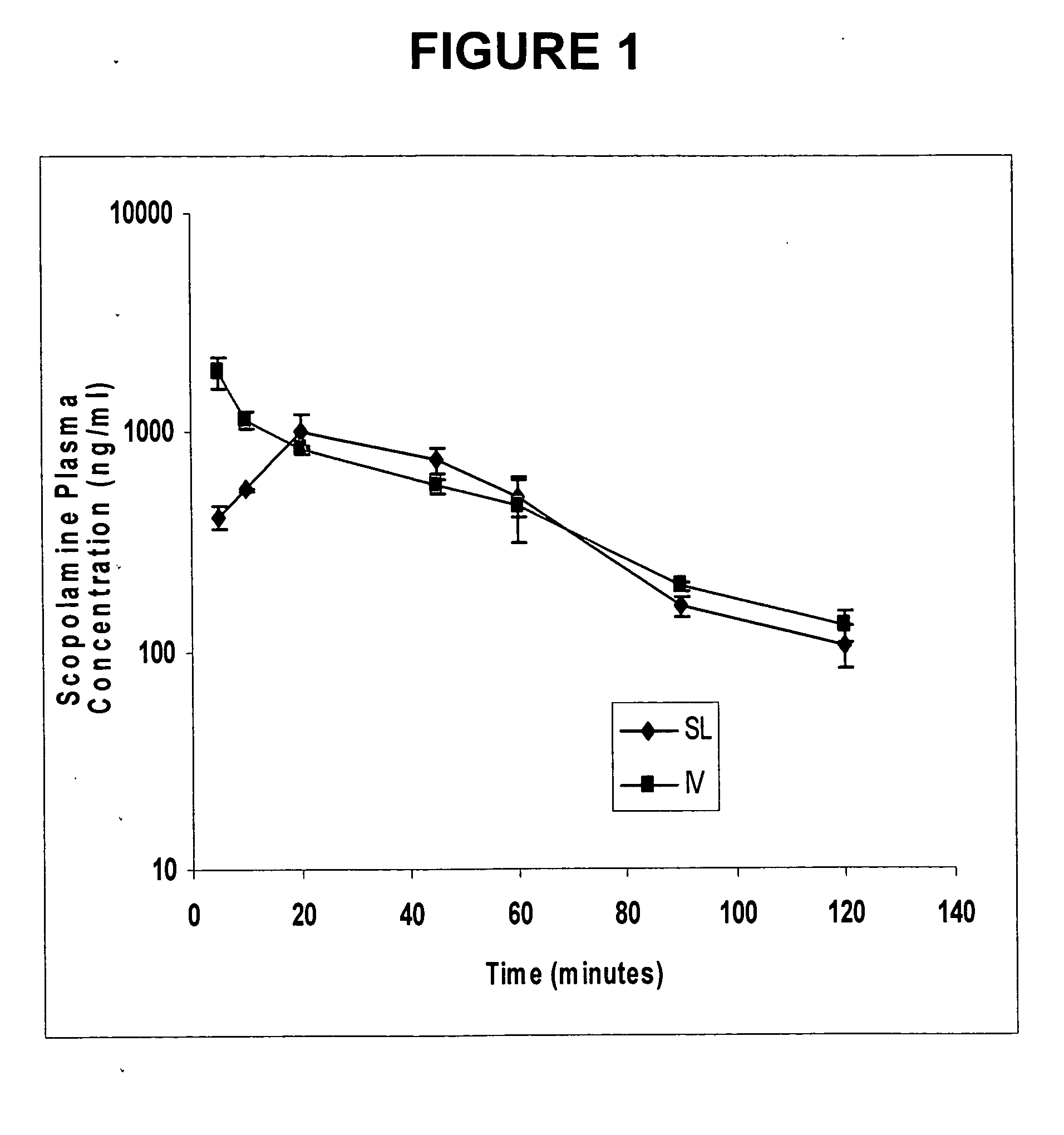
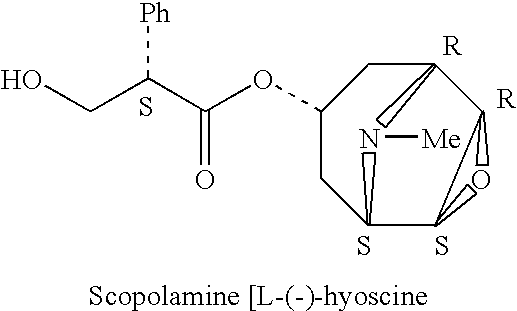
Scopolamine sublingual spray for the treatment of motion sickness
InactiveUS20060193784A1Quick reliefImprove solubilityBiocideAerosol deliveryMotion sicknessSublingual administration
This invention relates to a scopolamine spray for sublingual administration, used in the treatment and prevention of motion sickness, as well as the treatment and prevention of similar symptoms, such as nausea and vomiting, caused by conditions other than motion sickness. Also provided are methods of treatment, prevention and inhibition of these conditions and symptoms, as well as a metered dosage system for administration of the spray.
Owner:UNIV OF KENTUCKY RES FOUND
Sublingual Spray Formulation Comprising Dihydroartemesinin
InactiveUS20120157518A1Patient compliance is goodPrecise deliveryOrganic active ingredientsBiocideNasal cavityChemical compound
The invention provides pharmaceutical compositions for the treatment of neoplastic diseases, fluke infestations and Lyme disease, comprising compounds capable of providing dihydroartemesinin and a medium chain triglyceride formulated for transmucosal sublingual, buccal or nasal delivery, especially by a spray. Also provided are delivery devices containing the compositions.
Owner:LONDONPHARMA
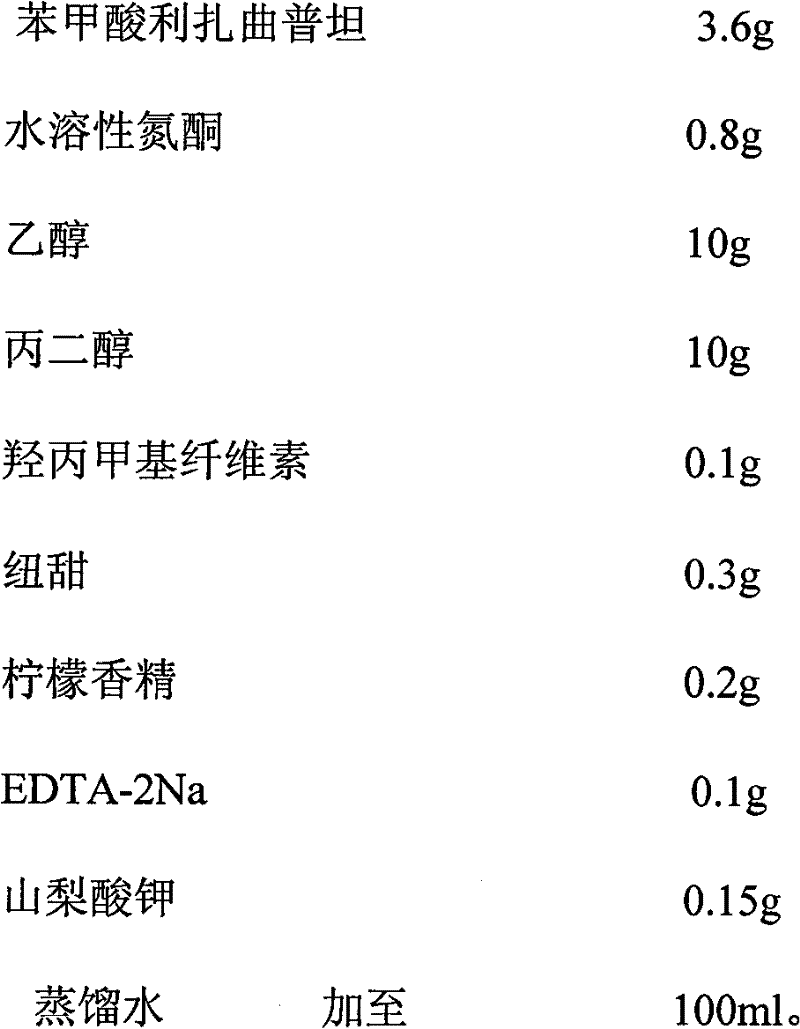
Rizatriptan benzoate oral spray
ActiveCN101856329BQuick effectImprove bioavailabilityOrganic active ingredientsNervous disorderOral medicationThird generation
The invention discloses a rizatriptan benzoate oral spray taking water as a solvent and containing the following components by content: 3.6-14.5 g / 100 ml of rizatriptan benzoate, 0.1-50 g / 100 ml of solubilizers, 0.05-5 g / 100 ml of sorbefacients and 0.01-3 g / 100 ml of flavoring agents. The rizatriptan benzoate oral spray sprays for drug administration through an oral cavity or a hypoglossis, and then drugs can be absorbed by mucosae of the oral cavity or the hypoglossis so as to avoid liver first-pass effect and food influence and realize fast absorption, quick effect and high bioavailability;in addition, the rizatriptan benzoate oral spray is convenient to use without being taken with water during drug administration and is especially suitable for patients who are not obedient to oral administration and injection drug administration; and besides, the rizatriptan benzoate oral spray is a liquid formulation, has no dust in the production process, and has easy labor protection and lowercost of mass production.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Diclofenac sublingual spray
The present invention is directed to diclofenac sublingual spray formulations. The present invention is also directed to methods for treating pain and inflammation by administering the formulations of the present invention to patients in need thereof.
Owner:BENUVIA OPERATIONS LLC
Sildenafil sublingual spray formulations
ActiveUS9662334B2Organic active ingredientsInorganic non-active ingredientsSildenafilMale sexual dysfunction
The invention is directed to sublingual spray formulations containing sildenafil. The invention is further directed to methods of treating male sexual dysfunction or pulmonary arterial hypertension by administering sublingual spray formulations containing sildenafil to patients in need of such treatments.
Owner:BENUVIA OPERATIONS LLC
Nimudipin undertongue spraying agent
InactiveCN1410061AImprove complianceAvoid side effectsOrganic active ingredientsAerosol deliveryMedicineNimodipine
A sublingual spray of nimodipine contains nimodipine (1-5 wt. portions), propanediol (10-50), polyethylene glycol-400 (10-30), BHT (0.001-0.01) and mint oil (0.001-0.02). Its advantages are high biological utilization rate, low dosage, and no first over effect.
Owner:张瑞琛 +1
Nimudipin undertongue spraying agent
InactiveCN1165304CImprove complianceAvoid side effectsOrganic active ingredientsAerosol deliveryMedicinePolyethylene glycol
A sublingual spray of nimodipine contains nimodipine (1-5 wt. portions), propanediol (10-50), polyethylene glycol-400 (10-30), BHT (0.001-0.01) and mint oil (0.001-0.02). Its advantages are high biological utilization rate, low dosage, and no first over effect.
Owner:张瑞琛 +1
Ondansetron sublingual spray formulation
The invention is directed to sublingual spray formulations containing ondansetron or a pharmaceutically acceptable salt thereof and water. The invention is further directed to methods for treating or preventing nausea and emesis associated with cancer treatments by administering sublingual spray formulations containing ondansetron or a pharmaceutically acceptable salt thereof to a patient in need thereof.
Owner:BENUVIA OPERATIONS LLC
Adrenaline sublingual spray for allergic shock as well as preparation method and application of adrenaline sublingual spray
ActiveCN113750046ASolve the problem of poor absorptionImprove stabilityOrganic active ingredientsAerosol deliveryDiseaseAdrenaline preparation
The invention belongs to the field of pharmaceutical preparations, and discloses an epinephrine sublingual spray for allergic shock and a preparation method thereof. The invention solves the problems of poor stability and delayed absorption of the existing adrenaline preparation. A patient can take the medicine at the first time when the allergic shock disease occurs, the medicine can rapidly penetrate through sublingual mucosa and enter blood circulation, the medicine effect can be achieved within 15 min, and the medicine can be used as a standing emergency medicine to replace an injection and has good clinical application prospects in the field of allergic shock treatment.
Owner:CHINA PHARM UNIV
Tosufloxacin tosylate sublingual spray for children and preparation method thereof
ActiveCN107375211BImprove use adaptabilityReduce dosageAntibacterial agentsOrganic active ingredientsPharmaceutical formulationVANILLA FLAVORING
The invention relates to a sublingual spray of tosufloxacin tosylate for children and a preparation method thereof, belonging to the field of pharmaceutical preparations. The sublingual spray of the present invention preferably comprises 100 parts of tosufloxacin tosufloxacin glucosyl-β-cyclodextrin inclusion compound, 0.3 part of cherry essence, 0.03 part of chlorobutanol, an appropriate amount of pH regulator, distilled water 100 parts, the present invention adopts glucosyl-β-cyclodextrin as clathrate material, has obtained the unexpected effect of solubilizing tosufloxacin tosylate, so that it can be used in the form of sublingual spray, The dosage form is flavored with cherry essence, so that the spray has a faint cherry fragrance, and the dosage is small, and the children have strong compliance, stable quality and remarkable effect.
Owner:CP PHARMA QINGDAO CO LTD
Neferine sublingual spraying agent and preparation method thereof
The invention provides a neferine sublingual spraying agent and a preparation method thereof. The neferine sublingual spraying agent can be quickly absorbed by the sublingual mucosa, can directly enter the blood, is free from liver first-pass effect, is convenient to take, causes less adverse reaction and has a good treatment effect.
Owner:宋金春 +2
Cannabidiol Sublingual Spray Formulations
InactiveUS20210137828A1Impurity levelHydroxy compound active ingredientsPharmaceutical delivery mechanismBiochemistryBioavailability
Sublingual cannabidiol formulations that are stable at room or refrigerated temperatures. It may also possess improved absorption, faster onset, increased bioavailability and preferred flavor. The dose range from 12.5 mg to 25 mg per spray.
Owner:LI HORNG SHIN JEN
Ketorolac Sublingual Spray Formulations
InactiveUS20160296498A1Organic active ingredientsPharmaceutical delivery mechanismKetorolacRoom temperature
The invention is directed to room temperature storage stable sublingual spray formulations containing ketorolac. The invention is further directed to methods of treating pain by administering sublingual spray formulations containing ketorolac to patients in need of such treatments.
Owner:INSYS DEV CO INC
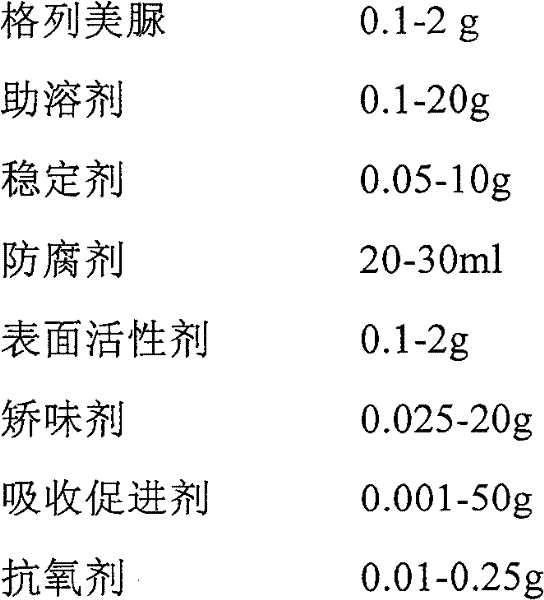
Glimepiride aqueous solution administration system and preparation method thereof
ActiveCN101658487BSolve the problem of extremely difficult to dissolveAvoid first pass effectMetabolism disorderSulfonylurea active ingredientsAdjuvantGlimepiride
The invention relates to a glimepiride aqueous solution administration system and a preparation method thereof, which belong to the technical field of medicaments. A glimepiride aqueous solution preparation consists of an active component glimepiride and a pharmaceutically acceptable adjuvant, and each 100 milliliters of the preparation contains 0.1 to 10 grams of the glimepiride, preferably 0.1 to 5 grams, and most preferably 0.1 to 2 grams. The glimepiride aqueous solution administration system and the preparation method have the advantages of successfully solving the problem that the glimepiride is extremely difficult to dissolve, and greatly improving the stability of a solution through a series of prescription screening works. The system or a composition performs administration in a sublingual spraying mode to avoid the action of alimentary canals, the reactions of gastrointestinal tracts and the liver first pass effect; besides, the system or the composition has quick response and high bioavailability, is particularly suitable for controlling the postprandial blood sugar, and can be used for treating the type 2 diabetes which cannot be appropriately controlled by dietary restriction and sports. The system or the composition also has the following advantages that: the use is convenient, the dosage can be adjusted as required, the compliance is good, the system or the composition is particularly suitable for old people and the patients who cannot swallow conveniently, the preparation process is simple and practicable and is suitable for industrial production, and the like.
Owner:BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
A kind of cyclobenzaprine hydrochloride sublingual spray and preparation method thereof
ActiveCN107496355BImprove applicabilityQuality improvementOrganic active ingredientsAntipyreticPeppermint FlavorDrugs preparations
The invention relates to a cyclobenzaprine hydrochloride sublingual spray and a preparation method thereof, belonging to the field of pharmaceutical preparations. The cyclobenzaprine hydrochloride sublingual spray of the present invention preferably comprises 15 parts of cyclobenzaprine hydrochloride-DM-β-cyclodextrin inclusion compound, 7 parts of vitamins, 3 parts of aspartame, 4 parts of peppermint essence, 0.3 part of acid, appropriate amount of pH regulator, 105 parts of distilled water, the present invention adopts DM-β-cyclodextrin as clathrate material to clathrate cyclobenzaprine hydrochloride to prepare sublingual spray, which is convenient for administration and rapid in action, and The dosage is small, the applicability to patients is strong, the quality is stable, and the effect is remarkable.
Owner:CP PHARMA QINGDAO CO LTD
Sublingual Spray Formulation Comprising Dihydroartemesinin
InactiveUS20160022629A1Reduced shelf lifeEliminate the effects ofOrganic active ingredientsBiocideNasal cavityMedium-chain triglyceride
The invention provides pharmaceutical compositions for the treatment of neoplastic diseases, fluke infestations and Lyme disease, comprising compounds capable of providing dihydroartemesinin and a medium chain triglyceride formulated for transmucosal sublingual, buccal or nasal delivery, especially by a spray. Also provided are delivery devices containing the compositions.
Owner:LONDONPHARMA
Sildenafil sublingual spray formulations
ActiveUS10111833B2Organic active ingredientsInorganic non-active ingredientsSildenafilMale sexual dysfunction
The invention is directed to sublingual spray formulations containing sildenafil. The invention is further directed to methods of treating male sexual dysfunction or pulmonary arterial hypertension by administering sublingual spray formulations containing sildenafil to patients in need of such treatments.
Owner:BENUVIA OPERATIONS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com