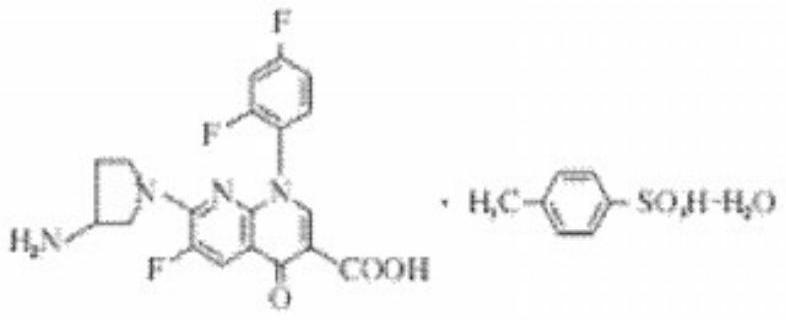
Tosufloxacin tosylate sublingual spray for children and preparation method thereof
A technology of tosufloxacin tosufloxacin tosufloxacin and spray, applied in the field of tosufloxacin tosufloxacin tosylate sublingual spray for children and its preparation, can solve problems such as inducing joint toxicity, and achieve strong compliance and stable quality , the effect of low dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Cyclodextrin inclusion solubilization tosufloxacin tosylate screening test
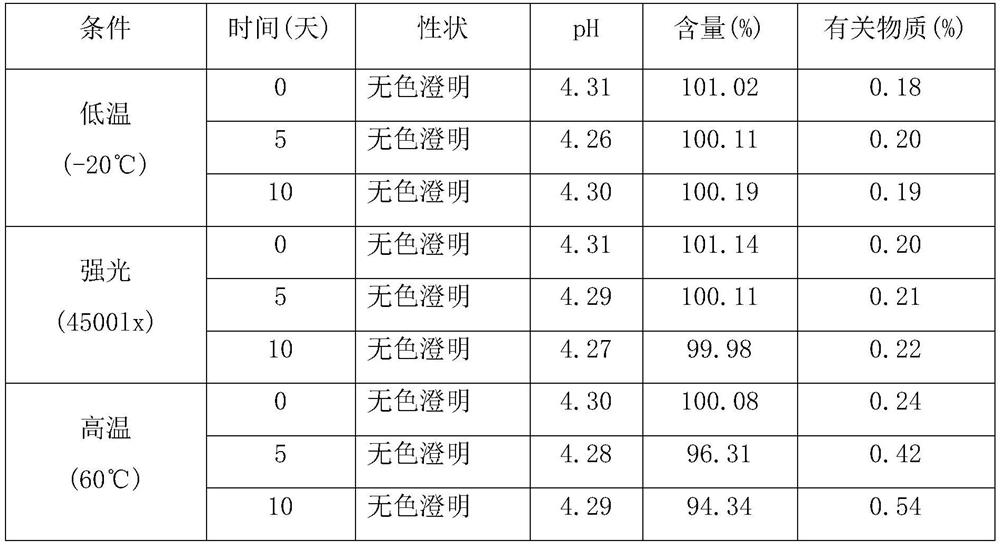
[0025] Separately prepare α-cyclodextrin, β-cyclodextrin, HP-β-cyclodextrin, DM-β-cyclodextrin and glucosyl-β-cyclodextrin aqueous solutions with the same concentration, and heat and stir at 55°C Add excess tosufloxacin tosylate, heat and stir for 48 hours, filter through a 0.45 μm microporous membrane, take the filtrate, and measure the content of tosufloxacin tosylate. The results are shown in Table 1.
[0026] Table 1 Solubilization of Tosufloxacin Tosylate by Different Cyclodextrins
[0027]
[0028] The results show that the inclusion of tosufloxacin tosylate with glucosyl-β-cyclodextrin can greatly improve its solubility.
Embodiment 2
[0029] Example 2 Preparation of Tosufloxacin Tosylate-Glucosyl-β-cyclodextrin Inclusion Compound 1
[0030] Take an appropriate amount of tosufloxacin tosylate, add an appropriate amount of glacial acetic acid to dissolve it, weigh the glucosyl-β-cyclodextrin inclusion compound according to the ratio of 1:3 tosufloxacin tosylate-β-cyclodextrin For β-cyclodextrin, add an appropriate amount of distilled water to dissolve it completely. Under the condition of heating and stirring in a water bath at 50°C, slowly add the tosufloxacin tosylate solution into the glucosyl-β-cyclodextrin aqueous solution, dropwise After completion, stir under the same conditions for an appropriate time to dissolve completely, evaporate the solvent to dryness by rotary evaporation, and dry the obtained solid under reduced pressure and vacuum to obtain the final product.
Embodiment 3
[0031] Example 3 Preparation of Tosufloxacin Tosylate-Glucosyl-β-cyclodextrin Inclusion Compound 2
[0032] Take an appropriate amount of tosufloxacin tosylate, add an appropriate amount of glacial acetic acid to dissolve it, and weigh the glucosyl-β-cyclodextrin inclusion compound according to the ratio of 1:4 tosufloxacin tosylate-β-cyclodextrin inclusion complex. For β-cyclodextrin, add an appropriate amount of distilled water to dissolve it completely. Under the condition of heating and stirring in a water bath at 55°C, slowly add the tosufloxacin tosylate solution into the glucosyl-β-cyclodextrin aqueous solution, dropwise After completion, stir under the same conditions for an appropriate time to dissolve completely, evaporate the solvent to dryness by rotary evaporation, and dry the obtained solid under reduced pressure and vacuum to obtain the final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com