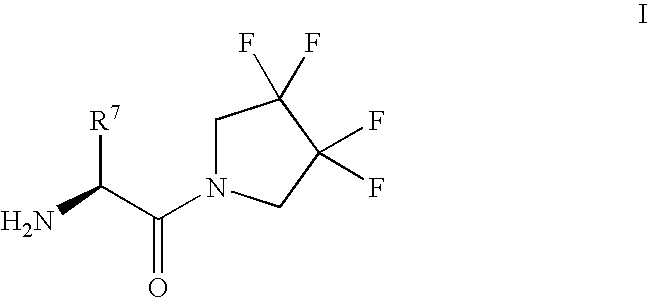
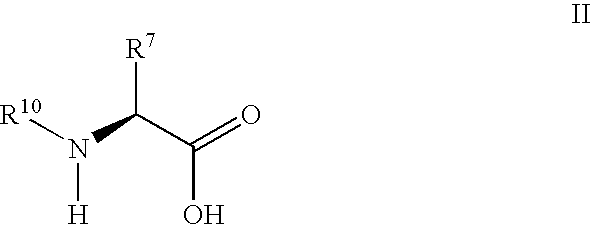
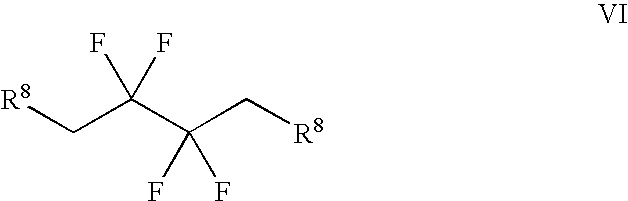
Synthesis of 3,3,4,4-tetrafluoropyrrolidine and novel dipeptidyl peptidase-IV inhibitor compounds
a technology of dipeptide iv inhibitor and synthesis method, which is applied in the field of synthesis of 3, 3, 4, 4tetrafluoropyrrolidine and novel dipeptide iv inhibitor compounds, can solve the problems of limiting their use, adding significantly to the overall morbidity and mortality attributable to those diseases, and few pharmacological agents available to reduce adiposity effectively and acceptably
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3,3,4,4-tetrafluoropyrrolidine Hydrochloride
[0063] Step 1: Trifluoro-methanesulfonic Acid 2,2,3,3-tetrafluoro-4-(trifl-uoro-methanesulfonyloxy)-butyl Ester.
[0064] To a cooled (about 0.degree. C.) solution of 2,2,3,3-tetrafluorobutanediol (15 grams, 93 mmol) and pyridine (19 mL, 230 mmol) in dichloromethane (250 mL), was added dropwise trifluoromethanesulfonic anhydride (34 mL, 200 mmol). After the addition, the mixture was stirred at about 0.degree. C. for about one hour, followed by stirring at room temperature for one additional hour, then diluted with dichloromethane, washed with water and brine, dried over magnesium sulfate, filtered and concentrated to near dryness, leaving a dichloromethane-containing oil. .sup.1H NMR (400 MHz, CDCl.sub.3) .delta. 4.82 (m). .sup.19F NMR (376 MHz, CDCl.sub.3) .delta.-120.83 (m, 4H), -74.38 (s, 6H).
[0065] Step 2: 1-Benzyl-3,3,4,4-tetrafluoro-pyrrolidine Hydrochloride
[0066] A solution of the crude trifluoro-methanesulfonic acid, 2,...
example 2
(2S,3S)-2-Amino-3-methyl-1-(3,3,4,4-tetrafluoro-pyrrolidin-1-yl)-pentan-1--one
[0069] Step 1: [(1S,2S)-2-Methyl-1-(3,3,4,4-tetrafluoro-pyrrolidine-1-carb-onyl)-butyl]-carbamic Acid Tert-Butyl Ester.
[0070] To a mixture of (L)-Boc-isoleucine (322 mg, 1.30 mmol), 3,3,4,4-tetrafluoro-pyrrolidine hydrochloride (300 mg, 1.67 mmol), hydroxybenzotriazole (225 mg, 1.67 mmol) and triethylamine (0.23 mL, 1.67 mmol) in dichloromethane (10 mL) was added 1-(-3-dimethylaminopropyl)-3-e-thylcarbodiimide hydrochloride (319 mg, 1.67 mmol). The mixture was stirred at room temperature overnight, diluted with ethyl acetate, washed with 2 N Hydrochloric acid, saturated sodium bicarbonate solution, water and brine, dried over magnesium sulfate and concentrated. The product was purified by flash-chromatography (4:1 ratio of hexane / ethyl acetate) and isolated as a white solid (415 mg, 86%).
[0071] Step 2: (2S, 3S)-2-Amino-3-methyl-1-(3,3,4,4-tetrafluoro-pyrrolidin--1-yl)-pentan-1-one Hydrochloride.
[0072] [(1S...
example 3
(S)-2-Amino-2-cyclohexyl-1-(3,3,4,4-tetrafluoro-pyrrolidin-1-yl)-ethanone
[0073] Step 1: (S)-[1-Cyclohexyl-2-oxo-2-(3,3,4,4-tetrafluoro-pyrrolidin-1--yl)-ethyl]-carbamic Acid Tert-Butyl Ester.
[0074] (S)-[1-cyclohexyl-2-oxo-2-(3,3,4,4-tetrafluoro-pyrrolidin-1-yl)-eth-yl]-carbamic acid tert-butyl ester was prepared from (L)-Boc-cyclohexylglycine and 3,3,4,4-tetrafluoropyrrolidine as analogously described in Step 1 of Example 1.
[0075] Step 2: (S)-2-Amino-2-cyclohexyl-1-(3,3,4,4-tetrafluoro-pyrrolidin--1-yl)-ethanone.
[0076] (S)-2-Amino-2-cyclohexyl-1-(3,3,4,4-tetrafluoro-pyrrolidin-1-yl)-et-hanone was obtained by hydrochloric acid treatment of (S)-[1-cyclohexyl-2-oxo-2-(3,3,4,4-tetrafluoro-pyrrolidin-1-yl)-ethyl]-ca-rbamic acid tert-butyl ester as analogously described in Step 2 of Example 1. (melting point: 278.degree. C.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com