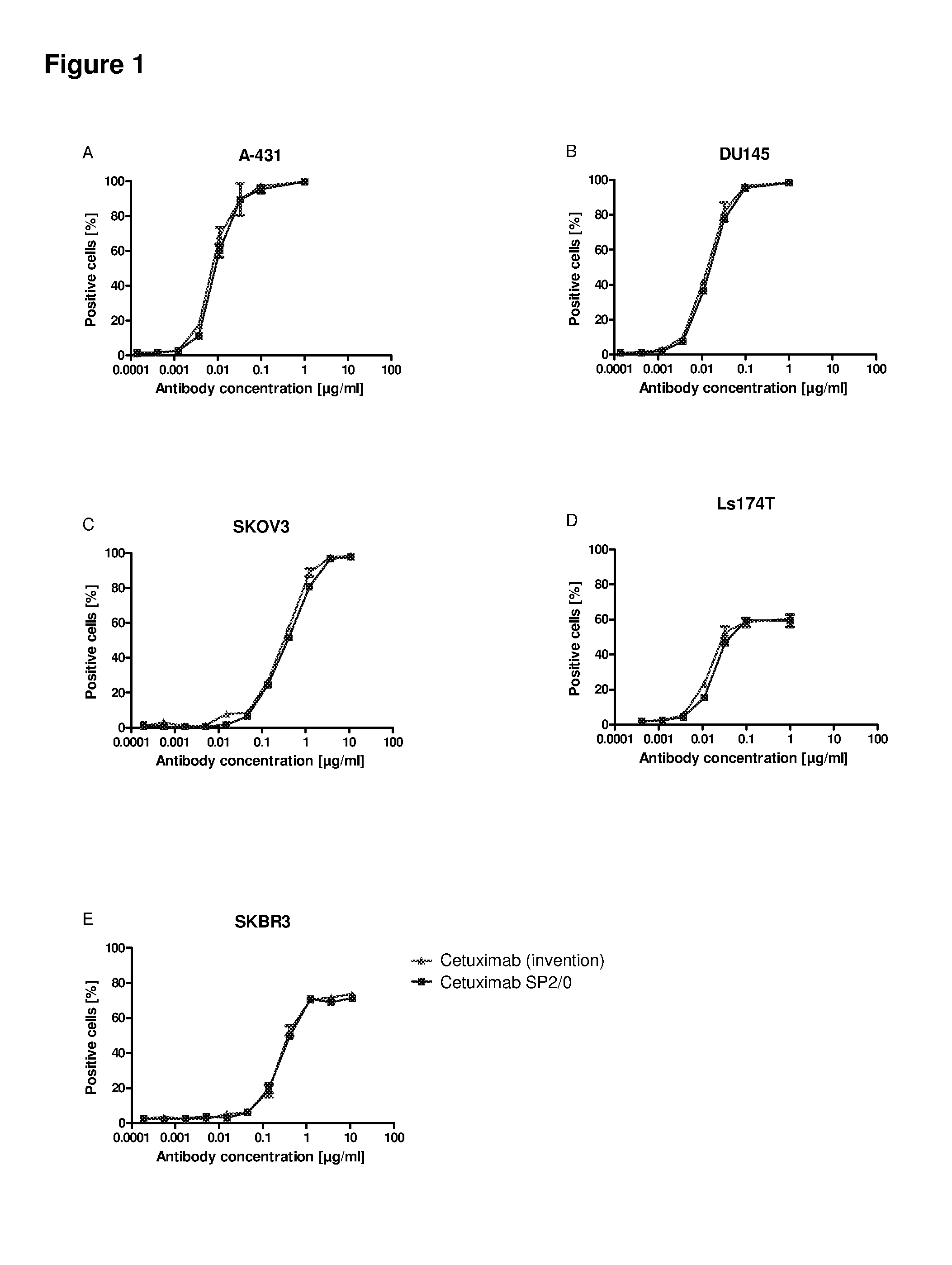
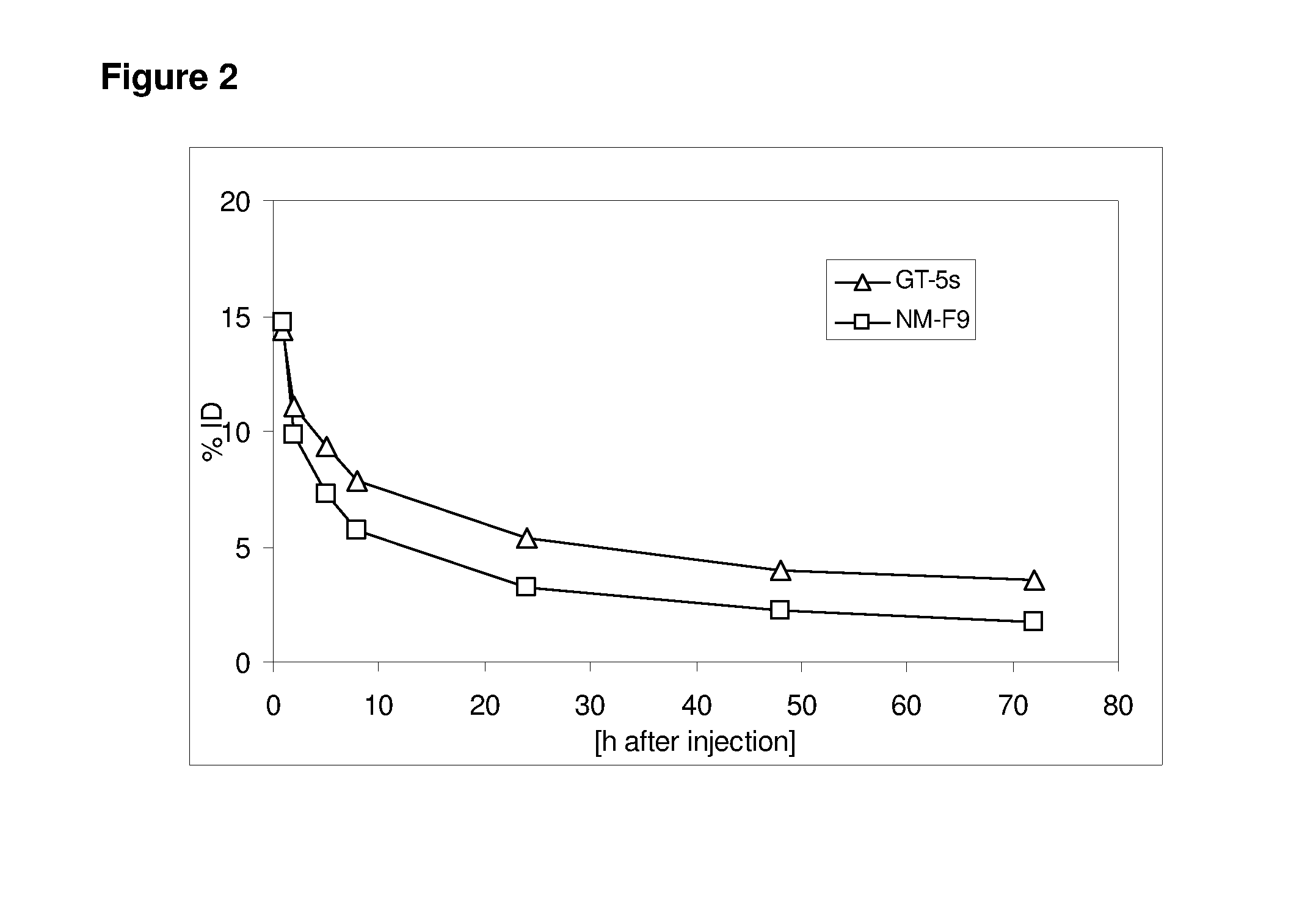
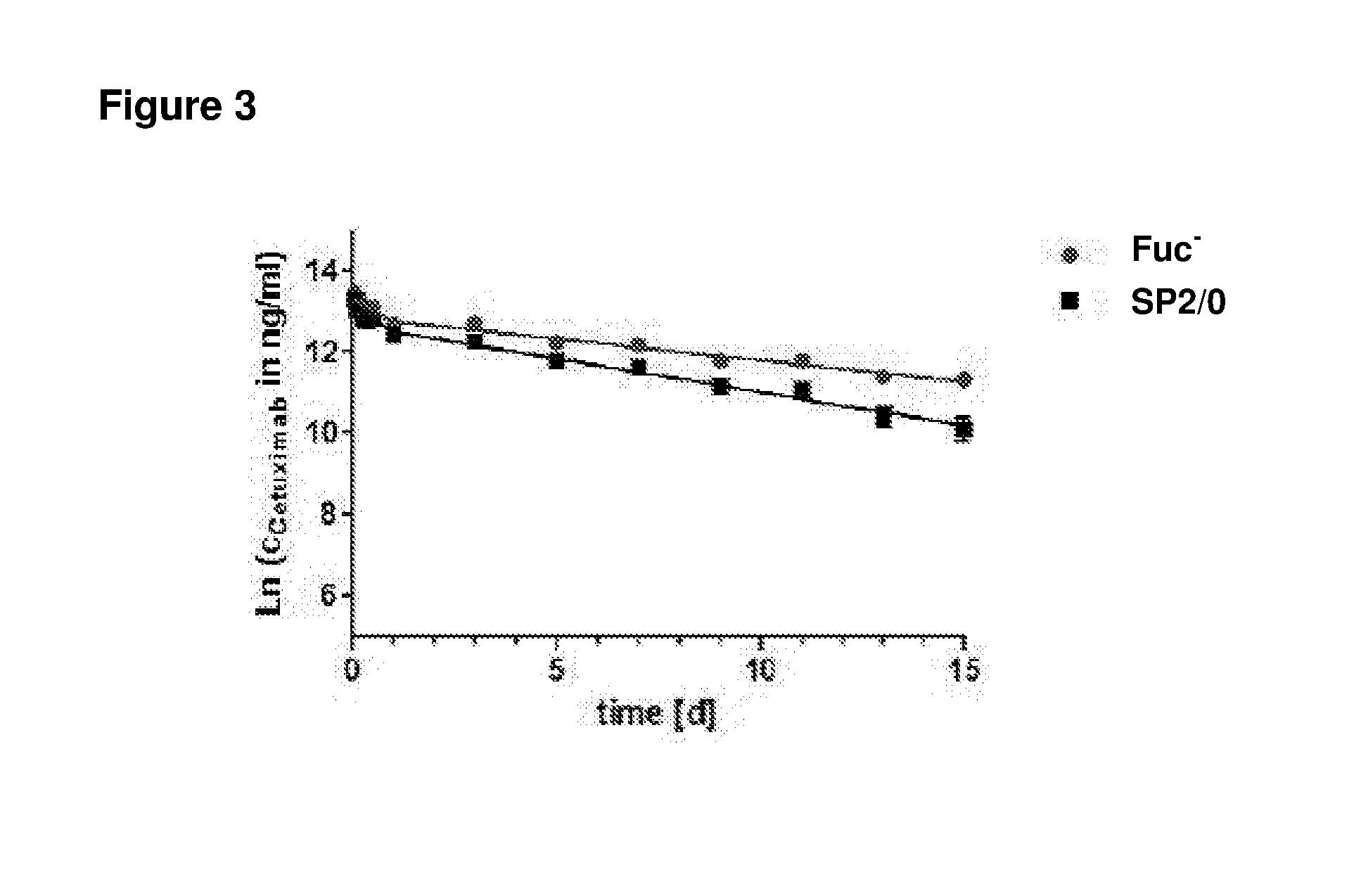
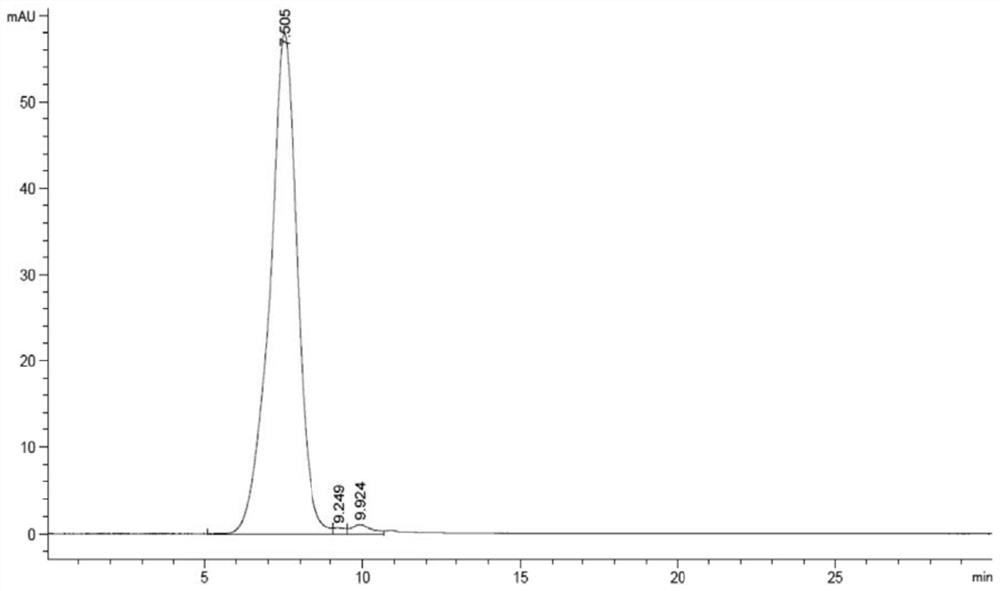
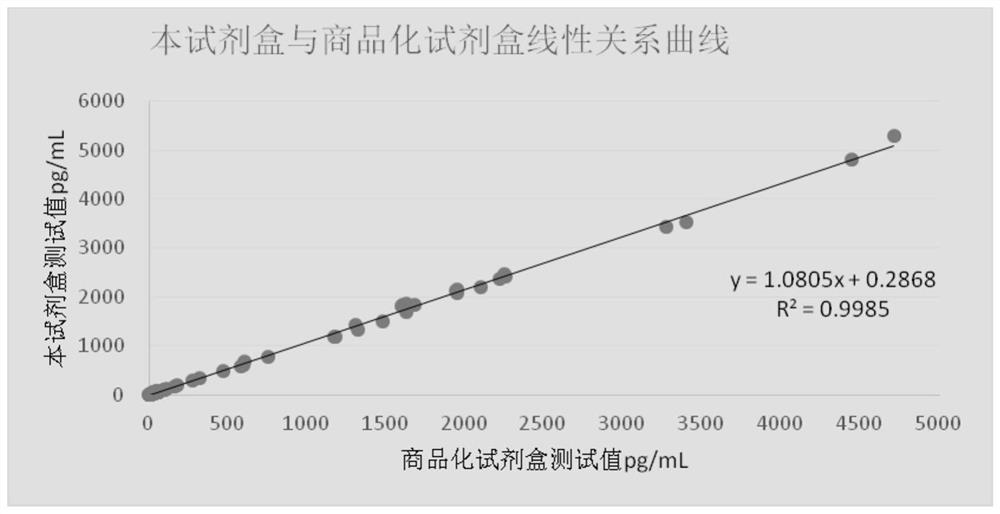
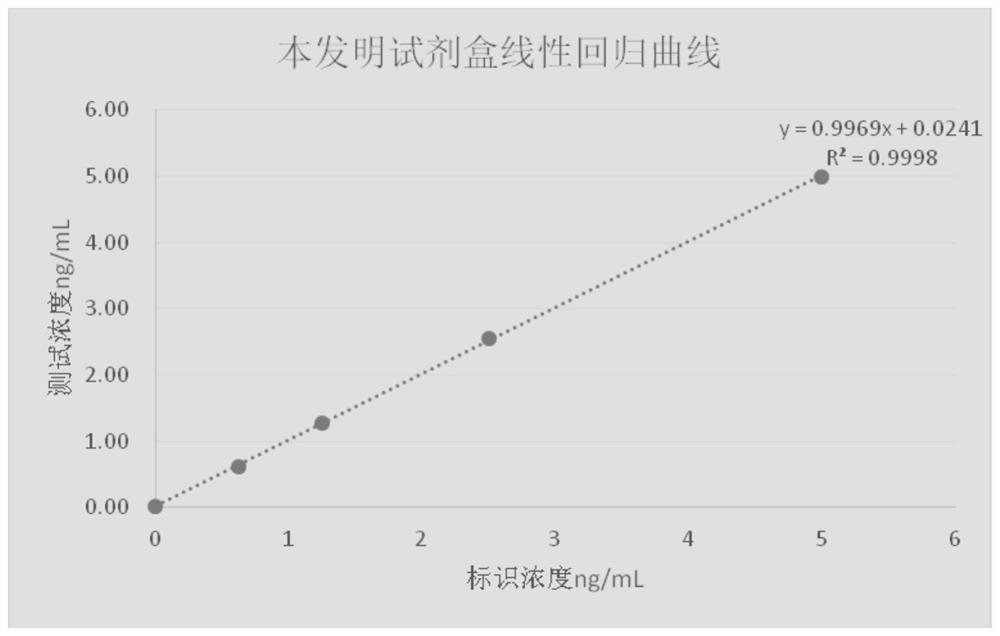
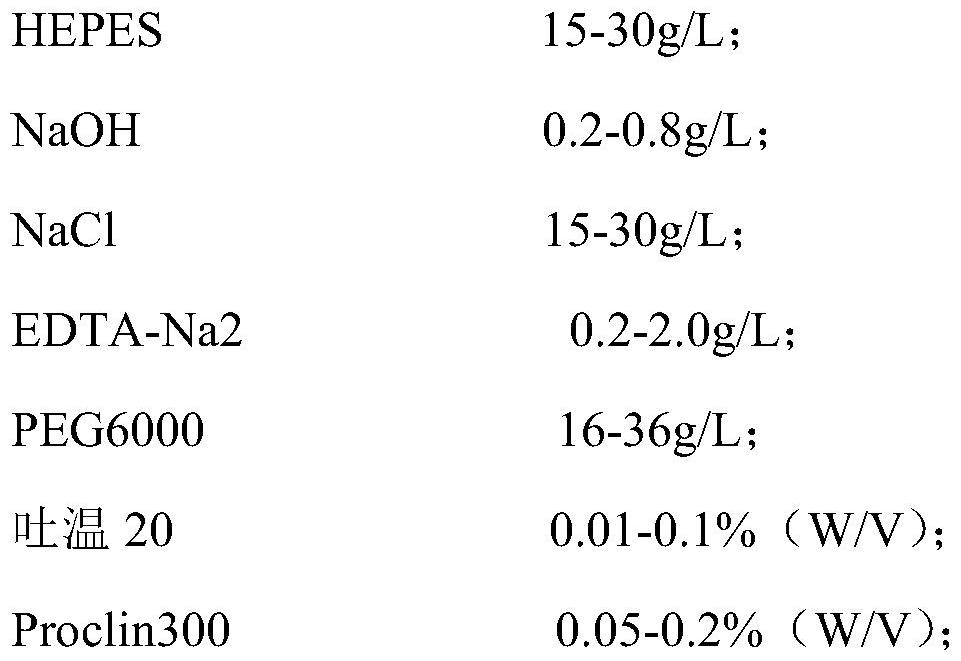
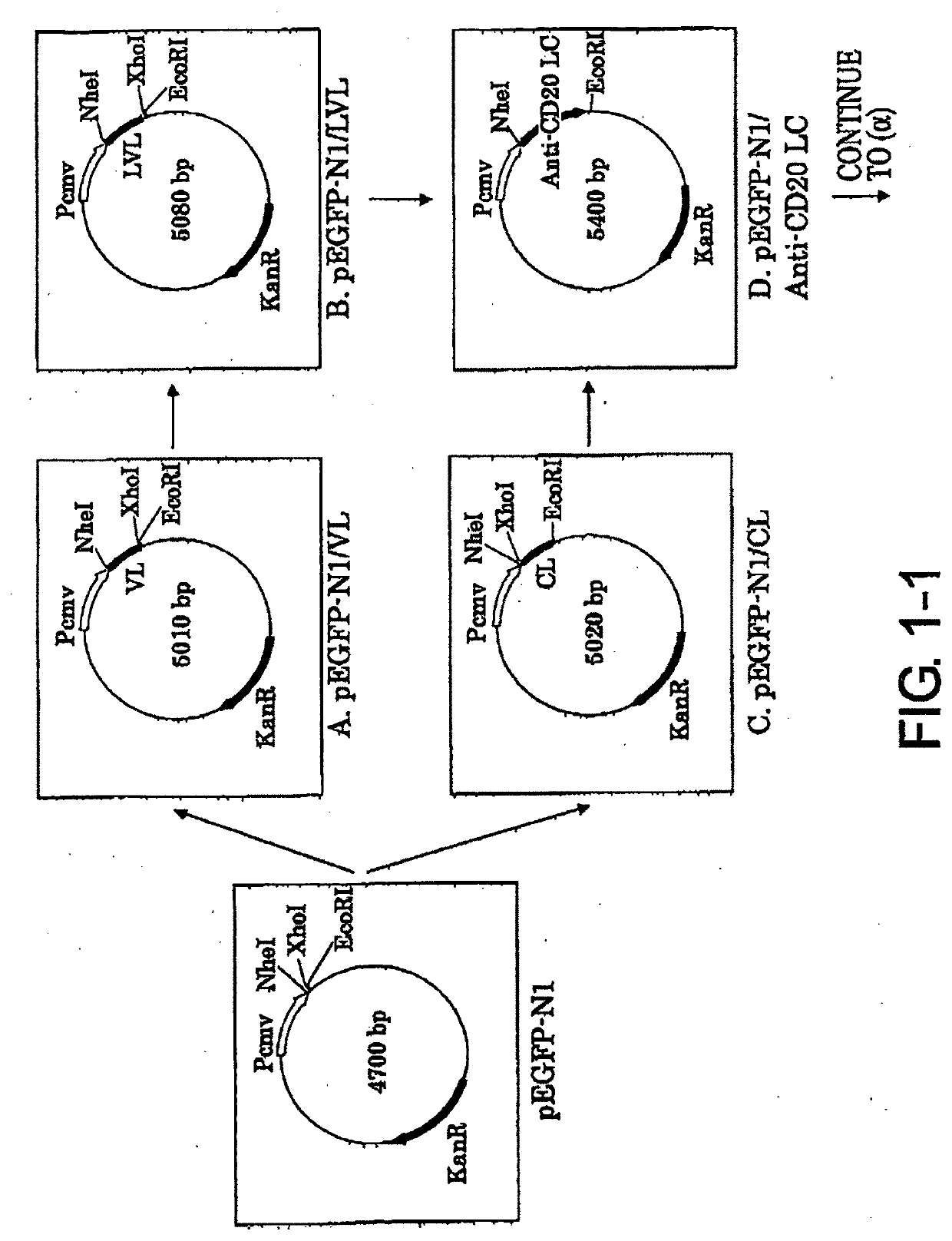
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40results about How to "High antibody activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
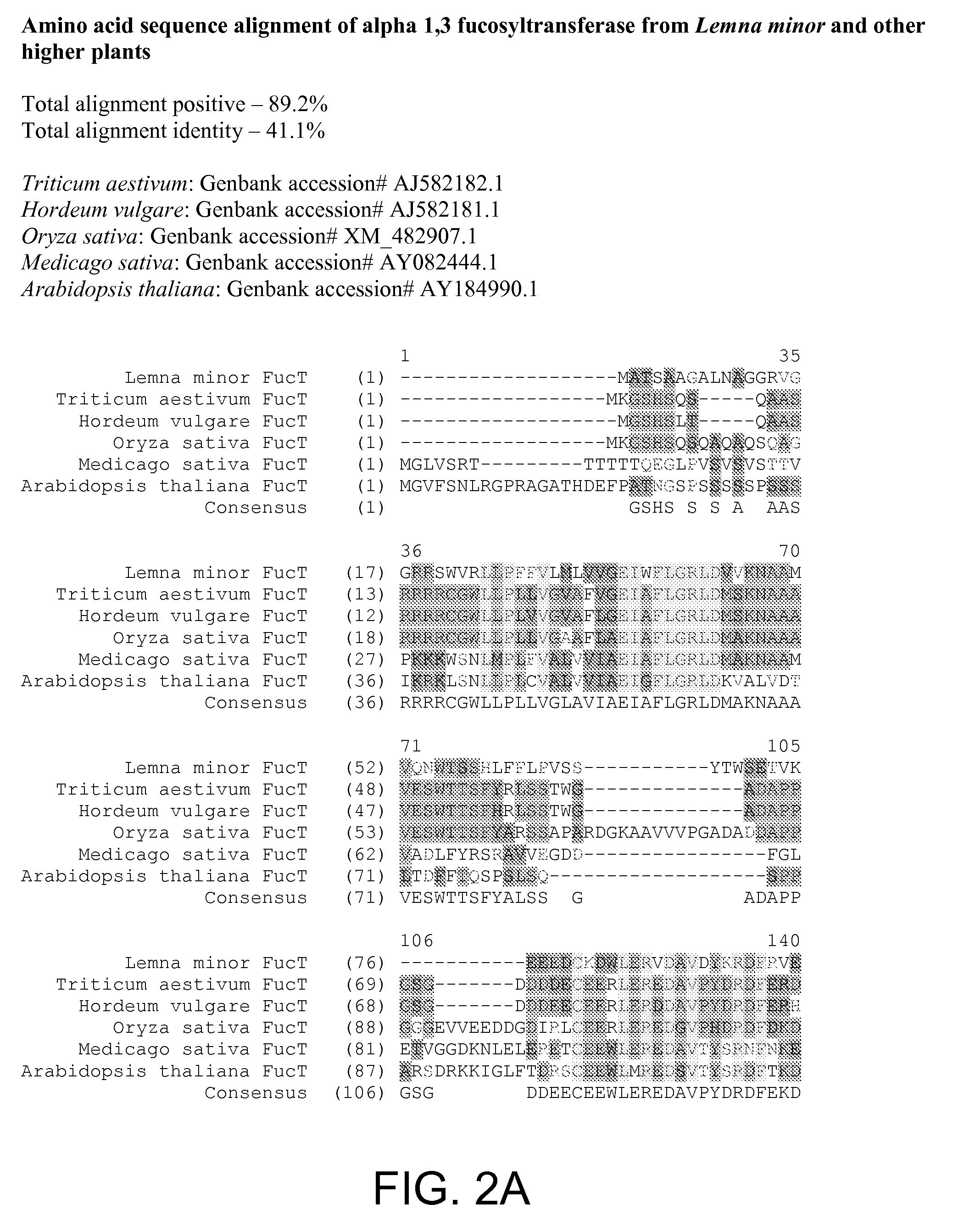
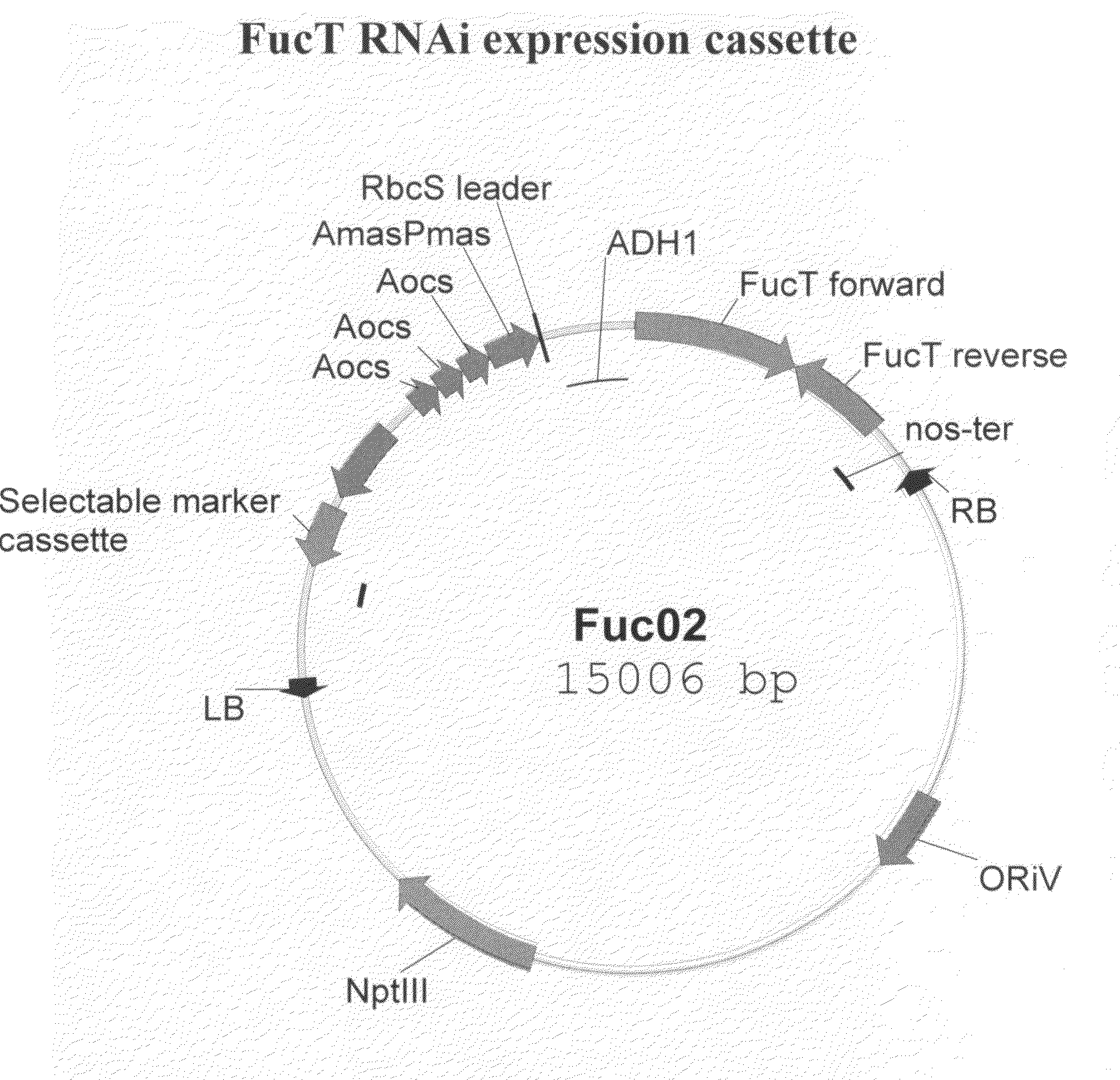
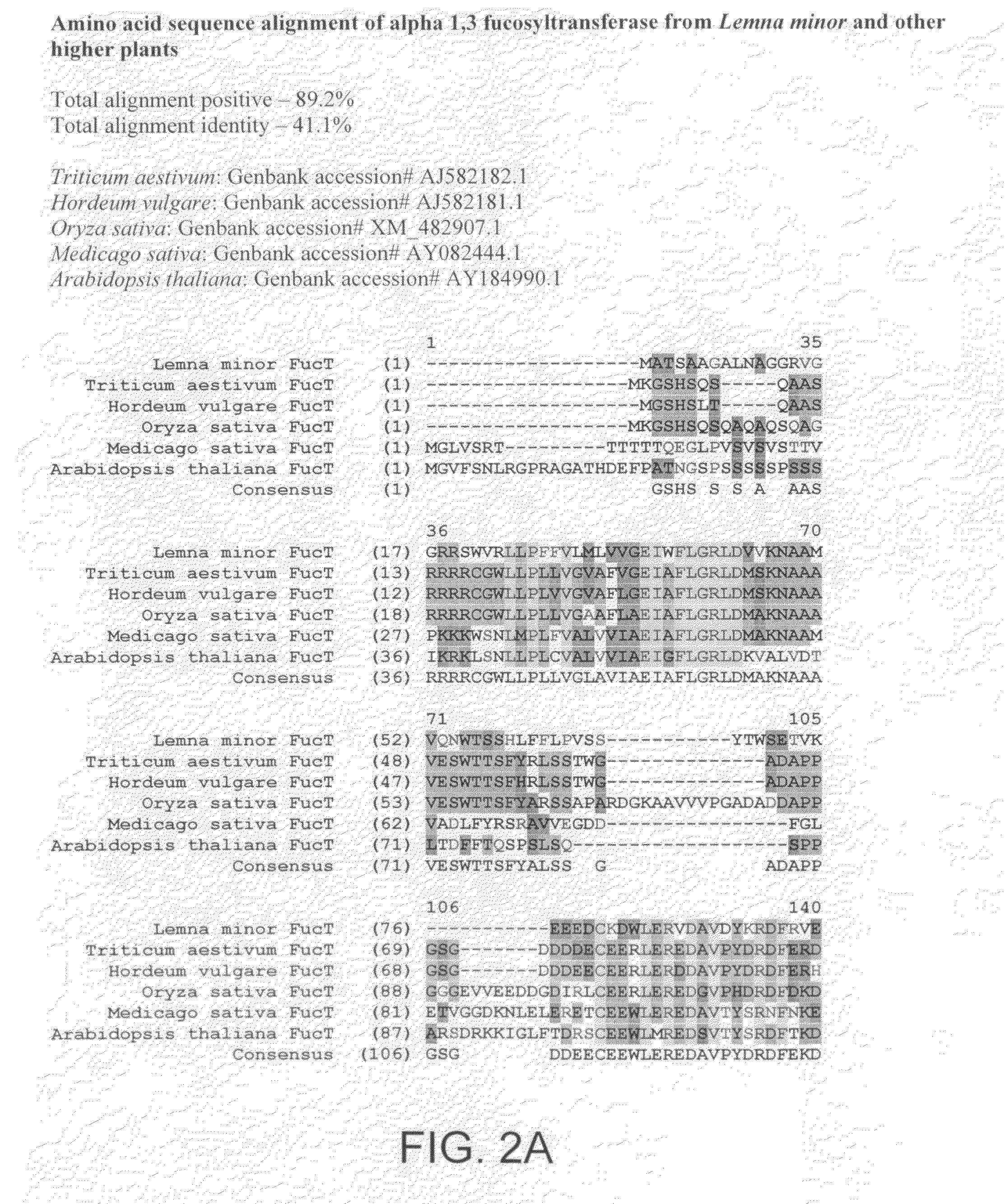
Compositions and methods for humanization and optimization of n-glycans in plants
InactiveUS20080066200A1Function increaseHigh antibody activityAntipyreticAnalgesicsGrowth plantGlycan
Methods for altering the N-glycosylation pattern of proteins in higher plants are provided. The methods comprise introducing into the plant a recombinant construct that provides for the inhibition of expression of α1,3-fucosyltransferase (FucT) and β1,2-xylosyltransferase (XylT) in a plant. Use of these constructs to inhibit or suppress expression of both of these enzymes, and isoforms thereof, advantageously provides for the production of endogenous and heterologous proteins having a “humanized” N-glycosylation pattern without impacting plant growth and development. Stably transformed higher plants having this protein N-glycosylation pattern are provided. Glycoprotein compositions, including monoclonal antibody compositions, having substantially homogeneous glycosylation profiles, and which are substantially homogeneous for the G0 glycoform, are also provided.
Owner:SYNTHON BIOPHARMACEUTICALS BV +1
Glycan-optimized Anti-cd20 antibodies
InactiveUS20090060921A1High antibody activityEnhanced effector functionImmunoglobulins against animals/humansVaccinesAntigenFucosylation
Glycan-optimized monoclonal antibodies that specifically bind CD20 antigen and which have improved effector function are provided. The anti-CD20 antibodies of the invention have a glycosylation pattern that results in an antibody composition having predominately the G0 glycoform, and thus comprise N-glycans that lack fucose (i.e., afucosylated) and galactose residues attached thereto. In some embodiments, these anti-CD20 antibodies comprise the light chain and heavy chain sequences of the rituximab anti-CD20 antibody, and thus represent afucosylated rituximab. Methods for producing these glycan-optimized anti-CD20 antibodies are also provided.
Owner:SYNTHON BIOPHARMACEUTICALS BV
Process for producing antibodies
InactiveUS20060269989A1High antibody activityPromote formationAnimal cellsFungiBispecific antibodyStereochemistry
Focusing on the fact that antibody molecules with one H chain are not secreted when using the “knobs-into-holes” method, the present inventors revealed that desired bispecific antibodies can be preferentially formed by first expressing the H and L chains of one arm, and then suppressing their expression, followed by expressing the H and L chains of the other arm so that first desired HL molecules (HaLa and HbLb) are constructed, and then the H chains are paired with each other (H2L2). The present invention was thus completed.
Owner:CHUGAI PHARMA CO LTD
Modified antibodies with enhanced biological activities
InactiveUS20090304715A1Enhanced ADCC activityEnhanced ADCC activity of modified antibodiesAntibody mimetics/scaffoldsAntibody ingredientsFc receptorFc(alpha) receptor
The present inventors generated modified antibodies in which several Fc domains are linked in tandem to the C terminus of the heavy chain, and modified antibodies in which Fc domains are linked in tandem via spacers, and measured the affinity for Fc receptors, CDC activity, and ADCC activity. A previous report indicated that CDC activity is not enhanced by linking multiple Fcs. However, the modified antibodies of the present invention exhibited enhanced ADCC activity. The methods of the present invention enable provision of antibody pharmaceuticals having a marked therapeutic effect.
Owner:TEIJIN PHARMA CO LTD +1
Immunoliposome inducing apoptosis into cell expressing death domain-containing receptor
InactiveUS20100209490A1Efficient drug targeting function to targetEffective therapeutic effectAntipyreticAnalgesicsDiseaseAutoimmune responses
The present invention relates to an immunoliposome preparation having a therapeutic effect on cancer, autoimmune disease, or inflammatory disease. Specifically, the present invention relates to an immunoliposome comprising, as a constituent, an antibody capable of inducing the apoptosis of cells expressing a death domain-containing receptor.
Owner:DAIICHI SANKYO CO LTD
Process for producing antibodies
InactiveUS8597911B2High antibody activityAnimal cellsFungiAntiendomysial antibodiesBispecific antibody
Focusing on the fact that antibody molecules with one H chain are not secreted when using the “knobs-into-holes” method, the present inventors revealed that desired bispecific antibodies can be preferentially formed by first expressing the H and L chains of one arm, and then suppressing their expression, followed by expressing the H and L chains of the other arm so that first desired HL molecules (HaLa and HbLb) are constructed, and then the H chains are paired with each other (H2L2). The present invention was thus completed.
Owner:CHUGAI PHARMA CO LTD
Pumpkin powder with blood sugar reducing effect and preparation method thereof
InactiveCN104543834AGood and stable hypoglycemic effectHigh antibody activityVitamin food ingredientsNatural extract food ingredientsVitamin CPuerarin
The invention discloses pumpkin powder with a blood sugar reducing effect. The pumpkin powder is characterized by consisting of the following components in percentage by weight: 10-50 percent of pumpkin powder, 0.4-3 percent of a mulberry extract, 0.4-3 percent of a puerarin extract, 0.3-2 permillage of an astragalus membranaceus extract, 2-12 percent of rhizoma anemarrhenae powder, 3-15 percent of ligustrum lucidum ait powder, 1-10 percent of purslane powder, 5-20 percent of resistant dextrin, 1-10 percent of L-arabinose, 1-6 percent of xylitol, 0.5-4 percent of xylooligosaccharide, 0.01-0.05 permillage of vitamin A, 0.01-0.05 permillage of vitamin B1, 0.01-0.05 permillage of vitamin B2, 0.01-0.05 permillage of vitamin B6, 0.01-0.05 permillage of folic acid, 1-5 permillage of vitamin C, 0.0001-0.0005 permillage of vitamin D, 0.1-0.5 permillage of vitamin E and the balance of citric acid. The pumpkin powder can be used for reducing the blood sugar of a diabetes patient and reducing the diabetic complications and has the effects of enhancing the immunity of the organism, resisting oxidization and promoting the organism to produce nutritional substances.
Owner:SHANDONG LONGLIVE BIO TECH CO LTD
Therapeutic agent for cancer, and method for treatment of cancer
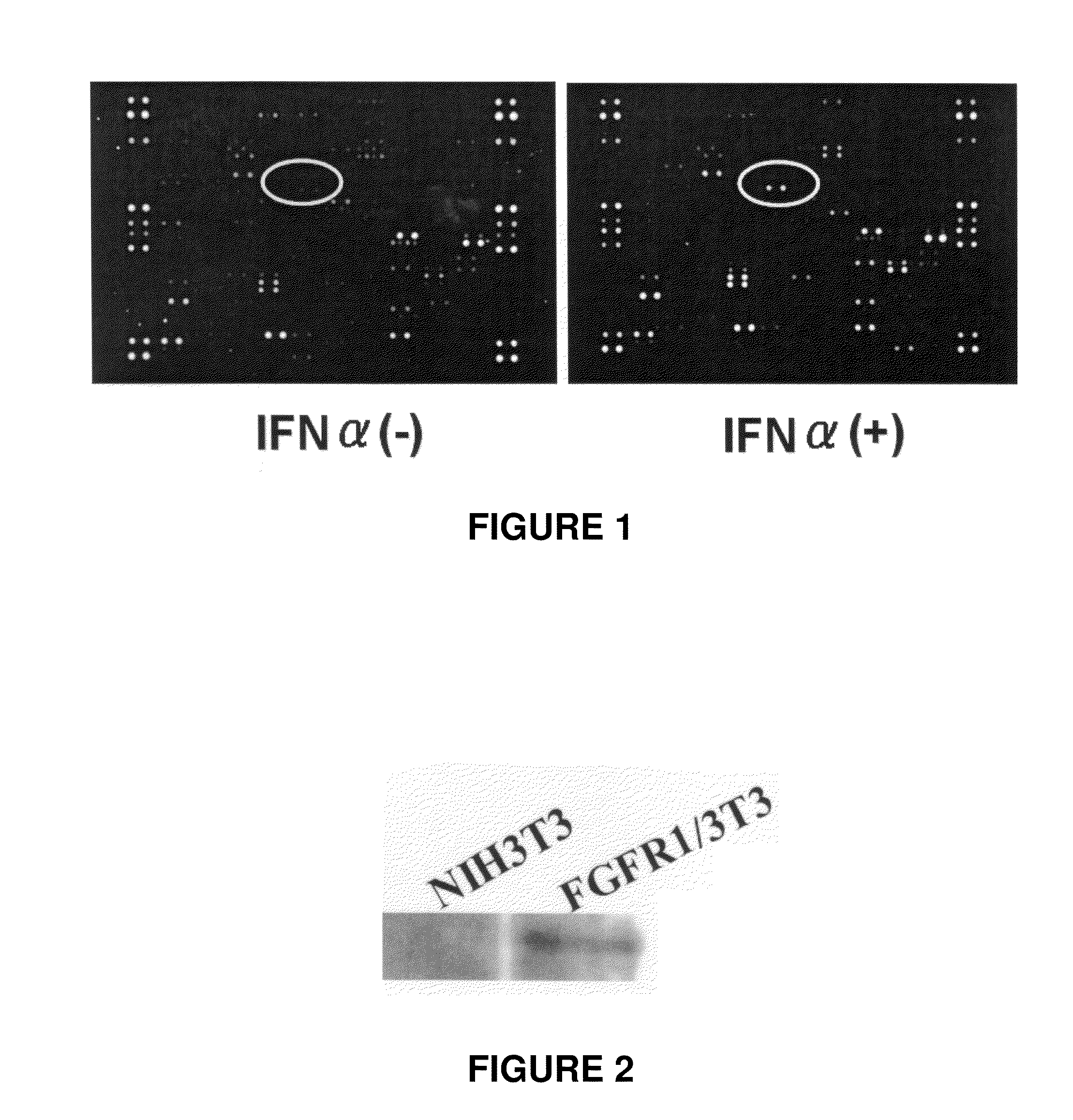
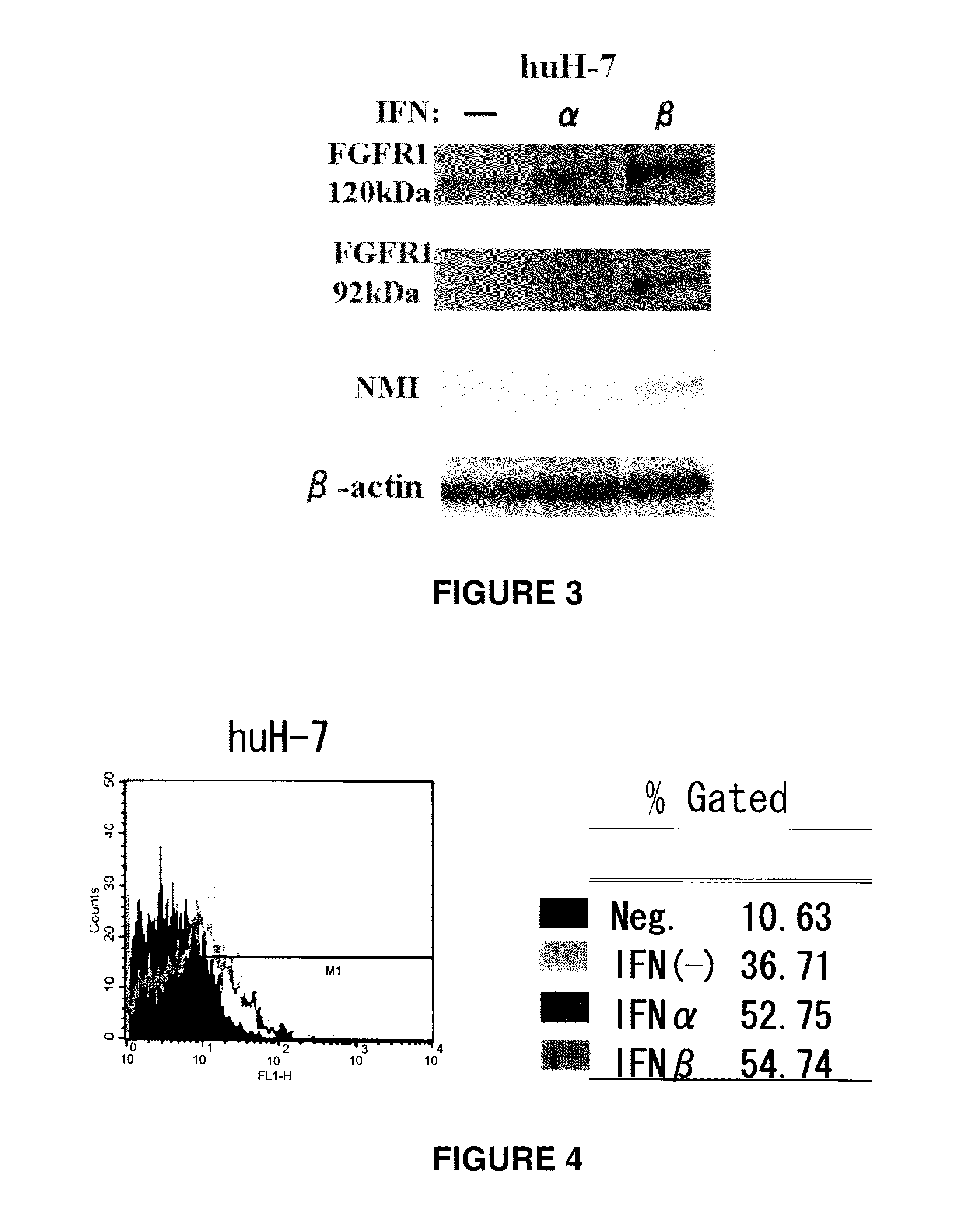
InactiveUS20110129524A1High expressionImprove aimingBiocidePeptide/protein ingredientsTherapeutic effectCancer therapy
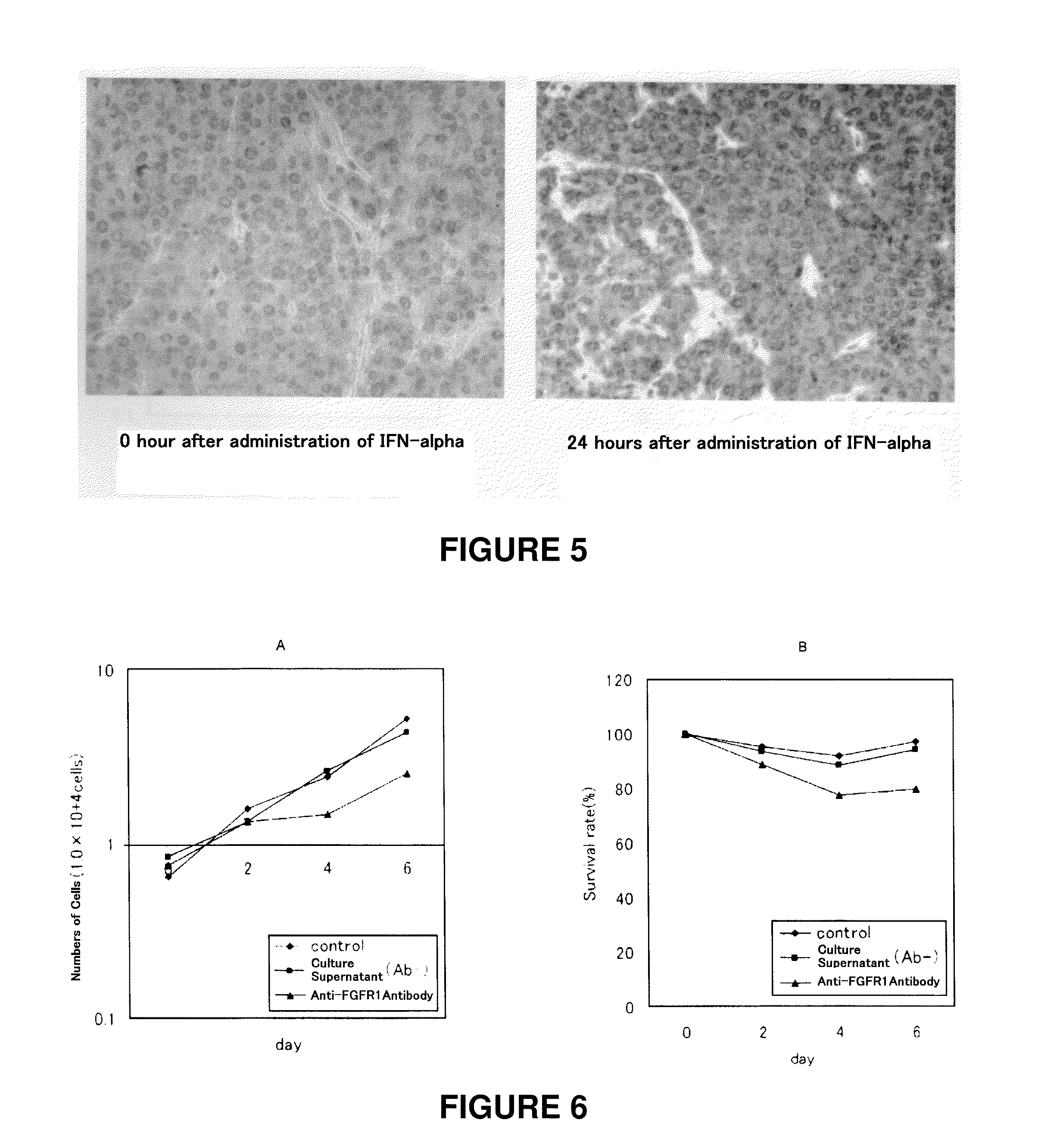
[Problems] To provide a technique which enables an effective antibody therapy for cancer which targets for FGFR1 without the need of using any effective antibody having high specificity and a potent cell-killing activity.[Means for Solving Problems] Disclosed are: a therapeutic agent for cancer, which comprises an enhancer of the expression of a fibroblast growth factor receptor-1 and an anti-fibroblast growth factor receptor-1 antibody; and a method for the treatment of cancer using the therapeutic agent.
Owner:THE UNIV OF TOKYO +1
Preparation method of fluorescence antibody for detecting Newcastle disease virus and solid-phase immunofluorescence detection assay kit
InactiveCN101975856AImprove stabilityImprove cross-linking efficiencySerum immunoglobulinsImmunoglobulins against virusesCross-linkMicrosphere
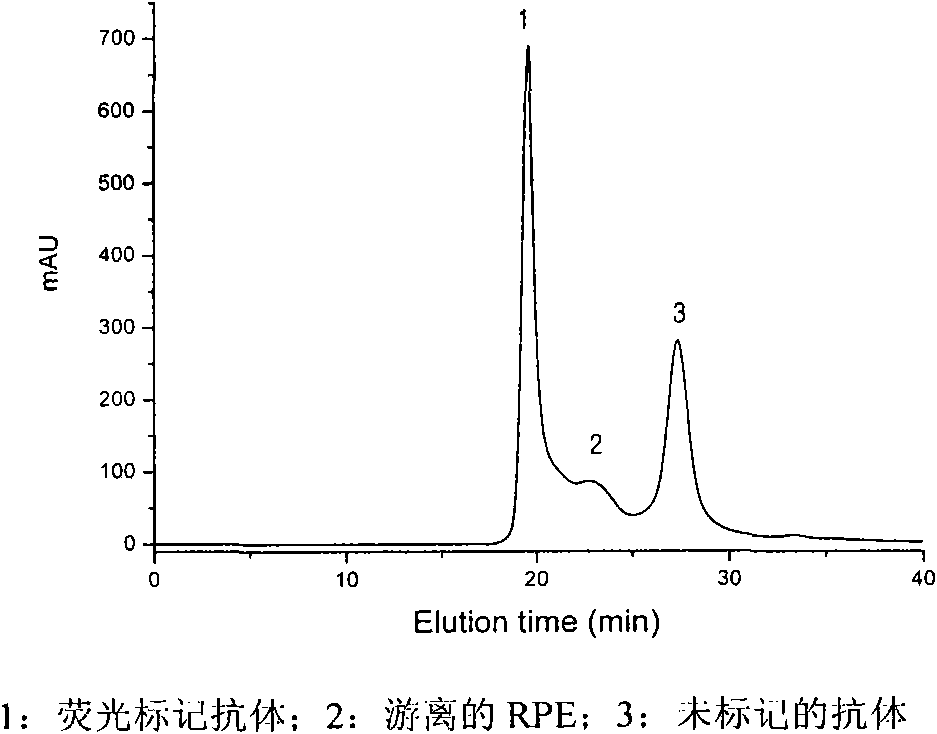
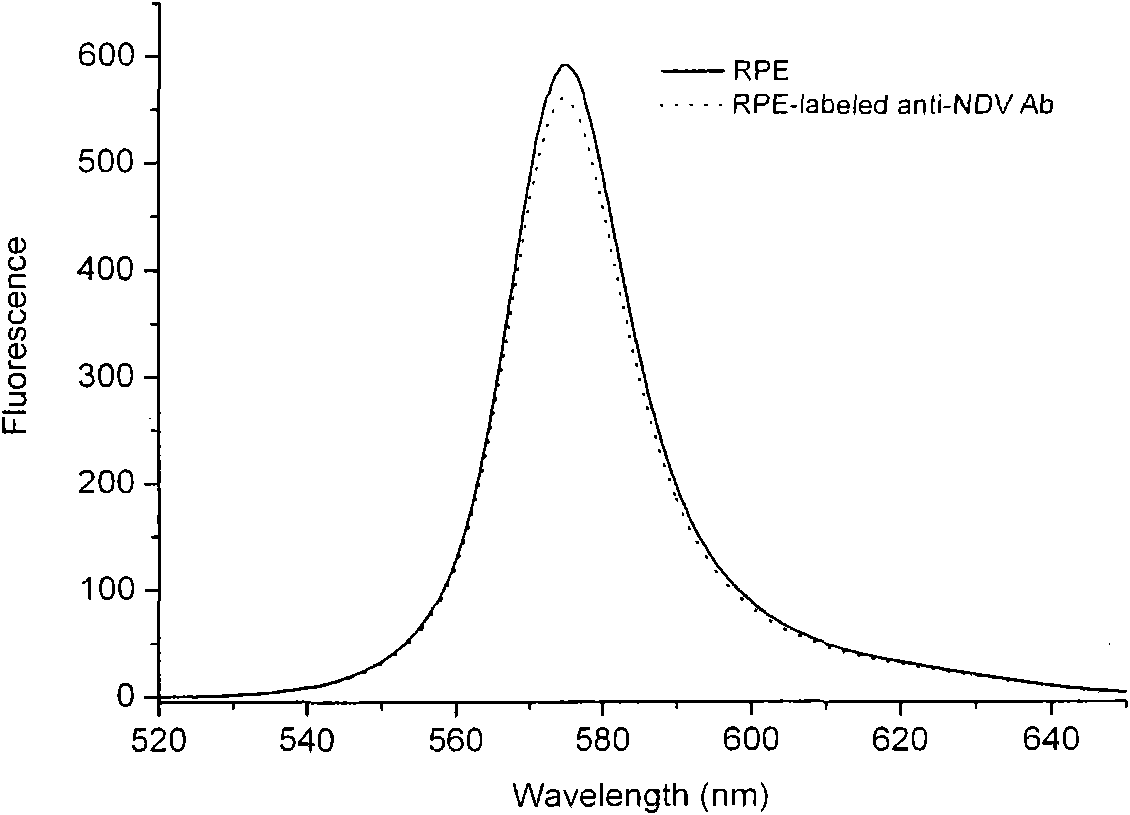
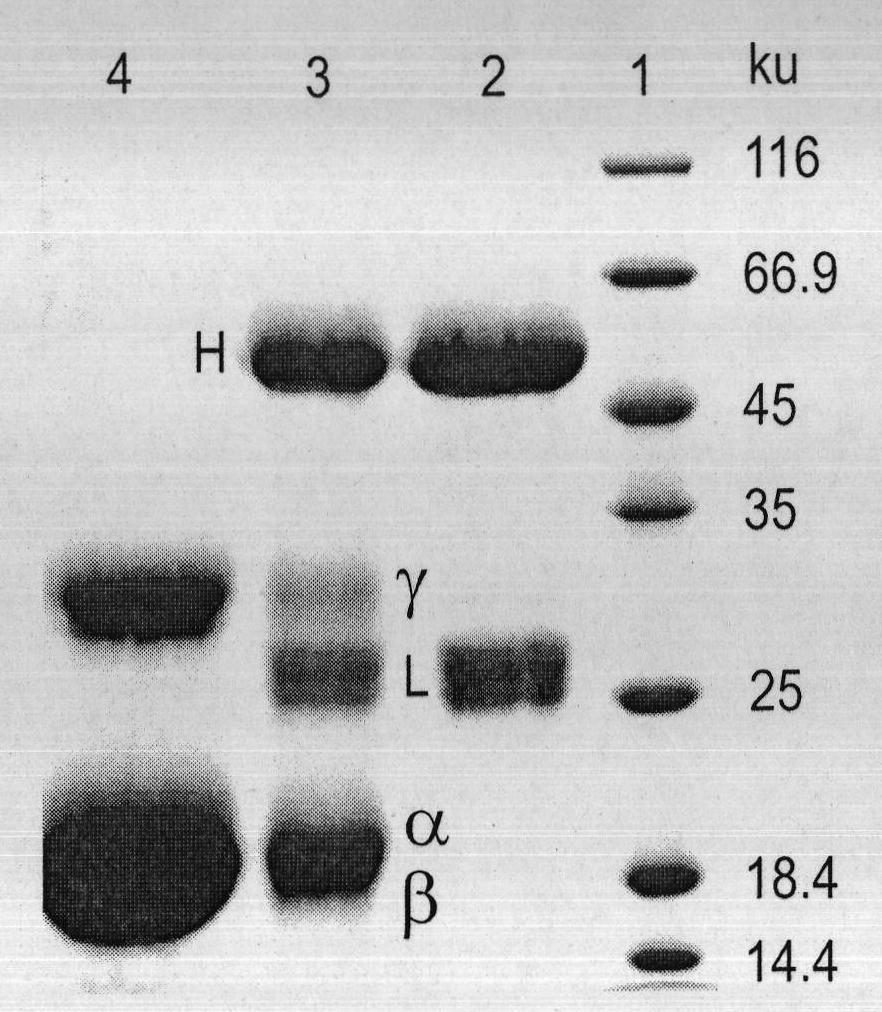
The invention relates to a preparation method of fluorescence antibody for detecting a Newcastle disease virus and a solid-phase immunofluorescence detection assay kit. The preparation method comprises the following steps of: respectively deriving R-phycoerythrin (RPE) and an antibody resisting the Newcastle disease virus (NDV) by using a cross-linking agent SPDP (N-succinimidyl-3-(2-pyridyldithiol) propionate), cross-linking the derivatives in a proper molar ratio, and purifying through HPLC (High Performance Liquid Chromatography) to prepare an RPE marked NDV fluorescence antibody. The solid-phase immunofluorescence detection assay kit is formed from the fluorescence antibody, an NDV-resisting antibody, an agarose microsphere, diluted hydrochloric acid and a washing liquid. The assay kit comprises the following detection flows of: coating an activated microsphere with the antibody, washing, combining with a sample to be measured, washing, combining with the fluorescence antibody, fully washing, exciting by blue and green light in a fluorescence microscope, observing and judging the result. The prepared fluorescence antibody has the advantages of high yield, high purity, bright orange fluorescence and good stability; and the assay kit has signal enrichment action by using a spherical carrier and can increase detection sensitivity. The invention is applicable to the rapid detection of the Newcastle disease virus.
Owner:QILU UNIV OF TECH
Method for preparing fluorescent antibody for detecting avian influenza virus and solid phase immunofluorescence detection kit
InactiveCN101957377AExcellent fluorescent dyeBright fluorescent dyeSerum immunoglobulinsImmunoglobulins against virusesCross-linkMicrosphere
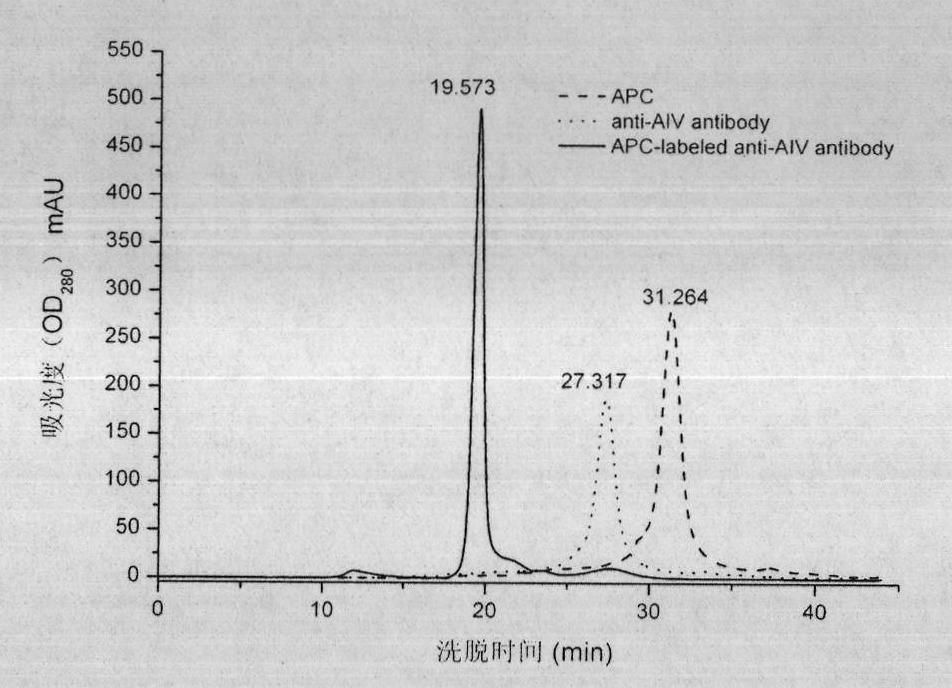
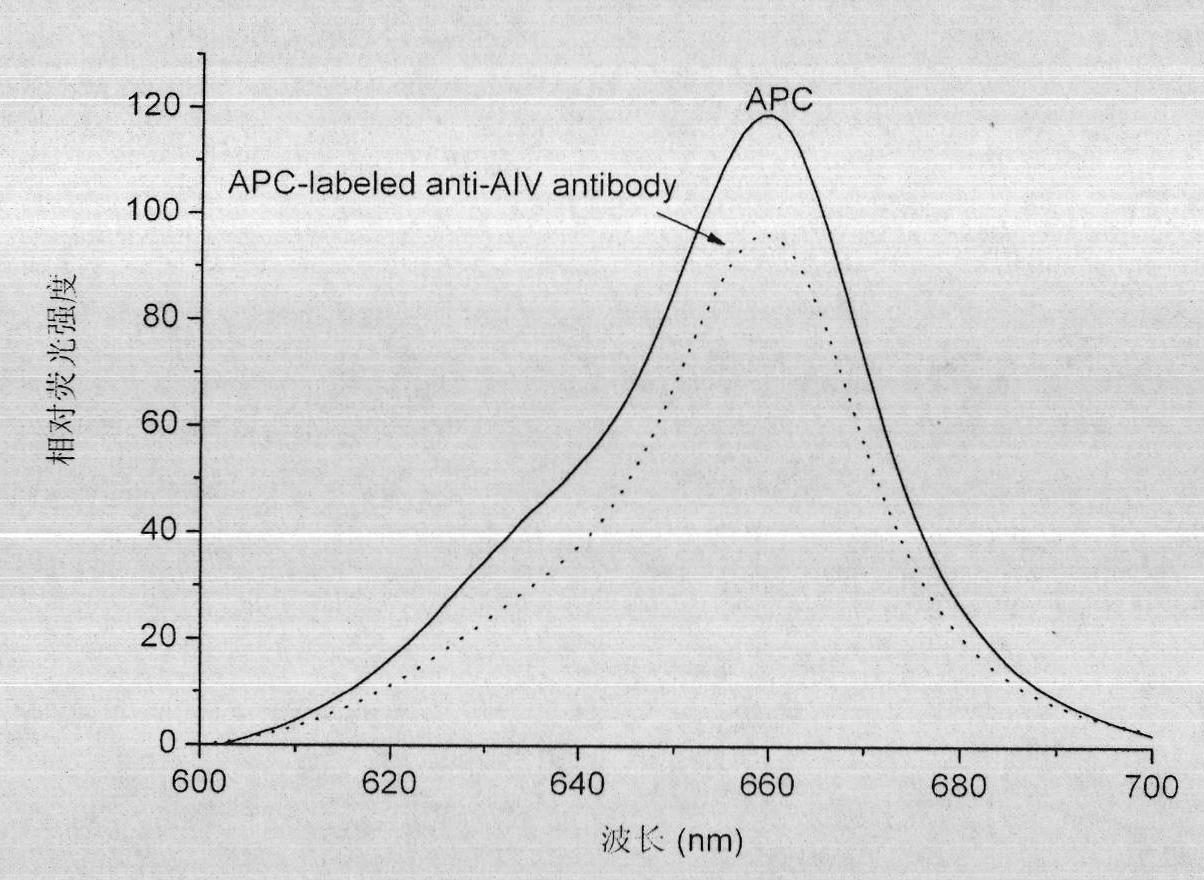
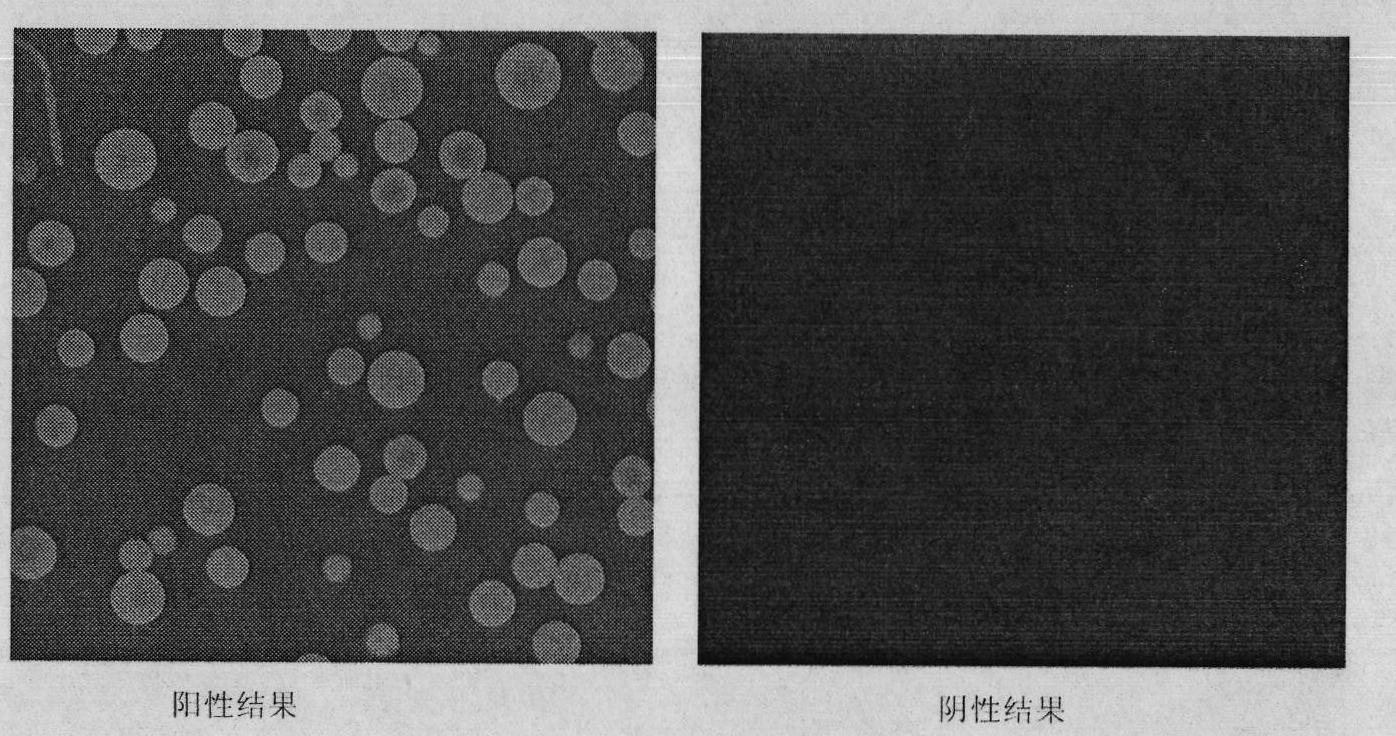
The invention relates to a method for preparing a fluorescent antibody for detecting avian influenza virus and a solid phase immunofluorescence detection kit. According to the invention, an avian influenza virus antibody is marked by allophycocyanin (APC) so as to prepare the avian influenza virus fluorescent antibody; the APC and the antibody are derived by a chemical cross-linking agent SPDP respectively; the derivatives are subjected to liquid phase cross-linking in a proper molar ratio; and then the fluorescent antibody is prepared by high pressure liquid chromatography purification. The solid phase immunofluorescence detection kit comprises the fluorescent antibody, CNBr activated agarose microspheres, the avian influenza virus antibody, cleaning solution and the like. The using method of the kit comprises the following steps of: activating a microsphere carrier and then coating the activated microsphere carrier by using the avian influenza virus antibody; cleaning; combining the microspheres coated with the antibody with a sample (an antigen) to be detected; combining the microspheres coated with the antibody with the fluorescent antibody after cleaning; removing the uncombined fluorescent antibody by cleaning; and observing under a fluorescence microscope and determining the result. The fluorescent antibody prepared by the method is characterized by high cross-linking efficiency, high purity, bright red fluorescence and stable performance. The microsphere solid phase carrier which is adopted by the kit can obviously improve fluorescent detection sensitivity, and is suitable for the quick detection of the avian influenza virus.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Anti-ror1 antibodies
ActiveUS20180142016A1Easy to understandEffectively prevent Wnt5a from bindingDipeptide ingredientsAntibody mimetics/scaffoldsNucleotideCancer therapy
The invention relates to antibodies, and in particular, to antibodies exhibiting specificity for Receptor tyrosine kinase-like Orphan Receptors (ROR), and to uses thereof, for example in the treatment of cancer. The invention extends to polynucleotide and polypeptide sequences encoding the antibodies, and therapeutic uses thereof, and to diagnostic kits comprising these molecules. The invention also extends to antibody-drug conjugates and to uses thereof in therapy.
Owner:EUREKA THERAPEUTICS INC
Complexes containing crosslinked avidin, analytical method with the use of crosslinked avidin and analytical reagents and kits
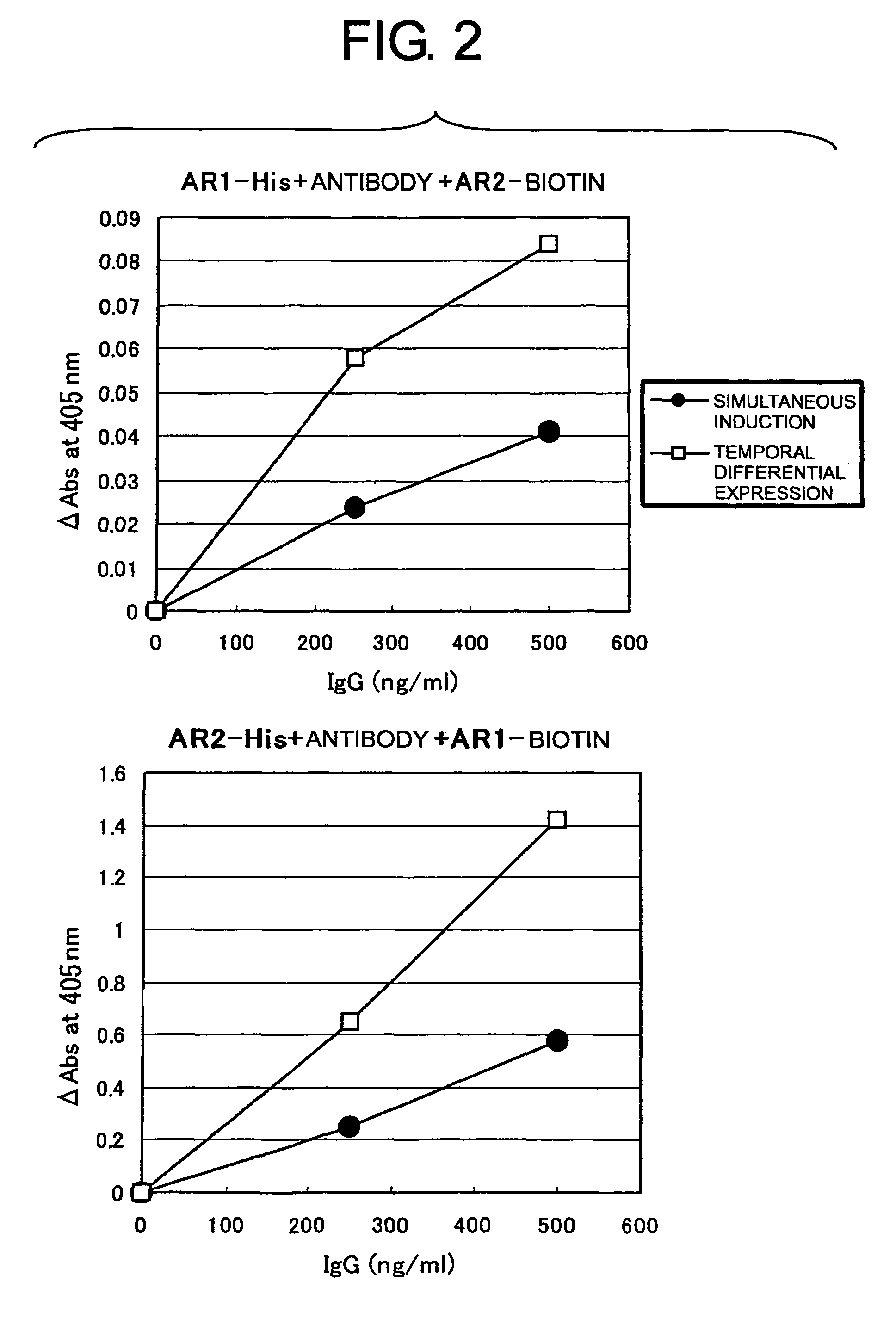
InactiveUS6787325B1High antibody activityMaintain relatively stableMicrobiological testing/measurementEnzymologyBiotin-binding proteinsAnalysis method
Provided are novel complexes containing a crosslinked avidin, an analyzing method and analyzing reagents and kits whereby a compound to be analyzed can be quickly, conveniently and accurately analyzed while taking advantage of the avidin-biotin reaction. The complexes contain at least two homogeneous or heterogeneous biotin-introduced products and one crosslinked avidin sandwiched therebetween. In the analyzing method, the homogeneous or heterogeneous biotin-introduced products and the crosslinked avidin are used. The analyzing reagent contains the crosslinked avidin. The analyzing kit contains the crosslinked avidin and a biotinylating agent.
Owner:MITSUBISHI CHEM MEDIENCE
Method for preparing prostate cancer antigen immunochromatography test paper based on hydrophobin high-efficiency fixed low-concentration antibody
InactiveCN106771143AHigh antibody activityEnhanced ability to capture antigensMaterial analysisAntigenNovel technique
The invention relates to a method for preparing prostate cancer antigen immunochromatography test paper based on a hydrophobin high-efficiency fixed low-concentration antibody. As exogenous biomolecules can be subjected to oriented immobilization of type-II hydrophobin, antibodies sprayed to a test paper nitrocellulose membrane can be arranged in an oriented manner, the 'Fab' ends of the antibodies are sufficiently exposed, the utilization rate of an antibody active site can be increased, the detection sensitivity can be improved, the amount of the antibodies can be reduced, the detection cost can be lowered, the nitrocellulose membrane is modified by using the hydrophobin, novel immunochromatography test paper based on the hydrophobin high-efficiency fixed low-concentration antibody can be prepared, the test paper comprises a sample pad and two conjugate pads, a detection line, the nitrocellulose membrane with the detection line and a quality control line antibody, an absorption pad and a bottom plate are simultaneously modified with the hydrophobin, and the method is a novel technique applicable to tumor marker detection.
Owner:TIANJIN UNIV
Stable troponin I detection kit
ActiveCN104142406AHigh antibody activityImprove accuracyDisease diagnosisBiological testingAntiendomysial antibodiesSucrose
The invention relates to a stable troponin I detection kit. The content of raw materials of each component of the stable troponin I detection kit is as follows: a component 1 comprises 25mmol / L glycine buffer solution with the pH value of 7.6, 150mmol / L sodium chloride and 1 percent of sucrose ester; a component 2 comprises 25mmol / L glycine buffer solution with the pH value of 7.6, 3 percent of sucrose, 8 percent of glycerinum, 0.1 percent of EDTA (ethylene diamine tetraacetic acid) and 3 percent of goat anti human TnI antibody cladded latex particles. The stable troponin I detection kit is good in accuracy and can be stably stored for one year at the temperature of 2 to 8 DEG C.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Fab-glycosylated antibodies
ActiveUS9359439B2Improved ability to induce different activity of immune systemHigh antibody activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSialic acidHalf-life
The present invention pertains to a method for controlling the circulation half-life of antibodies by adjusting the amount of sialic acid in the carbohydrates attached to the Fab part of the antibodies. Furthermore, the present invention provides antibodies having an increased circulation half-life.
Owner:GLYCOTOPE GMBH
Polypeptide, polypeptide derivative, and polypeptide-antibody complex and preparation and application of polypeptide
InactiveCN110551177ASimple preparation processReduce manufacturing costMammal material medical ingredientsAntibody ingredientsBispecific antibodyT cell
The invention discloses a polypeptide, and belongs to the field of biomedicine. In order to solve the problems of complicated production process, high cost and low targeting existing in existing methods for enhancing the targeting of T cells, the invention provides the polypeptide. The polypeptide can significantly enhance the targeting of immune cells. At the same time, the preparation process ofthe polypeptide is simple, and the cost is lower than the existing cost of preparing CAR-T cells or the existing cost of preparing bispecific antibody cells.
Owner:安徽瑞达健康产业有限公司
Milk product with specific immunity of anti-enterobacter sakazakii and the preparing method thereof
The invention relates to a method for preparing enterobacteria specific immunity milk product which contains enterobacteria specific IgY antibody. The preparation comprises that first cultivating single enterobacteria or various viruses, preparing single or composite antigen, then using the antigen to process high-immunity ejection into egg-laying bird, checking the immunity egg, preparing enterobacteria specific IgY or enterobacteria specific composite IgY, purifying, filtering into the final product, mixing the product with some milk powder, to obtain immunity milk product. The invention can effectively resolve the problem of infant milk powder that polluted by enterobacteria and salmonella.
Owner:SHENZHEN JASON INTELLIGENT BIOTECH CO LIMLTED PRC
Anti-ROR1 antibodies
ActiveUS11155615B2Simple structureEffectively prevent Wnt5a from bindingDipeptide ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesTyrosine
The invention relates to antibodies, and in particular, to antibodies exhibiting specificity for Receptor tyrosine kinase-like Orphan Receptors (ROR), and to uses thereof, for example in the treatment of cancer. The invention extends to polynucleotide and polypeptide sequences encoding the antibodies, and therapeutic uses thereof, and to diagnostic kits comprising these molecules. The invention also extends to antibody-drug conjugates and to uses thereof in therapy.
Owner:EUREKA THERAPEUTICS INC
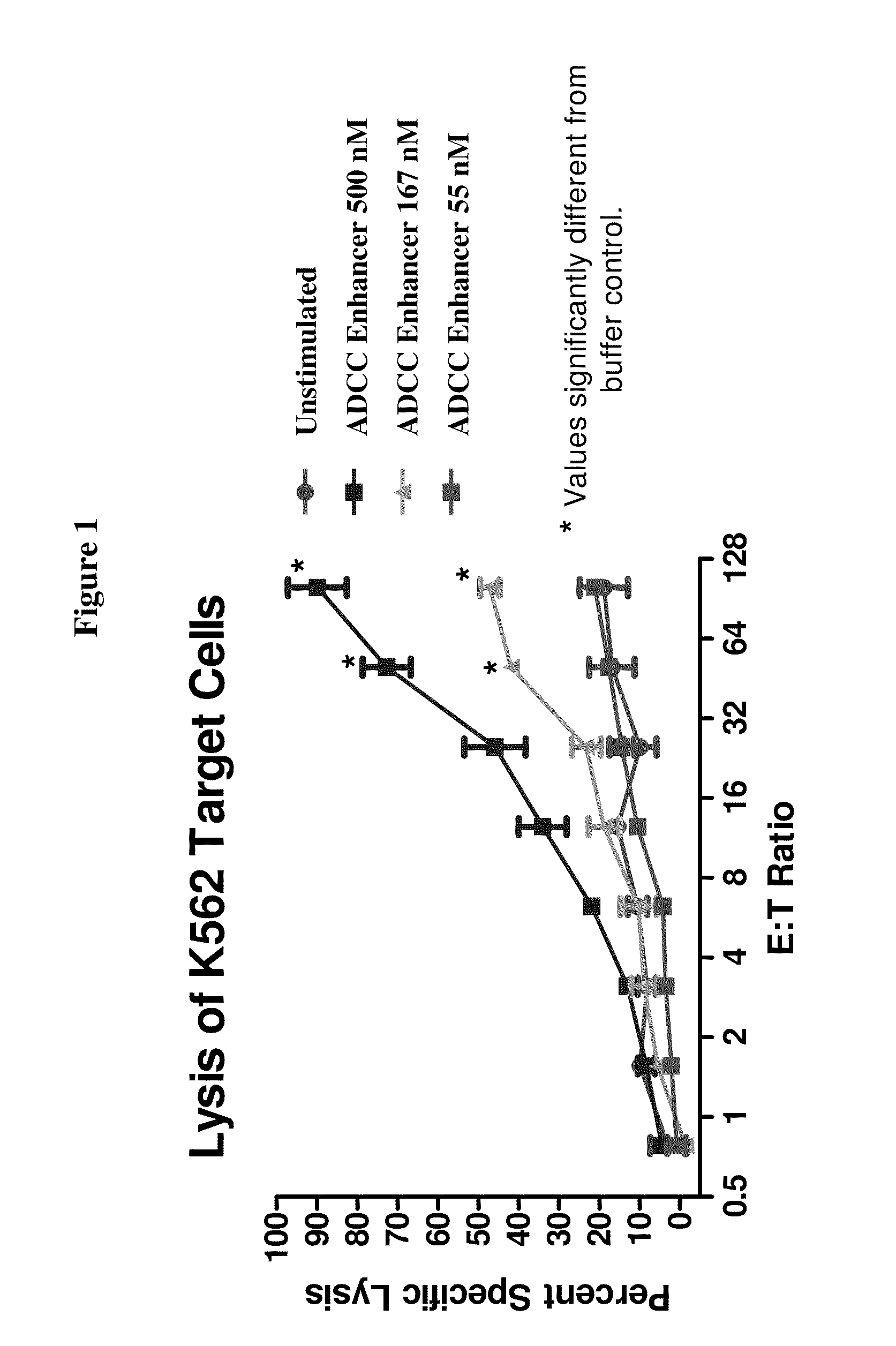
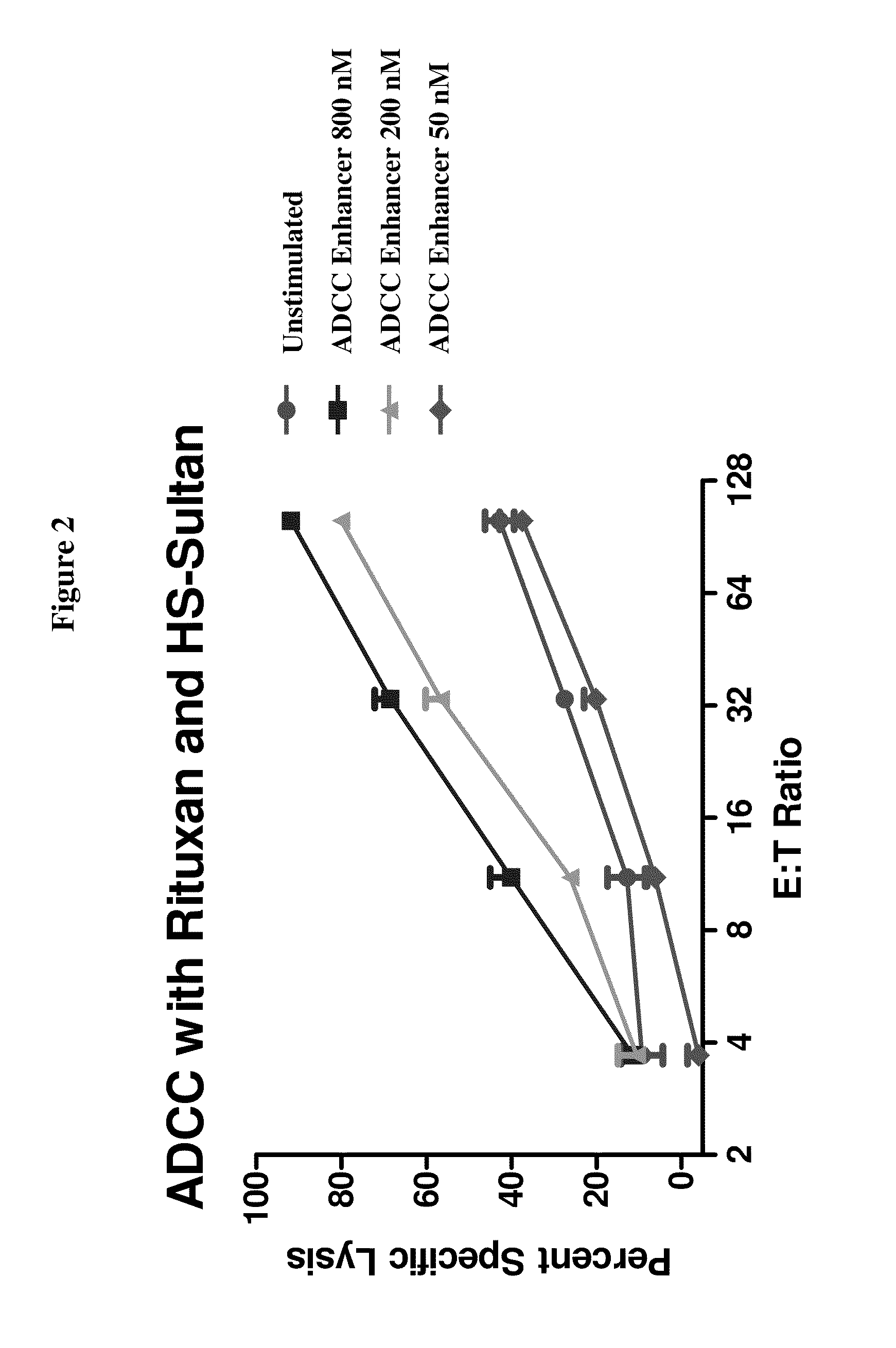
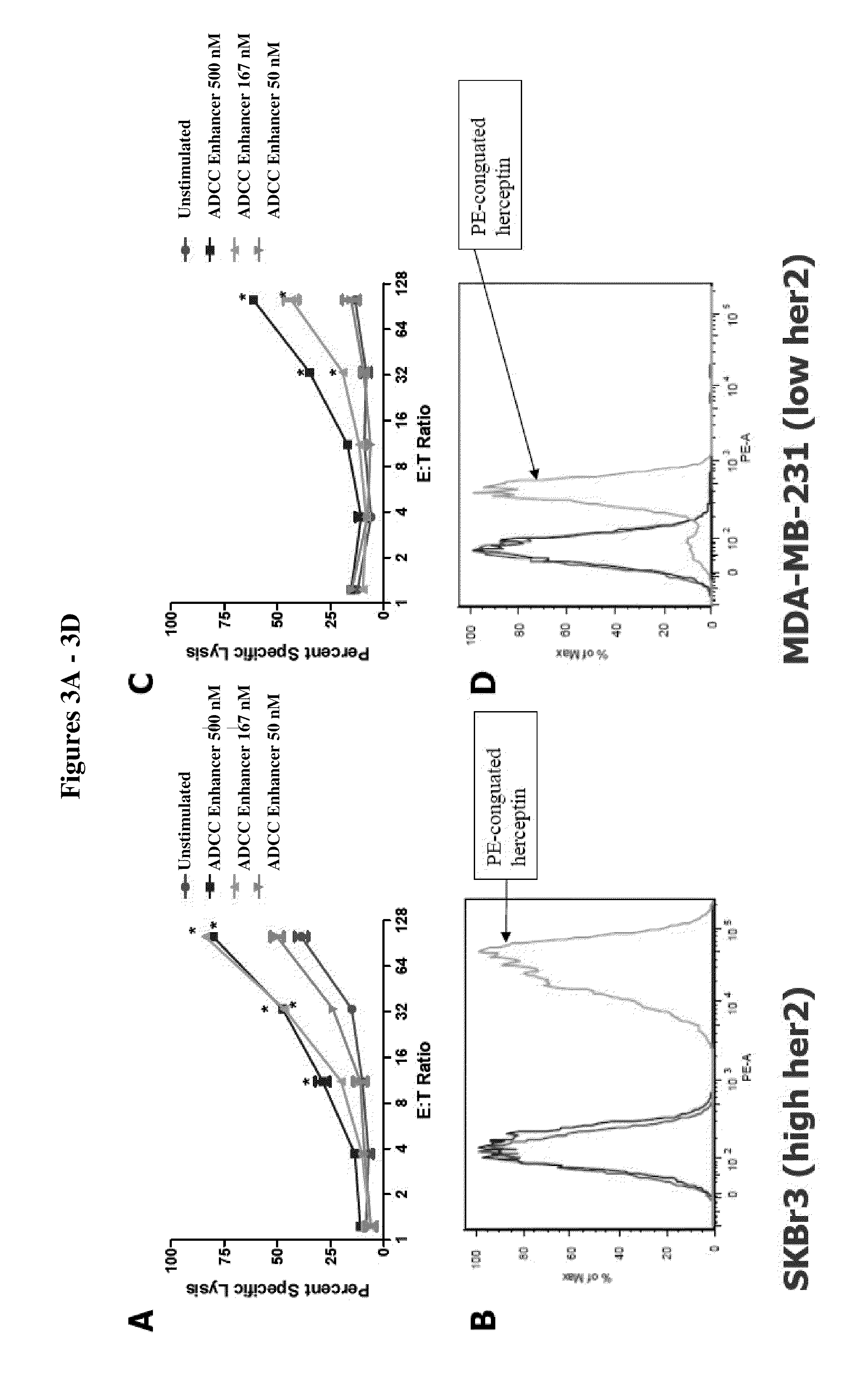
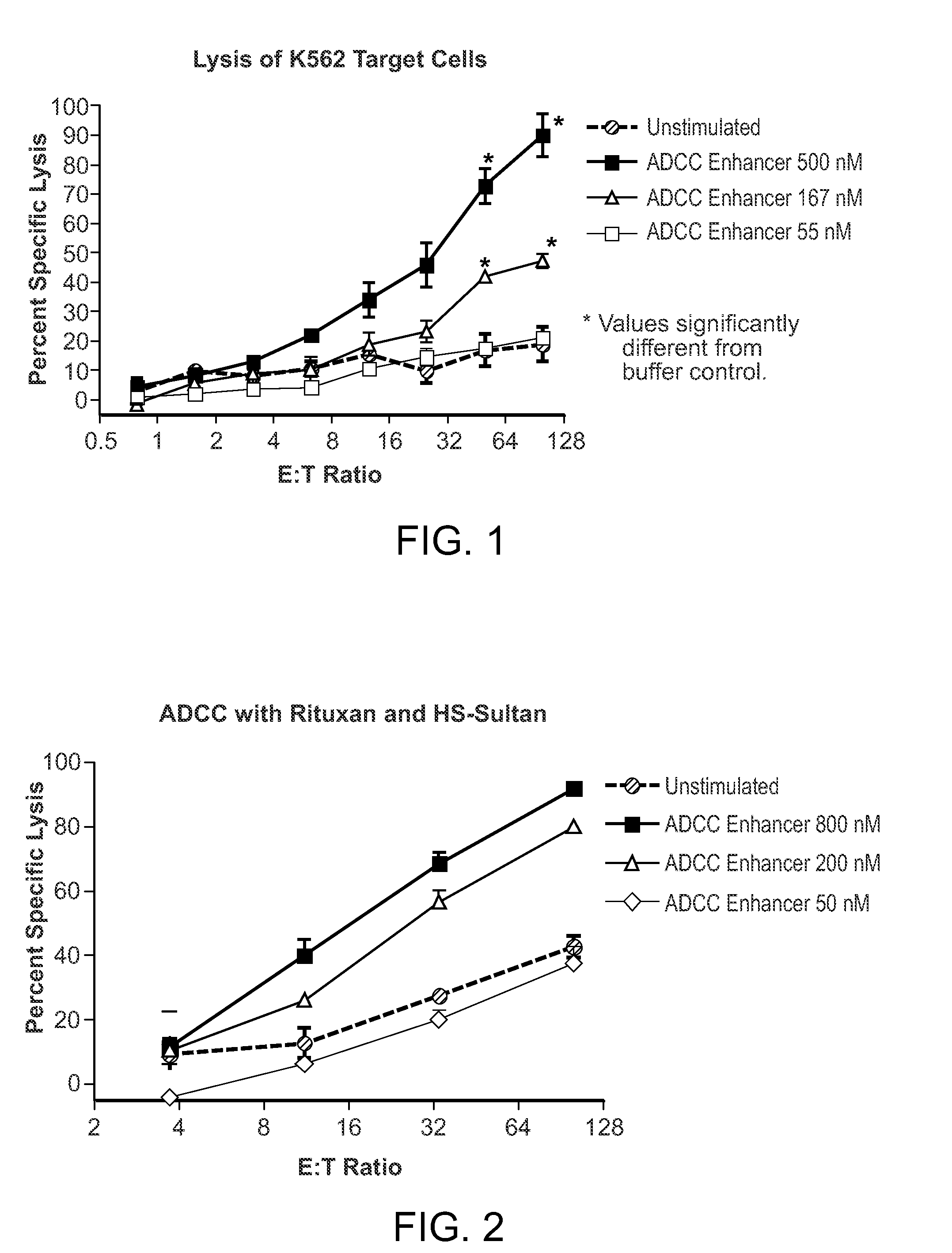
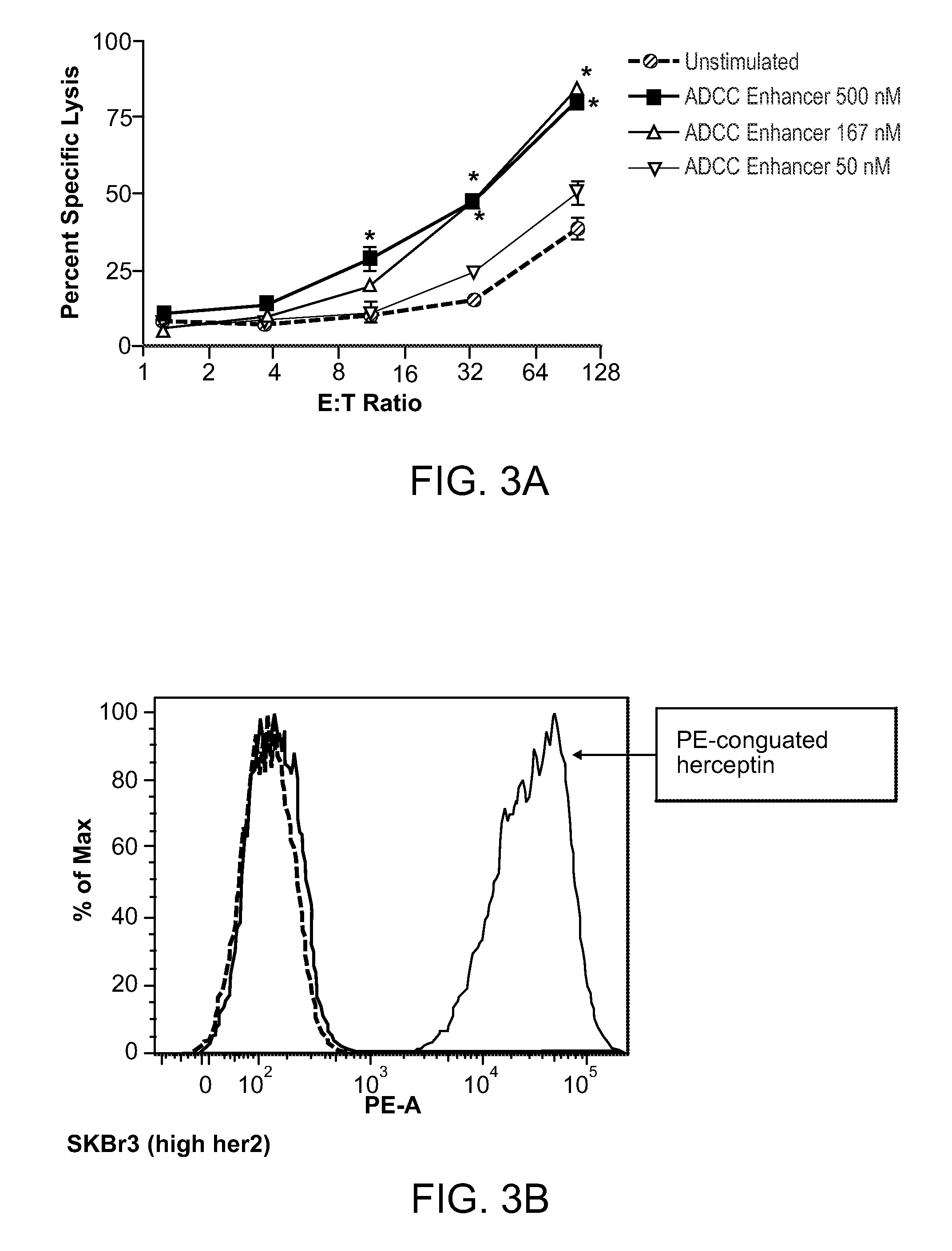
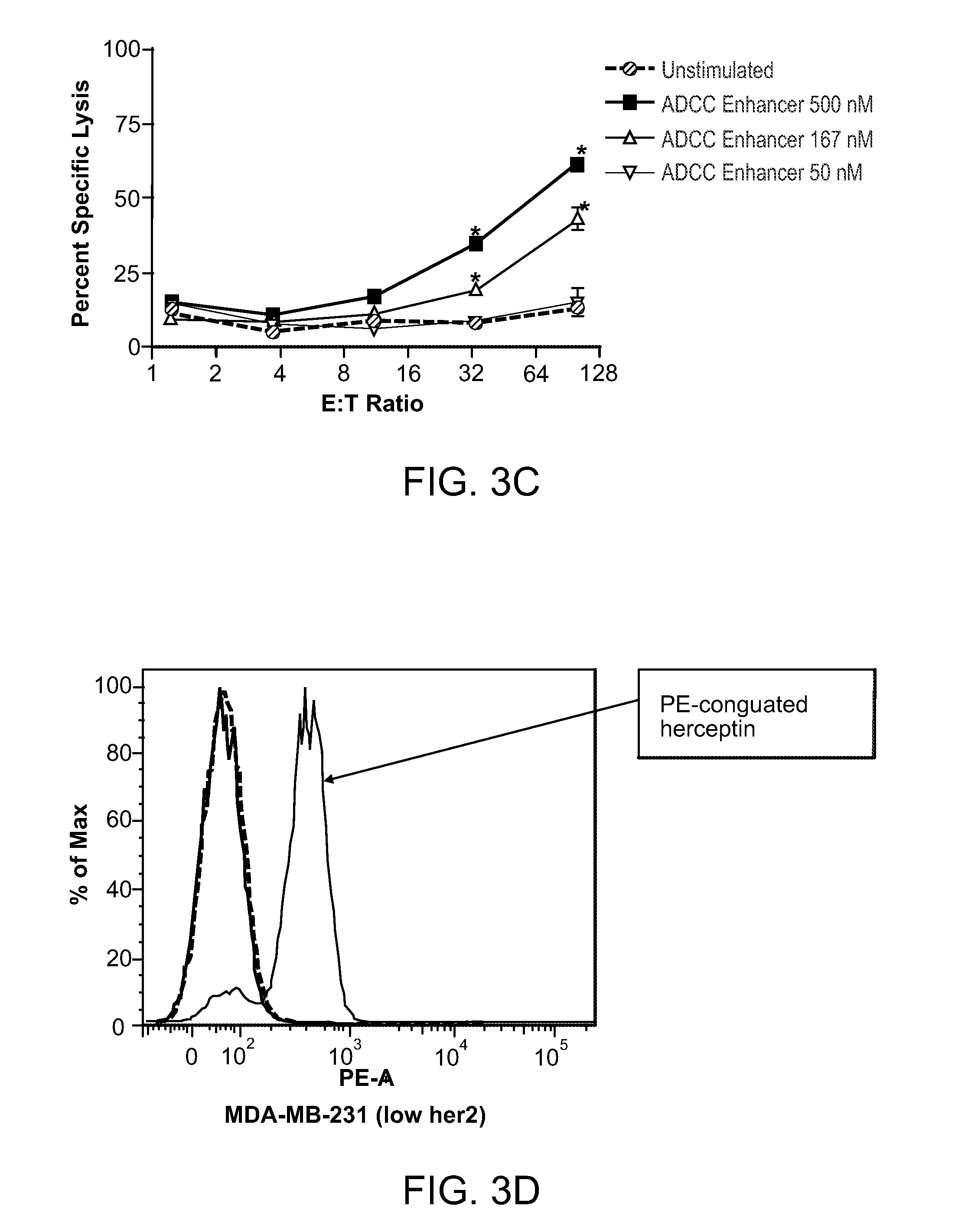
Methods Of Enhancing Antibody-Dependent Cellular Cytotoxicity
ActiveUS20120003213A1Enhance overall effectiveness of therapyIncrease efficiencyNervous disorderAntipyreticToxicityMonoclonal antibody therapy
The application relates to method of increasing antibody-dependent cellular cytotoxicity in a subject receiving therapeutic monoclonal antibody treatment. In some embodiments, methods are provided for administering to subject to a subject in need thereof a therapeutic antibody in conjunction with an ADCC enhancer molecule.
Owner:VENTIRX PHARMA
Preparation method of chicken adenovirus egg yolk antibody whole egg spray-dried powder
InactiveCN111632139ASpecific immune activity hazardDoes not cause deactivationPowder deliveryEgg immunoglobulinsAnimal scienceEggs per gram
The invention discloses a preparation method of chicken adenovirus egg yolk antibody whole egg spray-dried powder. The preparation method comprises the following steps: injecting an adenovirus vaccineinto laying hens, carrying out immunological detection on egg yolk antibodies in egg yolk, and collecting eggs when the agar diffusion titer of the egg yolk antibodies of adenovirus reaches 1:15 or above; and cleaning and disinfecting the collected eggs, beating the eggs under a sterile condition, adding water into the whole egg liquid, performing stirring, separately carrying out steps of yeastsugar removal and protein digestion by pepsase, and then carrying out warm bath, cooling, and carrying out spray drying to obtain the product. The whole egg antibody powder obtained by the preparationmethod provided by the invention is good in solubility, low in antibody loss and high in antibody activity, cost is greatly reduced, and the whole egg antibody powder has relatively good economic andsocial benefits.
Owner:QINGDAO RUNDA BIOTECH
Methods of enhancing antibody-dependent cellular cytotoxicity
InactiveUS20130236449A1Good treatment effectHigh activityNervous disorderAntipyreticTherapeutic antibodyCellular Cytotoxicity
Owner:VENTIRX PHARMA
Fab-glycosylated antibodies
ActiveUS20130209458A1Readily apparentImproved ability to induce different activity of immune systemImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSialic acidHalf-life
The present invention pertains to a method for controlling the circulation half-life of antibodies by adjusting the amount of sialic acid in the carbohydrates attached to the Fab part of the antibodies. Furthermore, the present invention provides antibodies having an increased circulation half-life.
Owner:GLYCOTOPE GMBH
Brewing type egg custard powder capable of rehabilitation and health preservation
InactiveCN105011211AEnhance phagocytosisImprove featuresNatural extract food ingredientsFood ingredient functionsAnimal scienceProduct processing
The invention relates to brewing type egg custard powder capable of rehabilitation and health preservation, and belongs to the field of processing of agricultural products. The brewing type egg custard powder disclosed by the invention is prepared through the following steps: using eggs as a main raw material, beating egg liquid, cooking the egg liquid through negative pressure, mechanically cutting the cooked egg liquid, allocating the cut egg liquid, performing self-assembly on the allocated egg liquid, drying the self-assembled egg liquid and packing the dried egg liquid to obtain convenient and instant egg custard powder. The brewing type egg custard powder disclosed by the invention is characterized in that the cooked egg liquid is mechanically cut, and the cut egg liquid, allocated functional factors and a gel additive are self-assembled and merged. The made egg custard powder is rich in nutrition and convenient to eat.
Owner:NANCHANG UNIV
IgM purification method
ActiveCN112409477AGood reproducibilityHigh antibody activityPeptide preparation methodsImmunoglobulinsProteinAntiendomysial antibodies
The invention relates to the technical field of antibodies, and particularly relates to an IgM purification method. The IgM purification method comprises the following steps of: a, providing a samplecontaining IgM; b, performing at least one time of protein precipitation treatment and at least one time of protein concentration treatment to obtain a protein precipitate; c, redissolving the proteinprecipitate, and removing impure protein by using an octanoic acid-ammonium sulfate method to obtain a crude extract containing the IgM; d, performing hydrophobic chromatography treatment, and collecting a penetrating substance; e, performing hydroxyl phosphate limestone chromatography, and collecting an eluate; and f, performing purification treatment by using affinity chromatography resin capturing IgG, and collecting a penetrating substance. The purity of the IgM in a composition obtained by purification according to the method is 95% or above, the product is stable, precipitates are not easy to appear after cryopreservation, and the antibody activity is good.
Owner:GUANGDONG FAPON BIOTECH CO LTD
A kind of anti-phosphatase 2cm monoclonal antibody and its application
InactiveCN106967690BHigh purityHigh antibody activityMicroorganism based processesDisease diagnosisAntiendomysial antibodiesGenetic engineering
The invention provides an anti-phosphatase 2Cm monoclonal antibody and also provides the preparation and application of the monoclonal antibody. A high-purity phosphatase 2Cm recombinant protein is obtained through a genetic engineering method. The most stable and most active hybridioma cell line of a phosphatase-secreting 2Cm protein antibody is screened out by using the recombinant protein, the preservation number of the hybridioma cell line is CGMCC NO.13818. The monoclonal antibody produced by the hybridioma cell line has high specificity and strong affinity, the content of rate-limiting enzyme phosphatase 2Cm of the BCAA metabolic process can be detected, and the anti-phosphatase 2Cm monoclonal antibody is used for auxiliary diagnosis of relevant diseases caused by BCKDH lack and can be also used for early screening of animal selection and breeding.
Owner:CHINA AGRI UNIV
A method for immobilizing hepatitis B antibody on the surface of medical equipment
The invention discloses a method for immobilizing hepatitis B antibody on the surface of a medical device, which mainly includes: 1) pretreatment of the surface of the device body, 2) preparation of holes, 3) post-treatment of the surface of the device body, and 4) process steps of fixing the antibody. It is characterized in that: the process steps of immobilizing antibodies are: treating the bracket with holes with citrate buffer solution; immobilizing hepatitis B antibody and incubating; storing at low temperature after drying; providing serum albumin nutrient buffer solution and incubating; cyclically variable temperature incubation ; fluorescently labeled and incubated; washed and allowed to dry. Compared with the prior art, the present invention has the advantages of high firmness of antibody immobilization, high activity of the antibody on the scaffold, and high detection accuracy.
Owner:巩晓东
IL-6 immunoturbidimetry detection kit prepared based on recombinant monoclonal antibody, and preparation method thereof
PendingCN112595853AHigh detection sensitivityHigh sensitivityBiological material analysisBiological testingPolyclonal antibodiesActive agent
The invention discloses an IL-6 immunoturbidimetry detection kit prepared based on a recombinant monoclonal antibody. The IL-6 immunoturbidimetry detection kit comprises a reagent R1 and a reagent R2.Two prepared anti-IL-6 antibody latex microsphere mother solutions are used as one component of reagent R2 of the kit, PEG 6000 is added to the reagent R1 according to a certain proportion, the reagent R1 is composed of a buffer solution, a surfactant, a stabilizer and a preservative, and the reagent R2 is composed of a buffer solution, a surfactant, a stabilizer, a preservative and an antibody.The invention also discloses a preparation method of the IL-6 immunoturbidimetry detection kit prepared on the basis of the recombinant monoclonal antibody. Compared with a similar kit for determiningIL-6 by latex enhanced immunotransmission turbidimetry, the kit disclosed by the invention adopts a mode of combining two anti-human IL-6 monoclonal antibodies, can be used for respectively identifying two antigen epitopes, has higher sensitivity than a single monoclonal antibody kit, and has better specificity than a polyclonal antibody kit.
Owner:ANHUI DAQIAN BIO ENG LIMITED
Modified Antibodies With Enhanced Biological Activities
InactiveUS20190367630A1Enhanced ADCC activityEnhanced ADCC activity of modified antibodiesAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsFc(alpha) receptorTherapeutic effect
Owner:TEIJIN PHARMA CO LTD +1
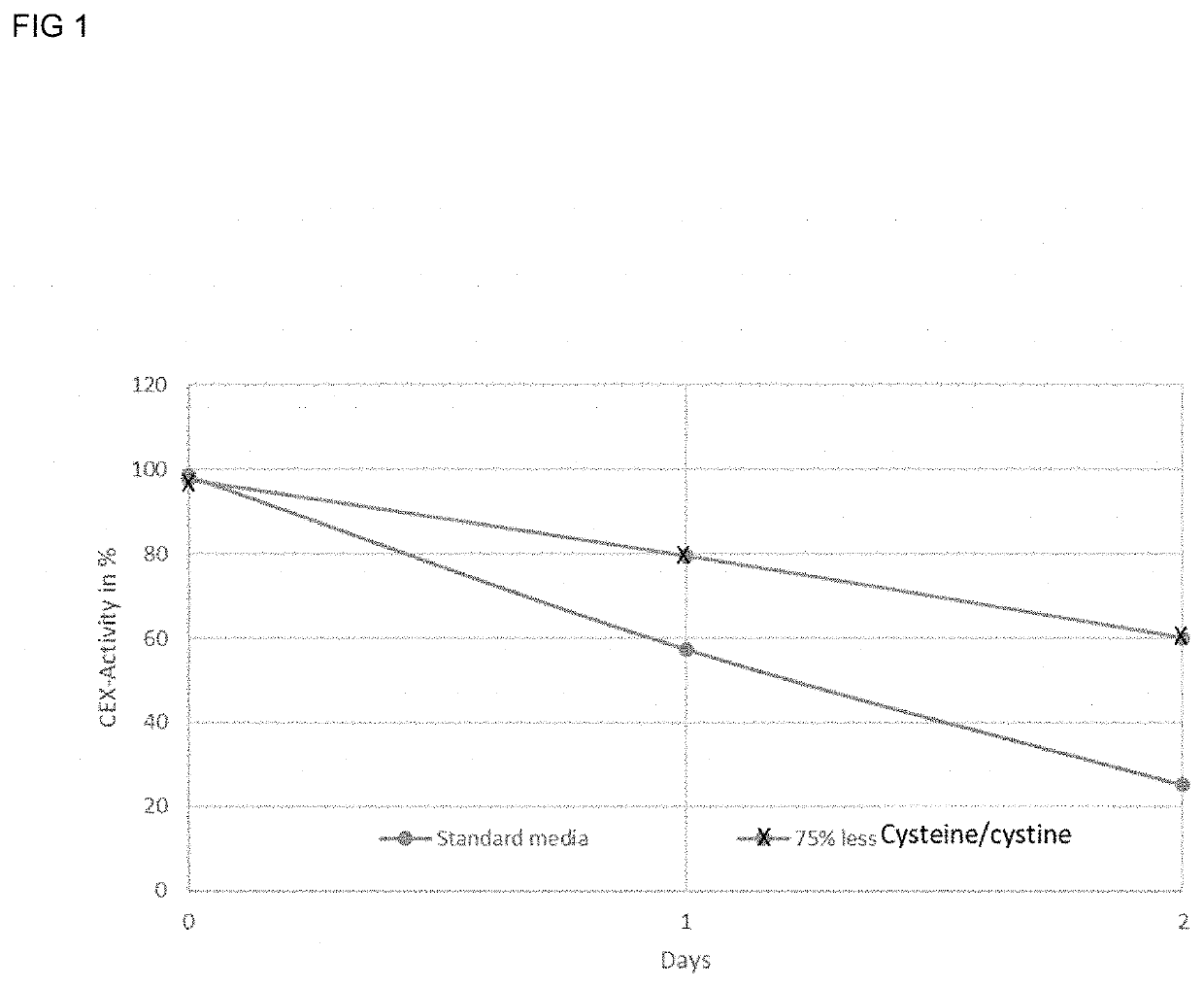
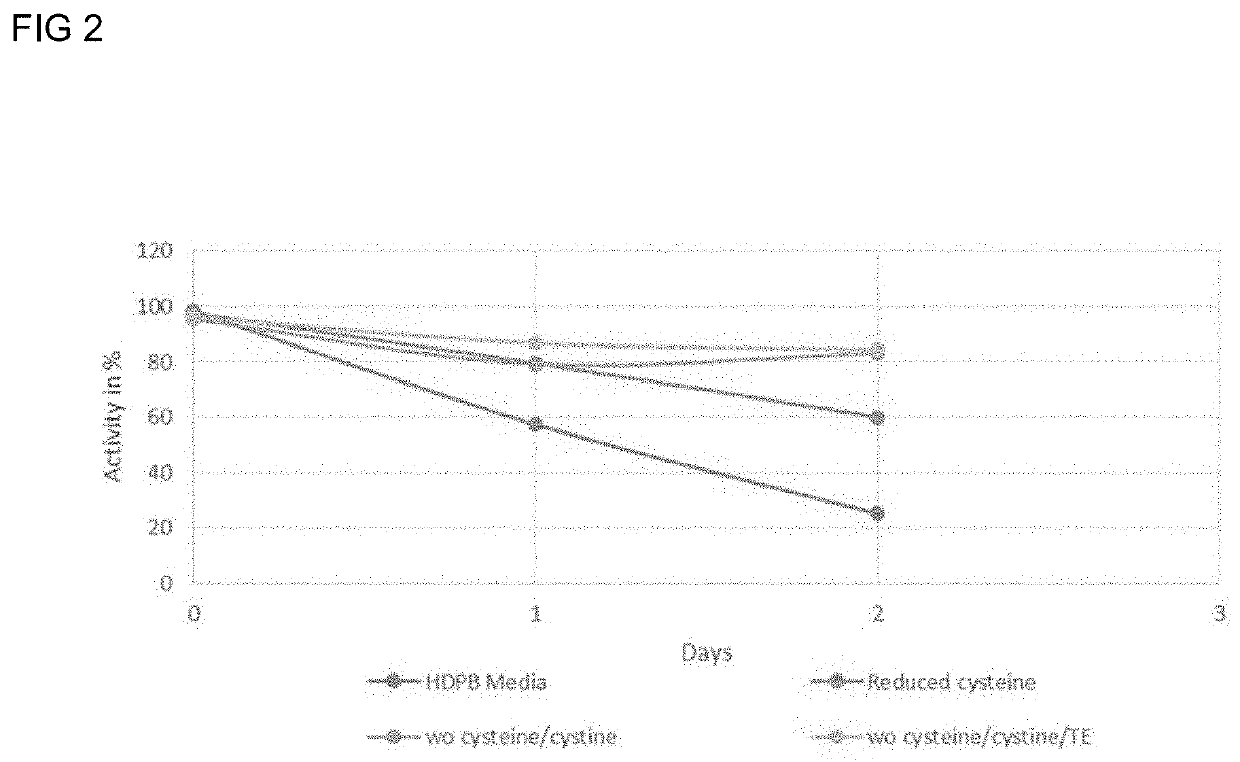
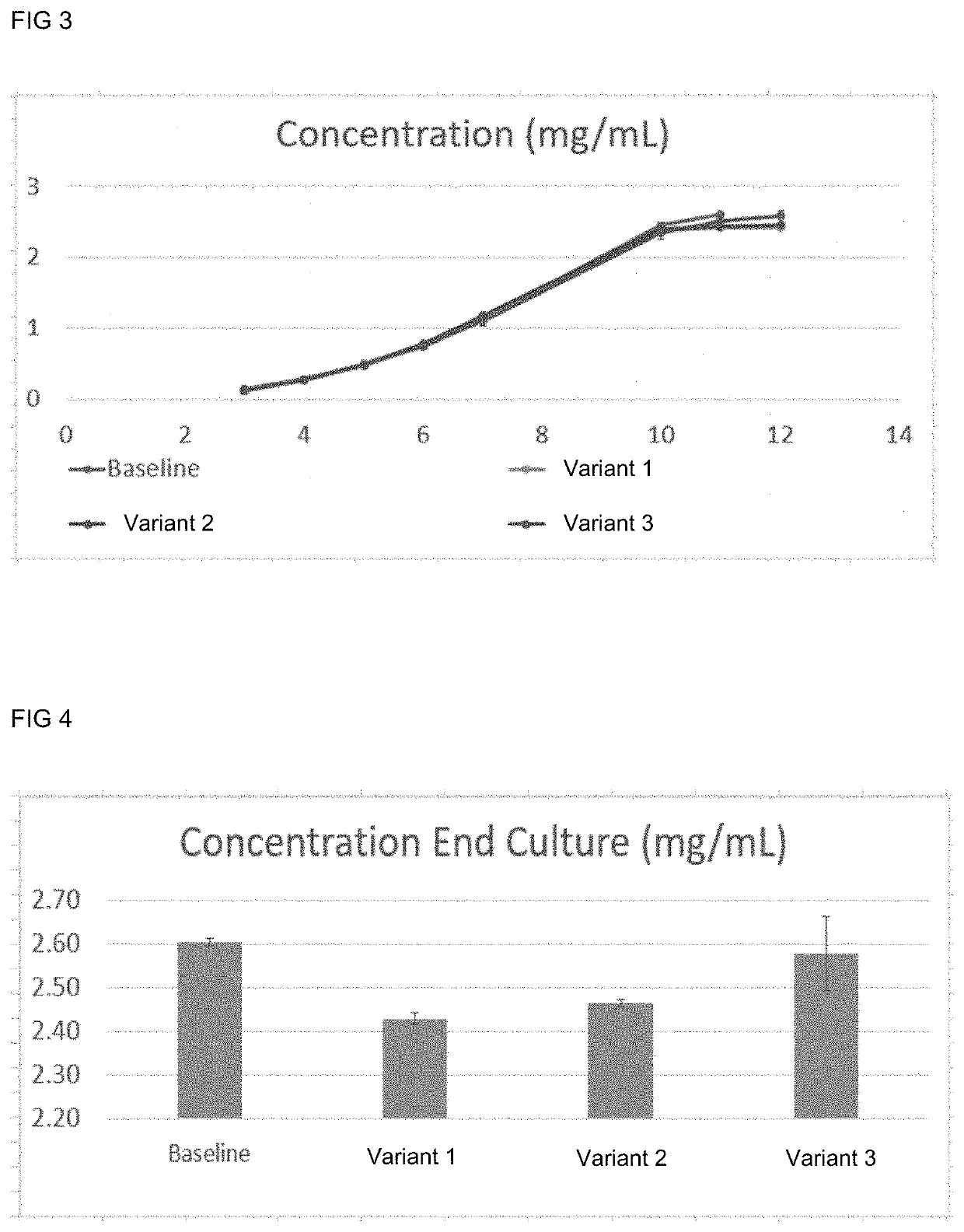
Methods for reducing the oxidation level of cysteine residues in a secreted recombinantly-expressed protein during cell culture
PendingUS20220251502A1Reduce concentrationReduce the amount requiredCulture processCell culture mediaSecukinumabAntiendomysial antibodies
The present disclosure relates to methods for reducing the oxidation level of cysteine residues in recombinant polypeptides such as anti-IL-17 antibodies during cell culture (e.g., a preparation of secukinumab antibodies) that have been recombinantly produced by mammalian cells. Also provided are purified preparations of recombinant polypeptides such as anti-IL-17 antibodies or antigen binding fragments thereof produced by such methods, e.g, purified preparations of secukinumab. Also provided are purified preparations of recombinant polypeptides produced by such methods wherein the level of active recombinant polypeptide in the preparation is high.
Owner:NOVARTIS AG
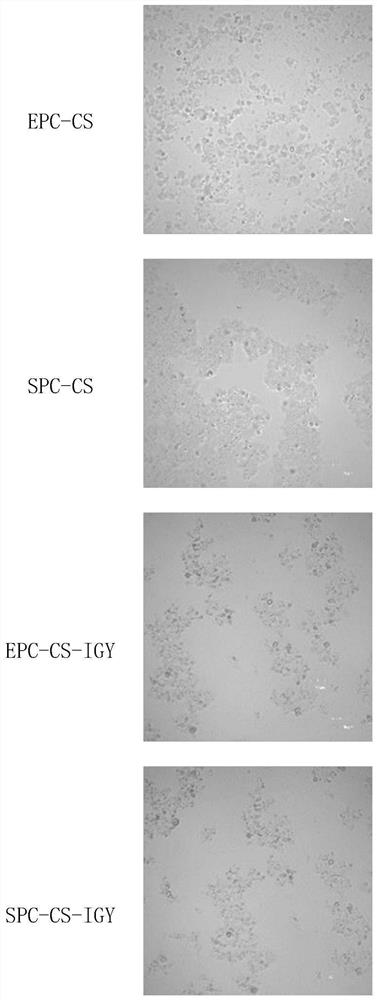
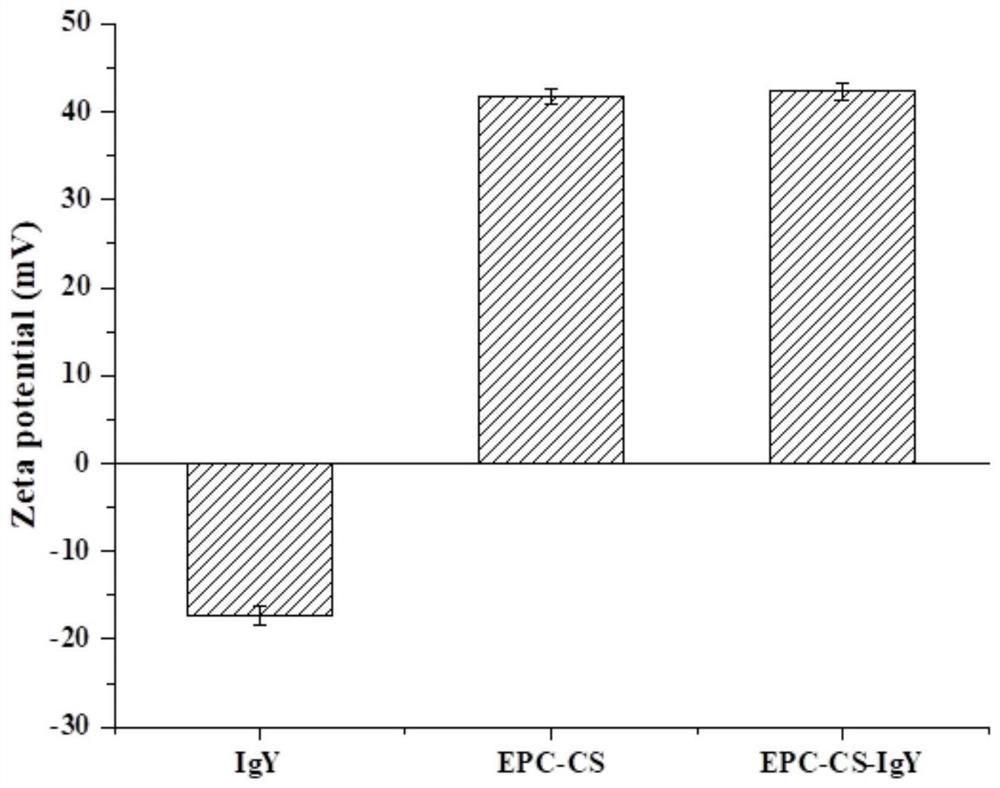
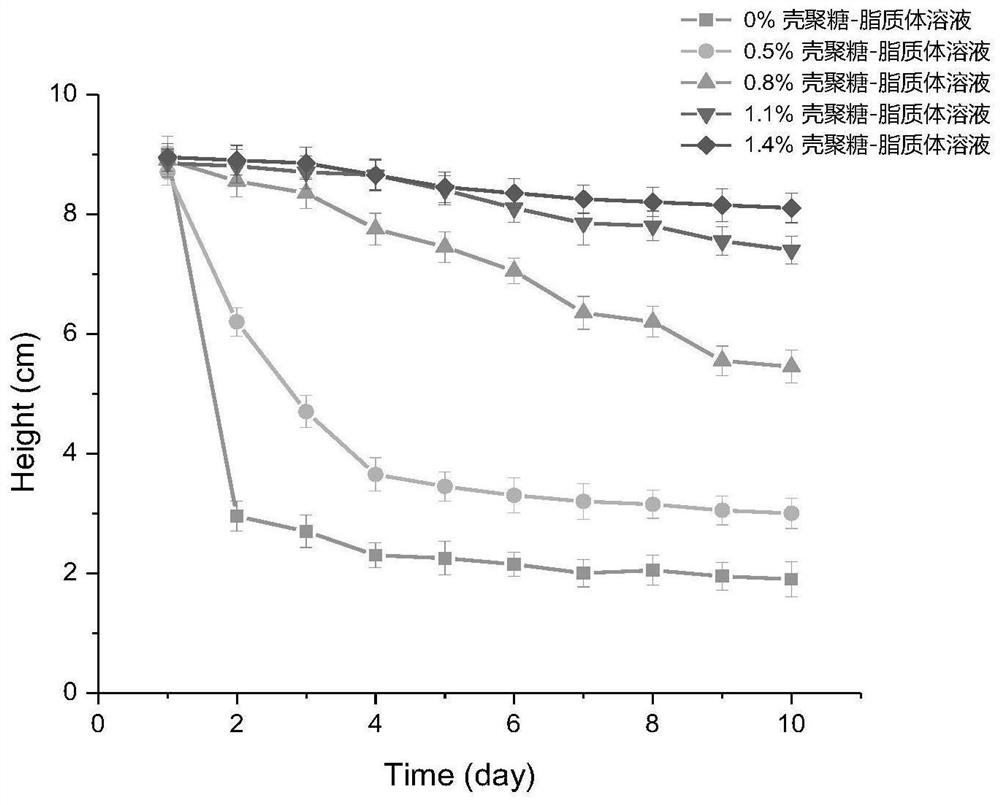
Chitosan liposome entrapped with yolk immunoglobulin and preparation method and application thereof
PendingCN113749259ANo residueChemically stableFood shapingFood ingredient as encapsulating agentMolecular biologyEthanolamine synthesis
The invention relates to a chitosan liposome entrapped with yolk immunoglobulin and a preparation method and application thereof. The liposome comprises the following components: lecithin, cholesterol, IGY and chitosan, wherein the liposome takes a phospholipid bilayer formed by the lecithin and the cholesterol as a liposome framework material; the IGY is embedded in an internal water phase of the liposome; the chitosan coats the inner layer surface and the outer layer surface of the phospholipid bilayer in the liposome; and the lecithin is selected from one of yolk lecithin, soybean lecithin, phosphatidylcholine and phosphatidyl ethanolamine. The liposome disclosed by the invention is high in stability and high in entrapment rate.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com