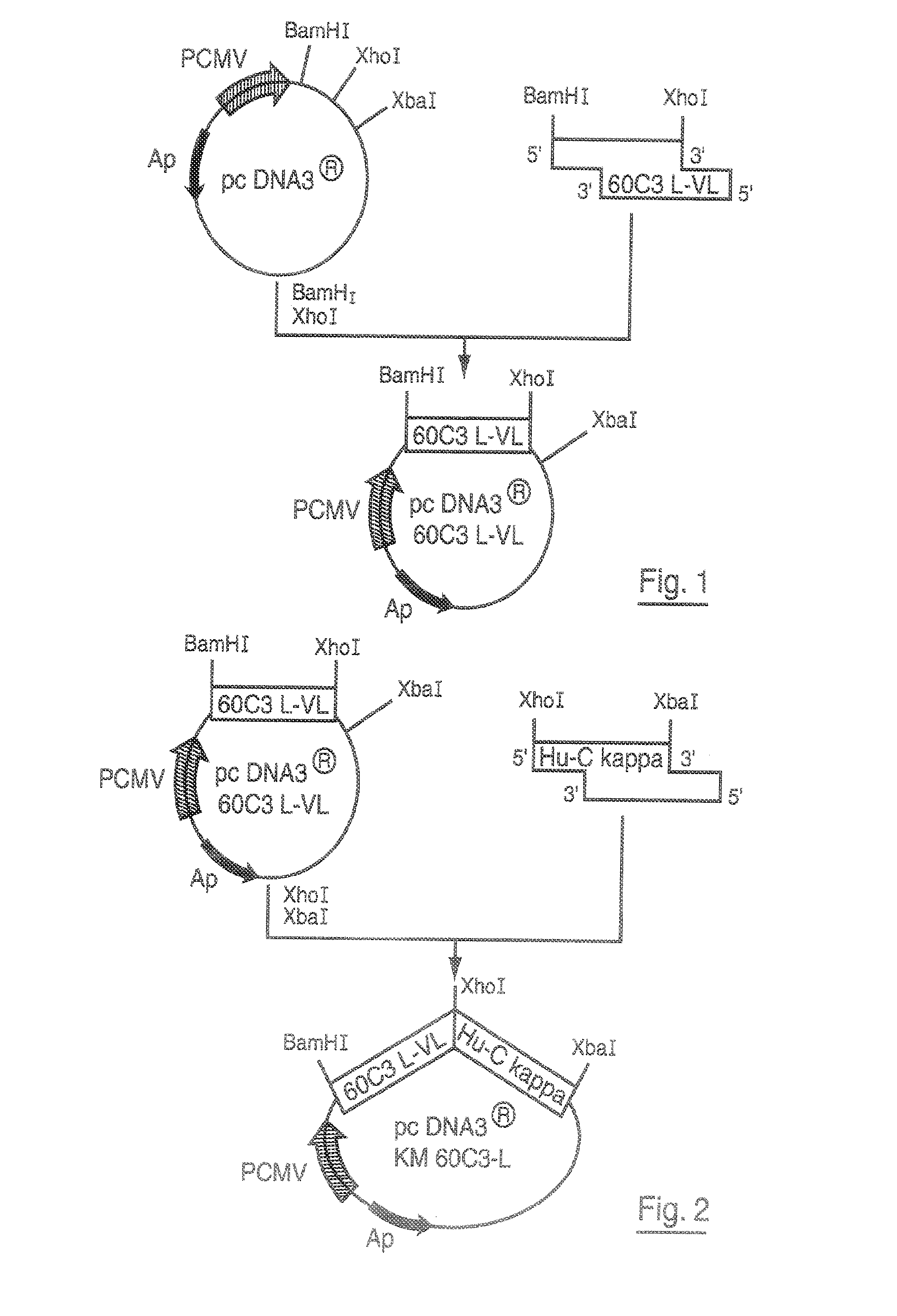
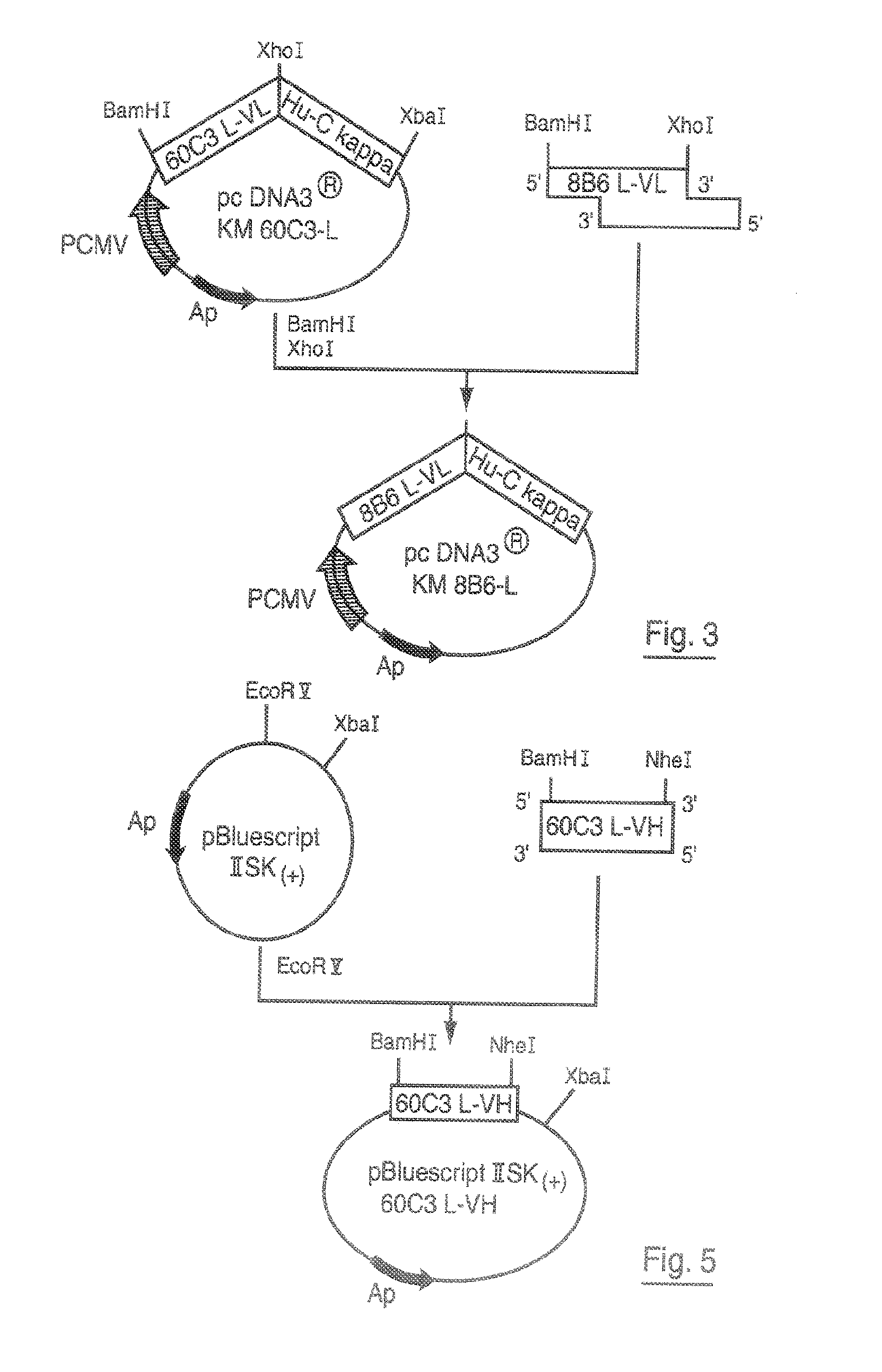
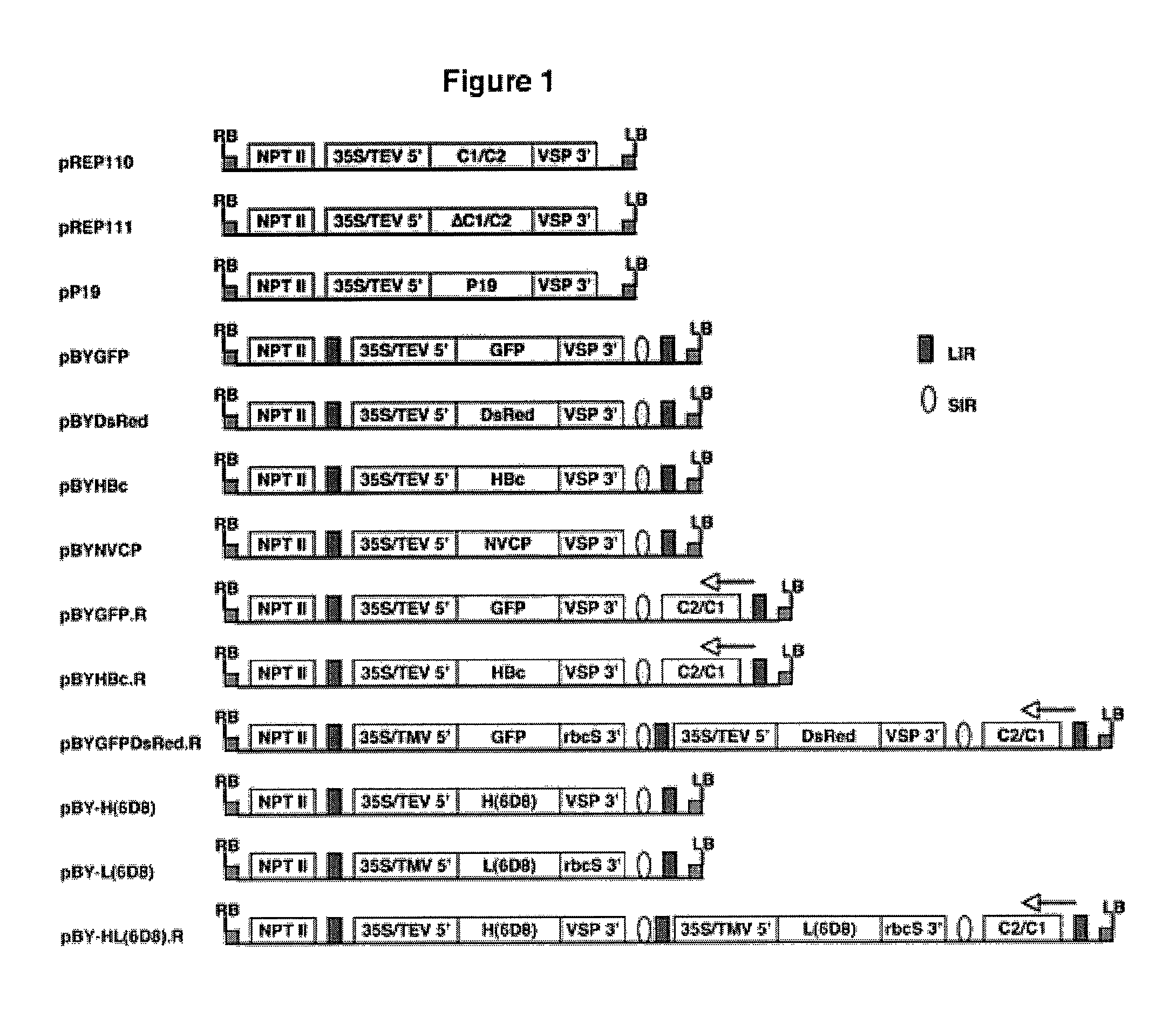
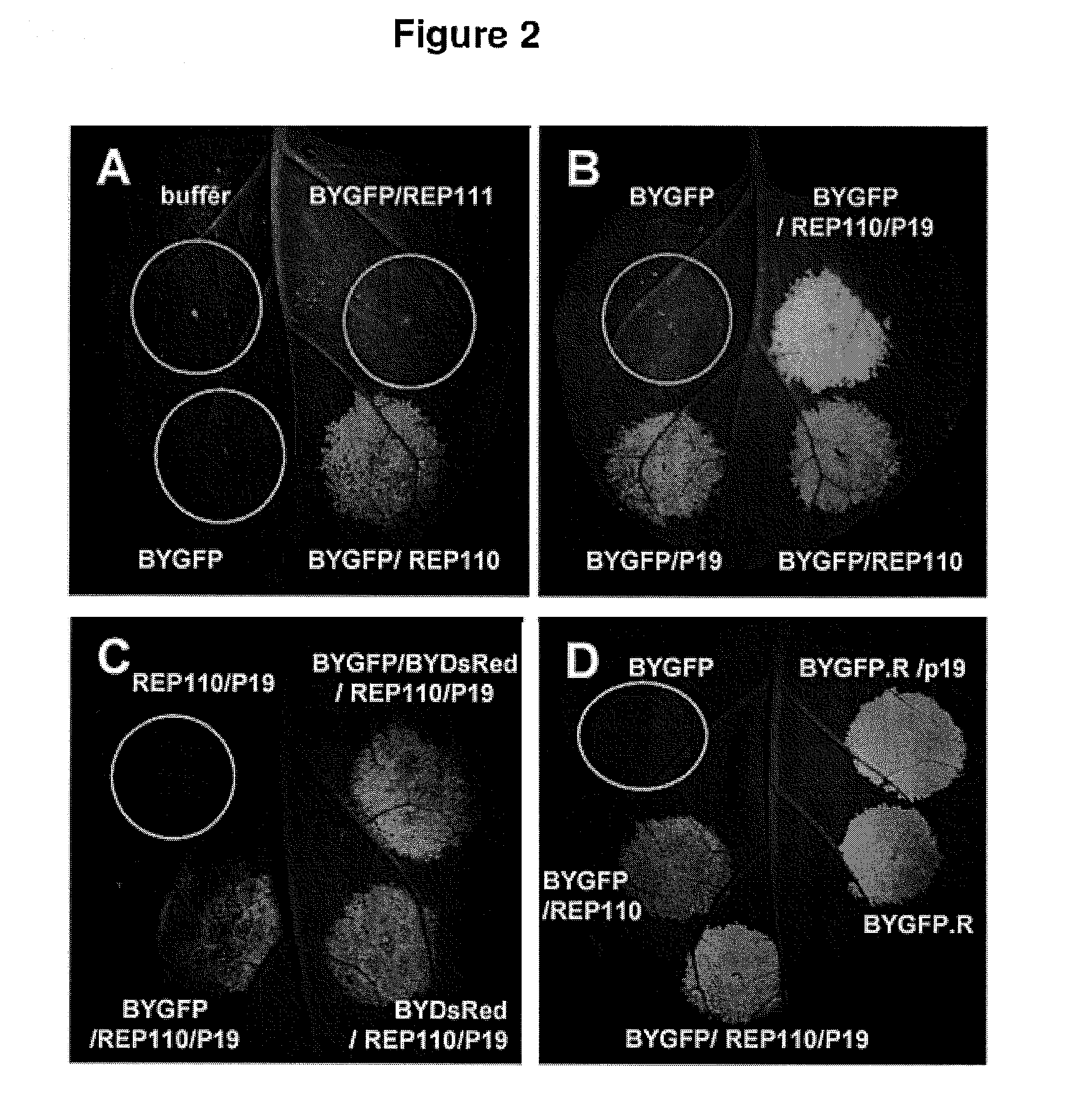
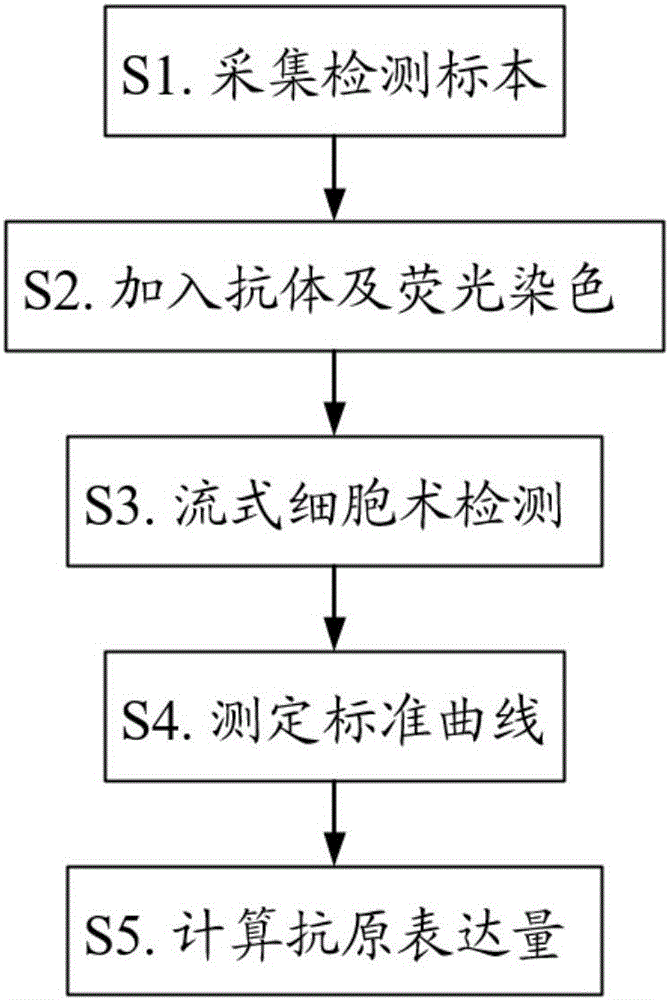
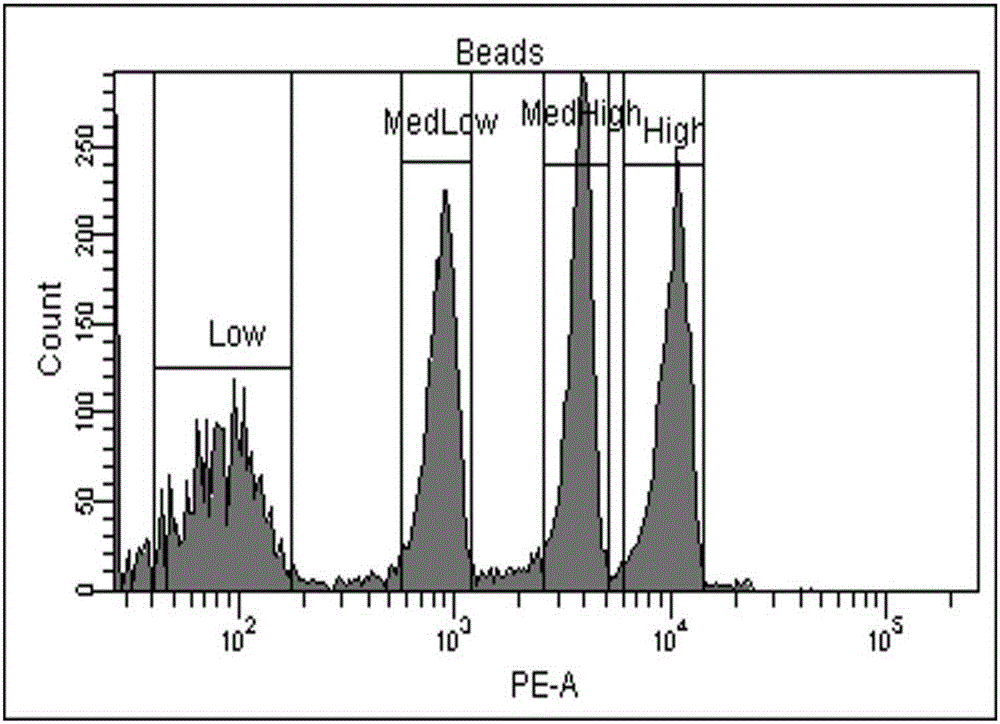
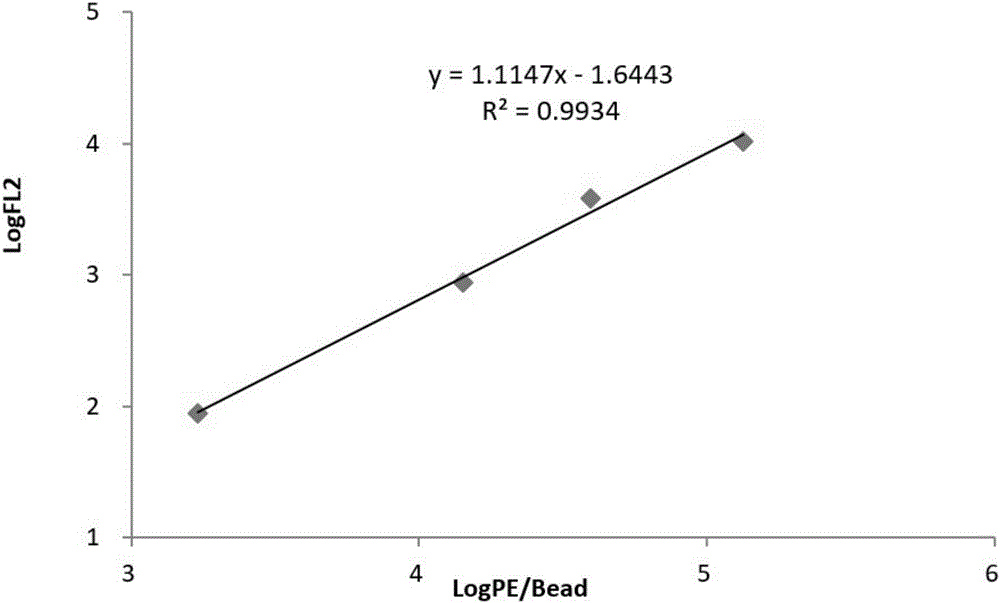
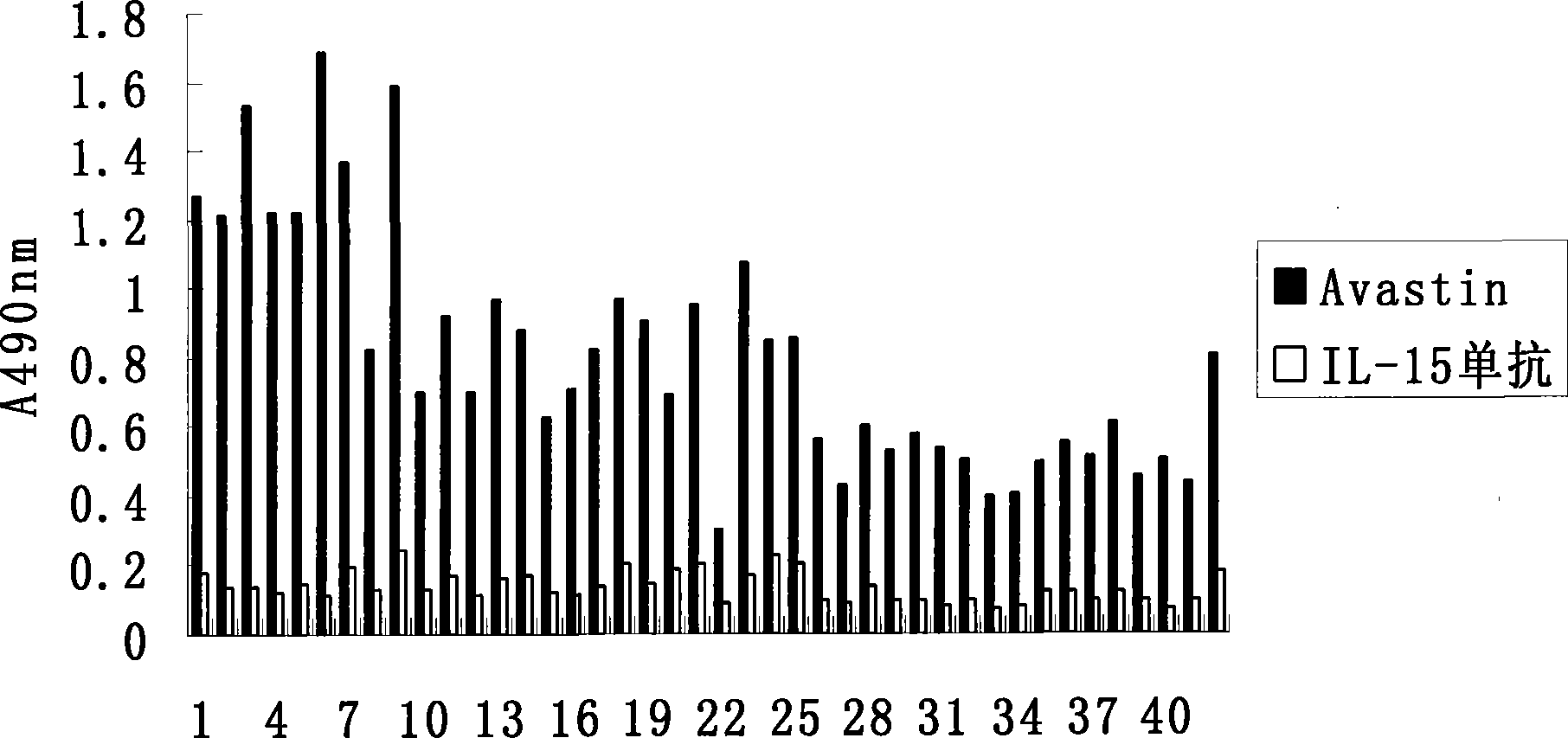
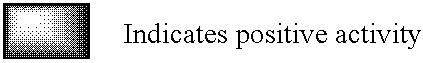Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Monoclonal antibody therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. More recently antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
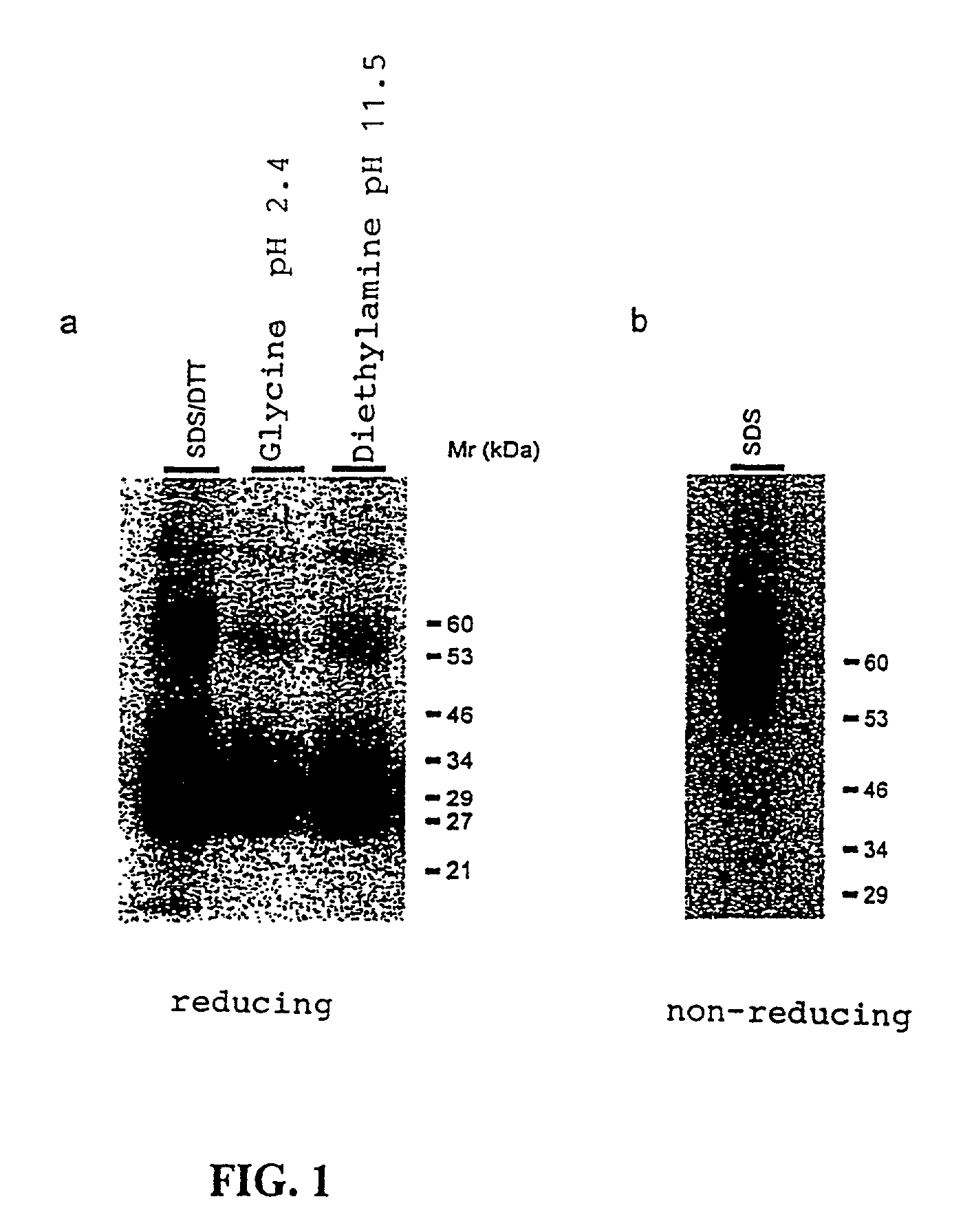
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS20070077246A1Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
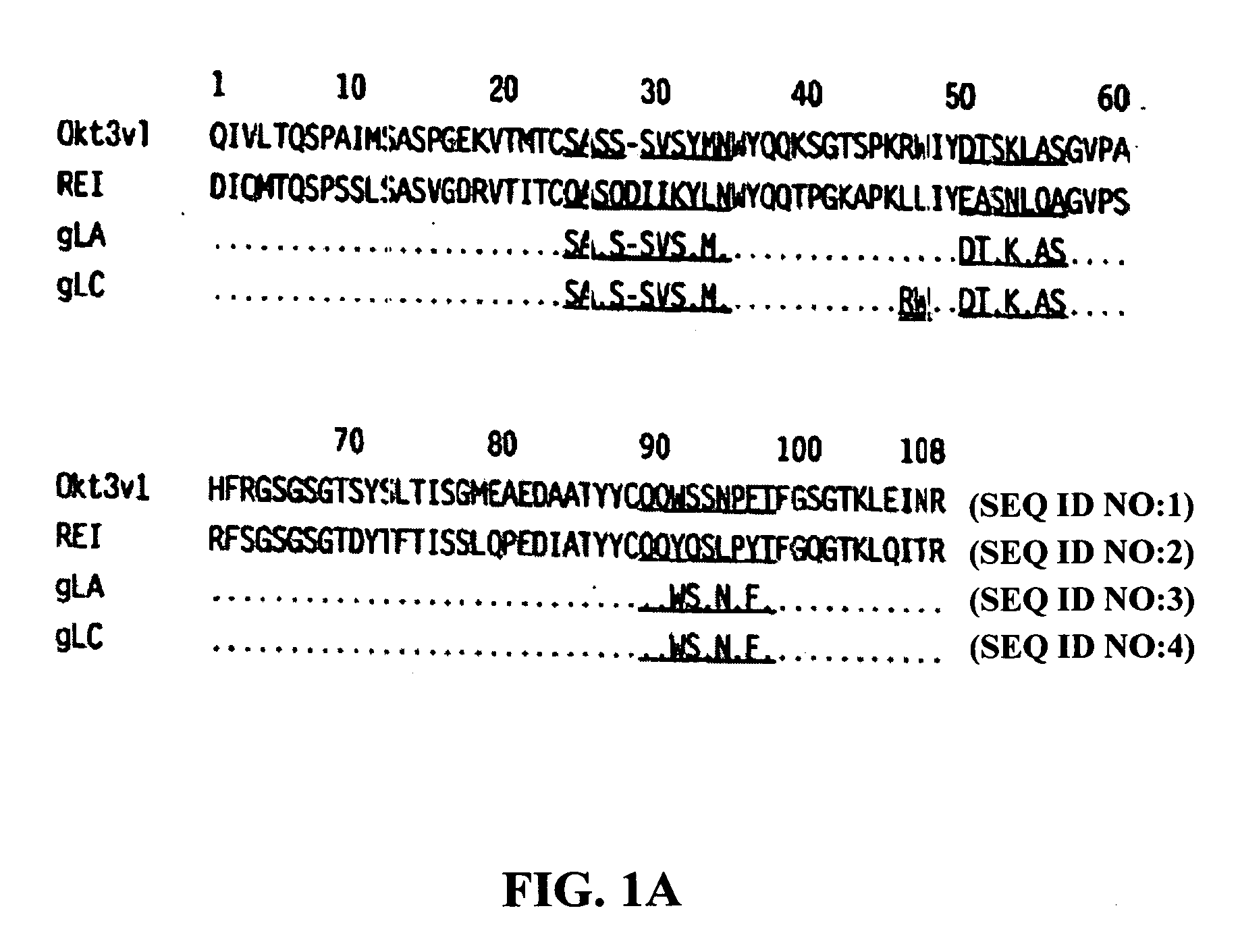
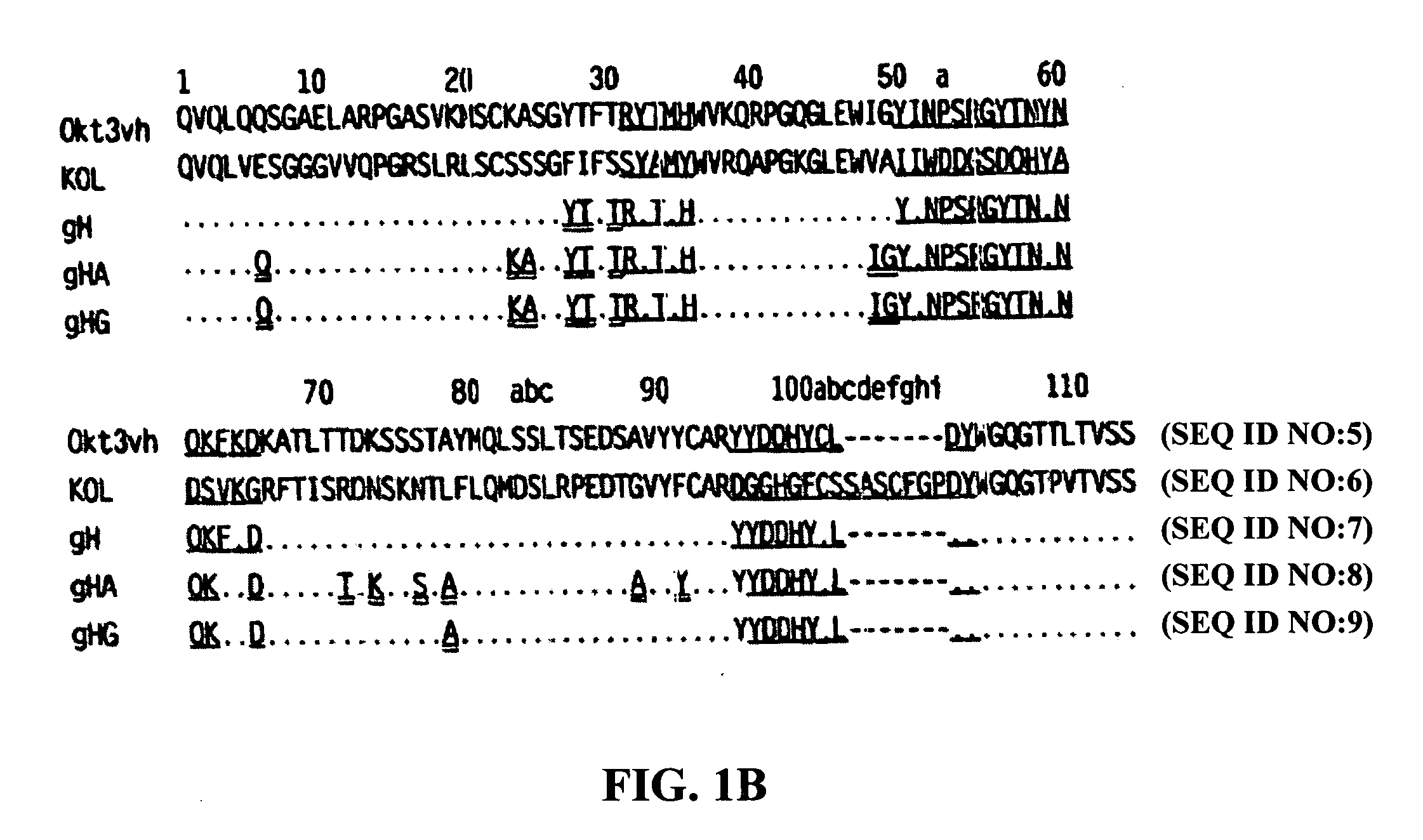
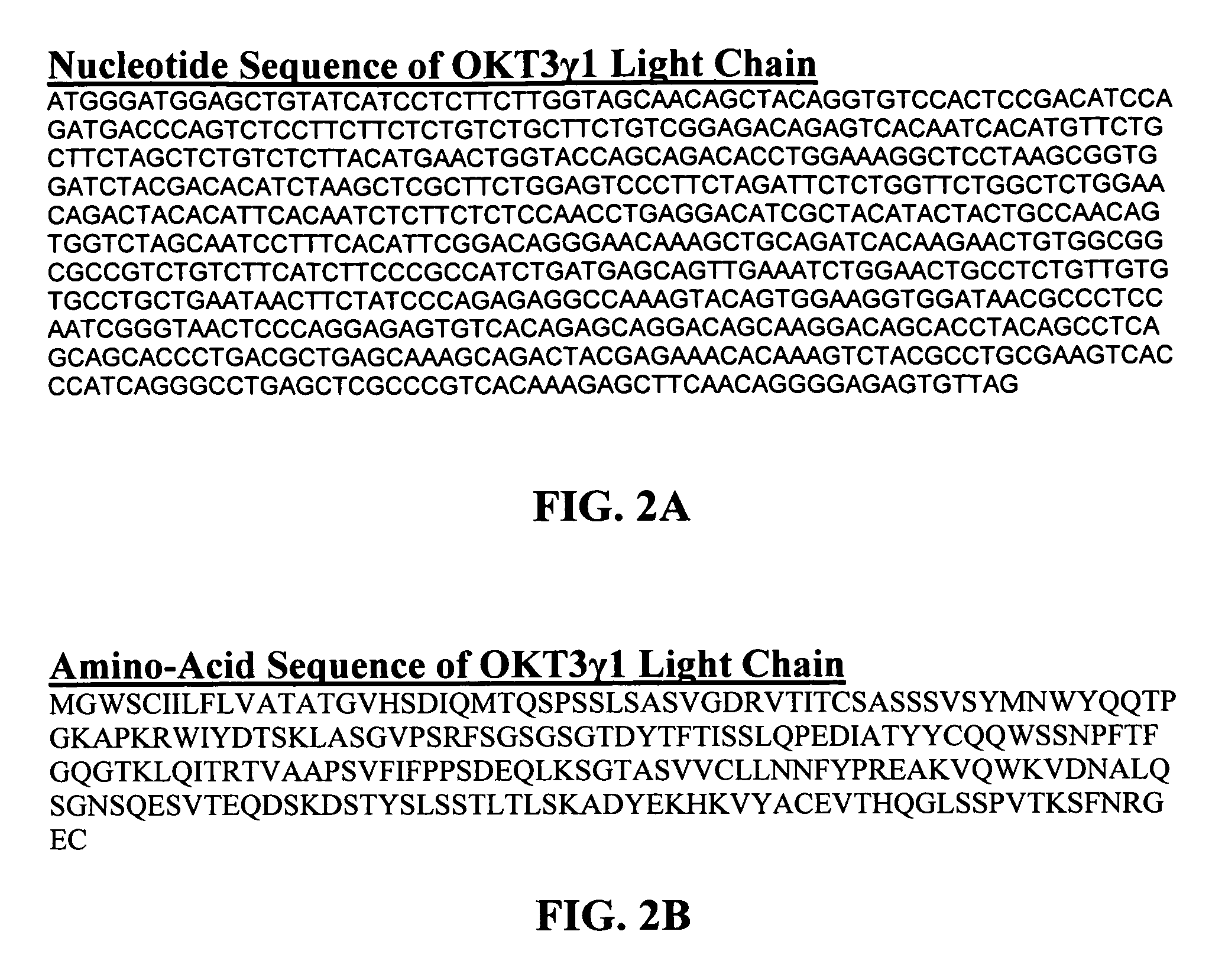
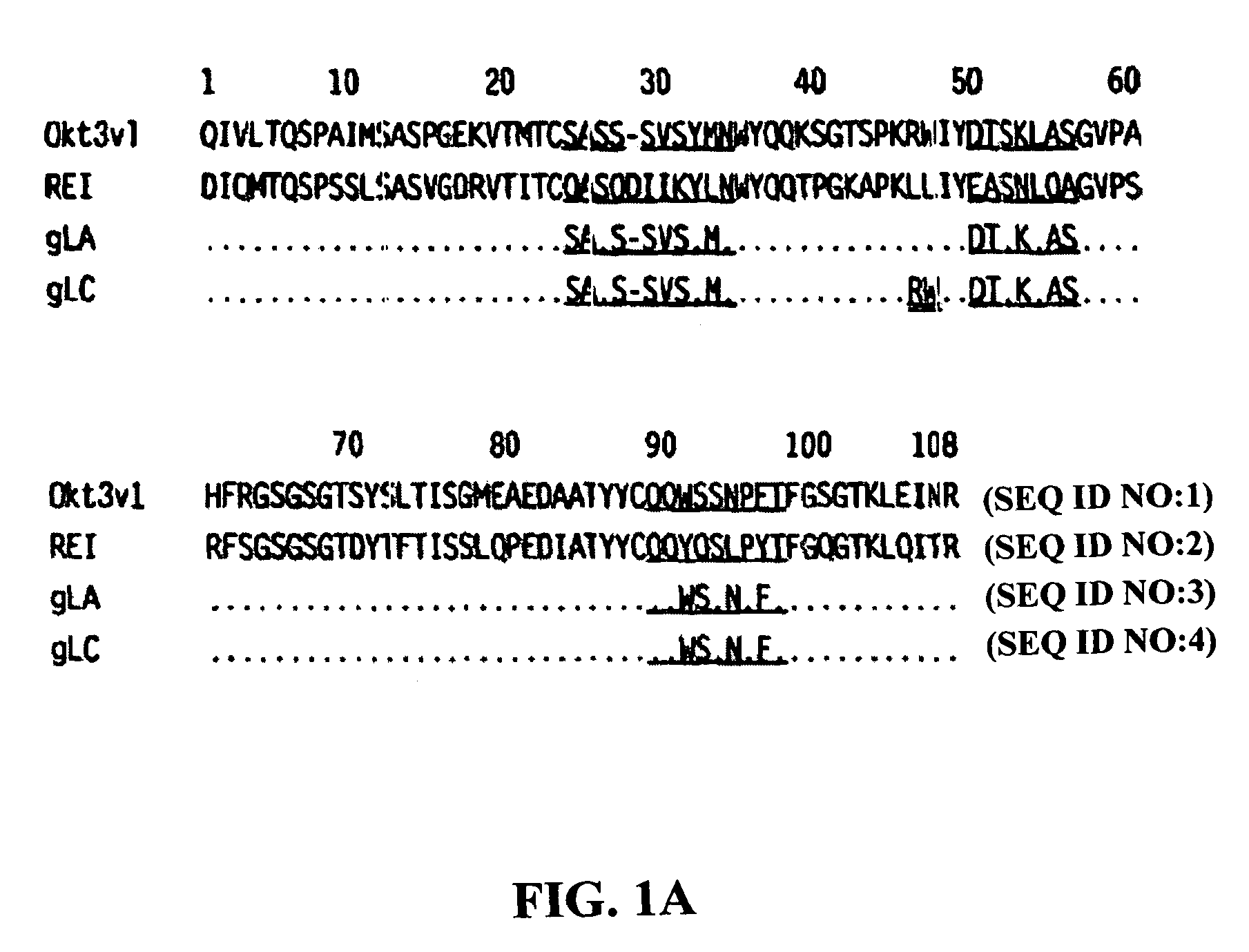
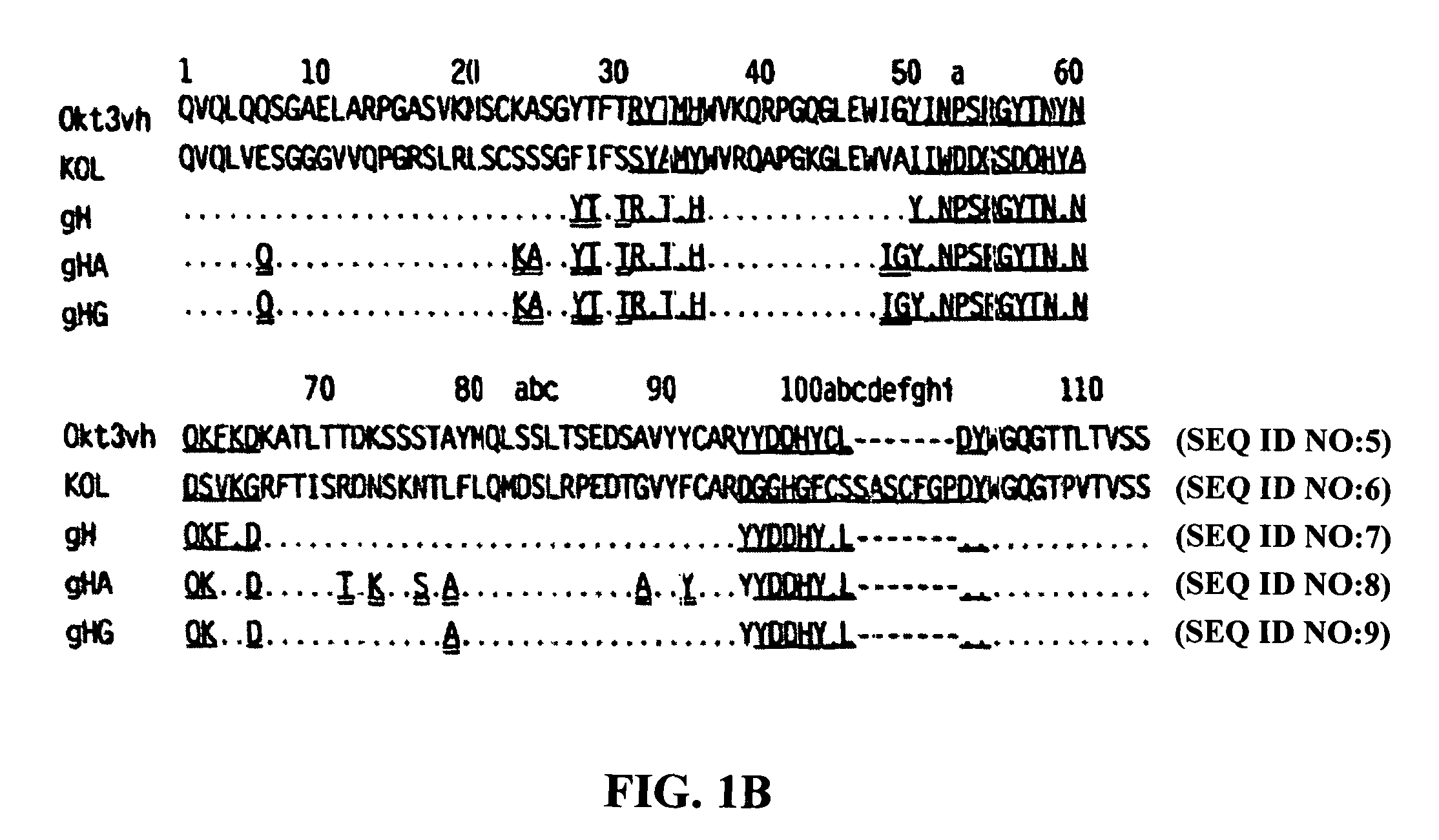
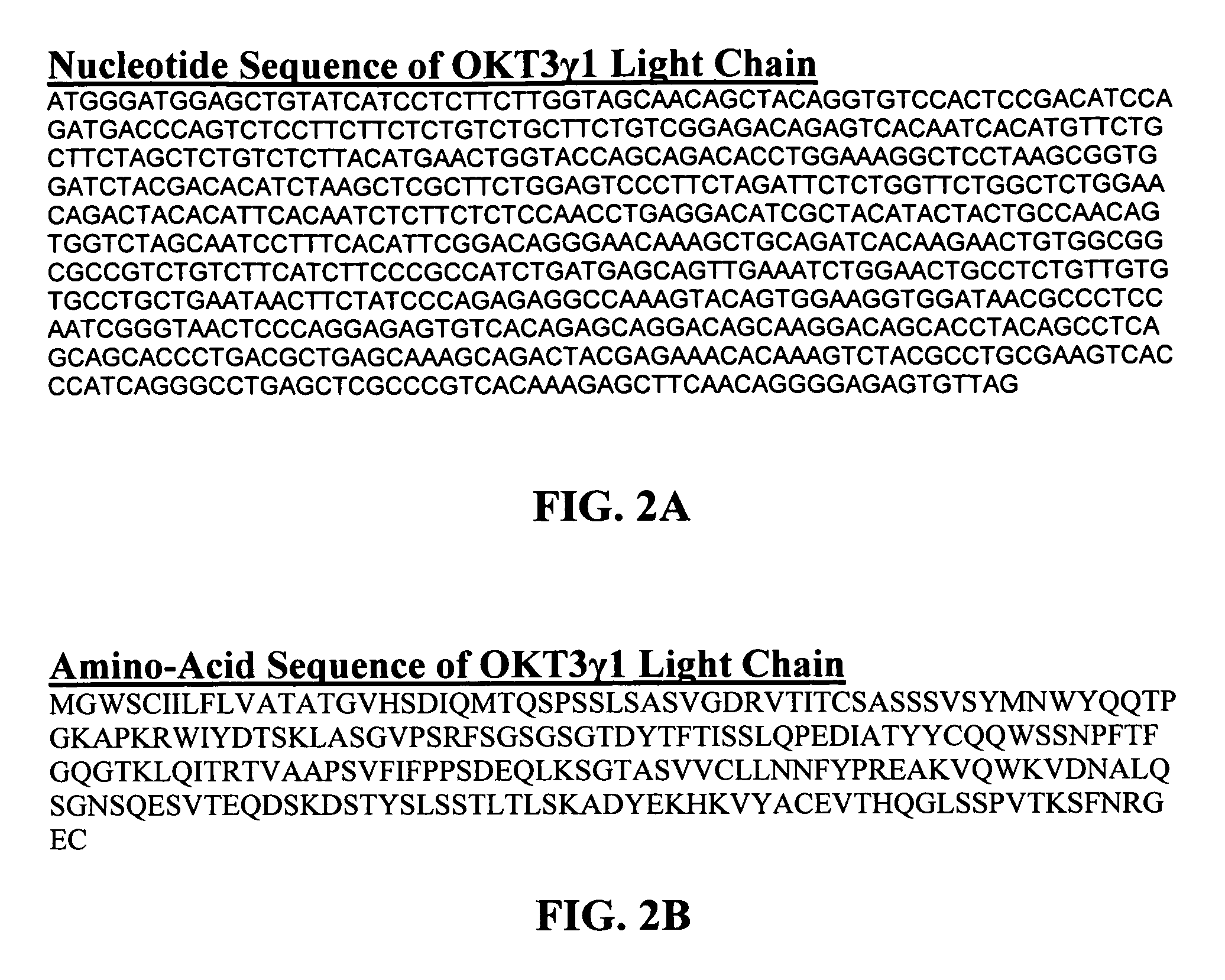
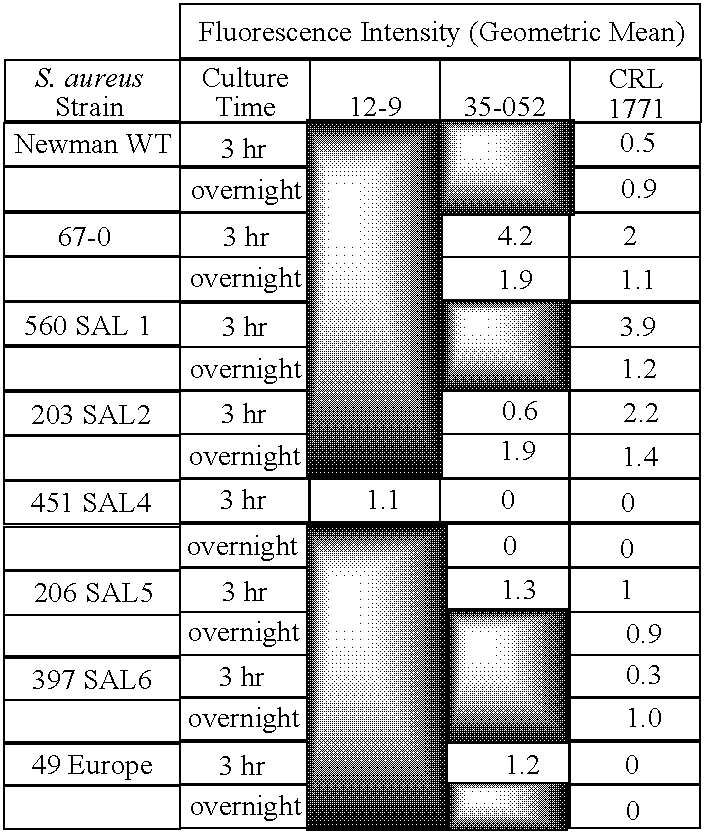
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
Monoclonal Antibody And Use Thereof
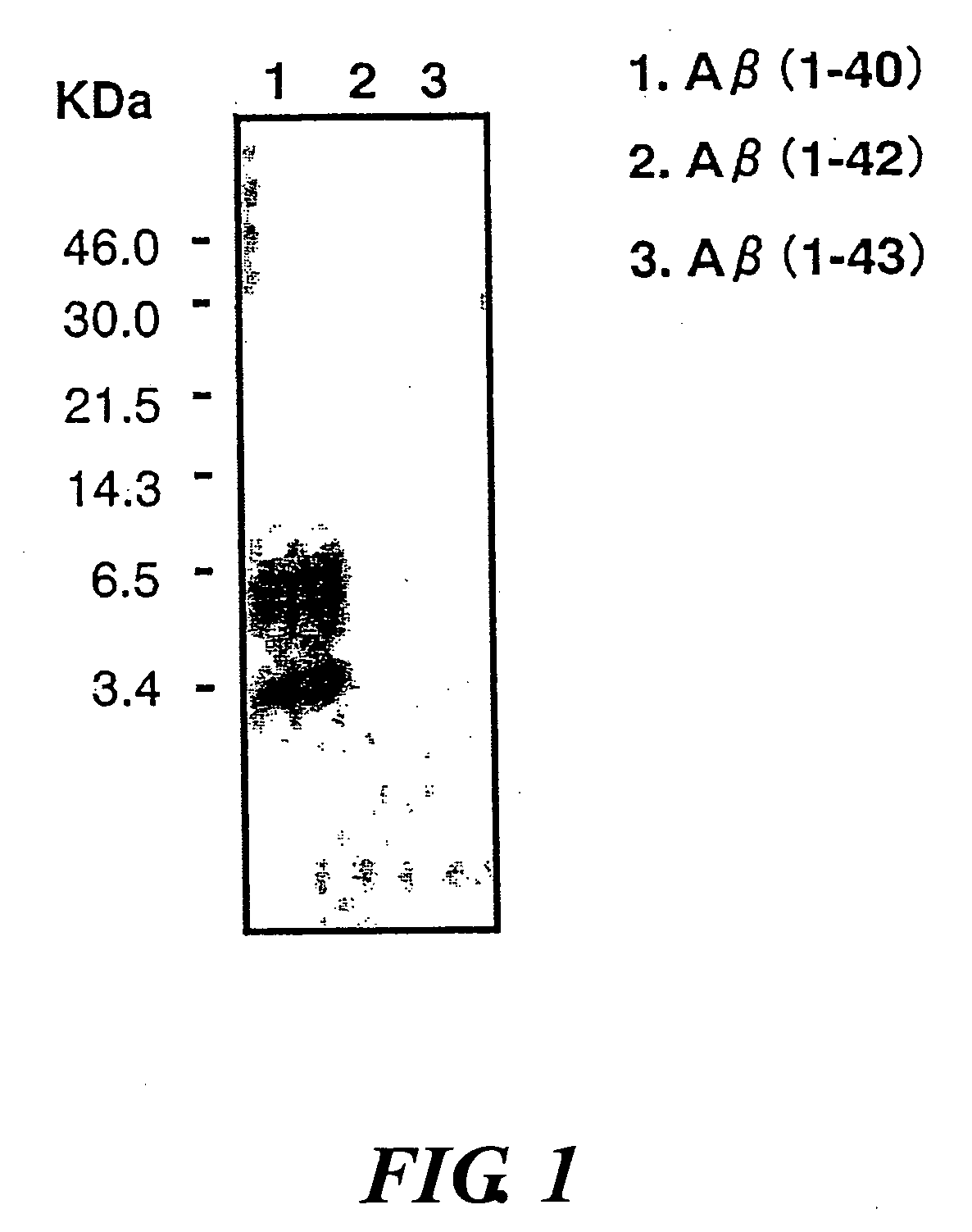
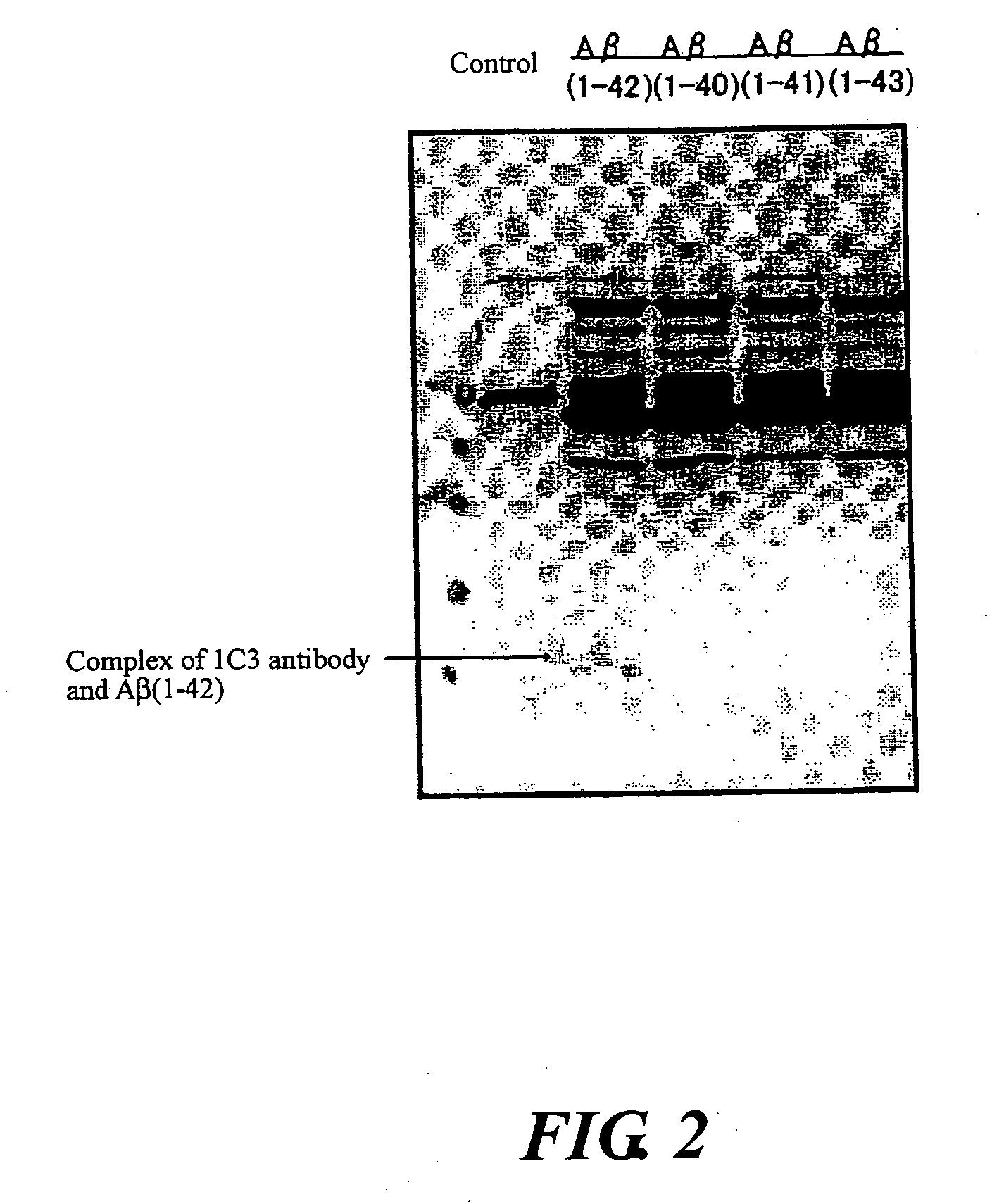
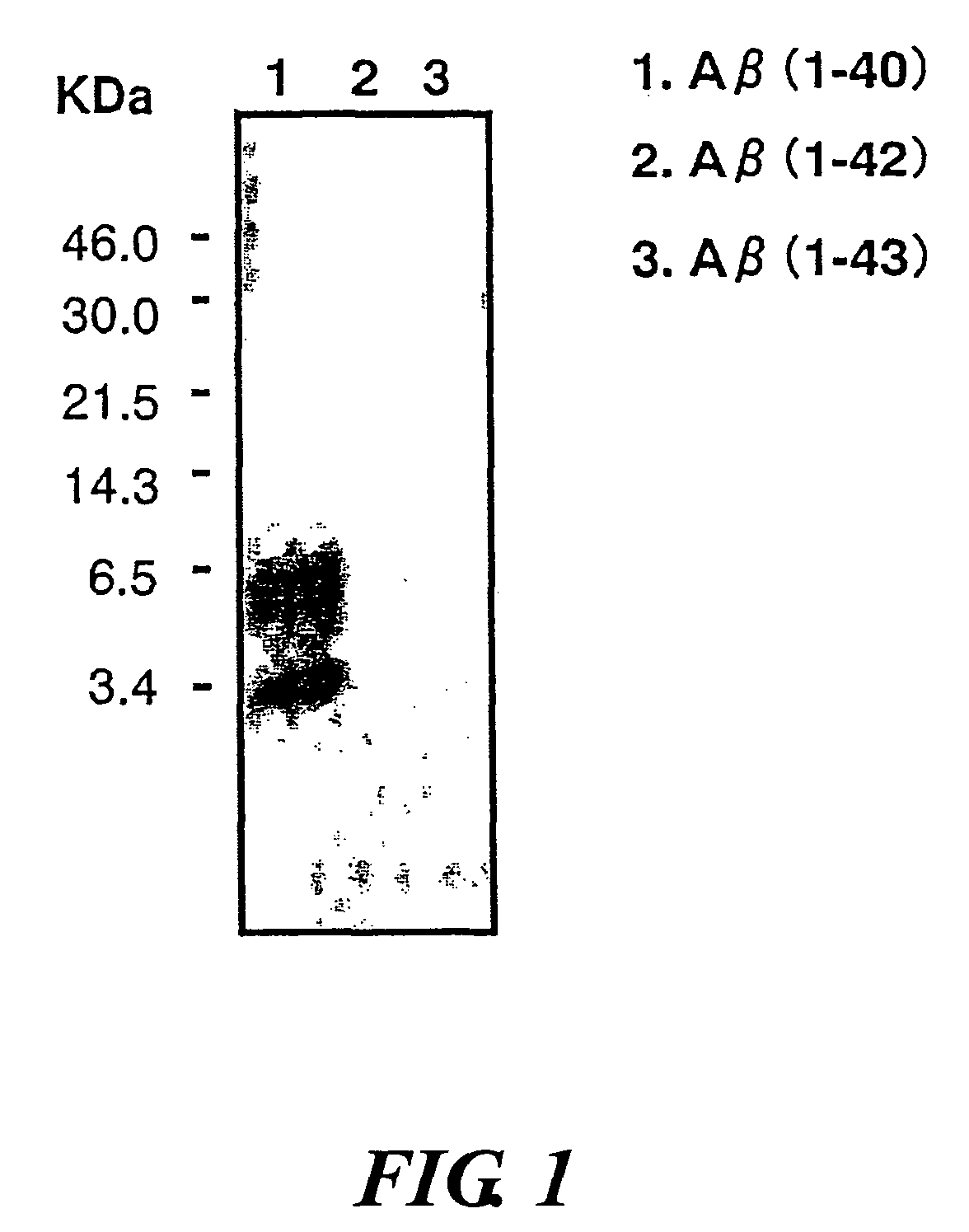
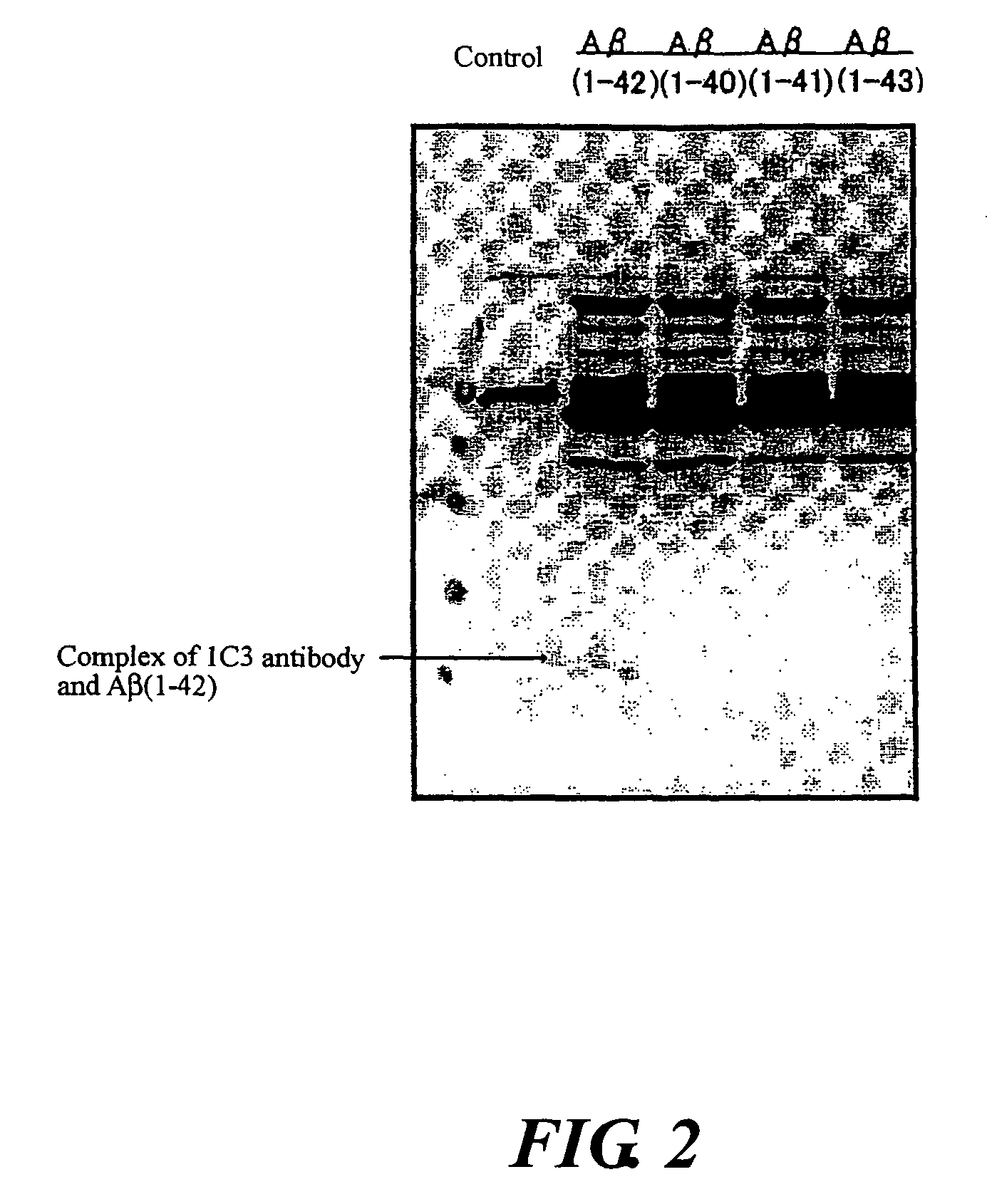
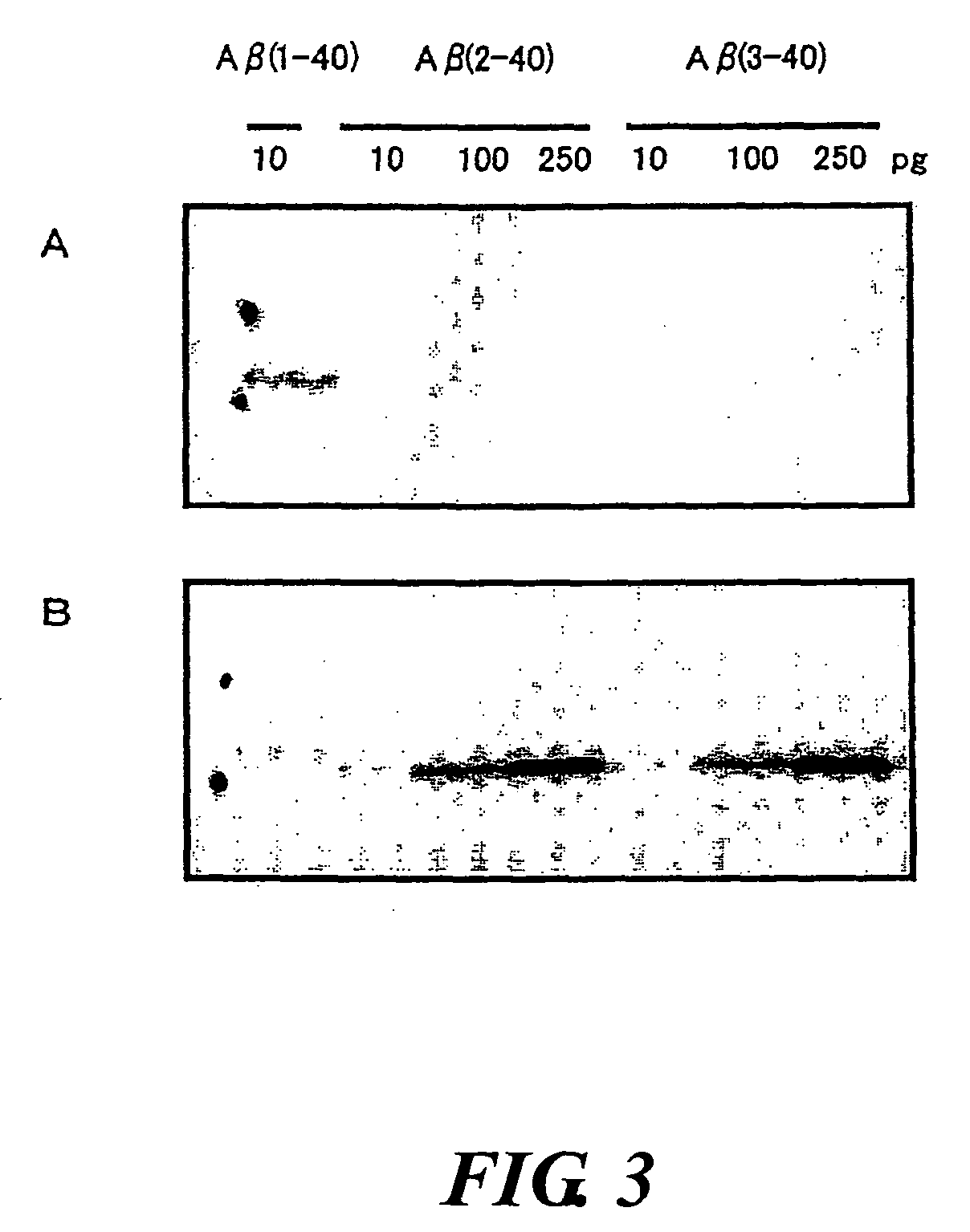
InactiveUS20080025988A1Treatment safetySuppress deposition of amyloidNervous disorderImmunoglobulins against animals/humansDiseaseAmyloid
An antibody capable of recognizing amyloid β while not recognizing amyloid β precursor proteins, and a method for using the same.A monoclonal antibody characterized by being capable of recognizing the N-terminus peptide of amyloid β while not recognizing amyloid β precursor proteins, an amyloid β assay kit, a therapeutic agent of Alzheimer's disease, and a method for treating Alzheimer's disease using the monoclonal antibody.
Owner:COLLATERAL AGENTS
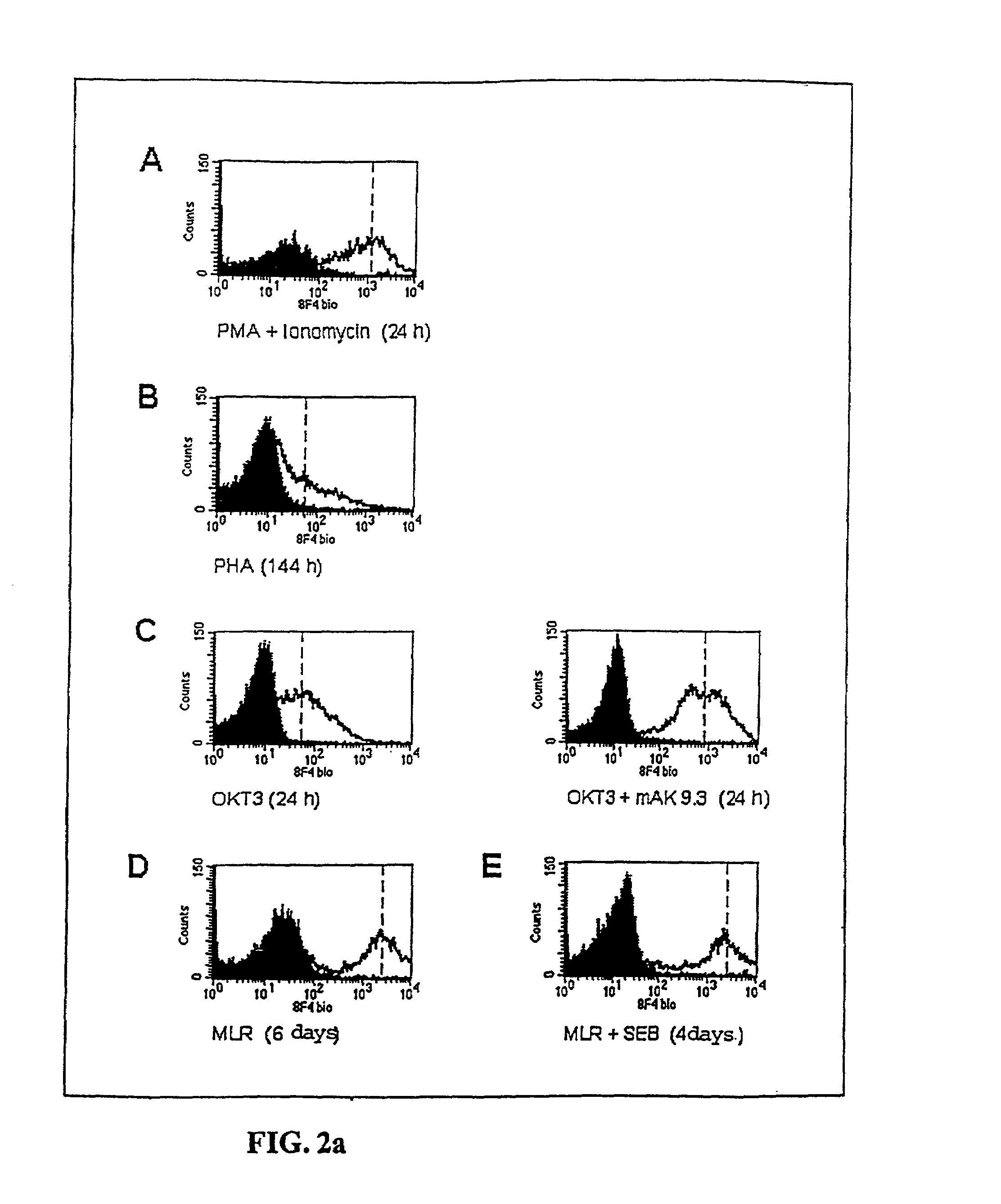
Methods for treatment of asthmatic disorders using a monoclonal antibody to 8F4 polypeptide
The present invention relates to methods and compositions for the prevention and treatment and prevention of immune system disorders, including cancer, AIDS, asthmatic disorders, autoimmune diseases, organ transplant rejection and chronic viral diseases such as HCV or HBV infections. The therapeutic methods of the invention comprise administering molecules that modulate the activity of 8F4, thereby modulating costimulation of T cells. The present invention further provides monoclonal antibodies against the 8F4 molecule and hybridoma cells which produce said monoclonal antibodies. Pharmaceutical compositions comprising molecules that modulate the activity of 8F4 are also provided.
Owner:BUNDESREPUBLIK DEUTSCHLAND
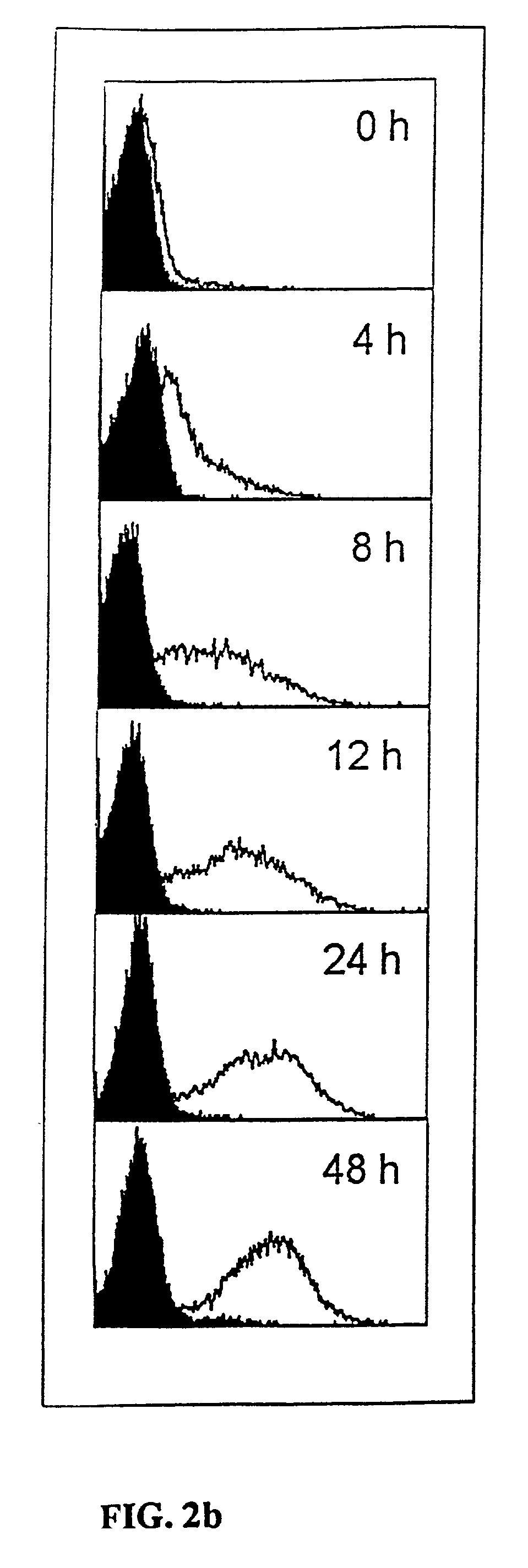
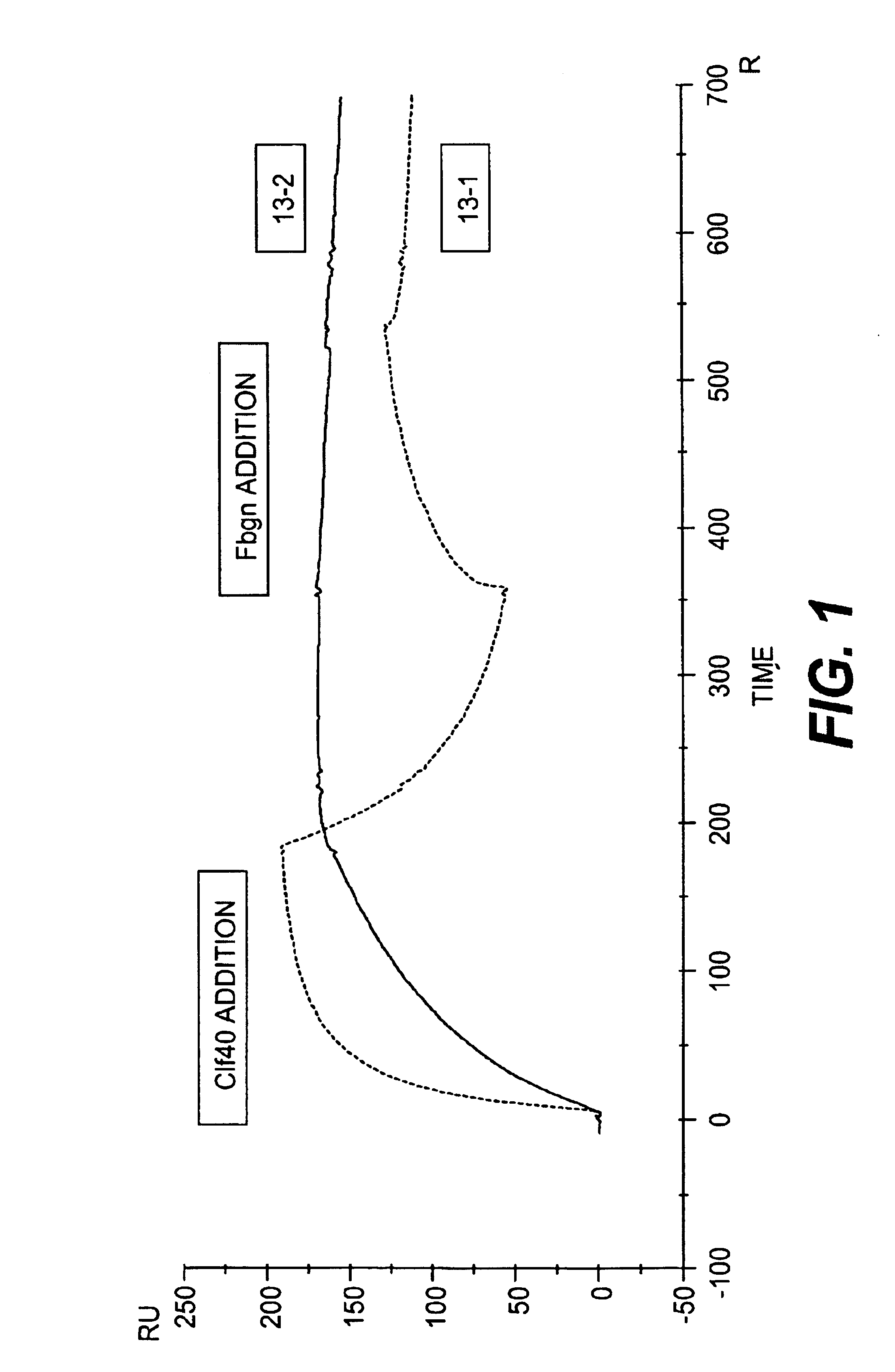
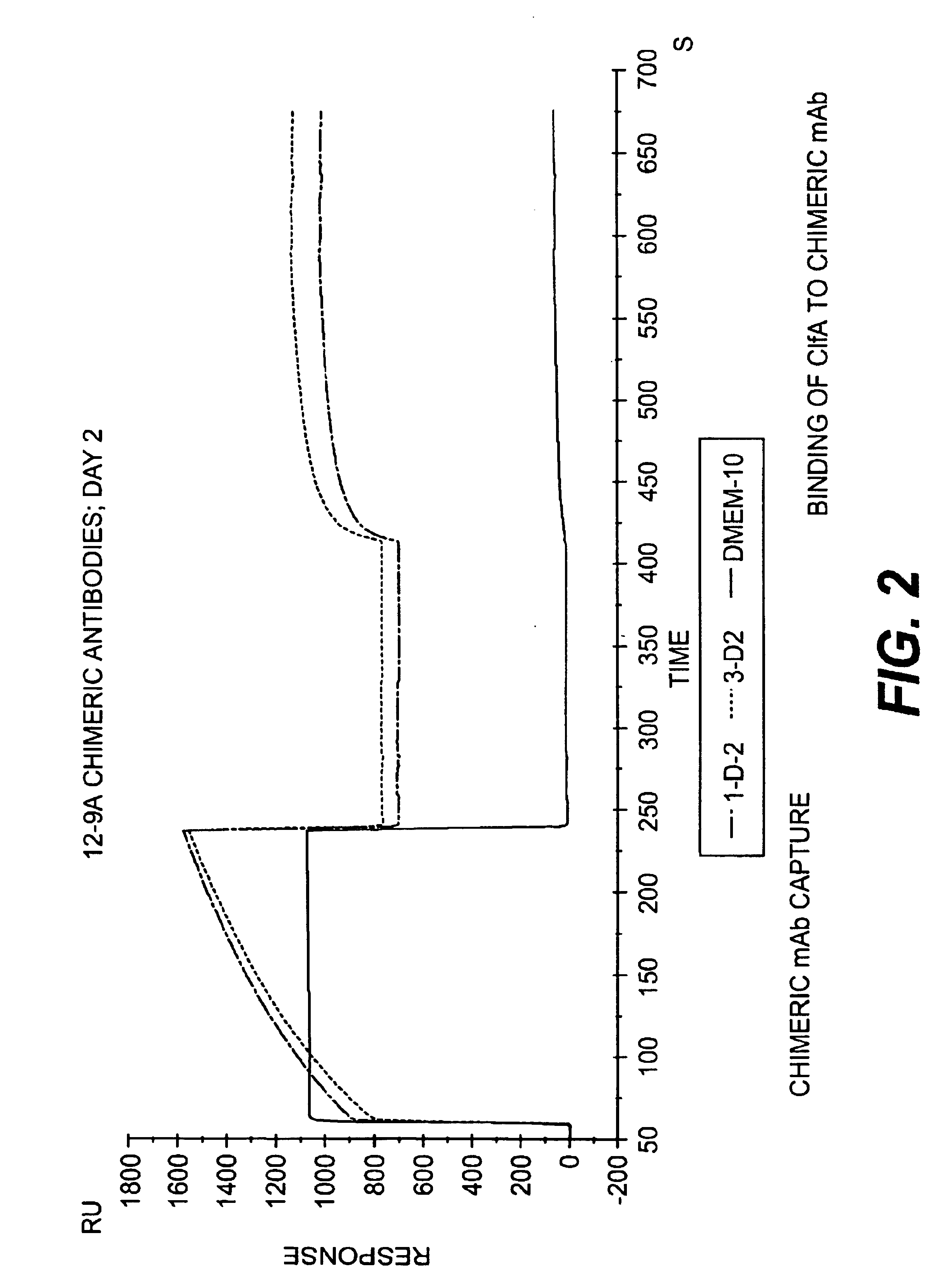
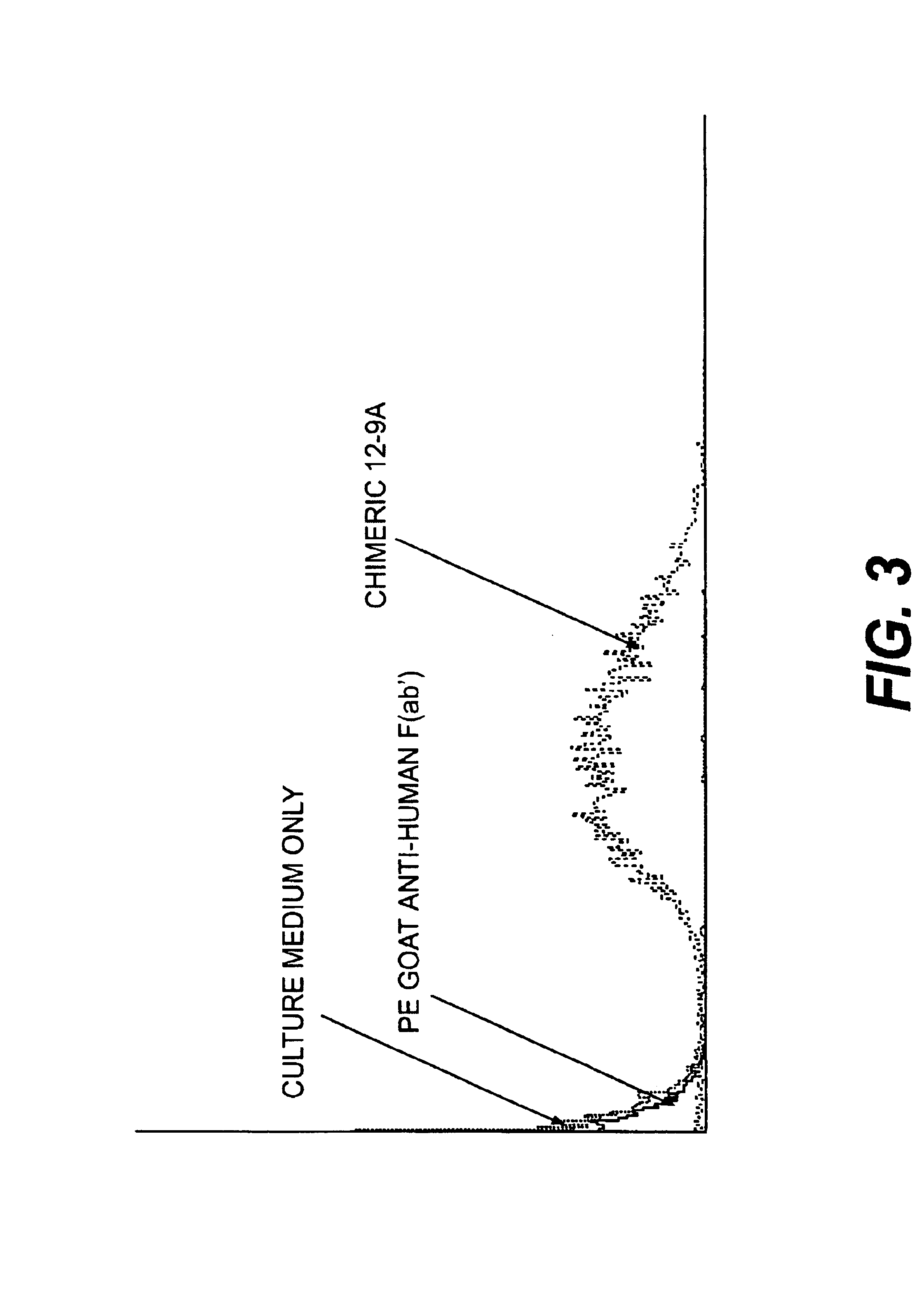
Monoclonal antibodies to the ClfA protein and method of use in treating or preventing infections
InactiveUS6979446B2Avoid stickingInhibiting or impairing the binding of the ClfA proteinAntibacterial agentsBacterial antigen ingredientsBacteroidesStaphylococcus cohnii
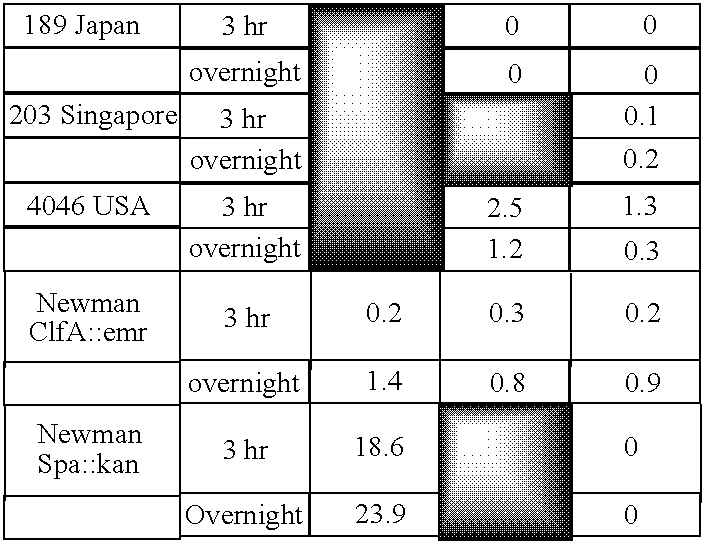
Monoclonal antibodies which can bind to the ClfA protein and which are generated from binding subdomains or active fragments of the ClfA protein from Staphylococcus aureus, including the active fragments proteins from its fibrinogen binding domain such as Clf40 protein, the Clf33 protein, or ClfA N3, are provided which can be useful in the treatment and protection against infection from staphylococcal bacteria such as Staphylococcus aureus. In addition, medical instruments can be treated using the monoclonal antibodies of the invention in order to reduce or eliminate the possibility of their becoming infected or further spreading the infection. In particular, the antibodies of the present invention are advantageous because they can prevent adherence of the bacteria to host cells by impairing or inhibiting the ability of S. aureus ClfA to bind to fibrinogen or fibrin, and thus can be utilized in methods or treating or preventing staphylococcal inventions.
Owner:INHIBITEX INC
Recombinant human Claudin18.2 tumor vaccine and preparation method thereof
InactiveCN101584860AImprove securitySimple manufacturing methodMicroorganism based processesAntibody medical ingredientsSide effectMouse Stomach
The invention belongs to the field of medicinal biotechnology, and in particular relates to a recombinant human Claudin18.2 tumor vaccine and a preparation method thereof. The invention aims to solve the problems of immunotherapy for stomach cancer, pancreatic cancer, esophagus cancer and metastatic and non-metastatic ovarian cancer, such as high after-excision recurrence rate, strong chemo-treatment and radiation treatment toxic side effect and high monoclonal antibody therapy cost. The invention adopts a technical proposal that the recombinant human Claudin18.2 tumor vaccine has a sequence of HMKSSQYIKANSKFIGEFDQWSTQDLYNNPVTAVFNYQGLWRSCVRESSGFTECRGYFTLLGLPAMLQAV. Animal experiments prove that rhClaudin18.2 fusion protein can induce high-titre neutralizing antibody in the bodies of tumor-bearing mice by over 1:10,000; the antibody can be combined with human KATOIII and PANC-1 tumor cells and mouse stomach cancer MFC and pancreatic cancer MPC-83 cells; and the protein serving as a tumor vaccine can suppress the growth of the stomach cancer MFC and pancreatic cancer MPC-83 cells in the bodies of the mice.
Owner:西安杰诺瓦生物科技有限公司
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS8663634B2Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticDiseaseAutoimmune responses
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
Monoclonal antibodies to the ClfA protein and method of use in treating or preventing infections
InactiveUS20050287164A1Avoid stickingInhibiting or impairing the binding of the ClfA proteinAntibacterial agentsAntibody mimetics/scaffoldsBacteroidesStaphylococcus cohnii
Monoclonal antibodies which can bind to the ClfA protein and which are generated from binding subdomains or active fragments of the ClfA protein from Staphylococcus aureus, including the active fragments proteins from its fibrinogen binding domain such as Clf40 protein, the Clf33 protein, or ClfA N3, are provided which can be useful in the treatment and protection against infection from staphylococcal bacteria such as Staphylococcus aureus. In addition, medical instruments can be treated using the monoclonal antibodies of the invention in order to reduce or eliminate the possibility of their becoming infected or further spreading the infection. In particular, the antibodies of the present invention are advantageous because they can prevent adherence of the bacteria to host cells by impairing or inhibiting the ability of S. aureus ClfA to bind to fibrinogen or fibrin, and thus can be utilized in methods or treating or preventing staphylococcal inventions.
Owner:INHIBITEX INC
Use of monoclonal antibodies specific to the o-acetylated form of gd2 ganglioside for the treatment of certain cancers
ActiveUS20150140023A1Limit its clinical developmentDifficult to handlePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCancer cellAcetylation
The monoclonal antibodies that only recognise the O-acetylated form of the GD2 ganglioside, or fragments of the antibody, for the diagnosis and the treatment of cancers in which the cells express the O-acetylated GD2, the antibody or the fragment recognising the O-acetylated GD2 molecules expressed by the tumoral cells and not recognising the GD2 molecules expressed at the surface of the peripheral nerves, in order to increase the specificity of the diagnosis and reduce the toxicity of the treatments. Also artificially modified antibodies advantageously used for treating and diagnosing cancers in which the cells express the O-acetylated GD2.
Owner:UNIV DE NANTES
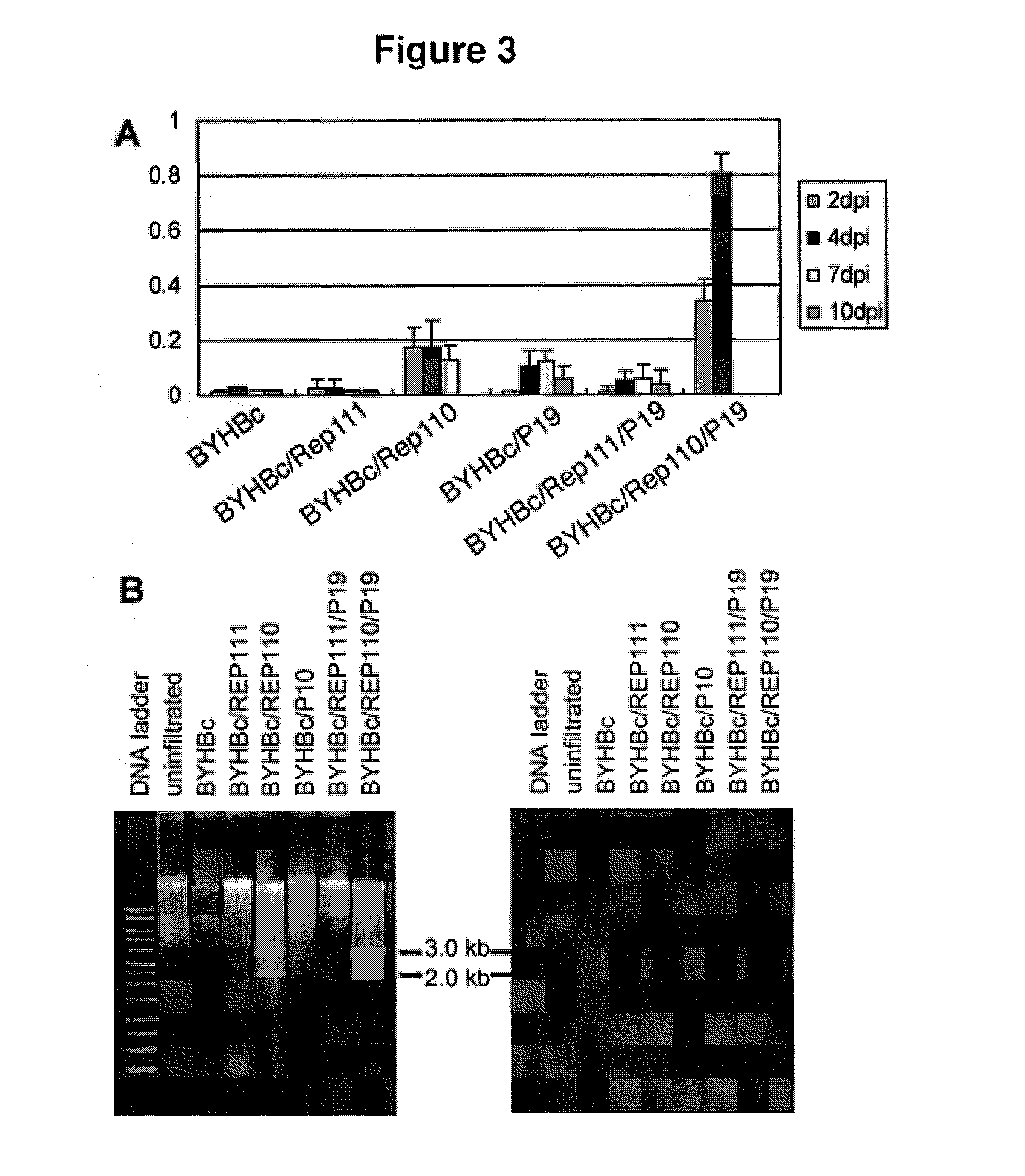
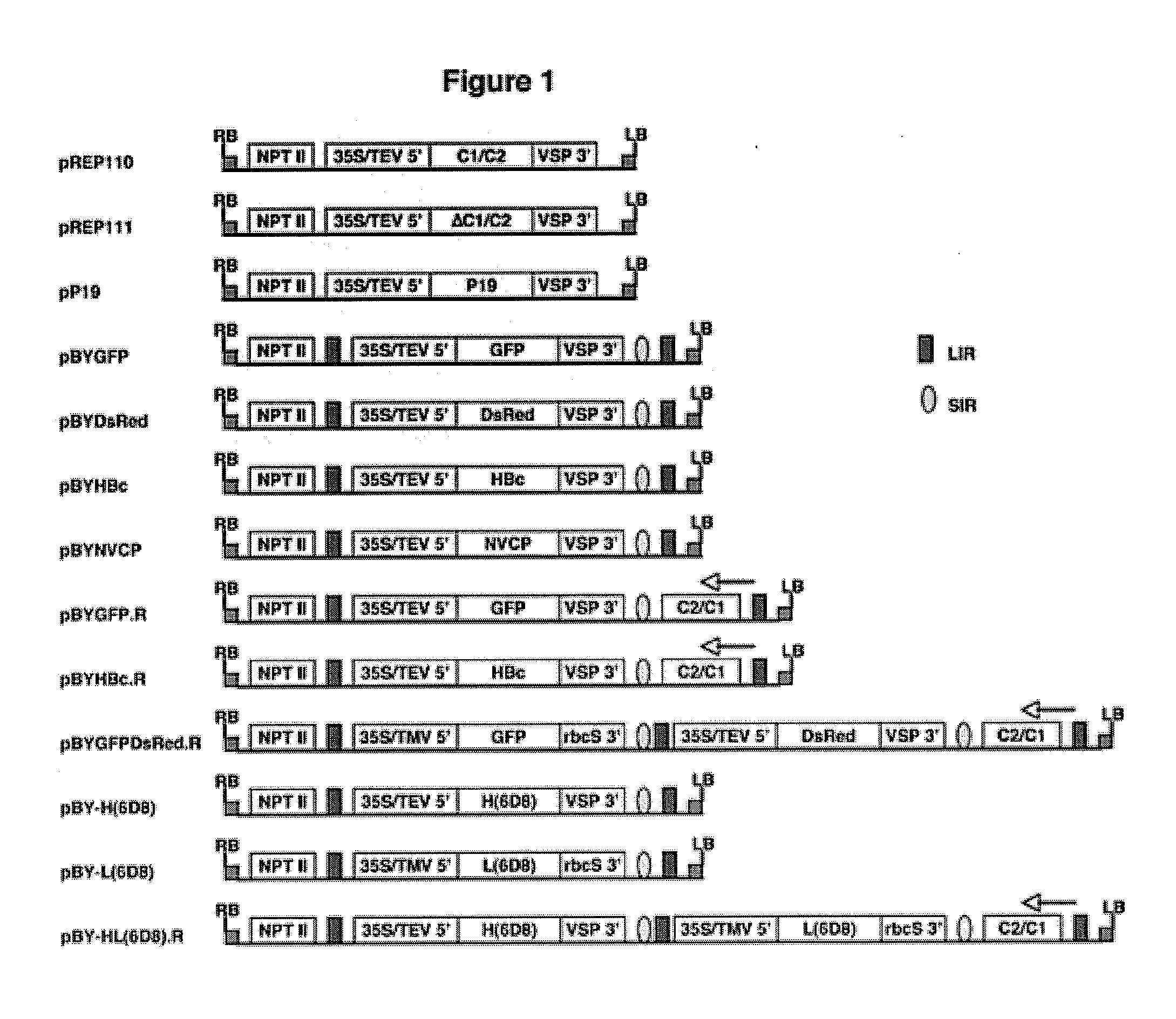
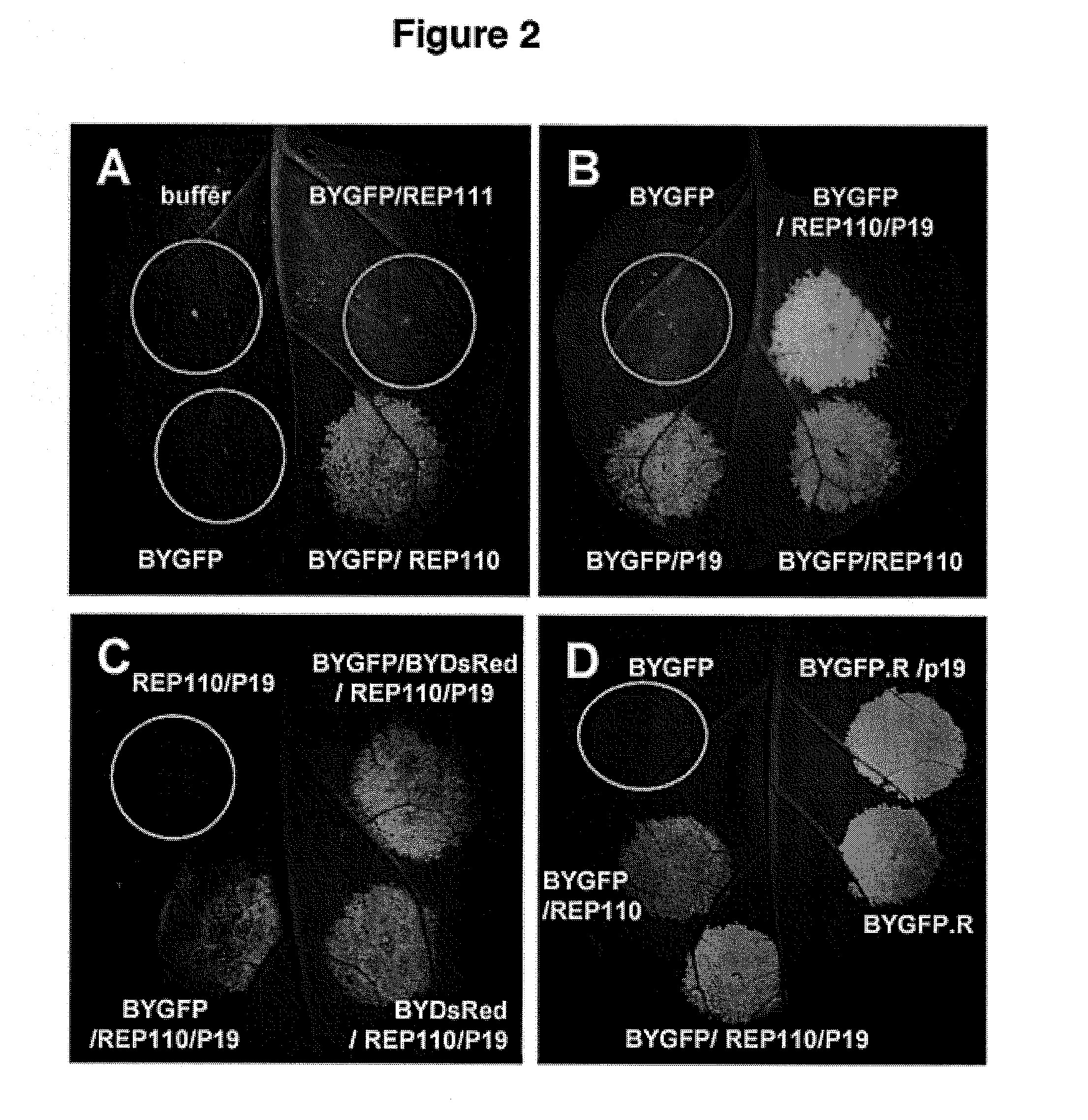
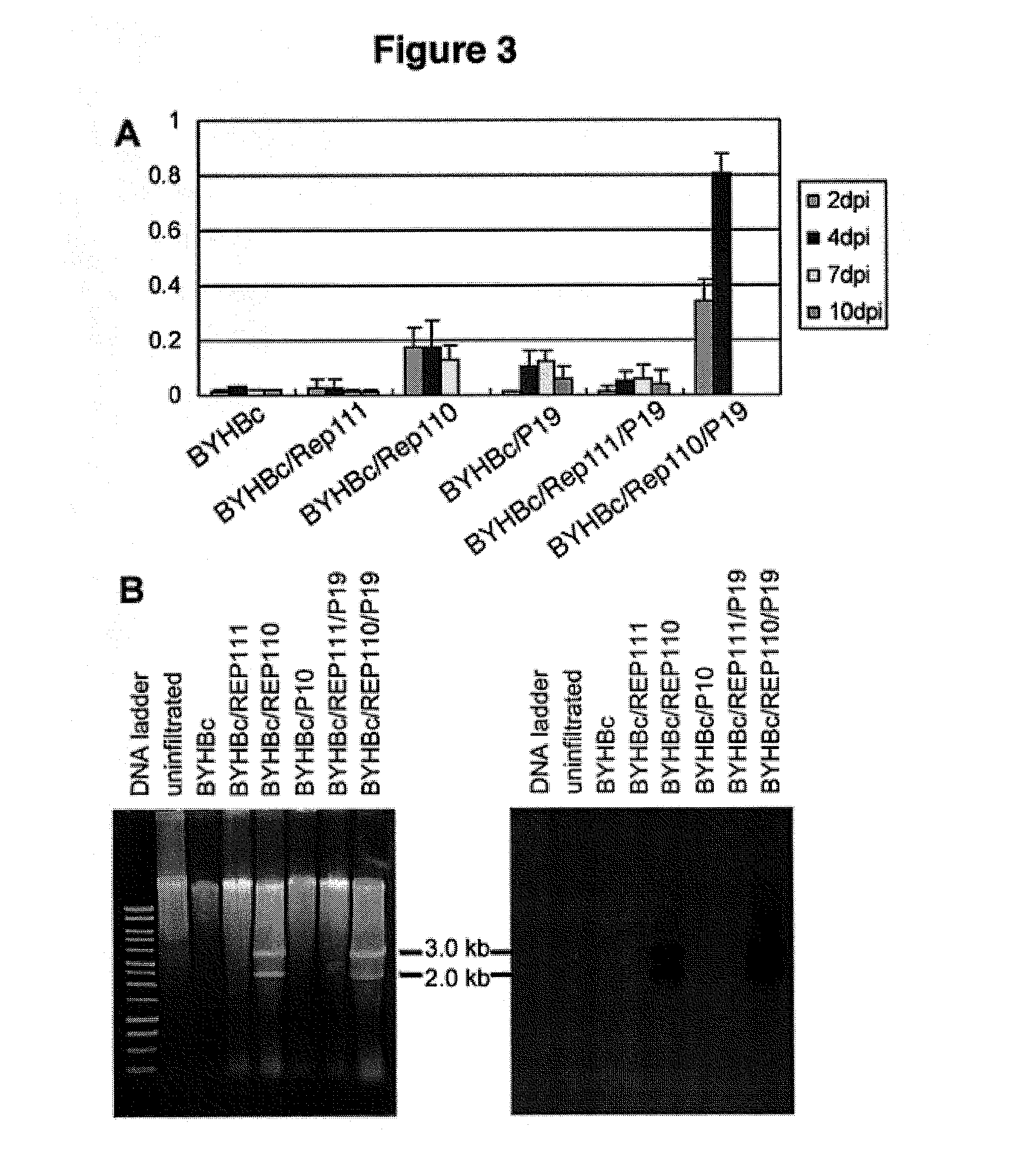
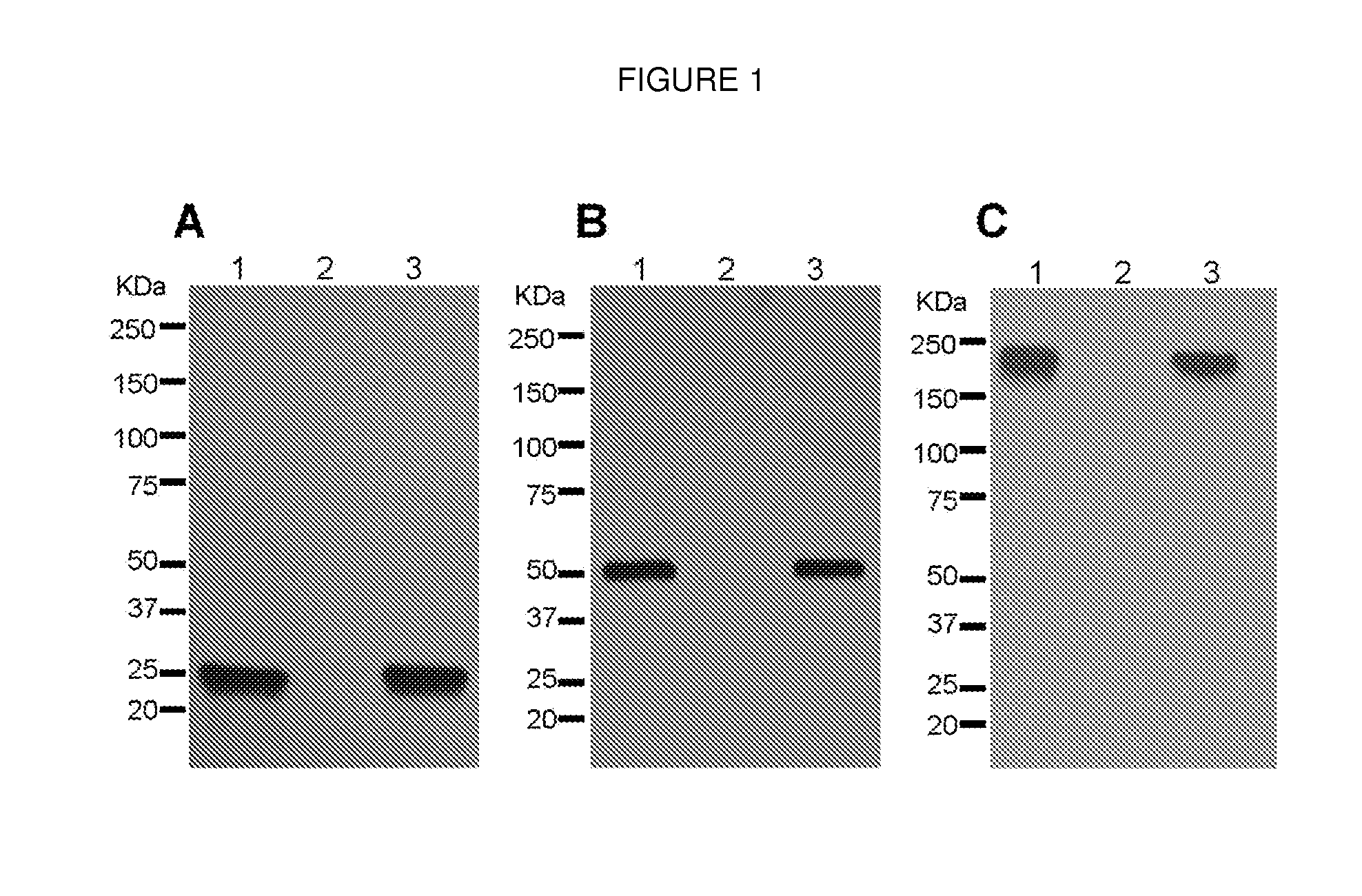
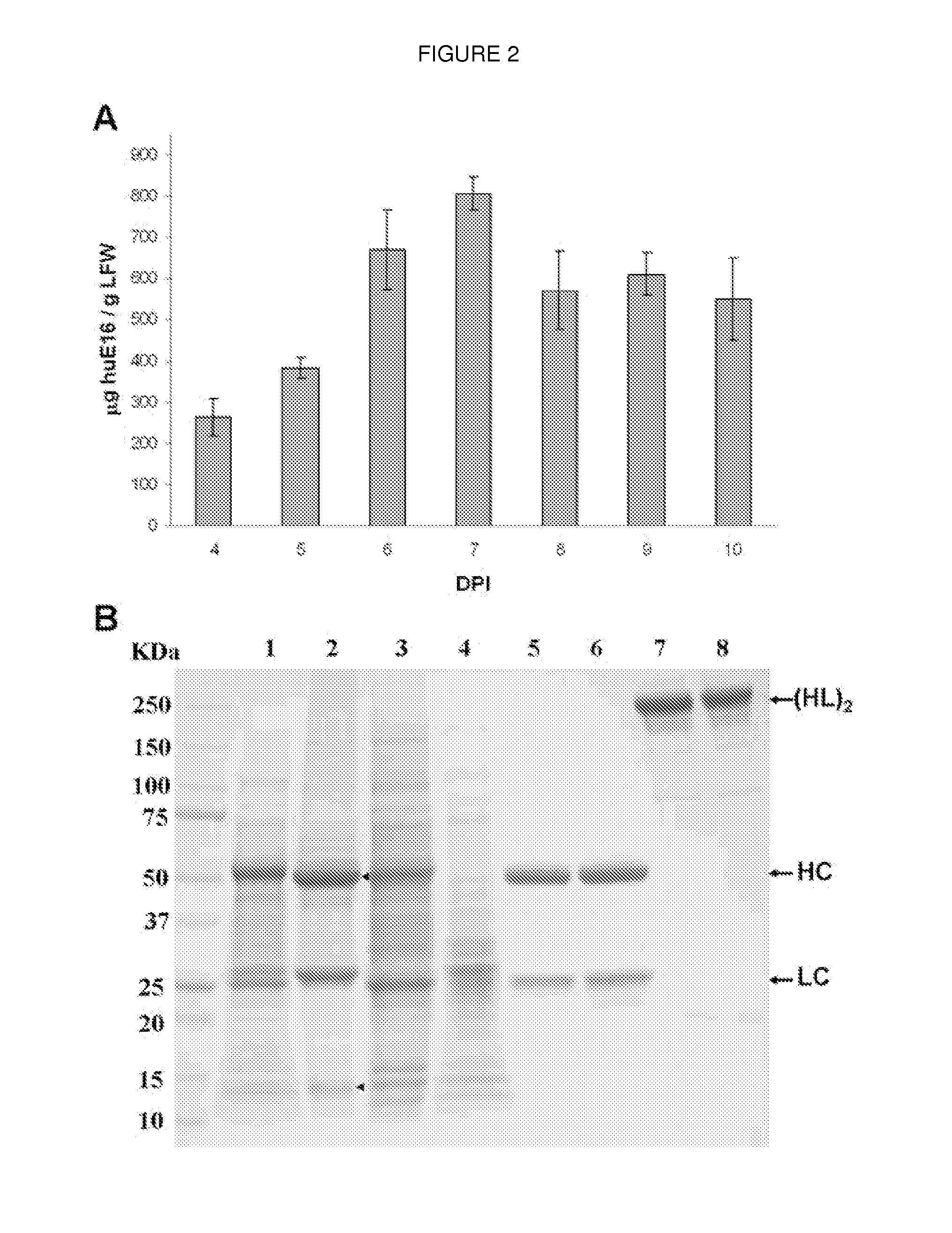
DNA replicon system for high-level rapid production of vaccines and monoclonal antibody therapeutics in plants
ActiveUS8513397B2Expand business applicationsSugar derivativesVaccinesPlant virusVirus-like particle
Plant viral vectors have great potential in rapid production of proteins, but no simple. Here a geminivirus-based system for high-yield and rapid production of oligomeric protein complexes, including virus-like particle (VLP) vaccines and monoclonal antibodies (mAbs) is described. In particular, a single vector that contains two non-competing replicons for transient expression in Nicotiana benthamiana leaves is described. The correct assembly of these subunit proteins into functional oligomeric structures (VLPs or full-size mAb) is also described. This system advances plant transient expression technology by eliminating the need for non-competing viruses, and thus, enhances the realistic commercial application of this technology for producing multiple-subunit protein complexes.
Owner:ARIZONA STATE UNIVERSITY
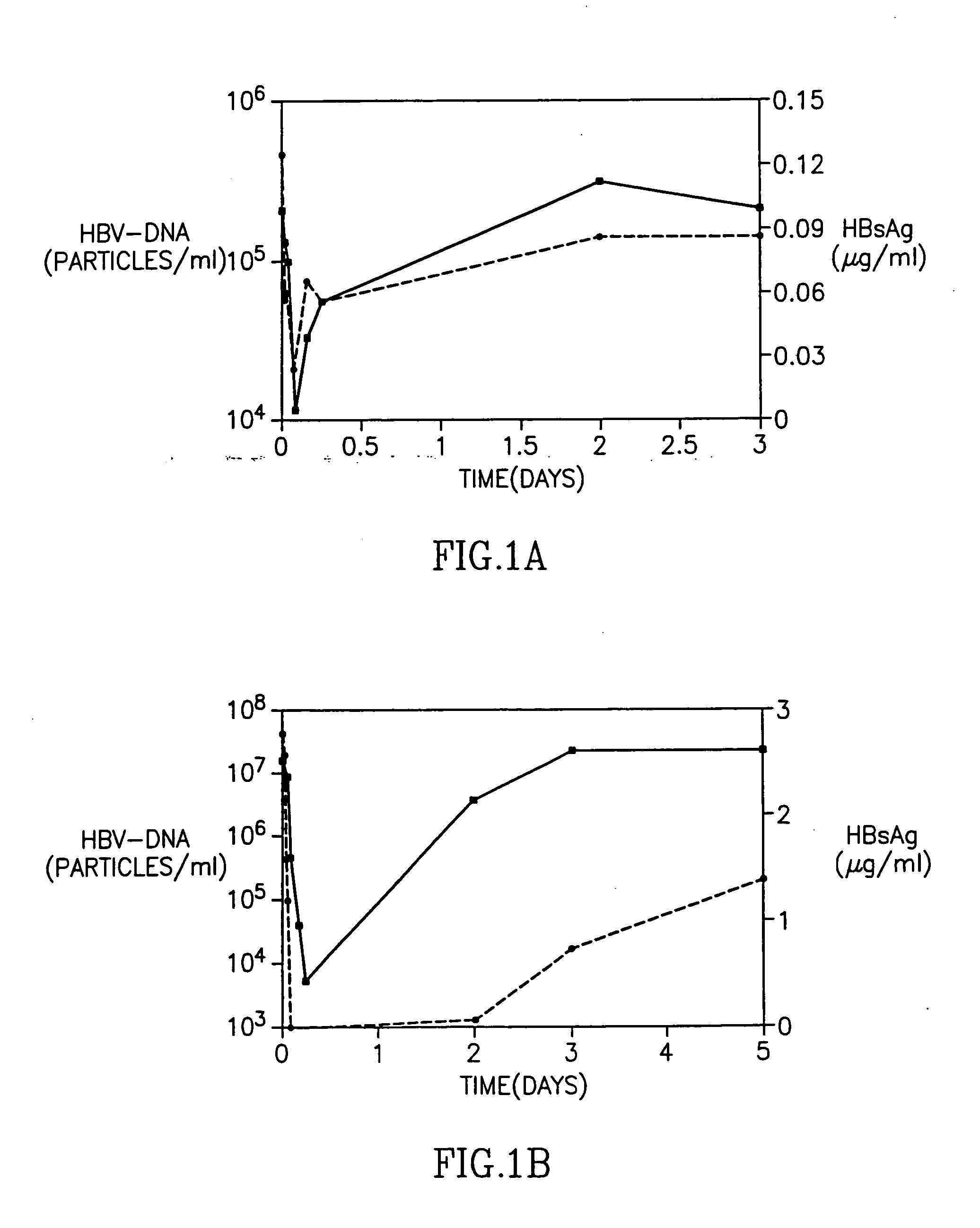
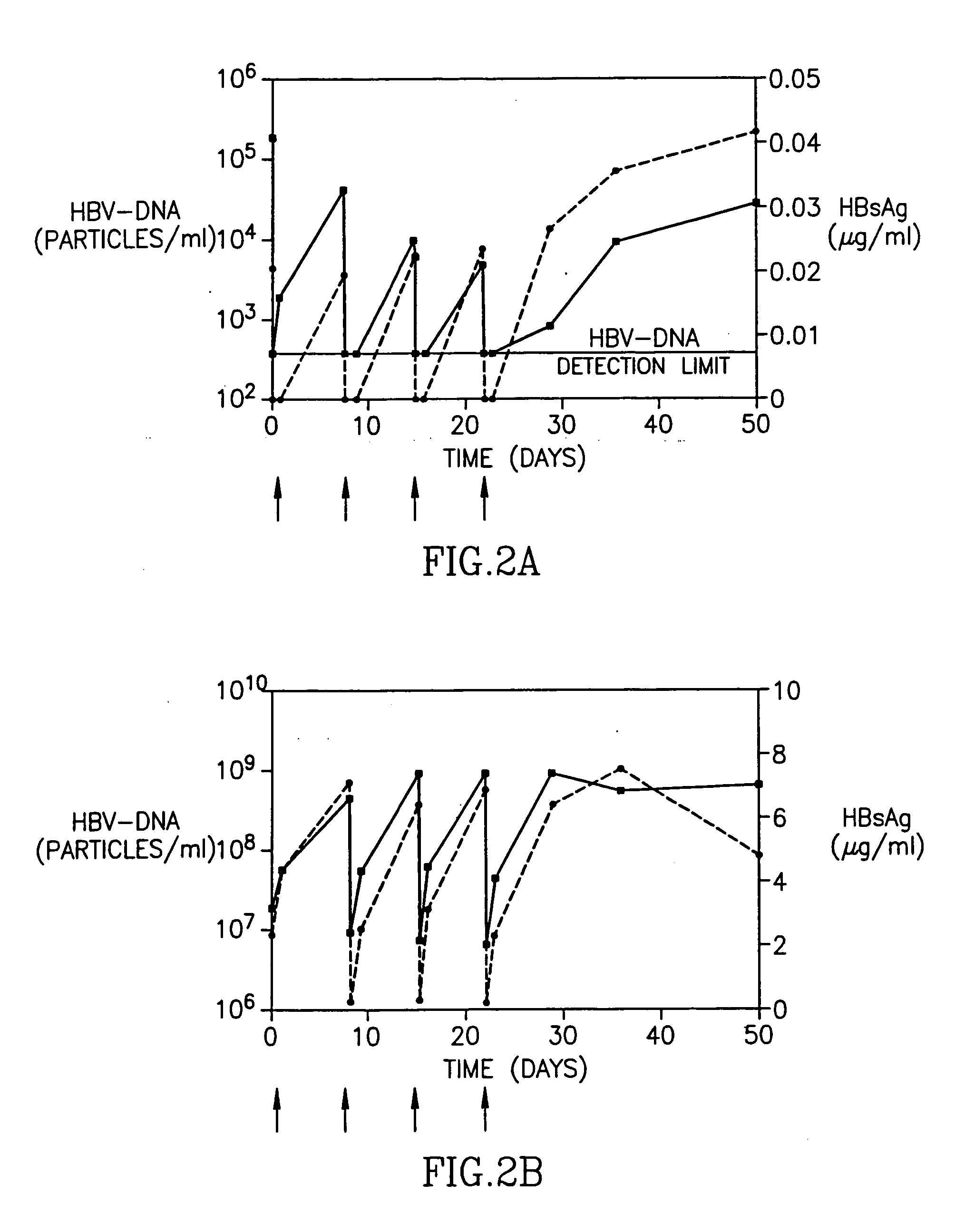
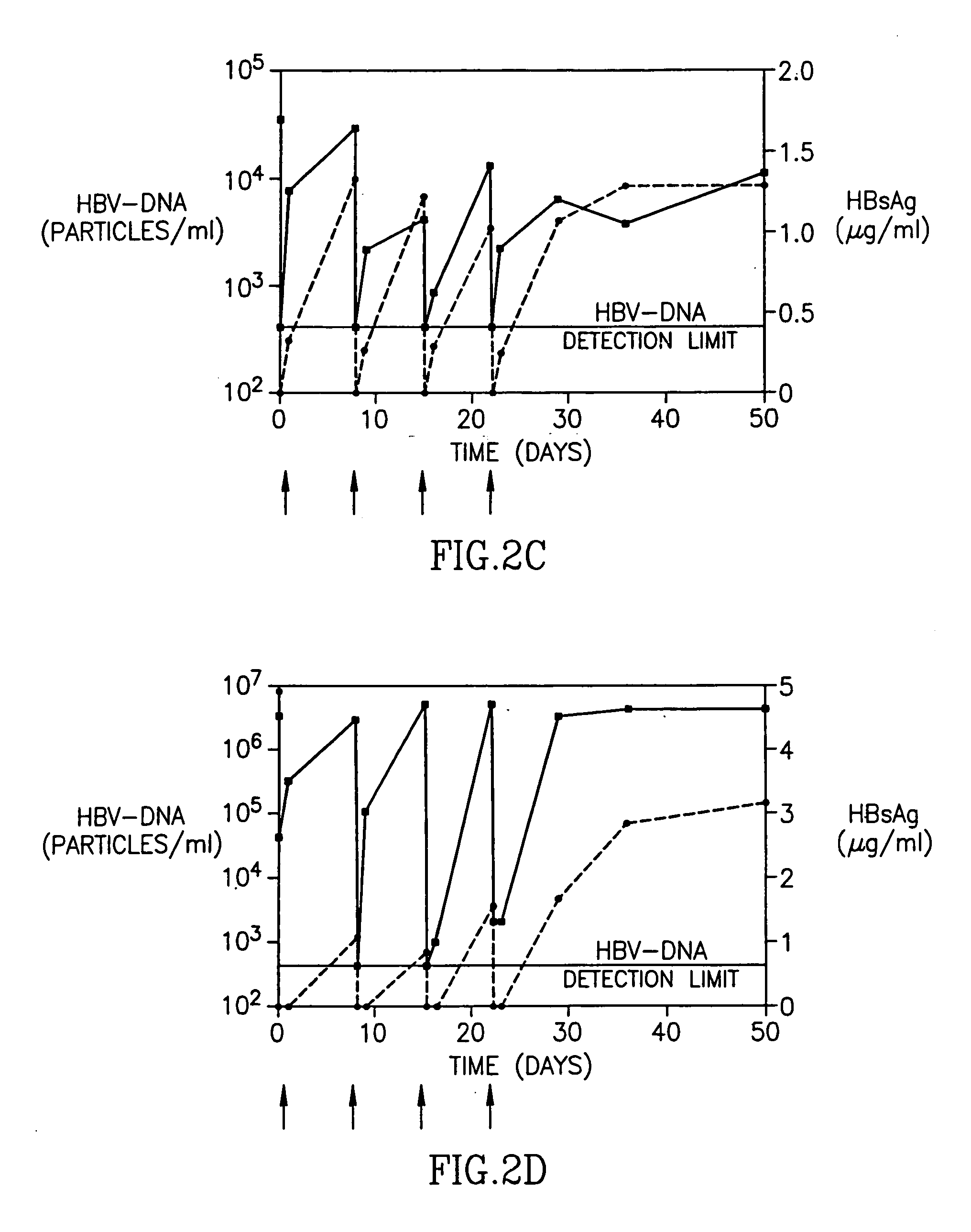
Treatment of hepatitis B virus infection with human monoclonal antibodies
InactiveUS20050260195A1Eliminate infectionMicrobiological testing/measurementImmunoglobulins against virusesHBsAgCyrtanthus elatus virus A
Disclosed is a pharmaceutical composition for the treatment or prevention of hepatitis B virus infection, comprising a 1:3 mixture of two fully human anti HBsAg monoclonal antibodies 19.79.5 and 17.1.41. Also disclosed are preferred modes of administration. The pharmaceutical composition can be given as a monotherapy or in combination with other anti viral agents.
Owner:XTL BIOPHARMLS
Hybrid tumor cell strain 1D7 capable of secreting high neutralizing activity canine distemper virus monoclonal antibody
InactiveCN102618503AHigh neutralization potencyGood treatment effectMicroorganism based processesImmunoglobulins against virusesAntiendomysial antibodiesCanine distemper virus CDV
The invention relates to a hybrid tumor cell strain capable of secreting high neutralizing activity CDV (canine distemper virus) monoclonal antibody. A hybrid tumor cell strain 1D7 is selected from a hybrid tumor cell bank containing 82 strains which secrete CDV monoclonal antibody, and is used for preparing mouse ascitic antibody which has neutralizing potency up to 106 to CDV. The hybrid tumor cell strain capable of secreting high neutralizing activity CDV monoclonal antibody is high in neutralizing potency and resistant to CDV strains in a wide range, and has evident treatment effect on CDattached dogs, minks or foxes in clinical treatment, and is safe without adverse side effects. The monoclonal antibody therapeutic agent prepared with the hybrid tumor cell strain has stable neutralizing potency after two years of storage, and is high in stability.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Hybridoma cell strain secreting high-neutralization activity infectious bursal disease virus (IBDV) monoclonal antibody
ActiveCN103232974AHigh neutralization potencyAnd high potencyMicroorganism based processesImmunoglobulins against virusesMonoclonalInfectious bursitis
The invention relates to a hybridoma cell strain secreting a high-neutralization activity infectious bursal disease virus (IBDV) monoclonal antibody, and belongs to the field of biotechnology. The hybridoma cell strain C128 is screened from an established bank of hybridoma cells secreting the IBDV monoclonal antibody, and a mice ascites monoclonal antibody prepared by the hybridoma cell strain has IBDV neutralization titer of 1010. The high-neutralization activity IBDV monoclonal antibody secreted by the hybridoma cell strain C128 has high neutralization titer and a wide IBDV strain resistance range, can be used for clinical treatment on IBD-infected animals, and has obvious curative effects, good safety and no adverse side reaction. A monoclonal antibody preparation prepared by the hybridoma cell strain C128 keeps neutralization titer after being preserved for 2 years and has good stability.
Owner:JIANGSU ACAD OF AGRI SCI
Monoclonal antibodies and use thereof
InactiveUS7807157B2Treatment safetyNervous disorderImmunoglobulins against animals/humansAmyloid betaProtein C
Owner:COLLATERAL AGENTS
DNA replicon system for high-level rapid production of vaccines and monoclonal antibody therapeutics in plants
ActiveUS20110262966A1Expand business applicationsConvenient lengthSugar derivativesVaccinesPlant virusVirus-like particle
Plant viral vectors have great potential in rapid production of proteins, but no simple. Here a geminivirus-based system for high-yield and rapid production of oligomeric protein complexes, including virus-like particle (VLP) vaccines and monoclonal antibodies (mAbs) is described. In particular, a single vector that contains two non-competing replicons for transient expression in Nicotiana benthamiana leaves is described. The correct assembly of these subunit proteins into functional oligomeric structures (VLPs or full-size mAb) is also described. This system advances plant transient expression technology by eliminating the need for non-competing viruses, and thus, enhances the realistic commercial application of this technology for producing multiple-subunit protein complexes.
Owner:ARIZONA STATE UNIVERSITY
Production of a Monoclonal Antibody Therapeutic Against West Nile Virus in Plants
InactiveUS20120329994A1Rapid productionMaintain good propertiesHybrid immunoglobulinsAntibody ingredientsTherapeutic antibodyAnti-West Nile virus IgG
The present invention describes the plant-based production of a therapeutic antibody against West Nile Virus.
Owner:ARIZONA STATE UNIVERSITY
Use of monoclonal antibodies specific to the O-acetylated form of GD2 ganglioside for treatment of certain cancers
ActiveUS8951524B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAcetylationPeripheral neuron
The invention relates to the use of monoclonal antibodies that only recognise the O-acetylated form of the GD2 ganglioside, or fragments of said antibody, for the diagnosis and the treatment of cancers in which the cells express the O-acetylated GD2, said antibody or said fragment recognising the O-acetylated GD2 molecules expressed by the tumoral cells and not recognising the GD2 molecules expressed at the surface of the peripheral nerves, in order to increase the specificity of the diagnosis and reduce the toxicity of the treatments. The invention also relates to artificially modified antibodies advantageously used for treating and diagnosing cancers in which the cells express the O-acetylated GD2.
Owner:UNIV DE NANTES
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS9056906B2Treating and preventing and slowing progression of and ameliorating symptomSlow and reduce autoimmunitySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
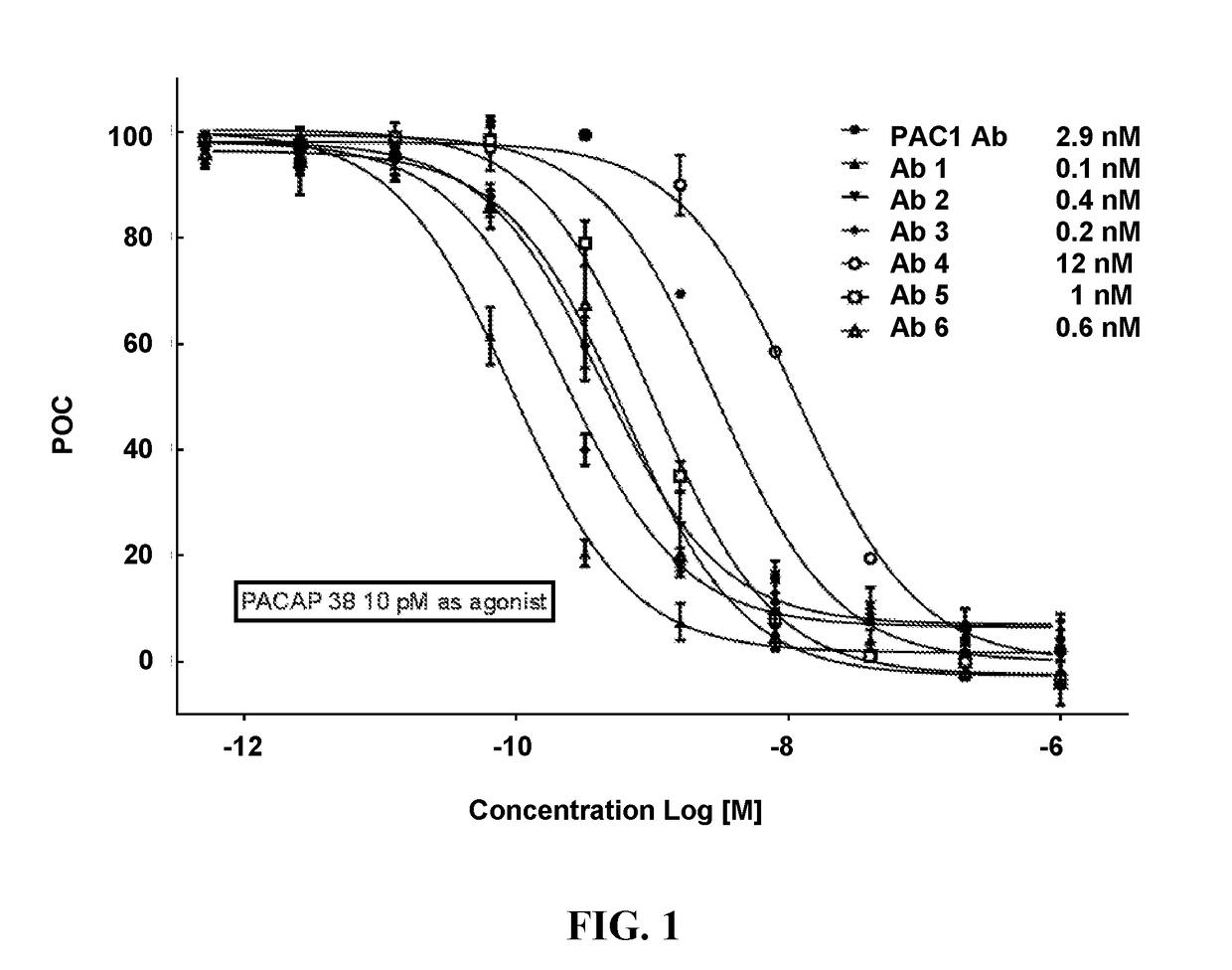
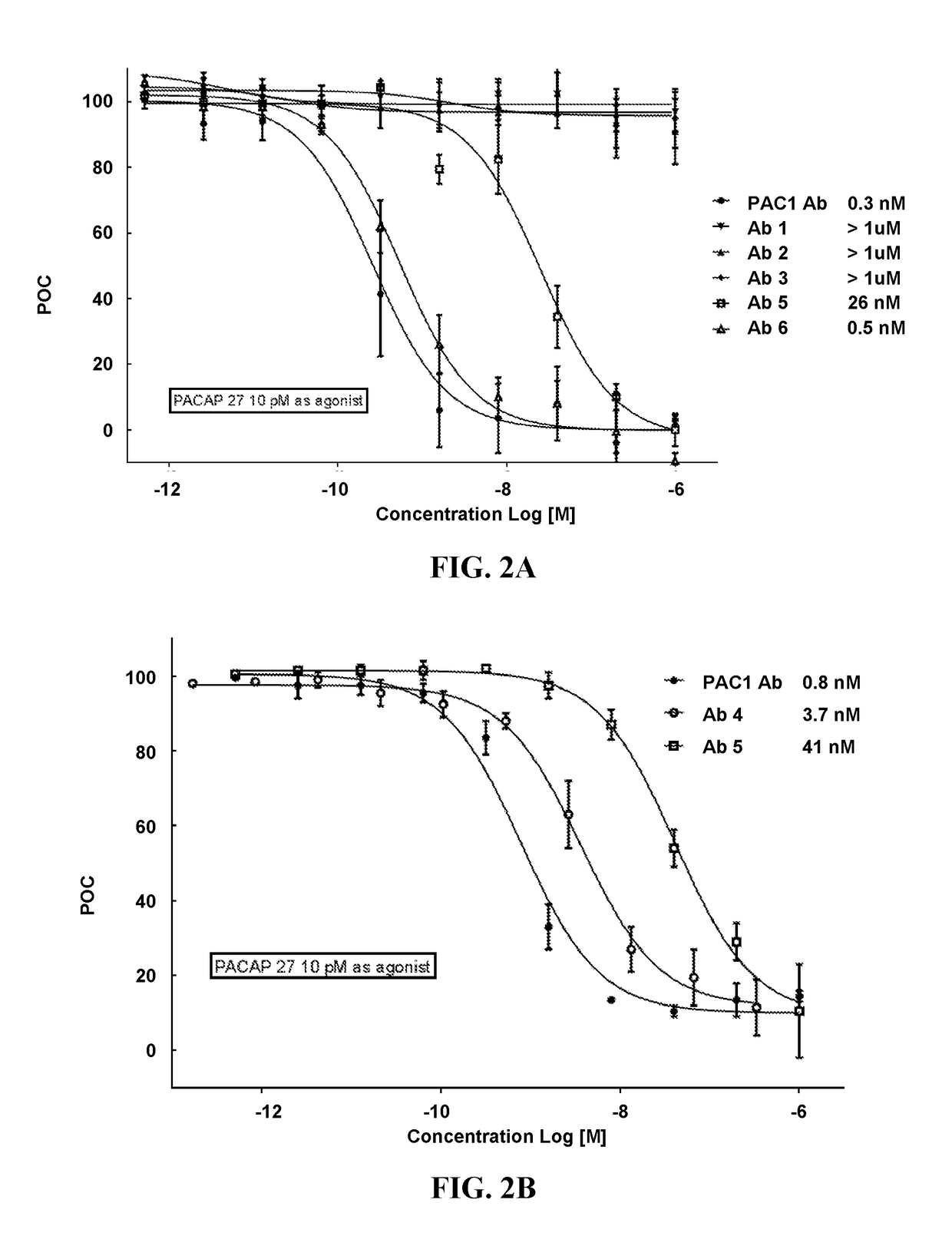
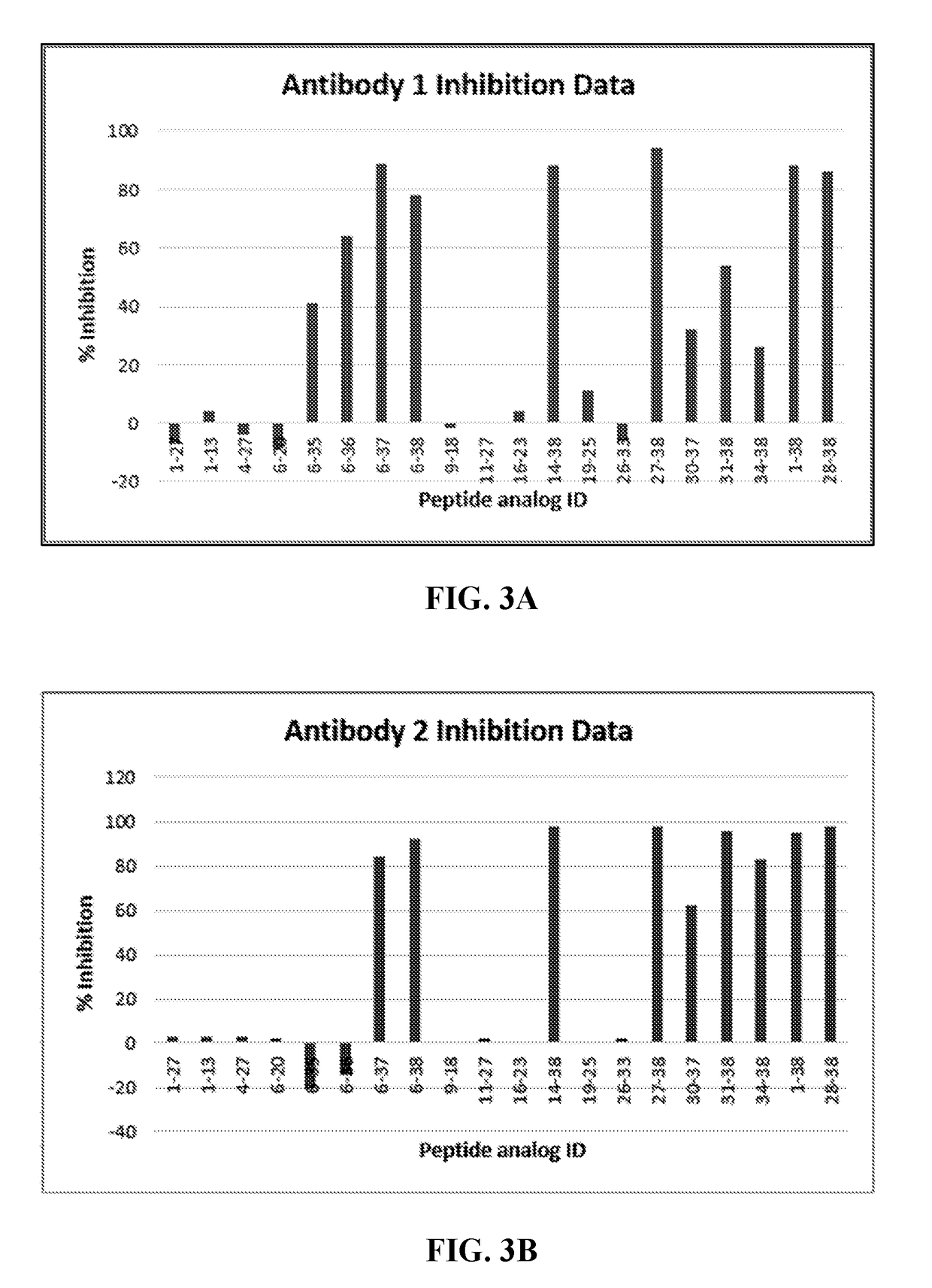
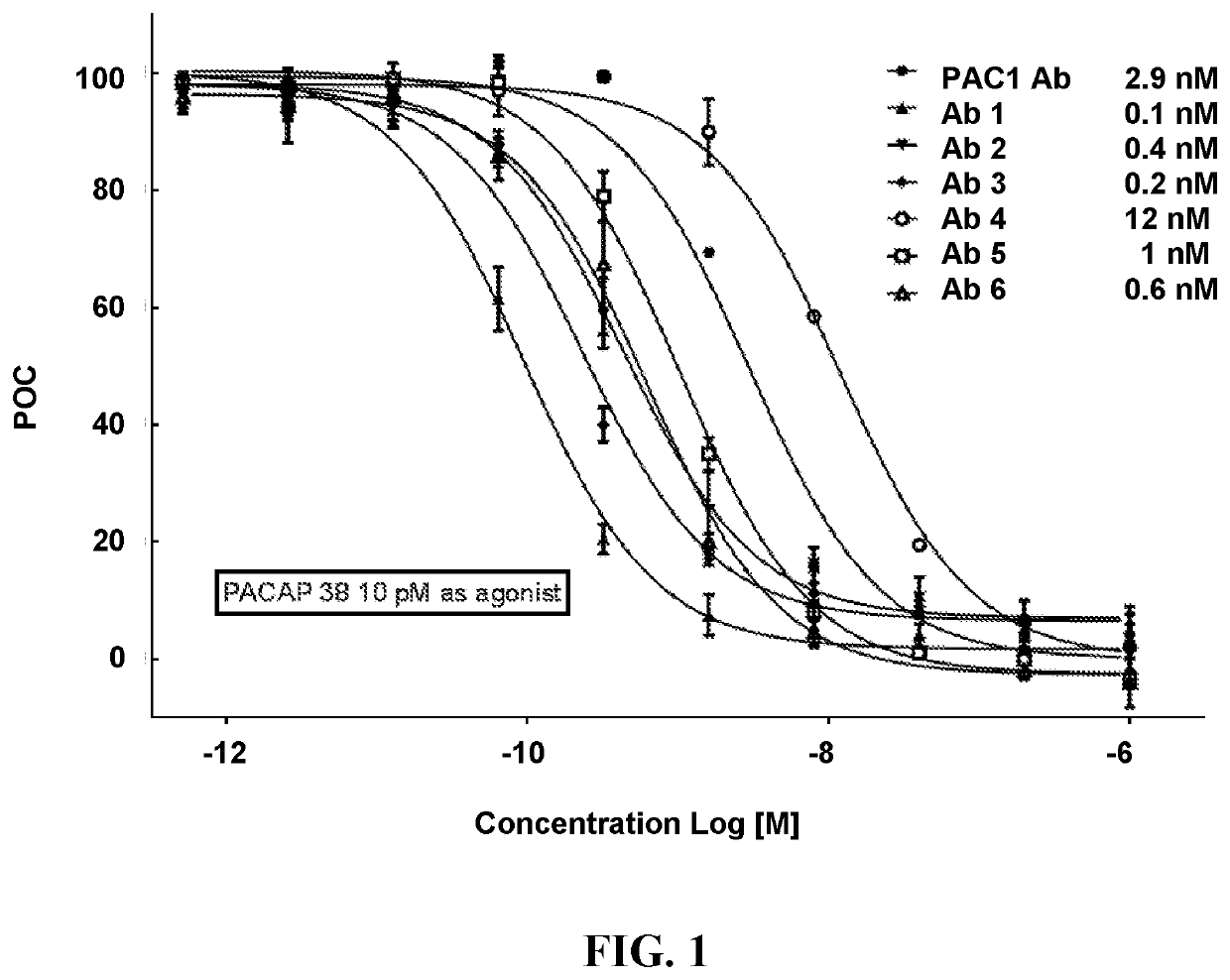
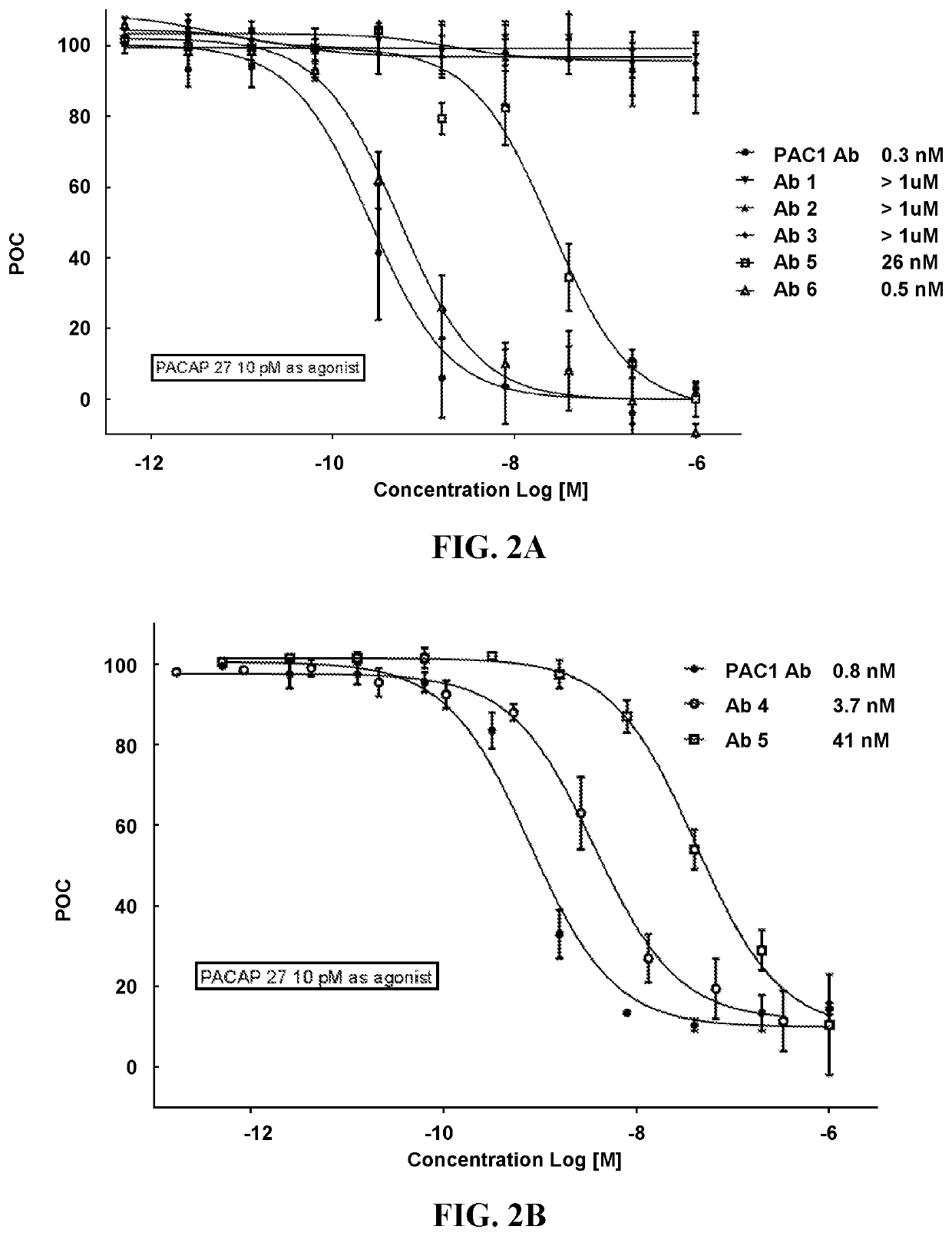
Pacap antibodies and uses thereof
ActiveUS20180362643A1Avoid adjustmentNervous disorderPharmaceutical delivery mechanismHeadachesCluster headache
The present invention relates to monoclonal antibodies that specifically bind to human pituitary adenylate cyclase activating polypeptide (PACAP) and pharmaceutical compositions comprising such antibodies. Methods of treating or preventing headache conditions, such as migraine and cluster headache, using the monoclonal antibodies are also described.
Owner:AMGEN INC
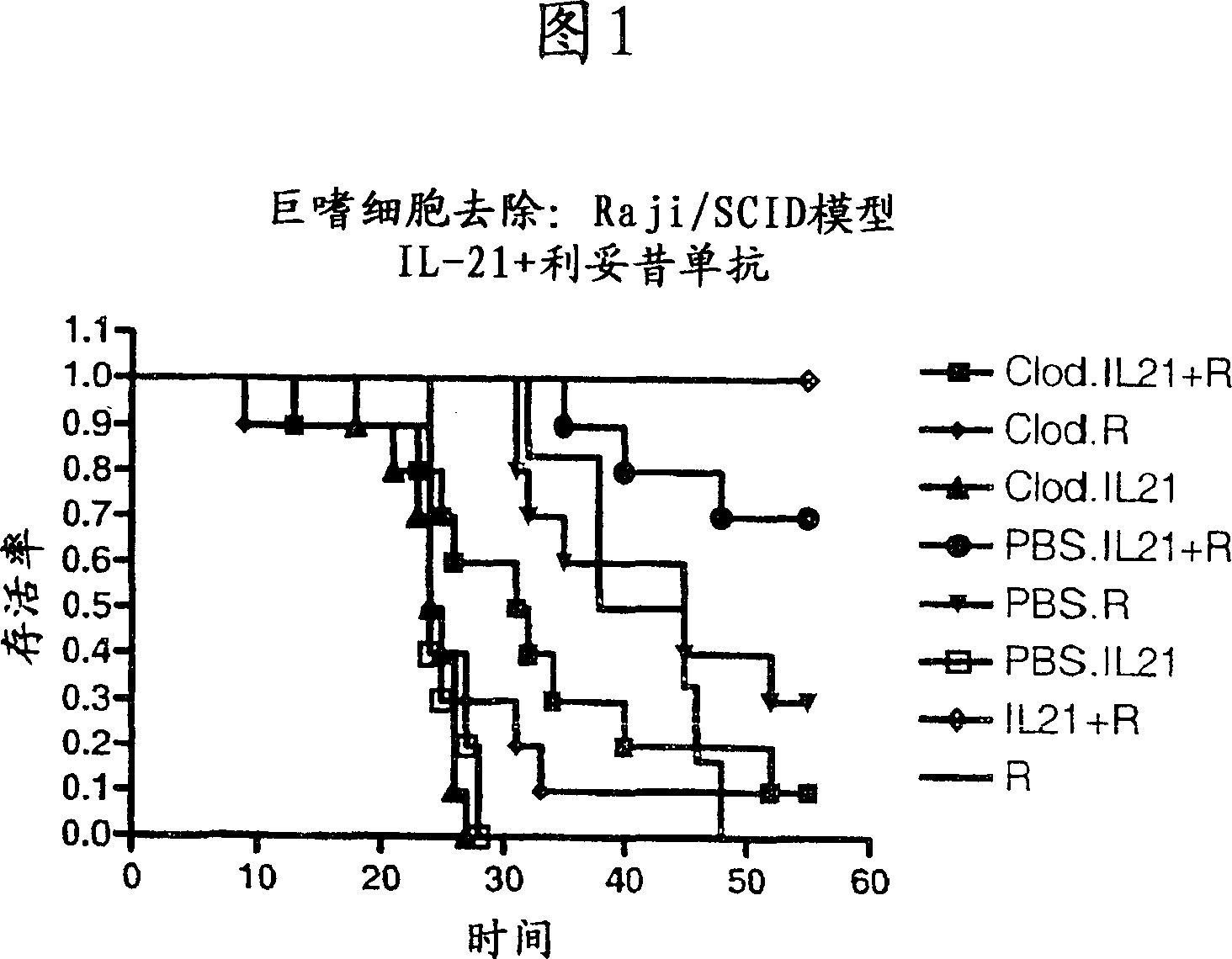
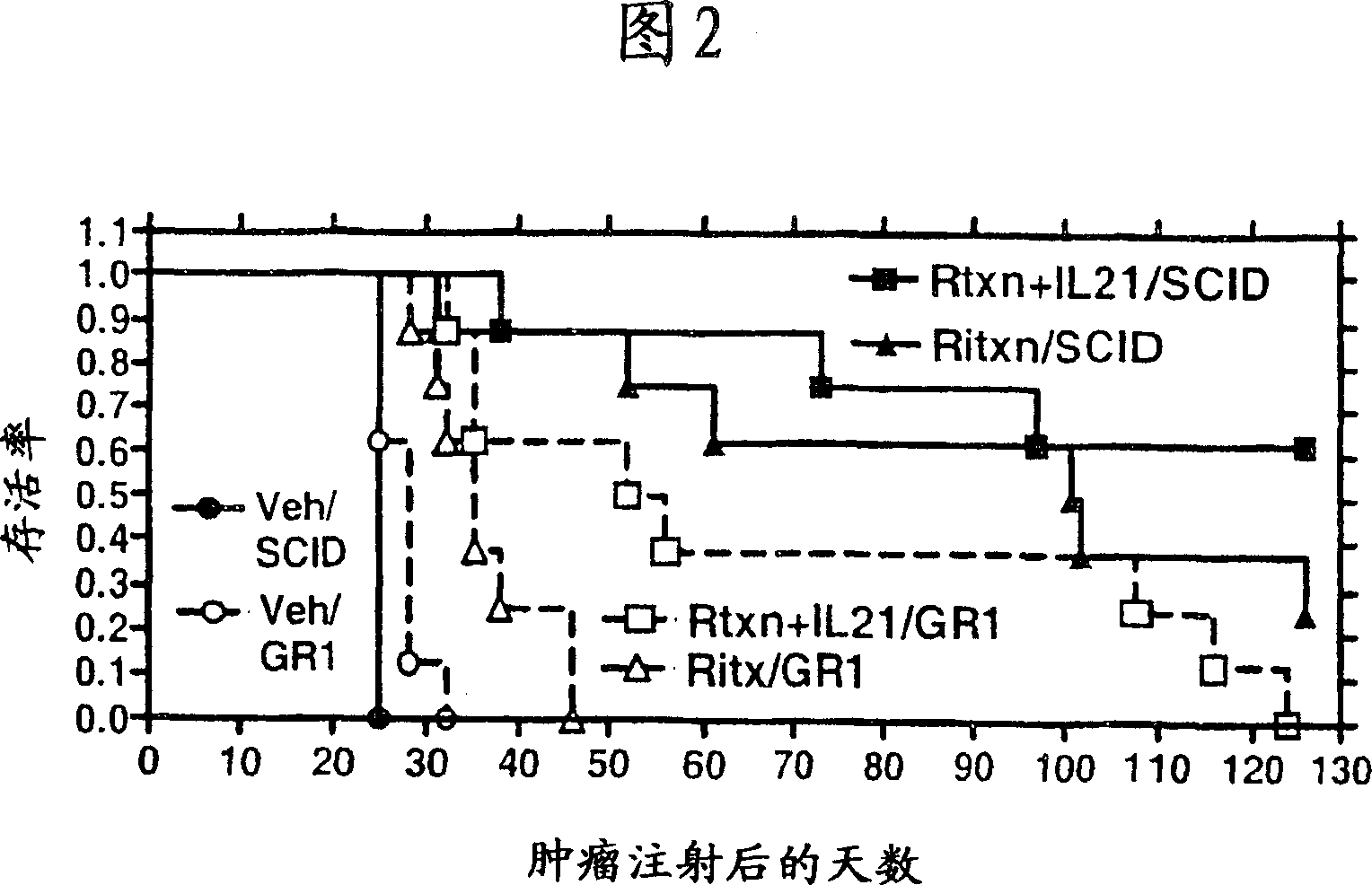
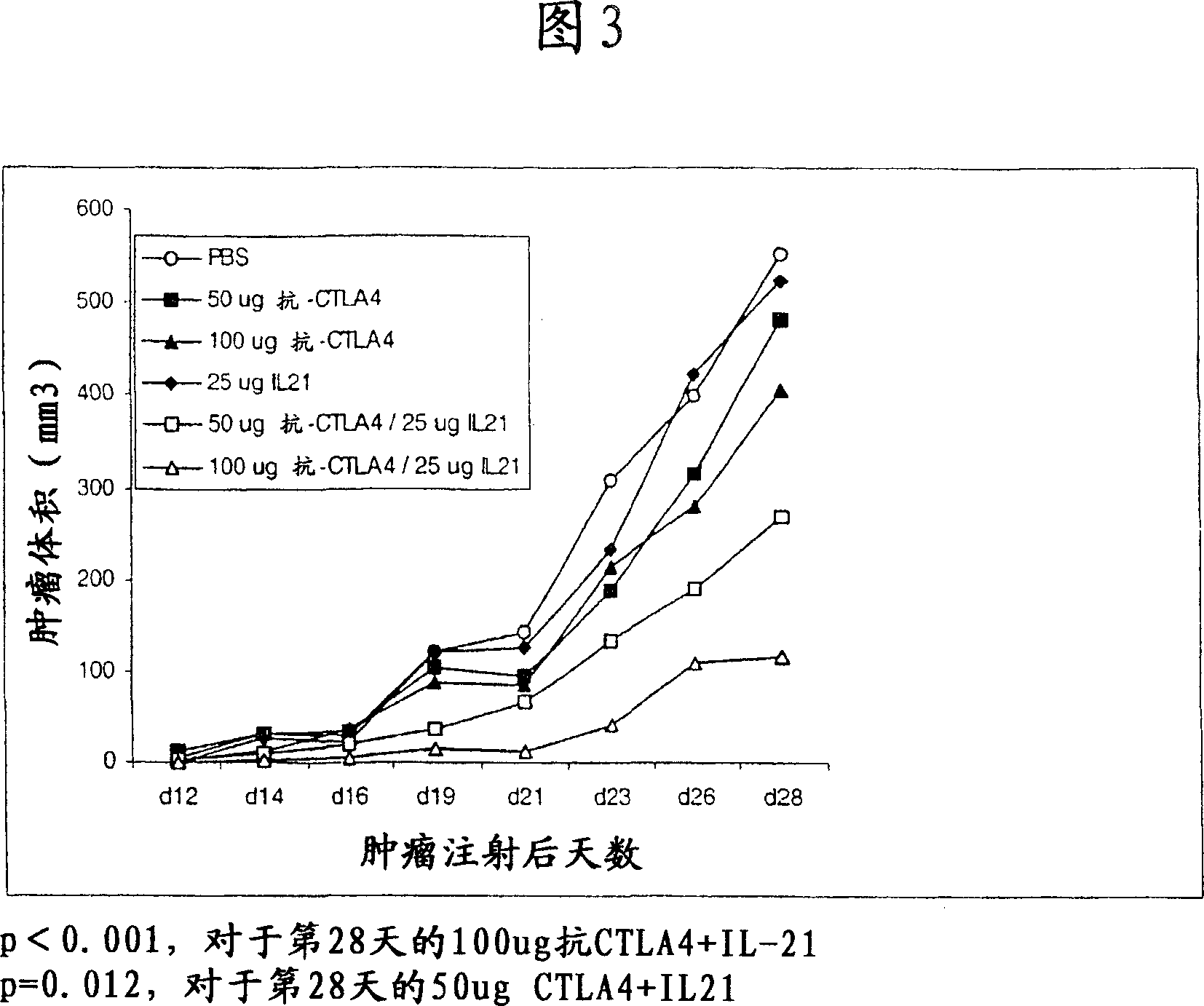
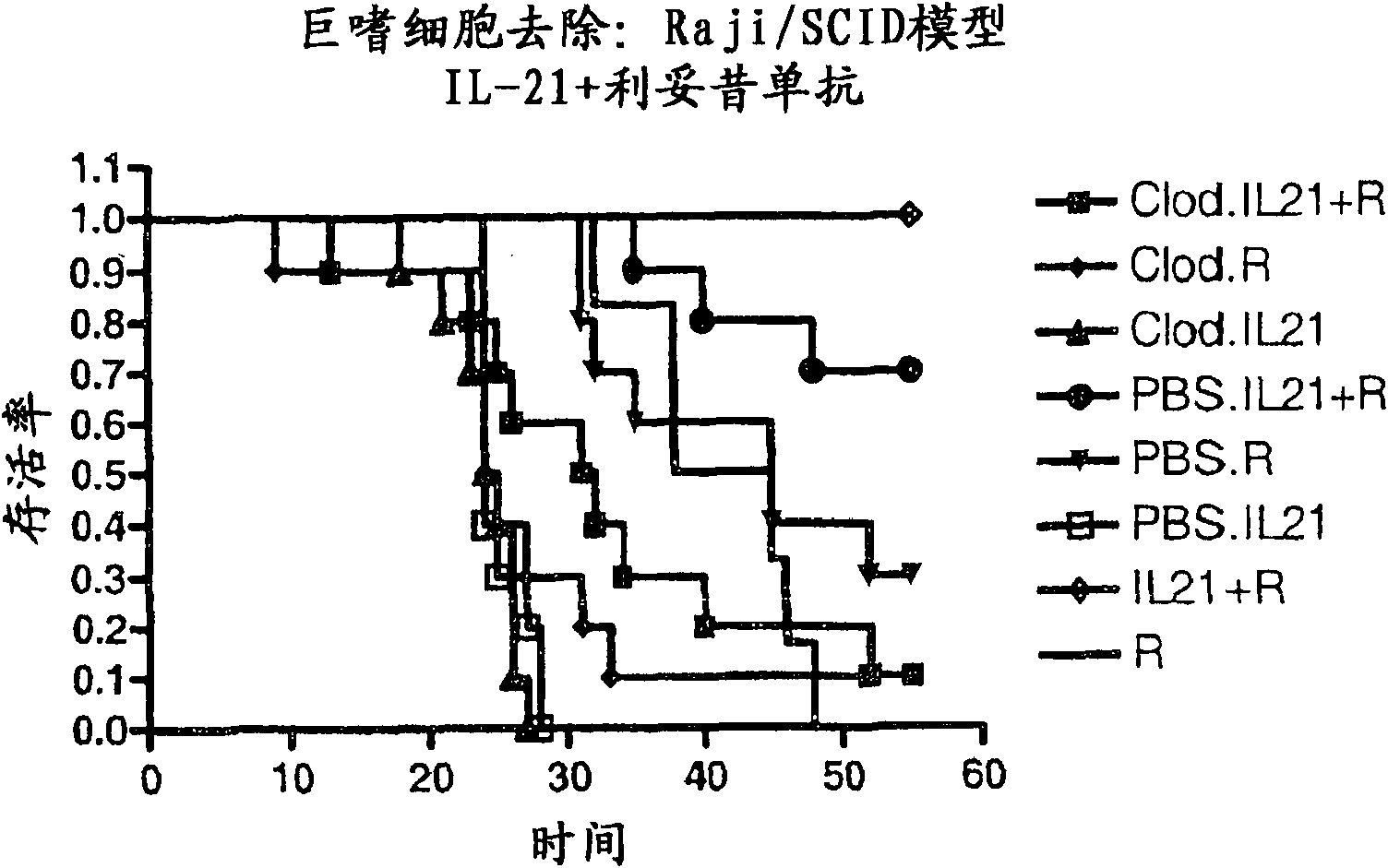
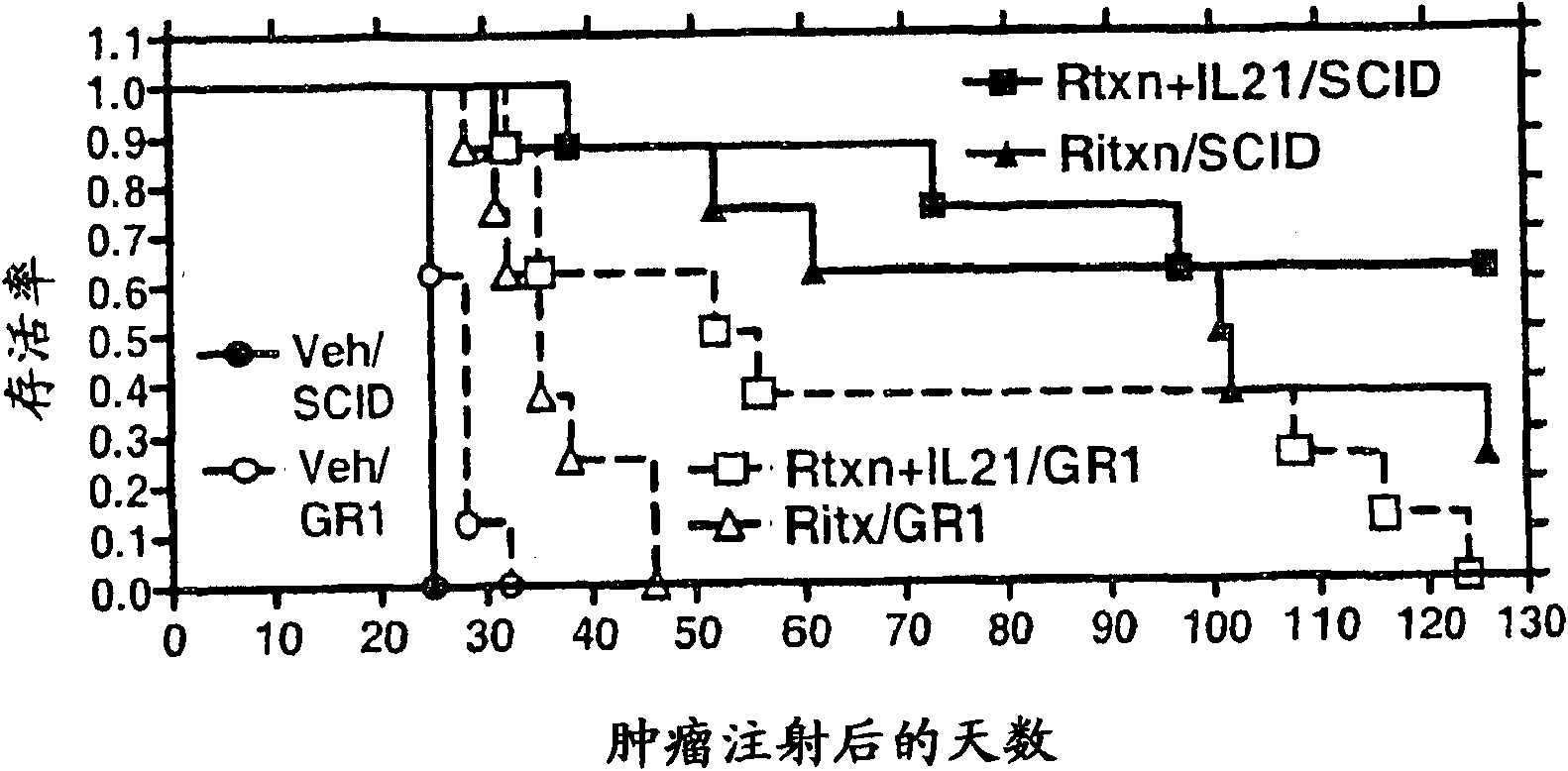
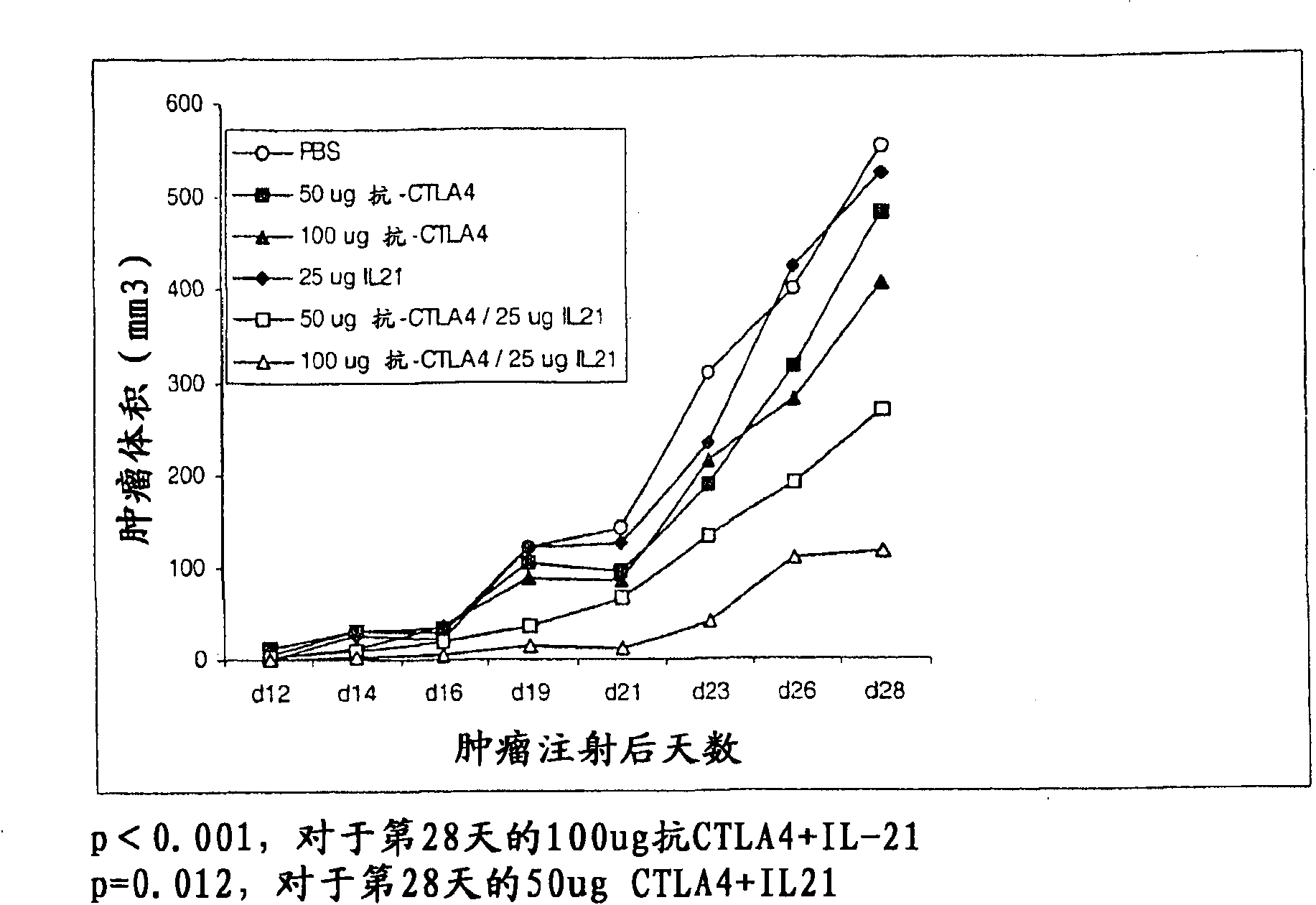
Methods of treating cancer using il-21 and monoclonal antibody therapy
A method of treating cancer by co-administering a therapeutic monoclonal antibody and IL-21 is described. Exemplary monoclonal antibodies that may be used are rituximab, trastuzumab, and anti-CTLA-4 antibodies. For patient populations resistant to monoclonal antibody therapy, relapsed after treatment with monoclonal antibodies, or in which enhanced IL-21 antitumor effects reduce toxicity associated with therapy with monoclonal antibodies, the The enhanced antitumor properties of the combination therapy described above are particularly useful.
Owner:ZYMOGENETICS INC
PACAP antibodies and uses thereof
ActiveUS10822408B2Nervous disorderPharmaceutical delivery mechanismAntiendomysial antibodiesAdenylyl Cyclases
Owner:AMGEN INC
Application of bone marrow mesenchymal stem cells combined with monoclonal antibodies in cancer treatment
InactiveCN112940126AInhibition of proliferative abilityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesCancer cell
The invention relates to application of bone marrow mesenchymal stem cells combined with monoclonal antibodies in cancer treatment. Monoclonal antibodies specifically aiming at the liver cancer are obtained through screening, the antibodies have a good effect of inhibiting proliferation of liver cancer cells Huh-7 and HepG2 at the same time, meanwhile it is confirmed in a mouse model test that the monoclonal antibodies have a good effect of inhibiting proliferation of tumors in a mouse body, and the antibodies and mesenchymal stem cells are combined for treating liver cancer to generate a synergistic effect, so that the antibodies have a relatively good application prospect.
Owner:北京广未生物科技有限公司
Methods of treating cancer using IL-21 and monoclonal antibody therapy
InactiveCN102188704APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAfter treatmentTreatment use
Methods for treating cancer by co-administering a therapeutic monoclonal antibody with IL-21 are described. Exemplary monoclonal antibodies that can be used are rituximab, trastuzumab and anti-CTLA-4 antibodies. The enhanced antitumor of the combination therapy is particularly useful for patient populations that are recalcitrant to monoclonal therapy, relapse after treatment with monoclonal antibodies or where the enhanced IL-21 antitumor effect reduces toxicities associated with treatment using the monoclonal antibodies.
Owner:ZYMOGENETICS INC
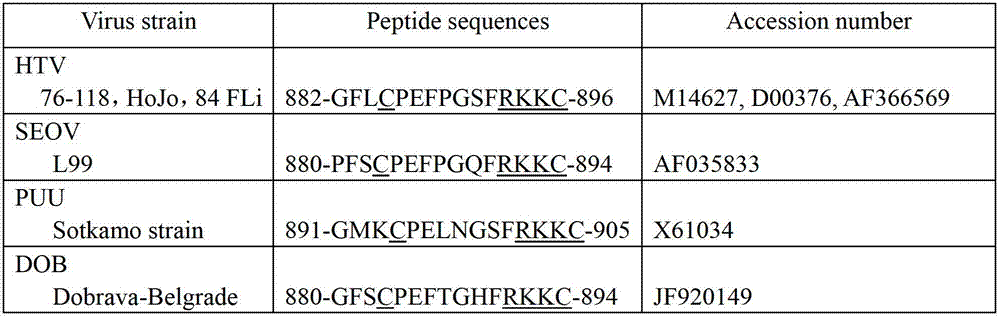
Murine original monoclonal antibody 3D8 identified hantaan virus glycoprotein neutralizing epitope peptide and application thereof
ActiveCN102731623ADirect determination of linear epitopesViral antigen ingredientsAntiviralsViral glycoproteinProtein target
The invention discloses a neutralizing monoclonal antibody 3D8 identified HTNV-GP specific B cell epitope and key amino acid residue sequence of the epitope for treating hemorrhagic fever with renal syndrome (HFRS) caused by hantaan virus (HTNV) infection. The B cell epitope has an amino acid sequence of 882GFLCPEFPGSFRKKC896. The epitope peptide can be applied to study of mechanism of 3D8 neutralizing monoclonal antibody in treatment of HFRS, or preparation of drug for treating HFRS and preparation of novel vaccine strain aiming at hantaan virus 76-118, or development of novel diagnostic kitfor hantaan virus 76-118 as a target protein. The invention has good prospects of development and application in the field of HFRS specific immunotherapy.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
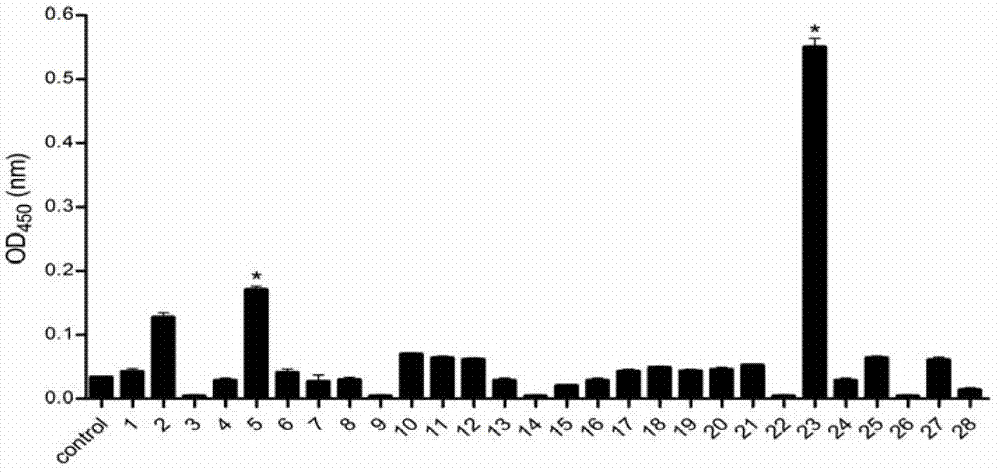
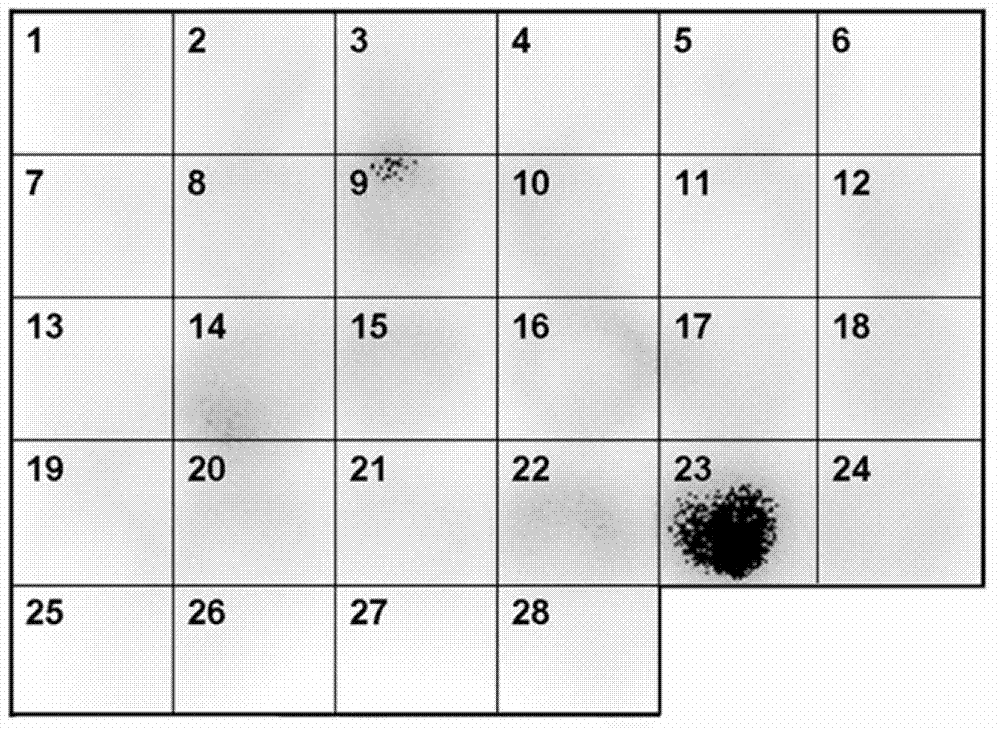
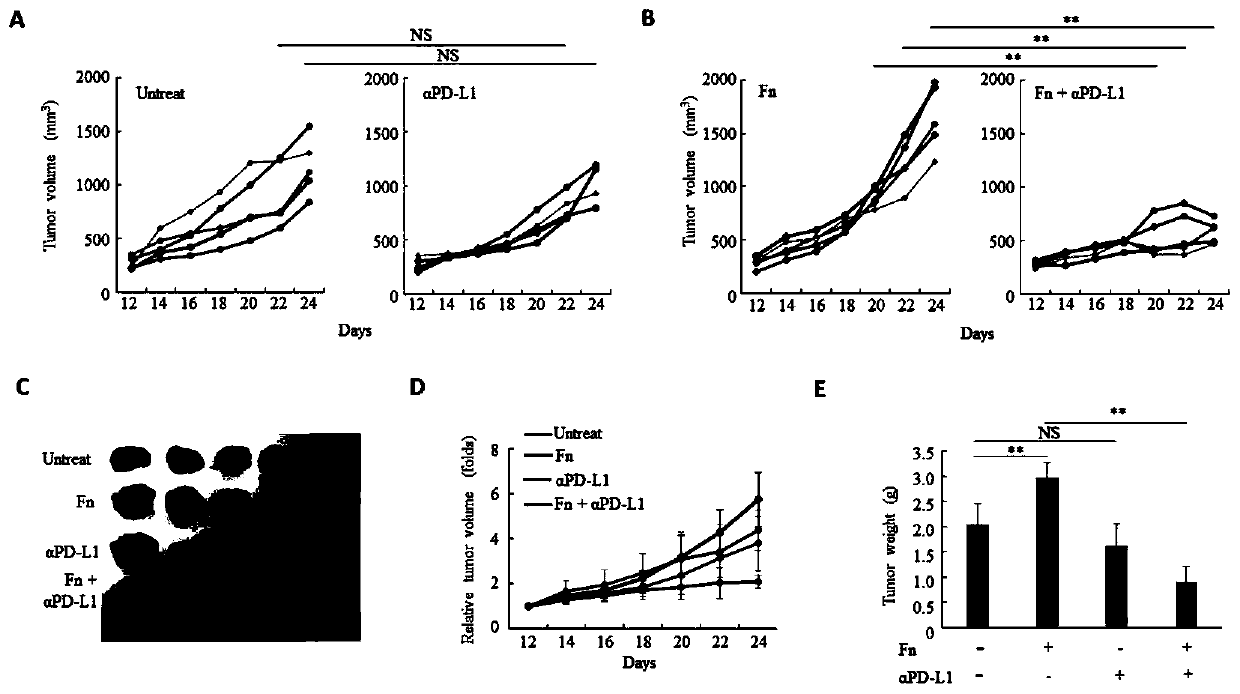
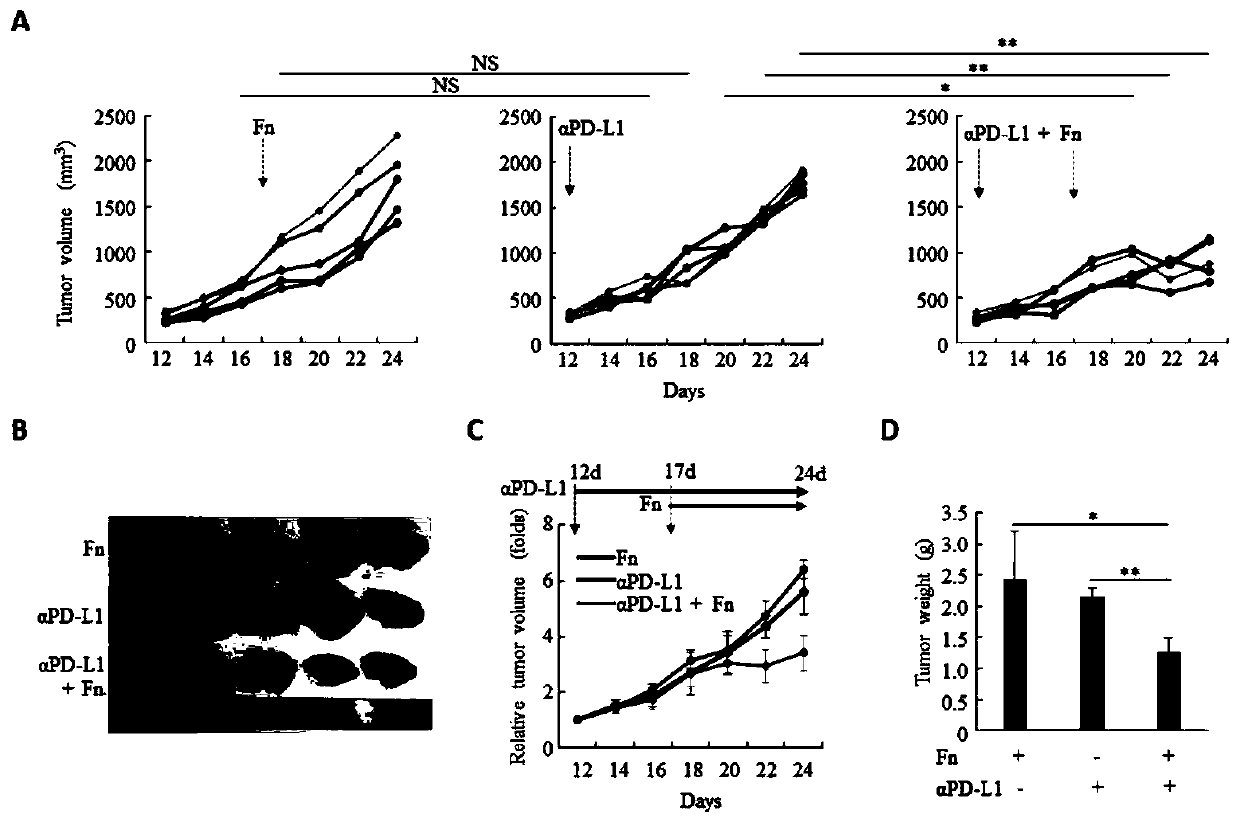
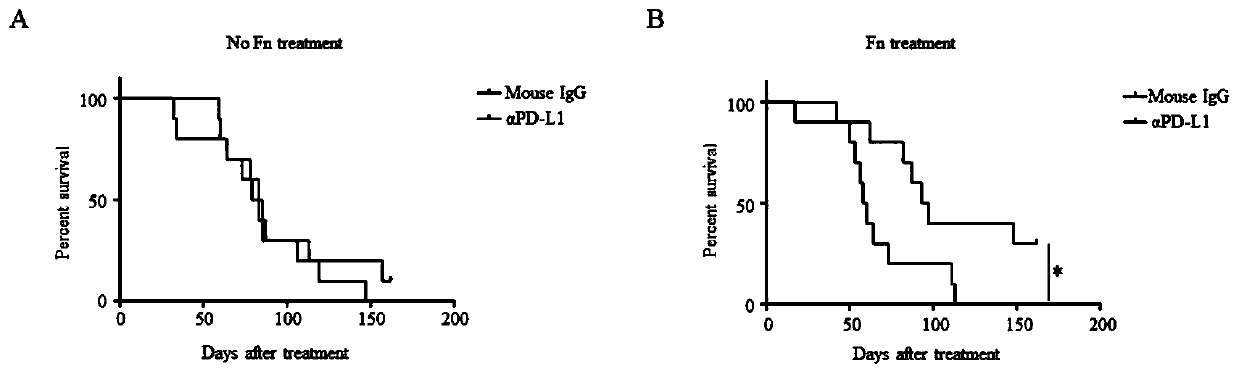
Fusobacterium nucleatum as marker to predict curative effect of PD-L1 antibody therapy of colorectal cancer and application of maker
ActiveCN109908342AGrowth inhibitionProlong lifeMicrobiological testing/measurementDigestive systemCurative effectPD-L1
The invention relates to a preparation containing Fusobacterium nucleatum and PD-L1 antibody and used for immunotherapy of colorectal cancer, application of the preparation, a Fusobacterium nucleatummarker to predict curative effect of PD-L1 antibody therapy of colorectal cancer, and application of the marker. The influence of Fusobacterium nucleatum upon PD-L1 antibody immunotherapy of colorectal cancer is studied deeply herein, and it is discovered that PD-L1 monoclonal antibody therapy is more effective to colorectal cancer in the presence of Fusobacterium nucleatum, while PD-L1 monoclonalantibody therapy helps evidently extend the lifetime of a mouse with colorectal cancer in the presence of Fusobacterium nucleatum; by discussing the mechanism of Fusobacterium nucleatum, it is discovered that Fusobacterium nucleatum can provide transcriptional control for the expression of PD-L1; after PD-L1 antibody therapy is carried out for colorectal cancer tissues in the presence of Fusobacterium nucleatum, the proportion of CD8 positive T cells is increased; all these above are good for the effect of PD-L1 antibody therapy. It is indicated that the presence or absence of Fusobacterium nucleatum is suitable for acting as a potential marker to judge the curative effect of PD-L1 monoclonal antibody therapy for the patient with colorectal cancer; Fusobacterium nucleatum and PD-L1 monoclonal antibody can form an immunotherapy preparation for patients with colorectal cancer.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
High-throughput single cell sorting using microbubble well arrays
InactiveUS20170074872A1Simple conditionsBiomass after-treatmentTissue/virus culture apparatusBiologyHeterogeneous population
The present invention provides a microfabricated device and methods for high throughput single cell screening of a heterogeneous population. The present invention is partly based upon but not limited to sorting by monitoring cell secreted factors that accumulate in time (hours, days, weeks) as cells are cultured in the microbubble well niche the architecture of which facilitate the accumulation. In certain embodiments, the device and method comprises a means to identify effective drugs for personalize therapeutics such as but not limited to discovery of monoclonal antibody therapeutics.
Owner:NIDUS BIOSCI LLC
Reagent kit and method for detecting expression quantities of antigens of acute myelogenous leukemia granulocytes
InactiveCN106290877AGuide targeted therapyRealize quantitative detection and analysisMaterial analysisProgenitorCellular antigens
The invention provides a reagent kit and a method for detecting the expression quantities of antigens of acute myelogenous leukemia granulocytes. Reagents of the reagent kit comprise percp-labeled CD45-resistant antibodies, FITC-labeled CD15-resistant antibodies and PE-labeled CD33-resistant antibodies. The reagent kit is applied to the method, and the expression quantities of the CD33 antigens of the granulocytes (non-stem / progenitor cells) in peripheral blood and bone marrow of AML (acute myelogenous leukemia) patients can be detected by the aid of flow cytometry. Compared with the traditional flow cytometry mainly for measuring target expression antigen cell proportions and qualitatively analyzing the target expression antigen cell proportions, the reagent kit and the method have the advantages that the expression of the CD33 antigens of the myelogenous leukemia granulocytes can be quickly and accurately quantitatively detected and analyzed by the aid of the reagent kit and the method, detection results can be used for evaluating curative effects of AML monoclonal antibody therapy, targeted therapy for AML by the aid of CD33 monoclonal antibody medicines can be effectively guided, and extremely important effects can be realized in related medical detection fields.
Owner:北京海思特医学检验实验室有限公司
Hybrid tumor cell strain 1D7 capable of secreting high neutralizing activity canine distemper virus monoclonal antibody
InactiveCN102618503BAnd high potencyGood treatment effectImmunoglobulins against virusesMicroorganism based processesSide effectAscitic fluid
The invention relates to a hybrid tumor cell strain capable of secreting high neutralizing activity CDV (canine distemper virus) monoclonal antibody. A hybrid tumor cell strain 1D7 is selected from a hybrid tumor cell bank containing 82 strains which secrete CDV monoclonal antibody, and is used for preparing mouse ascitic antibody which has neutralizing potency up to 106 to CDV. The hybrid tumor cell strain capable of secreting high neutralizing activity CDV monoclonal antibody is high in neutralizing potency and resistant to CDV strains in a wide range, and has evident treatment effect on CDattached dogs, minks or foxes in clinical treatment, and is safe without adverse side effects. The monoclonal antibody therapeutic agent prepared with the hybrid tumor cell strain has stable neutralizing potency after two years of storage, and is high in stability.
Owner:JIANGSU ACAD OF AGRI SCI
Vaccine based on simulating human blood vessel endothelial cell growth factor VEGF epitope and preparation method thereof
InactiveCN101429234APrevent proliferationInhibit migrationLibrary screeningPeptidesAntiangiogenesis TherapyVascular endothelium
The invention provides a vaccine based on an epitope simulating human vascular endothelial growth factor VEGF, as well as a preparation method thereof. A VEGF mimic epitope which is specifically affinitive with human-mouse chimeric monoclonal antibody Avastin is screened out by use of a phage random presentation technique, and the amino acid sequence of the mimic epitope is Asp-His-Thr-Leu-Tyr-Thr-Pro-Tyr-His-Thr-His-Pro; the mimic epitope has no homology with the protein sequence of VEGF. A vaccine which can induce a polypeptide epitope aiming at VEGF molecular autoantibody in vivo is constructed on the basis of the mimic epitope. The invention provides a strategy for developing and designing the tumor therapeutic vaccine, which is targeted at the VEGF. The VEGF is one of molecules which has the strongest effect of promoting vascular growth, and is an ideal target for resisting angiogenesis and treating tumors. Therefore, the vaccine replaces or replenishes monoclonal antibody passive immunotherapy with an active immunity mode, so as to lay foundations for overcoming the defects of monoclonal antibody therapy.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Use of antagonist anti-cd40 antibodies for treatment of chronic lymphocytic leukemia
InactiveCN1901937APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAntigen Binding Fragment
Methods of therapy for treating a subject for multiple myeloma are provided. The methods comprise administering a therapeutically effective amount of an antagonist anti-CD40 antibody or antigen-binding fragment thereof to a patient in need thereof. The antagonist anti-CD40 antibody or antigen-binding fragment thereof is free of significant agonist activity, but exhibits antagonist activity when the antibody binds a CD40 antigen on a human CD40-expressing cell. Antagonist activity of the anti- antibody or antigen-binding fragment thereof beneficially inhibits proliferation and / or differentiation of human CD40 expressing multiple myeloma cells.
Owner:CHIRON CORP +1
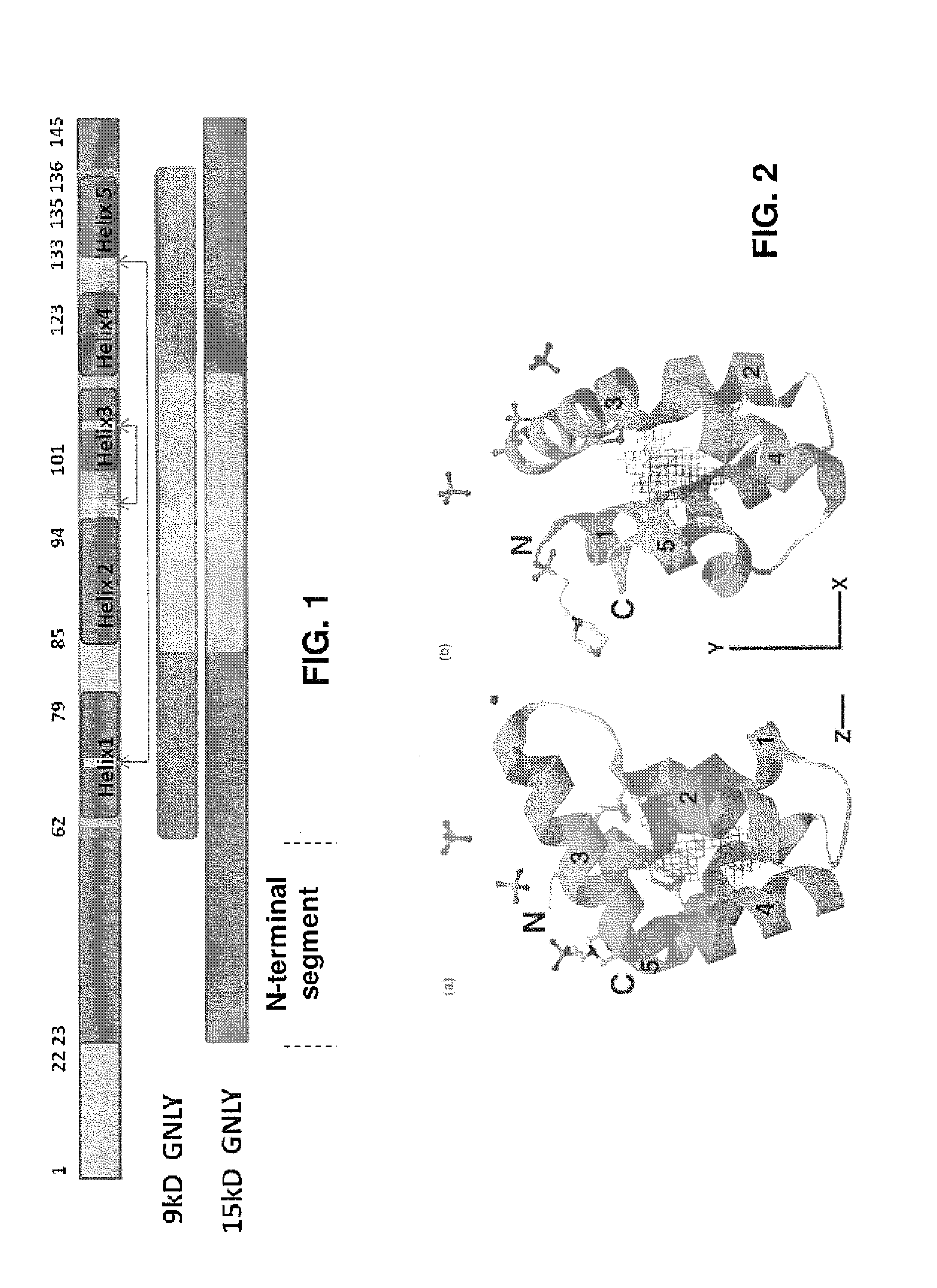
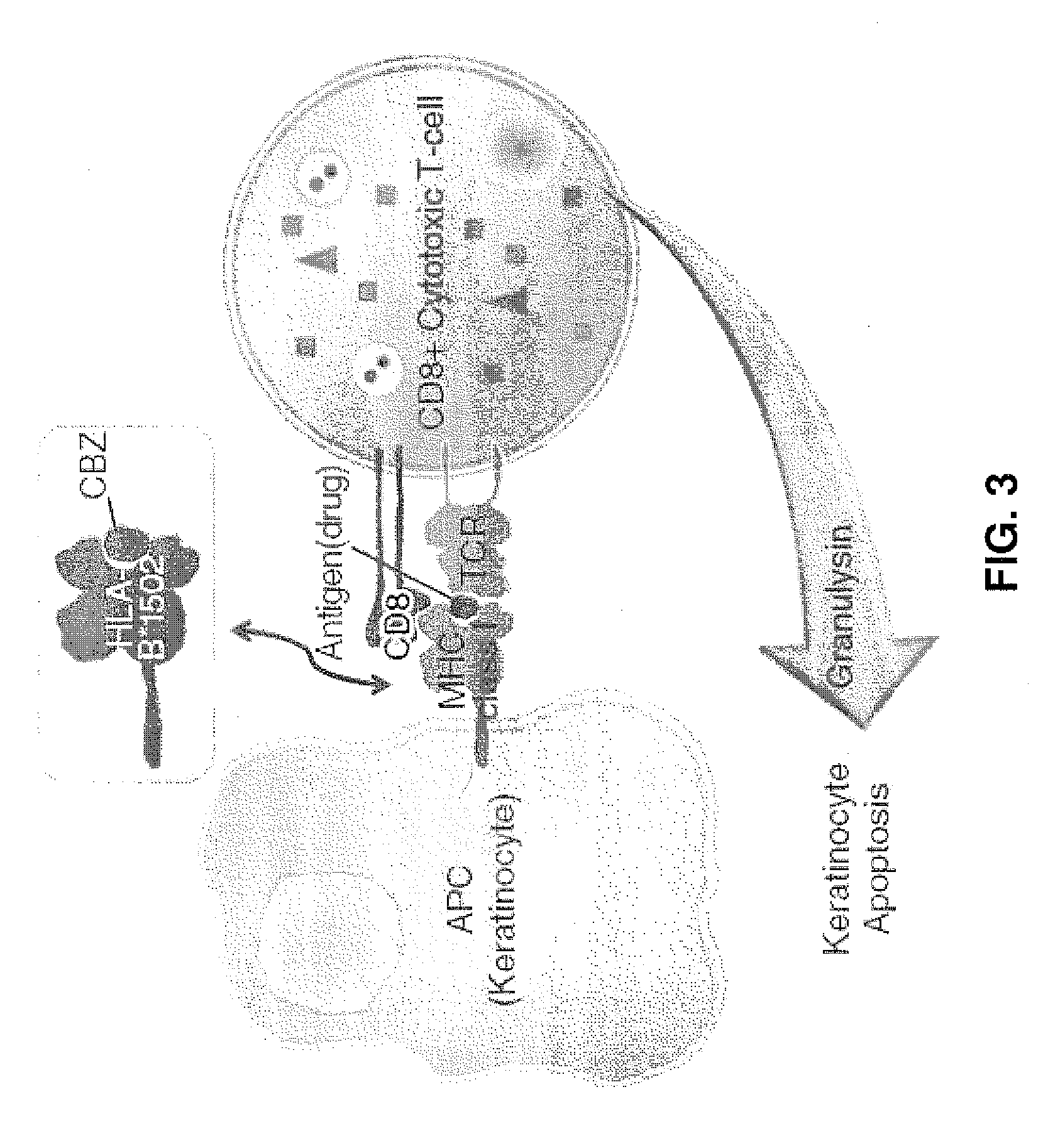
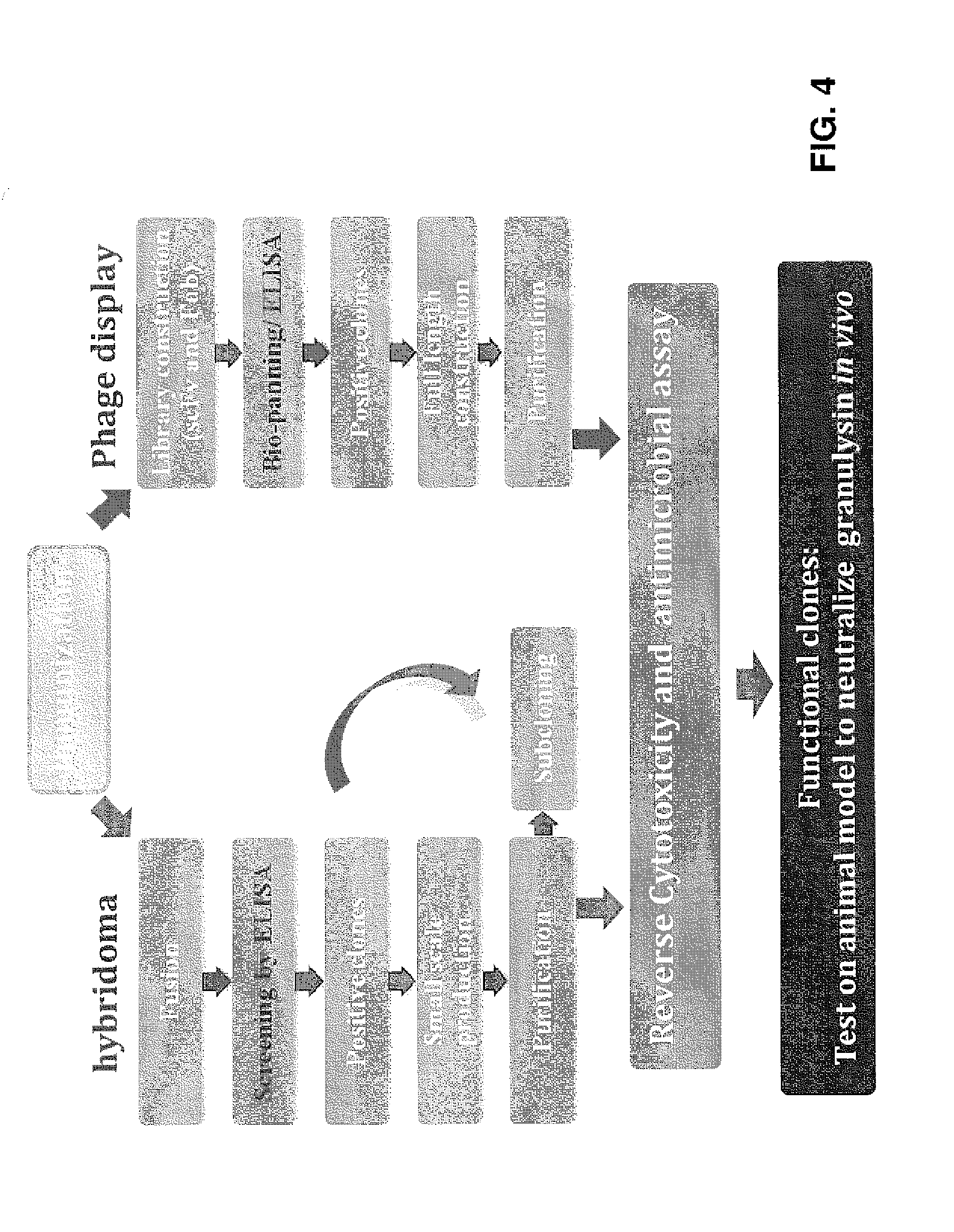
Anti-granulysin antibodies and methods of use thereof
ActiveUS20140186352A1Avoid cytotoxicityImmunoglobulins against animals/humansImmunological disordersEpitopeGranulysin
An anti-granulysin antibody, or an scFv or Fab fragment thereof, capable of binding to an epitope region from R64 to R113 of granulysin and capable of neutralizing an activity of granulysin. The antibody may contain a sequence selected from the sequences of SEQ ID NO:82 to SEQ ID NO:195, or the antibody may contain a sequence selected from the sequences of SEQ ID NO:39 to SEQ ID NO:76. The antibody may be a monoclonal antibody. A method for treating or preventing an unwanted immune response disorder includes administering to a subject in need thereof an effective amount of an anti-granulysin antibody capable of neutralizing the activity of granulysin. The unwanted immune response disorder may be SJS, TEN, or GVHD.
Owner:ACAD SINIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com