Method for preparing prostate cancer antigen immunochromatography test paper based on hydrophobin high-efficiency fixed low-concentration antibody
An immunochromatographic test strip and hydrophobin technology, which is applied in the field of clinical medical detection, can solve the problems of reduced utilization of epitope epitopes, reduced ability to capture antigens, and unsatisfactory detection effects, and can improve the ability to capture antigens and reduce antibodies. The effect of using the content and reducing the cost of testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] Preparation of quantum dot immunoprobe: dissolve water-soluble quantum dots, labeled antibody for prostate cancer, EDC (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride) in boric acid buffer solution, suspended at room temperature, activated the carboxyl group of the quantum dots, centrifuged, added the same amount of antibody to repeat the above process, and finally the quantum dots coupled with the antibody were blocked overnight with BSA.
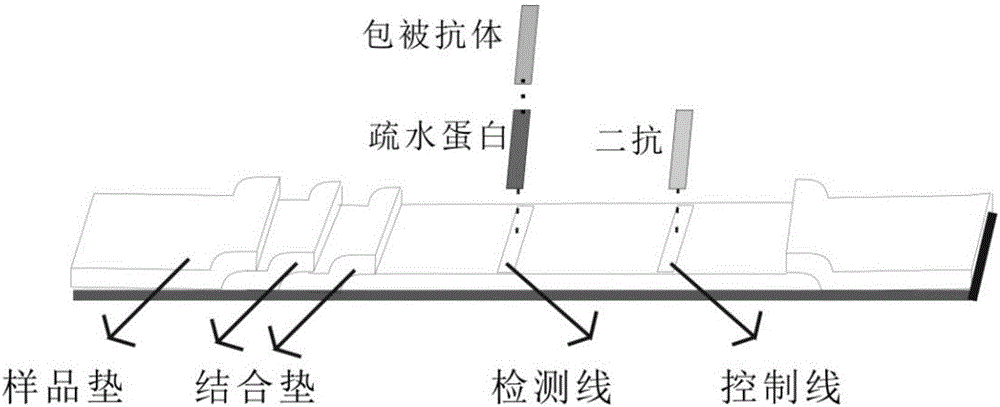
[0033] The assembly of the novel test strips with the help of hydrophobin-immobilized antibodies: common test strips are composed of sample pads, binding pads, nitrocellulose membranes, and water-absorbing pads. The test strips of the present invention are closely connected from left to right. It is a sample pad and a binding pad stacked together, in order to fully combine the antigen and the quantum dot-labeled antibody, such as figure 1 As shown, the number of sample pads in this technical invention is two. Different f...
Embodiment example 1
[0046] Preparation method of prostate cancer immunochromatographic test strips based on hydrophobin efficiently immobilizing low-concentration antibodies; 50 μg / ml hydrophobin is sprayed on the detection line position of nitrocellulose membrane, and the mass fraction ratio of quantum dots and EDC consumption is: 1:4000, the mass fraction ratio of quantum dots and antibodies is 1:16, the amount of BSA blocking solution is 1%, and the coating antibody concentration of PSA diluted with 0.01M PBS buffer with pH=7.4 is 0.2mg / ml Spray on the hydrophobin layer, and their overlapping area is used as the detection line area, drop 45 μl of standard antigen with a concentration of 5 ng / ml into the sample pool, do three repetitions, react for 15 minutes, and read the detection with a quantum dot immunofluorescence analyzer The indications of the line and the quality control line are the T value and the C value, and the average value of the T / C intensity of the detection signal obtained is ...
Embodiment example 2
[0048] Preparation method of prostate cancer immunochromatographic test strips based on hydrophobin efficiently immobilizing low-concentration antibodies; 50 μg / ml hydrophobin is sprayed on the detection line position of nitrocellulose membrane, and the mass fraction ratio of quantum dots and EDC consumption is: 1:4000, the mass fraction ratio of quantum dots and antibodies is 1:16, the amount of BSA blocking solution is 1%, and the coating antibody concentration of PSA diluted with 0.01M PBS buffer with pH=8 is 0.2mg / ml Spray on the hydrophobin layer, and their overlapping area is used as the detection line area, drop 45 μl of standard antigen with a concentration of 5 ng / ml into the sample pool, do three repetitions, react for 15 minutes, and read the detection with a quantum dot immunofluorescence analyzer The indications of the line and the control line are the T value and the C value, and the average value of the T / C intensity of the detection signal obtained is 0.165.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com