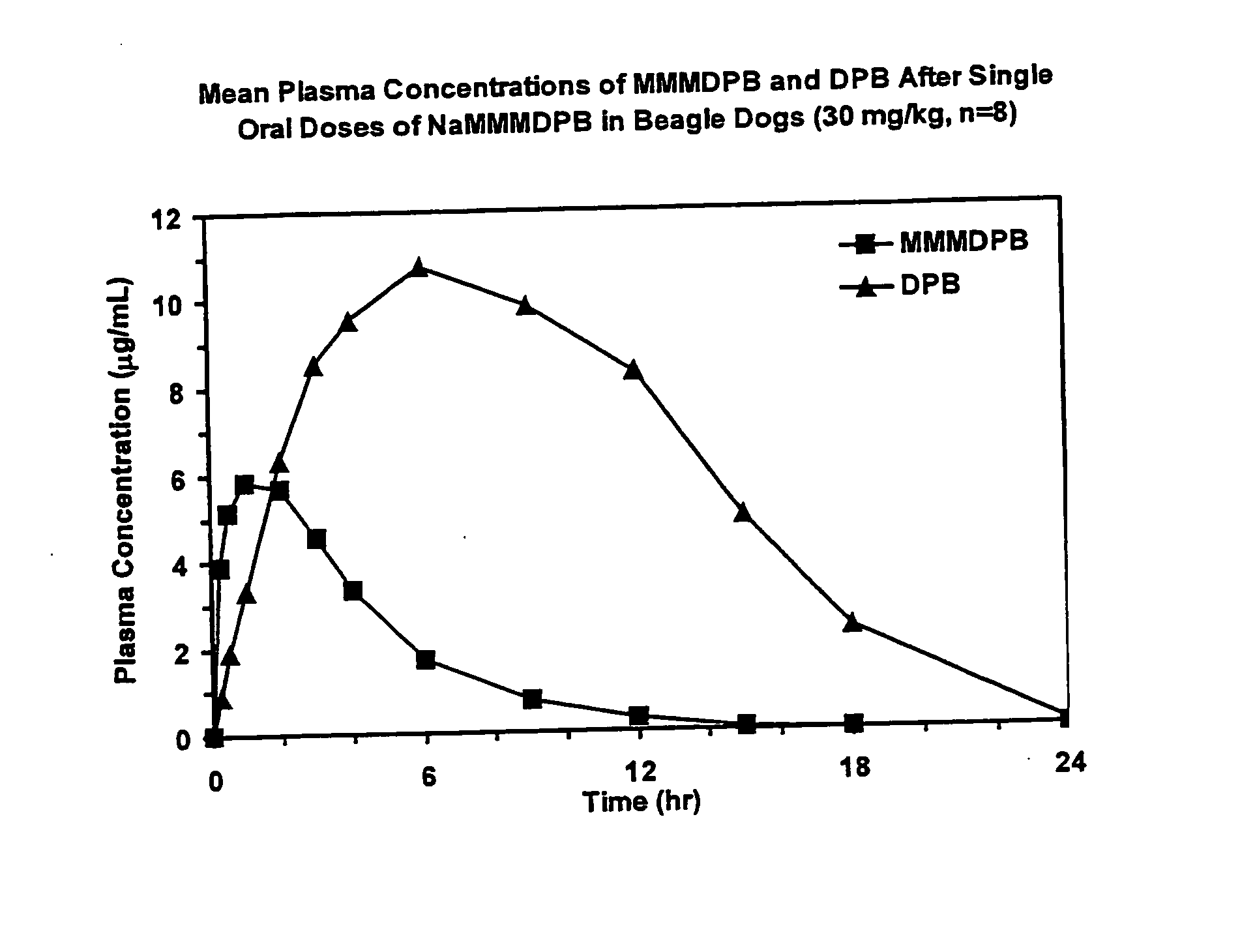
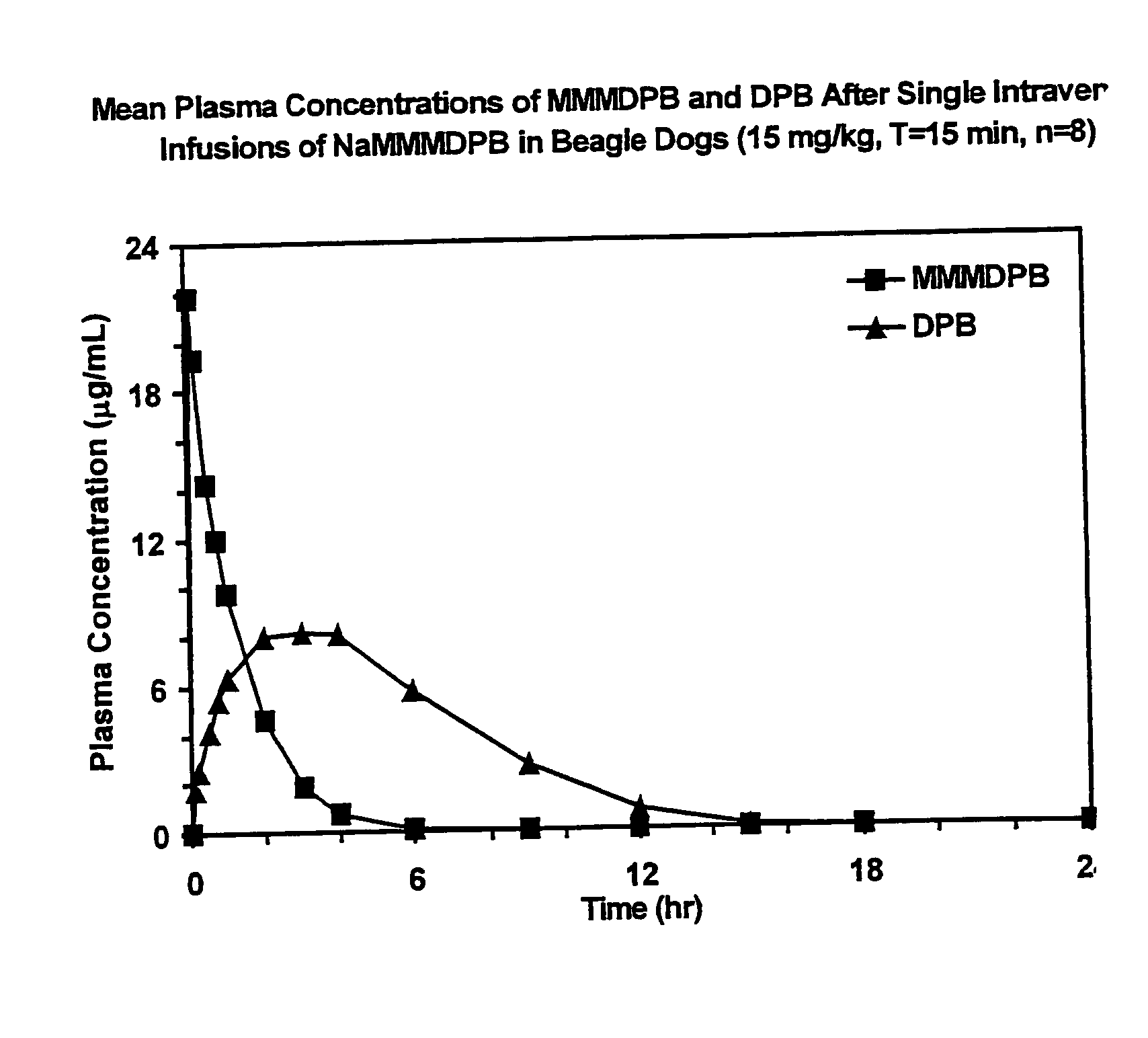
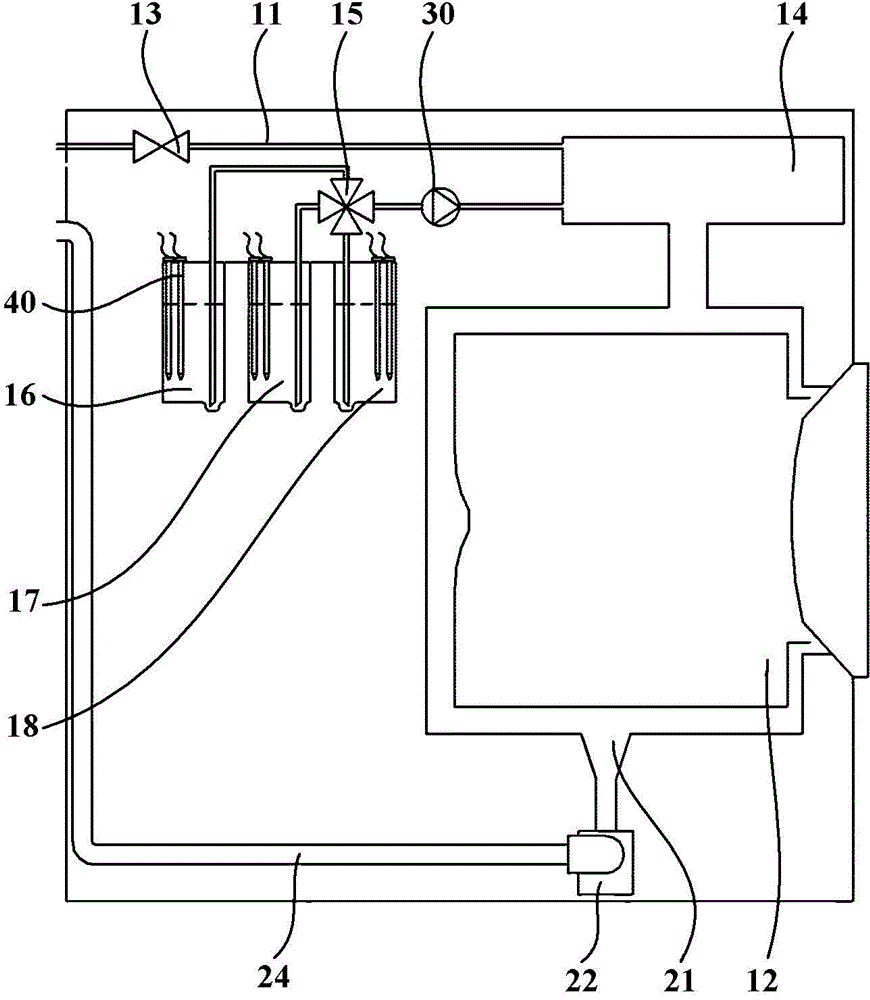
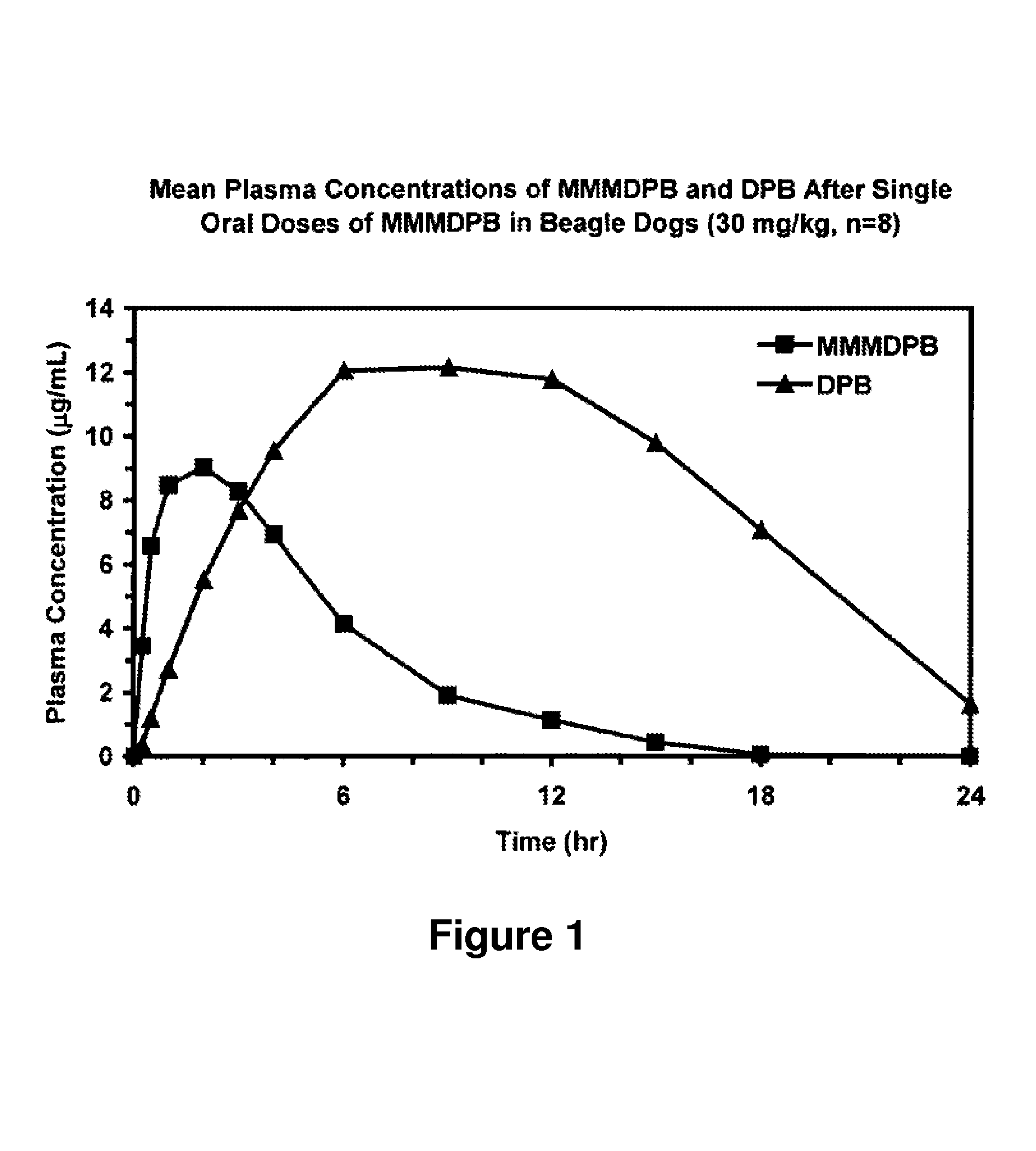
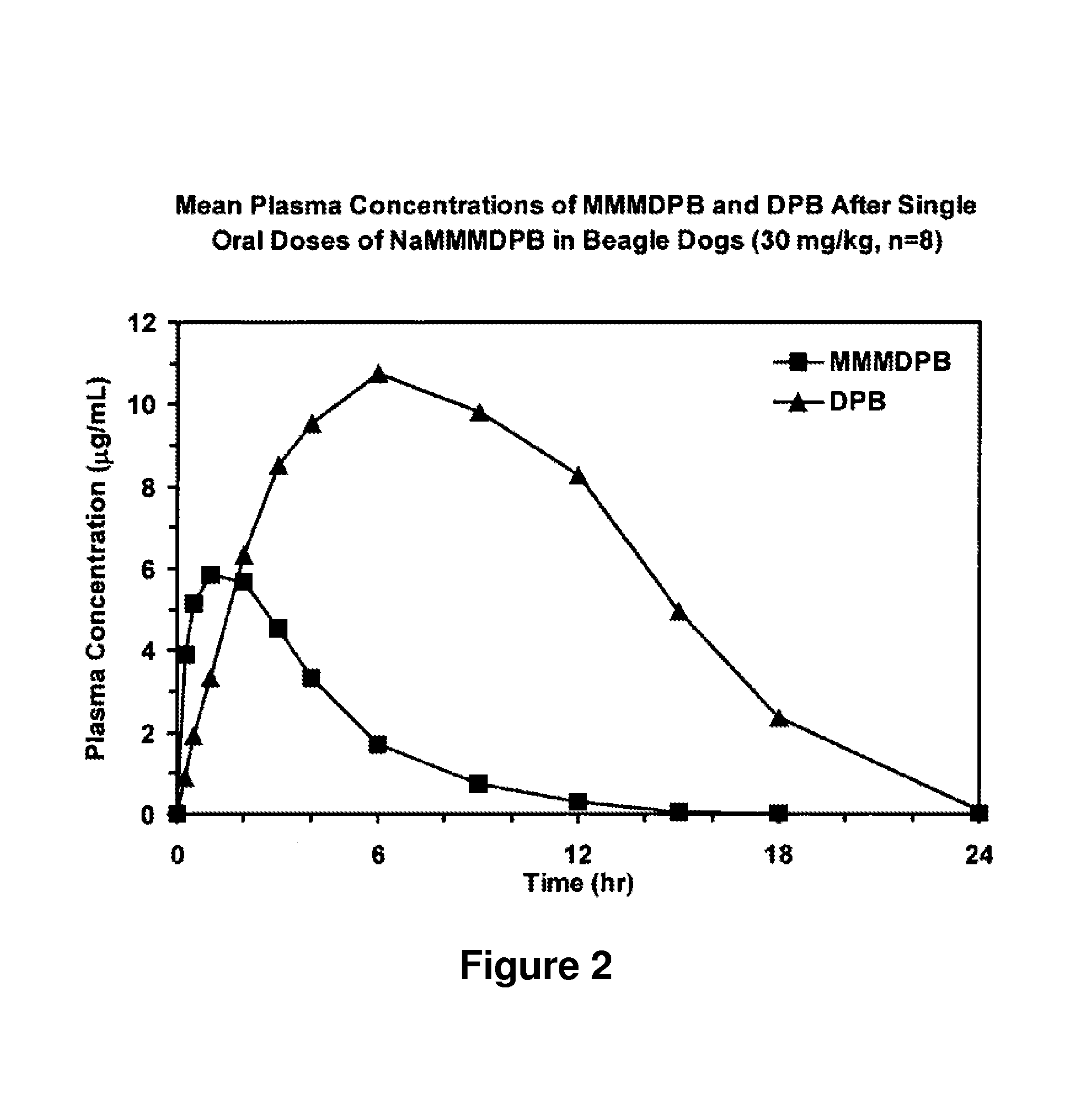
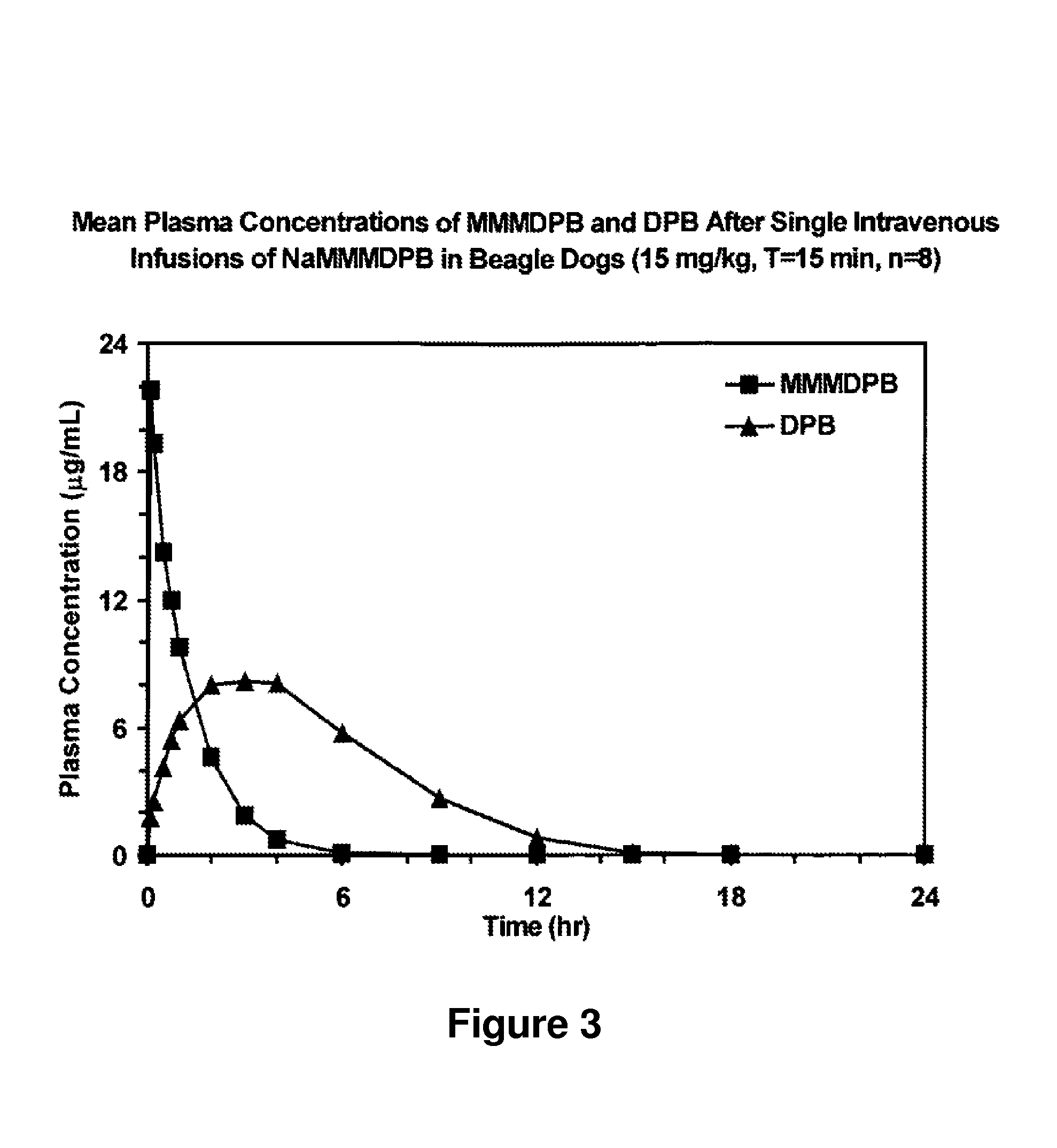
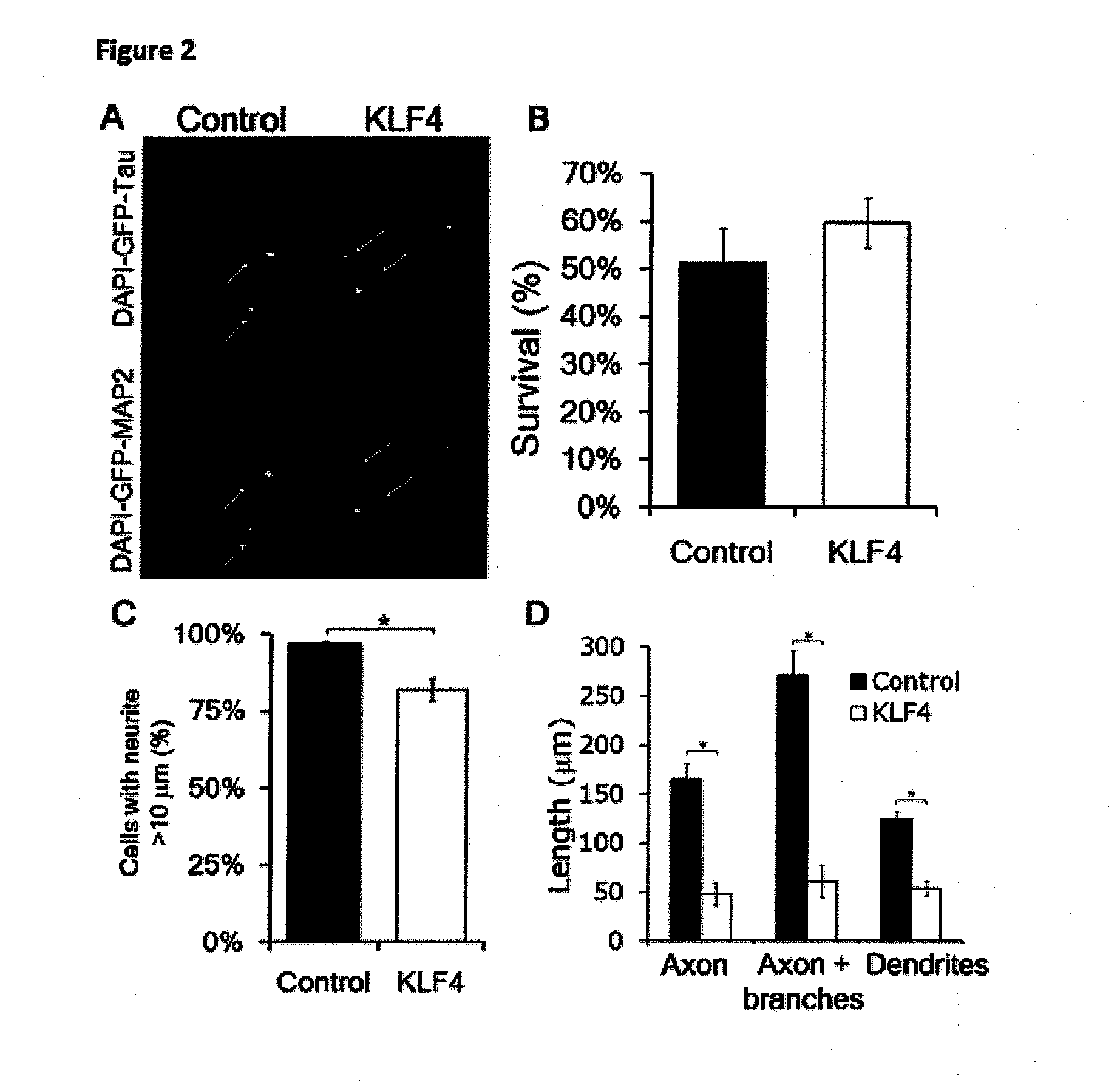
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45results about How to "Adequate dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
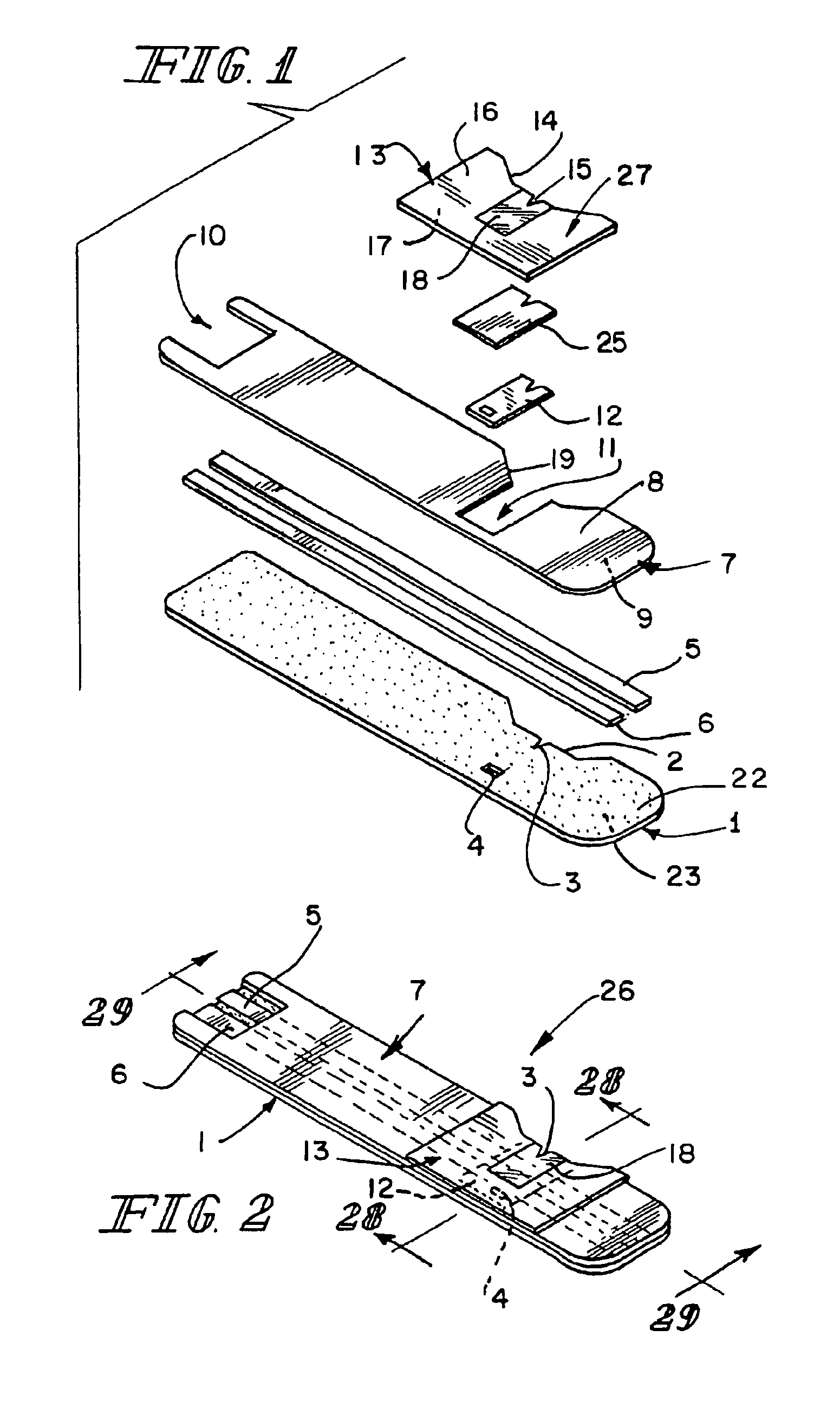
Inhaler
InactiveUS6880555B1Lifting efficiencyGood effectRespiratorsLiquid surface applicatorsInhalationMedicine
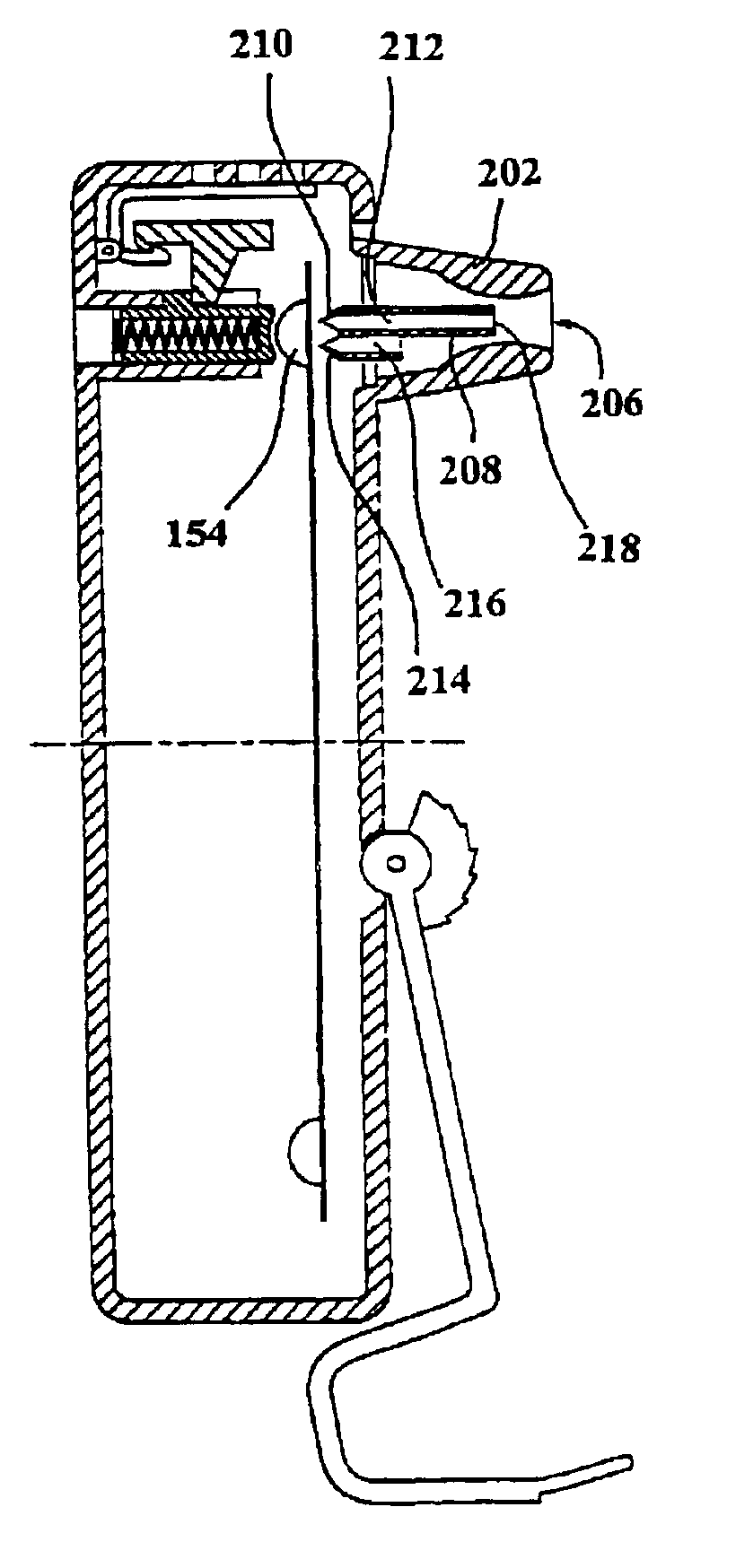
An inhaler for medicament in powder form with an opening intended for inhalation. The powder medicament is arranged in the inhaler in a number of enclosures, each enclosure including a specific dose of medicament. A member is provided for enabling access to the dose of medicament. The member is arranged and designed such that it is able to be inserted inside the enclosure and establish at least one outlet passage, between the interior of the enclosure and the inhalation opening, through which outlet passage the medicament is delivered to the patient upon inhalation.
Owner:SHL MEDICAL AG
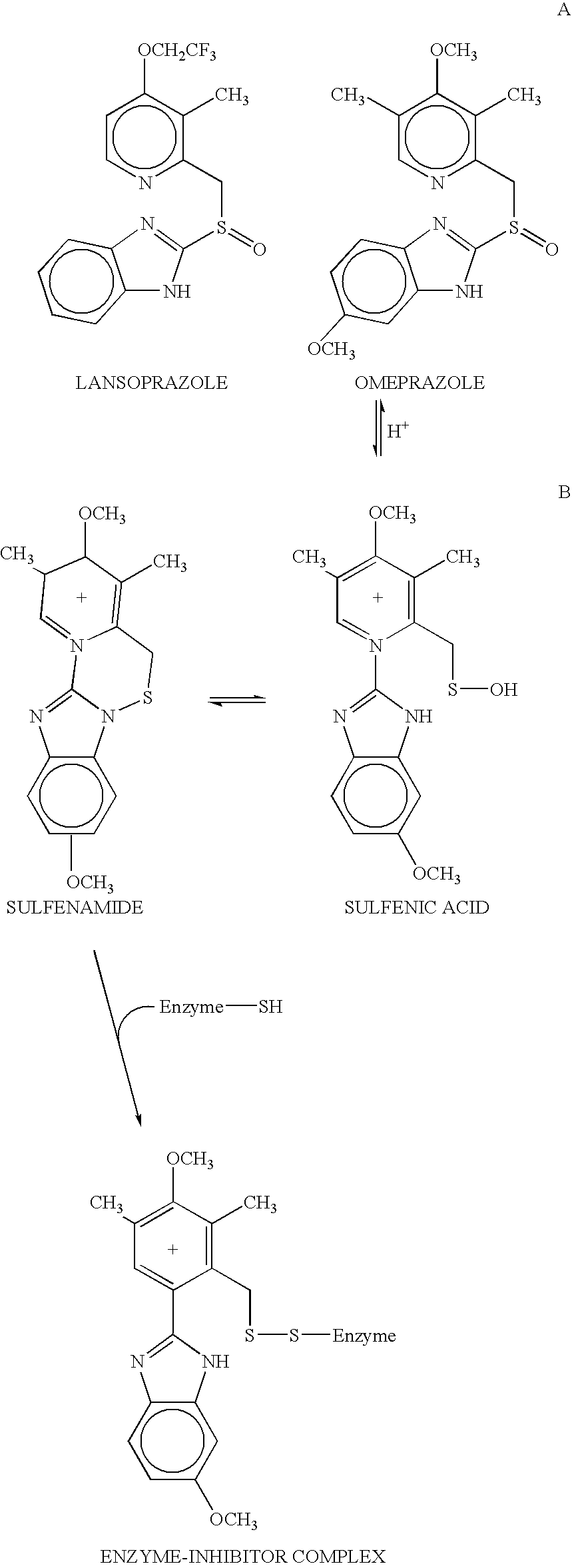
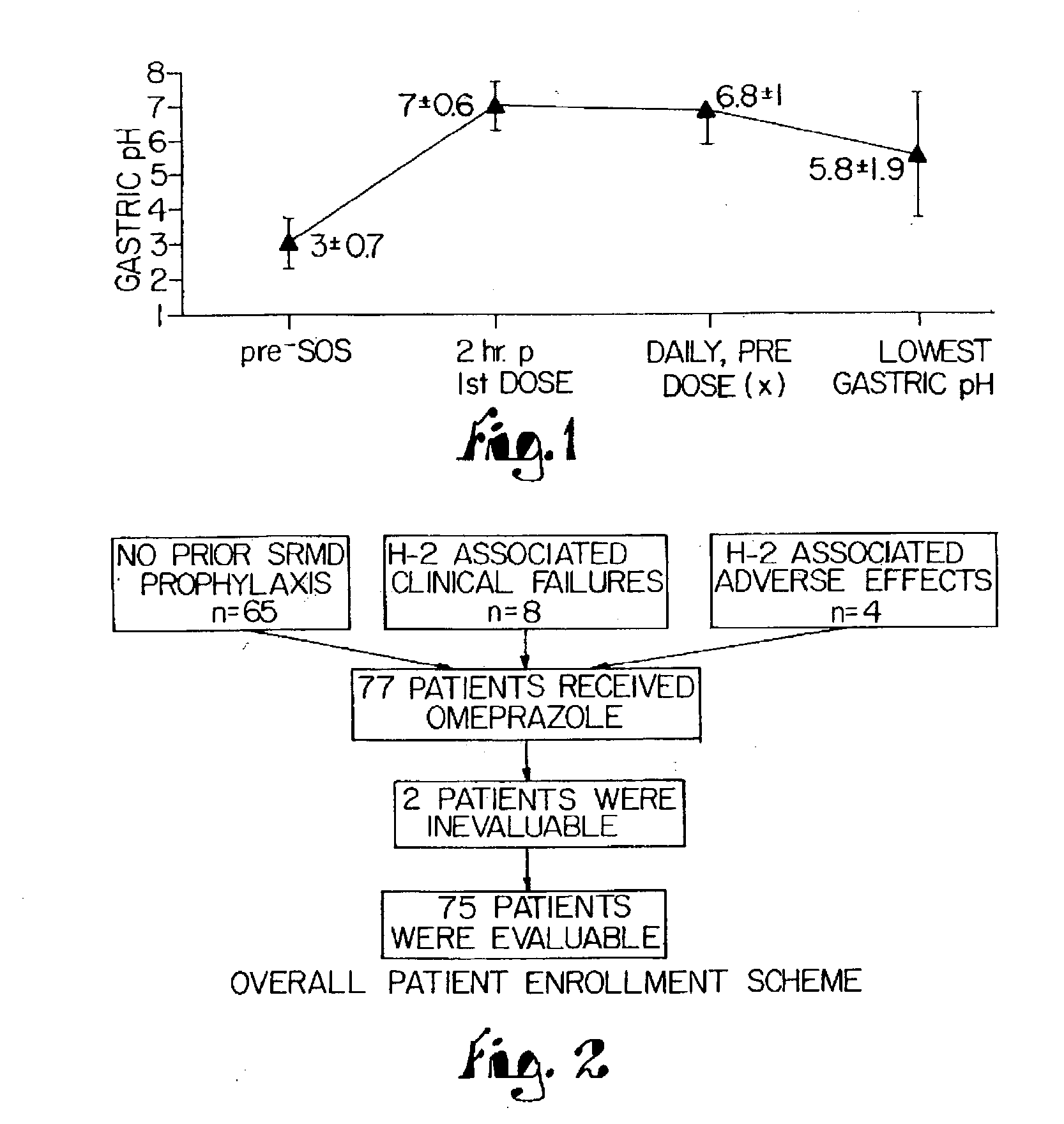
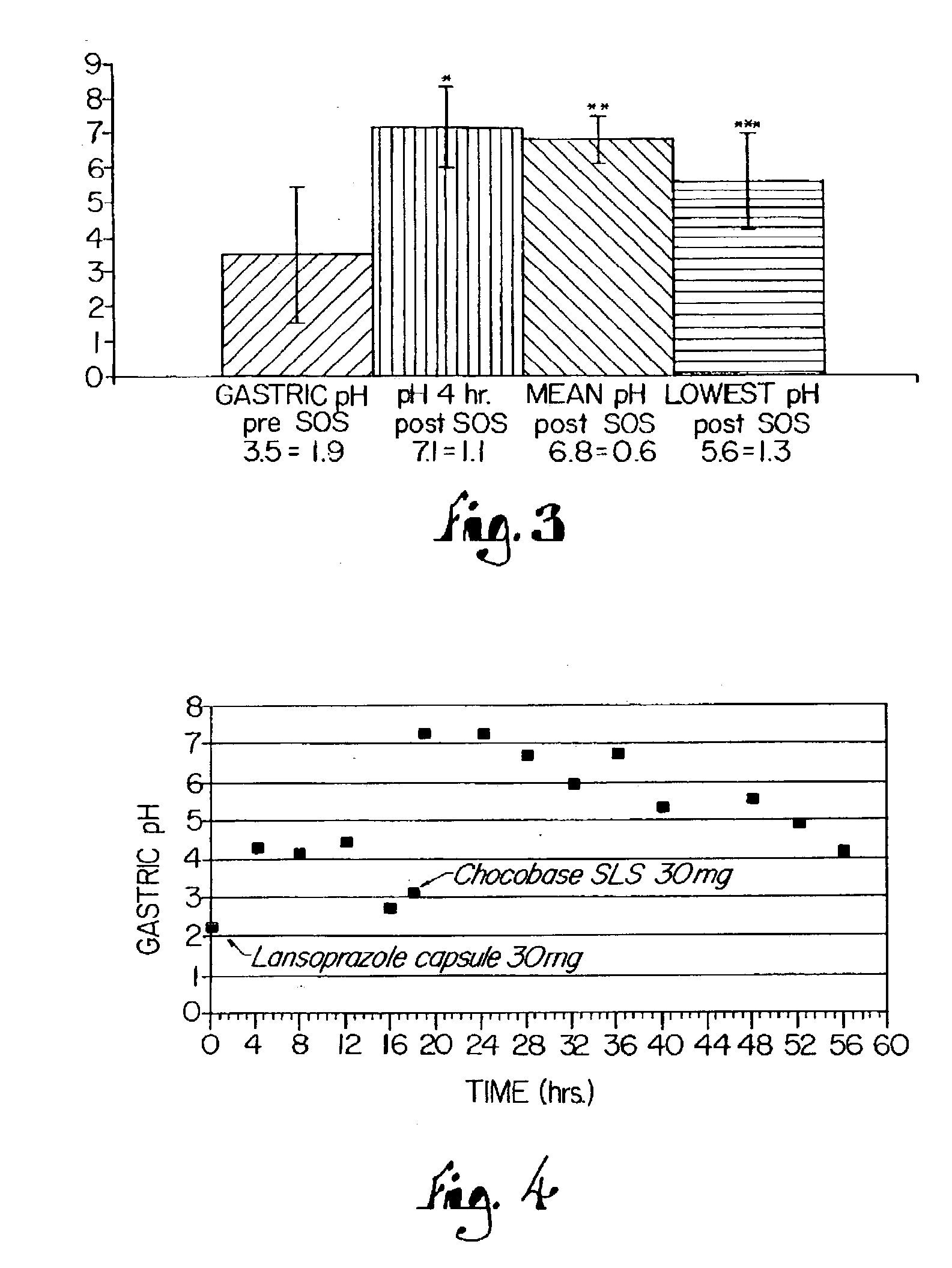
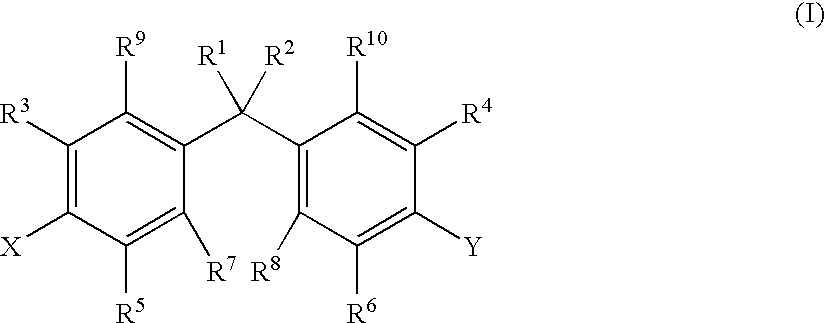
Novel substituted benzimidazole dosage forms and method of using same
InactiveUS20040048896A1Improve pharmacological activityGood effectBiocideOrganic chemistryDosage formBenzimidazole
Disclosed herein are compositions and methods for treating gastric acid disorders employing pharmaceutical compositions comprising a proton pump inhibitor (PPI) in a pharmaceutically acceptable carrier.
Owner:UNIVERSITY OF MISSOURI
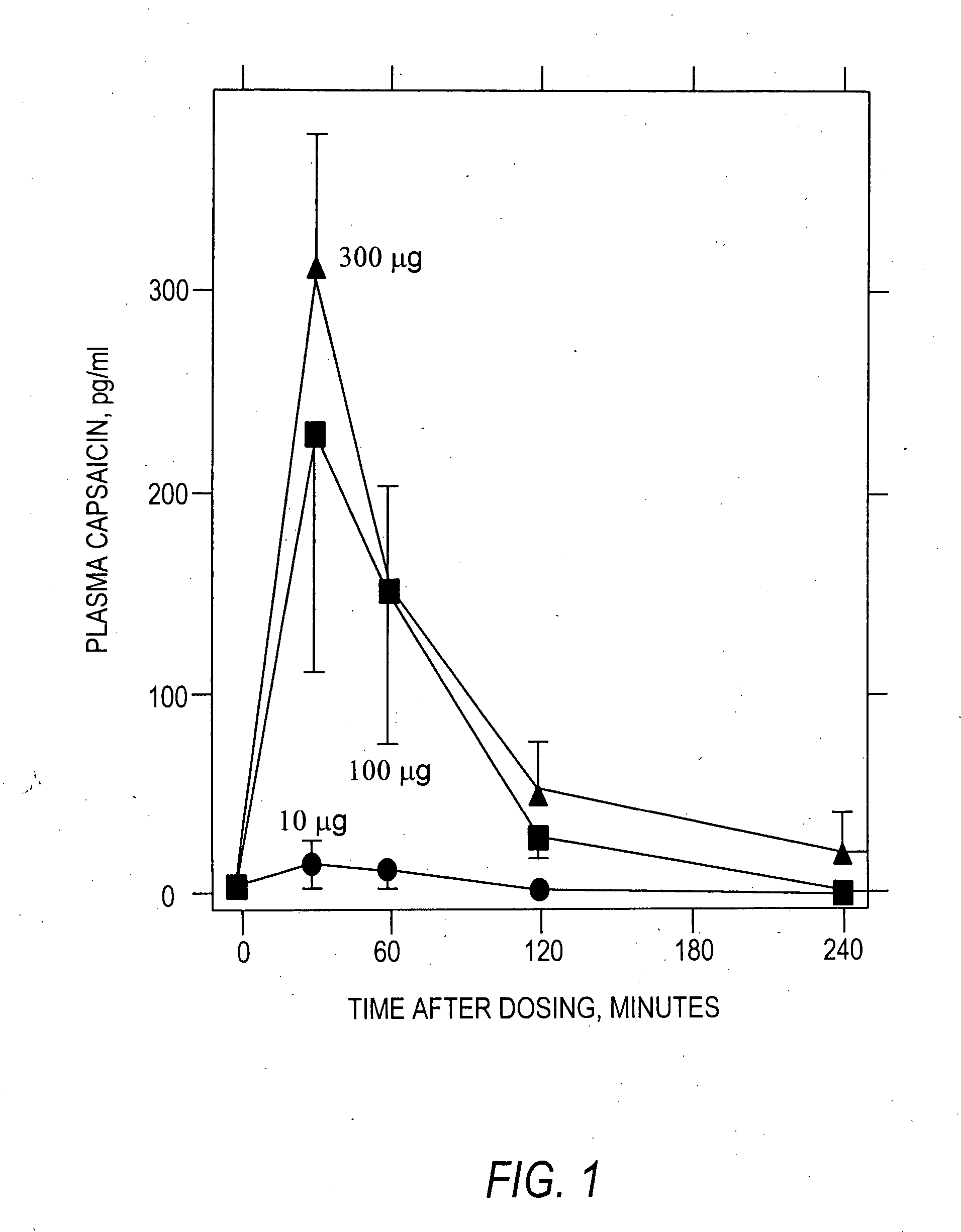
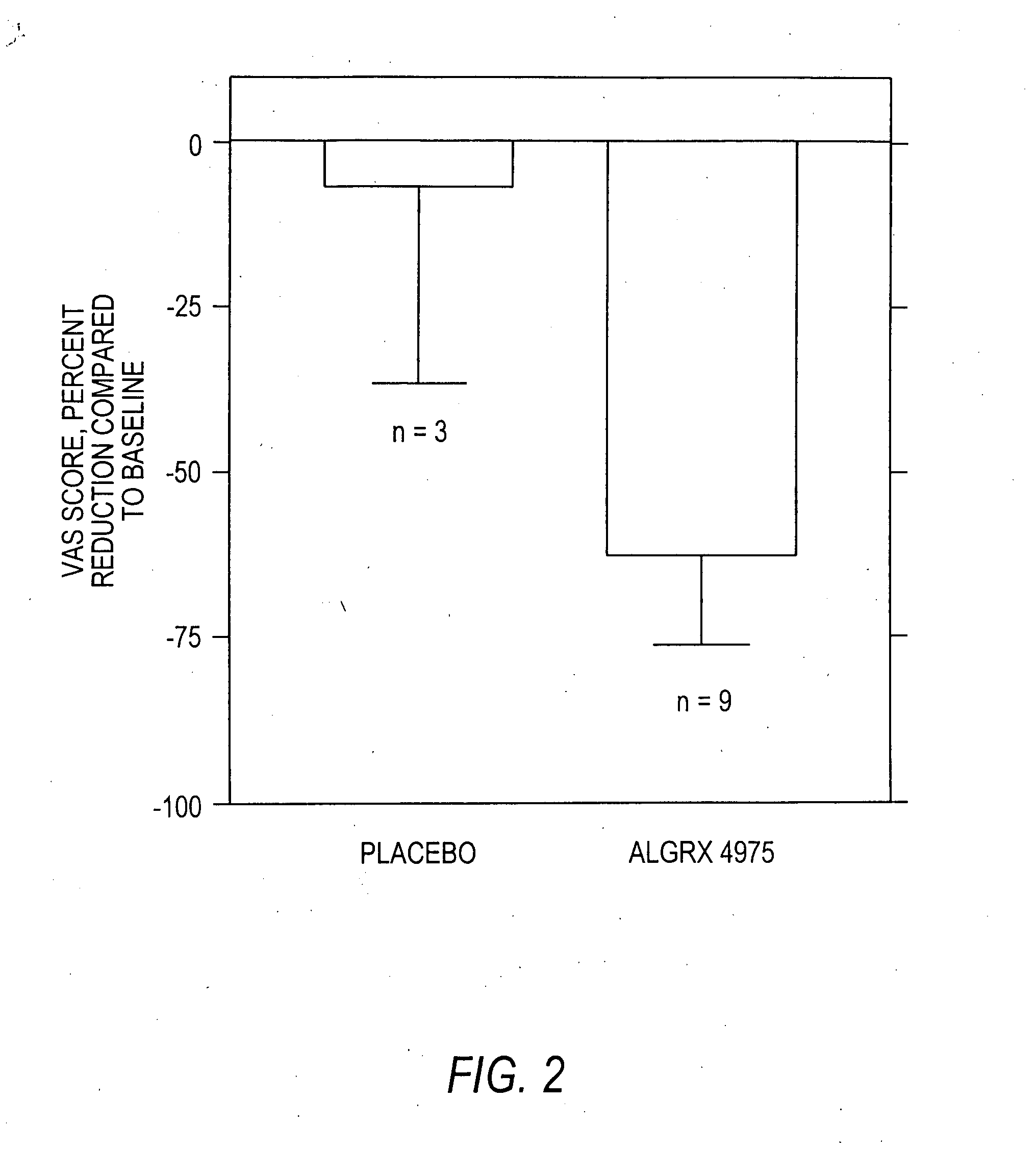
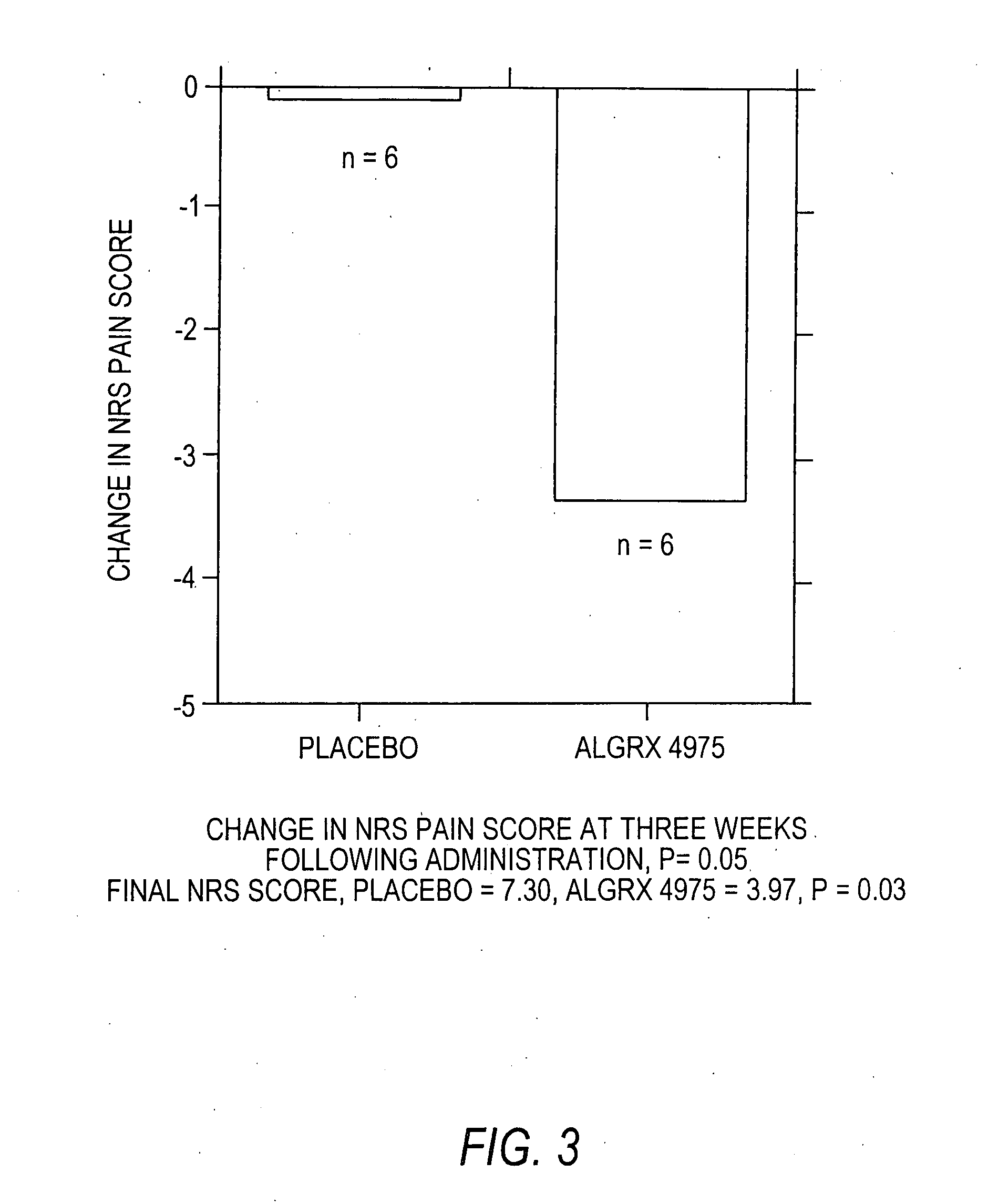
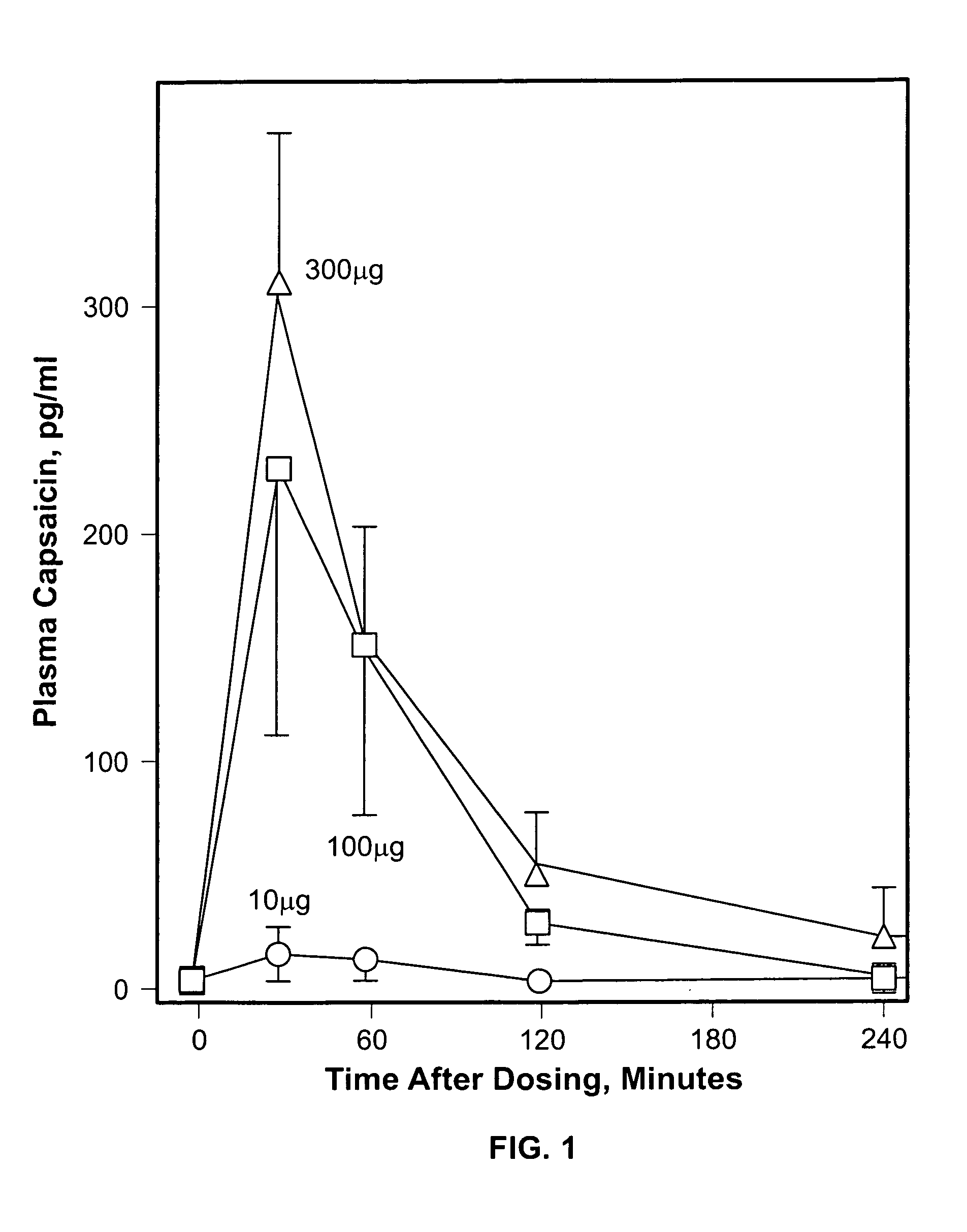
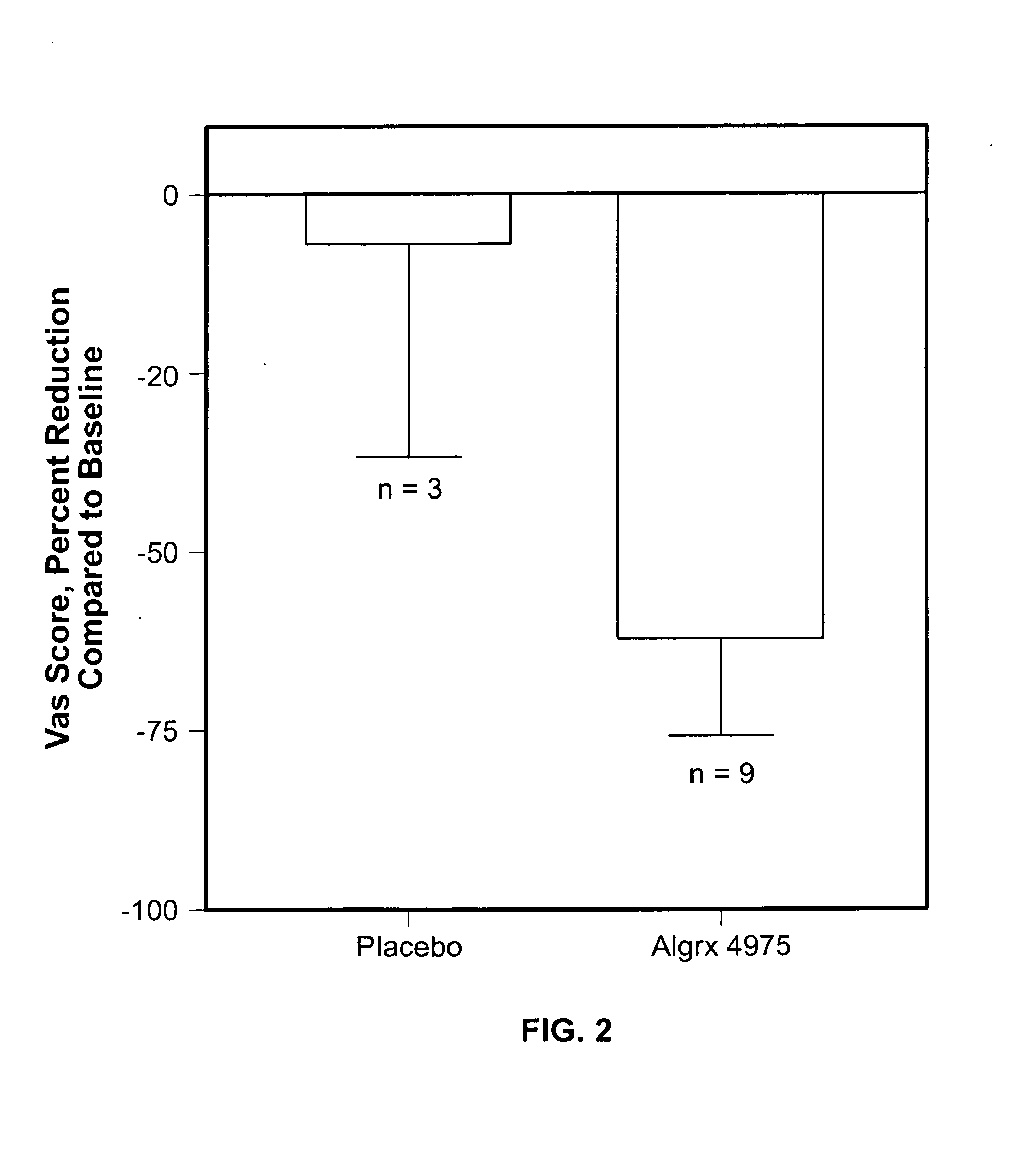
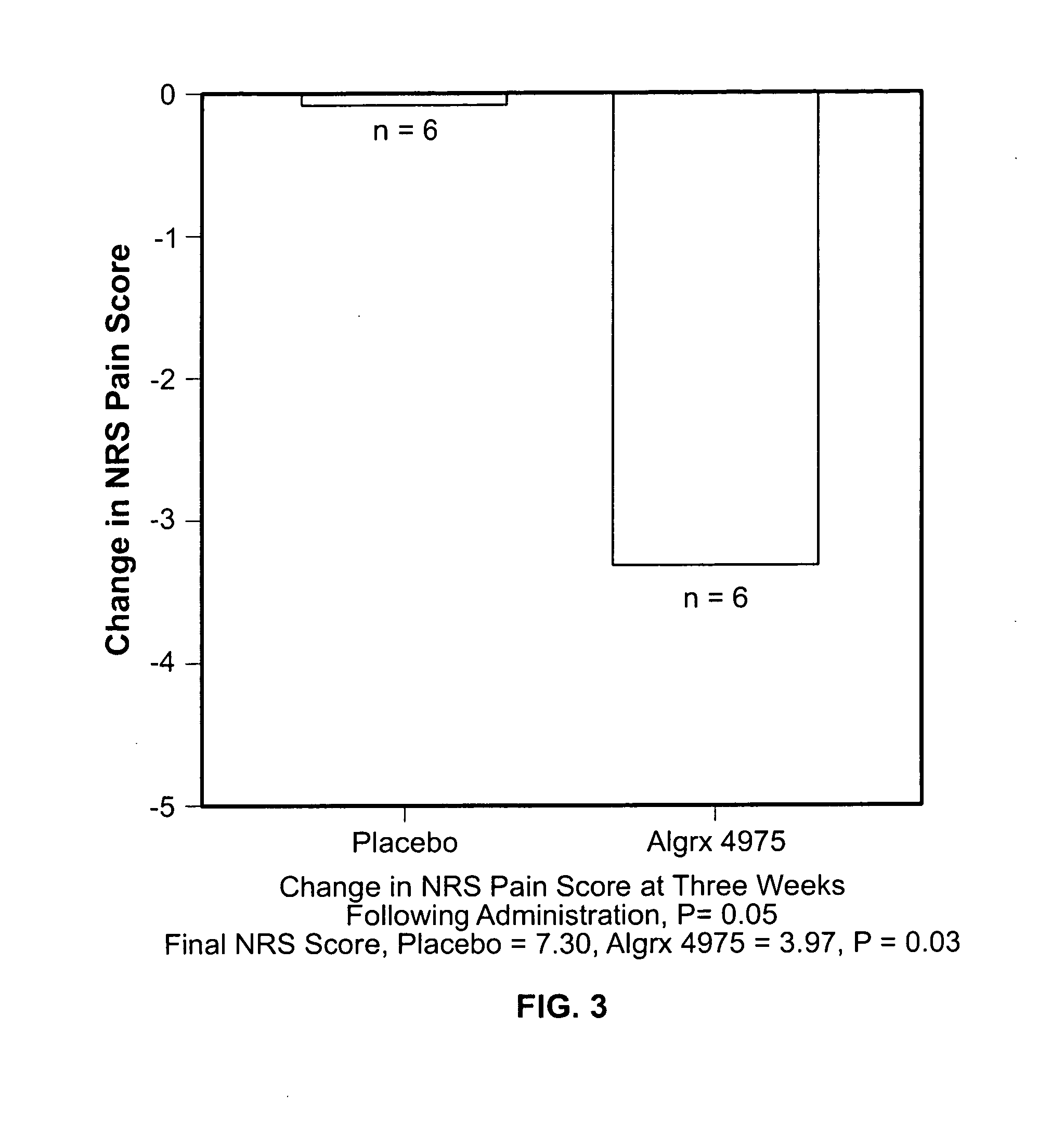
Injectable capsaicin
InactiveUS20050019436A1Reducing and eliminating painMinimizing adverse consequenceBiocideNervous disorderCapsaicinAnesthesia
The present invention provides compositions and methods for relieving pain at a site in a human or animal in need thereof by administering at a discrete site in a human or animal in need thereof a dose of capsaicin in an amount effective to denervate a discrete site without eliciting an effect outside the discrete location, the dose of capsaicin ranging from 1 μg to 3000 μg.
Owner:ALGORX PHARMA INC
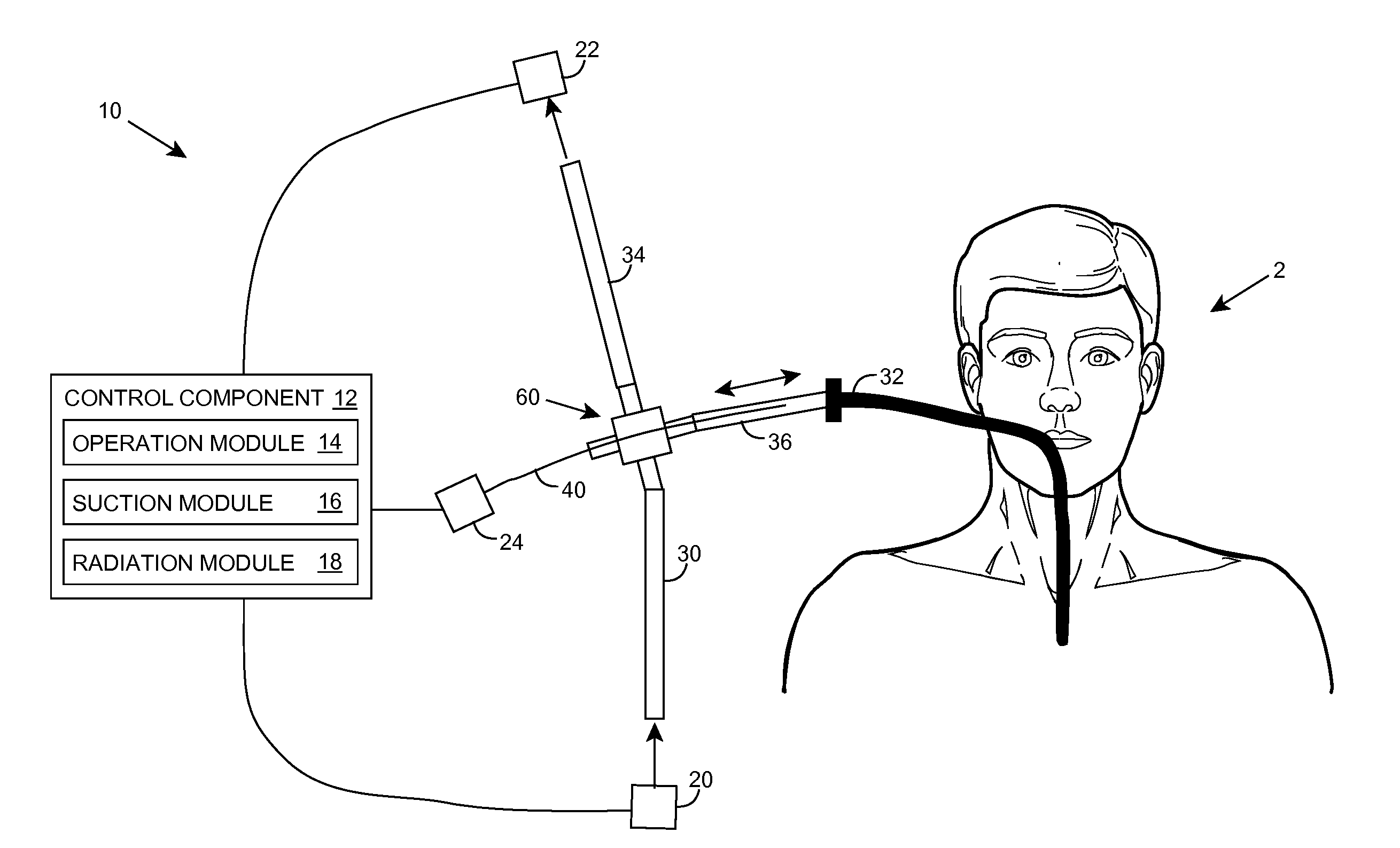
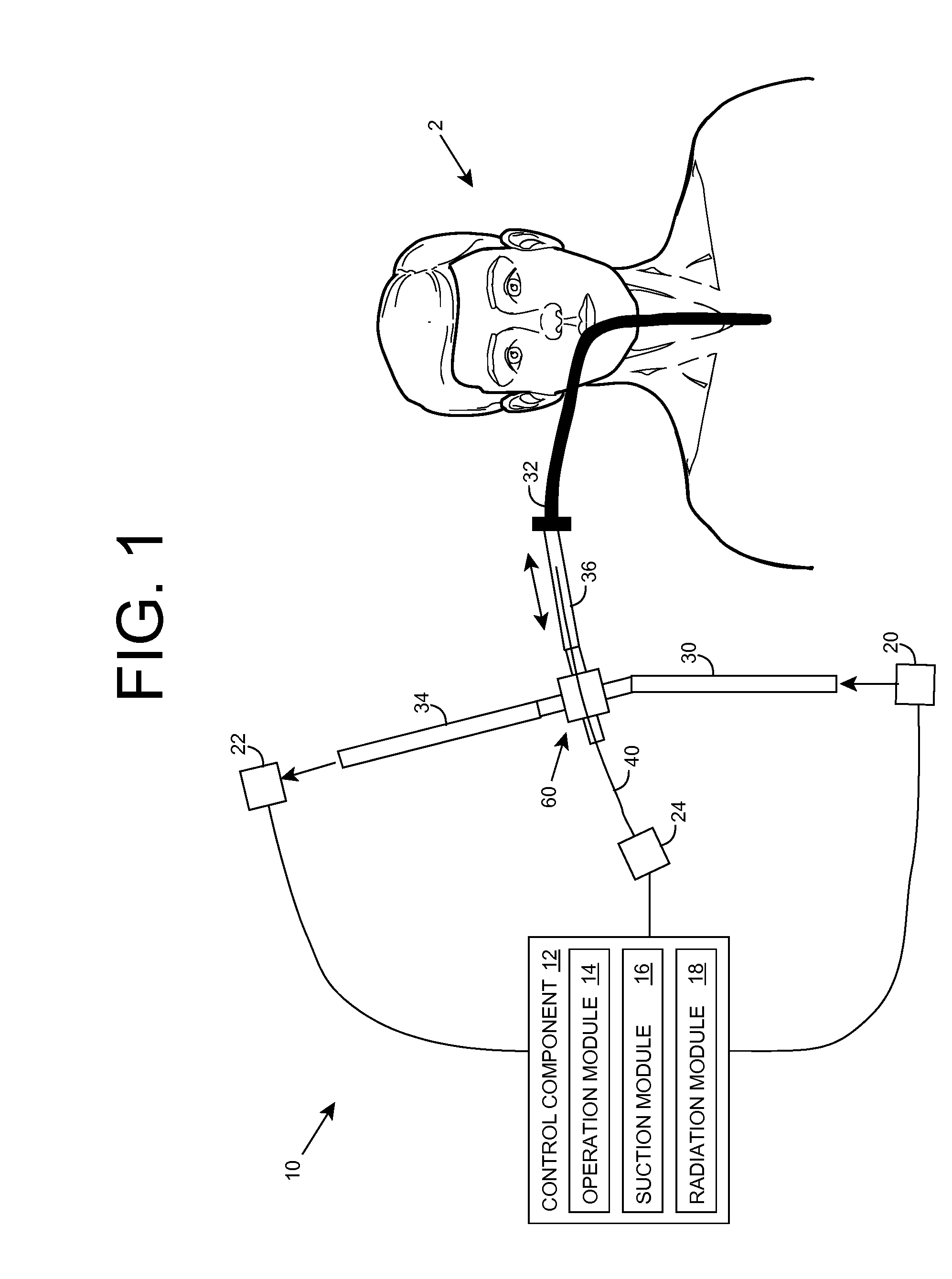
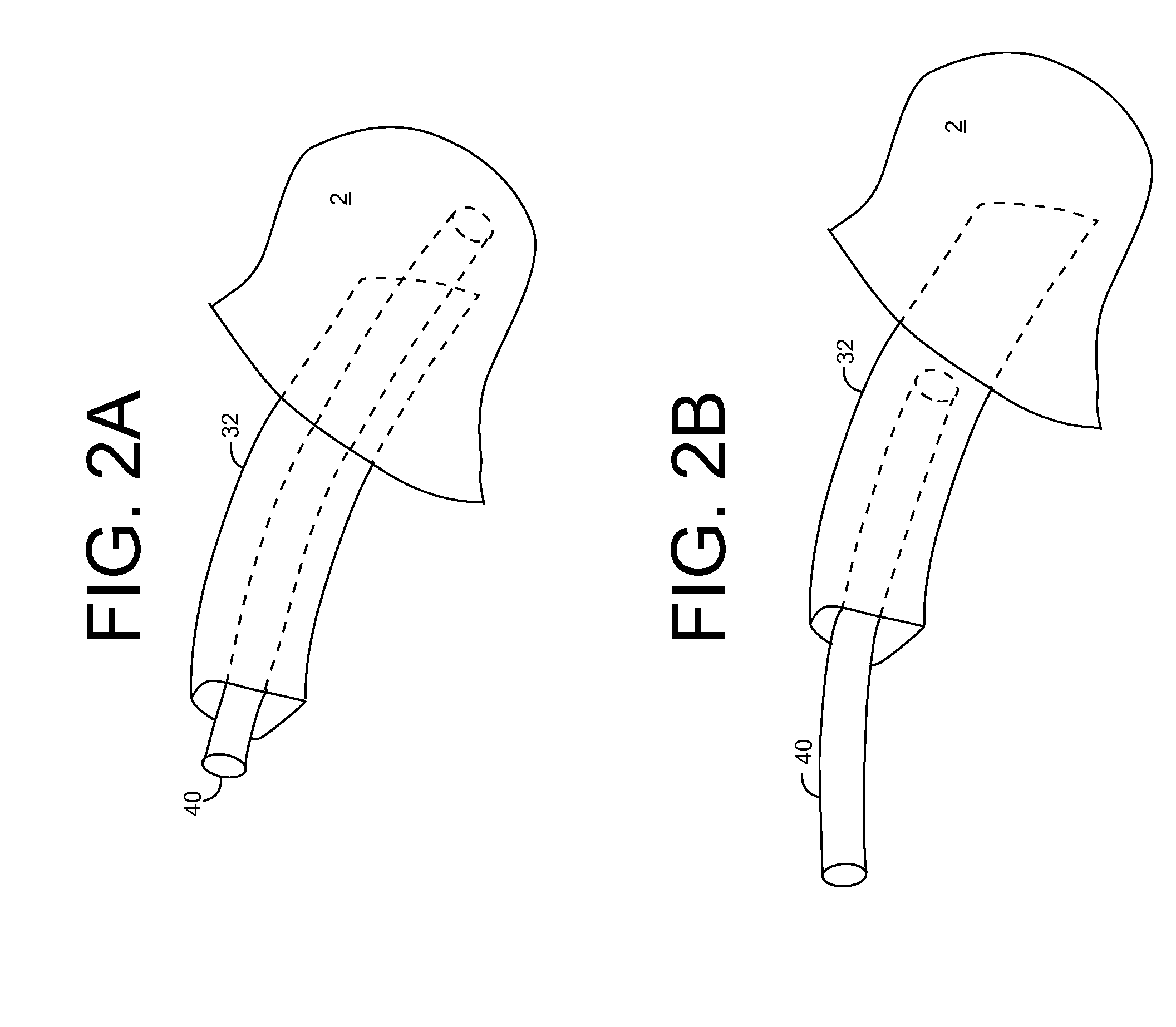
Ultraviolet radiation sterilization
ActiveUS20070187626A1Adequate doseEasy to cleanTracheal tubesRadiation pyrometryUltravioletUltraviolet radiation
A solution for sterilizing one or more hollow components of a device, such as a medical device, is provided. Ultraviolet radiation having one or more predominant wavelength(s) and a sufficient dose is generated and directed to an interior side of the hollow component(s). The predominant wavelength(s) is / are selected to harm one or more target organisms that may be present on the interior side. The ultraviolet radiation can be delivered by a structure that is periodically inserted and retracted into the hollow component. The structure can be configured to provide additional cleaning capability, such as suction, for removing matter that may be present in the hollow component.
Owner:SENSOR ELECTRONICS TECH
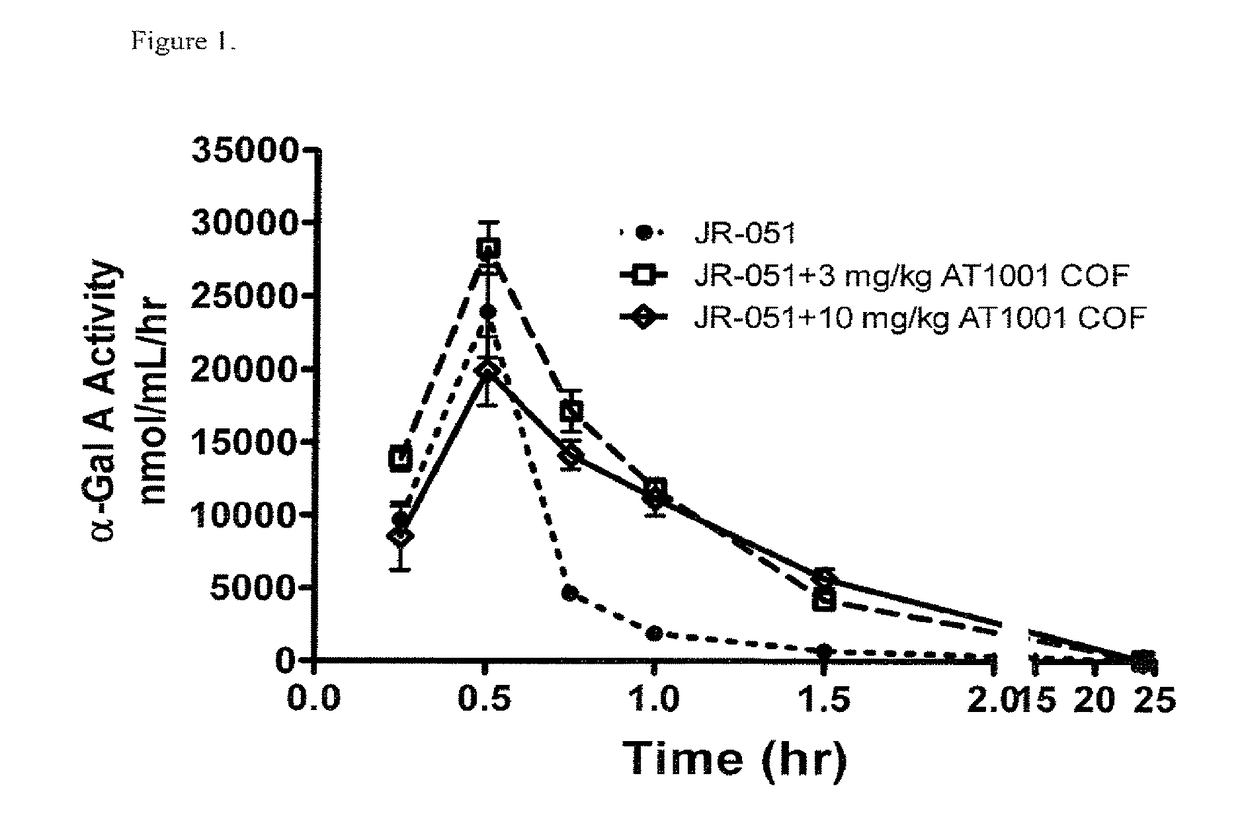
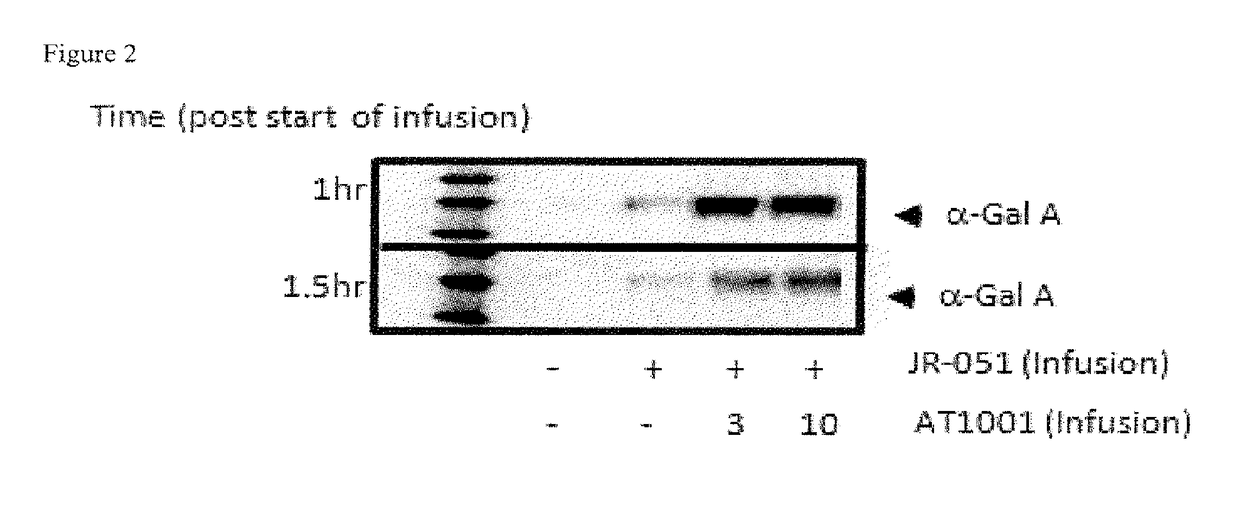
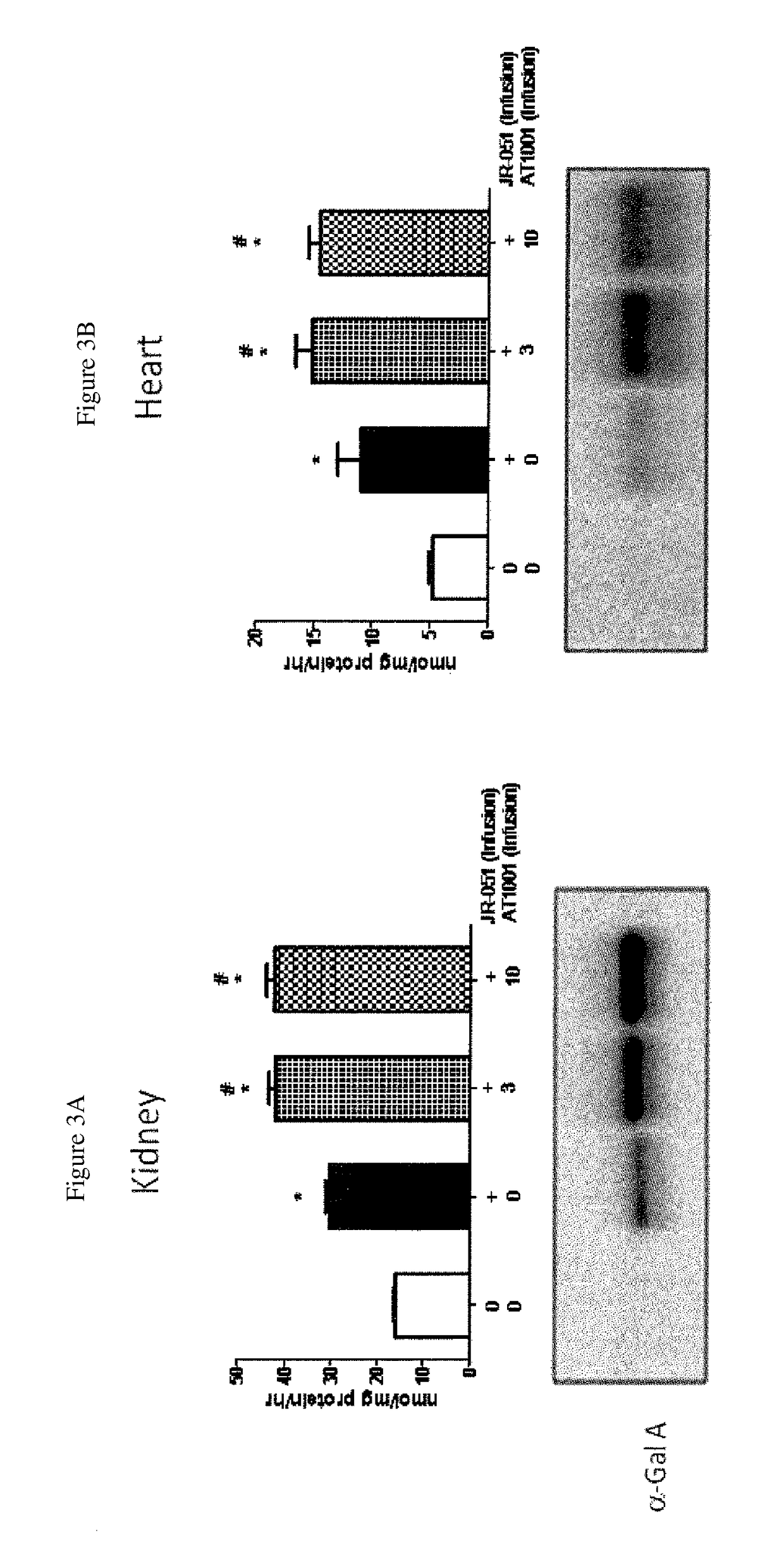
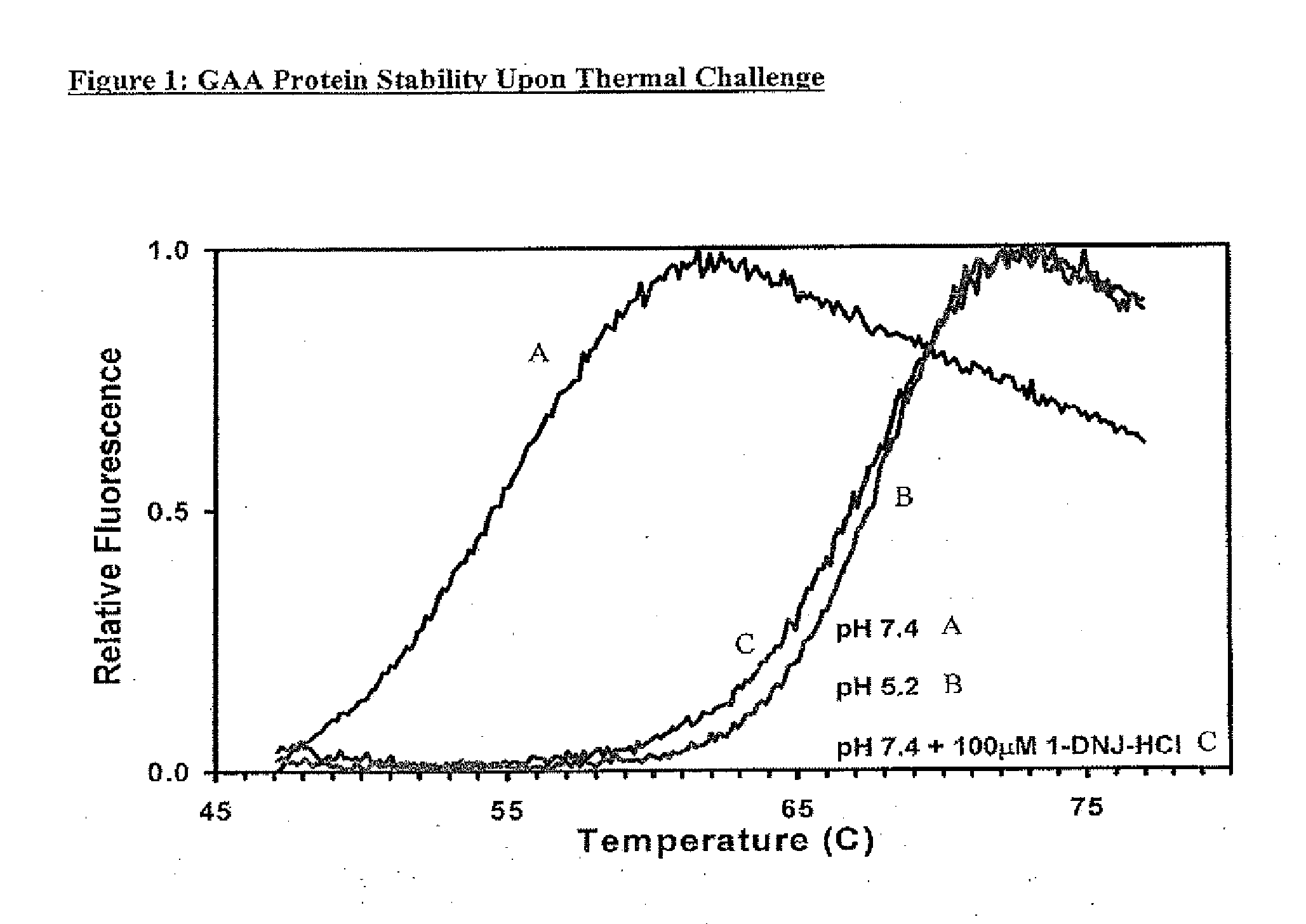
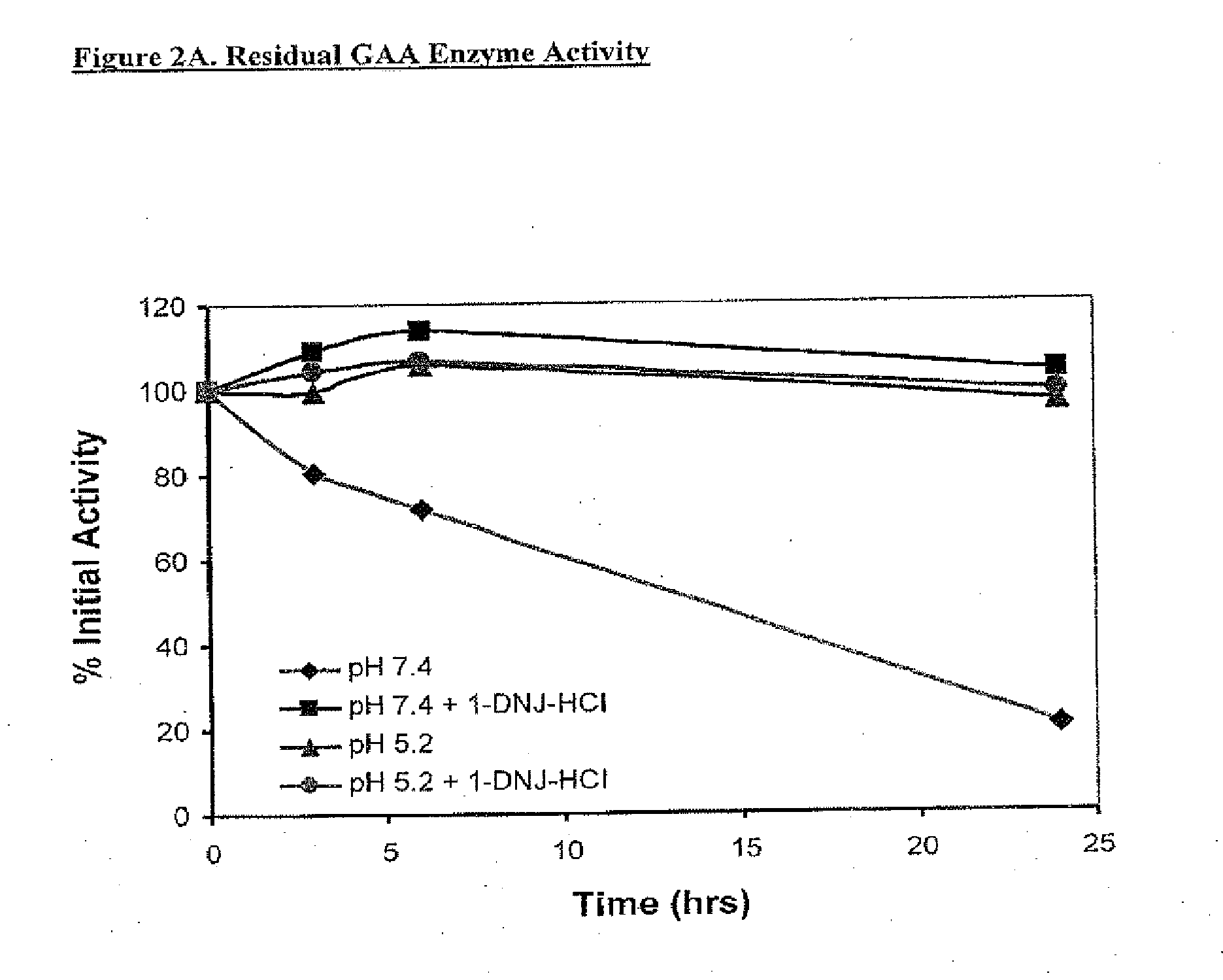
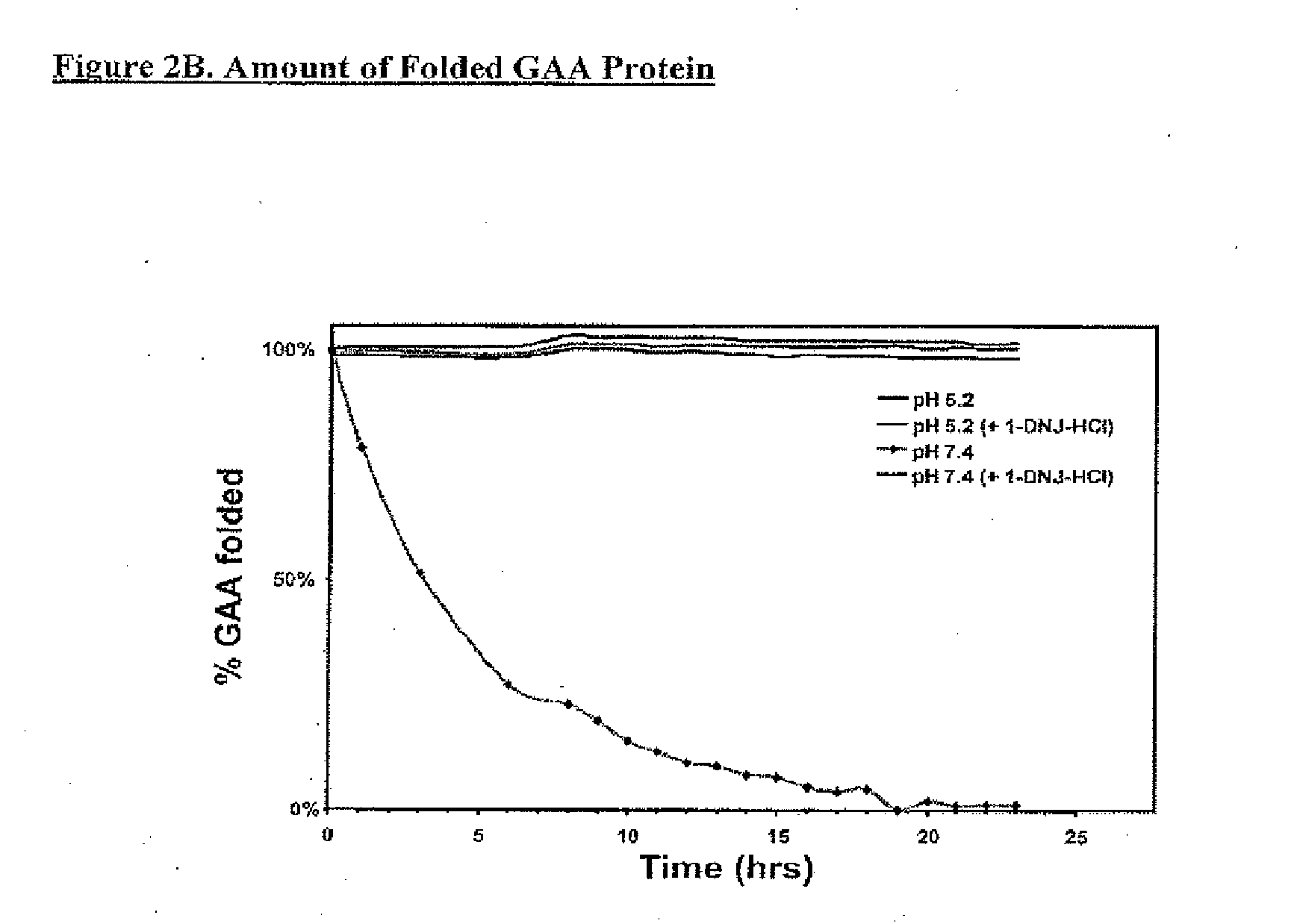
Alpha-galactosidase A and 1-deoxygalactonojirimycin co-formulation for the treatment of fabry disease
ActiveUS10155027B2Reduce deliveryAdequate dosePeptide/protein ingredientsPharmaceutical delivery mechanismActive siteIn vivo
The present application provides compositions comprising α-galactosidase A in combination with an active site-specific chaperone for the α-galactosidase A, and methods for treating Fabry disease in a subject in need thereof, that includes a method of administering to the subject such compositions. The present application also provides methods for increasing the in vitro and in vivo stability of an α-galactosidase A enzyme formulation. The present application also provides methods for treating Fabry disease using intravenous administration of 1-deoxygalactonojirimycin.
Owner:AMICUS THERAPEUTICS INC
Methods for Reprogramming Adult Somatic Cells and Uses Thereof
InactiveUS20100135970A1Easy to produceReduce probabilityBiocideGenetic material ingredientsSomatic cellSomatic portion
As described below, the present invention features methods for reprogramming somatic cells and related therapeutic compositions and methods.
Owner:STEWARD RES & SPECIALTY PROJECTS
Prevention of thrombotic disorders with active vitamin D compounds or mimics thereof
InactiveUS20070037779A1Great therapeutic efficacyReduce or avoid effectBiocideOrganic active ingredientsHigh dosesThrombotic disorder
The present invention relates to a method for preventing, treating, or ameliorating thrombotic disorders in an animal comprising administering to the animal an active vitamin D compound or a mimic thereof. According to the invention, the active vitamin D compound or the mimic thereof may be administered by HDPA so that high doses of the active vitamin D compound or the mimic thereof can be administered to an animal without inducing severe symptomatic hypercalcemia. The invention also relates a method for preventing, treating, or ameliorating thrombotic disorders in an animal comprising administering to the animal an active vitamin D compound or a mimic thereof in combination with one or more other therapeutic agents.
Owner:NOVACEA INC
Electrochemical biosensor test strip
InactiveUSRE42924E1Easy to identifyEnsure correct executionImmobilised enzymesBioreactor/fermenter combinationsElectrochemical biosensorPolyethylene oxide
An electrochemical biosensor test strip with four new features. The test strip includes an indentation for tactile feel as to the location of the strips sample application port. The sample application port leads to a capillary test chamber, which includes a test reagent. The wet reagent includes from about 0.2% by weight to about 2% by weight polyethylene oxide from about 100 kilodaltons to about 900 kilodaltons mean molecular weight, which makes the dried reagent more hydrophilic and sturdier to strip processing steps, such as mechanical punching, and to mechanical manipulation by the test strip user. The roof of the capillary test chamber includes a transparent or translucent window which operates as a “fill to here” line, thereby identifying when enough test sample (a liquid sample, such as blood) has been added to the test chamber to accurately perform a test. The test strip may further include a notch located at the sample application port. The notch reduces a phenomenon called “dose hesitation”.
Owner:ROCHE OPERATIONS +1
Photon therapy device
InactiveUS20090216219A1Sufficient timeImprove skinSurgical instrument detailsLight therapyPhotonicsAtomic physics
The invention provides a controller unit for controlling the emission of at least on photon emitted source, the controller unit includes a central processing unit pre-programmed with selectable pre-programmed photonic emission protocols. The photonic emission protocols include parameters for regulating photonic emission from photon emitting sources, with the protocols having a pulsed emission mode for about 30% of the duration of each protocol, and a continuous emission mode for about 70% of the duration of each protocol, with a pulse rate selected from a range of frequencies of between 120 Hz and 20,000 Hz when in the pulsed emission mode.
Owner:PHOTON THERAPY SYST
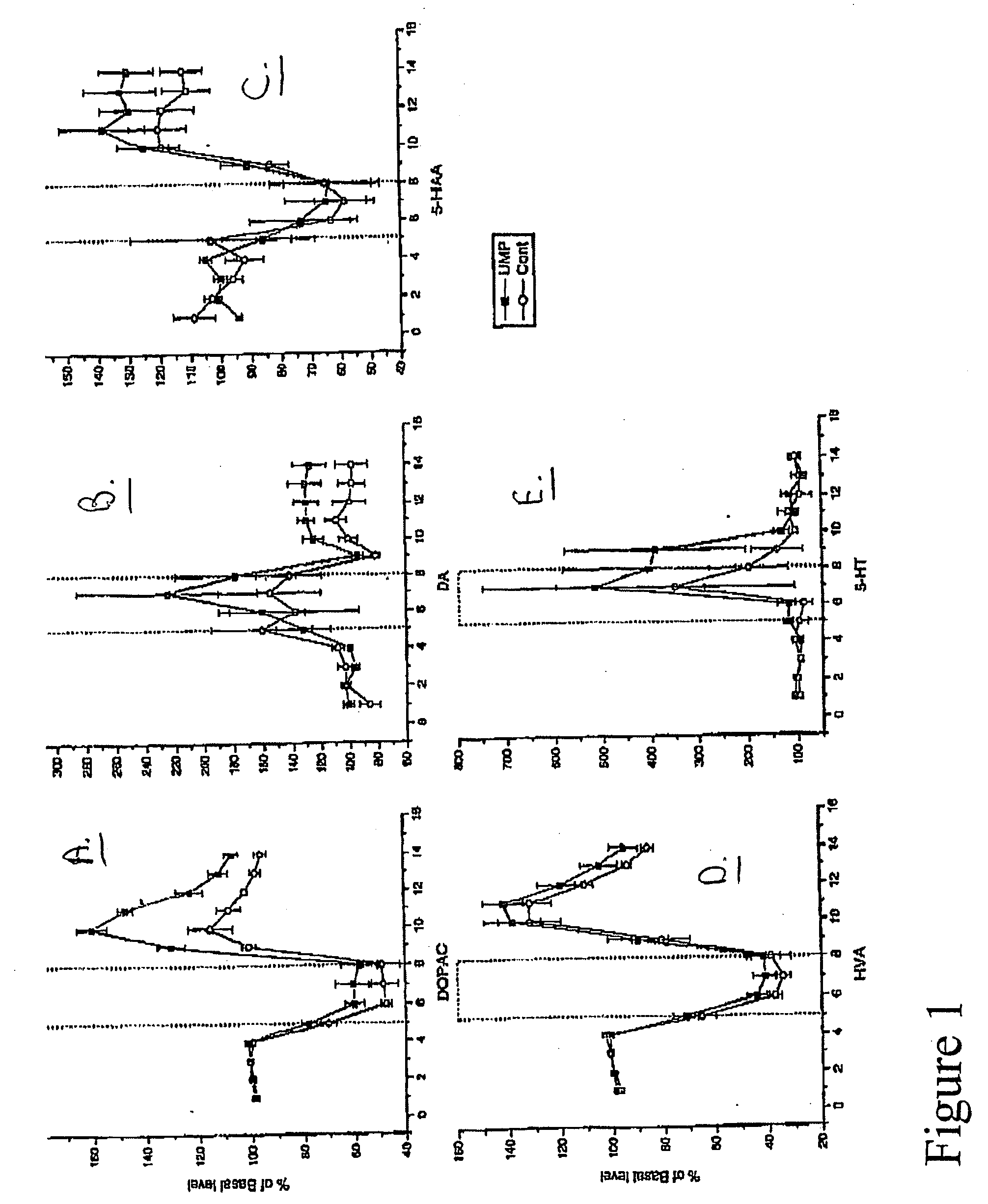
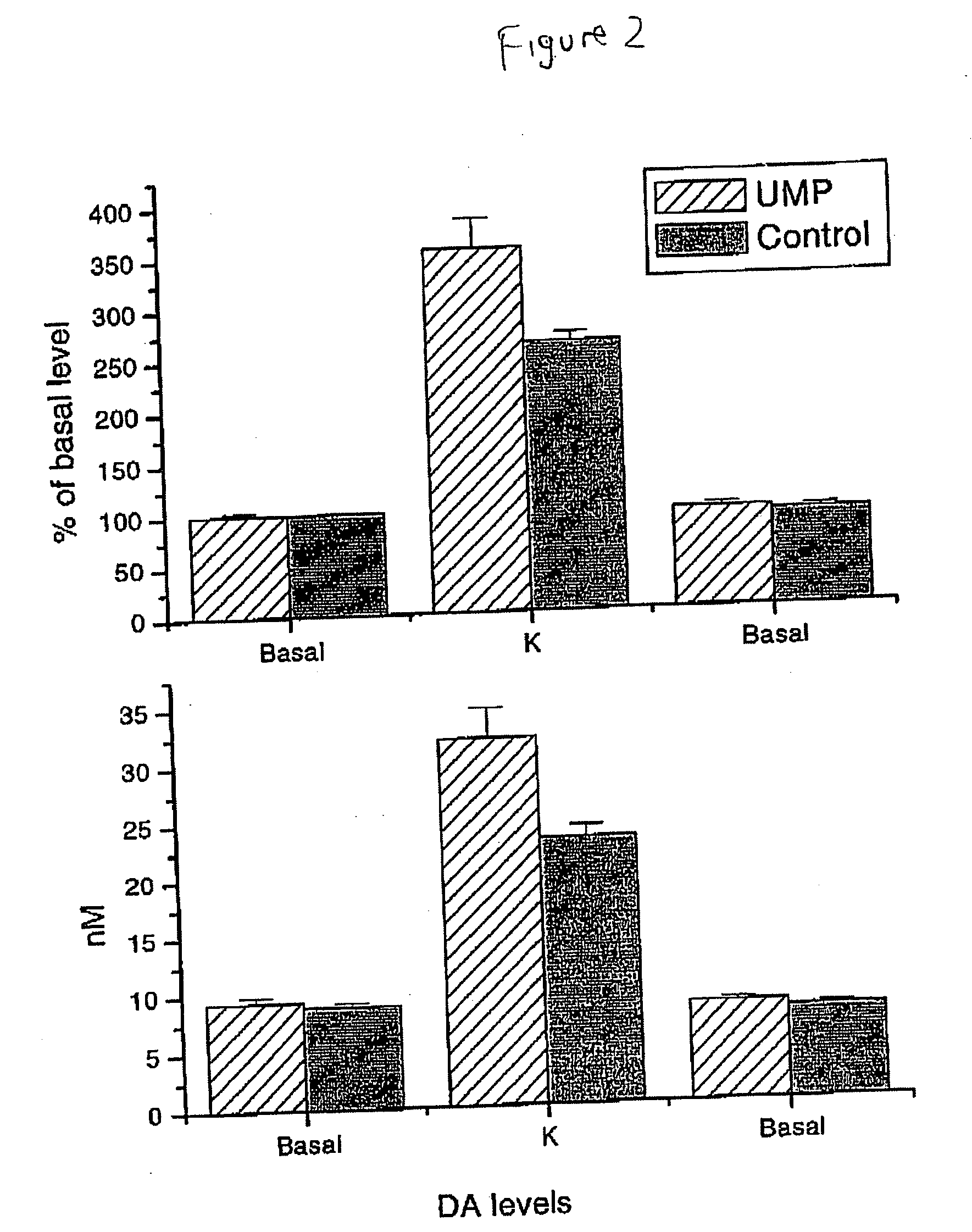
Uridine effects on dopamine release
ActiveUS20060025376A1Increase secretionReduce incidenceBiocideNervous disorderSecretionUridine monophosphate
The present invention provides methods for increasing secretion of dopamine and other neurotransmitters and treating or reducing the incidence of diseases involving decreased secretion of dopamine and other neurotransmitters, e.g. Parkinson's disease, comprising administering to the subject a uridine or a source thereof, and compositions for treating or reducing an incidence of Parkinson's disease, comprising a uridine, a uridine monophosphate, or a source thereof.
Owner:MASSACHUSETTS INST OF TECH
Electrochemical biosensor test strip
InactiveUSRE42560E1Easy to identifyEnsure correct executionImmobilised enzymesBioreactor/fermenter combinationsPunchingPolyethylene oxide
Owner:ROCHE DIABETES CARE INC +1
Method of enhancing the control of viruses on skin
Owner:DIAL CORPORATION
Injectable capsaicin
The present invention provides compositions and methods for relieving pain at a site in a human or animal in need thereof by administering at a discrete site in a human or animal in need thereof a dose of capsaicin in an amount effective to denervate a discrete site without eliciting an effect outside the discrete location, the dose of capsaicin ranging from 1 μg to 3000 μg.
Owner:SOFINNOVA VENTURE PARTNERS VII
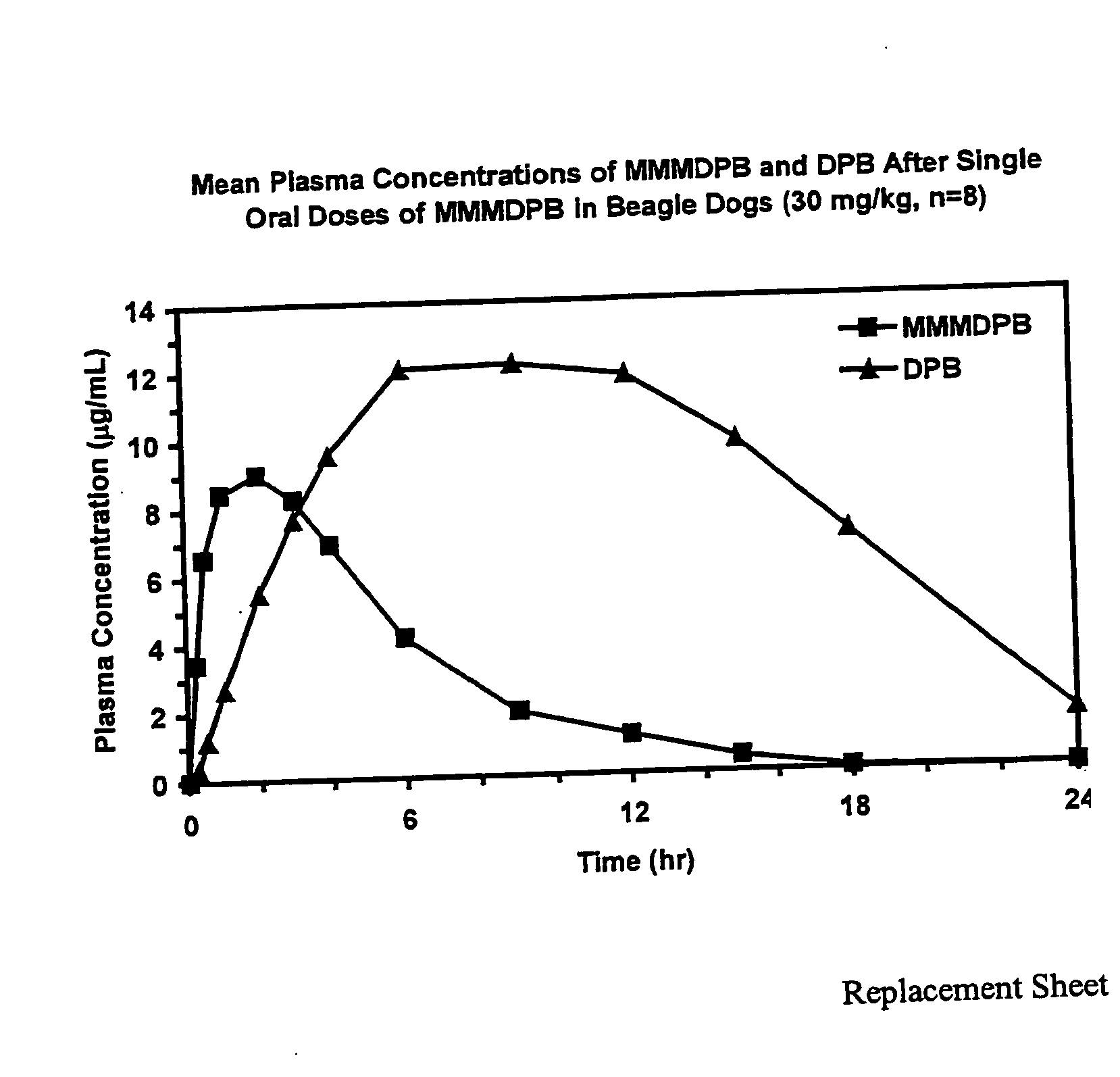
Composition and method for improved bioavailability and enhanced brain delivery of 5,5-diphenyl barbituric acid
InactiveUS20060122208A1Enhanced and efficient deliveryAdequate doseBiocideOrganic active ingredientsBioavailabilitySodium salt
The present invention relates to a composition and a method of delivering a barbituric acid derivative to the central nervous system of a mammal in need of treatment for neurological conditions. In particular, the present invention relates to a method of administering an oral dosage form of a sodium salt of 5,5-diphenyl barbituric acid to enhance the bioavailability of 5,5-diphenyl barbituric acid and brain delivery of same.
Owner:TARO PHARMA INDS
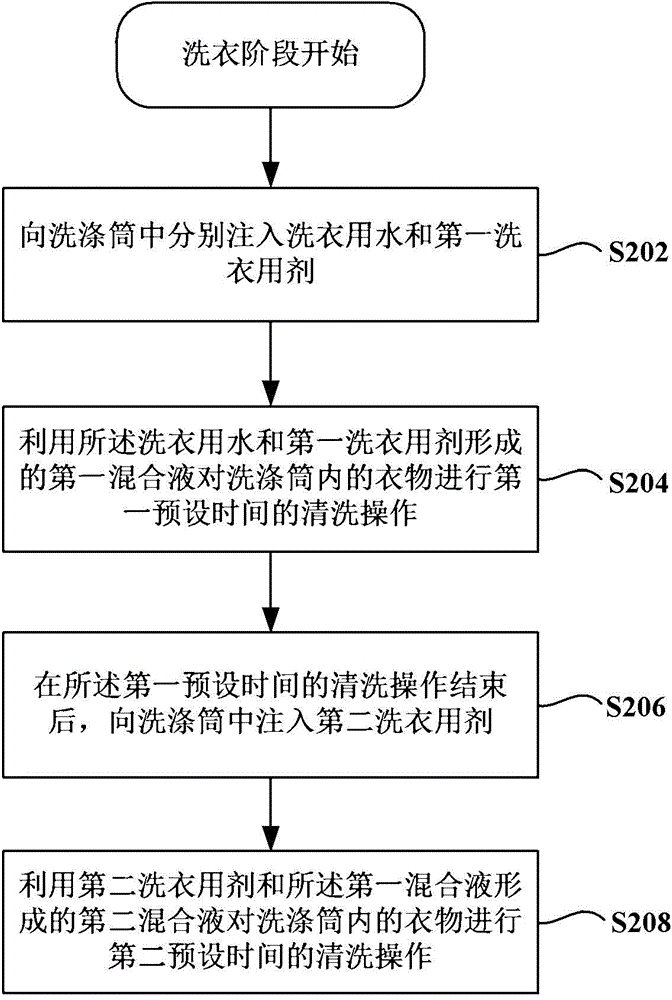
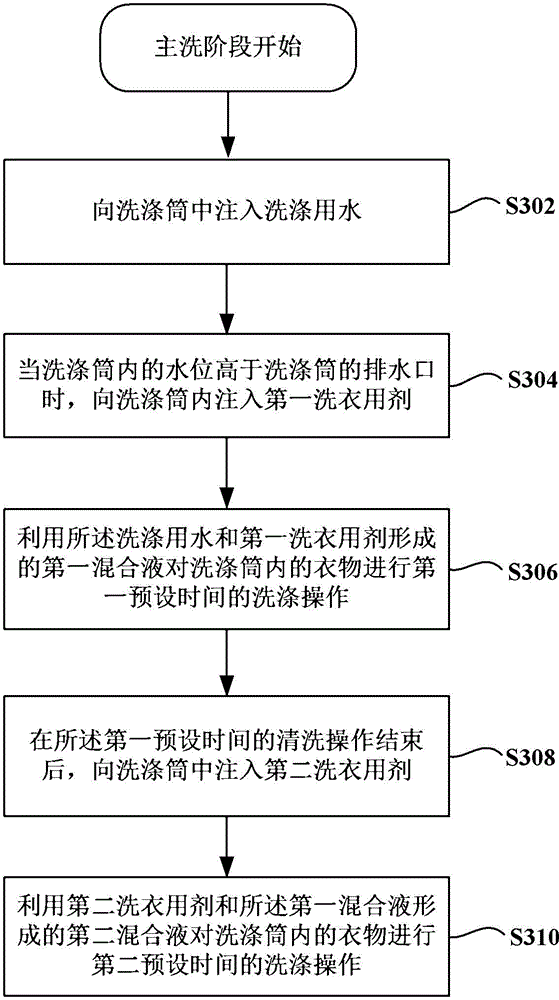
Washing machine and control method thereof
ActiveCN105624984AEasy to washImprove nursing effectOther washing machinesControl devices for washing apparatusEngineeringProcess engineering
The invention provides a washing machine and a control method thereof. The washing machine comprises a washing cylinder, a laundry detergent box set and a metering pump. The laundry detergent box set at least comprises a first laundry detergent box for storing first laundry detergent and a second laundry detergent box for storing second laundry detergent. The metering pump is configured in a way that during a washing phase of the washing program of the washing machine, the metering pump pumps the first laundry detergent from the first laundry detergent box to the washing cylinder, first mixed liquor formed by the first laundry detergent and laundry water in the washing cylinder is used for cleaning clothes for first preset time; after the cleaning operation in the first preset time is finished, the metering pump pumps the second laundry detergent from the second laundry detergent box to the washing cylinder, and second mixed liquor formed by the second laundry detergent and the first mixed liquor is used for cleaning the clothes for second preset time. When the washing machine cleans fabrics, the decontamination effect is good, and the fabrics do not fade.
Owner:QINGDAO HAIER SMART TECH R & D CO LTD
Compound magnesium sulfate wet-dressing agent and preparation method thereof
The invention discloses a compound magnesium sulphate wet packing and a preparation method thereof. The compound magnesium sulphate wet packing consists of 20 to 50 percent of magnesium sulphate, 0.1 to 4 percent of lidocaine, the balance being surplus water for injection and medical carrier. The preparation method comprises the steps of soaking the medical carrier in compound magnesium sulphate solution for thirty to sixty minutes after being made into sheets and being disinfected and sterilized, sterilizing the medical carrier with steam for thirty to forty-five minutes, and then putting the medical carrier into a plastic bag to be sealed and packed to prepare the compound magnesium sulphate wet packing. The compound magnesium sulphate wet packing can effectively prevent chemotherapy phlebitis; and the clinical trials show that through the compound magnesium sulphate wet packing, the effective rate of caring and treating swelling and pain caused by edema on local subcutaneous tissue when chemotherapy drugs are instilled in the vein of the patient is over 90 percent, thereby reducing the pain of the patient effectively and improving the quality of life of the patient.
Owner:贾国平
Sterilized magnesium sulfate dressing preparation
The invention relates to sterilized magnesium sulfate wet dressing agent. It is formed by medical using magnesium sulfate aqueous solution and carrier. The former accounts for 20-50wt%, and can be made by high temperature sterilizing after sand stick filtering with 0.50-0.80mum aperture. The latter can be the water absorption paper towel, gauze, or medical using dressing. It can be used to cure local subcutaneous tissue swelling ache. The using method includes the following steps: dressing it along the vein direction, changing for every 30-60min until to the dripping off, stopping the wet dressing after withdrawing the needle for 1-2h. The user can understand clearly through the direction for use on the packaging bag which can solve the problems of carryover, using inconvenient, and infection.
Owner:天津市海卫益嘉医疗用品有限公司
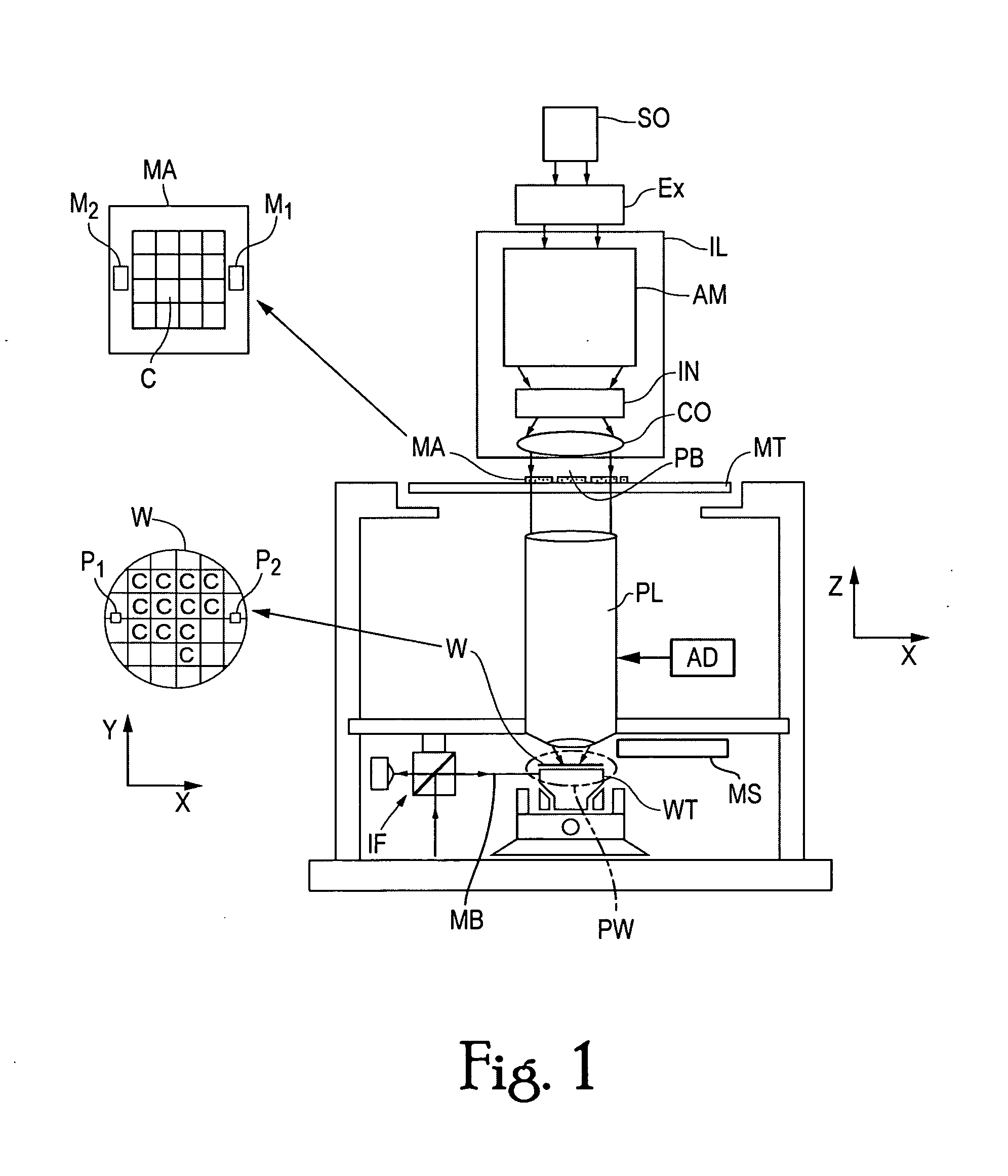
Method and system for enhanced lithographic alignment
InactiveUS20070212652A1Enhance the imageAdequate doseSemiconductor/solid-state device detailsSolid-state devicesMaterials scienceHard mask
A method for alignment mark preservation includes a step of preparing a lower alignment mark structure on a substrate. In one configuration of the invention, the alignment mark structure includes a lower trench. In a further step, a hard mask coating is applied to a substrate that includes the alignment marks. Preferably, the hard mask material is an amorphous carbon material. In a further step, a selected portion of the hard mask located above the lower alignment mark structure is exposed to a dose of radiation. In one aspect of the invention, the surface of regions of the hard mask coating that receive the dose of radiation become elevated with respect to other regions of the hard mask surface. For those elevated regions of the hard mask that are aligned with an underlying alignment mark trench, the elevated regions serve as an alignment mark that preserves the original horizontal position of the underlying alignment mark.
Owner:ASML NETHERLANDS BV
Prevention of Thrombotic Disorders with Active Vitamin D Compounds or Mimics Thereof
InactiveUS20080069814A1Reduce and avoid unwantedReduce and avoid and adverse effectOrganic active ingredientsBiocideDiseaseMedicine
The present invention relates to a method for preventing, treating, or ameliorating thrombotic disorders in an animal comprising administering to the animal an active vitamin D compound or a mimic thereof. According to the invention, the active vitamin D compound or the mimic thereof may be administered by HDPA so that high doses of the active vitamin D compound or the mimic thereof can be administered to an animal without inducing severe symptomatic hypercalcemia. The invention also relates a method for preventing, treating, or ameliorating thrombotic disorders in an animal comprising administering to the animal an active vitamin D compound or a mimic thereof in combination with one or more other therapeutic agents.
Owner:NOVACEA INC
High Concentration Alpha-Glucosidase Compositions for the Treatment of Pompe Disease
ActiveUS20150044194A1Improve stabilityMaintain relatively stableOrganic active ingredientsNervous disorderHigh concentrationReactive site
The present application provides for compositions comprising high concentrations of acid α-glucosidase in combination with an active site-specific chaperone for the acid α-glucosidase, and methods for treating Pompe disease in a subject in need thereof, that includes a method of administering to the subject such compositions. The present application also provides methods for increasing the in vitro and in vivo stability of an acid α-glucosidase enzyme formulation.
Owner:AMICUS THERAPEUTICS INC
Composition and method for improved bioavailability and enhanced brain delivery of 5,5-diphenyl barbituric acid
InactiveUS7683071B2Reduce deliveryAdequate doseOrganic active ingredientsBiocideBioavailabilitySodium salt
The present invention relates to a composition and a method of delivering a barbituric acid derivative to the central nervous system of a mammal in need of treatment for neurological conditions. In particular, the present invention relates to a method of administering an oral dosage form of a sodium salt of 5,5-diphenyl barbituric acid to enhance the bioavailability of 5,5-diphenyl barbituric acid and brain delivery of same.
Owner:TARO PHARMA INDS
Klf family members regulate intrinsic axon regeneration ability
InactiveUS20120225084A1Adequate doseSure easyNervous disorderPeptide/protein ingredientsNeuronAxon growth
This invention relates, e.g., to a method for promoting CNS axon regeneration, comprising (1) inhibiting the expression or activity in a neuron of one or more of the members of the Krüppel-like transcription factor (KLF) family that suppress axon growth (e.g., KLF 1, 2, 3, 4, 5, 9, 12, 13, 14, 15 and / or 16), and / or (2) stimulating the expression or activity in a neuron of one or more of the members of the KLF family that promote axon growth (e.g., KLF 6 and / or 7).
Owner:UNIV OF MIAMI
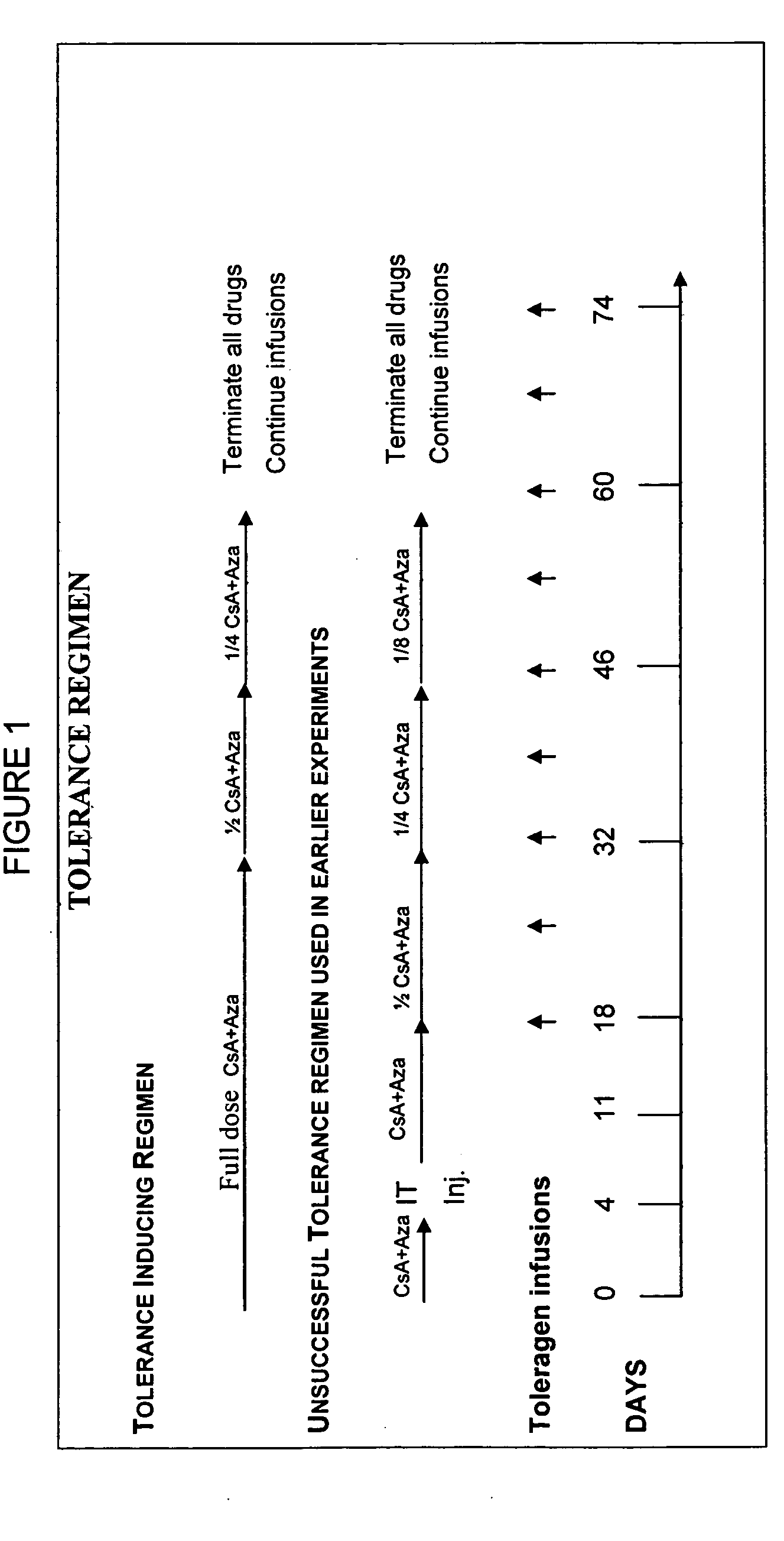
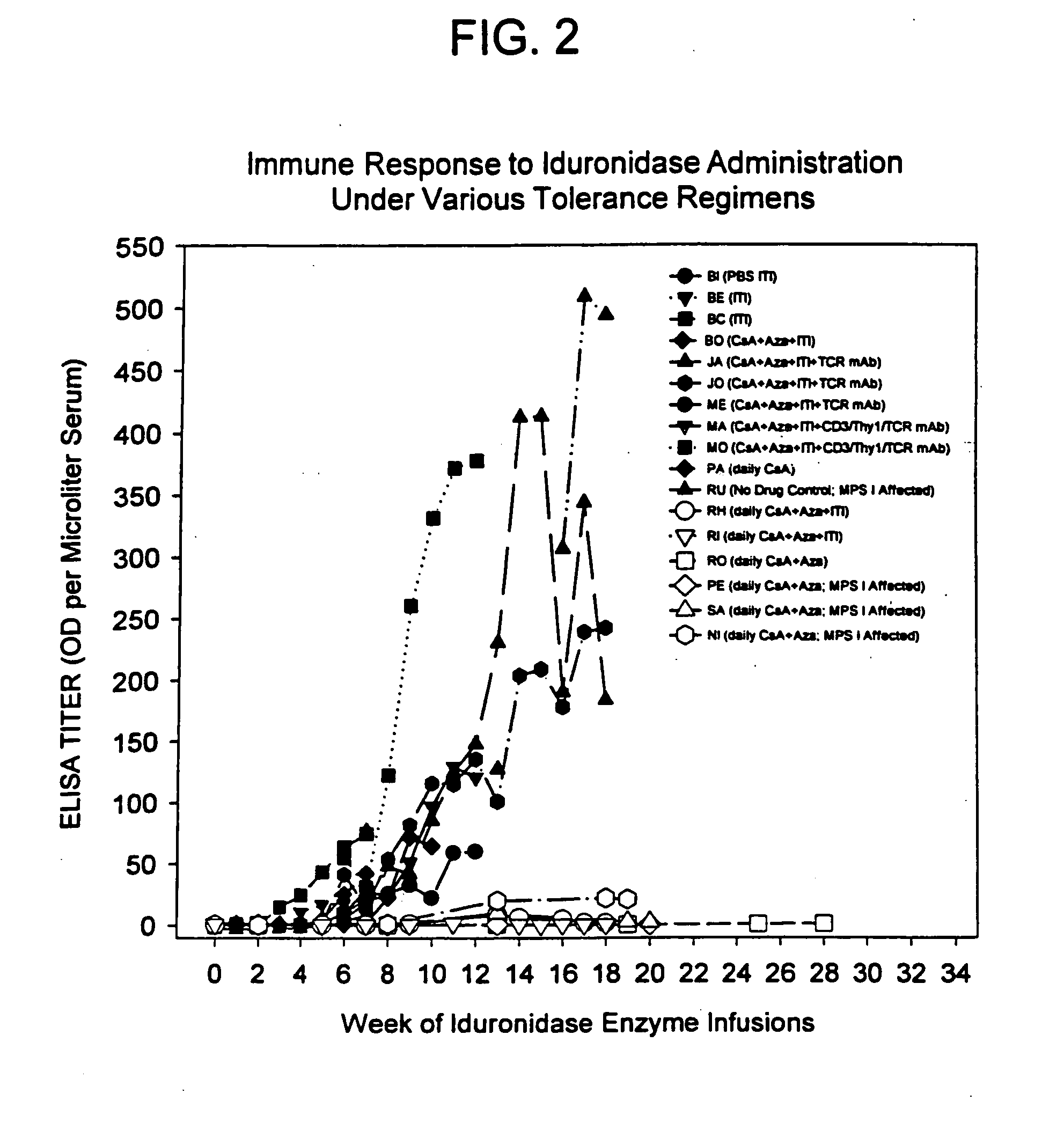
Induction of antigen specific immunologic tolerance
Antigen specific immune tolerance is induced in a mammalian host by administration of a toleragen in combination with a regimen of immunosuppression. The methods optionally include a preceding conditioning period, where immunosuppressive agents are administered in the absence of the toleragen. After the tolerizing regimen, the host is withdrawn from the suppressive agents, but is able to maintain specific immune tolerance to the immunogenic epitopes present on the toleragen. Optimally, the toleragen will have high uptake properties that allow uptake in vivo at low concentrations in a wide variety of tolerizing cell types.
Owner:KAKKIS EMIL D +4
Programmable mask and method for fabricating biomolecule array using the same
InactiveUS7362387B2Facilitate strippingHigh densityMaterial nanotechnologySequential/parallel process reactionsHigh densityUltraviolet lights
A programmable mask used in a photolithography process for fabricating a biomolecule array and a method for fabricating a biomolecule array using the same are disclosed. Particularly, a TFT-LCD type programmable mask for selectively transmitting incident light in accordance with an electrical signal applied thereto and a method for fabricating a biomolecule array using the same are provided. The ultraviolet light is selectively illuminated to a sample substrate so that the biomolecule array having high density can be fabricated.
Owner:ELECTRONICS & TELECOMM RES INST
17-hydroxyprogesterone ester-containing oral compositions and related methods
InactiveUS20170035781A1Effectively delivered orallyHigh percent w/w loadingPowder deliveryOrganic active ingredients17α-HydroxyprogesteroneDosage form
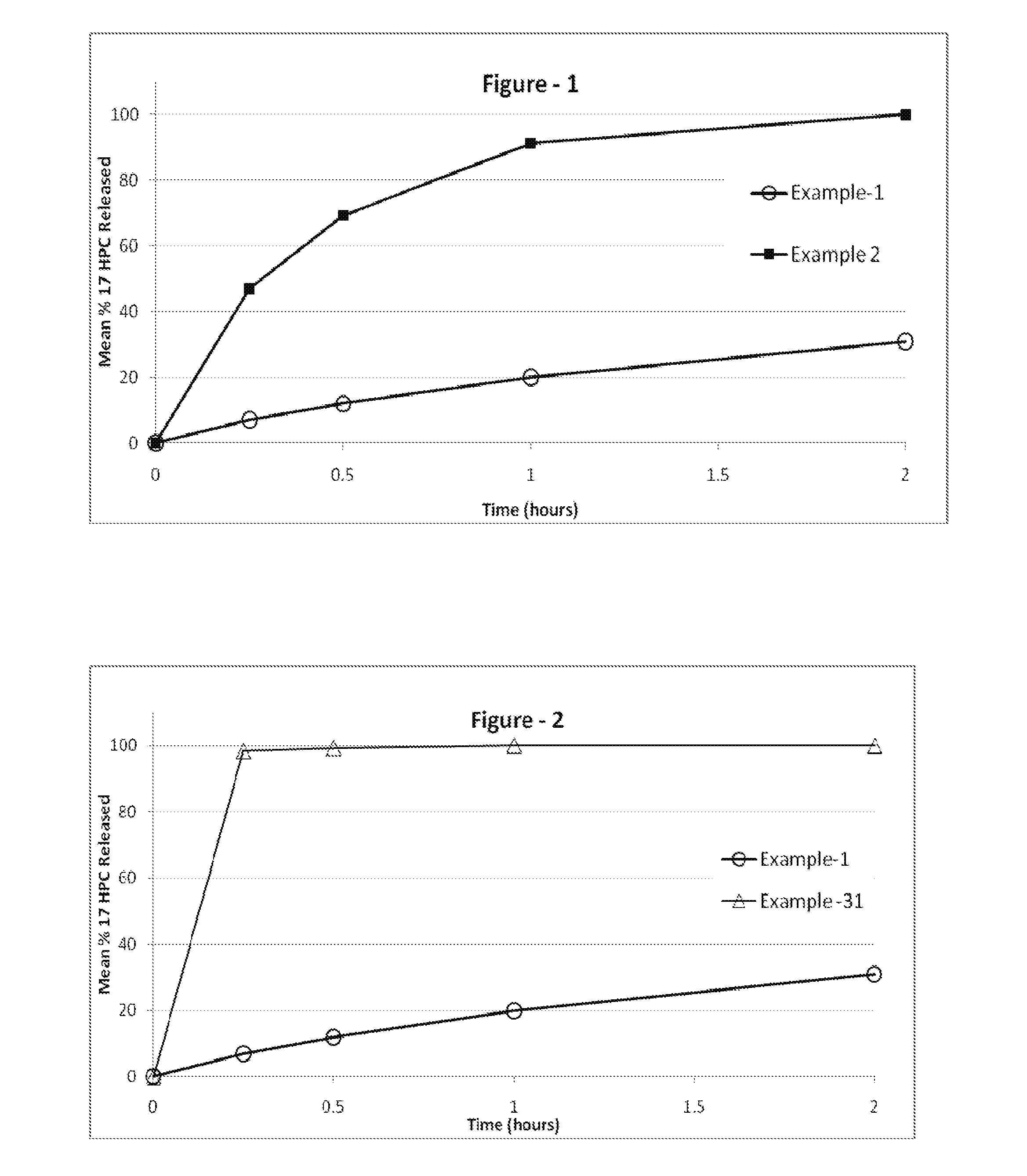
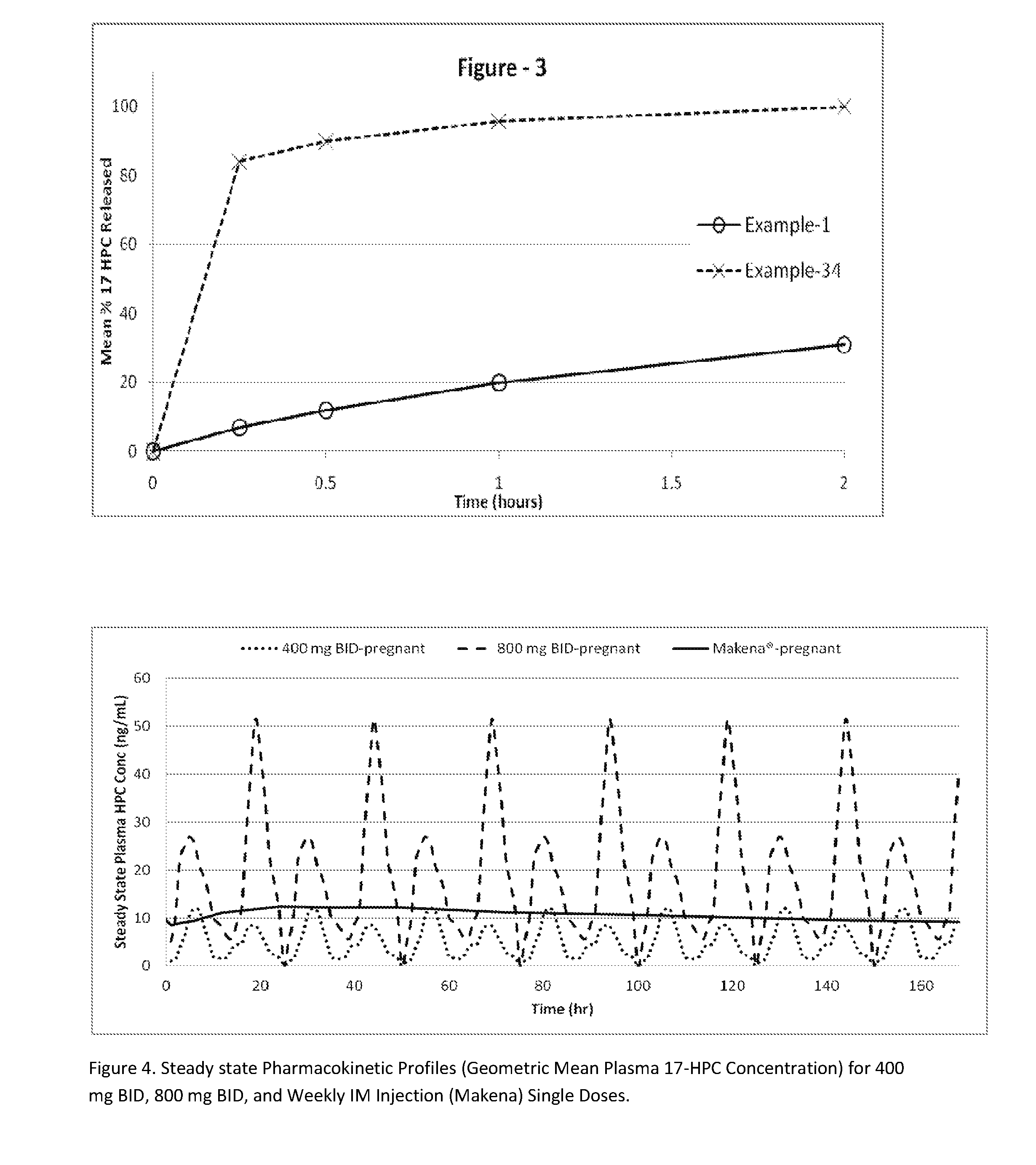
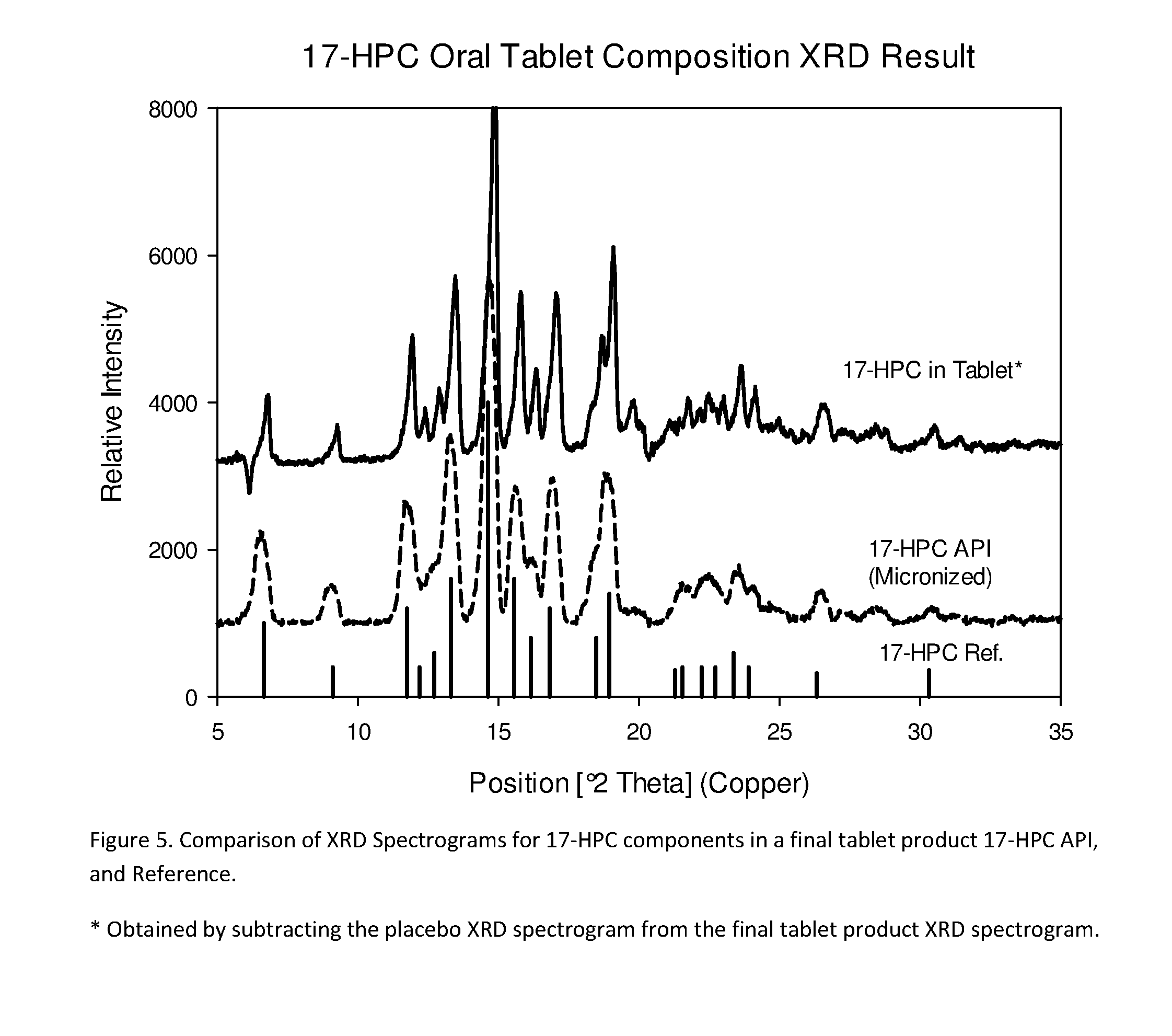
The present invention provides for bioavailable oral dosage forms containing esters of 17-hydroxyprogesterone as well as related methods. The oral dosage forms can be formulated for pregnancy support and can include a therapeutically effective amount of an ester of 17-hydroxyprogesterone and a pharmaceutically acceptable carrier. In another embodiment, a pharmaceutically acceptable oral dosage form for pregnancy support is provided. The pharmaceutically acceptable oral dosage can include a therapeutically effective amount of an ester of 17-hydroxyprogesterone and a pharmaceutically acceptable carrier. The oral dosage form can, when measured using a USP Type-II dissolution apparatus in 900 mL of deionized water with 0.5 (w / v) of sodium lauryl sulfate at 50 RPM at 37° C., release at least 20 wt % of the dose of the ester of 17-hydroxyprogesterone after 60 minutes, or in the alternative release at least 20 wt % more after 60 minutes than an equivalently dosed oral dosage form without the carrier.
Owner:LIPOCINE
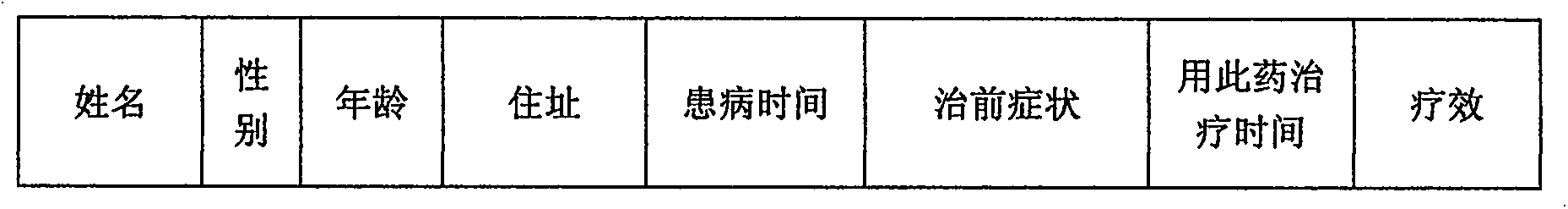
Medicine for treating rheumatoid bone diseases and preparation method thereof
InactiveCN103055232ALittle side effectsReduce joint painAnthropod material medical ingredientsAntipyreticMonkshoodsMyrrh
The invention relates to a medicine, and in particular relates to a medicine for treating rheumatoid bone diseases and a preparation method thereof. A medicine bag comprises the following medicines by weight: 10-15 g of szechuan lovage rhizome, 10-15 g of radix angelicae, 15-20 g of radix clematidis, 5-10 g of radix aconiti agrestis, 5-10 g of monkshood, 5-10 g of fructus liquidambaris, 10-15 g of cinnamon, 10-20 g of cortex acanthopanacis, 10-15 g of frankincense, 10-15 g of myrrh, 10-15 g of dried body of ground beetle, 10-15 g of Japanese snailseed root, 10-15 g of rhizoma acori graminei, 10-15 g of galangal, 15-20 g of ramulus rubi, 20-30 g of Tibetan hair cream, and 20-30 g of tinospora sinensis merr. The medicinal liquor comprises the following medicines by weight: 20-30 g of boschniakia rossica, 10-15 g of Tibetan saussurea involucrata, 10-15 g of saffron crocus, 20-30 g of fructus teminaliae billaricae, 20-30 g of Tibetan Fritillaria thun-bergli, 10-20 g of Tibetan lithospermum, and the balance of 60% alcoholic solution with the volume fraction of 500ml.
Owner:兰明
Treatment, prevention and amelioration of pulmonary disorders associated with chemotherapy or radiotherapy with active vitamin D compounds or mimics thereof
InactiveUS20090069276A1Preventing and treating and ameliorating pulmonary disorderAdequate doseBiocideOrganic active ingredientsDrugHigh doses
The present invention relates to a method for preventing, treating or ameliorating pulmonary disorders in a patient receiving a chemotherapeutic or radiotherapeutic agent or treatment comprising administering to the patient a pharmaceutical composition comprising an effective amount of active vitamin D compound or a mimic thereof. According to the invention, the active vitamin D compound, or the mimic thereof, may be administered by HDPA so that high doses of the active vitamin D compound can be administered to an animal without inducing severe symptomatic hypercalcemia.
Owner:NOVACEA INC
Self-lighting device for a cigarette
InactiveUS20140113239A1Effective lightingEasy to carryExothermal chemical reaction heat productionCigar manufactureMajor and minorEngineering
Owner:ETIENNE LACROIX TOUS ARTIFICES
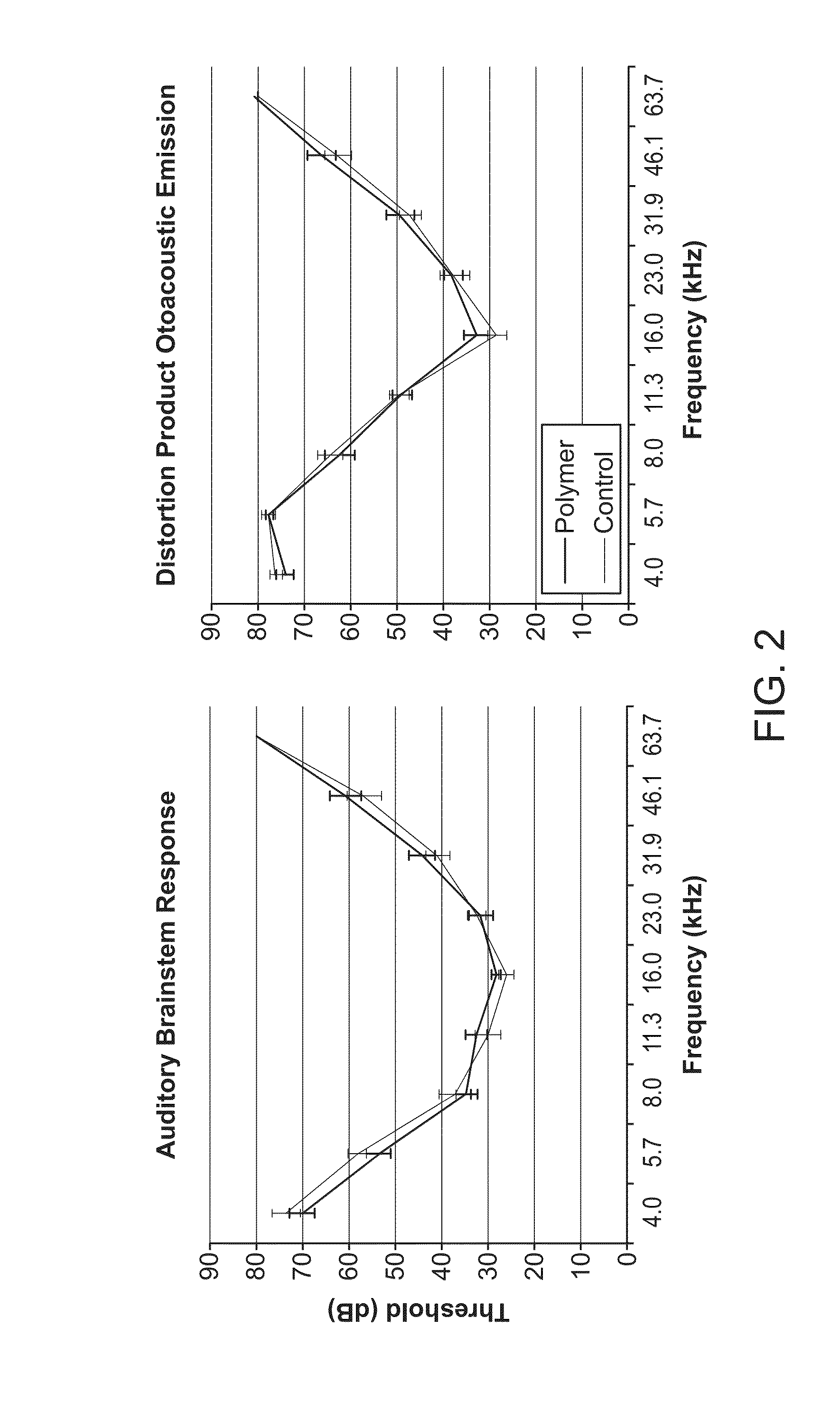
Modulation of Heparin-binding Epidermal Growth Factor Activity for Tympanic Membrane Healing
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
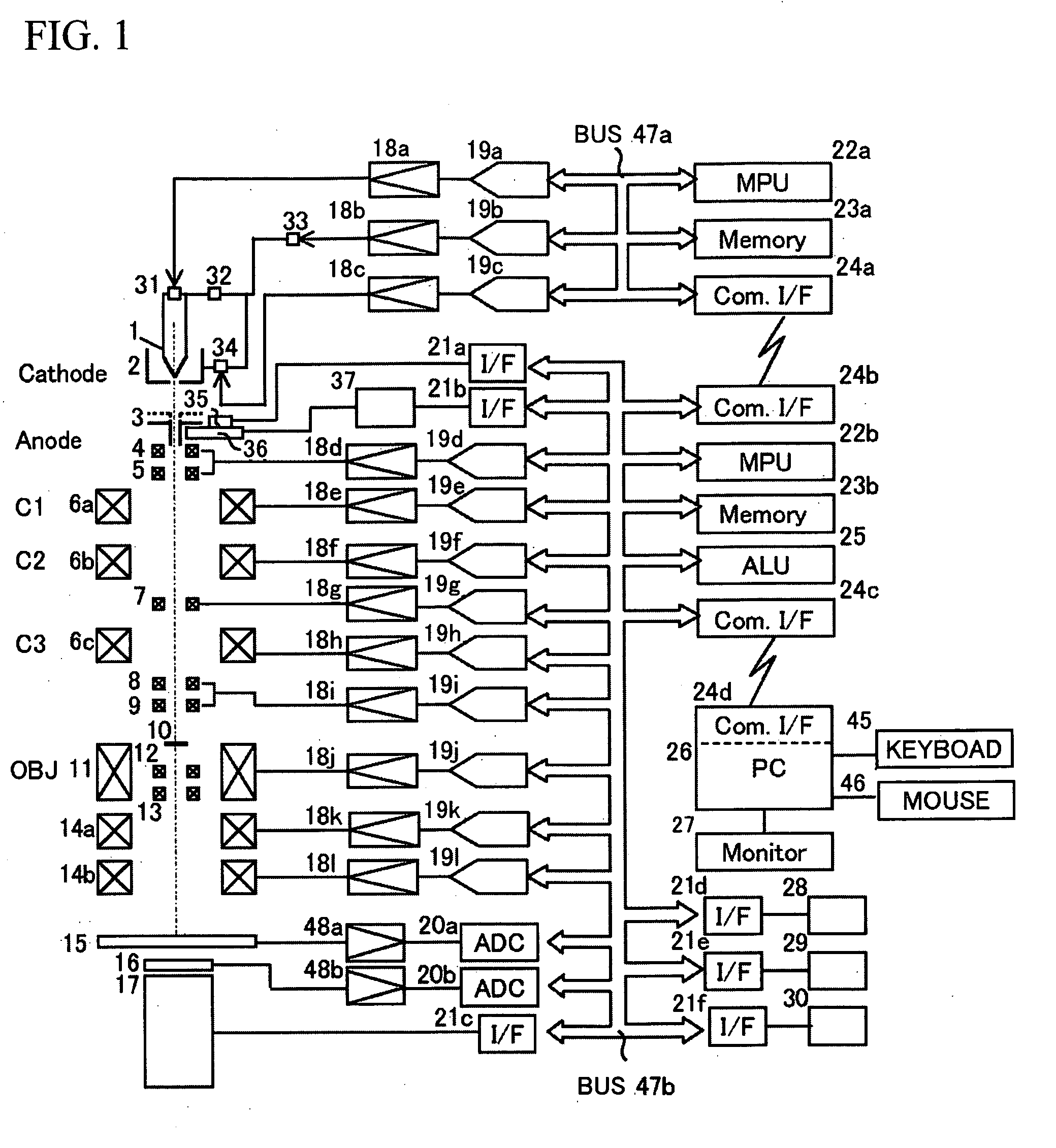
Charged particle beam device
InactiveUS20090218507A1Labor savingShorten the timeThermometer detailsStability-of-path spectrometersOptical axisLight beam
The present invention provides a charged particle beam device which can effectively restrain misalignment of an optical axis even if a position of an anode is changed. The present invention is a charged particle beam device comprising a cathode provided with a charged particle source which emits a charged particle, an anode which applies an electric field to the emitted charged particle, a charged particle beam deflector which deflects an orbit of a charged particle beam having passed the anode, and a charged particle beam detector which detects the charged particle beam from a sample to which the charged particle is irradiated, wherein a distance changing mechanism which changes a distance between the cathode and the anode, corresponding to a charged particle amount emitted from the charged particle source and a deflection amount control mechanism which detects a condition of the deflector under which the charged particle dose detected from the sample scanned by deflecting the charged particle beam in the changed distance becomes a desired size and controls deflection of the deflector at sample measurement on the basis of the condition are provided.
Owner:HITACHI HIGH-TECH CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com