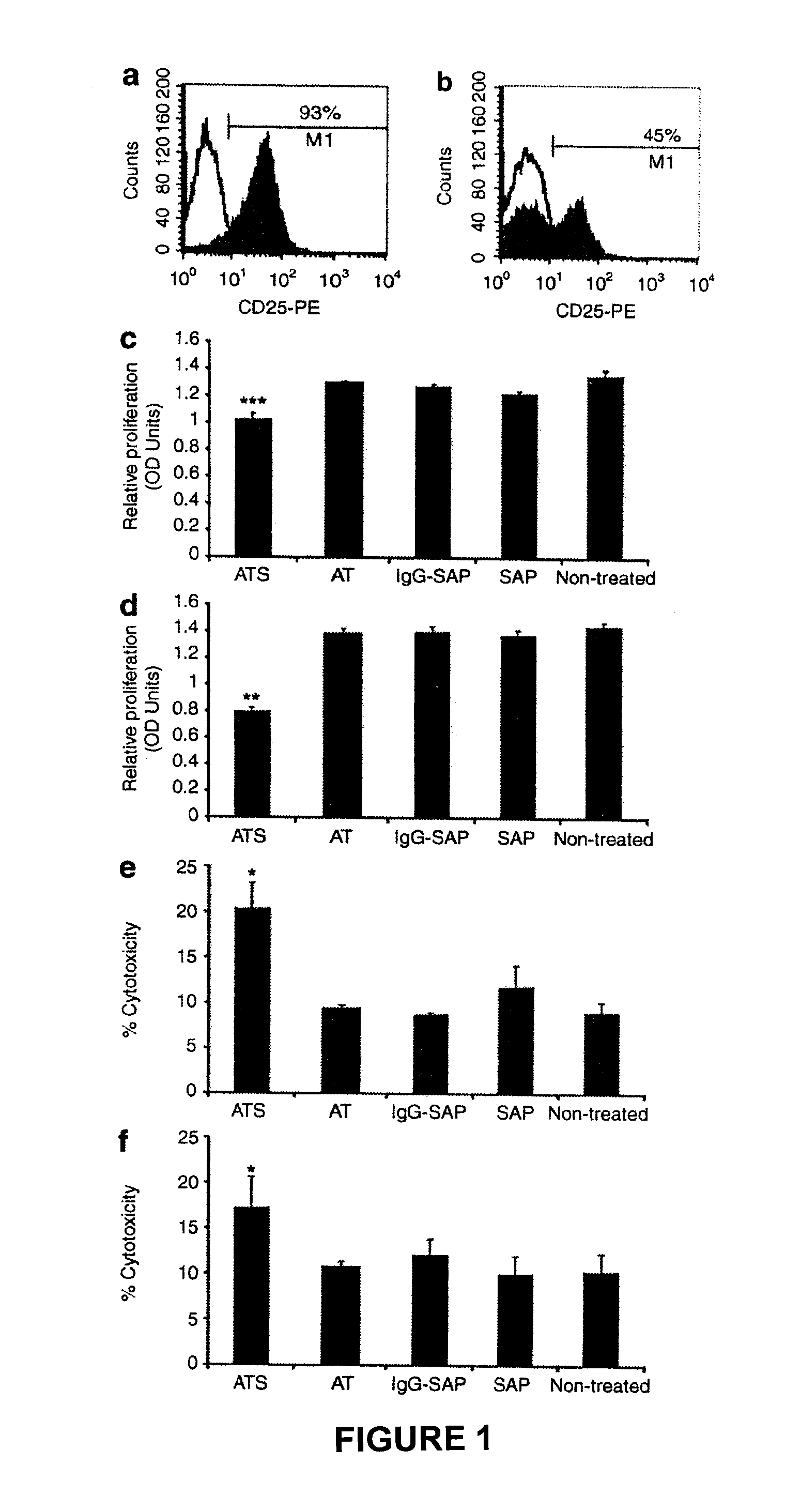
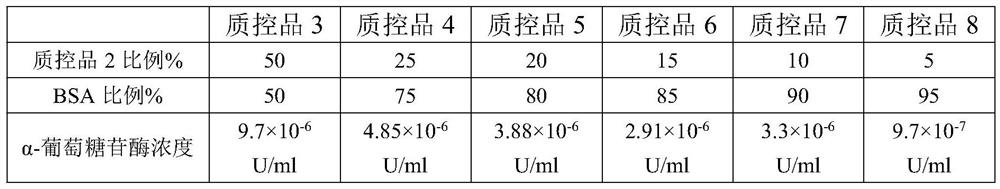
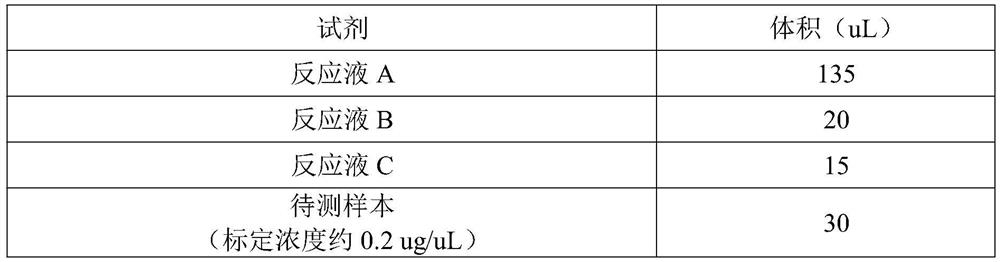
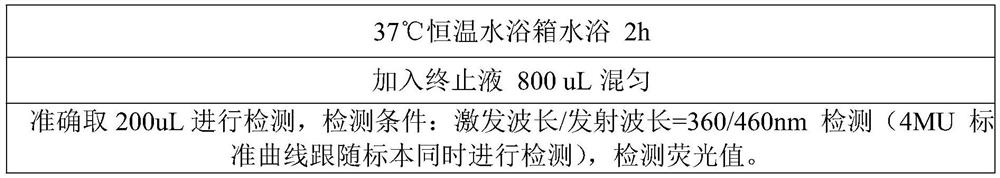
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Fabry disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A rare genetic disease caused by the deficiency of an enzyme called alpha-galactosidase A.
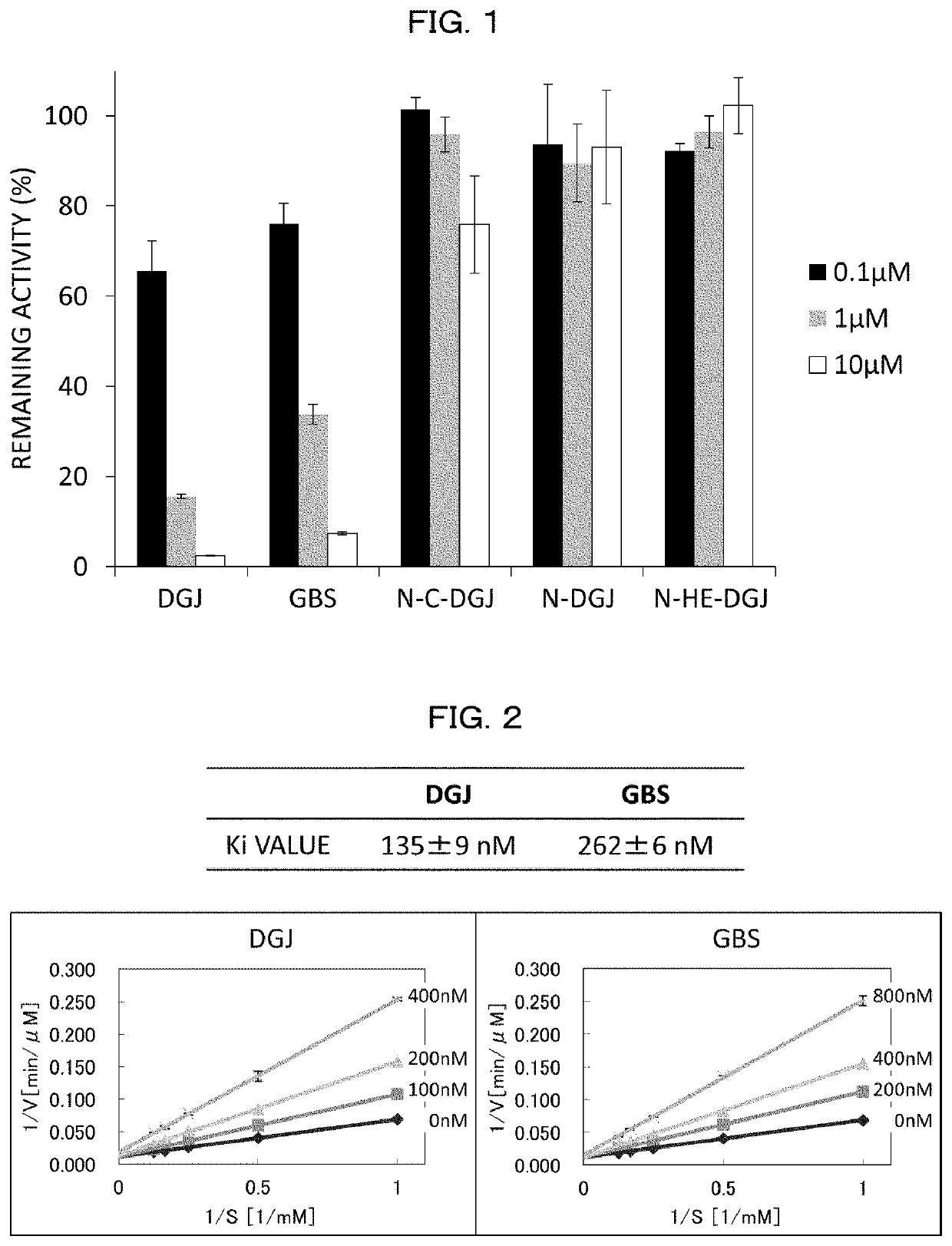
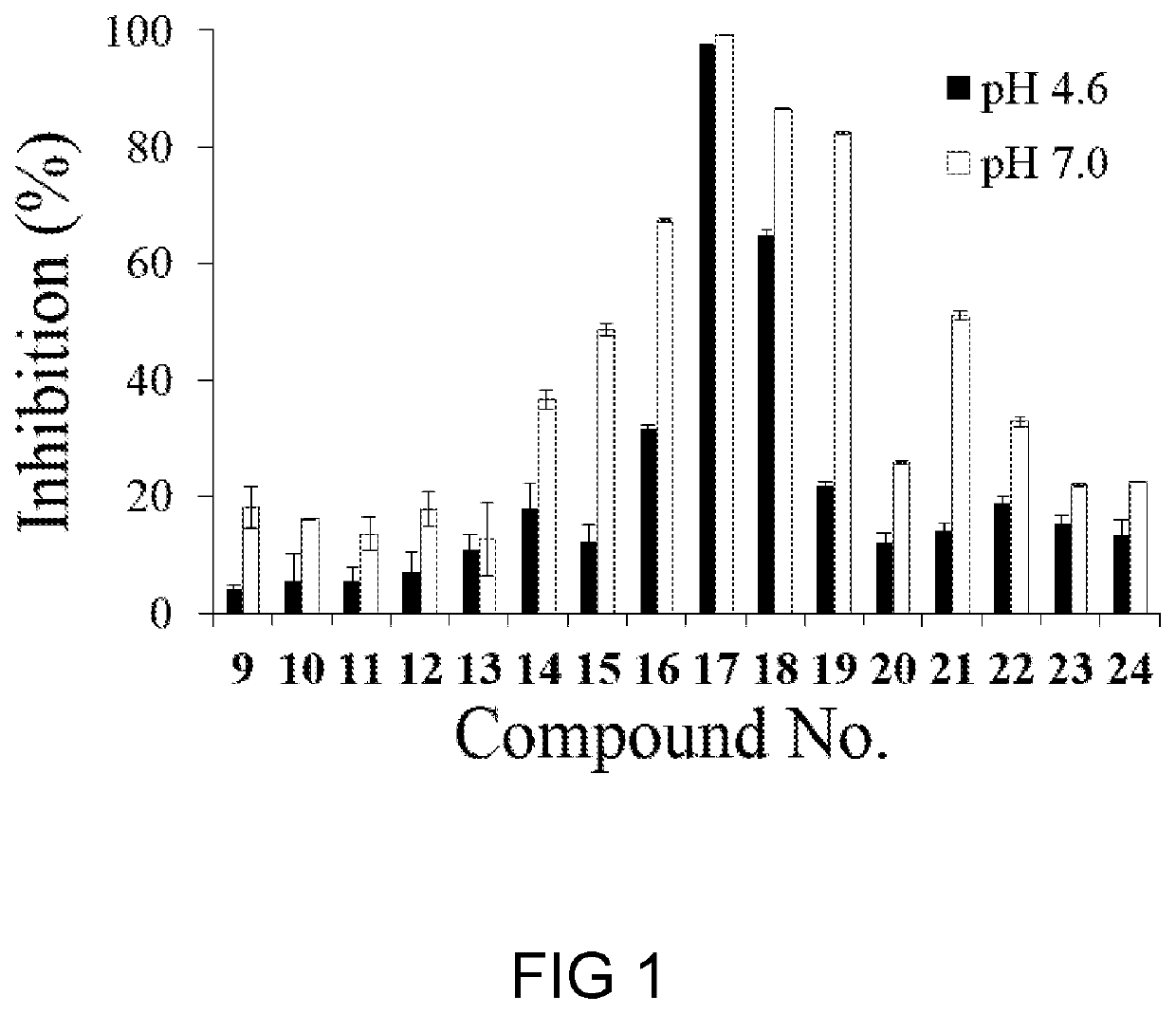
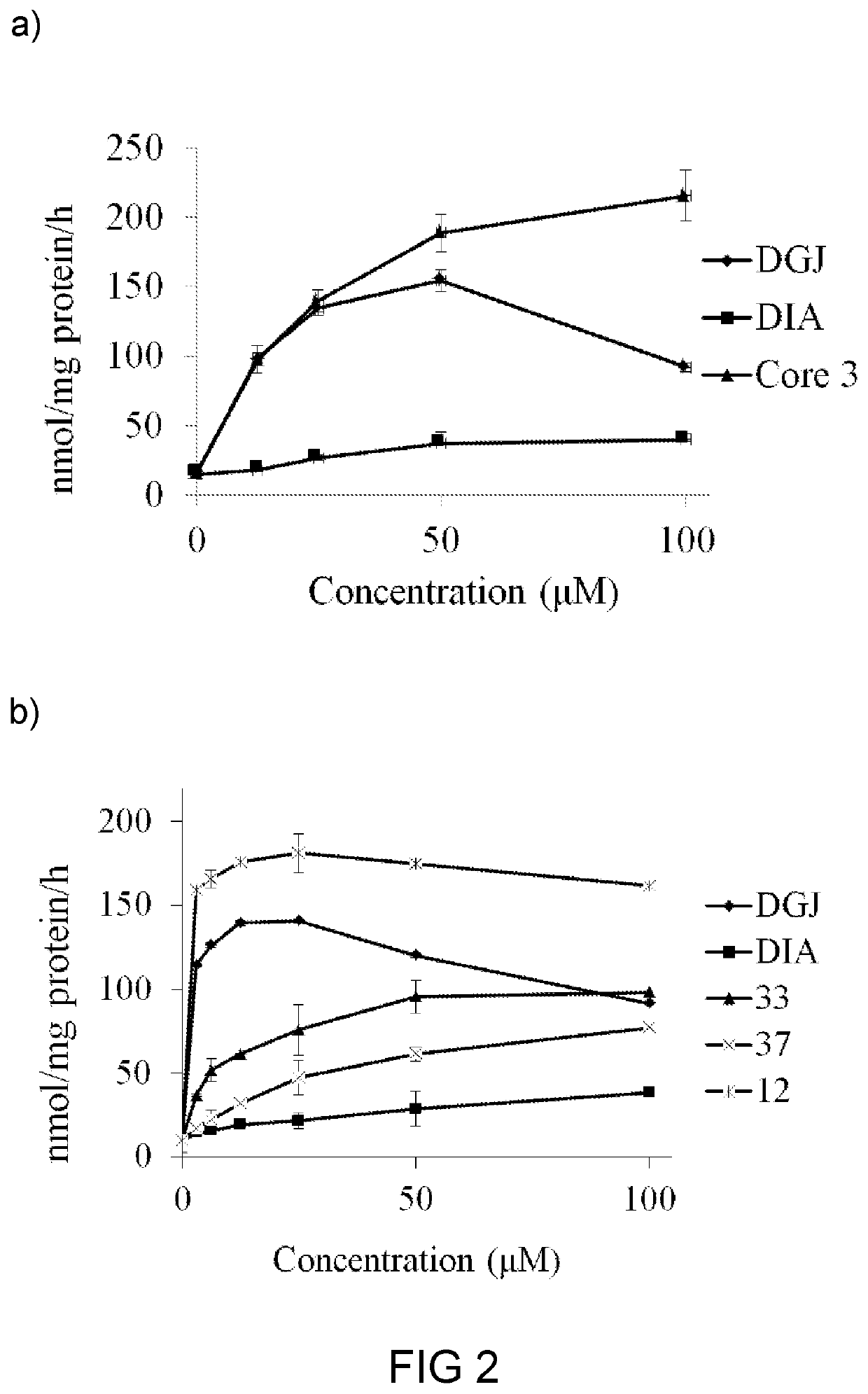
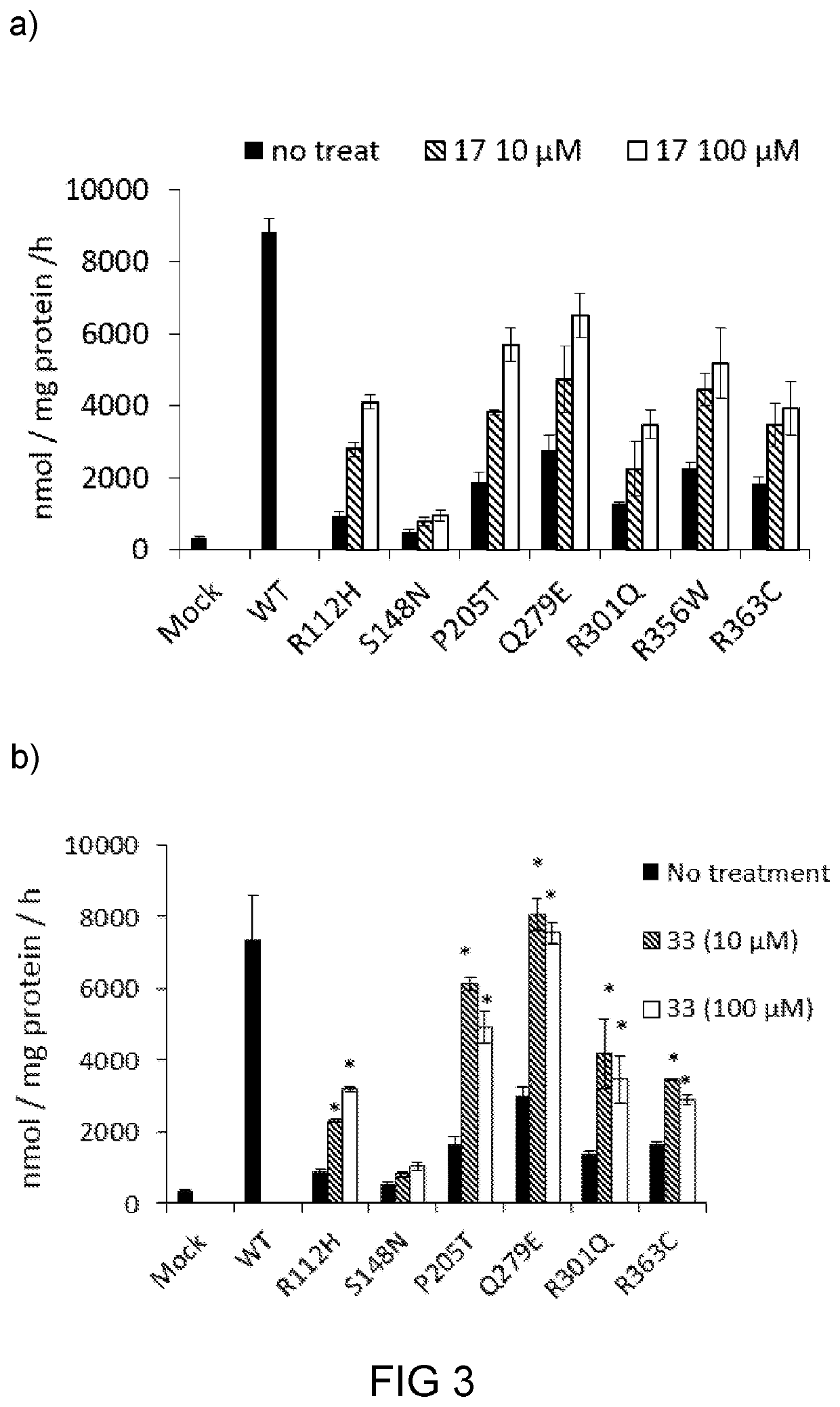
Glucosylceramide synthase inhibitors and therapeutic methods using the same
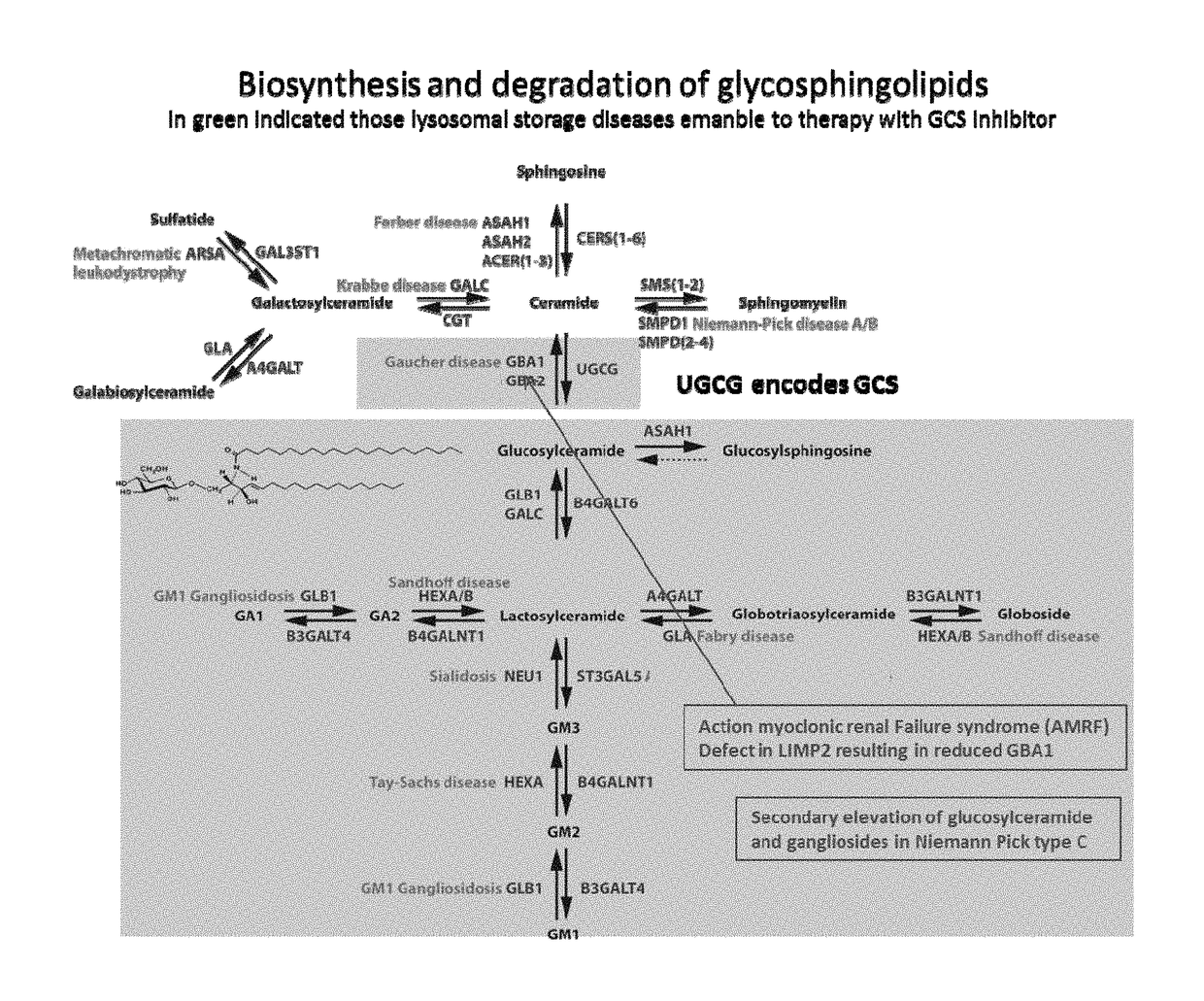
Glucosylceramide synthase inhibitors and compositions containing the same are disclosed. Methods of using the glucosylceramide synthase inhibitors in the treatment of diseases and conditions wherein inhibition of glucosylceramide synthase provides a benefit, like Gaucher disease and Fabry disease, also are disclosed.
Owner:RGT UNIV OF MICHIGAN
Assays for diagnosing and evaluating treatment options for Fabry disease
Provided are in vitro and in vivo methods for determining whether a patient with Fabry disease will respond to treatment with a specific pharmacological chaperone.
Owner:AMICUS THERAPEUTICS INC +1
Methods of enhancing lysosomal storage disease therapy by modulation of cell surface receptor density
InactiveUS7658916B2Promote absorptionUptake of extracellular lysosomal enzymes by cells can be increasedBiocidePeptide/protein ingredientsLysosomeFabry disease
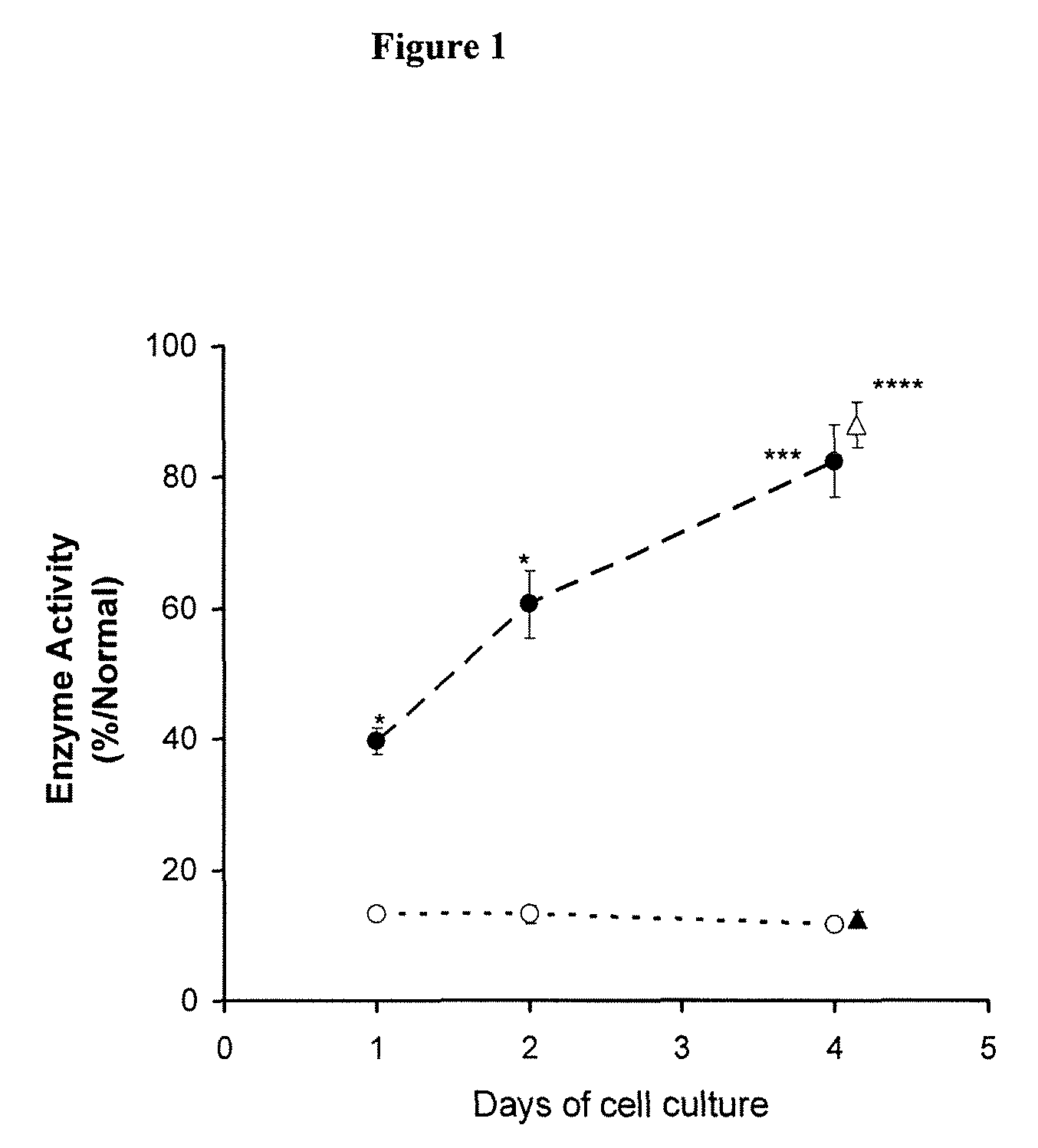
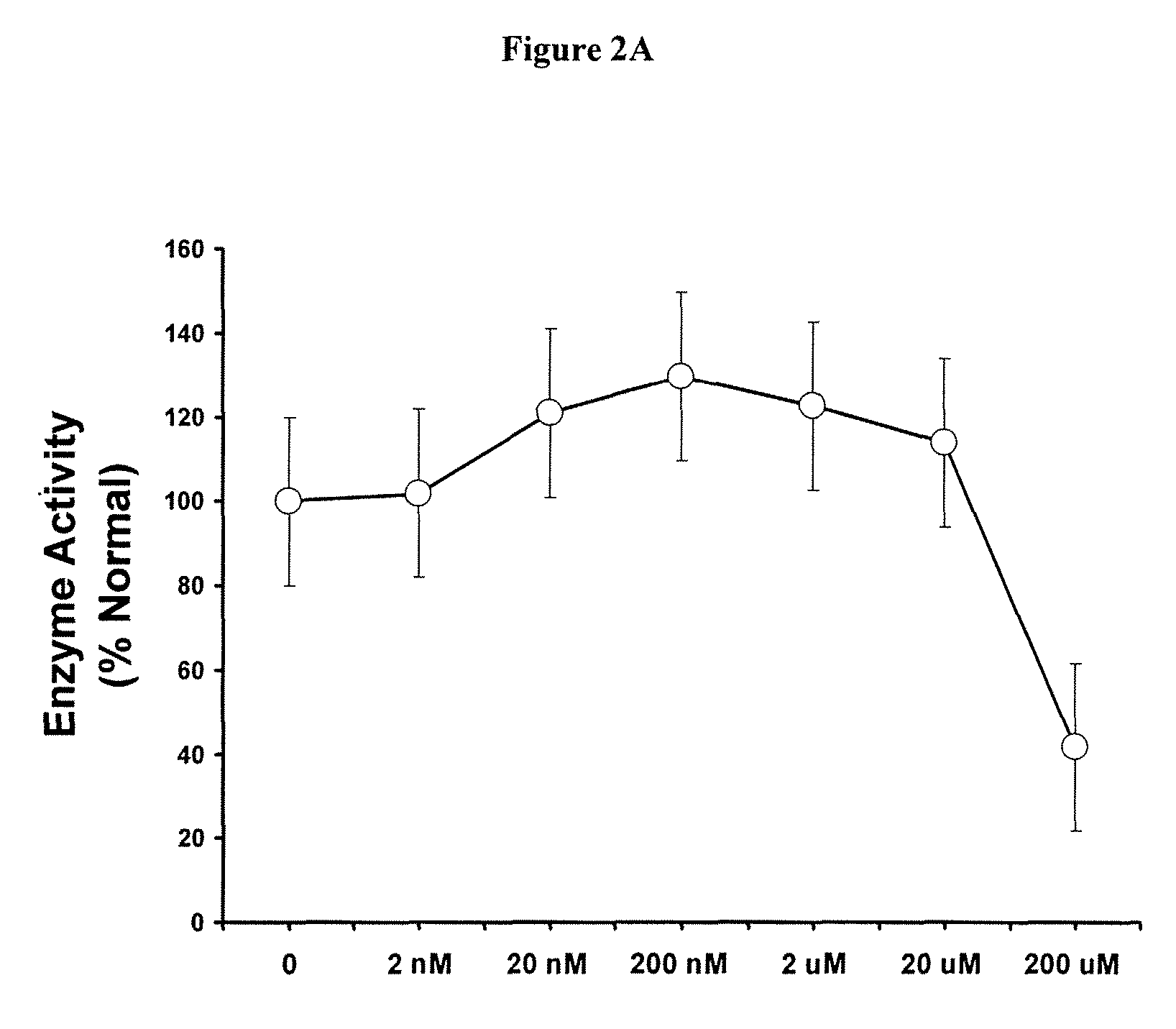
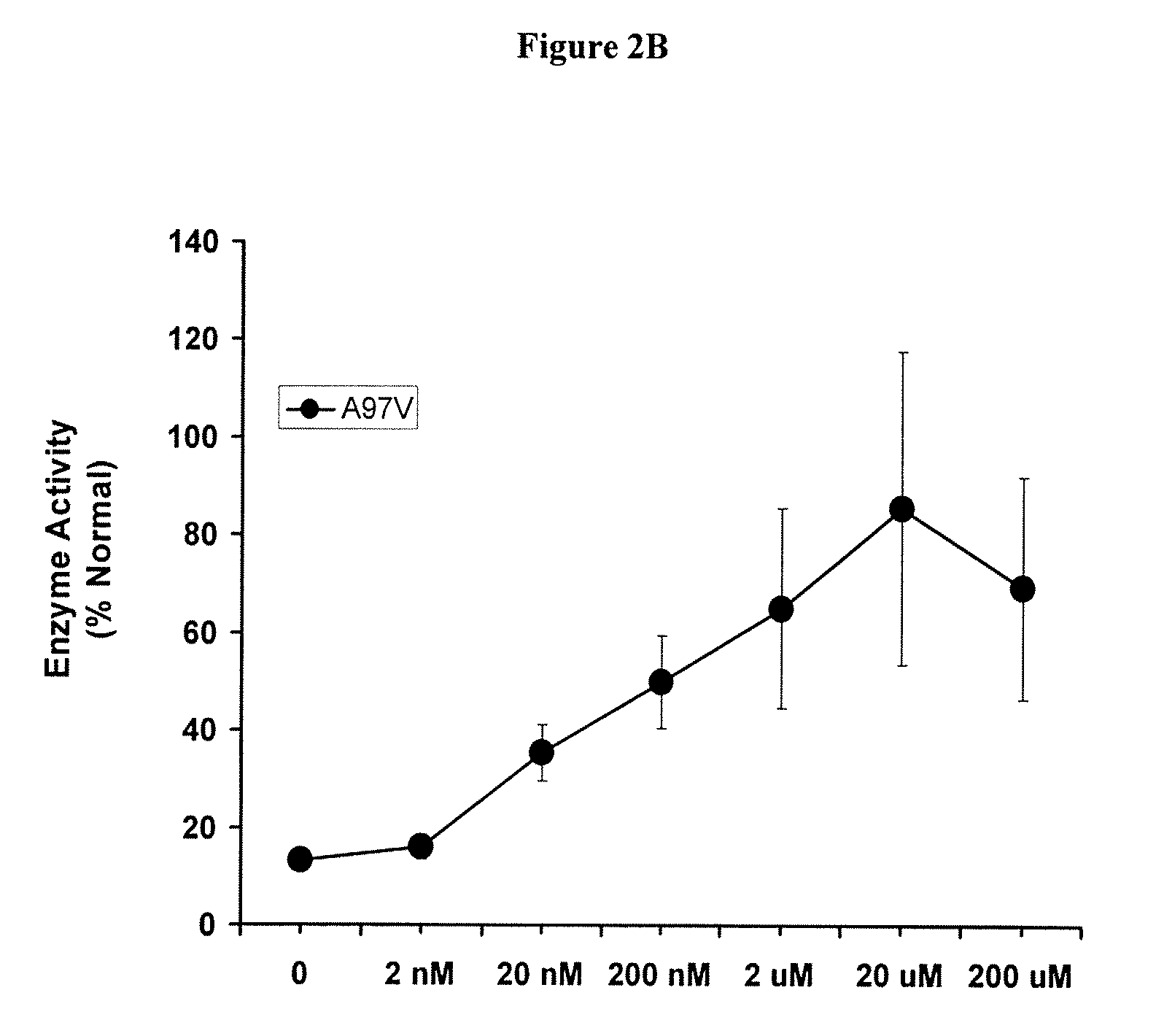
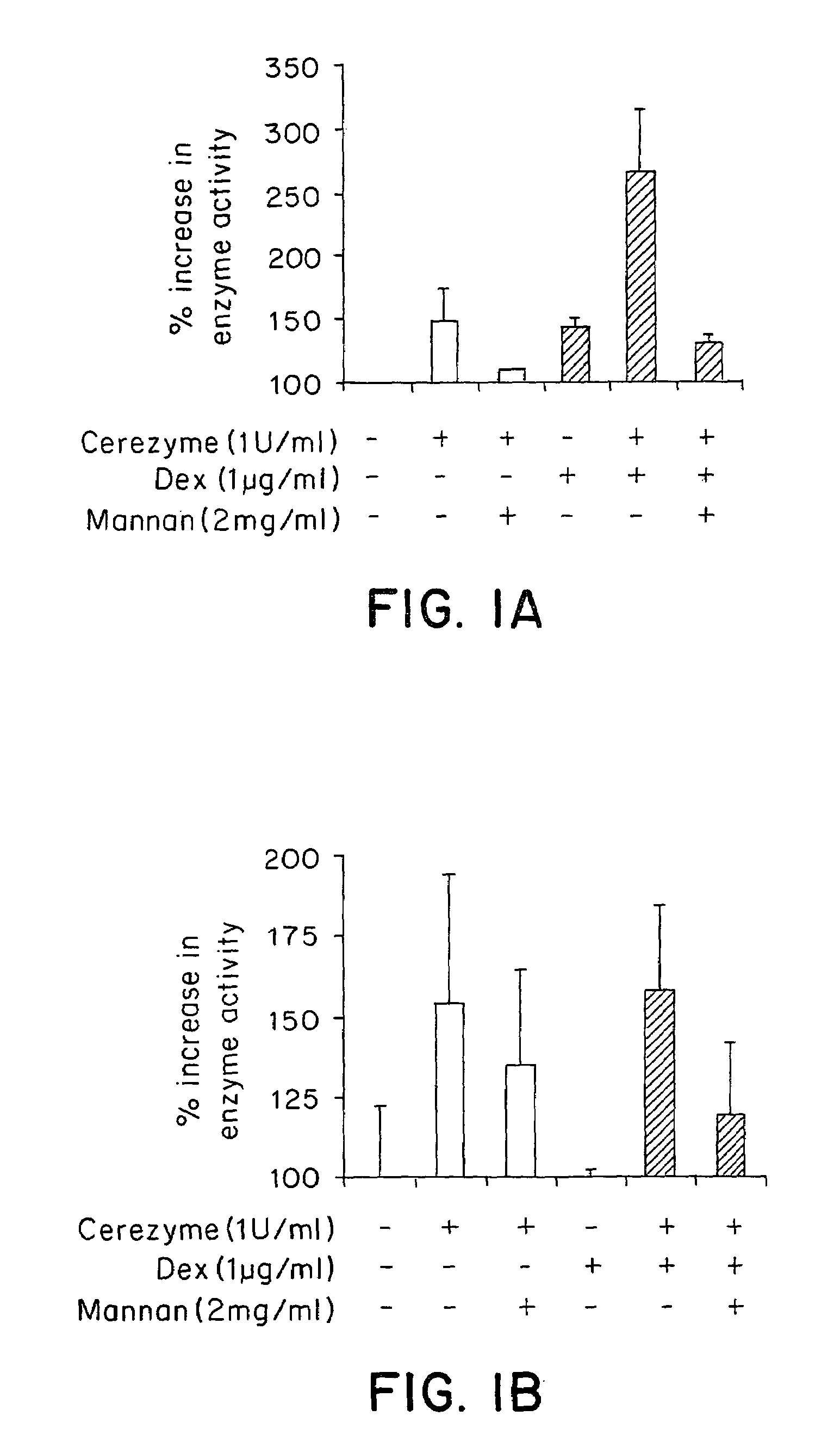
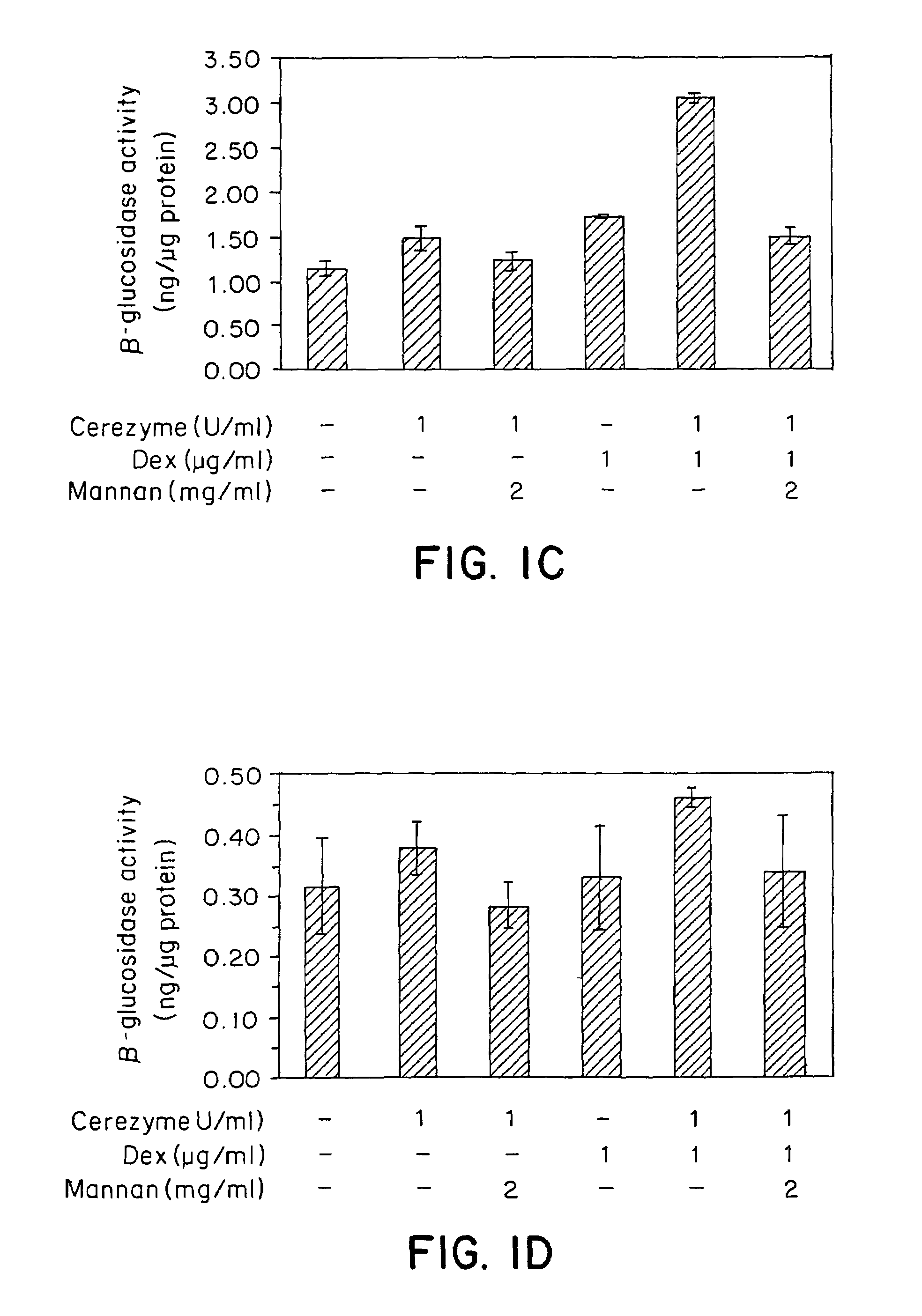
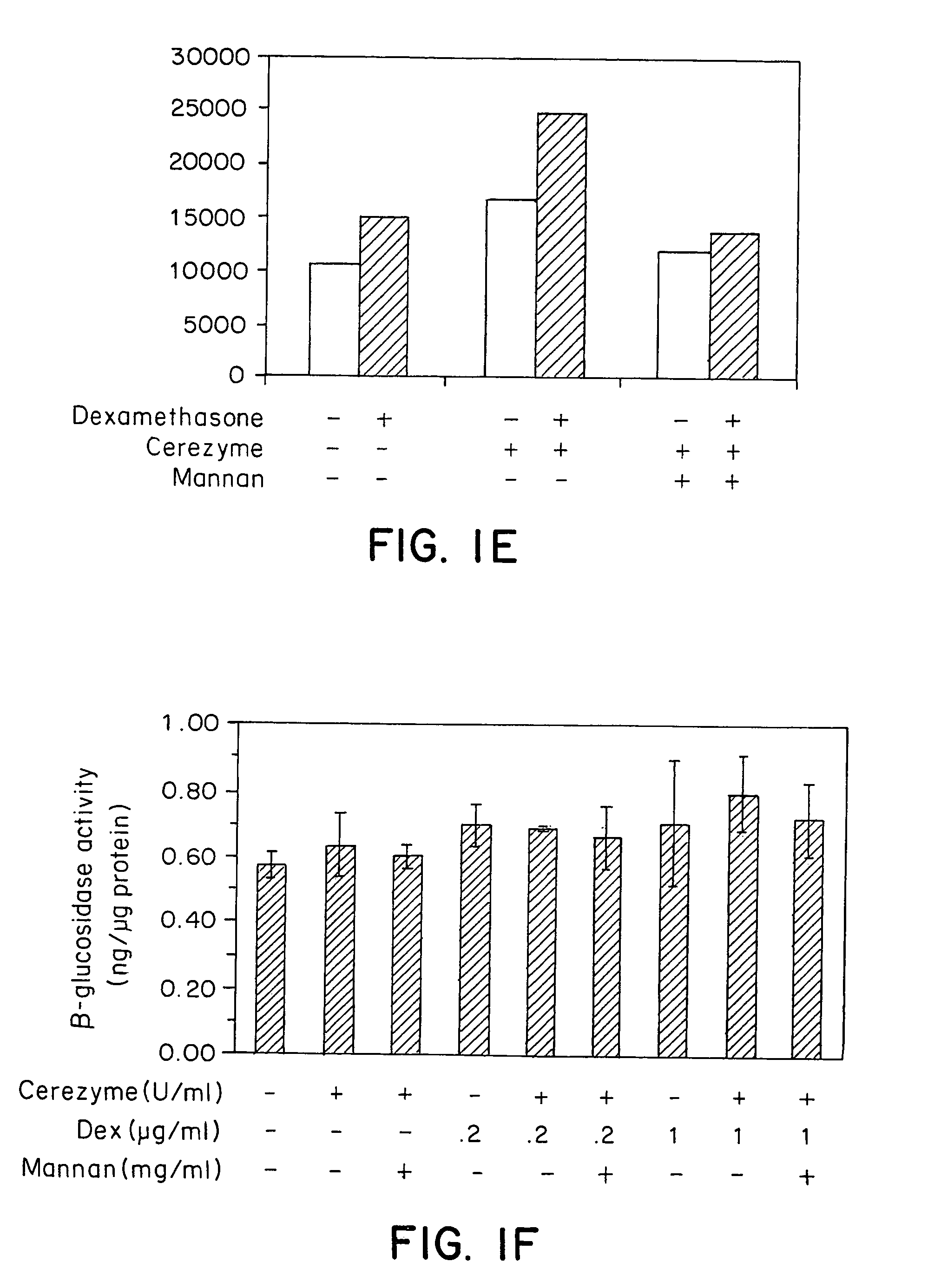
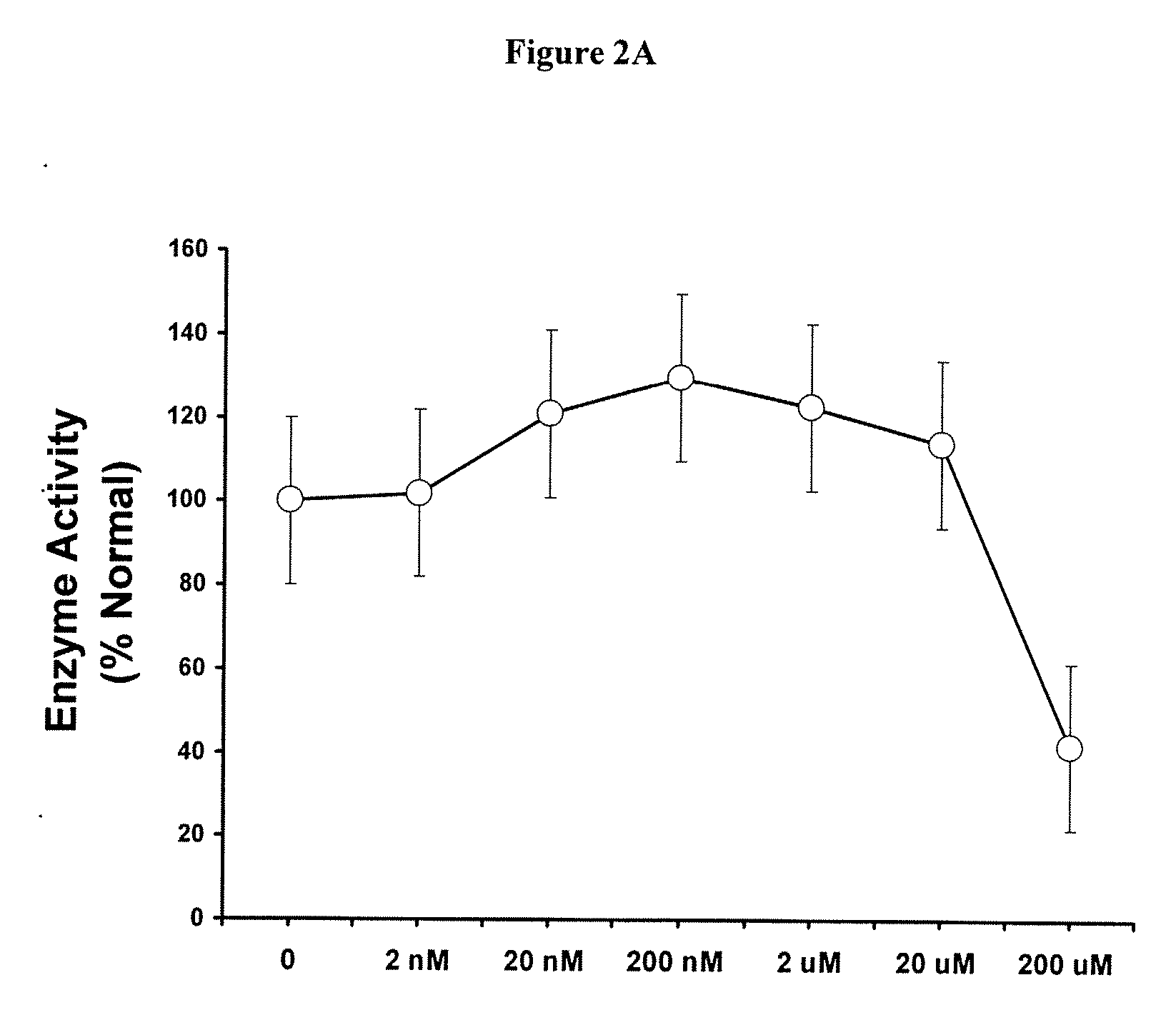
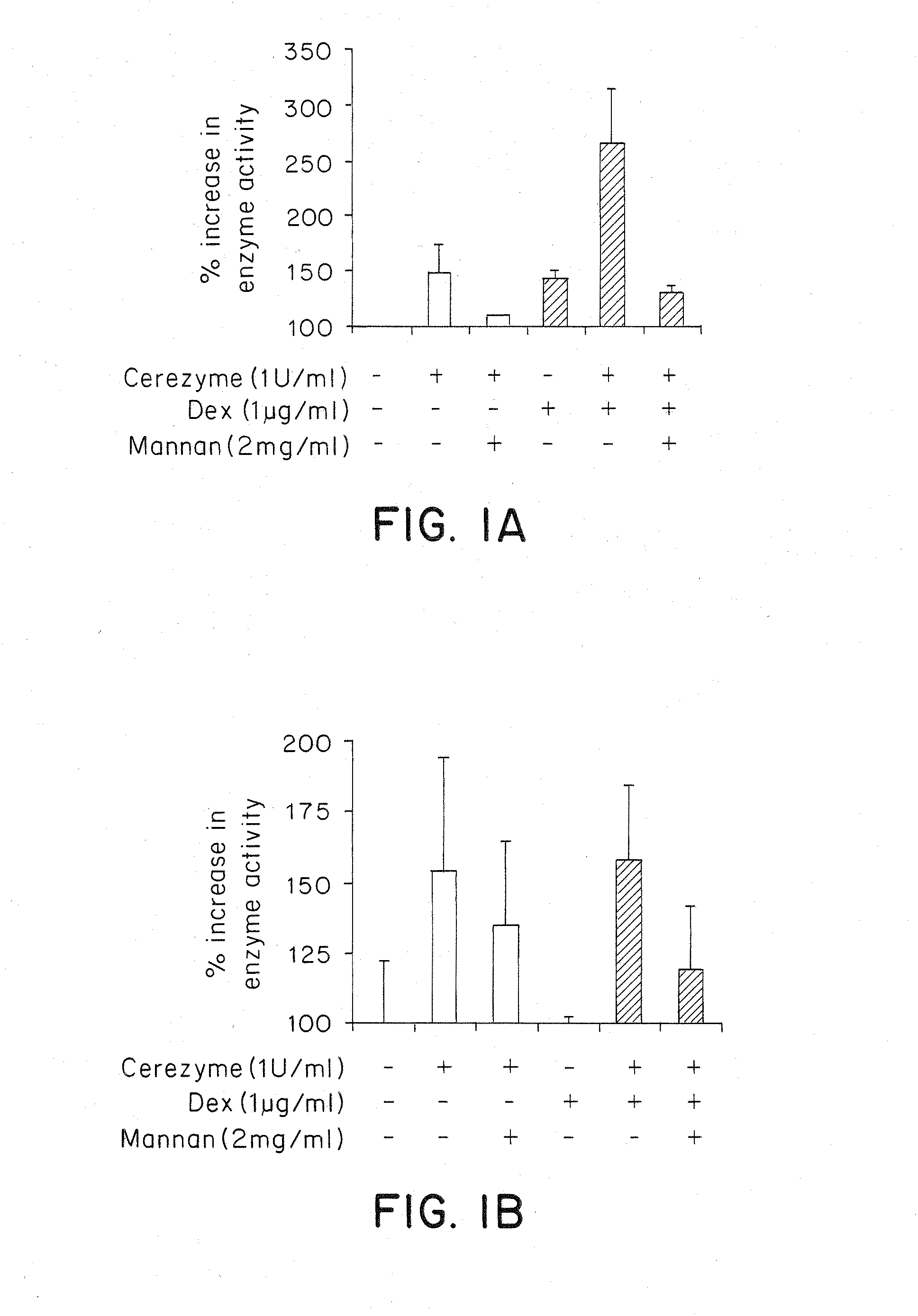
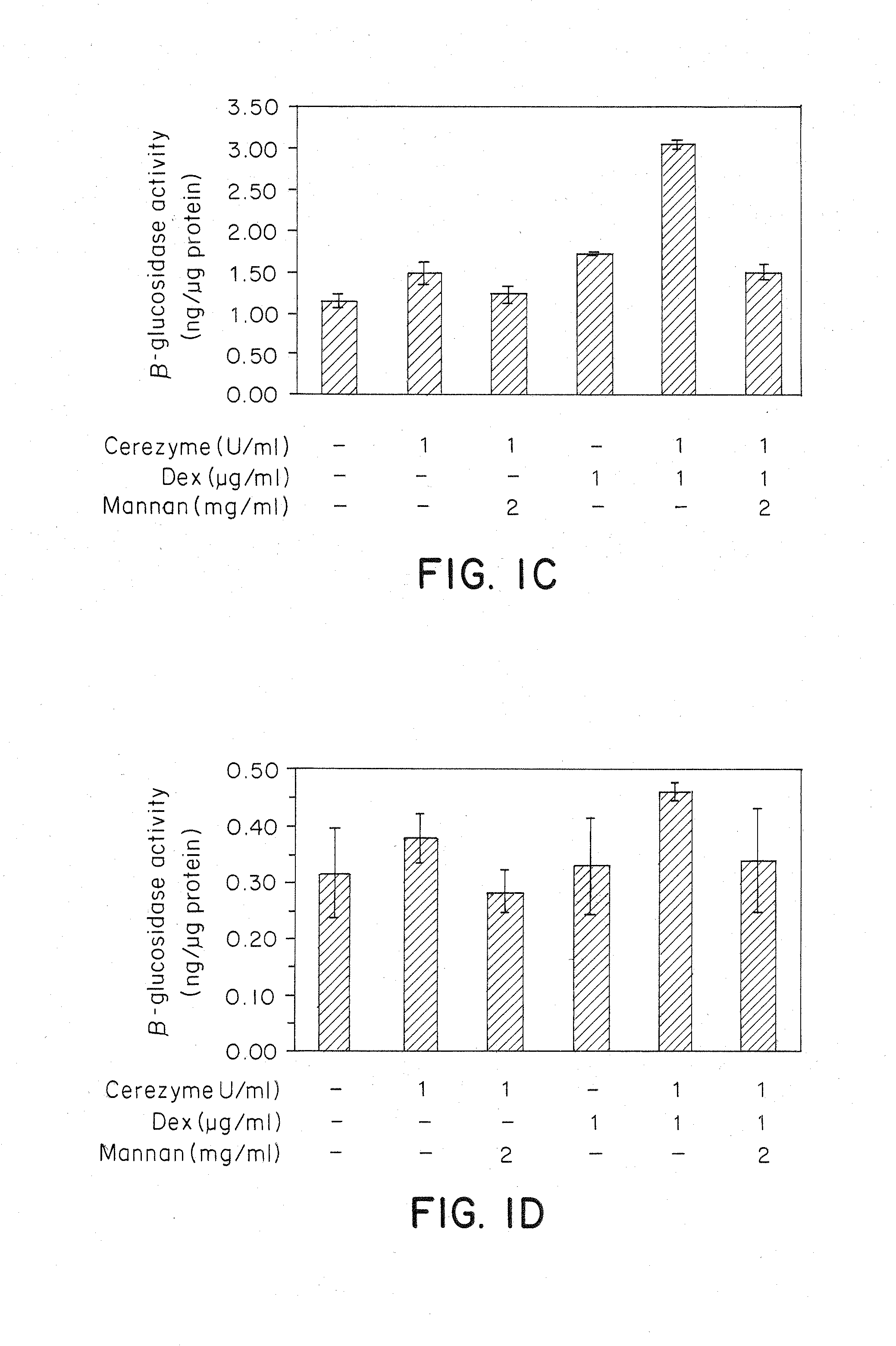
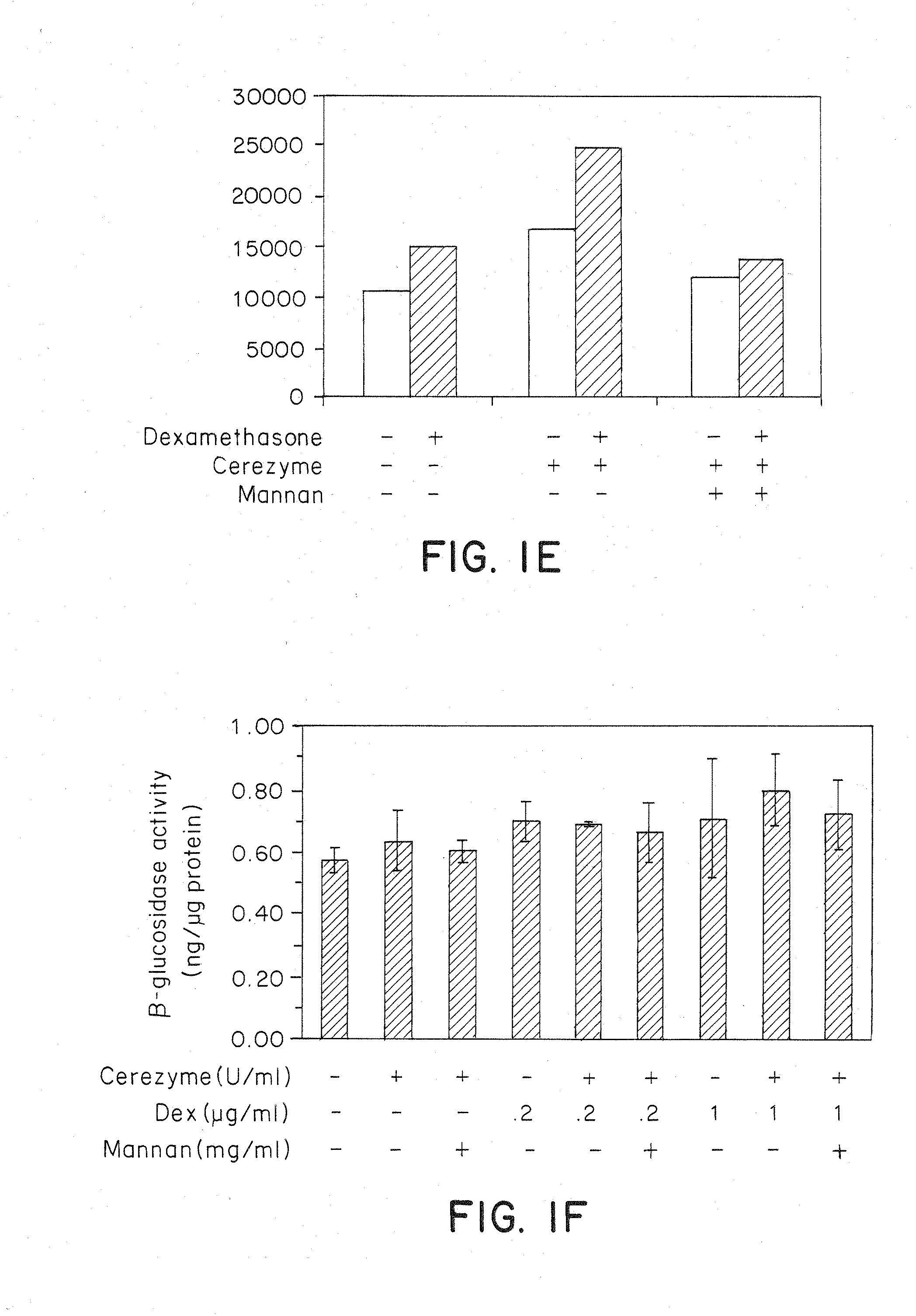
Methods of modulating uptake of extracellular lysosomal enzymes by administering a pharmaceutical agent and methods of treating a lysosomal storage disease (such as Gaucher disease, Pompe disease, Fabry disease or Niemann-Pick disease) or enhancing enzyme replacement therapy or gene therapy, comprising administering a pharmaceutical agent such as dexamethasone, glucose or insulin, are provided.
Owner:GENZYME CORP
Assays For Diagnosing And Evaluating Treatment Options For Fabry Disease
Provided are in vitro and in vivo methods for determining whether a patient with Fabry disease will respond to treatment with a specific pharmacological chaperone.
Owner:AMICUS THERAPEUTICS INC +1
Methods of enhancing lysosomal storage disease therapy by modulation of cell surface receptor density
InactiveUS20100143297A1Uptake of extracellular lysosomal enzymes by cells can be increasedIncreases the target organ uptake of glucocerebrosidaseBiocideElcosanoid active ingredientsLysosomeFabry disease
Methods of modulating uptake of extracellular lysosomal enzymes by administering a pharmaceutical agent and methods of treating a lysosomal storage disease (such as Gaucher disease, Pompe disease, Fabry disease or Niemann-Pick disease) or enhancing enzyme replacement therapy or gene therapy, comprising administering a pharmaceutical agent such as dexamethasone, glucose or insulin, are provided.
Owner:GENZYME CORP
Compositions and methods for treatment of lysosomal storage disorders
InactiveUS20110293585A1Reduces and prevents break downBiocideSugar derivativesLipid formationLysosome
Compositions and methods for treating lysosomal storage diseases are disclosed. Lysosomal dysfunction is usually the result of deficiency of a single enzyme necessary for the metabolism of lipids, glycoproteins (sugar containing proteins) or mucopolysaccharides which are fated for breakdown or recycling. The compositions contain triplex-forming molecules which can be used to induce site-specific homologous recombination in mammalian cells when combined with donor DNA molecules, by stimulating cellular DNA synthesis, recombination, and repair mechanisms. The methods are particular useful for correcting point mutations in genes associated with lysosomal storage diseases such as Gaucher's disease, Fabry disease, and Hurler syndrome. Methods for determining the frequency of target gene repair and assessing the restoration of the enzymatic activity of corrected polypeptides are also disclosed. Ex vivo and in vivo methods of gene correction in patients are also provided.
Owner:YALE UNIV
Stabilized alpha-galactosidase and uses thereof
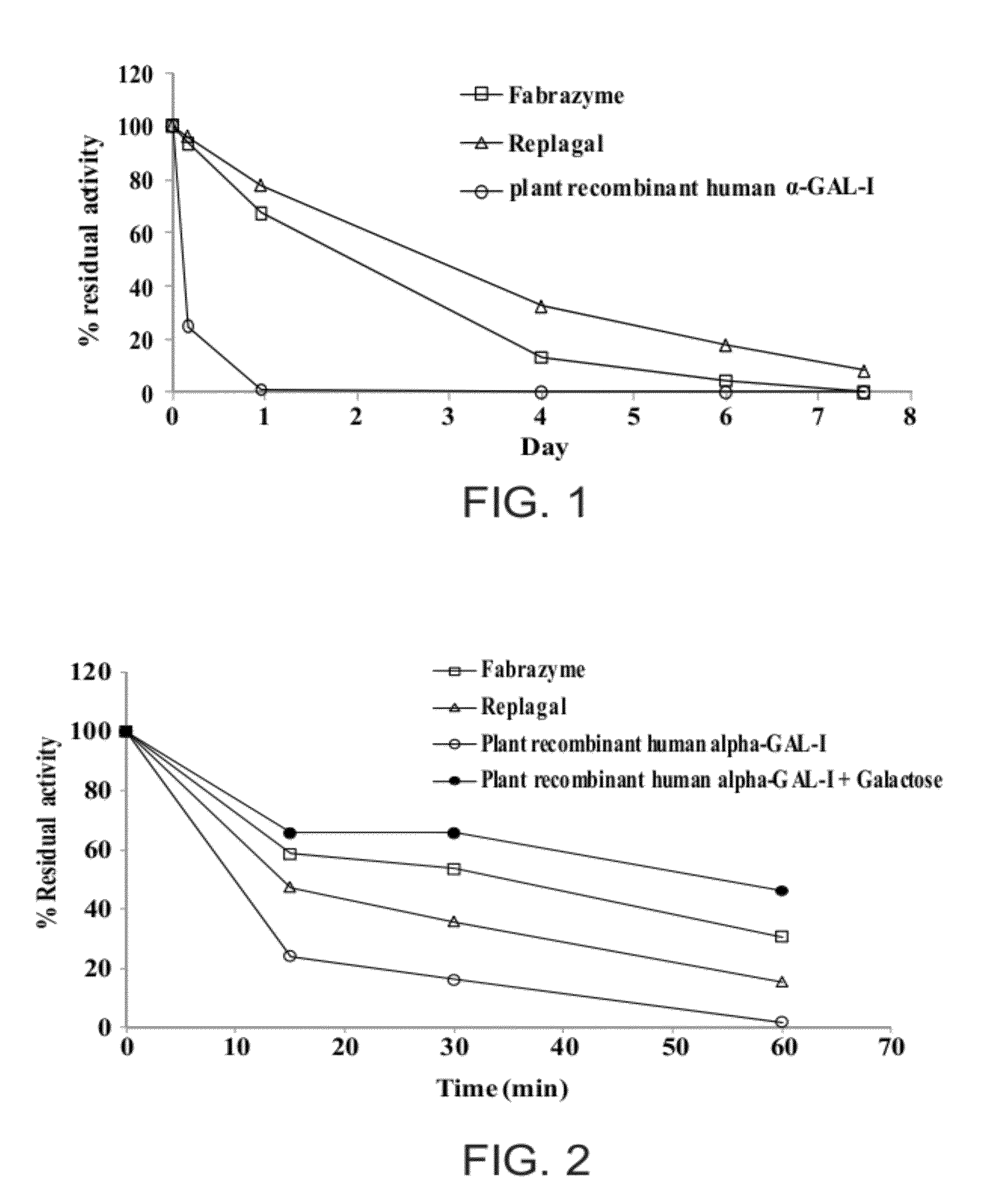
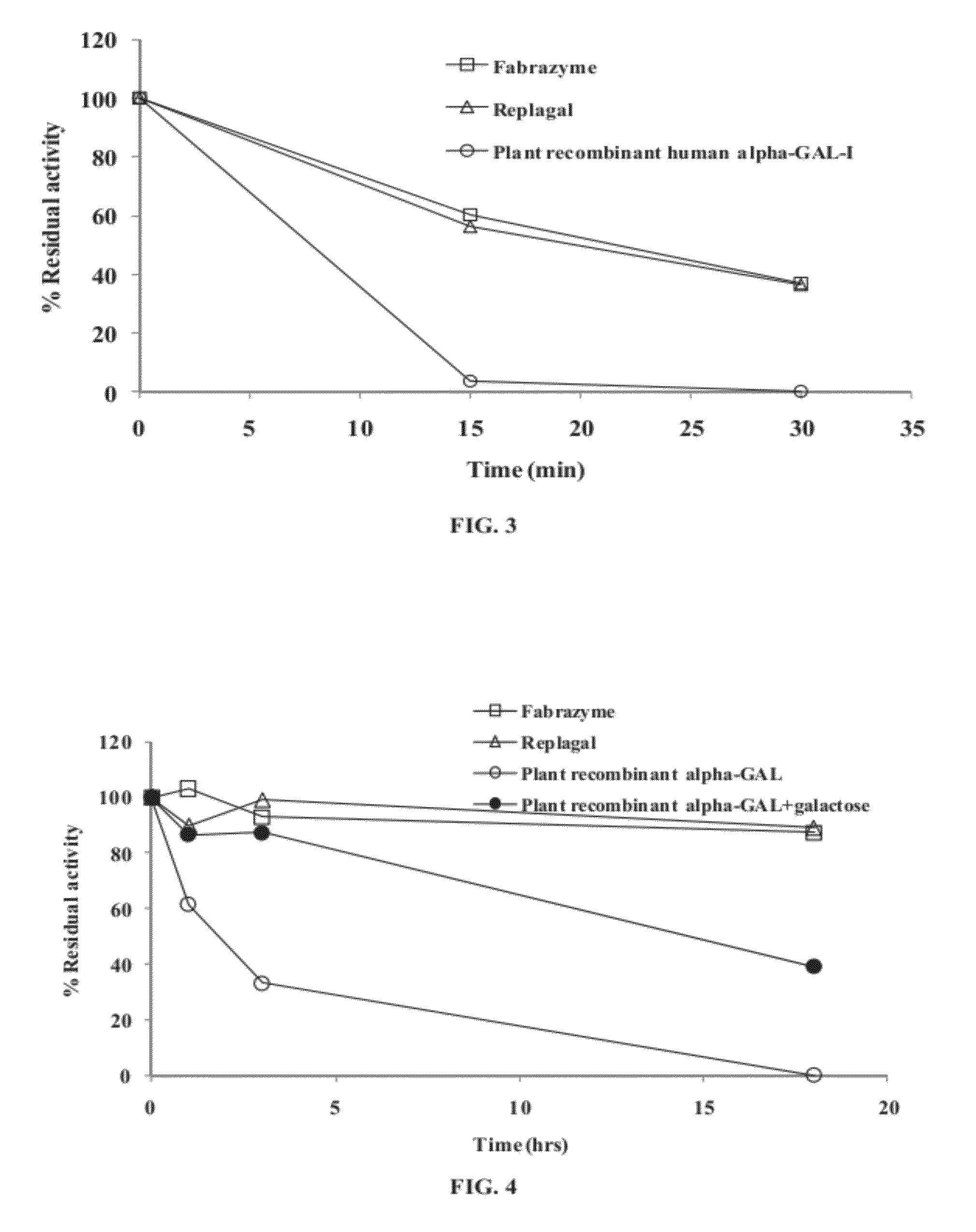
Multimeric protein structures comprising at least two alpha-galactosidase monomers being covalently linked to one another via a linking moiety are disclosed herein, as well a process for preparing same, and methods of treating Fabry disease via administration of a multimeric protein structure. The disclosed multimeric protein structures exhibit an improved performance, in terms of enhanced activity and / or a longer lasting activity under both lysosomal conditions and in a serum environment.
Owner:PROTALIX
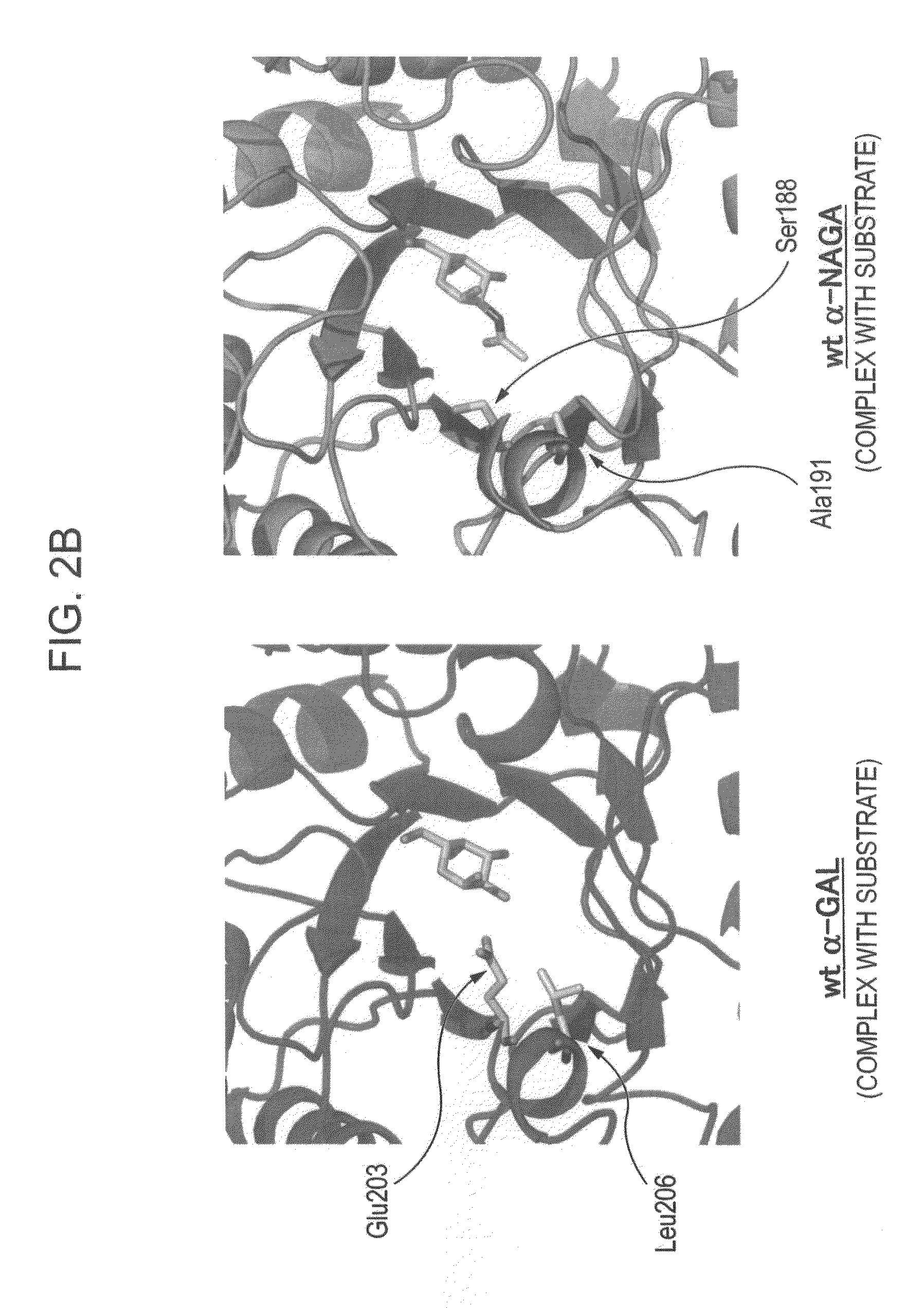
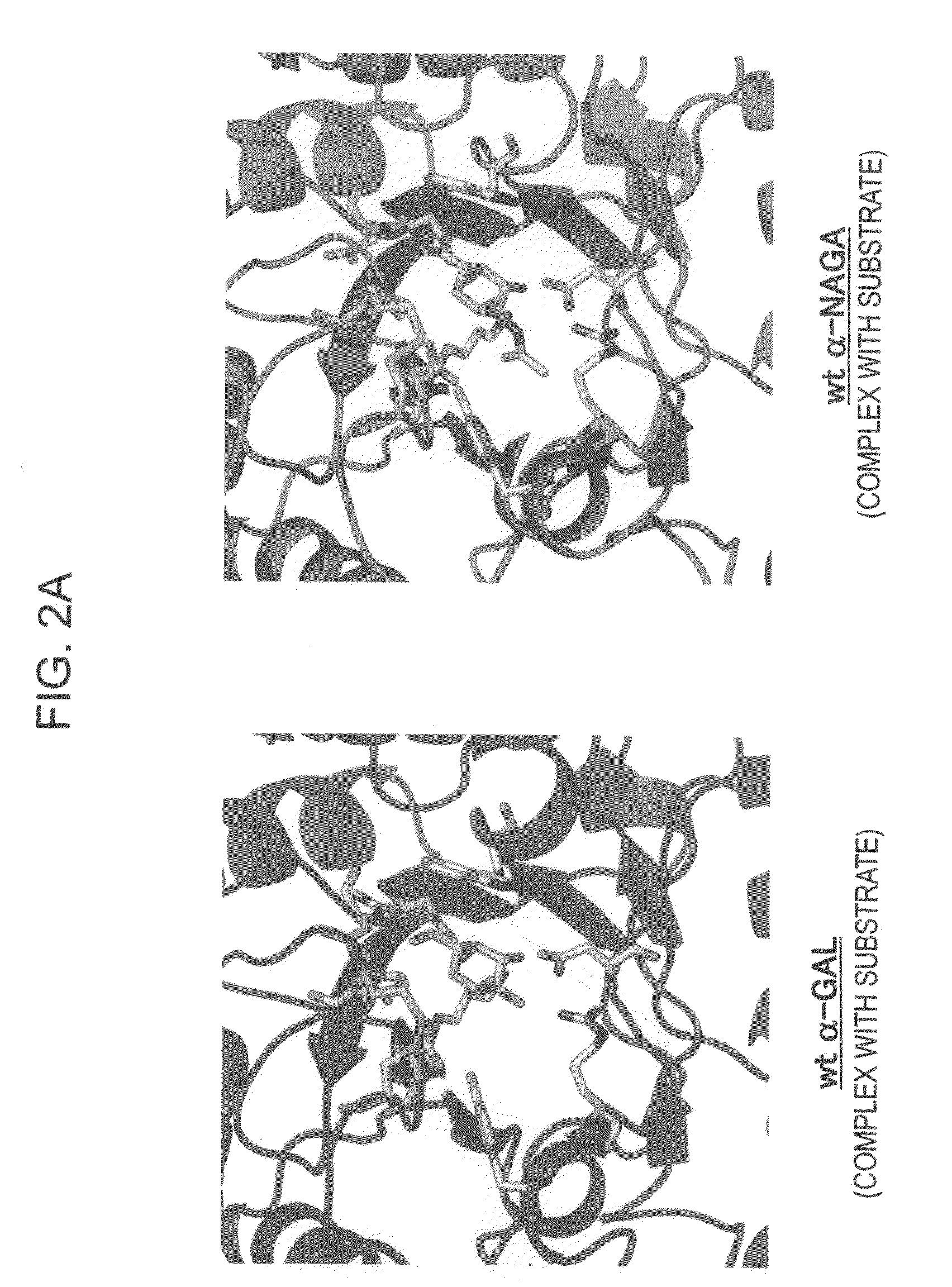
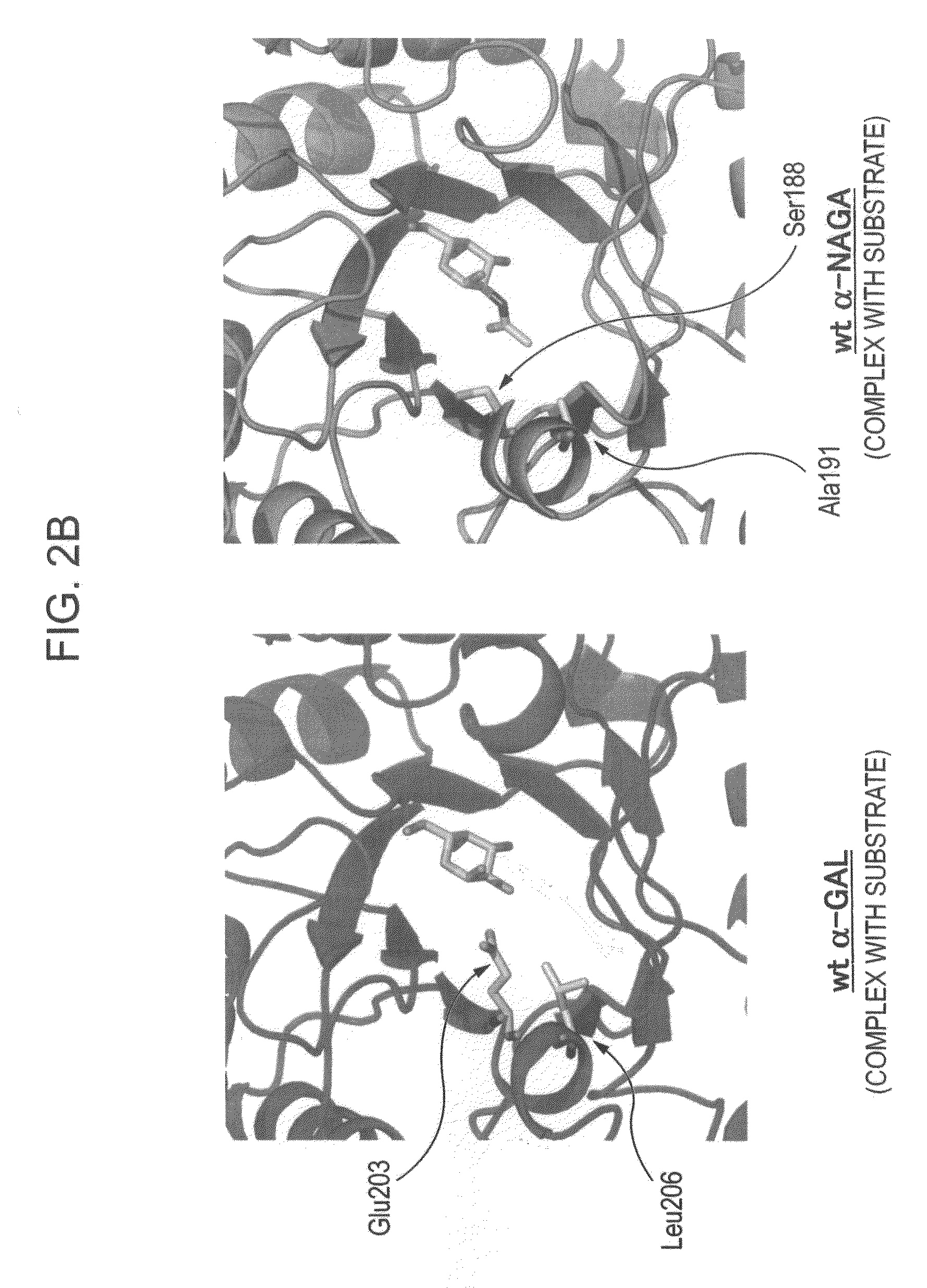
Highly functional enzyme having α-galactosidase activity
ActiveUS7935336B2Improve stabilityEasily incorporated into cellSenses disorderNervous disorderSide effectFabry disease
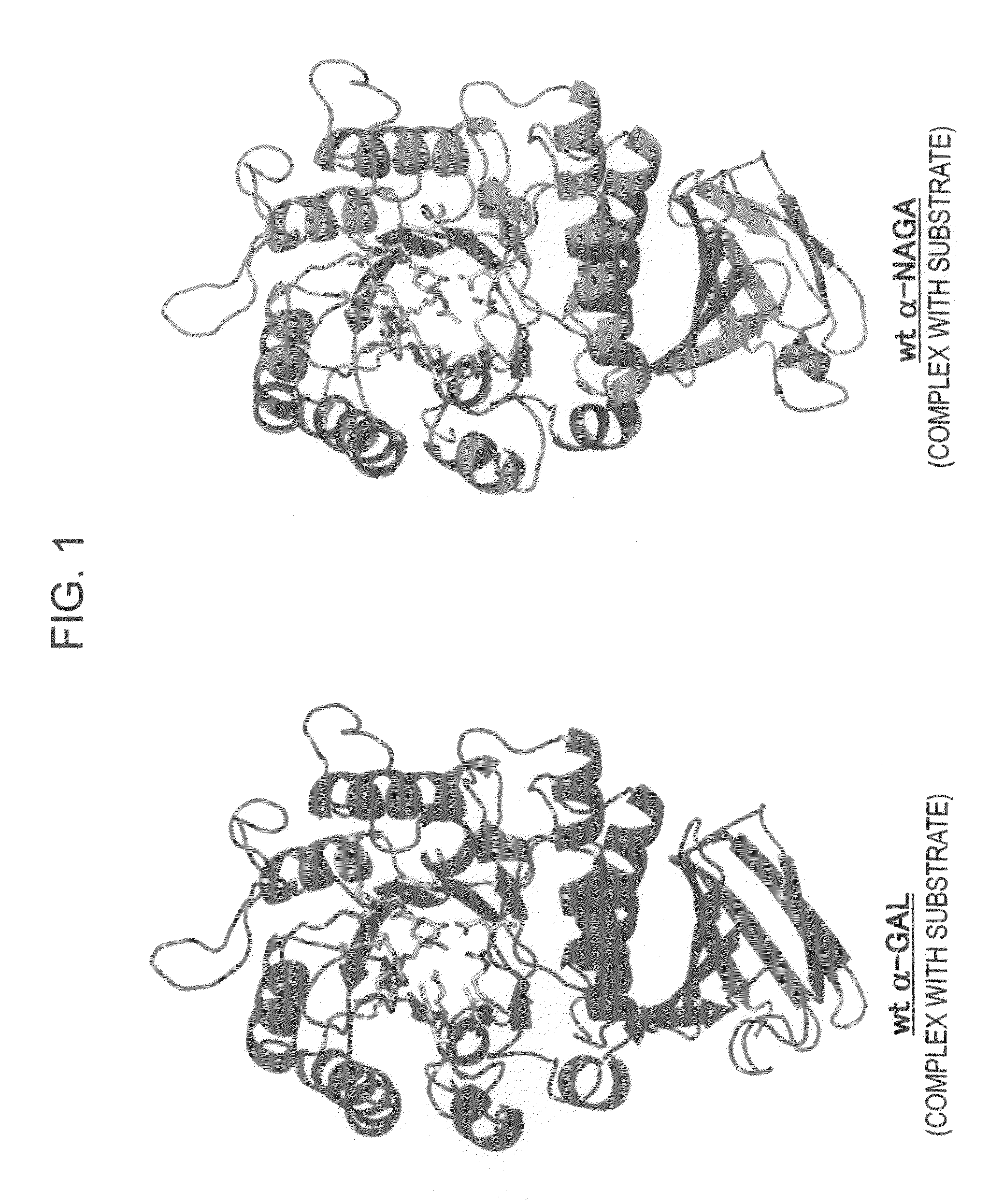
The present invention provides, as an enzyme which can be used for enzyme replacement therapy for Fabry disease, a protein having α-galactosidase activity, which shows no allergic adverse side effect, shows a high stability in blood, and can be easily incorporated into a cell of an affected organ. The protein of the present invention is a protein which has acquired α-galactosidase activity by changing the structure of the active site of wild-type human α-N-acetylgalactosaminidase.
Owner:ALTIF LAB
Quantitation of gl3 in urine
InactiveUS20120283290A1Quantitative precisionReduce restrictionsBiocideSugar derivativesBacteriuriaFabry disease
The present invention is directed to the quantitation of GL3 in human urine which can be used for the diagnosis of Fabry disease as well as for the assessment of treatment efficacy thereof.
Owner:AMICUS THERAPEUTICS INC
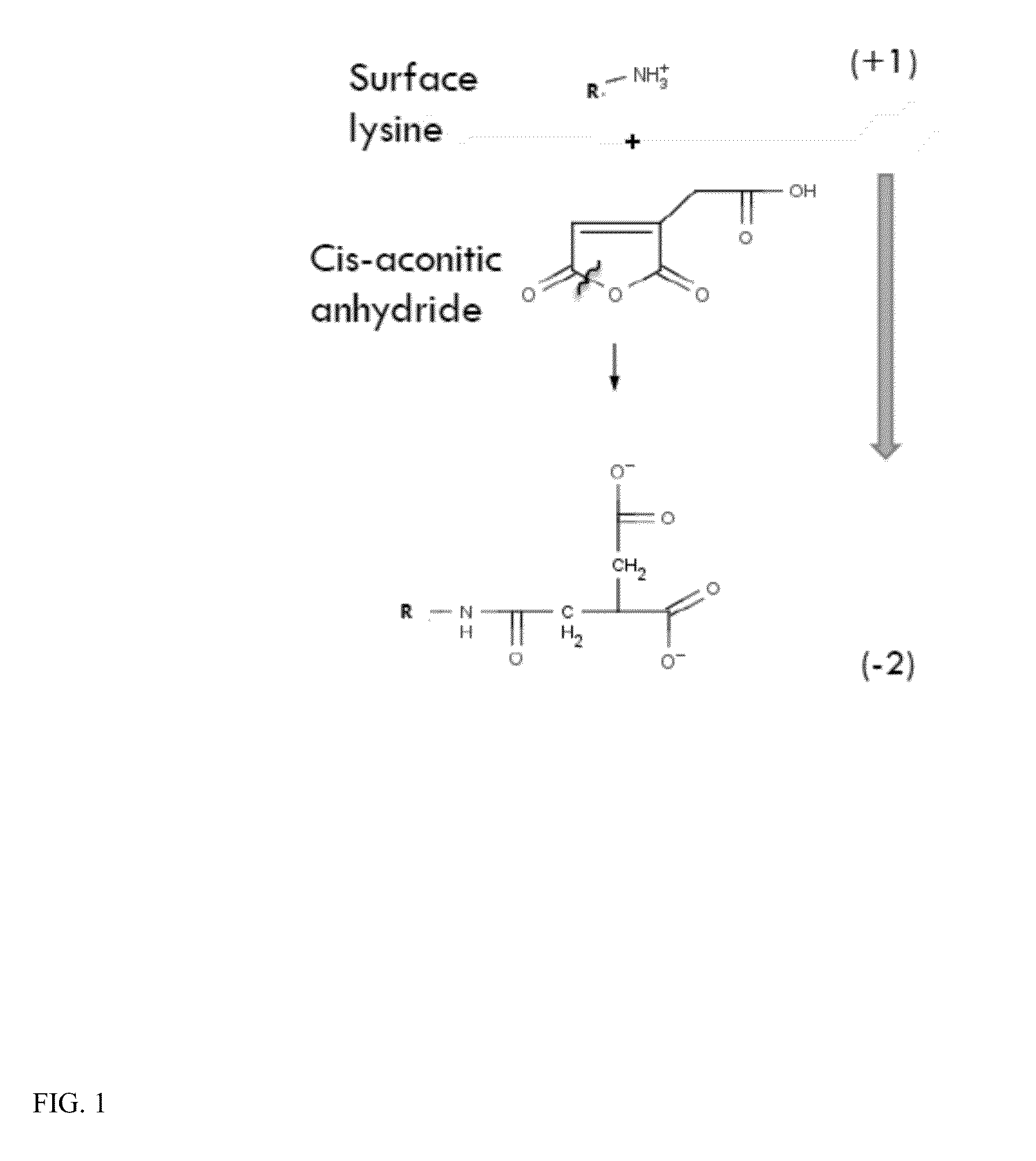
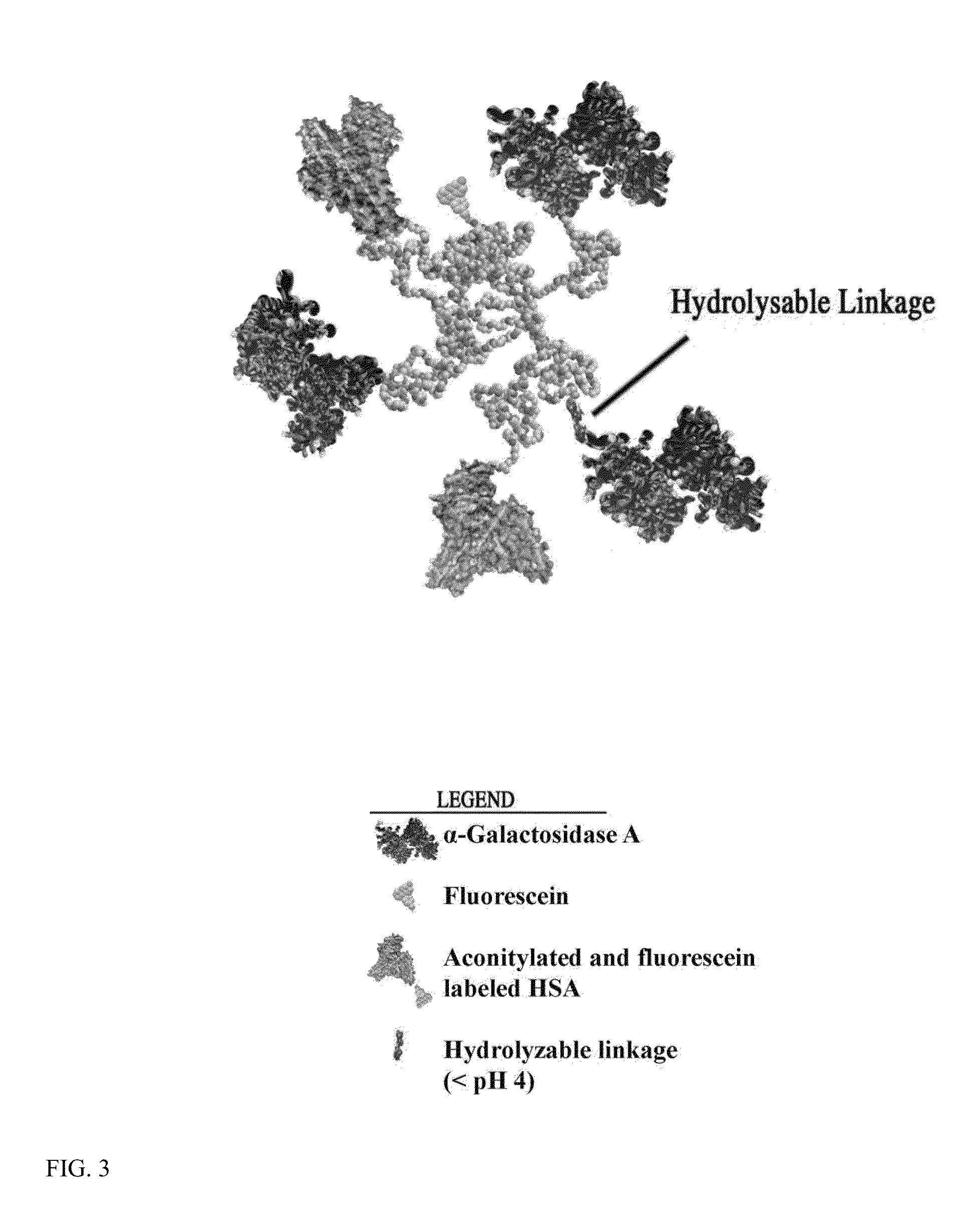
Scavenger receptor uptake for fabry disease enzyme replacement therapy
ActiveUS20140050666A1Ultrasonic/sonic/infrasonic diagnosticsBiocideScavenger receptor ligandLysosome
The present invention relates to a composition comprising a lysosomal enzyme conjugated to a negatively charged scavenger receptor ligand. In some embodiments, the lysosomal enzyme is conjugated to the scavenger receptor ligand by way of a linker. The present invention also relates to a composition comprising lysosomal enzyme encapsulated by a liposome, said liposome externally comprising a negatively charged scavenger receptor ligand. The invention further encompasses a method of treating a lysosomal storage disease with the compositions listed above. The invention further encompasses a method of treating a lysosomal storage disease with an acylated, acetylated, or aconitylated lysosomal enzyme.
Owner:RES FOUND THE CITY UNIV OF NEW YORK
Methods and compositions for delivering enzymes and nucleic acid molecules to brain, bone and other tissues
Disclosed are methods for delivering an enzyme to a subject's brain or bone. The methods include administering a hyaluronidase to the subject and administering the enzyme to the subject. The hyaluronidase and the enzyme are administered to the subject under conditions effective to deliver the enzyme to the subject's brain or bone. Compositions and kits which include hyaluronidase and an enzyme are also disclosed, as are methods for increasing blood-brain barrier permeability in a subject. Also disclosed are methods, compositions, and kits for delivering genes or other nucleic acid molecules to a subject's brain or bone, as well as methods, compositions, and kits for delivering enzymes to a subject's tissues. The methods, compositions, and kits are disclosed as being useful in treating or preventing a variety of enzyme deficiency diseases, such as those affecting brain and / or bone, e.g., as Canavan's disease, Fabry disease, Gaucher's disease, various forms of mucopolysaccharidosis (e.g., Hurler's syndrome, Scheie syndrome, Hurler-Scheie syndrome, Sanfillippo A syndrome, Morquio A syndrome, Morquio B syndrome, etc.), Niemann-Pick disease, Schindler disease, and Pompe disease.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Methods and oral formulations for enzyme replacement therapy of human lysosomal and metabolic diseases
InactiveUS20160346363A1Improve the quality of lifeReduce conveniencePeptide/protein ingredientsMetabolism disorderAcid alpha-glucosidaseLysosome
The invention provides methods, compositions and kits for enzyme replacement therapy as well as molecular constructs, cells, tissues and plants suitable for expressing recombinant enzymes. Similarly, the invention provides methods for recombinantly producing and orally administering certain metabolic or lysosomal enzymes such as acid alpha glucosidase (GAA) alone, in a pharmaceutical composition or with an activator protein (AGA). Also, the invention provides methods for treating a glycogen storage disease type II (GSDII) or acid maltase deficiency (AMD) or Pompe disease or Fabry disease.
Owner:NEW YORK UNIV
Proteins having acquired A-galactosidase activity
ActiveUS8569032B2Easy to carryAltered substrate specificitySenses disorderSugar derivativesSide effectWild type
The present invention provides a pharmaceutical composition comprising a protein having α-galactosidase activity for treating Fabry disease, which causes no allergic side effect, which is highly stable in blood (plasma) and which can readily be taken up by a cell of an affected organ. The pharmaceutical composition for treating Fabry disease of the invention comprises, for example, a protein which acquires an α-galactosidase activity through alteration of the structure of the active site of wild-type human α-N-acetylgalactosaminidase.
Owner:ALTIF LAB +1
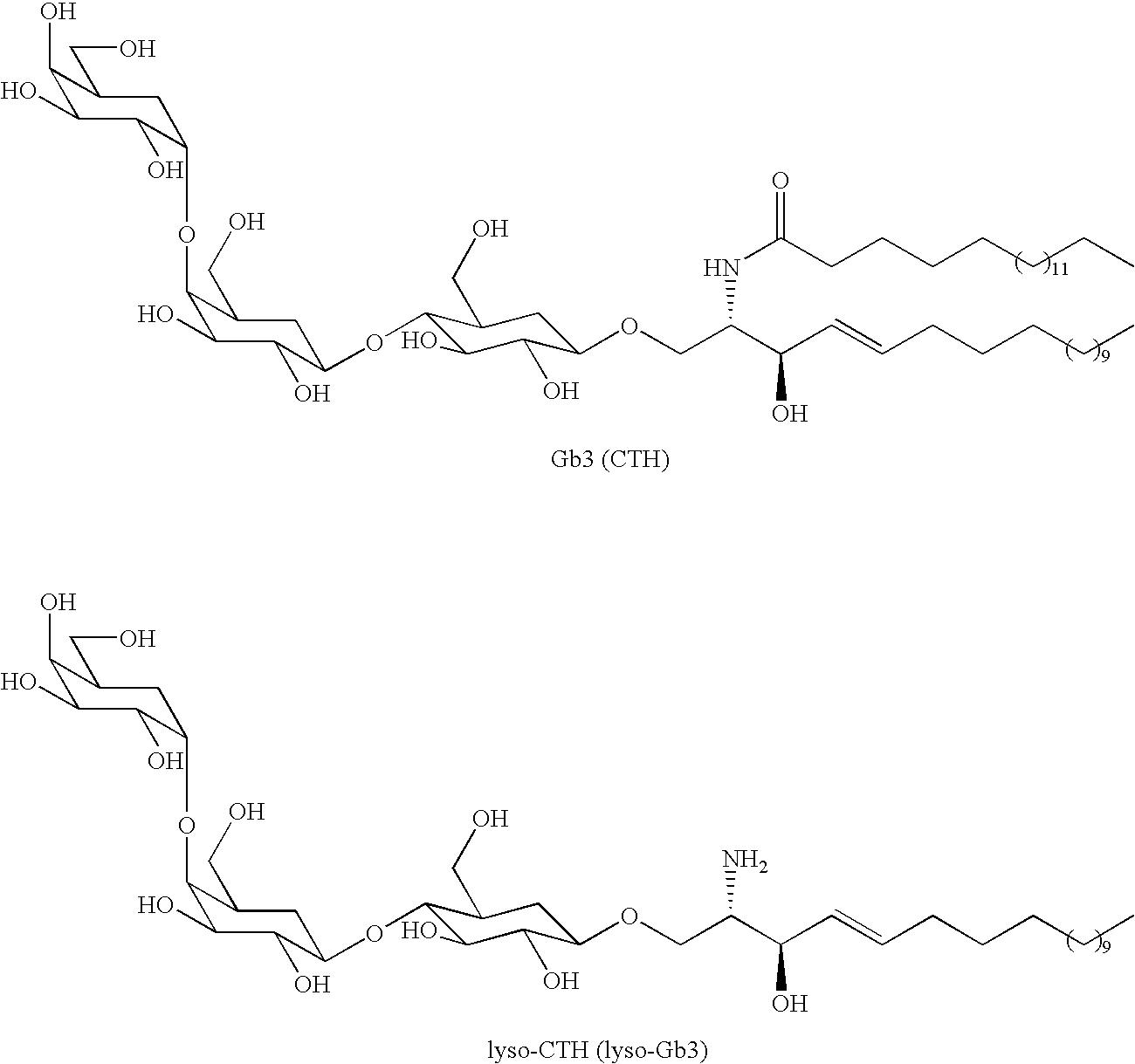
Diagnostic marker for fabry disease
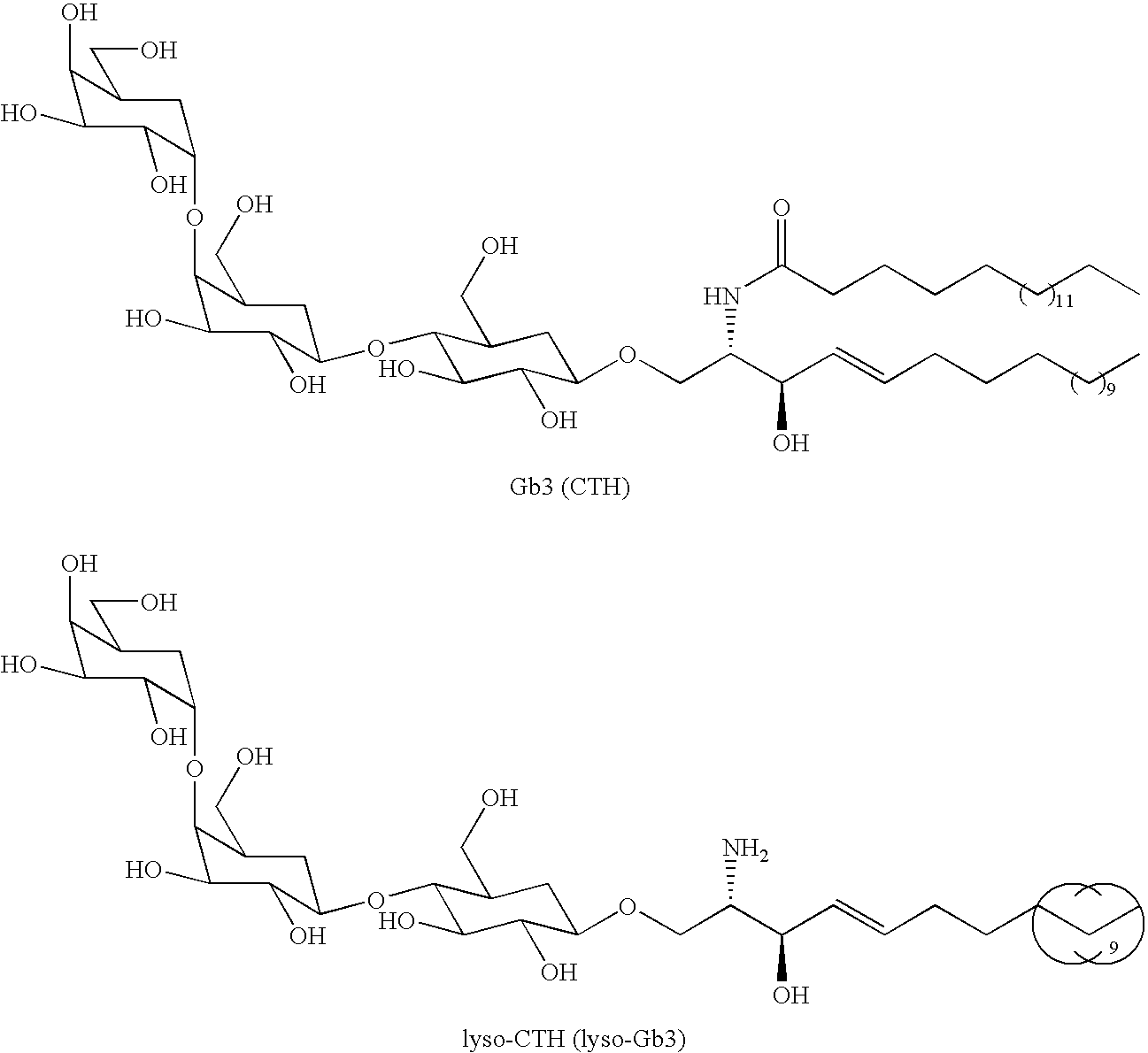
InactiveUS20100047844A1Sugar derivativesMicrobiological testing/measurementFabry diseaseCeramide Trihexosides
The present invention is in the field of Fabry disease and concerns a pathogenic factor allowing diagnosis of Fabry disease. In particular lyso-ceramide trihexosamide (lyso-CTH) has been found to function as a diagnostic marker for Fabry disease.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Glucosylceramide synthase inhibitors and therapeutic methods using the same
Glucosylceramide synthase inhibitors and compositions containing the same are disclosed. Methods of using the glucosylceramide synthase inhibitors in the treatment of diseases and conditions wherein inhibition of glucosylceramide synthase provides a benefit, like Gaucher disease and Fabry disease, also are disclosed.
Owner:RGT UNIV OF MICHIGAN
Glucosylceramide synthase inhibitors and therapeutic methods using the same
Glucosylceramide synthase inhibitors and compositions containing the same are disclosed. Methods of using the glucosylceramide synthase inhibitors in the treatment of diseases and conditions wherein inhibition of glucosylceramide synthase provides a benefit, like Gaucher disease and Fabry disease, also are disclosed.
Owner:RGT UNIV OF MICHIGAN
Stabilized alpha-galactosidase and uses thereof
Multimeric protein structures comprising at least two alpha-galactosidase monomers being covalently linked to one another via a linking moiety are disclosed herein, as well a process for preparing same, and methods of treating Fabry disease via administration of a multimeric protein structure. The disclosed multimeric protein structures exhibit an improved performance, in terms of enhanced activity and / or a longer lasting activity under both lysosomal conditions and in a serum environment.
Owner:普罗塔里克斯有限公司
Novel Highly Functional Enzyme Having Modified Substrate-Specificity
ActiveUS20100166728A1Improve stabilityEasily incorporated into cellSenses disorderNervous disorderFabry diseaseActive site
The present invention provides, as an enzyme which can be used for enzyme replacement therapy for Fabry disease, a protein having α-galactosidase activity, which shows no allergic adverse side effect, shows a high stability in blood, and can be easily incorporated into a cell of an affected organ. The protein of the present invention is a protein which has acquired α-galactosidase activity by changing the structure of the active site of wild-type human α-N-acetylgalactosaminidase.
Owner:ALTIF LAB
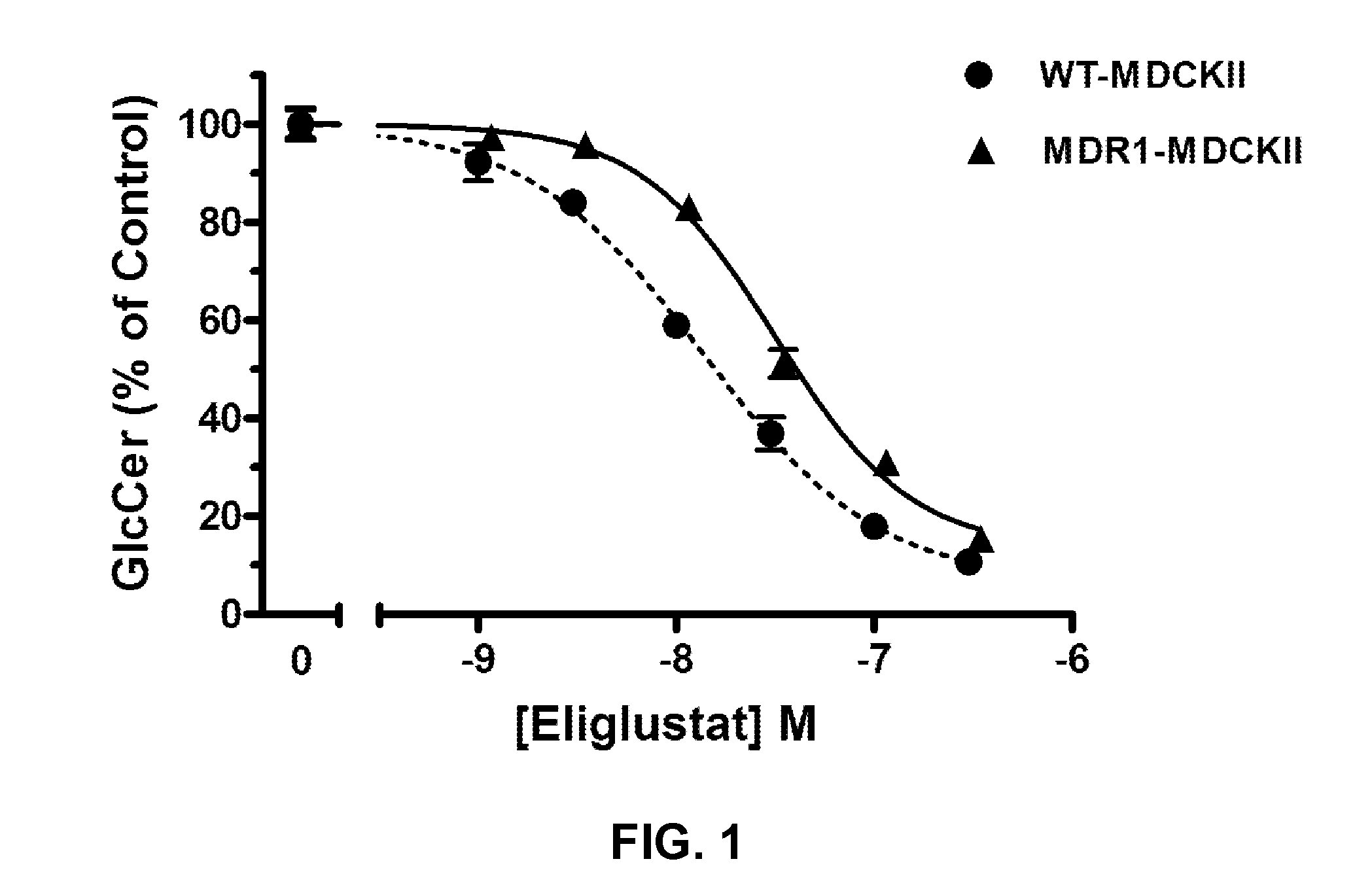
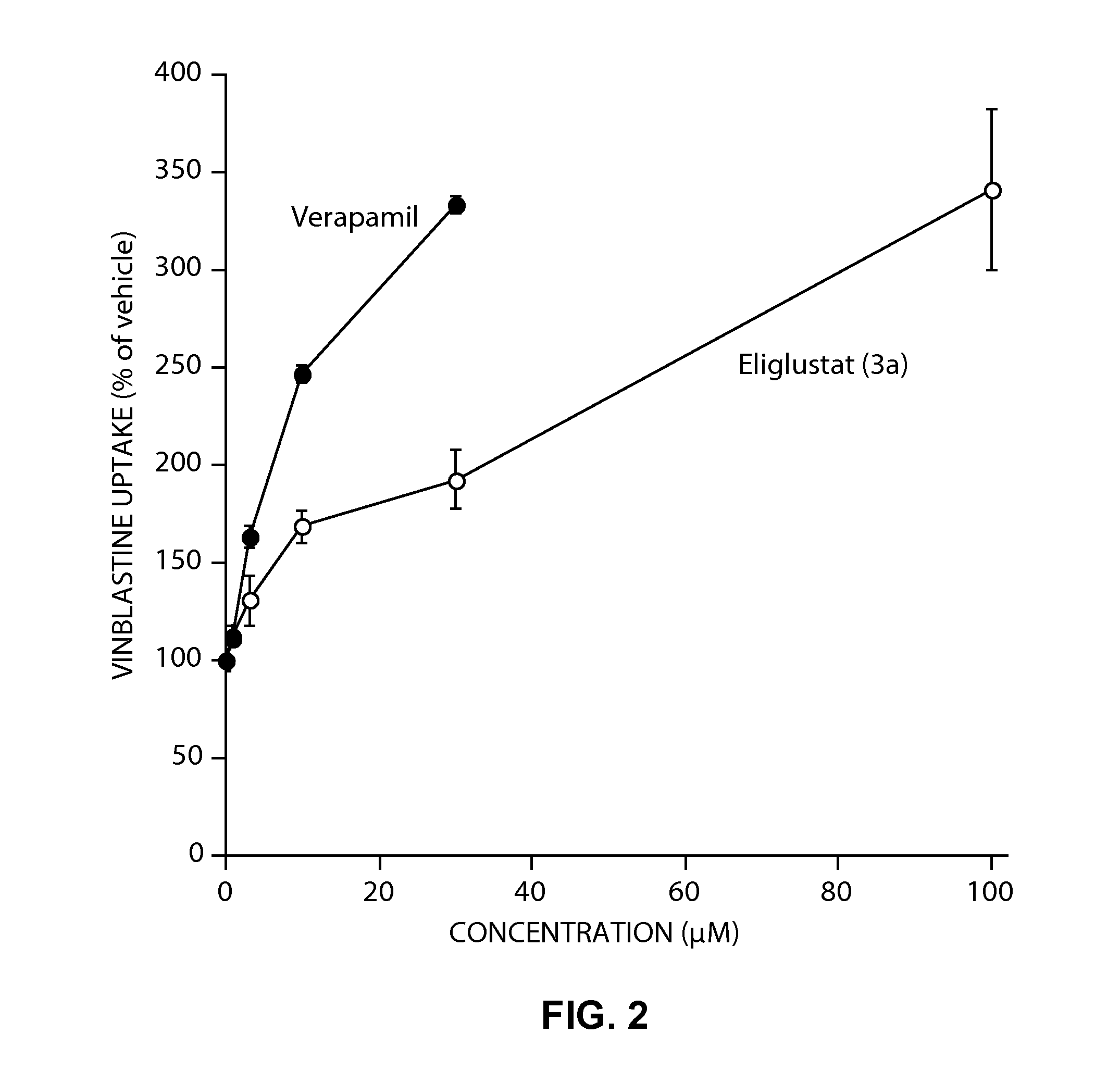
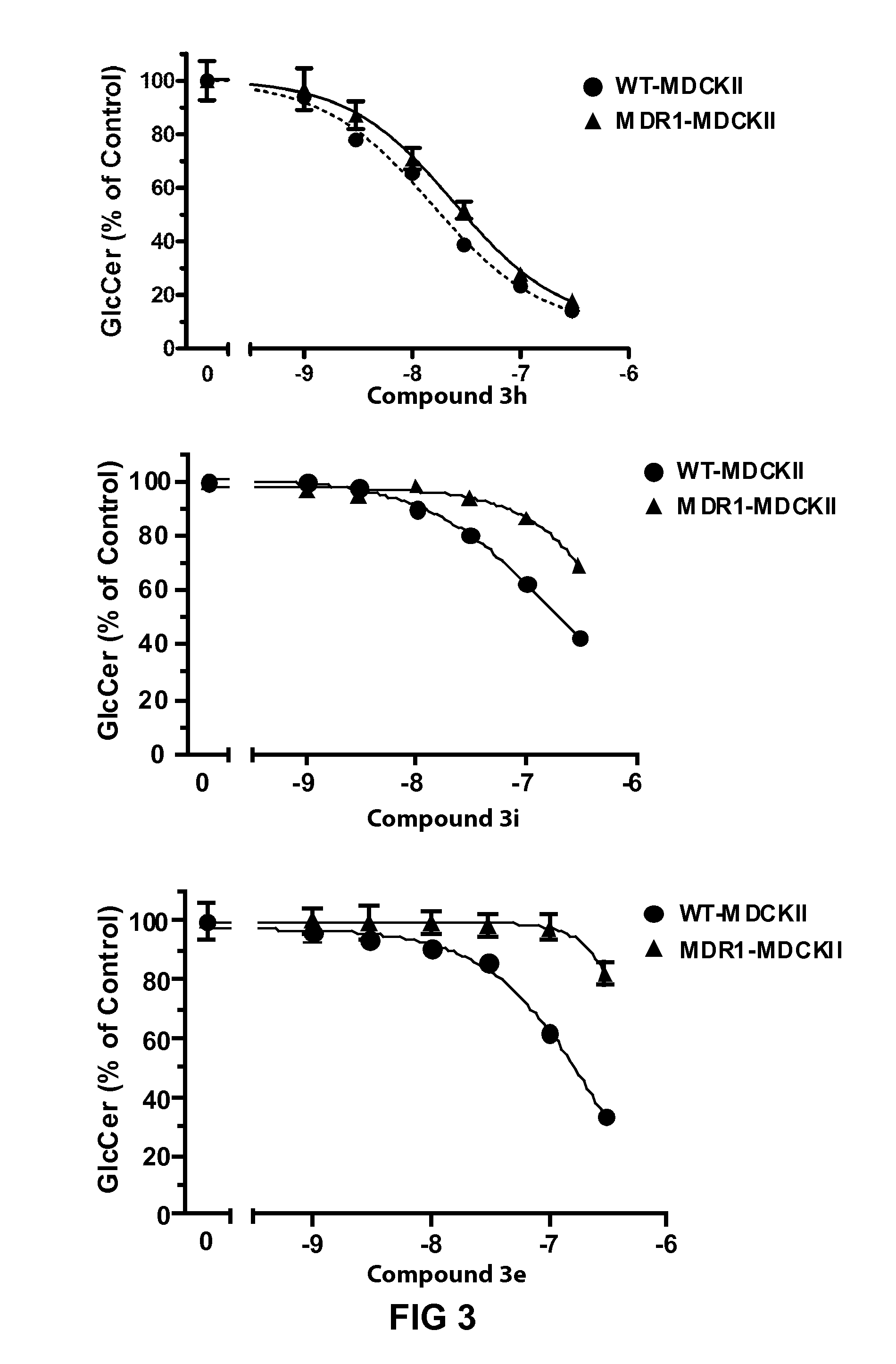
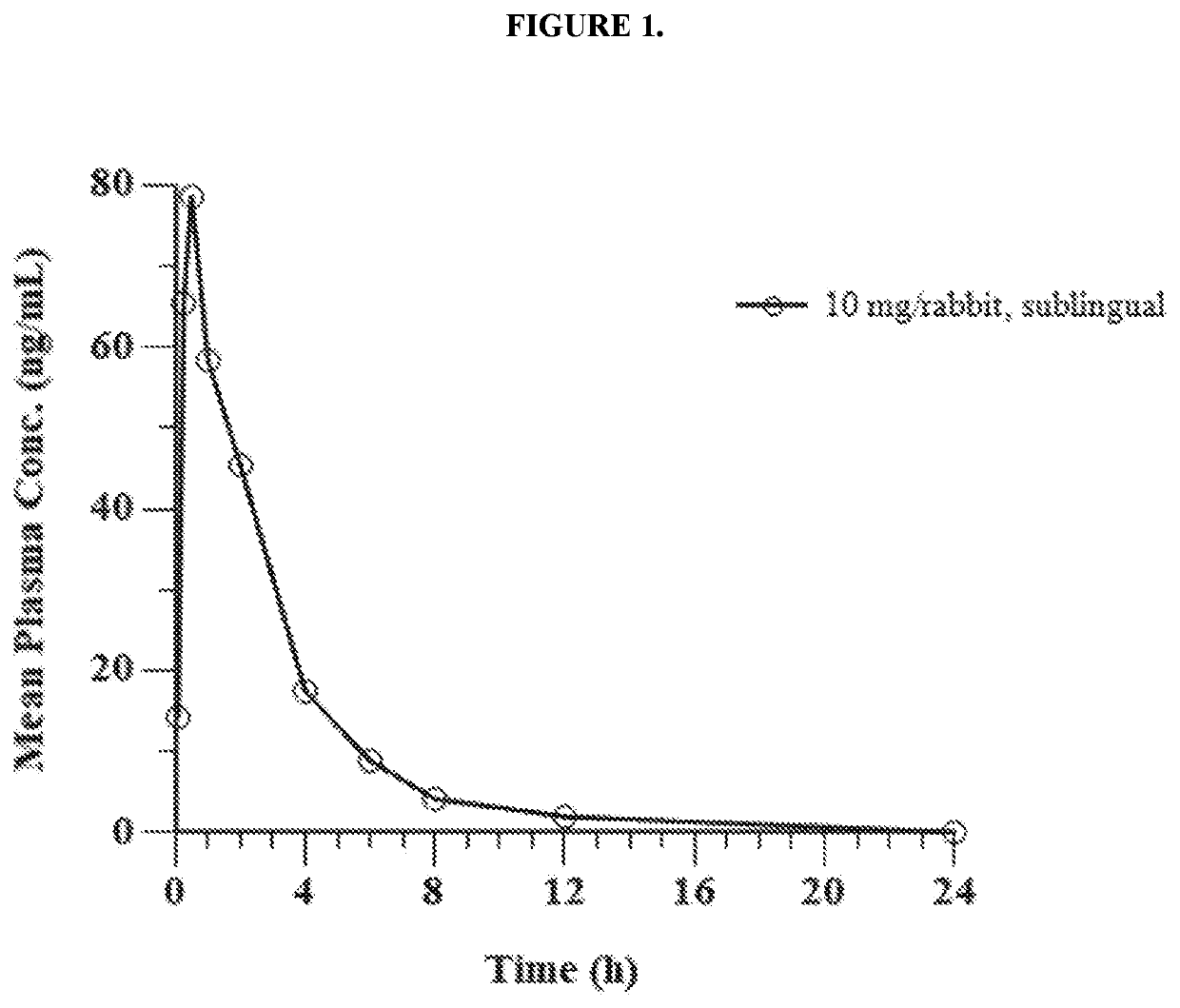
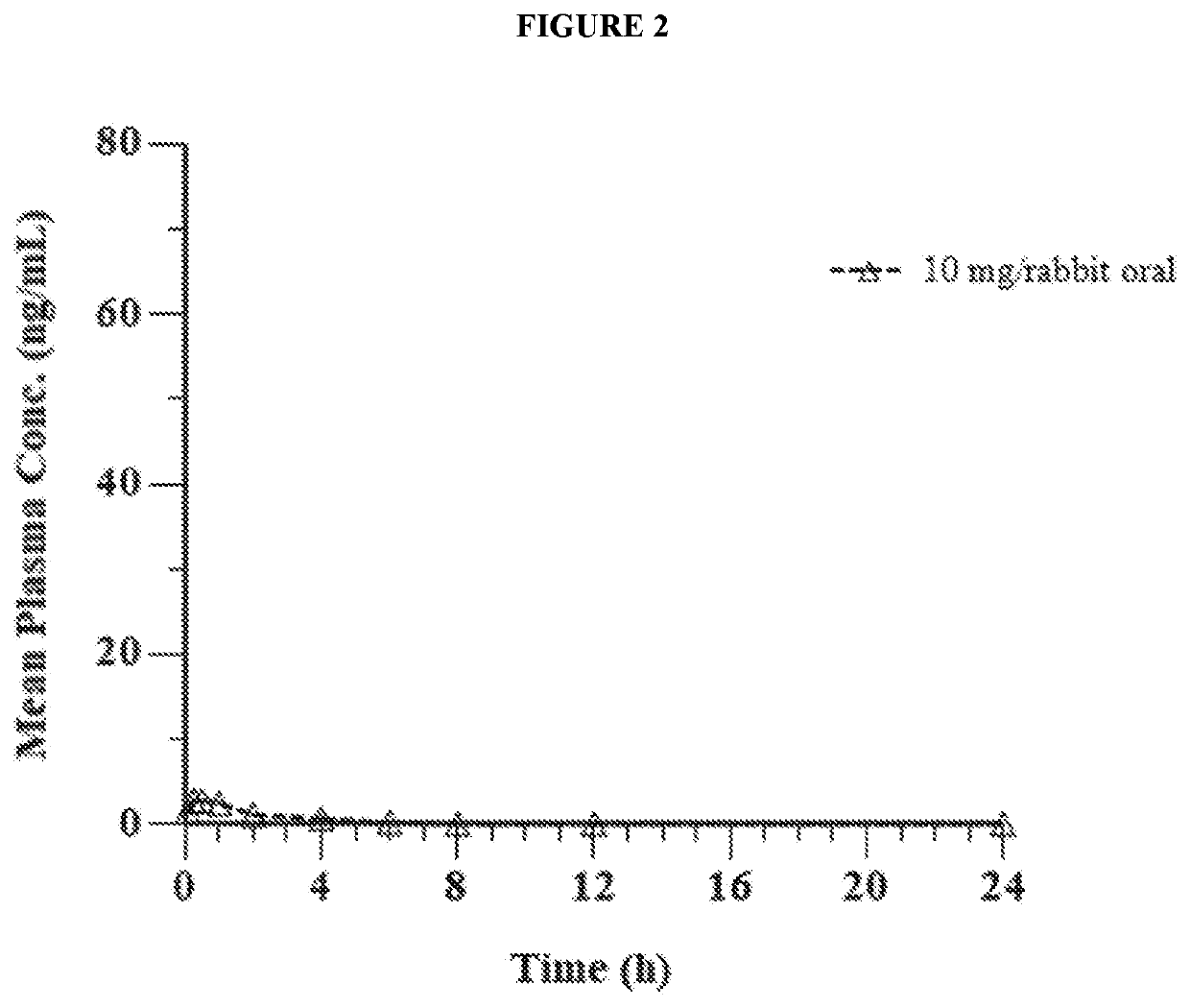
Pharmaceutical composition comprising eliglustat
PendingUS20200222310A1Improve bioavailabilityImproved pharmacokinetic profileOrganic active ingredientsPharmaceutical non-active ingredientsSchindler diseaseEliglustat
The present invention relates to a pharmaceutical composition comprising glucosylceramide synthase inhibitor and a one or more pharmaceutically acceptable excipients. The present invention specifically relates to a sublingual pharmaceutical composition of eliglustat or a pharmaceutically acceptable salt thereof and a one or more pharmaceutically acceptable excipients. Moreover, the present invention further relates to a pharmaceutical composition of eliglustat or a pharmaceutically acceptable salt thereof which is used in the treatment of individual with lysozymal storage diseases selected from the group consisting of, Gaucher disease, Sphingolipidoses, Farber disease, Krabbe disease, Fabry disease, Schindler disease, Tay-Sachs disease and Niemann-Pick disease.
Owner:KASHIV BIOSCIENCES LLC
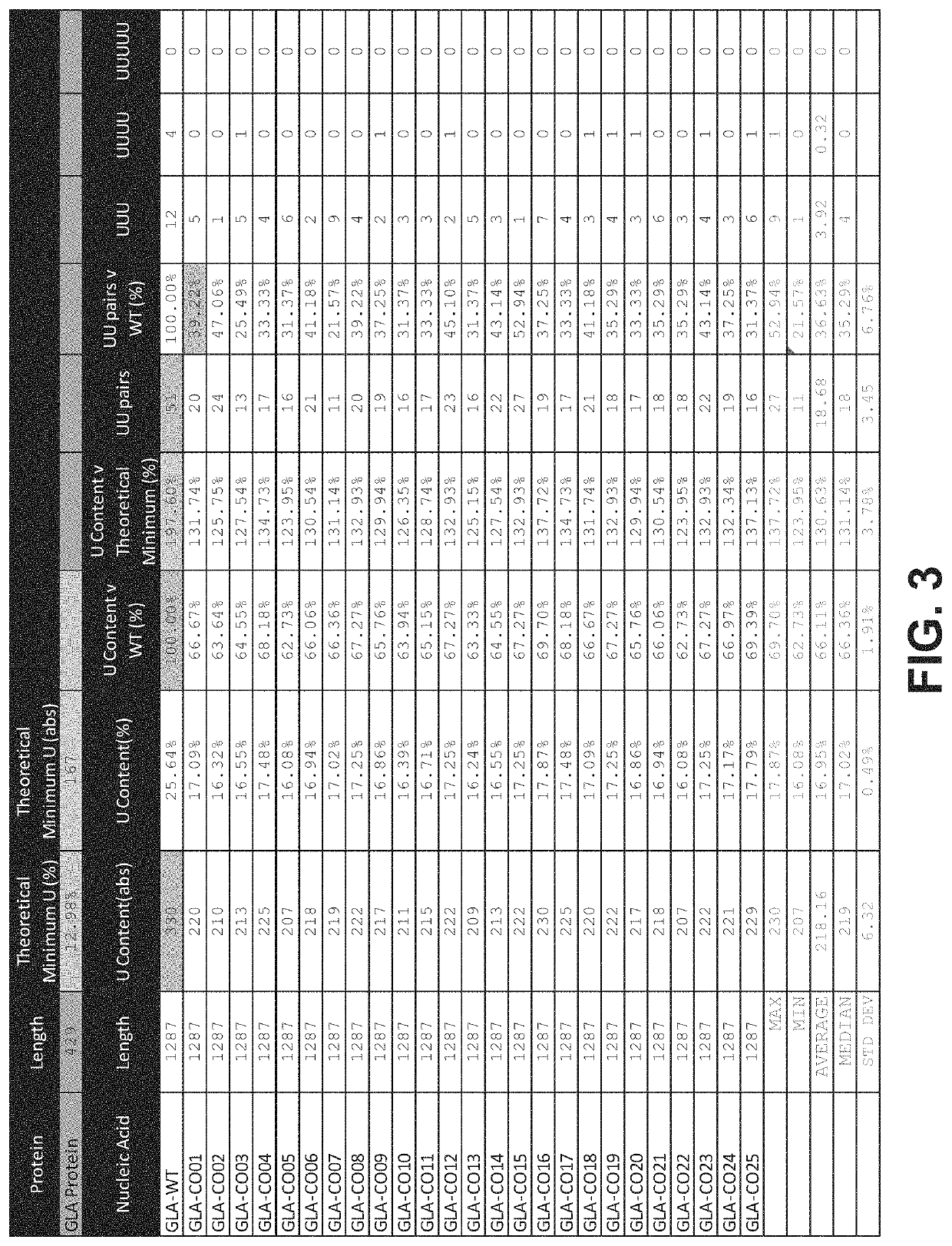
Polynucleotides encoding alpha-galactosidase a for the treatment of fabry disease
ActiveUS20200149052A1Minimize unwanted immune activationImprove translation efficiencyPeptide/protein ingredientsMetabolism disorderMetaboliteNanoparticle
The invention relates to mRNA therapy for the treatment of Fabry disease. mRNAs for use in the invention, when administered in vivo, encode human the α-galactosidase A (GLA), isoforms thereof, functional fragments thereof, and fusion proteins comprising GLA. mRNAs of the invention are preferably encapsulated in lipid nanoparticles (LNPs) to effect efficient delivery to cells and / or tissues in subjects, when administered thereto. mRNA therapies of the invention increase and / or restore deficient levels of GLA expression and / or activity in subjects. mRNA therapies of the invention further decrease levels of toxic metabolites associated with deficient GLA activity in subjects, namely Gb3 and lyso-Gb3.
Owner:MODERNATX INC
N-(5-((aryl or heteroaryl)methyloxy)pentyl)-substituted iminosugars as inhibitors of glucosylceramide synthase
Deoxynojirimycin and deoxygalactonojirimycin derivatives according to the present invention are N-alkylated D-galacto, D-gluco- or L-ido-deoxynojirimycin with a linear methyloxypentyl group bearing various sidegroups and a non-fused bicyclic aromatic group (“X”) on the methyloxy-carbon. These compounds display an increased inhibitory potency towards GCS, and / or an increased inhibitory potency towards GBA2, and / or a decreased inhibitory potency towards GBA1, relative to known deoxynojirimycin derivatives of the same (D-gluco, L-ido or D-galacto) configuration. Therefore, compounds of the present invention are effective in the treatment of diseases which are associated with an irregular level of cytosolic or lysosomal glucosylceramide and / or higher glycosphingolipids, such as a lysosomal storage disorder, such as Gaucher disease, Fabry disease, Tay-Sachs disease, Sandhoff disease, GM1 gangliosidosis, Sialidosis, Niemann Pick disease type C and AMRF, or a symptom of one of the diseases collectively classed as metabolic syndrome, such as obesity, insulin resistance, hyperlipidemia, hypercholesterolemia, polycystic kidney disease, type II diabetes and chronic inflammation, or a neurodenegerative disorder, such as Parkinson disease or Lewy-body dementia.
Owner:LEIDEN UNIVERSITY +1
Treatment of fabry disease
InactiveUS20100144008A1Promote degradationReduce concentrationPeptide/protein ingredientsDrug compositionsFabry diseasePathogenic factor
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Vector encoding therapeutic polypeptide and safety elements to clear transduced cells
InactiveUS20160074533A1Efficient removalReduce riskBiocideAntibody mimetics/scaffoldsPhosphorylationThymidylate kinase
A composition comprising: a stably integrating delivery vector; a modified mammalian thymidylate kinase (tmpk) activator polynucleotide wherein the modified mammalian tmpk polynucleotide encodes a modified mammalian tmpk polypeptide that increases phosphorylation of a prodrug relative to phosphorylation of the prodrug by wild-type mammalian tmpk polypeptide to a drug; and / or a targeting polynucleotide encoding a cell surface polypeptide that selectively binds a toxic binding agent. The disclosure also relates to use of these compositions in methods of treatment of diseases such as Fabry disease.
Owner:UNIV HEALTH NETWORK
Detection kit for alpha-galactosidase activity
PendingCN112176026AQuick assistRapid Auxiliary DiagnosisMicrobiological testing/measurementBiological material analysisVenous bloodFabry disease
The invention discloses a detection kit for alpha-galactosidase activity, and belongs to the field of biochemical detection. The detection kit comprises a reaction solution, a precipitant, a stop solution, a linear standard substance and a reference substance, wherein the linear standard substance is a 4-methylumbelliferone solution, and the reaction solution is a 4-methylumbelliferone acyl-alpha-D-galactoside solution. The kit can rapidly and accurately carry out in-vitro detection on the activity of alpha-galactosidase in a human blood sample, and can achieve the purpose of rapidly, conveniently and accurately assisting in diagnosing Fabry disease patients in batches by detecting a collected filter paper dry blood slice sample or a venous blood sample; and the kit is high in detection sensitivity, good in selectivity, strong in anti-interference capability, good in reproducibility, simple and convenient to operate, good in product stability and easy to use clinically.
Owner:诺道中科(北京)生物科技有限公司
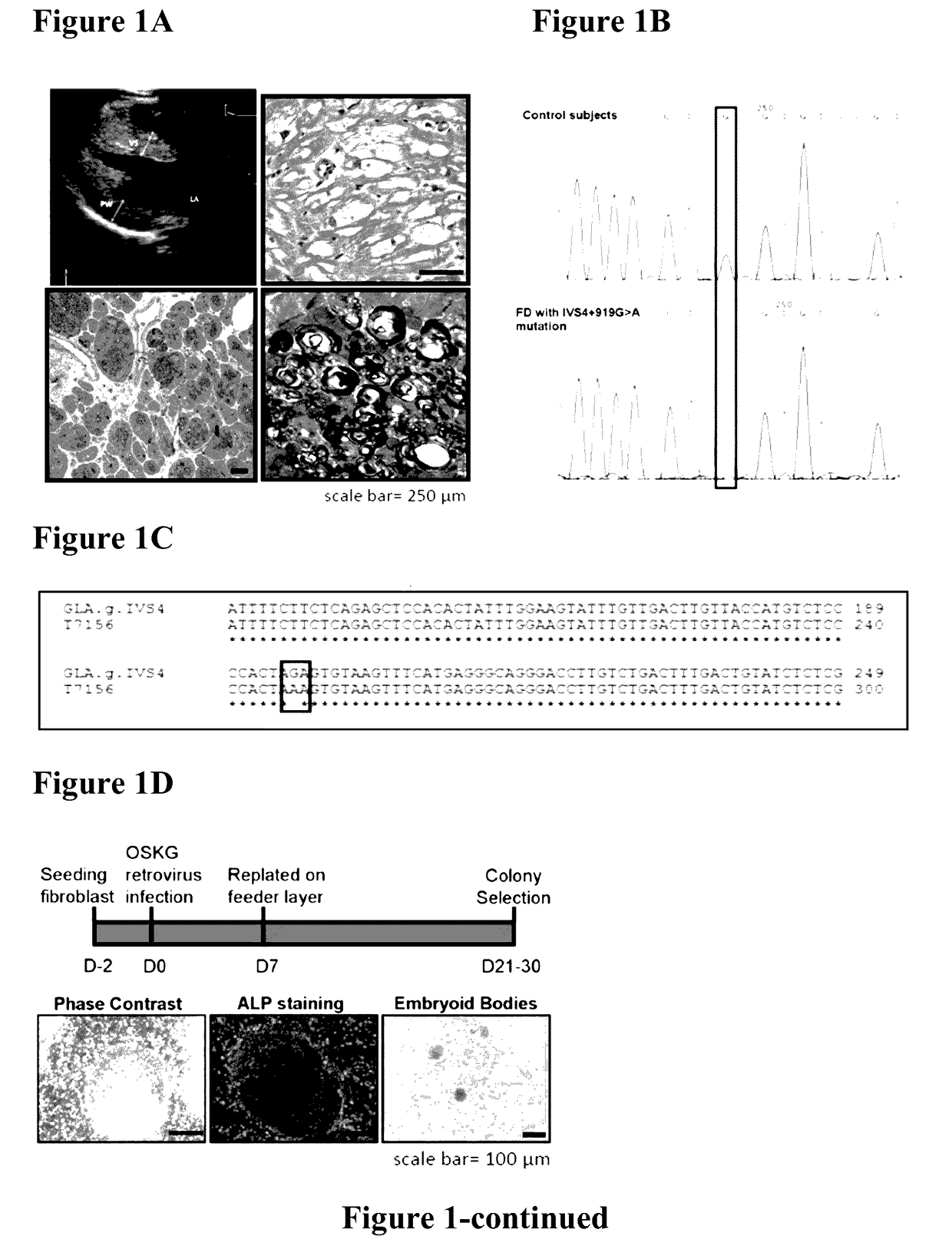
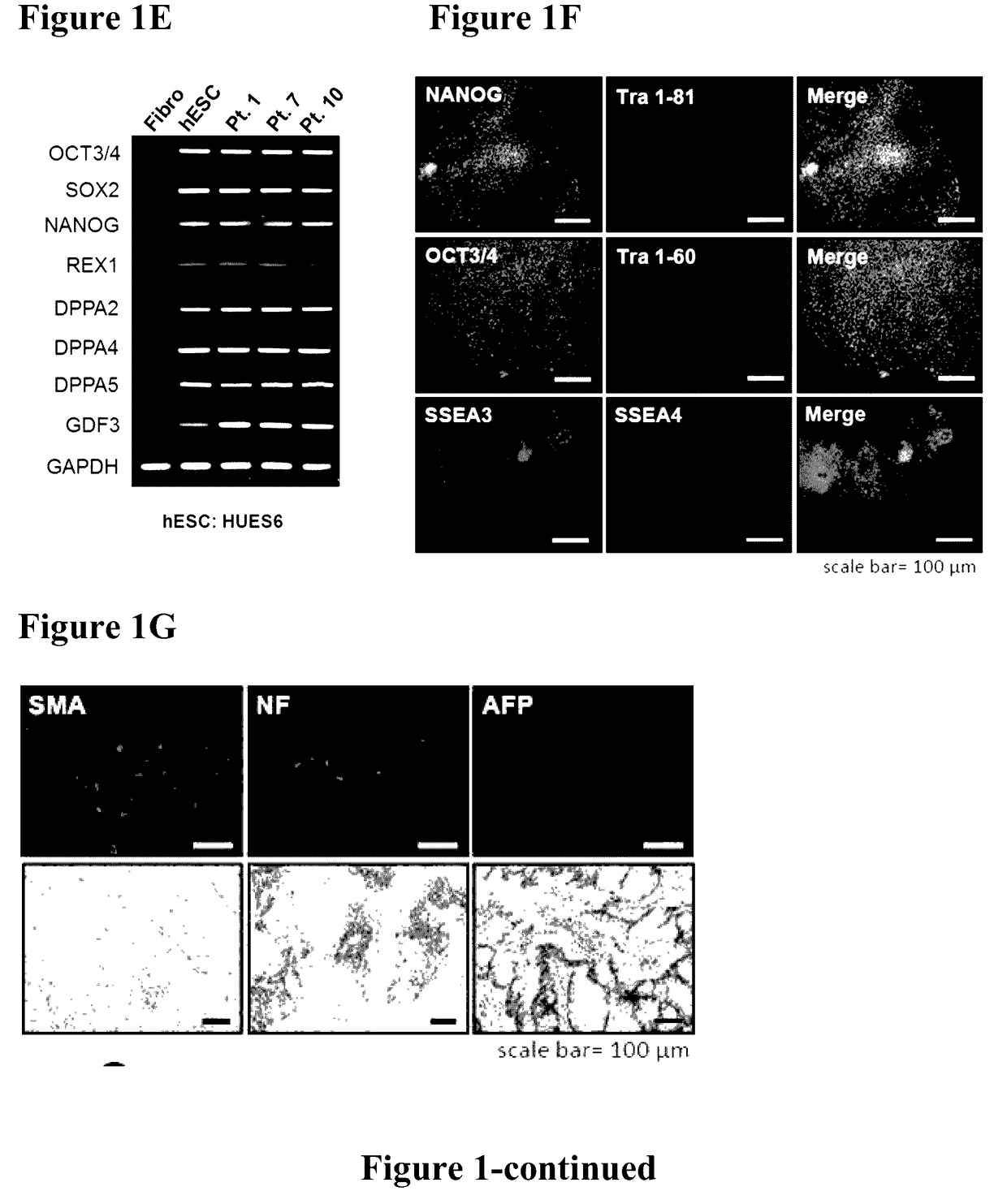
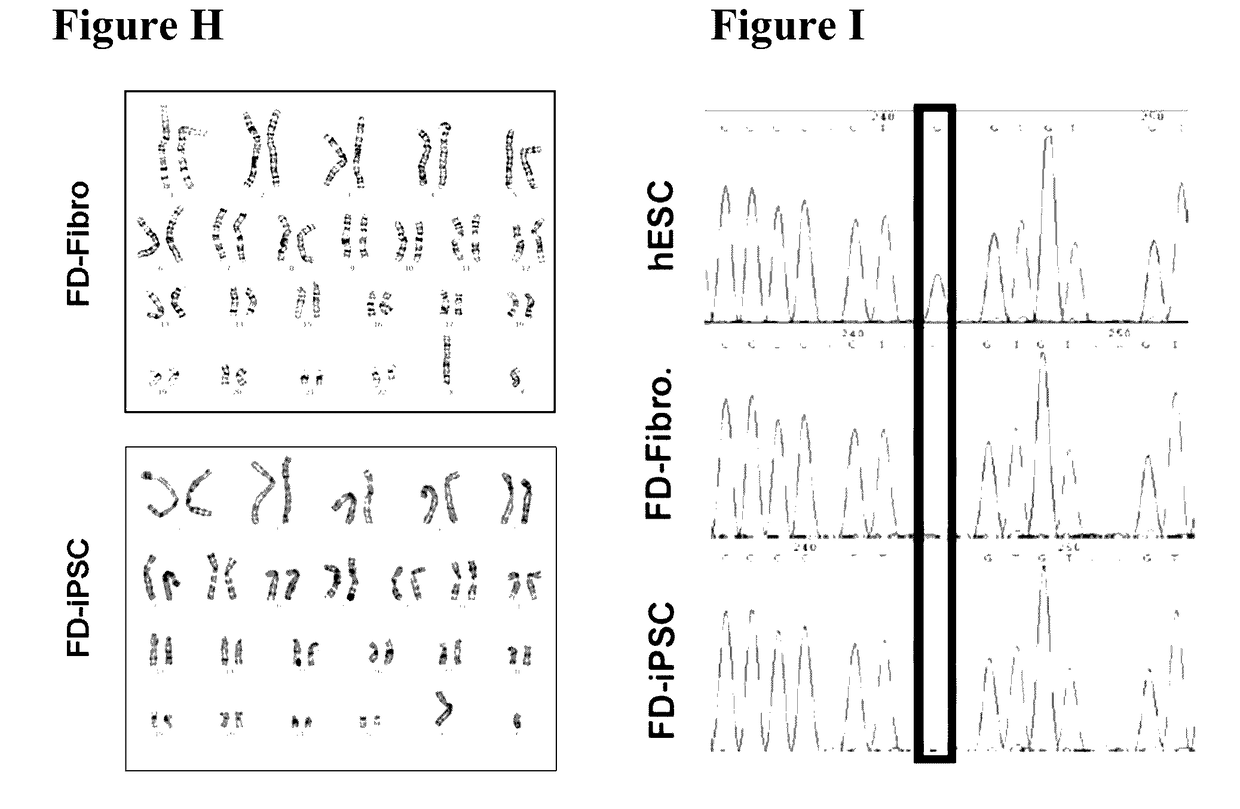
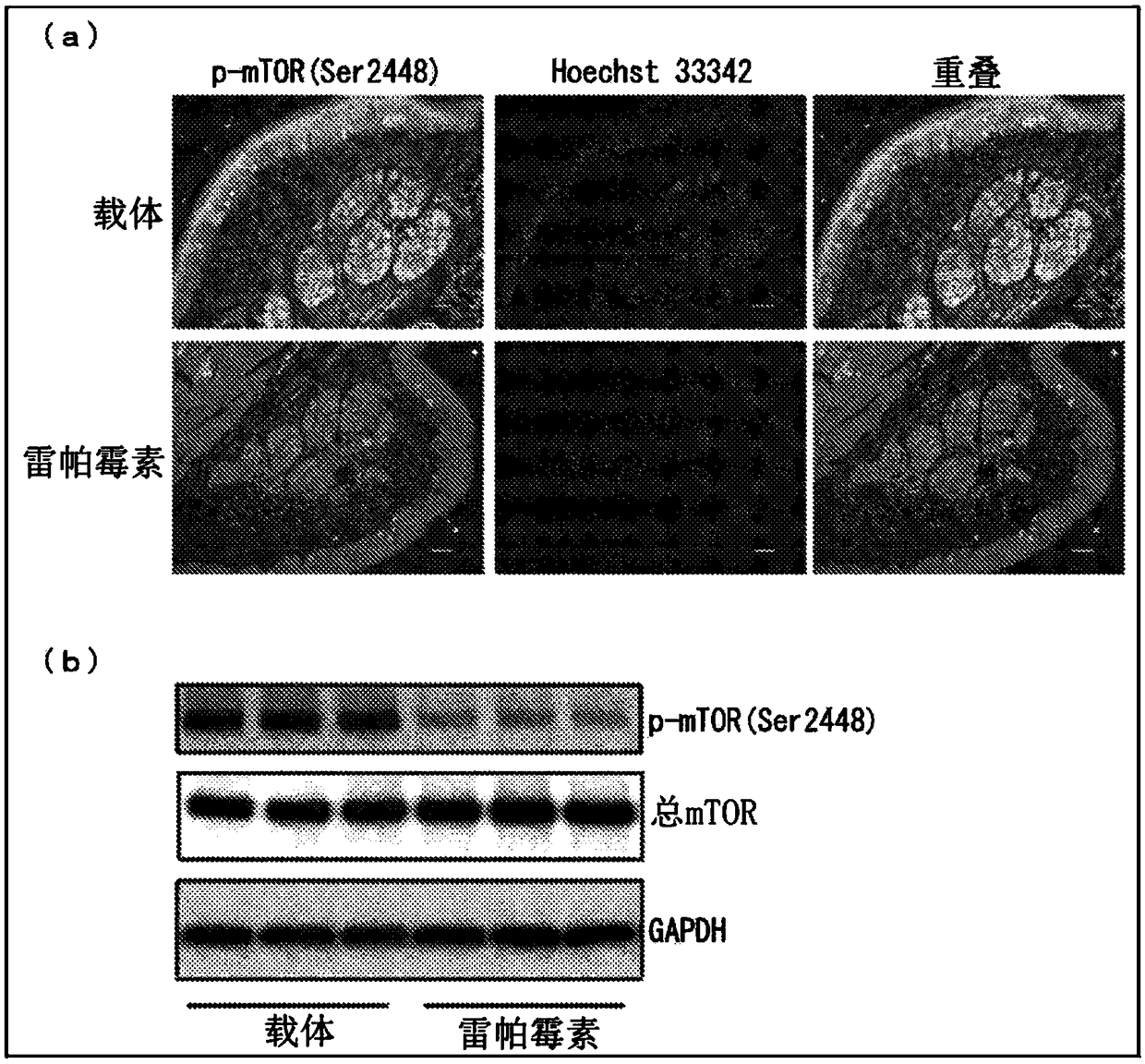
Method for preparing induced pluripotent stem cells
InactiveUS20170130205A1Improve the situationOrganic active ingredientsPeptide/protein ingredientsFabry diseaseSomatic cell
The present invention relates to a novel method for preparing induced pluripotent stem cells (iPSCs) by introducing four genes, Oct-4, Sox2, Klf4, and Glial, into somatic cells. The present invention also relates to the iPSCs produced by the aforementioned method. Also provided is a process of drug selection for a heritable genetic disease by use of the iPSCs produced by the aforementioned method. In particular, wherein the inherited disease is Fabry disease. The present invention also relates to a method for treating Fabry-associated myocardiopathy in a subject in need thereof, and a method for determining prognosis in a subject with Fabry-associated myocardiopathy.
Owner:VETERANS GEN HOSPITAL TAIPEI
Methods and compositions for the treatment of fabry disease
The present disclosure provides expression constructs comprising a GLA transgene encoding the at least one alpha-Gal A protein for use in expressing alpha-Gal A proteins and preventing, inhibiting or treating Fabry disease or one or more symptoms associated with Fabry disease.
Owner:SANGAMO BIOSCIENCES INC
Adeno-associated viral vectors for treatment of Fabry disease and uses thereof
PendingCN114507692AVirus peptidesPharmaceutical delivery mechanismFabry diseaseNucleotide sequencing
The invention discloses a recombinant adeno-associated virus (AAV) vector, which comprises an AAVX capsid and a vector genome, and the vector genome comprises a nucleotide sequence for coding functional alpha-galactosidase A and a regulatory sequence for guiding the expression of the alpha-galactosidase A sequence in a host cell. The invention also discloses application of the recombinant adeno-associated virus vector to treatment of Fabry disease.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
Agent for treating fabry disease, analgesic for external use and perspiration accelerator
InactiveCN108697691APain relief and reliefSpeed up sweatingOrganic active ingredientsNervous disorderPerspirationFabry disease
Owner:OSAKA UNIV
Pharmaceutical combination for treatment of fabry disease and use thereof
InactiveUS20200115698A1Easy to usePeptide/protein ingredientsMetabolism disorderFabry diseasePharmaceutical drug
The present invention provides a pharmaceutical combination for treatment of Fabry disease and use thereof. The present invention relates to a pharmaceutical combination for treating Fabry disease, and the pharmaceutical combination includes (i) a protein which has mutations in an amino acid sequence of α-N-acetylgalactosaminidase and has α-galactosidase activity and (ii) an active site specific chaperone.
Owner:MEIJI PHARMA UNIVERSITY
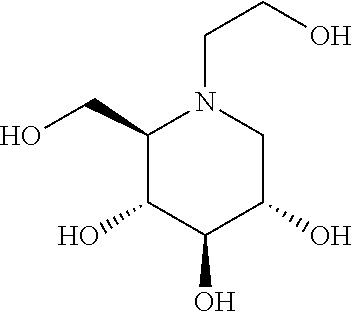
Treatment of Fabry disease
ActiveUS10995067B2Ameliorate and alleviate and mitigate and prevent symptomAmeliorate ,Organic chemistryPeptide/protein ingredientsLysosomeChemical compound
Disclosed herein are novel uses of a polyhydroxylated pyrrolidine for the manufacture of a medicament for treating Fabry disease (FD). Accordingly, the present disclosure provides a method of treating a subject having or suspected of having FD. The method includes the step of, administering to the subject a therapeutically effective amount of a compound of formula (I), a salt, an ester or a solvate thereof, wherein: R1 is H, or C1-3 amine optionally substituted with —COR2; R2 is alkyl or alkene optionally substituted with cycloalkyl or phenyl having at least one substituent selected from the group consisting of, halo, alkyl, haloalkyl, and alkoxyl; so as to ameliorate, alleviate mitigate and / or prevent symptoms associated with the FD. According to preferred embodiments of the present disclosure, the compound of formula (I) is a chaperon of a mutated human lysosomal α-galactosidase A (α-Gal A).
Owner:ACAD SINIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com