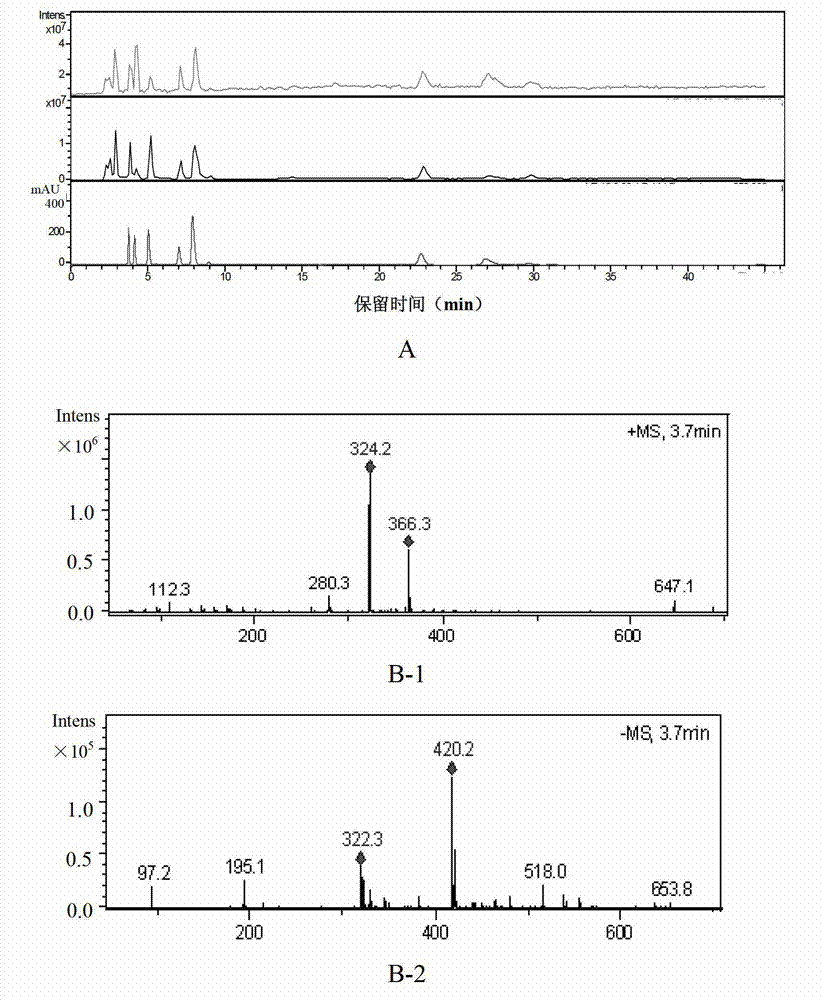
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Uridine monophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Uridine monophosphate (UMP), also known as 5′-uridylic acid (conjugate base uridylate), is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid with the nucleoside uridine. UMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase uracil; hence, it is a ribonucleotide monophosphate. As a substituent or radical its name takes the form of the prefix uridylyl-. The deoxy form is abbreviated dUMP. Covalent attachment of UMP (e.g. to a protein such as adenylyltransferase) is called uridylylation (or sometimes uridylation).
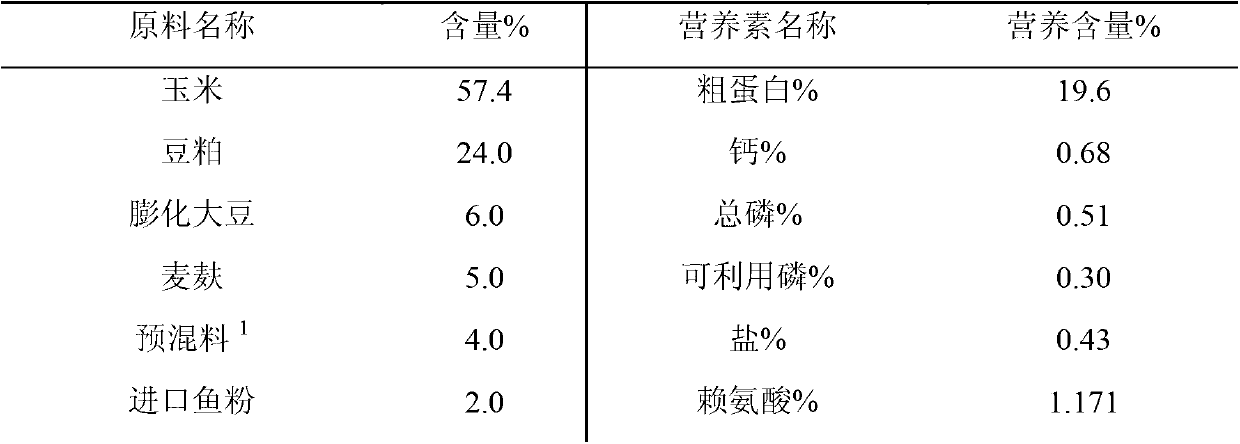
Weaned piglet nucleotide feed additive
InactiveCN102987169AImprove antioxidant capacityEnhance immune functionAnimal feeding stuffBiotechnologyGuanosine monophosphate
The invention discloses a weaned piglet nucleotide feed additive. The weaned piglet nucleotide feed additive comprises, by weight, 0.8 to 1.3 of adenosine monophosphate 5'-AMP, 0.3 to 0.8 of cytidine monophosphate 5'-CMP, 1.1 to 1.5 of guanosine monophosphate 5'-GMP and 0.7 to 1.4 of uridine monophosphate 5'UMP. The invention further discloses a preparation method and an application of the feed additive. According to the weaned piglet nucleotide feed additive, stress reaction of weaned piglets can be effectively relieved, the piglet grazing rate and the feed utilization efficiency are improved, the oxidation resistance and the immune function of weaned piglets are improved apparently, the organic resistance is enhanced, and accordingly, important significances are provided for modern scientific, intensive and large-scale pig production.
Owner:NANJING BIOTOGETHER
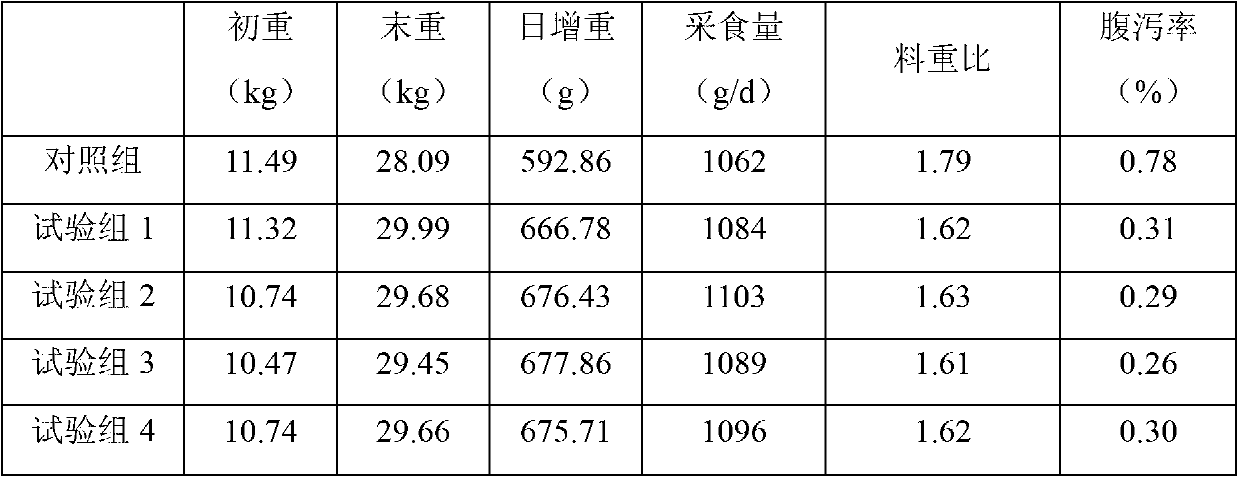
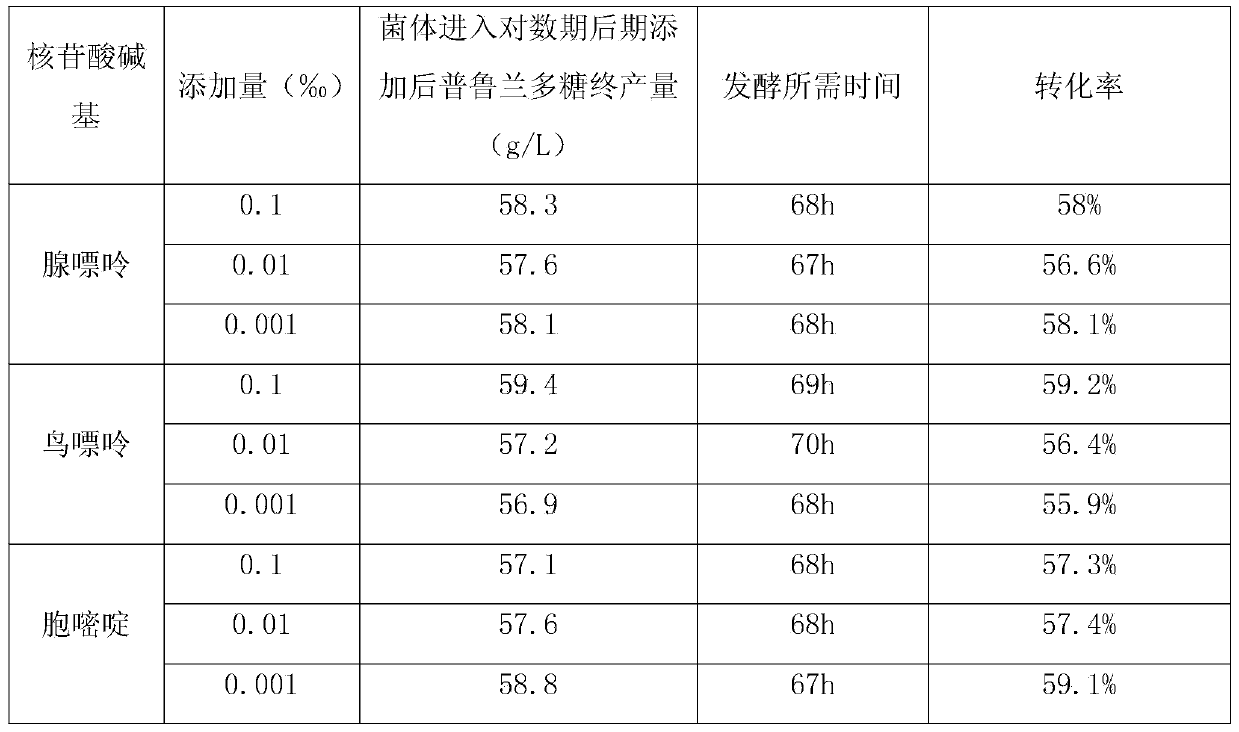
Method for increasing pulullan yield
InactiveCN103695500ALow costImprove conversion rateMicroorganism based processesFermentationBiotechnologyPullulan
The invention discloses a method for increasing pulullan yield by sub-sectional adding of growth factors in a fermentation process, and belongs to the technical field of biological fermentation engineering. The method comprises the following steps: producing pulullan from aureobasidium pullulans CGMCC NO.7055 in a fermentation manner; adding 0.003-0.005% of vitamin B1 when bacteria growth is at the beginning of the logarithmic phase; and adding 0.002-0.004% of uridine monophosphate when the bacteria growth is at the early stage of a stable stage, so as to induce and facilitate accumulation of secondary metabolite, namely the pulullan. The growth factors are specifically added according to the characteristics of sugar production in different growth periods and fermentation processes, so that the method is convenient and fast to operate and has an obvious effect; the fermentation period is greatly shortened, the transformation rate of a substrate is increased, and the cost of the pulullan is reduced.
Owner:天津北洋百川生物技术有限公司
Method of preparing phosphate
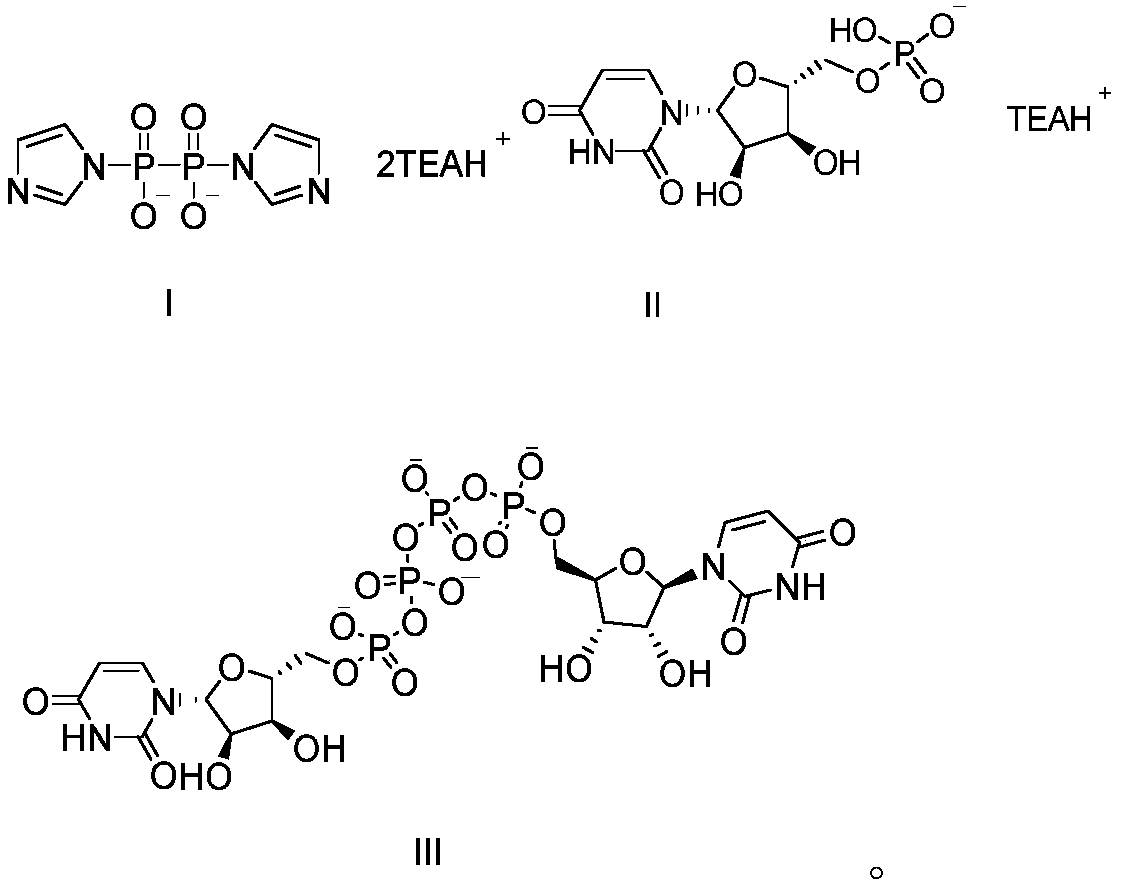
The invention provides a method of preparing a phosphate. The method comprises the following step: enabling a pyrophosphate active compound expressed by formula II to react with uridine monophosphate expressed by a formula III or a salt thereof in a hydrophilic solvent under the action of a bimetallic ion composite catalyst to obtain P1,P4-bis (5'-uridine group) tetraphosphate expressed by formula I. In the formula II, X is imidazolyl, N-methyl imidazolyl, or 1, 2, 4-triazolyl; and the bimetallic ions in the bimetallic ion composite catalyst are a combination of any two of Zn2+, Mn2+, Mg2+, Fe2+, Fe3+ and Al3+. The method of preparing a phosphate employs a bimetallic catalytic system and can achieve high-efficiency and easy separation preparation of diquafosol.
Owner:CHANGCHUN PUHUA PHARMA
Method for preparing uridine diphosphate
InactiveCN1962875AReduce the burden onShorten the timeMicroorganism based processesFermentationYeastPhosphoric acid
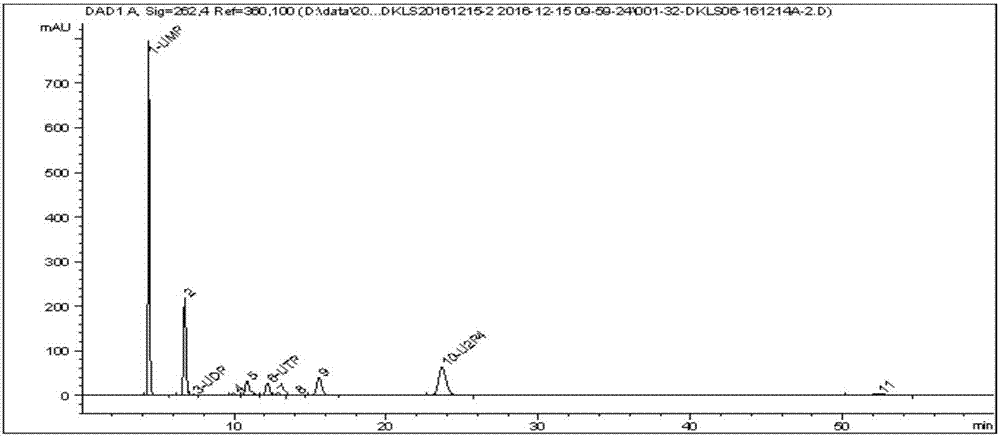
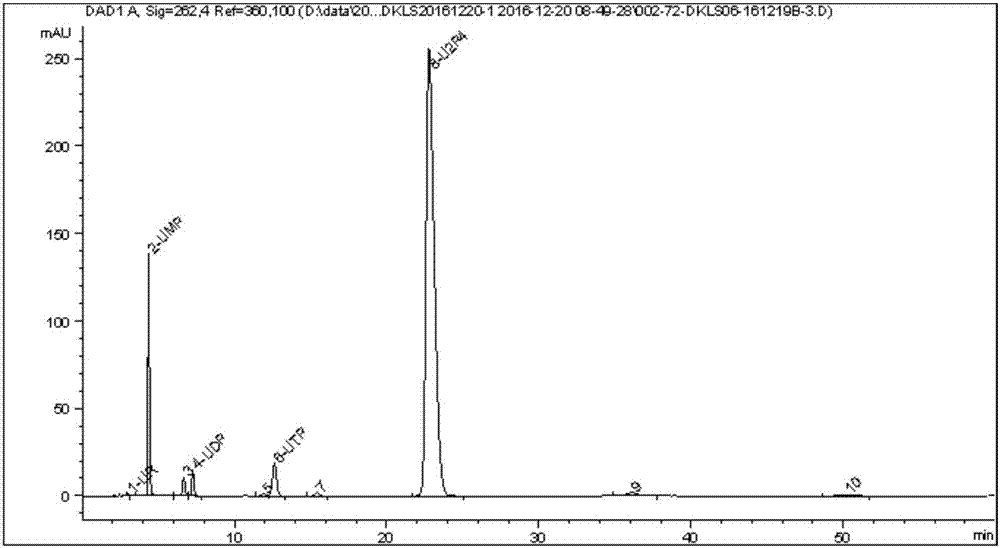
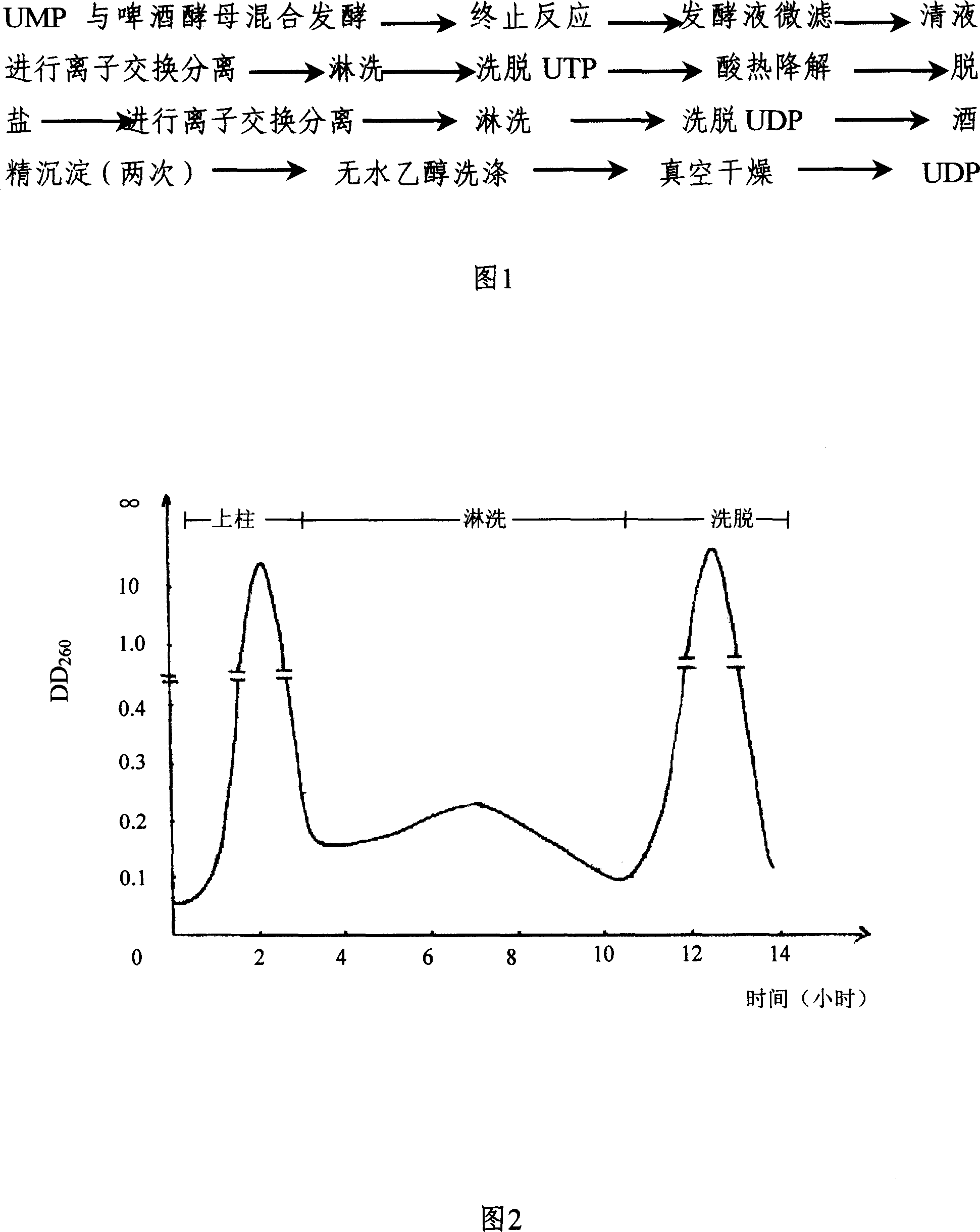
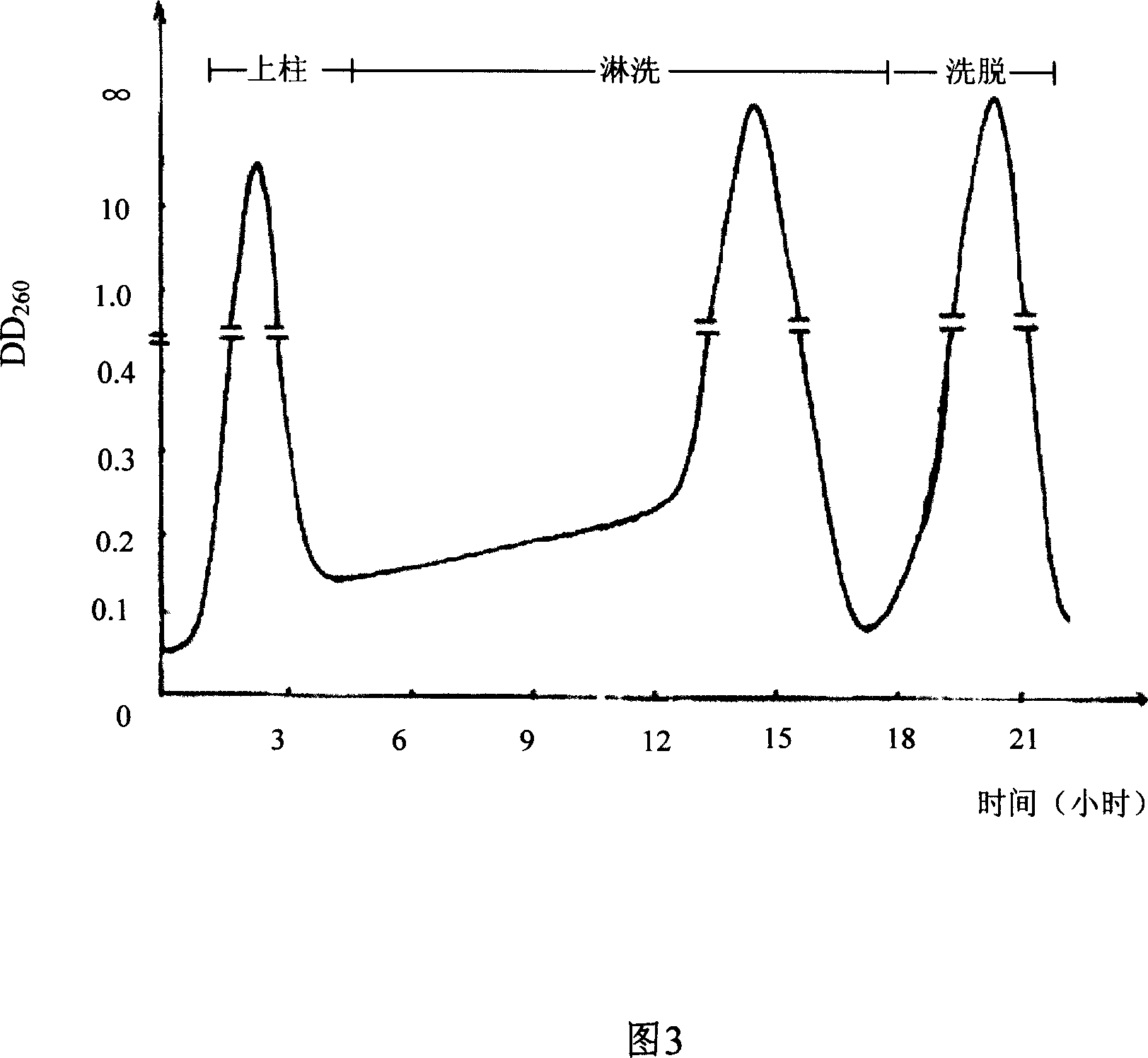
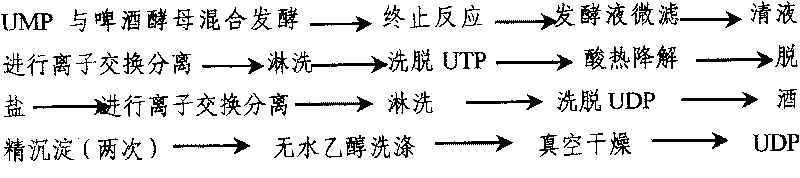
The invention discloses a preparing method of uridine diphosphite, which comprises the following steps: blending uridine monophosphate and beer yeast to ferment; terminating fermenting to obtain the ferment liquid of uridine triphosphate; predisposing ferment liquid; separating and purifying; proceeding acid heat to decompose purified uridine triphosphate; filtering; separating; refining. The invention shortens the predisposing time by two thirds and separating purifying time by one third, which makes receiving rate by over 30%.
Owner:北京燕京中科生物技术有限公司
Nucleotide probiotic capsule for delaying senescence
ActiveCN113615837AAvoid Oxidative DamageExtend your lifeOrganic active ingredientsDigestive systemBiotechnologyAdenosine 5 monophosphate
The invention discloses a nucleotide probiotic capsule for delaying senescence, and belongs to the technical field of medicine and health care. The nucleotide probiotic capsule comprises a capsule shell and a content, wherein the content comprises four exogenous nucleotides and five probiotics; the exogenous nucleotides comprise 5'-cytidine monophosphate (5'-CMP), 5'-adenosine monophosphate (5'-AMP), 5'-disodium uridine monophosphate (5'-UMPNa2) and 5'-disodium guanylate (5'-GMPNa2); the probiotics comprise lactobacillus acidophilus, lactobacillus plantarum, lactobacillus casei and the like; and the content of the probiotics is greater than 1 * 10 <7>CFU / g. The nucleotide probiotic capsule for delaying senescence can remove free radicals in a body, inhibit oxidative damage of the free radicals to the body and prolong the cell life, has multiple health care effects of delaying senescence, prolonging life, improving immunity, regulating intestinal flora and the like, is convenient to carry and take, and is a health care food suitable for middle-aged and elderly people.
Owner:陈玉松
Controlling the texture of high-protein nutritional compositions comprising micellar casein
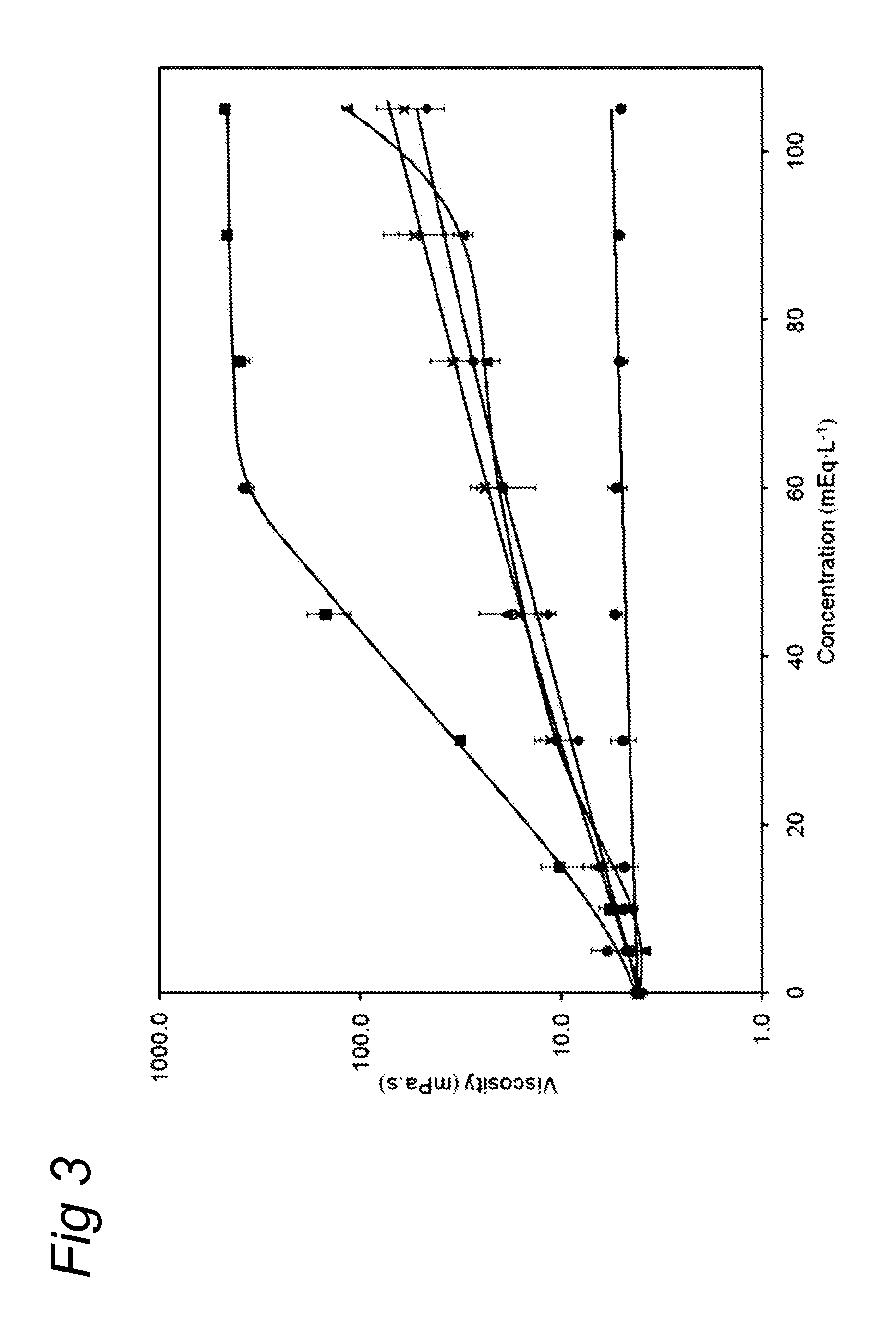
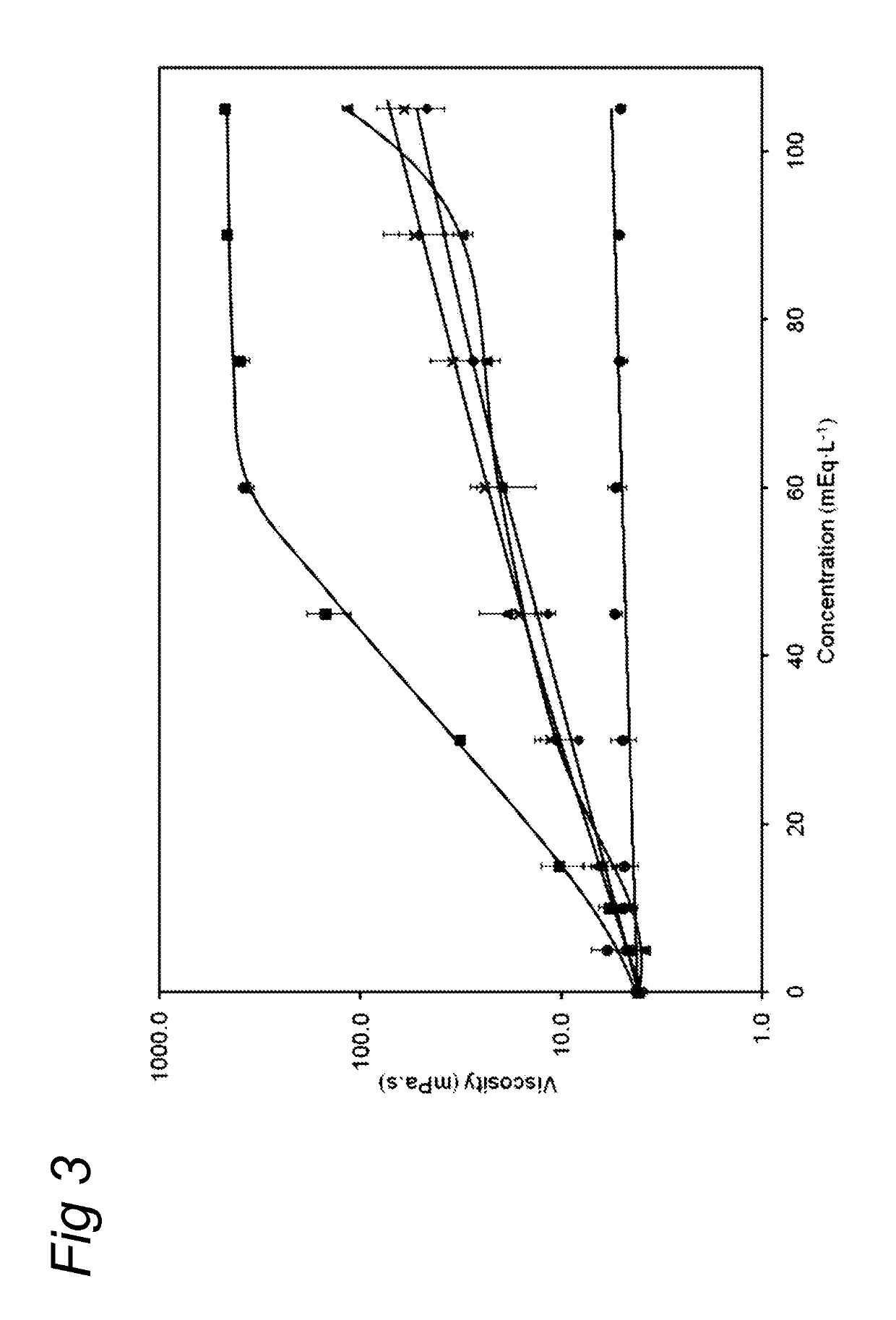
ActiveUS20130065824A1Low viscosityReduce consumptionPeptide/protein ingredientsMetabolism disorderHigh concentrationCitrate salt
Medical dairy products are highly concentrated in proteins and minerals. Formulation of such products is challenging, since viscosities can easily increase during processing and storage. It was found that using one or more chelating agents selected from the group consisting of a phosphoric acid, citric acid, a soluble phosphate salt, a soluble citrate salt, or a mixture thereof, the viscosity and the transparency of an aqueous micellar casein composition, comprising 6 to 20 g / 100 ml of micellar casein and having a pH of about 6 to 8 could be controlled independently of each other. It was found that products become more viscous after addition of phytate, citrate, or orthophosphate, and that the viscosity depends on concentration and type of phosphate. Addition of hexametaphosphate leads to gel formation. In contrast, high concentrations of uridine monophosphate can be added without significantly affecting the viscosity.
Owner:NV NUTRICIA
Simplified representative unicellular whole-genome database creating method and application thereof
InactiveCN108148830AReduce error rateMicrobiological testing/measurementLibrary creationGenome databaseSmall cell
The invention provides a better and suitable simplified representative unicellular whole-genome database creating method and application thereof. On one hand, the method can be effectively applied totrace samples or samples with smaller cell population to perform simplified genome database creation by optimizing primers and amplifying steps; on the other hand, by means of enzyme correction treatment, an enzyme treating step capable of specifically resecting uridine monophosphate can be applied to a simplified genome unicellular sample amplifying method, and amplification error rate can be remarkably reduced. Compared with the prior art, the method creatively applies an optimized and improved unicellular database creating scheme to simplified genome database creation of the trace samples or the samples with the smaller cell population; meanwhile, compared with the prior art, the method has the advantages of higher efficiency, simpleness, practicability, accuracy, small loss, low cost,good method repeatability, low error rate, great suitability for the simplified genome unicellular whole-genome amplification of the trace samples or the samples with the smaller cell population and ability in widening sample application ranges; meanwhile, the error rate is reduced, and detection accuracy is improved.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
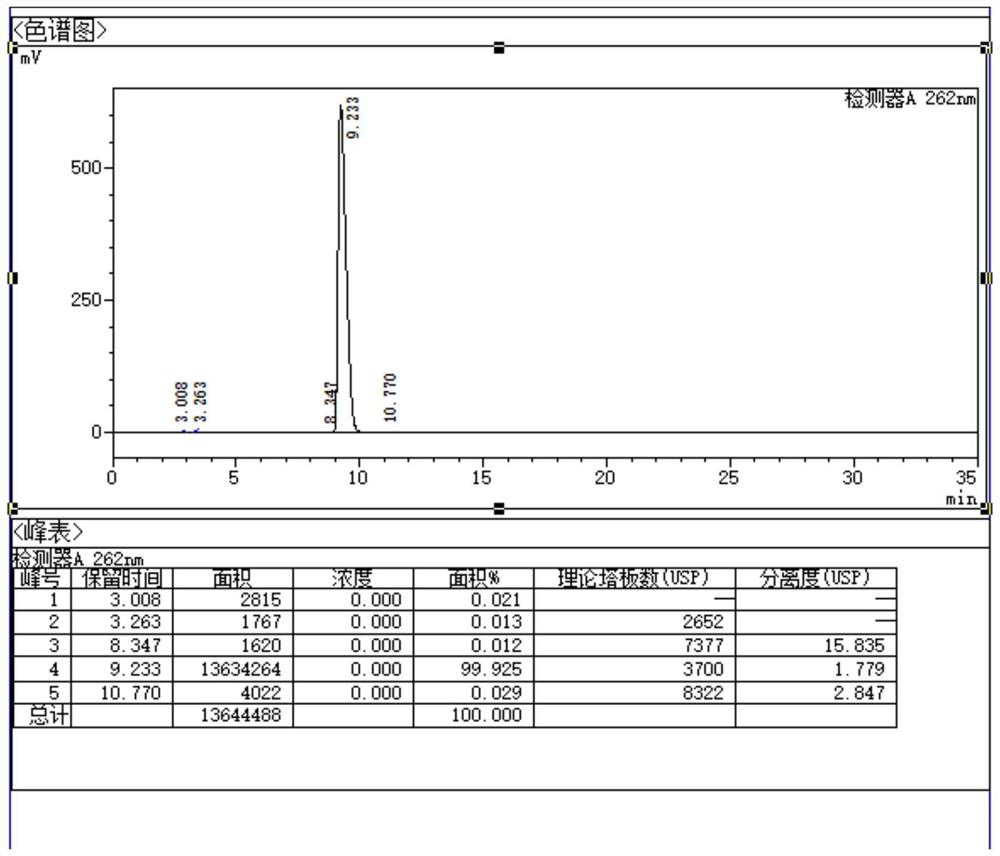
Quality control method for ribonucleic acid II for injection
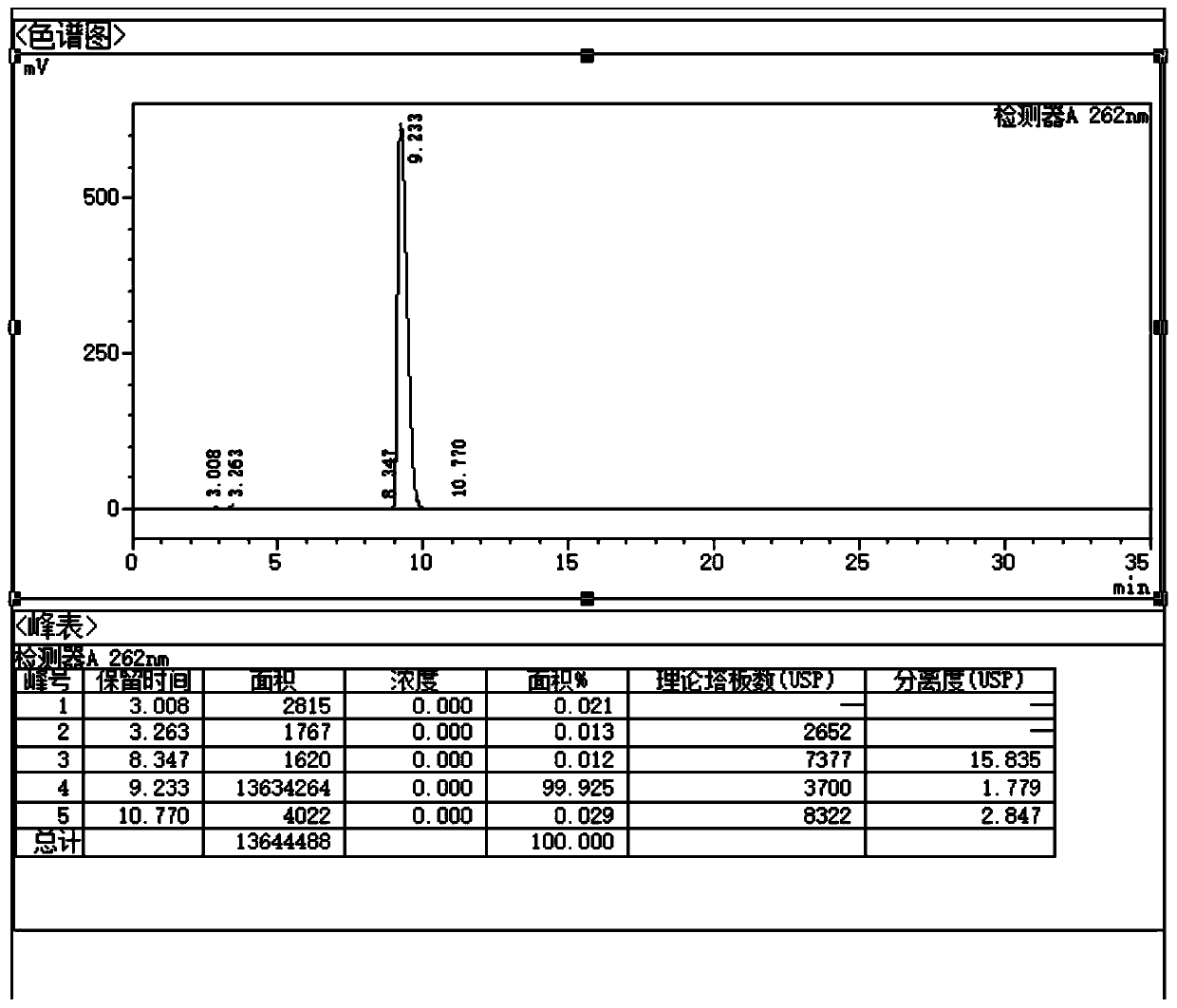
ActiveCN102818867AHigh technology contentImprove securityComponent separationInternational marketHydrolysis
The invention discloses a quality control method for ribonucleic acid II for injection. The method comprises the following steps that the nucleic acid enzyme hydrolysis solution of a substance to be measured is subjected to high-performance liquid chromatogram analysis, an adopted chromatographic column is an Agilent ZORBAX SB-AQC18 chromatographic column, and a flowing phase is a mixture of a formic acid solution and an acetonitrile solution; after the analysis, if the substance to be measured is determined to contain five substances as follows: cytidylate, uridine monophosphate, guanine nucleotide, guanosine and adenosine, the substance to be measured is the ribonucleic acid II for injection or is the ribonucleic acid II for injection as a candidate; and if not, the substance to be measured is not the ribonucleic acid II for injection or is not the ribonucleic acid II for injection as the candidate. A high-performance liquid chromatographic technique is utilized, and the strong-specificity quality control method for the ribonucleic acid II for injection is established. The method has important meanings on increasing the technological content of the medicine, increasing the safety and effectiveness, reducing the cost, enlarging the production scale, increasing the market occupancy, and going forward to the international market.
Owner:JILIN AODONG PHARMACEUTICAL INDUSTRY GROUP YANJI CO LTD
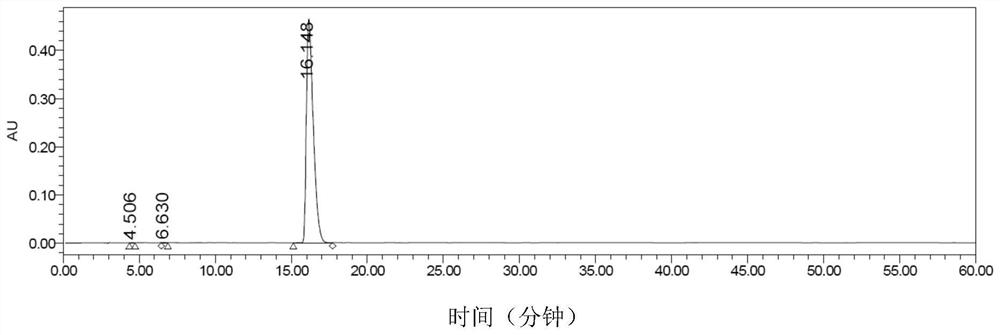
Preparation method of phosphate ester
ActiveCN110590887AHigh yieldRaw materials are cheap and readily availableSugar derivativesSugar derivatives preparationPhosphateTM compound
The invention discloses a preparation method of phosphate ester, and relates to the technical field of pharmaceutical chemicals. Uridine monophosphate or salt thereof and a pyrophosphoric acid activecompound are used, and under the action of a metal ion catalyst Ca<2+>, Mn<2+> or Mg<2+>, high-purity U2P4 can be obtained with high yield in large-scale industrial production.
Owner:江苏金殳医药科技有限公司
Primer composition for detecting harmful gene of deficiency of uridine monophosphate synthase of cattle, kit with primer composition and application of kit
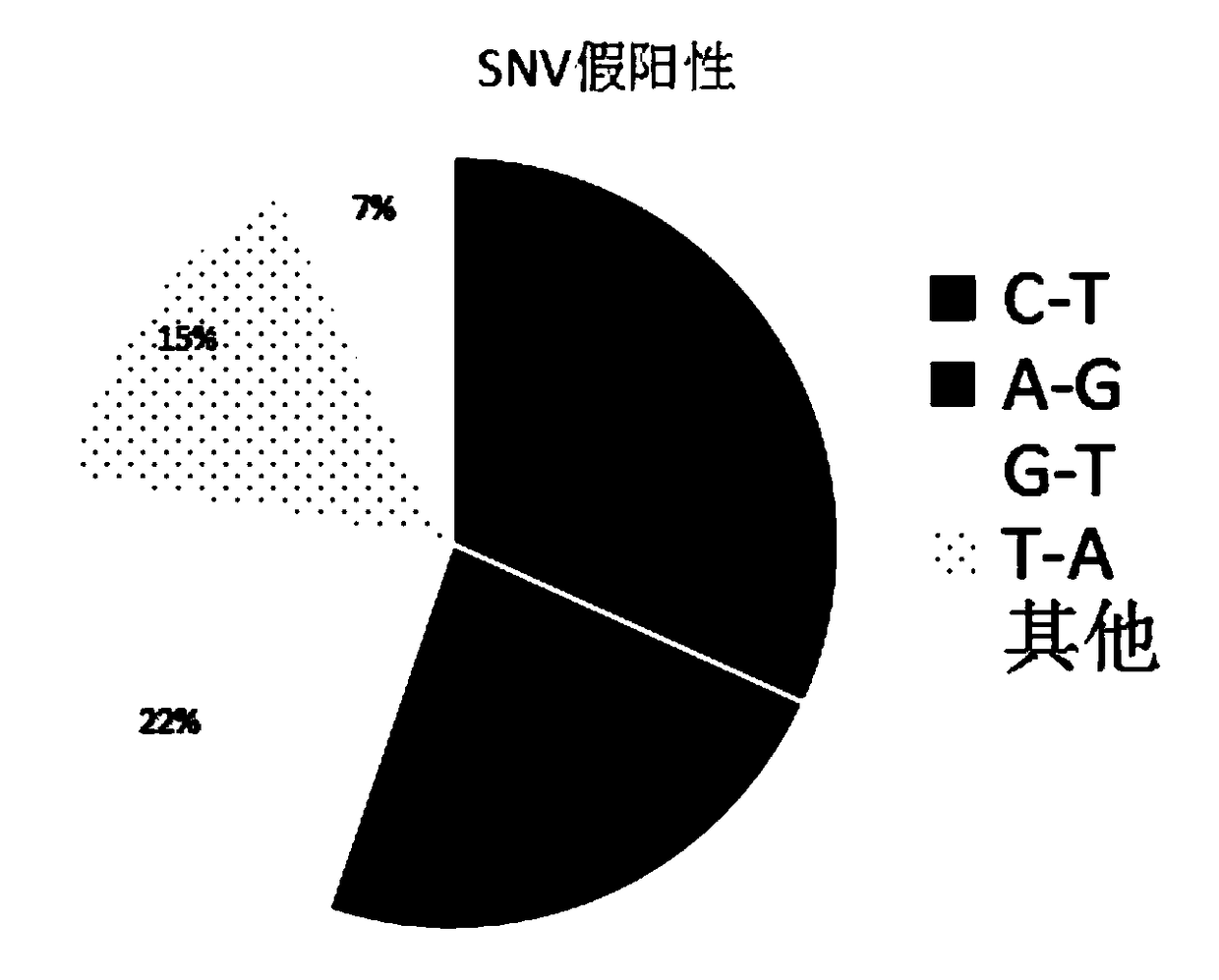
ActiveCN103627804AStrong amplification specificityHigh amplification efficiencyMicrobiological testing/measurementDNA/RNA fragmentationNucleotide sequencingUridine monophosphate
The invention discloses a primer composition for detecting a harmful gene of deficiency of uridine monophosphate synthase of cattle, a kit with the primer composition and an application of the kit. The primer composition disclosed by the invention is composed of a primer group A and a primer group B, wherein the primer group A is composed of a primer 1 and a primer 2, the primer group B is composed of a primer 3 and a primer 4, and the nucleotide sequences of the primer 1, the primer 2, the primer 3 and the primer 4 are respectively shown as SEQIDNO. 1-4. The invention also provides the kit with the primer composition. The method for applying the kit disclosed by the invention to the detection of the harmful gene of the deficiency of uridine monophosphate synthase of cattle comprises the steps of extracting the complete set of DNA (Deoxyribonucleic acid) in cattle blood as a template to carry out nested PCR (Polymerase Chain Reaction) amplification to obtain a PCR product, and sequencing the obtained PCR product so as to directly know about the basic group change on a mutation site according to a sequenced result, thereby ensuring the accuracy of the result and meeting the requirements of a detecting technology for characteristics such as high speed, precision, high throughput and the like.
Owner:SOUTH CHINA AGRI UNIV
Chilled fresh meat freshness marker and screening and prediction model fitting method and application thereof
ActiveCN111830181AAccurate detectionHigh resolutionComponent separationCharacter and pattern recognitionBiotechnologyMetabolite
The invention discloses a metabonomics-based chilled fresh meat freshness marker and a screening and prediction model fitting method and application thereof. The marker is prepared from indole-3-formaldehyde, uridine monophosphate, phenylmercaptouric acid, gluconic acid, tyramine and serine-phenylalanine. The prediction model is: Y=3.964+1.97E<-7>X1- 4.22E<-7>X2-3.37E<-7>X3+8.80E<-8>X4+1.26E<-8>X5-5.57E<-7>X6. According to the method, an Agilent 1290 UHPLC is connected with a Q Exactive Orbitrap high-resolution mass spectrum in series, the method has higher resolution, more substances can be detected more accurately, metabolites in the preservation process of the chilled fresh chicken can be illustrated more comprehensively, and the obtained result is more reliable.
Owner:YANGZHOU UNIV
Recombinant microorganism for producing uridine and method for producing uridine
PendingCN113755414ARealize large-scale industrial productionReduce manufacturing costBacteriaMicroorganism based processesRibonucleosidePhosphorylation
The invention provides a recombinant microorganism for producing uridine and a method for producing uridine by using the recombinant microorganism. Wherein the uridine is degraded and knocked out by using genes, and the genes encode ribonucleoside hydrolase, uridine phosphorylase, nucleoside phosphorylase, cytidine / uridine kinase and nucleoside transporter protein. Meanwhile, overexpression is carried out on key enzymes in a uridine biosynthetic pathway, including cytidine triphosphate pyrophosphorylase for degrading cytidine triphosphate to cytidine monophosphate and uridine monophosphate phosphorylase for catalyzing uridine monophosphate to uridine. In addition, a pyrimidine nucleoside pathway is subjected to genetic engineering modification, and feedback inhibition of a synthetic pathway is relieved. The recombinant strain can reach the uridine yield of 20 g / L or above in a 5-liter fermentation tank through a biological fermentation method, industrial mass production can be achieved, meanwhile, the uridine production cost is low, pollution is reduced, the method is green and environmentally friendly, and the method has high application and popularization value.
Owner:SUZHOU BIOSYNTHETICA CO LTD
Screening method and application of uridine monophosphate modified protein in mitochondria
InactiveCN111693715AComponent separationBiological testingMass Spectrometry-Mass SpectrometryTotal protein
The invention discloses a screening method and an application of uridine monophosphate modified protein in mitochondria. The method is characterized in that biotin-labeled UTP (Biotin-16-UTP) is usedas a raw material, under the action of uridine monophosphate transferase SelO, total mitochondrial protein is subjected to UMP modification reaction, and the protein modified by UMP further has a biotin label, subsequently, the modified protein is screened out by using a biotin-streptavidin Pull-down technology, and finally, the modified protein and a modification site are determined by mass spectrometry. The method is advantaged in that a simple and feasible experimental method is developed, proteins which can be modified by UMP are screened from mitochondrial total proteins, and an importantresearch means is provided for signal transduction mediated by SelO proteins and post-translational modification in mitochondria.
Owner:INST OF BASIC MEDICINE OF SAMS
Oligo dT primer and method for constructing cDNA library
ActiveCN104388426AImprove applicabilityIncrease success rateLibrary creationProtein nucleotide librariesCDNA libraryUracil nucleotide
The invention relates to the field of molecular biology and provides an Oligo dT primer. The Oligo dT primer contains single-chain DNA molecules having a continuous dT sequence, wherein the DNA molecules contain recognition sites used for cutting; and the recognition sites used for cutting refer to uridine monophosphate, deoxyinosine or phosphorothioate bond. The invention also provides a method for constructing a cDNA library on the basis of the Oligo dT primer. The Oligo dT primer disclosed by the invention is high in applicability and can be applied to multiple different library construction schemes; the initiation sites at the polyA tails of mRNA molecules can be accurately positioned; and according to the method for constructing the cDNA library disclosed by the invention, all the mRNA molecules in samples can be specifically reflected in the constructed cDNA library.
Owner:盛司潼
Compound nucleotide immunoenhancer for paralichthys olivaceus
The invention relates to a compound nucleotide immunoenhancer for paralichthys olivaceus, which is composed of the following substances in percentage by weight: 55-65% of cytidylate, 5-15% of uridine monophosphate, 5-15% of thymidine phosphorylase, 1-5% of hypoxanthine nucleotide and 10-20% of bioactive peptide. The compound nucleotide immunoenhancer disclosed by the invention is capable of obviously improving the ingestion and the growth of paralichthys olivaceus, and remarkably improving the cellular immunity, the humoral immunity, the immune gene expression quantity, the disease resistance and the like of paralichthys olivaceus. Moreover, the compound nucleotide immunoenhancer can also be directly added in a feed for paralichthys olivaceus, and orally fed; and the compound nucleotide immunoenhancer has the advantages of being free from toxic and side residues, free from drug resistance, and pollution-free to environment.
Owner:QINGDAO AGRI UNIV
Product and method for supporting uridine homeostasis
InactiveUS9968629B2Avoid renal clearanceMaintain sufficiencyNervous disorderPhosphorous compound active ingredientsUridine monophosphateNeurological disorder
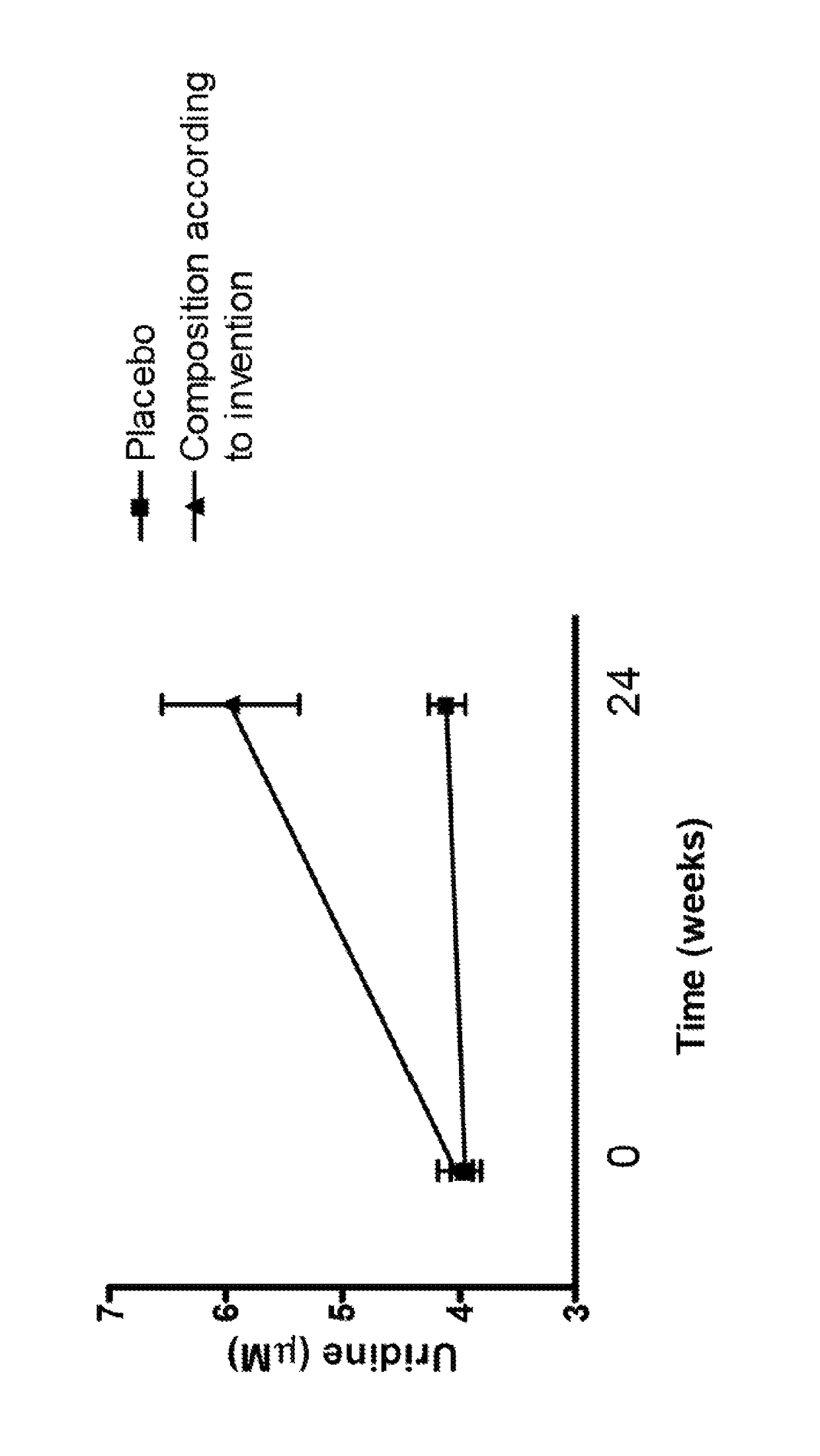
This invention pertains to the use of an uridine source, preferably uridine monophosphate, for increasing, controlling and / or maintaining fasting plasma uridine concentrations in a range of 4 to 8 μM in a subject in need thereof, comprising administering to said subject a composition comprising 300-900 mg of said uridine source daily for a period of at least 4 weeks. In particular, the use of an uridine source is intended forelderly and / or subjects suffering from neurological disorders such as Alzheimer's Disease and dementia syndromes.
Owner:NV NUTRICIA
Method for producing uridine diphosphate glucose and special engineering bacteria for method
ActiveCN112239771ARaw materials are easy to getLow costBacteriaMicroorganism based processesSucrose synthetaseUridine Diphosphoglucose
The invention discloses a method for producing uridine diphosphate glucose and special engineering bacteria for the method. The method for producing the uridine diphosphate glucose comprises the following steps of by using uridine monophosphate and sucrose as raw materials, producing the uridine diphosphate glucose under the action of the engineering bacteria, wherein the engineering bacteria arerecombinant bacteria for expressing functional protein and are obtained by introducing a gene for encoding the functional protein into starting bacteria, and the functional protein comprises polyphosphokinase, uridine monophosphate kinase and sucrose synthase. The method is of great significance to industrial production of the uridine diphosphate glucose.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
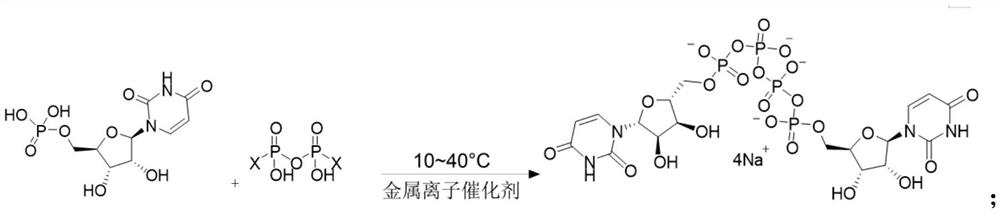
Preparation method of P<1>,P<4>-di(uridine 5'-)tetraphosphate
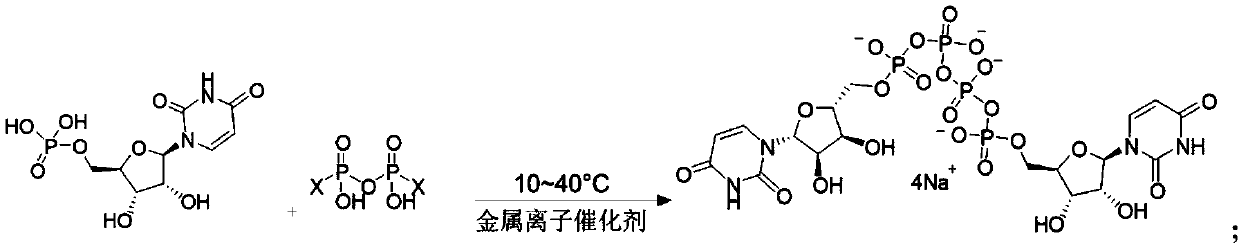
ActiveCN111116694AHigh yieldHigh puritySugar derivativesSugar derivatives preparationPhosphateCombinatorial chemistry
The invention relates to a preparation method of P<1>,P<4>-di(uridine 5'-)tetraphosphate. The method comprises the following steps: under the action of a metal salt catalyst, imidazole triethylamine pyrophosphate shown in formula I and uridine monophosphate triethylamine salt shown in formula II react in N,N-dimethylformamide, and P<1>,P<4>-di(uridine 5'-)tetraphosphate shown in formula III is obtained.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Method for preparing uridine diphosphate
InactiveCN1962875BShorten fermentation timeReduce the burden onMicroorganism based processesFermentationYeastPhosphoric acid
The invention discloses a preparing method of uridine diphosphite, which comprises the following steps: blending uridine monophosphate and beer yeast to ferment; terminating fermenting to obtain the ferment liquid of uridine triphosphate; predisposing ferment liquid; separating and purifying; proceeding acid heat to decompose purified uridine triphosphate; filtering; separating; refining. The invention shortens the predisposing time by two thirds and separating purifying time by one third, which makes receiving rate by over 30%.
Owner:北京燕京中科生物技术有限公司
A method for producing uridine diphosphate glucose and its special engineering bacteria
ActiveCN112239771BRaw materials are easy to getLow costBacteriaMicroorganism based processesSucrose synthetaseGlucose phosphate
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Determination of 5'-nucleotidase activity and its diagnostic reagent kit of 5'-nucleotidase
InactiveCN1778948AStrong specificityLess susceptible to interferenceMicrobiological testing/measurementNucleotidaseAbsorbance
The invention is about a method of measuring the activation of 5í»-phosphonuclease, and it also concerns the reagent box of 5í»-phosphonucleasediagnosis. This invention belongs to the field of medical testing and measuring technology. The reagent box is consisted of buffer solution, uridine monophosphate, reduced coenzyme, uridine phosphorylase, dihydrothymine dehyddrogenase and stabilizer. Firstly, we cause an enzyme-coupled reaction through mixing the sample and the reagent according to a certain proportion of volume; secondly, put the final reactant under the biochemical analyzer and test the absorbance variational situation (speed) of dominant wavelength; then we can get theactivation of 5í»-phosphonuclease. By using this invention, we can get the necessary measuring result with high sensitiveness and fine precision through biochemical analyzer, and the result would not be contaminated by material of internal and exogenous sources. Thus, this method can be conveniently promoted and applied.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Uridine effects on dopamine release
The present invention provides methods for increasing secretion of dopamine and other neurotransmitters and treating or reducing the incidence of diseases involving decreased secretion of dopamine and other neurotransmitters, e.g. Parkinson's disease, comprising administering to the subject a uridine or a source thereof, and compositions for treating or reducing an incidence of Parkinson's disease, comprising a uridine, a uridine monophosphate, or a source thereof.
Owner:MASSACHUSETTS INST OF TECH
Reagent kit for sifting carrier of bovine uridine monophosphate ribozyme deletion disease
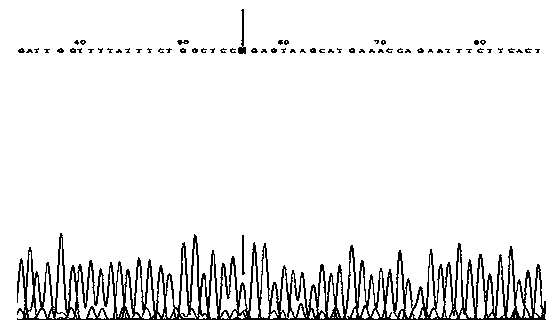
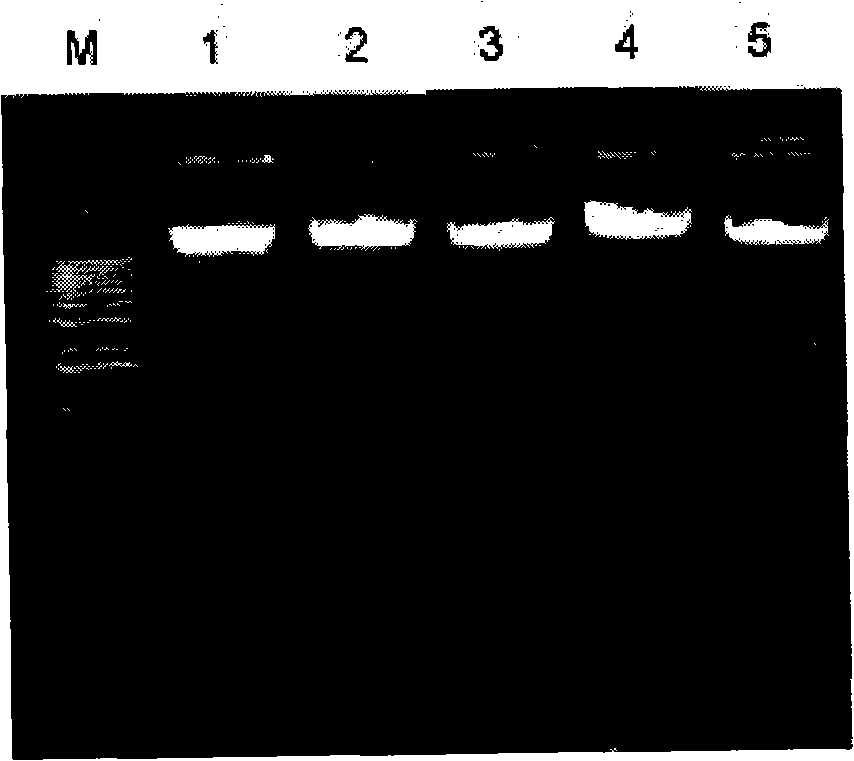
InactiveCN101270393AReduce workloadImprove accuracyMicrobiological testing/measurementGenotypeBiology
The invention discloses a reagent box of screening the carrier of missing uridine monophosphate ribozyme of ox. The reagent box comprises a PCR primer pair and an endonuclease AvaI. The PCR primer pair comprises a primer pair comprising a deoxyribonucleotide with the sequence 1 in the sequence table and a deoxyribonucleotide with the sequence 2 in the sequence table. In the detection method of DUMPS heterozygote on herb based on genome DNA, the PCR-RFLP method is utilized to directly classify the genotype of Holstein cow, the method is not affected by animal age, sex and gene expression time, no radioactivity is used, no collection of comparison sample is required, and not only the determination work is reduced, but also the determination accuracy is promoted. Using the method to screen the carrier of missing uridine monophosphate ribozyme does not need special instrument and is favorable for the requirement of the conventional laboratory detection.
Owner:CHINA AGRI UNIV
A kind of preparation method of phosphoric acid ester
ActiveCN110590887BHigh yieldRaw materials are cheap and readily availableSugar derivativesSugar derivatives preparationPhosphoric Acid EstersPtru catalyst
Owner:江苏金殳医药科技有限公司
p 1 ,p 4 The preparation method of two (uridine 5'-) tetraphosphate
ActiveCN111116694BHigh yieldHigh puritySugar derivativesSugar derivatives preparationPtru catalystPhosphate
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD
Quality control method for ribonucleic acid II for injection
ActiveCN102818867BHigh technology contentImprove securityComponent separationInternational marketHydrolysis
The invention discloses a quality control method for ribonucleic acid II for injection. The method comprises the following steps that the nucleic acid enzyme hydrolysis solution of a substance to be measured is subjected to high-performance liquid chromatogram analysis, an adopted chromatographic column is an Agilent ZORBAX SB-AQC18 chromatographic column, and a flowing phase is a mixture of a formic acid solution and an acetonitrile solution; after the analysis, if the substance to be measured is determined to contain five substances as follows: cytidylate, uridine monophosphate, guanine nucleotide, guanosine and adenosine, the substance to be measured is the ribonucleic acid II for injection or is the ribonucleic acid II for injection as a candidate; and if not, the substance to be measured is not the ribonucleic acid II for injection or is not the ribonucleic acid II for injection as the candidate. A high-performance liquid chromatographic technique is utilized, and the strong-specificity quality control method for the ribonucleic acid II for injection is established. The method has important meanings on increasing the technological content of the medicine, increasing the safety and effectiveness, reducing the cost, enlarging the production scale, increasing the market occupancy, and going forward to the international market.
Owner:JILIN AODONG PHARMACEUTICAL INDUSTRY GROUP YANJI CO LTD
Controlling the texture of high-protein nutritional compositions comprising micellar casein
ActiveUS10264806B2Low viscosityReduce consumptionMetabolism disorderFood ingredient as chelating agentHigh concentrationPhosphoric acid
Medical dairy products are highly concentrated in proteins and minerals. Formulation of such products is challenging, since viscosities can easily increase during processing and storage. It was found that using one or more chelating agents selected from the group consisting of a phosphoric acid, citric acid, a soluble phosphate salt, a soluble citrate salt, or a mixture thereof, the viscosity and the transparency of an aqueous micellar casein composition, comprising 6 to 20 g / 100 ml of micellar casein and having a pH of about 6 to 8 could be controlled independently of each other. It was found that products become more viscous after addition of phytate, citrate, or orthophosphate, and that the viscosity depends on concentration and type of phosphate. Addition of hexametaphosphate leads to gel formation. In contrast, high concentrations of uridine monophosphate can be added without significantly affecting the viscosity.
Owner:NV NUTRICIA
Fluorescence labeling-based uridine monophosphate acidification detection method
PendingCN111781178AShorten experiment timeFluorescence/phosphorescenceMass Spectrometry-Mass SpectrometryFluorescent imaging
The invention discloses a fluorescence labeling-based uridine monophosphate acidification detection method. The method comprises the following steps of: adding YdiU protein and a substrate into a reaction system, mixing the substances uniformly, then adding 1 microliter of CY3-X-UTP, and carrying out UMPylation modification reaction at 30-40DEG C for 1h to obtain UMPylation modified protein with aCY3 label; after adding the UMPylation modified protein into a sample loading buffer solution, using 12% SDS-PAGE gel electrophoresis; and carrying out fluorescence imaging on the buffer solution, and carrying out imaging observation under 565nm emission after 553nm excitation. The method is used for qualitatively identifying the protein possibly subjected to uridine monophosphate modification, can determine a specific modification site by combining mass spectrometry, and can be used for screening an inhibitor of targeted uridine monophosphate transferase.
Owner:INST OF BASIC MEDICINE OF SAMS
Primer composition for detecting harmful gene of deficiency of uridine monophosphate synthase of cattle, kit with primer composition and application of kit
ActiveCN103627804BImprove amplification specificityHigh amplification efficiencyMicrobiological testing/measurementDNA/RNA fragmentationNucleotide sequencingUridine monophosphate
The invention discloses a primer composition for detecting a harmful gene of deficiency of uridine monophosphate synthase of cattle, a kit with the primer composition and an application of the kit. The primer composition disclosed by the invention is composed of a primer group A and a primer group B, wherein the primer group A is composed of a primer 1 and a primer 2, the primer group B is composed of a primer 3 and a primer 4, and the nucleotide sequences of the primer 1, the primer 2, the primer 3 and the primer 4 are respectively shown as SEQIDNO. 1-4. The invention also provides the kit with the primer composition. The method for applying the kit disclosed by the invention to the detection of the harmful gene of the deficiency of uridine monophosphate synthase of cattle comprises the steps of extracting the complete set of DNA (Deoxyribonucleic acid) in cattle blood as a template to carry out nested PCR (Polymerase Chain Reaction) amplification to obtain a PCR product, and sequencing the obtained PCR product so as to directly know about the basic group change on a mutation site according to a sequenced result, thereby ensuring the accuracy of the result and meeting the requirements of a detecting technology for characteristics such as high speed, precision, high throughput and the like.
Owner:SOUTH CHINA AGRI UNIV
Determination of 5'-nucleotidase activity and its diagnostic reagent kit of 5'nucleotidase
InactiveCN1778951AStrong specificityLess susceptible to interferenceMicrobiological testing/measurementNucleotidaseAbsorbance
The invention is about a method of measuring the activation of 5í»-phosphonuclease, and it also concerns the reagent box of 5í»-phosphonucleasediagnosis. This invention belongs to the field of medical testing and measuring technology. The reagent box is consisted of buffer solution, uridine monophosphate, oxidized coenzyme, uridine nucleotide, ribose1-dehyddrogenase and stabilizer. Firstly, we cause an enzyme-coupled reaction through mixing the sample and the reagent according to a certain proportion of volume; secondly, put the final reactant under the biochemical analyzer and test the absorbance variational situation (speed) of dominant wavelength; then we can get the activation of 5í»-phosphonuclease. By using this invention, we can get the necessary measuring result with high sensitiveness and fine precision through biochemical analyzer, and the result would not be contaminated by material of internal and exogenous sources. Thus, this method can be conveniently promoted and applied.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com